Summary
Biological diversity often arises as organisms adapt to new ecological conditions (i.e., ecological opportunities) or colonize suitable areas (i.e., spatial opportunities). Cases of geographical expansion followed by local ecological divergence are well described; they result in clades comprising ecologically heterogeneous subclades. Here, we show that the desert ant genus Cataglyphis likely originated in open grassland habitats in the Middle East ∼18 million years ago and became a taxon of diverse species specializing in prey of different masses. The genus then colonized the Mediterranean Basin around 9 million years ago. The result was the rapid accumulation of species, and the appearance of local assemblages containing species from different lineages that still displayed ancestral foraging specialties. These findings highlight that, in Cataglyphis, ecological diversification preceded geographical expansion, resulting in a clade composed of ecologically homogeneous subclades.
Subject areas: Ecology, Biological sciences, Evolutionary ecology
Graphical abstract
Highlights
-
•
Cataglyphis ants appeared ∼18 MA, likely in open habitats in the Middle East
-
•
Genus lineages accumulated slowly and were different in worker head width ∼9 MA
-
•
Aridification of the Mediterranean Basin promoted genus geographical expansion
-
•
The emerging species conserved ancestral worker head width and foraging ecology
Ecology; Biological sciences; Evolutionary ecology
Introduction
Evolutionary radiation is an event during which there is species proliferation within a taxonomic clade. It is a phenomenon commonly thought to be stimulated by the appearance of new ecological or spatial opportunities.1,2 Ecological opportunities arise when unoccupied niches with novel abiotic and biotic conditions become available3 and can lead to the emergence of multiple, ecologically distinct species from a shared ancestor. Competition plays a crucial role herein, promoting the initial divergence process and driving subsequent niche partitioning. Some well-known examples of these dynamics include Darwin’s finches in the Galapagos4 and cichlids in the African Great Lakes.5 In both cases, the emergence of distinct ecological conditions appears to encourage rapid species accumulation and results in clades composed of phenotypically divergent species. Spatial opportunities, on the other hand, occur when existing species colonize new areas with suitable abiotic and biotic conditions or when the species’ current habitat becomes fragmented.2,6 Geographical isolation may then spur the emergence of multiple species with similar ecological requirements, whose coexistence is precluded by competition. Such dynamics are illustrated by North American woodland salamanders7 and cold-climate lizards.8 In these cases, the accumulation of ecologically equivalent species was apparently facilitated by geographical isolation, leading to clades predominantly composed of allopatric species.
Although ecological and spatial opportunities represent distinct pathways for evolutionary radiation, evidence is mounting that they could act in tandem, as seen in various continental-scale events (e.g., Australian lizards9; soil diatoms,10 and Saxifragales flowering plants11). During these events, spatial opportunities occurred first and prompted species diversification via geographical isolation. Next, new species appeared by adapting to local ecological conditions while in situations of sympatry or following secondary contacts (ecological diversification). Ecological and spatial opportunities may also jointly shape biological diversification, as seen in caviomorph rodents12 and Espeletiinae plants.13 In the literature, there are numerous descriptions of circumstances in which spatial opportunities preceded or co-occurred with ecological opportunities; such situations seem to typically produce clades containing ecologically heterogeneous subclades. In contrast, to our knowledge, there is no reported example of ecological opportunities preceding spatial opportunities.
Here, we describe the case of Cataglyphis desert ants, a genus for which ecological diversification was followed by spatial expansion and species diversification. There are approximately 100 species of Cataglyphis organized into 9 species groups; their geographical distribution spans from western Europe to central Asia.14 Research suggests that Cataglyphis originated about 20 MA (range: 30–10 MA)15 in the Middle East,16 a region that was then featured by open grassland habitats.17,18,19 At present, all Cataglyphis species are found in open, arid habitats and share extensive ecological similarities. They are thermophilic scavengers: workers leave the colony during the hottest hours of the day, search individually for heat-stricken arthropods, and return single food items to the nest.20 This unique ecological niche allows Cataglyphis species to limit competitive interactions with ecologically dominant species from other genera.21
However, competition for food resources may be intense within the genus. In ants, worker size can shape competition by placing constraints on the size of items that individuals can transport and process.22,23 In Cataglyphis, worker size is highly similar within species groups but significantly different between species groups. Accordingly, phylogeographic studies have shown that species from the same species group rarely co-occur24,25,26,27; instead, instances of co-existence involve species from different species groups (pers. obs.). These observations suggest that worker size could have had a pivotal role in ecological divergence in Cataglyphis and could thus be an informative trait when investigating evolutionary radiation within the genus.
In this study, we explored the evolutionary radiation of Cataglyphis desert ants using a comprehensive approach. We utilized comparative phylogenetic methods, biogeographic analyses, morphological measurements, and field observations to achieve six objectives. First, we constructed a phylogeny and inferred lineage divergence times for Cataglyphis employing data from 36 species belonging to the 6 main Cataglyphis species groups found in the Mediterranean Basin and Middle East. Second, we characterized the dynamics of lineage diversification; we hypothesized that species accumulated rapidly after the genus originated in the open grassland habitats of the Middle East (∼20 MA),17,18,19 with a second round of rapid speciation occurring in response to the aridification of the Mediterranean Basin (∼11–5 MA).19,28,29 Third, we explored the biogeographical history of the genus: we expected that the genus was able to effectively exploit the aridification of the Mediterranean Basin, resulting in its ancestral lineages moving westward between 11 and 5 MA. Fourth, we performed field research to test whether differences in worker head width, a proxy for worker size, could facilitate coexistence among Cataglyphis species by partitioning food resources. Fifth, we evaluated evolutionary patterns of worker head width, hypothesizing that head width diversification may have occurred early on and thus allowed the genus to fill available ecological niches shortly after it arose in the Middle East. Sixth, using model comparisons, we tested whether interspecific competition within Cataglyphis could have promoted divergence in worker head width and species geographical distributions over evolutionary time.
Results
Objective 1: Phylogeny construction and inference of divergence times
First, we constructed a multilocus phylogeny of 36 species representing the 6 main species groups of the genus (Figure 1A). Our DNA dataset consisted of 2,294 ultra-conserved elements (UCEs),30 whose mean sequence length was 1,159 bp (Data S1 and S2). There was a high degree of concordance and support for the topologies recovered based on maximum-likelihood analyses (i.e., concatenated, by-locus, and SWSC-EN partitioning strategies)31 and coalescent-based estimators32 (Figures S1–S4). As expected, the analyses all confirmed the presence of six monophyletic species groups within the genus. Moreover, we found that Cataglyphis is paraphyletic because it includes Rossomyrmex, a result that is consistent with a previous phylogenetic analysis.16
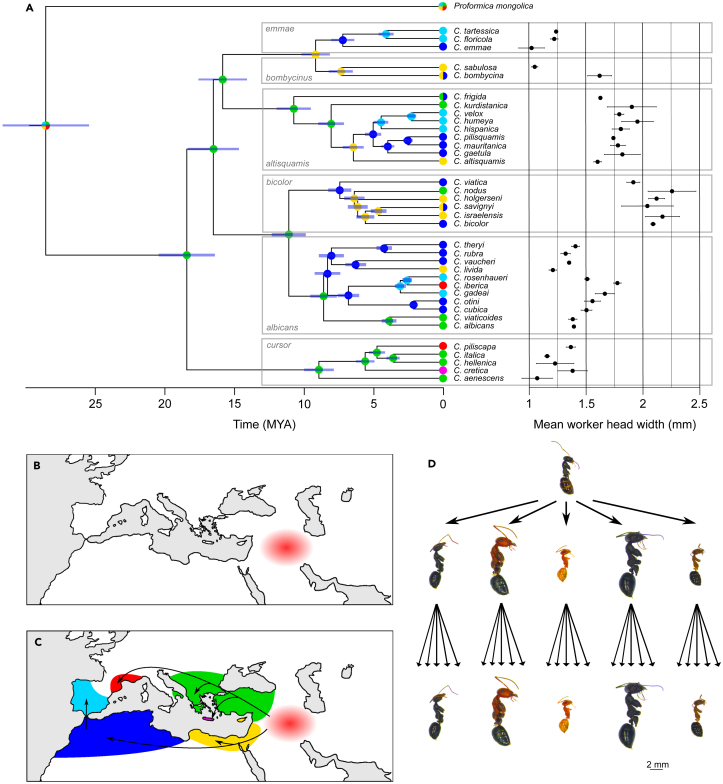
Figure 1.
Phylogenetic relationships, biogeographical history, worker head width, and species diversification in Cataglyphis
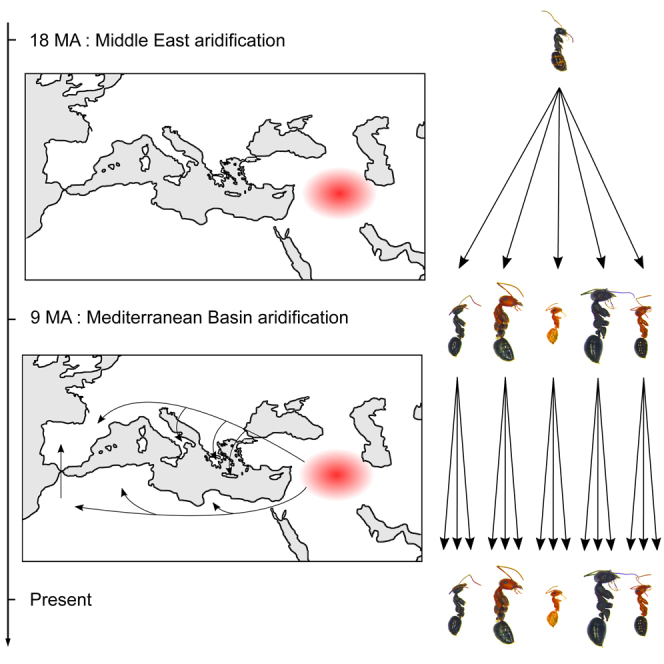
(A) The 36 Cataglyphis species included in this study represent 6 monophyletic species groups: emmae, bombycina, altisquamis, bicolor, albicans, and cursor. The genus originated ∼18 MA and, at ∼9 MA, began to spread from the area around modern-day Türkiye (green) to the areas around modern-day Israel (yellow) and northern African (dark blue). It reached the Iberian Peninsula (light blue) ∼5 MA. Southern France (red) and Crete (mauve) were colonized by two and one species, respectively. The 95% highest posterior density intervals for node age are indicated (blue bars). Mean worker head width (mm) varies among Cataglyphis species but is conserved within species groups. For each species, the standard deviation of worker head width among colonies is shown (black bars).
(B) Cataglyphis originated in arid regions that emerged in the Middle East ∼18 MA (in red).
(C) From the Middle East (in red), Cataglyphis ants colonized the southern and northern Mediterranean Basin beginning at ∼9 MA (black arrows), when these regions began to experience aridification. The color code is the same as in Figure 1A.
(D) Illustration of worker morphology diversification in Cataglyphis, a process that started early in genus history. When members of the genus began colonizing the Mediterranean Basin (∼9 MA), the new species that arose conserved the worker head widths (a proxy for worker size) of their ancestors. The scale is the same for all the pictures.
Using primary and secondary node calibrations33 (Data S3), we discovered that the genus Cataglyphis originated in the early Miocene (mean = 18.5 MA, 95% highest posterior density interval = 16.5–20.5 MA; Figure 1A; Figure S5). This dating estimate and those for the outgroups are consistent with findings from recent phylogenetic studies.15,34,35 During this period, open grassland habitats were present in the Middle East,17,18,19 where the genus seems to have emerged.16
Objective 2: Characterization of lineage diversification
We evaluated lineage diversification dynamics for Cataglyphis using a lineage-through-time (LLT) plot.36 The LTT plot revealed a pattern of early gradual lineage diversification (Figure 2A). The diversification rate increased suddenly ∼9 MA and then progressively slowed (MCCR test: γ = −3.55, p = 0.02, number of simulations = 1000). These dynamics are further supported by our comparison of multiple lineage diversification models,37,38 which point to a significant upturn in diversification ∼9.2 MA. The underlying cause could be increased speciation or reduced extinction accompanied by greater clade carrying capacity (Data S4). This rise in diversification was seen in most species groups (Figure 1A).
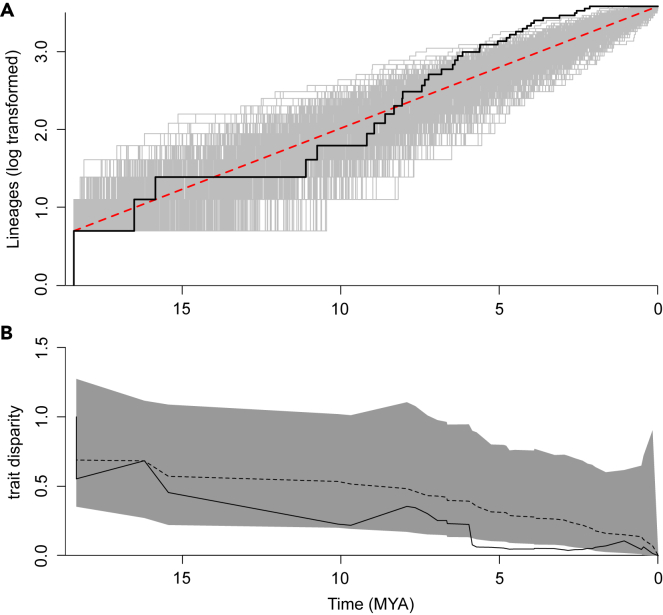
Figure 2.
Lineage diversification and disparities in worker head width over time in Cataglyphis
(A) Lineage diversification significantly accelerated ∼9 MA (black line). The results of the 1,000 iterations of the pure-birth-process model are indicated (gray lines), as is the linear increase in species number (red dashed line).
(B) Disparities in worker head width remained low within clades over evolutionary time, a pattern that was especially marked after the rapid increase in species number that started ∼9 MA (solid black line; rank envelope test: p = 0.025; p interval = 0.023–0.046). Also shown are the expectations arising from the null model: trait disparity (dashed black line) with its 95% confidence interval (solid gray area).
These results do not support our hypothesis that Cataglyphis species rapidly accumulated after the genus arose in the open grasslands of the Middle East. Instead, rapid species diversification seems to have been a response to the aridification of the Mediterranean Basin during the late Miocene (∼11–5 MA).19,28,29
Objective 3: Evaluating the geographical origin of rapid lineage diversification
To test whether the above trend in species diversification coincided with the spread of Cataglyphis from the Middle East to the Mediterranean Basin, we reconstructed the ancestral distributions of the genus’ lineages39 based on 1,128 observations of species presence (Data S5). Six biogeographical regions were inferred from species distributions40 (Figures 1A and C; Data S6). As predicted, our biogeographic analyses (Data S7) indicated that Cataglyphis probably began colonizing the Mediterranean, moving from east to west, around 9 MA (Figures 1A–C). The results suggest the cursor species group spread northward, from modern-day Türkiye to Crete and southern France. In contrast, species groups albicans, bicolor, altisquamis, bombycinus, and emmae moved south, from Türkiye to Israel, northern Africa and, later, the Iberian Peninsula. The colonization of the latter by Cataglyphis most likely followed the appearance of a land bridge connected to northern Africa during the Messinian salinity crisis and the subsequent, sudden reopening of the Straits of Gibraltar (∼5.33 MA).26,41
These findings support the idea that a spatial opportunity, namely the aridification of the Mediterranean Basin, triggered geographical spread and rapid species diversification in Cataglyphis ∼9 MA.
Objective 4: Assessing the ecological significance of worker head width
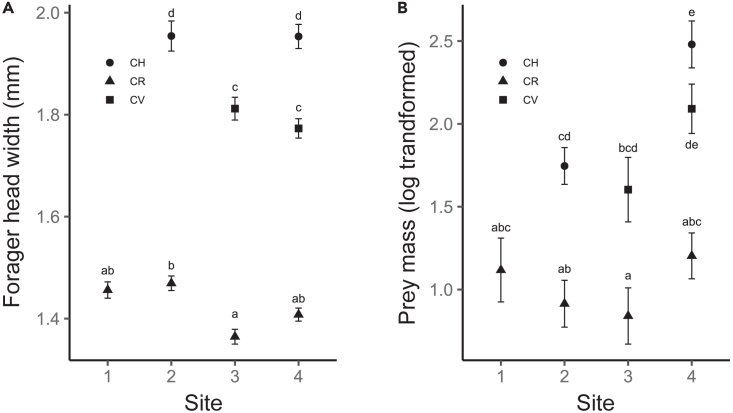
In nature, Cataglyphis species with different worker head widths frequently co-occur, while those with similar worker head widths do not. We conducted field research to determine whether differences in worker head width were related to food resource partitioning among species in the genus. To this end, we studied the relationship between prey mass and worker head width at four sites hosting three Cataglyphis species with distinct worker head widths: Cataglyphis rosenhaueri (mean worker head width within colonies = 1.51 ± 0.07 mm; range: 1.1–1.8 mm), Cataglyphis velox (1.79 ± 0.04 mm; range: 1.0–2.3 mm), and Cataglyphis hispanica (1.81 ± 0.08 mm; range: 1.0–2.5 mm). Within the sites, species differed in mean forager head width (Figure 3A) and mean prey mass (Figure 3B) (Data S8 and S9). In addition, mean forager head width was positively correlated with mean prey mass (linear regression: slope coefficient ±SE = 2.07 ± 0.45, p = 0.004, number of populations studied across sites and species = 8): the small-headed C. rosenhaueri gathered the smallest prey, while the large-headed C. hispanica collected the largest prey.
Figure 3.
Field observations of forager head width and prey mass for three coexisting Cataglyphis species
(A) Forager head width varied significantly among the three species. Across all sites, it was large for C. hispanica (CH, circles), medium for C. velox (CV, squares), and small for C. rosenhaueri (CR, triangles). However, at the colony level, worker head width was similar for C. hispanica and C. velox (see text). Standard errors are indicated (black bars). The p-value for each comparison can be found in Data S9.
(B) Prey mass (mg) was small for C. rosenhaueri, medium for C. velox, and large for C. hispanica across all sites. Standard errors are indicated (black bars). The p-value for each comparison can be found in Data S9.
Thus, worker head width is strongly tied to resource use and likely reduces interspecific competition among Cataglyphis ants via niche partitioning.
Objective 5: Exploring evolutionary patterns in worker head width
We explored the diversification of mean worker head width in 36 Cataglyphis species using phylogenetic comparative methods (Figure 1A; Data S10). We found a strong phylogenetic signal for worker head width (Blomberg’s K = 1.76, randomization procedure—1,000 iterations, p = 0.001; Pagel’s λ = 1.08, p = 0.001),36 which signifies that more closely related species displayed a greater degree of trait similarity than expected based on the null model. A disparity-through-time (DTT) analysis42 confirmed that disparity within clades contributed little to total clade disparity over evolutionary time (rank envelope test; p = 0.025; p interval = 0.023–0.046) (Figure 2B). Moreover, disparity within clades decreased over evolutionary time; indeed, the rate of evolution for worker head width decreased over time (node height test: df = 33, t = −3.053, p = 0.004; Figure S6).43 Notably, disparity within clades was particularly low starting at ∼6 MA, during the burst of species accumulation, supporting that emerging species retained the worker head width of their ancestors. Ancestral state estimation unveiled that ancestors of the different species groups were already distinguishable based on their worker head width (cursor’s ancestor worker head width = 1.28 mm; albicans = 1.51 mm; bicolor = 1.93 mm; altisquamis = 1.63 mm; emmae = 1.24 mm; bombycinus = 1.32 mm).
Collectively, our findings (objectives 2–5) indicate that, in Cataglyphis, spatial opportunities promoted species diversification. The eventual result was the emergence of morphologically homogeneous species groups (Figure 1D), whose members each arose from a common ancestor. Within each group, worker head width is highly similar and reflects the shared ancestral value. Among groups, worker head with is strikingly different because ancestral values were strikingly different.
Objective 6: Exploring the effects of interspecific competition over evolutionary time
Finally, we explored whether interspecific competition could have affected patterns of worker head width and species geographical distribution in Cataglyphis over evolutionary time. To this end, we compared the results of multiple evolutionary models that included or excluded competition37,43,44 (see STAR Methods). The MC model45 takes into account trait values and geographical overlap. It provided the best fit for our data (Data S11), indicating that competition likely had a strong influence on worker head width evolution in the genus, and affected species geographical distribution.
Because within-colony variation in worker head width (worker polymorphism) can affect the range of prey collected, we investigated the evolution of worker head width variation using the same phylogenetic comparative methods as for mean worker head width. Our results show that the evolution of worker head width variation appears to be random in Cataglyphis (Data S12, Figures S7 and S8). Thus, over evolutionary time, worker polymorphism does not appear to have been affected by interspecific competition.
Discussion
Our study strongly suggests that evolutionary radiation in Cataglyphis desert ants was the product of ecological diversification followed by geographical expansion. These findings concur with the idea that Cataglyphis originated approximately ∼18 MA, likely in open grassland habitats in the Middle East (Figures 1A and 1B).16,17,18,19 Ants in the genus developed a range of adaptations, including heightened physiological thermotolerance and elongated legs, which enabled them to become thermophilic scavengers. Initially, species diversification occurred at a slow pace. Around 9 MA, Cataglyphis lineages had diverged in worker head width, reflecting that a difference in foraging ecology had developed. Ath this time, a spatial opportunity presented itself: new arid habitats became available in the Mediterranean Basin,19,28,29 which seemingly promoted the geographical expansion of the genus and triggered a surge in species diversification. Each lineage gave rise to new species, which formed morphologically homogeneous species groups in which the ancestral value for worker head width was retained. Next, the rate of species diversification progressively declined. At present, Cataglyphis remains a clade of several morphologically homogeneous species groups that occur in the Mediterranean Basin and beyond. Geographical overlap is rare within species groups but frequent between species groups, a pattern that is likely linked to worker size and food resource partitioning.
Within the species groups, species diversification was accompanied by minimal ecological divergence. The mechanisms underlying this niche conservatism remain unclear,6 although past research suggests that intrinsic or extrinsic factors may commonly prevent species from undergoing ecological shifts. Intrinsic factors may include physicochemical, developmental, and genetic constraints, while extrinsic factors may include dispersal limitations, competition, and predation.6,46 Evidence suggests that intrinsic factors are unlikely to be acting in the case of Cataglyphis. Indeed, in the genus, worker head width is greatly variable, and many species are polymorphic, producing small and large workers. Additionally, Cataglyphis species display pronounced diversity in social organization and reproductive strategies.20
Extrinsic causes seem more likely and are inherent to our explanation of the observed patterns. Our results suggest that ancestral values of worker head width were conserved within species groups because the Mediterranean Basin was simultaneously colonised by species that already differed in worker head width. As species with larger worker head widths spread geographically, they consistently coexisted with species with smaller worker head widths. The reverse was also true. As a result, species were less likely to develop smaller or larger worker head widths over evolutionary time. Instead, resource competition may have acted to prevent the coexistence of species having similar worker head widths (i.e., species of the same species group) within the same geographical area. Evidence for this hypothesis comes from the biogeographical species distribution patterns seen in this study (support for the MC model) and from previous studies conducted at smaller spatial scales that have highlighted the predominance of allopatric species distributions within species groups.24,25,26,27 Thus, in the evolutionary radiation of this genus, it may be the precedence of worker head width diversification over the geographic expansion that caused the current homogeneous species groups.
Interspecific competition has traditionally been considered the hallmark of ant ecology47 and a key driver of ant morphological adaptations.22 Recent research supports a more tempered view: other forces, including predation, parasitism, and habitat disturbance have been reported to affect the evolutionary trajectories of worker morphology48 and the assemblage of communities.47 Cataglyphis ants are behaviorally subordinate, display low levels of aggressiveness, and avoid resource competition by foraging under thermal conditions that other genera cannot tolerate.21 However, our study indicates that, within Cataglyphis, interspecific competition has had large macroevolutionary implications for both species geographical distributions and worker head width. The reasons for these findings are an open question. Worker head width may be a key trait in Cataglyphis because it affects many aspects of desert life. Indeed, worker size has impacts in domains beyond food resource partitioning; it also influences temporal foraging patterns and levels of heat resistance.49,50 Larger workers are more heat tolerant and can remain active during the hottest period of the day. Furthermore, all Cataglyphis species are thermophilic scavengers, and it could be that only a few traits are responsible for finer-scale ecological niche differentiation within the genus. However, even rare, coexistence of closely related Cataglyphis species does occur, supporting that other traits than worker head width may still promote sympatry in the genus. For instance, in central Tunisia, the close relatives C. bicolor and Ciona savignyi have partially overlapping distributions.51 These species display similarities in worker size, foraging strategies, and nest site preference. Yet, when they occur in sympatry, nest site specialization is observed: the more dominant C. bicolor establishes nests in nutritionally richer microhabitats than does the more subordinate C. savignyi. Thus, the coexistence of these two species is facilitated by microhabitat segregation, where C. savignyi is able to tolerate lower-quality conditions.
Our results also reveal that mean worker head width, but not worker head width variation (polymorphism), reduced resource competition over evolutionary time in Cataglyphis ants. However, such result does not preclude worker polymorphism from helping to somewhat limit interspecific competition. Worker polymorphism allows for more flexible exploitation of food resources because colonies can gather food items of different shapes and sizes. Species may be better able to compete under variable environmental conditions if they have polymorphic workers. Indeed, we observed that C. rosenhaueri foragers have smaller head widths when they coexist with the medium-headed C. velox versus the large-headed C. hispanica (Figure 2A). Similarly, in the polymorphic C. bicolor, foragers have smaller size in Greece than in Tunisia, where they coexist with the small C. albicans.52 The world’s arid regions harbor the highest densities of polymorphic ants,53 perhaps because worker polymorphism can reduce interspecific competition and because the diversity of food resources is limited (for desert ants, essentially seeds and dead arthropods). However, our findings indicate that niche partitioning can be strongly tied to mean worker head width, and we therefore call for more research that focuses on this trait.
Limitations of the study
Our study utilized data from 36 of the approximately 56 Cataglyphis species that occur in the Mediterranean Basin. As a result, we might have missed instances of co-occurrence for species of the same species group, which could have potentially have affected our macroevolutionary analyses. However, we believe that our results are reliable since previous phylogenetic and geographical studies have reported that such instances of co-occurrence are indeed rare. Furthermore, our biogeographic analyses could have been affected by the fact that there are around 100 Cataglyphis species in total, whose range spans from the Mediterranean Basin to China; we did not include any species from Asia (∼15–20 species). It is worth noting that a prior study that did include Asian species identified the same region of origin for the genus as we did (the Middle East).16 Future research should include the full range of Cataglyphis species to more thoroughly unravel the evolutionary history of this genus.
STAR★Methods
Key resources table
| REAGENT or RESOURCE | SOURCE | IDENTIFIER |
|---|---|---|
| Deposited data | ||
| SPAdes assembly contigs, concatenated UCE matrices, tree files, and the UCE bait sequence file | This study | Dryad: https://doi.org/10.5061/dryad.sbcc2frck |
| Raw Illumina reads – deposited to NCBI | This study | NCBI BioProject: PRJNA1087578 and Data S1 |
| Experimental models: Organisms/strains | ||
| Cataglyphis species | This study | Data S1 |
| Software and algorithms | ||
| PHYLUCE pipeline | Faircloth | https://phyluce.readthedocs.io/en/latest/ |
| AMAS | Borowiec | https://github.com/marekborowiec/AMAS |
| IQ-TREE v. 2.0 | Minh et al.31 | http://www.iqtree.org/ |
| SWSC-EN | Tagliacollo and Lanfear54 | https://github.com/Tagliacollo/PFinderUCE-SWSC-EN |
| ASTRAL-III | Zhang et al.28 | https://github.com/smirarab/ASTRAL |
| Newick utilities | Junier and Zdobnov55 | https://github.com/tjunier/newick_utils |
| Clocklikeness script | Borowiec et al.34 | https://github.com/marekborowiec/metazoan_phylogenomics |
| BEAST v. 2.6.2 | Bouckaert et al.33 | https://www.beast2.org/ |
| Infomap Bioregions | Edler et al.40 | https://www.mapequation.org/bioregions/ |
Resource availability
Lead contact
Further information and requests for resources and reagents should be directed to and will be fulfilled by the lead contact, Nathan Lecocq de Pletincx (nathan.lecocq@outlook.com).
Materials availability
This study did not generate new unique reagents.
Data and code availability
-
•
Raw Illumina reads have been deposited at the NCBI Sequence Read Archive and are publicly available as of the date of publication. Accession numbers are listed in the Data S1; see key resources table. SPAdes assembly contigs, concatenated UCE matrices, tree files, and the UCE bait sequence file have been deposited at Dryad: https://doi.org/10.5061/dryad.sbcc2frck). DOI is given in the key resources table.
-
•
This paper does not report original code.
-
•
Any additional information required to reanalyse the data reported in this paper is available from the lead contact upon request.
Experimental model and study participant details
The ant genus Cataglyphis (RRID:NCBITaxon_47732) contains ∼100 described species organised into 9 species groups.14,56 Its geographical range includes the arid habitats of the Palearctic, from western Europe and Africa to central Asia. Between 2009 and 2021, we collected specimens for 36 out of the 56 Cataglyphis species found around the Mediterranean Basin (Data S1); they were representative of the genus’ 6 main species groups. We sampled entire colonies to fully characterise their worker size distributions. To control for variation in worker size due to colony ontogeny, we only collected mature colonies (i.e., those producing sexuals). Samples included individuals at all stages of development (eggs, larvae, nymphs as well as adult workers and sexuals).
Method details
Dataset
We used worker head width as a proxy for worker size.57 We specifically measured maximum head width (MHW), which includes the eyes. MHW is an ant trait that is meaningful, widely used, highly repeatable, and markedly variable in polymorphic species.58 We used a sample-size-corrected coefficient of variation (CV∗), which is the ratio of the median absolute deviation of worker MHW (MAD) to median worker MHW (Mdn)59:
CV∗ values climb as worker polymorphism increases. Furthermore, this estimator performs better than others in the case of non-normal distributions,59 which are common in Cataglyphis ants.57
We measured MHW for 40 randomly chosen workers per colony, which is the number of workers at which CV∗ plateaued.57 Four colonies per species were used for the 18 species characterised in previous research,57 and 2 colonies per species were used for the 18 other species sampled in this study. In C. bombycina, the helper caste is dimorphic, composed of workers and soldiers. We excluded the latter from our analyses. Measurements were performed to the nearest 0.01 mm using a MZ6 stereomicroscope (Leica Microsystems, Wetzlar, Germany).
Objective 1: Phylogeny construction and inference of divergence times
Ultra-conserved elements data generation
To generate a genome-scale dataset of all 36 Cataglyphis species, we employed ultra-conserved elements (UCE) phylogenomics,60 an approach that uses targeted enrichment of conserved genomic regions. We sent tissue samples to Rapid Genomics, Florida, USA, where the DNA was extracted. The genomic libraries were then prepared and enriched using 31,829 baits targeting 2,590 UCE loci conserved across ants (Hymenoptera 2.5Kv2).30 Sequencing was performed utilising an Illumina NovaSeq 2x150 system. We used one to five individuals per species.
We cleaned and processed the resulting reads using PHYLUCE software v. 1.7.1.61 We trimmed raw reads for adapter contamination using Illumiprocessor v. 2.0,62 which incorporates Trimmomatic.63 Trimmed reads were assembled de novo into contigs using SPAdes.64
Next, we added assemblies for 11 outgroup species (Data S2) that were obtained from previous studies.34,35 To identify and extract UCE contigs from the bulk set of contigs for all 47 species, we employed a PHYLUCE program (match_contigs_to_probes) that uses Lastz v. 1.065 to match probe sequences to contig sequences and to create a database of hits. Our min-identity and min-coverage settings were 80. We aligned each UCE locus using MAFFT v. 7.130b66 and the default algorithm setting in PHYLUCE (FFT-NS-i). We tested different trimming strategies: (i) no trimming, (ii) edge trimming, and (iii) trimming internal and external alignment regions using GBLOCKS,67 implemented with reduced stringency parameters (b1:0.5, b2:0.5, b3:12, b4:7). Based on the descriptive statistics generated by PHYLUCE and AMAS,68 we decided to use the GBLOCKS strategy in further analyses because it achieved a good compromise between the number of informative sites and the percentage of missing data (Data S3). We then concatenated all the loci to form a supermatrix and filtered the alignments for taxon occupancy, requiring the loci to be found in 0, 50, 75, 85, 90, 95, and 100% of the taxon. For each of the seven supermatrices, we generated descriptive statistics using PHYLUCE and AMAS. For further analyses, we decided to use the locus set filtered for 75% taxon occupancy because it achieved a good compromise between the number of informative sites and the percentage of missing data (Data S3). The resulting matrix contained 2,294 loci (mean length = 1,159 bp), 560,008 informative sites, and a gaps/missing data level of 18.5%.
Phylogeny inference
We assessed the robustness of our phylogenetic inference methods using a range of sensitivity analyses. First, we performed an unpartitioned phylogenetic analysis with IQ-TREE v. 2.0.31 We selected GTR+F+G4 as the model of sequence evolution and performed 1,000 ultrafast bootstrap replicates (UFB)69 and 1,000 replicates of the SH-like approximate likelihood-ratio test.70 We employed the GTR+F+G4 model because using more complex models does not negatively impact the phylogenetic results.71 We did not utilise the proportion of invariant sites (+I) model with gamma distributed rates (+G) for among-site rate heterogeneity because these parameters cannot be collectively optimised.72
Second, we tested the effect of partitioning the concatenated data matrix by locus and with the sliding-window site characteristics method based on site entropy (SWSC-EN).54 Given the high number of partitions obtained using SWSC-EN, we implemented ModelFinder273 within IQ-TREE to merge the data subsets, using the rclusterf algorithm (set to check only the top 10% of merging schemes), the corrected Akaike information criterion (AICc), and the GTR+F+G4 model. For both partitioning strategies, we inferred a maximum-likelihood tree using IQ-TREE and the same parameters as for the concatenated analysis, except that we employed the -spp option to link branch lengths.
Finally, we estimated a coalescent-based species tree using ASTRAL-III.32 Our first step was to estimate individual gene trees via IQ-TREE. Then, for each gene tree, we performed model testing and generated 1,000 UFB replicates. Across all the gene trees, we collapsed nodes whose UFB support was less than or equal to 10% using Newick utilities.55 We then fed the collapsed gene trees into ASTRAL-III for species tree inference.
Divergence time inference
To estimate divergence times, we simplified the analysis by creating data subsets and by constraining the tree search. To create the data subsets, we ranked loci by their clocklikeness using an R script74 and concatenated the alignments of the corresponding 100 best-performing loci (i.e., those with the smallest variation in root-to-tip lengths). To constrain the tree search, we used the best tree from the concatenated analysis (root = Gnamptogenys simulans). We made it ultrametric by performing a quick dating analysis with the chronos function in R.75,76
We estimated the time-measured tree using BEAST v. 2.6.2.33 We employed the HKY+G model of sequence evolution, a strict clock model, and a yule model for the tree prior. Using more complex models did not affect the results (data not shown). Tree topology search parameters were turned off. We calibrated the tree using three fossil records and one form of secondary calibration (Data S4). The age of the crown group, Formicinae (all species except Gnamptogenys simulans), was constrained using the New Jersey Cretaceous amber fossil †Kyromyrma neffi,77 while the age of the clade containing Nylanderia terricola and Paratrechina longicornis was constrained using the Priabonian Baltic amber fossil †Nylanderia pygmaea.78 Priabonian Baltic amber fossils †Formica were used to constrain the age of the Formicini node (represented by the clade containing all Cataglyphis species, Formica fuscocinerea, Iberoformica subrufa, Polyergus vinosus, Proformica mongolica, and Rossomyrmex minuchae) because recent studies suggest that these fossils should be included in the ancestral Formicini lineage rather than in the extant genus Formica.15,34 Our form of secondary calibration was to employ Lasiini as a crown group (represented by the clade containing Nylanderia terricola, Paratrechina longicornis, Lasius arizonicus, and Myrmecocystus pyramicus) because it displays consistent dating across different studies.15,35,79,80 We executed two independent Markov chain Monte Carlo (MCMC) runs that each comprised 75 million generations, where sampling occurred every 100,000 generations. We evaluated MCMC convergence and mixing properties using the BEAST programs Log Combiner v. 1.10.4 and Tracer v. 1.7.181 to ensure that the combined effective sample size (ESS) values associated with the estimated parameters were all greater than 200. Finally, a maximum clade credibility (MCC) tree was retrieved from the posterior distribution of the trees using the BEAST programs Log Combiner v. 1.10.4 and Tree Annotator v. 1.10.4; we discarded the first 10% of the trees sampled from the posterior distribution as burn-in. All phylogenies were edited for clarity in FigTree v. 1.4.4.82
Objective 2: Characterisation of lineage diversification
We used several methods to investigate lineage diversification. First, we computed the γ statistic,83 a metric that reflects declines in diversification rates over time. Significant negative γ values indicate a slowdown in speciation rate moving toward the present. We used the Monte Carlo constant-rate (MCCR) γ-test to assess metric significance and to account for incomplete clade sampling.36
Second, we assessed lineage diversification dynamics using a lineage-through-time (LTT) plot in association with the MCC tree.36 For our null model, we simulated 1,000 trees assuming a pure birth process.
Third, we compared the fit of different diversification models using AICc84: (i) a pure birth model37; (ii) several birth-death models with constant, linear, or exponential variation in λ (speciation rate) and μ (extinction rate)37; (iii) diversity-dependent models incorporating K (clade carrying capacity); and (iv) diversity-dependent models incorporating estimated shifts in time for λ, μ, and/or K.38 We included models that allowed shifts in diversification parameters (λ, μ, K) because the LTT plot revealed a shift in lineage diversification rate.
Objective 3: Evaluating the geographical origin of rapid lineage diversification
We discretised the contemporary ranges of Cataglyphis ants using Infomap Bioregions40 and 1,128 records of occurrence for extant species obtained from various sources, including AntMaps85 and Cataglyphis studies24,25,86 (Data S5). Six bioregions were defined (Data S6). Each species was classified as being present or absent in each bioregion. We then used the R package BioGeoBEARS39 to infer the biogeographical history of the genus by evaluating six models: the dispersal–extinction–cladogenesis model (DEC),87 an ML version of the dispersal-vicariance model (DIVA-like),88 and an ML version of the Bayesian analysis of biogeography model (BayArea-like).89 In addition, we also tested models that included founder-event speciation (+j). Proformica mongolica was used as an outgroup representing the actual distribution of genus Proformica.
Objective 4: Assessing the ecological significance of worker head width
We explored the relationship between worker head width and resource utilisation in Cataglyphis species in situ. We focused on three species that are known to coexist and that have workers of contrasting sizes: C. rosenhaueri (small-headed workers), C. velox (medium-headed workers), and C. hispanica (large-headed workers) (see text). We carried out our field observations at four sites with varying species combinations. Site 1 hosted only C. rosenhaueri; site 2 hosted C. rosenhaueri and C. hispanica; site 3 hosted C. rosenhaueri and C. velox; and site 4 hosted C. rosenhaueri, C. hispanica, and C. velox. The sites were separated by a few kilometers. At each site, between 10 am and 4 pm, we collected foragers returning to the nest with naturally captured prey during 2–4 periods of 25–45 min. Sampling was carried out over 10 non-consecutive days at each site in alternation in July, a month during which Cataglyphis activity levels are high. For each forager collected, head width and prey mass were measured. We compared forager head width and prey mass among species within and among sites using a crossed ANOVA. Prey mass was log transformed to meet the assumption of data normality.
Objective 5: Exploring evolutionary patterns in worker head width
First, we examined whether the evolution of morphological traits (worker head width and worker polymorphism) departed from the null model of Brownian motion using a disparity-through-time (DTT) analysis. We employed a rank envelope method to compare the empirical data with the null model’s expectations,42 which were generated from 1,000 iterations of trait distributions simulated under Brownian motion. The explanation of convergent evolution is supported if trait disparities are predominantly found within subclades. In contrast, the explanation of divergent evolution is supported if trait disparities occur among subclades. In other words, morphological differences should be greater between than within subclades.
Second, we performed the univariate node height test, which checks for accelerations or decelerations in trait evolution rates via phylogenetically independent contrasts and the heights of the respective nodes.90 We also performed an ancestral state reconstruction analysis to obtain estimates of the worker head width of the ancestors of each species group.
Objective 6: Exploring the effects of interspecific competition over evolutionary time
To explore the role of competition over evolutionary time, it has proven fruitful to look for phylogenetic signals in trait data and evaluate support for phenotypic models that incorporate competition.91 We explored whether competition had influenced the evolution of worker morphology by estimating the phylogenetic signal for worker head width across the phylogeny using Blomberg’s K (Blomberg et al. 2003) and Pagel’s λ (Pagel 1999) statistics. Phylogenetic signals are expected to be large for competition-driven radiation events.91 Next, we compared the fit of different diversification models using the AICc.84 We tested a range of models that do not account for interspecific competition: the Brownian motion model92; the Ornstein-Uhlenbeck model of adaptation to an optimum trait value93; the early-burst model94; the delta, kappa, and lambda models that transform the tree using a single parameter prior to model fitting95; a white noise model that assumes the data come from a single normal distribution with no covariance structure among species43; and models that incorporate time shifts, using the time shifts estimated in the lineage diversification models.96 We also tested three models that do account for interspecific competition: two diversity-dependent models (Ddlin and Ddexp), in which the rate of evolution varies as a positive linear, negative linear, or exponential function of lineage number in the reconstructed phylogeny,97 and the matching competition (MC) model, in which the trait values of competing lineages repel each other.98 The MC model is process based and designed to directly test for the role of competition in trait evolution. It requires identifying lineages that are likely to encounter one another throughout clade history, which requires information about species’ past and present geographical distributions. We thus used the results of the biogeographic analyses as input, after discarding the outgroup, Proformica mongolica.
Quantification and statistical analysis
We performed all statistical analyses using the R packages phytools,36 RPANDA,37 DDD,38 Geiger,43 BioGeoBEARS,39 mvMORPH,44 and ape.76 Significance level was set to p < 0.05.
For objective 2, we computed the γ statistic and used the Monte Carlo constant-rate (MCCR) γ-test to assess metric significance and to account for incomplete clade sampling (see Results). We compared the fit of different diversification models using AICc. The best models were selected based on ΔAICc < 2 (see Data S4).
For objective 3, we compared the fit of different biogeographic models using AICc. The best models were selected based on ΔAICc < 2 (see Data S7).
For objective 4, we compared forager head width and prey mass among species within and among sampling sites using a crossed ANOVA. Prey mass was log transformed to meet the assumption of data normality (Shapiro-Wilk test). Sample sizes can be found in Data S8.1. p-values for the ANOVAs can be found in Data S9. We performed a linear regression between population mean forager head width and mean prey mass (see Results).
For objective 5, we estimated the phylogenetic signal for worker head width using Blomberg’s K (Blomberg et al. 2003) and Pagel’s λ (Pagel 1999) statistics (see Results). We examined whether the evolution of morphological traits (worker head width and worker polymorphism) departed from the null model of Brownian motion using a disparity-through-time (DTT) analysis. We employed a rank envelope method to compare the empirical data with the null model’s expectations, which were generated from 1,000 iterations of trait distributions simulated under Brownian motion (see Results). We performed a univariate node height test (see Results). We compared the fit of different diversification models using AICc. The best models were selected based on ΔAICc < 2 (see Data S11 and S12).
Acknowledgments
We thank Hugo Darras, Abraham Hefetz, Alexandre Kuhn, Laurianne Leniaud, Rémy Perez, and Quentin Willot for their help in the field; Patrick Mardulyn and Jonathan Romiguier for their helpful comments on previous versions of the manuscript; ICTS-RBD (Spain) and Département des Eaux et Forêts - Ministère de l’Agriculture, de la Pêche Maritime, du Développement Rural et des Eaux et Forêts (Morocco, Decisions 23/2019, 11/2021 and 17/2022) for providing us with field facilities; and Claudie Doums for kindly furnishing samples of C. aenescens. This work was financially supported by the Belgian F.R.S. - FNRS (grant no. T.0140.18) and by the Université Libre de Bruxelles (Actions Blanches program). N.L. received funding in the form of a PhD fellowship (F.R.S. - FNRS).
Author contributions
N.L. and S.A. came up with and designed the study. A.T., C.K., K.K., N.L., S.A., and X.C. collected the samples. X.C. performed the field experiments. N.L. performed the lab experiments. N.L. analyzed the data. N.L. and S.A. wrote the article.
Declaration of interests
The authors declare no competing interests.
Published: April 30, 2024
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.isci.2024.109852.
Supplemental information
A. Number of workers of each species collected in each site during the field experiment.
B. Size of foragers and weight of their associated prey for each species in each site in the field experiment.
A. P-values for comparison within and among sites of Cataglyphis species forager head width.
B. P-values for comparison within and among sites of the weight of preys collected by Cataglyphis species.
References
- 1.Simões M., Breitkreuz L., Alvarado M., Baca S., Cooper J.C., Heins L., Herzog K., Lieberman B.S. The evolving theory of evolutionary radiations. Trends Ecol. Evol. 2016;31:27–34. doi: 10.1016/j.tree.2015.10.007. [DOI] [PubMed] [Google Scholar]
- 2.Lambert J.W., Reichard M., Pincheira-Donoso D. Live fast, diversify non-adaptively: evolutionary diversification of exceptionally short-lived annual killifishes. BMC Evol. Biol. 2019;19:10–13. doi: 10.1186/s12862-019-1344-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stroud J.T., Losos J.B. Ecological opportunity and adaptive radiation. Annu. Rev. Ecol. Evol. Syst. 2016;47:507–532. [Google Scholar]
- 4.Grant P.R., Grant B.R. Princeton University Press; 2014. 40 Years of Evolution: Darwin’s Finches on Daphne Major Island. [Google Scholar]
- 5.Seehausen O. African cichlid fish: a model system in adaptive radiation research. Proc. Biol. Sci. 2006;273:1987–1998. doi: 10.1098/rspb.2006.3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Czekanski-Moir J.E., Rundell R.J. The ecology of nonecological speciation and nonadaptive radiations. Trends Ecol. Evol. 2019;34:400–415. doi: 10.1016/j.tree.2019.01.012. [DOI] [PubMed] [Google Scholar]
- 7.Kozak K.H., Weisrock D.W., Larson A. Rapid lineage accumulation in a non-adaptive radiation: phylogenetic analysis of diversification rates in eastern North American woodland salamanders (Plethodontidae: Plethodon) Proc. Biol. Sci. 2006;273:539–546. doi: 10.1098/rspb.2005.3326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reaney A.M., Saldarriaga-Córdoba M., Pincheira-Donoso D. Macroevolutionary diversification with limited niche disparity in a species-rich lineage of cold-climate lizards. BMC Evol. Biol. 2018;18:1–12. doi: 10.1186/s12862-018-1133-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blom M.P.K., Horner P., Moritz C. Convergence across a continent: adaptive diversification in a recent radiation of Australian lizards. Proc. Biol. Sci. 2016;283 doi: 10.1098/rspb.2016.0181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pinseel E., Janssens S.B., Verleyen E., Vanormelingen P., Kohler T.J., Biersma E.M., Sabbe K., Van de Vijver B., Vyverman W. Global radiation in a rare biosphere soil diatom. Nat. Commun. 2020;11:2382. doi: 10.1038/s41467-020-16181-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Folk R.A., Stubbs R.L., Mort M.E., Cellinese N., Allen J.M., Soltis P.S., Soltis D.E., Guralnick R.P. Rates of niche and phenotype evolution lag behind diversification in a temperate radiation. Proc. Natl. Acad. Sci. USA. 2019;116:10874–10882. doi: 10.1073/pnas.1817999116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Álvarez A., Arévalo R.L.M., Verzi D.H. Diversification patterns and size evolution in caviomorph rodents. Biol. J. Linn. Soc. 2017;121:907–922. [Google Scholar]
- 13.Pouchon C., Fernández A., Nassar J.M., Boyer F., Aubert S., Lavergne S., Mavárez J. Phylogenomic analysis of the explosive adaptive radiation of the Espeletia complex (Asteraceae) in the tropical Andes. Syst. Biol. 2018;67:1041–1060. doi: 10.1093/sysbio/syy022. [DOI] [PubMed] [Google Scholar]
- 14.Agosti D. Review and reclassification of Cataglyphis (Hymenoptera, Formicidae) J. Nat. Hist. 1990;24:1457–1505. [Google Scholar]
- 15.Boudinot B.E., Borowiec M.L., Prebus M.M. Phylogeny, evolution, and classification of the ant genus Lasius, the tribe Lasiini and the subfamily Formicinae (Hymenoptera: Formicidae) Syst. Entomol. 2022;47:113–151. [Google Scholar]
- 16.Sanllorente O., Lorite P., Ruano F., Palomeque T., Tinaut A. Phylogenetic relationships between the slave-making ants Rossomyrmex and their Proformica hosts in relation to other genera of the ant tribe Formicini (Hymenoptera: Formicidae) J. Zool. Syst. Evol. Res. 2018;56:48–60. [Google Scholar]
- 17.Strömberg C.A., Werdelin L., Friis E.M., Saraç G. The spread of grass-dominated habitats in Turkey and surrounding areas during the Cenozoic: phytolith evidence. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2007;250:18–49. [Google Scholar]
- 18.Edwards E.J., Osborne C.P., Strömberg C.A.E., Smith S.A., C4 Grasses Consortium. Bond W.J., Christin P.A., Cousins A.B., Duvall M.R., Fox D.L., et al. The origins of C4 grasslands: integrating evolutionary and ecosystem science. Science. 2010;328:587–591. doi: 10.1126/science.1177216. [DOI] [PubMed] [Google Scholar]
- 19.Strömberg C.A. Evolution of grasses and grassland ecosystems. Annu. Rev. Earth Planet Sci. 2011;39:517–544. [Google Scholar]
- 20.Boulay R., Aron S., Cerdá X., Doums C., Graham P., Hefetz A., Monnin T. Social life in arid environments: the case study of Cataglyphis ants. Annu. Rev. Entomol. 2017;62:305–321. doi: 10.1146/annurev-ento-031616-034941. [DOI] [PubMed] [Google Scholar]
- 21.Cerdá X., Retana J., Manzaneda A. The role of competition by dominants and temperature in the foraging of subordinate species in Mediterranean ant communities. Oecologia. 1998;117:404–412. doi: 10.1007/s004420050674. [DOI] [PubMed] [Google Scholar]
- 22.Traniello J.F.A. Foraging strategies of ants. Annu. Rev. Entomol. 1989;34:191–210. [Google Scholar]
- 23.Dornhaus A., Powell S., Lach L., Parr C.L. Foraging and defence strategies. Ant Ecol. 2010;29:210–230. [Google Scholar]
- 24.Eyer P.A., Seltzer R., Reiner-Brodetzki T., Hefetz A. An integrative approach to untangling species delimitation in the Cataglyphis bicolor desert ant complex in Israel. Mol. Phylogenet. Evol. 2017;115:128–139. doi: 10.1016/j.ympev.2017.07.024. [DOI] [PubMed] [Google Scholar]
- 25.Eyer P.A., Hefetz A. Cytonuclear incongruences hamper species delimitation in the socially polymorphic desert ants of the Cataglyphis albicans group in Israel. J. Evol. Biol. 2018;31:1828–1842. doi: 10.1111/jeb.13378. [DOI] [PubMed] [Google Scholar]
- 26.Villalta I., Amor F., Galarza J.A., Dupont S., Ortega P., Hefetz A., Dahbi A., Cerdá X., Boulay R. Origin and distribution of desert ants across the Gibraltar Straits. Mol. Phylogenet. Evol. 2018;118:122–134. doi: 10.1016/j.ympev.2017.09.026. [DOI] [PubMed] [Google Scholar]
- 27.Darras H., Kuhn A., Aron S. Evolution of hybridogenetic lineages in Cataglyphis ants. Mol. Ecol. 2019;28:3073–3088. doi: 10.1111/mec.15116. [DOI] [PubMed] [Google Scholar]
- 28.Zhang Z., Ramstein G., Schuster M., Li C., Contoux C., Yan Q. Aridification of the Sahara desert caused by Tethys Sea shrinkage during the Late Miocene. Nature. 2014;513:401–404. doi: 10.1038/nature13705. [DOI] [PubMed] [Google Scholar]
- 29.Saarinen J., Mantzouka D., Sakala J. Nature Through Time: Virtual Field Trips Through the Nature of the Past. Springer, Cham. 2020. Aridity, Cooling, Open Vegetation, and The Evolution of Plants and Animals During the Cenozoic; pp. 83–107. [Google Scholar]
- 30.Branstetter M.G., Longino J.T., Ward P.S., Faircloth B.C. Enriching the ant tree of life: enhanced UCE bait set for genome-scale phylogenetics of ants and other Hymenoptera. Methods Ecol. Evol. 2017;8:768–776. [Google Scholar]
- 31.Minh B.Q., Schmidt H.A., Chernomor O., Schrempf D., Woodhams M.D., Von Haeseler A., Lanfear R. IQ-TREE 2: new models and efficient methods for phylogenetic inference in the genomic era. Mol. Biol. Evol. 2020;37:1530–1534. doi: 10.1093/molbev/msaa015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang C., Rabiee M., Sayyari E., Mirarab S. ASTRAL-III: polynomial time species tree reconstruction from partially resolved gene trees. BMC Bioinf. 2018;19:15–30. doi: 10.1186/s12859-018-2129-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bouckaert R., Vaughan T.G., Barido-Sottani J., Duchêne S., Fourment M., Gavryushkina A., Heled J., Jones G., Kühnert D., De Maio N., et al. BEAST 2.5: An advanced software platform for Bayesian evolutionary analysis. PLoS Comput. Biol. 2019;15 doi: 10.1371/journal.pcbi.1006650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Borowiec M.L., Cover S.P., Rabeling C. The evolution of social parasitism in Formica ants revealed by a global phylogeny. Proc. Natl. Acad. Sci. USA. 2021;118 doi: 10.1073/pnas.2026029118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.van Elst T., Eriksson T.H., Gadau J., Johnson R.A., Rabeling C., Taylor J.E., Borowiec M.L. Comprehensive phylogeny of Myrmecocystus honey ants highlights cryptic diversity and infers evolution during aridification of the American Southwest. Mol. Phylogenet. Evol. 2021;155 doi: 10.1016/j.ympev.2020.107036. [DOI] [PubMed] [Google Scholar]
- 36.Revell L.J. phytools: an R package for phylogenetic comparative biology (and other things) Methods Ecol. Evol. 2012;3:217–223. [Google Scholar]
- 37.Morlon H., Lewitus E., Condamine F.L., Manceau M., Clavel J., Drury J. RPANDA: an R package for macroevolutionary analyses on phylogenetic trees. Methods Ecol. Evol. 2016;7:589–597. [Google Scholar]
- 38.Etienne R.S., Haegeman B. DDD: Diversity-Dependent Diversification. R package version. 2020;4.3 [Google Scholar]
- 39.Matzke N.J. Trait-dependent dispersal models for phylogenetic biogeography, in the R package BioGeoBEARS. Integr. Comp. Biol. 2016;56 [Google Scholar]
- 40.Edler D., Guedes T., Zizka A., Rosvall M., Antonelli A. Infomap bioregions: interactive mapping of biogeographical regions from species distributions. Syst. Biol. 2017;66:197–204. doi: 10.1093/sysbio/syw087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Achalhi M., Münch P., Cornée J.J., Azdimousa A., Melinte-Dobrinescu M., Quillévéré F., Drinia H., Fauquette S., Jiménez-Moreno G., Merzeraud G., et al. The late Miocene Mediterranean-Atlantic connections through the north Rifian corridor: new insights from the Boudinar and Arbaa Taourirt basins (northeastern Rif, Morocco) Palaeogeogr. Palaeoclimatol. Palaeoecol. 2016;459:131–152. [Google Scholar]
- 42.Murrell D.J. A global envelope test to detect non-random bursts of trait evolution. Methods Ecol. Evol. 2018;9:1739–1748. [Google Scholar]
- 43.Pennell M.W., Eastman J.M., Slater G.J., Brown J.W., Uyeda J.C., FitzJohn R.G., Alfaro M.E., Harmon L.J. geiger v2. 0: an expanded suite of methods for fitting macroevolutionary models to phylogenetic trees. Bioinformatics. 2014;30:2216–2218. doi: 10.1093/bioinformatics/btu181. [DOI] [PubMed] [Google Scholar]
- 44.Clavel J., Escarguel G., Merceron G. mvMORPH: an R package for fitting multivariate evolutionary models to morphometric data. Methods Ecol. Evol. 2015;6:1311–1319. [Google Scholar]
- 45.Drury J., Clavel J., Manceau M., Morlon H. Estimating the effect of competition on trait evolution using maximum likelihood inference. Syst. Biol. 2016;65:700–710. doi: 10.1093/sysbio/syw020. [DOI] [PubMed] [Google Scholar]
- 46.Crisp M.D., Cook L.G. Phylogenetic niche conservatism: what are the underlying evolutionary and ecological causes? New Phytol. 2012;196:681–694. doi: 10.1111/j.1469-8137.2012.04298.x. [DOI] [PubMed] [Google Scholar]
- 47.Cerdà X., Arnan X., Retana J. Is competition a significant hallmark of ant (Hymenoptera: Formicidae) ecology. Myrmecological News. 2013;18:131–147. [Google Scholar]
- 48.Wills B.D., Powell S., Rivera M.D., Suarez A.V. Correlates and consequences of worker polymorphism in ants. Annu. Rev. Entomol. 2018;63:575–598. doi: 10.1146/annurev-ento-020117-043357. [DOI] [PubMed] [Google Scholar]
- 49.Cerdá X., Retana J. Alternative strategies by thermophilic ants to cope with extreme heat: individual versus colony level traits. Oikos. 2000;89:155–163. [Google Scholar]
- 50.Perez R., Benbachir M., Decroo C., Mascolo C., Wattiez R., Aron S. Cataglyphis desert ants use distinct behavioral and physiological adaptations to cope with extreme thermal conditions. J. Therm. Biol. 2023;111 doi: 10.1016/j.jtherbio.2022.103397. [DOI] [PubMed] [Google Scholar]
- 51.Dietrich B., Wehner R. Sympatry and allopatry in two desert ant sister species: how do Cataglyphis bicolor and C. savignyi coexist? Oecologia. 2003;136:63–72. doi: 10.1007/s00442-003-1245-0. [DOI] [PubMed] [Google Scholar]
- 52.Wehner R., Harkness R.D., Schmid-Hempel P. Fischer; 1983. Foraging Strategies in Individually Searching Ants. [Google Scholar]
- 53.La Richelière F., Muñoz G., Guénard B., Dunn R.R., Economo E.P., Powell S., Sanders N.J., Weiser M.D., Abouheif E., Lessard J.P. Warm and arid regions of the world are hotspots of superorganism complexity. Proc. Biol. Sci. 2022;289 doi: 10.1098/rspb.2021.1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tagliacollo V.A., Lanfear R. Estimating improved partitioning schemes for ultraconserved elements. Mol. Biol. Evol. 2018;35:1798–1811. doi: 10.1093/molbev/msy069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Junier T., Zdobnov E.M. The Newick utilities: high-throughput phylogenetic tree processing in the UNIX shell. Bioinformatics. 2010;26:1669–1670. doi: 10.1093/bioinformatics/btq243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bolton B. AntCat. An online catalog of the ants of the world. 2012. https://www.antcat.org/
- 57.Lecocq de Pletincx N., Dellicour S., Aron S. The evolution of ant worker polymorphism correlates with multiple social traits. Behav. Ecol. Sociobiol. 2021;75:1–11. [Google Scholar]
- 58.Jaffé R., Kronauer D.J.C., Kraus F.B., Boomsma J.J., Moritz R.F.A. Worker caste determination in the army ant Eciton burchellii. Biol. Lett. 2007;3:513–516. doi: 10.1098/rsbl.2007.0257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ospina R., Marmolejo-Ramos F. Performance of some estimators of relative variability. Front. Appl. Math. Stat. 2019;5:43. [Google Scholar]
- 60.Faircloth B.C., McCormack J.E., Crawford N.G., Harvey M.G., Brumfield R.T., Glenn T.C. Ultraconserved elements anchor thousands of genetic markers spanning multiple evolutionary timescales. Syst. Biol. 2012;61:717–726. doi: 10.1093/sysbio/sys004. [DOI] [PubMed] [Google Scholar]
- 61.Faircloth B.C. PHYLUCE is a software package for the analysis of conserved genomic loci. Bioinformatics. 2016;32:786–788. doi: 10.1093/bioinformatics/btv646. [DOI] [PubMed] [Google Scholar]
- 62.Faircloth B.C. 2013. Illumiprocessor: A Trimmomatic Wrapper for Parallel Adapter and Quality Trimming. [DOI] [Google Scholar]
- 63.Bolger A.M., Lohse M., Usadel B. Trimmomatic: a flexible trimmer for Illumina sequence data. Bioinformatics. 2014;30:2114–2120. doi: 10.1093/bioinformatics/btu170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bankevich A., Nurk S., Antipov D., Gurevich A.A., Dvorkin M., Kulikov A.S., Lesin V.M., Nikolenko S.I., Pham S., Prjibelski A.D., et al. SPAdes: a new genome assembly algorithm and its applications to single-cell sequencing. J. Comput. Biol. 2012;19:455–477. doi: 10.1089/cmb.2012.0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Harris R.S. Pennsylvania State University, State College; 2007. Improved Pairwise Alignment of Genomic DNA. [Google Scholar]
- 66.Katoh K., Standley D.M. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol. Biol. Evol. 2013;30:772–780. doi: 10.1093/molbev/mst010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Talavera G., Castresana J. Improvement of phylogenies after removing divergent and ambiguously aligned blocks from protein sequence alignments. Syst. Biol. 2007;56:564–577. doi: 10.1080/10635150701472164. [DOI] [PubMed] [Google Scholar]
- 68.Borowiec M.L. AMAS: a fast tool for alignment manipulation and computing of summary statistics. PeerJ. 2016;4 doi: 10.7717/peerj.1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hoang D.T., Chernomor O., Von Haeseler A., Minh B.Q., Vinh L.S. UFBoot2: improving the ultrafast bootstrap approximation. Mol. Biol. Evol. 2018;35:518–522. doi: 10.1093/molbev/msx281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Guindon S., Dufayard J.F., Lefort V., Anisimova M., Hordijk W., Gascuel O. New algorithms and methods to estimate maximum-likelihood phylogenies: assessing the performance of PhyML 3.0. Syst. Biol. 2010;59:307–321. doi: 10.1093/sysbio/syq010. [DOI] [PubMed] [Google Scholar]
- 71.Abadi S., Azouri D., Pupko T., Mayrose I. Model selection may not be a mandatory step for phylogeny reconstruction. Nat. Commun. 2019;10:934. doi: 10.1038/s41467-019-08822-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yang Z. Oxford University Press; 2006. Computational Molecular Evolution. [Google Scholar]
- 73.Kalyaanamoorthy S., Minh B.Q., Wong T.K.F., Von Haeseler A., Jermiin L.S. ModelFinder: fast model selection for accurate phylogenetic estimates. Nat. Methods. 2017;14:587–589. doi: 10.1038/nmeth.4285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Borowiec M.L., Lee E.K., Chiu J.C., Plachetzki D.C. Extracting phylogenetic signal and accounting for bias in whole-genome data sets supports the Ctenophora as sister to remaining Metazoa. BMC Genom. 2015;16:1–15. doi: 10.1186/s12864-015-2146-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.R Core Team . R: A language and environment for statistical computing. R Foundation for Statistical Computing; Vienna, Austria: 2018. https://www.R-project.org/ [Google Scholar]
- 76.Paradis E., Schliep K. ape 5.0: an environment for modern phylogenetics and evolutionary analyses in R. Bioinformatics. 2019;35:526–528. doi: 10.1093/bioinformatics/bty633. [DOI] [PubMed] [Google Scholar]
- 77.Grimaldi D., Agosti D. A formicine in New Jersey Cretaceous amber (Hymenoptera: Formicidae) and early evolution of the ants. Proc. Natl. Acad. Sci. USA. 2000;97:13678–13683. doi: 10.1073/pnas.240452097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Perkovsky E.E. Tropical and Holarctic ants in Late Eocene ambers. Vestnik zoologii. 2016;50:111–122. [Google Scholar]
- 79.Blaimer B.B., Brady S.G., Schultz T.R., Lloyd M.W., Fisher B.L., Ward P.S. Phylogenomic methods outperform traditional multi-locus approaches in resolving deep evolutionary history: a case study of formicine ants. BMC Evol. Biol. 2015;15:1–14. doi: 10.1186/s12862-015-0552-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Economo E.P., Narula N., Friedman N.R., Weiser M.D., Guénard B. Macroecology and macroevolution of the latitudinal diversity gradient in ants. Nat. Commun. 2018;9:1778. doi: 10.1038/s41467-018-04218-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rambaut A., Drummond A.J., Xie D., Baele G., Suchard M.A. Posterior summarization in Bayesian phylogenetics using Tracer 1.7. Syst. Biol. 2018;67:901–904. doi: 10.1093/sysbio/syy032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rambaut A. Figtree, a graphical viewer of phylogenetic trees [Internet] 2014. http://tree.bio.ed.ac.uk/software/figtree
- 83.Pybus O.G., Harvey P.H. Testing macro–evolutionary models using incomplete molecular phylogenies. Proc. Biol. Sci. 2000;267:2267–2272. doi: 10.1098/rspb.2000.1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Burnham K.P., Anderson D.R. Multimodel inference: understanding AIC and BIC in model selection. Socio. Methods Res. 2004;33:261–304. [Google Scholar]
- 85.Guénard B., Weiser M.D., Gomez K., Narula N., Economo E.P. The Global Ant Biodiversity Informatics (GABI) database: synthesizing data on the geographic distribution of ant species (Hymenoptera: Formicidae) Myrmecological News/Osterreichische Gesellschaft fur Entomofaunistik. 2017;24:83–89. [Google Scholar]
- 86.Kiran K., Karaman C. Additions to the ant fauna of Turkey (Hymenoptera, Formicidae) Zoosystema. 2020;42:285–329. [Google Scholar]
- 87.Ree R.H., Smith S.A. Maximum likelihood inference of geographic range evolution by dispersal, local extinction, and cladogenesis. Syst. Biol. 2008;57:4–14. doi: 10.1080/10635150701883881. [DOI] [PubMed] [Google Scholar]
- 88.Ronquist F. Dispersal-vicariance analysis: a new approach to the quantification of historical biogeography. Syst. Biol. 1997;46:195–203. [Google Scholar]
- 89.Landis M.J., Matzke N.J., Moore B.R., Huelsenbeck J.P. Bayesian analysis of biogeography when the number of areas is large. Syst. Biol. 2013;62:789–804. doi: 10.1093/sysbio/syt040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Freckleton R.P., Harvey P.H. Detecting non-Brownian trait evolution in adaptive radiations. PLoS Biol. 2006;4 doi: 10.1371/journal.pbio.0040373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Aristide L., Morlon H. Understanding the effect of competition during evolutionary radiations: an integrated model of phenotypic and species diversification. Ecol. Lett. 2019;22:2006–2017. doi: 10.1111/ele.13385. [DOI] [PubMed] [Google Scholar]
- 92.Felsenstein J. Maximum-likelihood estimation of evolutionary trees from continuous characters. Am. J. Hum. Genet. 1973;25:471–492. [PMC free article] [PubMed] [Google Scholar]
- 93.Butler M.A., King A.A. Phylogenetic comparative analysis: a modeling approach for adaptive evolution. Am. Nat. 2004;164:683–695. doi: 10.1086/426002. [DOI] [PubMed] [Google Scholar]
- 94.Harmon L.J., Losos J.B., Jonathan Davies T., Gillespie R.G., Gittleman J.L., Bryan Jennings W., Mooers A.Ø., McPeek M.A., Moreno-Roark F., Near T.J., et al. Early bursts of body size and shape evolution are rare in comparative data. Evolution. 2010;64:2385–2396. doi: 10.1111/j.1558-5646.2010.01025.x. [DOI] [PubMed] [Google Scholar]
- 95.Pagel M. Inferring the historical patterns of biological evolution. Nature. 1999;401:877–884. doi: 10.1038/44766. [DOI] [PubMed] [Google Scholar]
- 96.O'Meara B.C. Evolutionary inferences from phylogenies: a review of methods. Annu. Rev. Ecol. Evol. Syst. 2012;43:267–285. [Google Scholar]
- 97.Weir J.T., Mursleen S. Diversity-dependent cladogenesis and trait evolution in the adaptive radiation of the auks (Aves: Alcidae) Evolution. 2013;67:403–416. doi: 10.1111/j.1558-5646.2012.01786.x. [DOI] [PubMed] [Google Scholar]
- 98.Nuismer S.L., Harmon L.J. Predicting rates of interspecific interaction from phylogenetic trees. Ecol. Lett. 2015;18:17–27. doi: 10.1111/ele.12384. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A. Number of workers of each species collected in each site during the field experiment.
B. Size of foragers and weight of their associated prey for each species in each site in the field experiment.
A. P-values for comparison within and among sites of Cataglyphis species forager head width.
B. P-values for comparison within and among sites of the weight of preys collected by Cataglyphis species.
Data Availability Statement
-
•
Raw Illumina reads have been deposited at the NCBI Sequence Read Archive and are publicly available as of the date of publication. Accession numbers are listed in the Data S1; see key resources table. SPAdes assembly contigs, concatenated UCE matrices, tree files, and the UCE bait sequence file have been deposited at Dryad: https://doi.org/10.5061/dryad.sbcc2frck). DOI is given in the key resources table.
-
•
This paper does not report original code.
-
•
Any additional information required to reanalyse the data reported in this paper is available from the lead contact upon request.