Abstract
Introduction and importance
Giant ovarian cysts are rare and usually pose significant diagnostic challenges especially in adolescents and young adults. There is limited report of such cases reported in existing literature with hardly any cases published from the Sub-Sharan African region.
Case presentation
We present the case of a 24-year-old young woman who reported to our gynaecology clinic on the 23rd of January 2023 with a year's history of a progressively increasing abdominopelvic mass. She was successfully managed surgically and made smooth recovery.
Clinical discussion
Based on the history and examination findings, confirmed the diagnosis clinically with abdomino-pelvic ultrasound scan, removed the tumour surgically and undertook histopathological studies to confirm a benign disease. To the best of our knowledge, our successful management of this patient is the first case of such a huge borderline ovarian tumour reported in Ghana and the Sub-Saharan African region to inform clinicians on safe surgical management in our context.
Conclusion
Our successful management of this giant mucinous BOT reiterates the fact that in the absence of precise prognostic marker of malignancy, clinicians should always balance the oncologic safety of the patient against less radical treatment modality.
Keywords: Ovarian, Cyst, Mucinous, Borderline, Tumour, Intraepithelial, Carcinoma, Young, Woman
Highlights
-
•
Giant ovarian cysts have become rare due early presentation and availability of modern imaging modalities.
-
•
They pose significant diagnostic challenges especially in adolescents and young adults.
-
•
Data is limited especially from Sub-Sharan African region.
-
•
We successfully managed the giant mucinous borderline ovarian tumour with about 25litres in a 24year old woman.
-
•
Clinicians should balance the oncologic safety of the patient against less radical treatment modality.
1. Introduction
Ovarian cysts are defined by their sizes and histopathological composition. Giant ovarian cyst are cysts >10 cm in diameter on radiological assessment [1]. Clinically, a cyst palpable above the umbilicus is defined as a giant ovarian cyst. Giant ovarian cysts have become rare due early presentation and widespread availability of modern imaging modalities. Large cysts are usually rare and usually pose diagnostic challenges especially in adolescents and young adults. Serous tumors are the most common cystic neoplasms of the ovary, 60 % of which are benign serous cystadenoma [1,2]. Surgical management is the preferred treatment for extra-large ovarian cysts [2,3]. There is limited report of such cases reported in existing literature with hardly any cases published from the Sub-Sharan African region. This work has been reported in line with the SCARE criteria [12].
2. Case presentation
We present the case of a 24-year-old young woman who reported to the gynaecology clinic at our Facility on the 23rd of January 2023 with a year's history of a progressively increasing abdominopelvic mass. She had been diagnosed with a small ovarian cyst about 18 months' prior, which was managed conservatively. There was no significant associated pain with the mass, and there was no vomiting or weight loss. She however noticed increasing abdominal girth a year prior to presentation and had sought help in various health facilities and herbal clinics without a definite solution, and hence her visit to our Facility. She had no other significant past medical or surgical history, attained menarche at 12 years and had regular 30 day menstrual cycles out of which she bled 4-5 days with associated dysmenorrhea. Her last menstrual period (LMP) was on the 11th of January 2023.
Our initial assessment revealed a fully conscious young woman who looked unwell, not pale and anicteric. She was in mild respiratory distress (respiratory rate 20 cycles per minute), tachycardic (pulse 108 beats per minute) but had a normal blood pressure of 118/74 mmHg. Her erect posture depicted significant lordosis due to the prolonged severe abdominal distension. There was also bilateral pitting pedal edema up to the groin. Her abdominal examination revealed a grossly distended abdomen, tense and mildly tender with a Visual Analog Scale of 4 out of 10. There was no definite mass palpable due to the tension of the distension.
Abdominopelvic ultrasound scan showed a large cystic abdominal mass arising from the pelvis with echogenic fluid noted with no septations, internal echoes and papillary excrescences. There was no ascites or sonographic evidence of metastasis, pelvic or para-aortic lymphadenopathy detected. A subsequent abdomino-pelvic MRI findings were similar to the ultrasound scan findings. A complete blood count results were normal, renal and liver function tests were also normal. The tumour markers were essentially normal save an elevated cancer antigen 125 (CA-125) of 54.3u/ml. Electrocardiogram, chest x-ray and Doppler ultrasound scan of the lower limbs were all essentially normal.
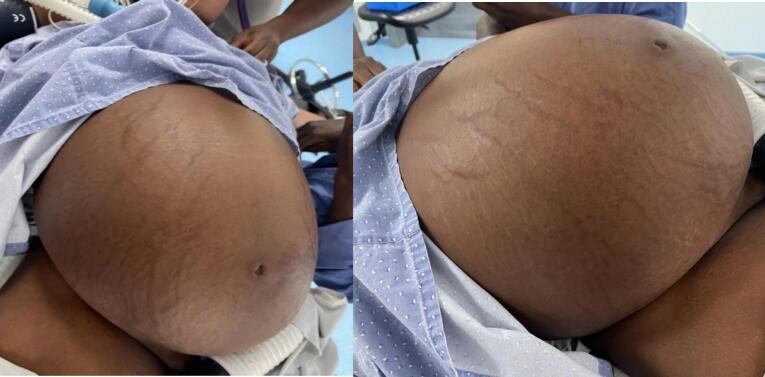
Hence, the clinical radiological diagnosis of a large cystic ovarian abdominopelvic mass was made with a suspicion of a cystadenoma or cystadenocarcinoma, with a differential diagnosis of a large mesenteric cyst (Fig. 1).
Fig. 1.
a and 1: Tense abdominal distension.
3. Management
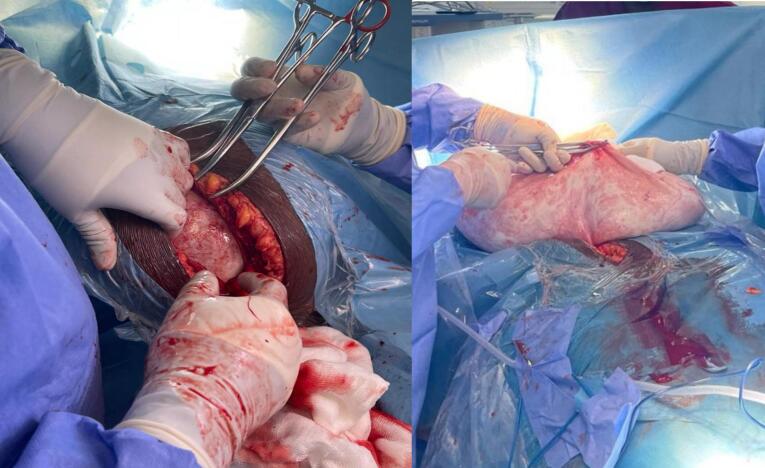
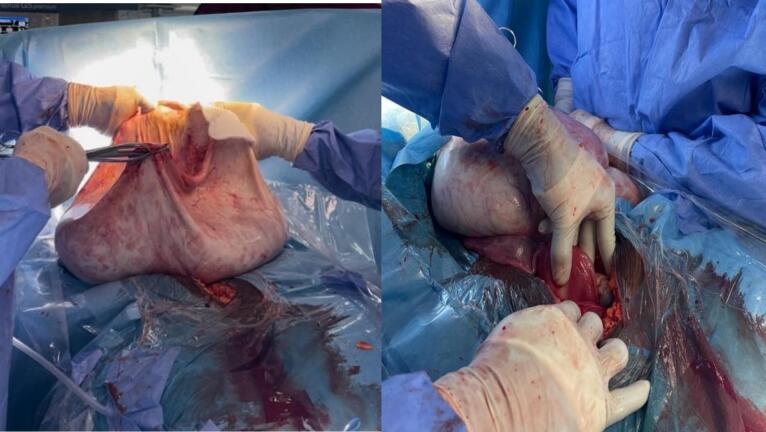
She was appropriately counselled on the diagnosis and need for surgery to which she agreed. Client was subsequently prepared for laparotomy under general anaesthesia. Pre-operative preparations included an anaesthetic assessment, psychological counselling and appropriate pre-operative hydration. Although the abdominal mass measured approximately 50 cm in the longitudinal dimension, a midline sub-umbilical incision was placed (Fig. 2a). There was no ascites and all peritoneal surfaces were free of tumour seedlings. The mass could be palpated completely with a smooth, regular capsular surface. About 20 l of serosanguinous fluid was drained through a 2 cm window made on the anterior aspect of the cyst wall (Fig. 2b). After the initial drainage, there was significant decompression of the cyst. The cyst sac (capsule) (Fig. 3a) was delivered through the incision with another 3 to 4 l of fluid in situ.
Fig. 2.
(a) abdominal incision (b)2 cm opening in the anterior cyst wall.
Fig. 3.
(a) Cyst wall (b) normal uterus left tube and ovary.
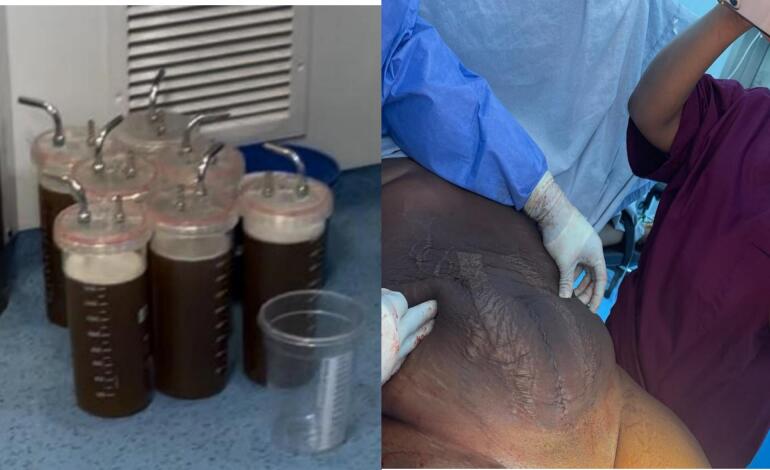
The total volume of serosanguinous cystic fluid drained was 24 l (Fig. 4a). The uterus, left fallopian tube and ovary appeared grossly normal. A right salpingo ophorectomy was done and there was no evidence of ovarian or extra ovarian residual disease, extra pelvic or peritoneal involvement. There were no palpable para-aortic or pelvic lymph nodes. She made satisfactory post-operative recovery. The immediate post-operative period was relatively uneventful, however, she had to undergo 2 weeks of physiotherapy to regain her normal ambulatory posture due to an exaggerated lumbar lordosis. The surgery was performed by a Consultant Obstetrician Gynaecologist assisted by two Medical Officers and the peri-operative nurse.
Fig. 4.
(a) Serosanguinous fluid cyst content (b) Repaired (closed) abdominal incision.
Client made a full recovery and by the second post-operative review (40 days' post-surgery), she was fully fit and had resumed her regular work. The histopathological examination of the specimen reported a Mucinous Borderline Tumour With Intraepithelial Carcinoma. There was no histological evidence of microinvasion or capsular involvement. She had 2 subsequent follow-up reviews at three months intervals with transvaginal ultrasound scans done on both occasions which revealed a normal left ovary and uterus. There was no evidence of recurrent disease in the left ovary.
This work has been reported in line with the SCARE criteria [12].
4. Discussion
We reported the case of a 24-year-old woman who was successfully managed for a giant ovarian cyst. Giant ovarian cysts are rare and potentially pose both diagnostic and management challenges. There are very few reports of ovarian cysts with fluid volumes >20 l.
Although ovarian cysts are seen in children, girls and women of all ages, giant ovarian cysts have become uncommon in contemporary gynaecology [1]. This is attributable to more regular physical examination by women; better, easily accessible imaging techniques and promptness to seek help with abdominal discomfort, pain or distension [1].
Ovarian cysts rarely reach a large size and are generally asymptomatic at early stages and cause symptoms only after reaching enormous dimensions. These symptoms include progressive abdominal distension, nonspecific abdominal pain, constipation, early satiety, vomiting and increased frequency of micturition [2,3]. Extra-large benign and malignant cysts of the ovary are uncommon and present diagnostic and management challenges as seen in this case. According to Gershenson et al., 30 % of patients with BOTs are asymptomatic prior to diagnosis, and while up to 60 % would present with non-specific symptoms such as abdominal pain, abdominal distention, nonspecific vaginal bleeding, and dyspareunia [13]. Another study reported 84 % oof patients with BOT having these non-specific symptoms lasting an average of 6 months before diagnosis [14].
.The patient in this case reported had been diagnosed of a small ovarian cyst nearly two years earlier which was managed conservatively. She began experiencing symptoms of abdominal distension and pain when the mass caused worsening abdominal distension. Our differential diagnosis of a large mesenteric cyst reflected a diagnostic challenge and the appropriate incision for the surgery also posed a management challenge in the case of our patient.
Diagnosis: clinical usg/mri, tumour marker,
Generally, preoperative diagnosis of BOTs has remained a difficult challenge, making clinician s to depend on speculative diagnosis based on clinical manifestations, imaging findings and results of serum tumour markers [15]. However, the final diagnosis needs to be supported by a pathological diagnosis encompassing the unusual degree of epithelial cell proliferation, and the existence of microinvasion [15].
.Diagnostic imaging modalities including transvaginal ultrasound (TVUS), magnetic resonance imaging (MRI), and computed tomography (CT) are useful in presumptively diagnosing BOTs with reported sensitivities and specificities of 77.0 % and 83.0 % (TVUS), 85 % and 74 % (MRI), respectively [16]. Regarding the use ultrasound imaging, some studies have limited value with a reported accuracy of approximately 69 % [17]. The absence of typical morphological features in BOTs, on ultrasound makes it difficult to distinguish them from benign or malignant ovarian tumors [18]. Similarly, the value of CT and MRI features in differentiating BOTs from malignant tumors appear limited because of the tumour's solid components and thickness oof their septations [19]. Our patient's preoperative evaluation involved both abdomeno-pelvic ultrasound scan and MRI.
The place of tumour markers has remained under consideration globally. One study reported that, when compared with a single tumour marker, such as cancer antigen 125 (CA125), and human epididymis protein-4 (HE4) and cancer antigen 19–9 (CA19–9), the risk algorithm for ovarian cancer (ROMA, combined CA125, HE4 and being in menopausal state) was found to be the best way to distinguish malignant and borderline tumors from benign tumors [20].
.Other researchers also indicated a combination of the malignant tumour risk index, CA125, ultrasound and Menopausal state was the best means to distinguish between borderline and benign ovarian tumors [17] [18]. Using the cutoff value of RMI1, RMI2 and RMI as 200, the sensitivities were 80 %, 90 %, 80 %, respectively, and the specificities are 86.4 %, 82.6 %, and 86.4 %, respectively. At the cutoff value of 450, RMI 4 has a sensitivity of 86.8 % and a specificity of 91.0 % [21]. Our patient's CA125 was moderately elevated to 54.3u/ml.
Many problems are linked with surgical care of these giant ovarian cysts including significant hypotension following evacuation of the cyst, heart failure, respiratory failure, intestinal distention, and hypovolemic shock [4,5]. Appropriate pre-operative assessment and preparation is required prior to the commencement of the surgery. This was done in this case to reduce the risk of occurrence of complications in this patient presented.
Most giant ovarian tumors are serous cystadenomas, however, mucinous tumors are also quite commonly diagnosed giant ovarian tumors [5]. In this case, a diagnosis of mucinous borderline tumour with intraepithelial carcinoma was made on histology. Borderline ovarian tumors (BOT) represent a heterogeneous group of noninvasive tumors of uncertain malignant potential with characteristic histological features [5]. They are a group of epithelial tumors acknowledged by the International Federation of Gynaecology and Obstetrics (FIGO) in 1961 and adopted by the World Health Organization (WHO) in 1973 [5]. They make up about 15 %–20 % of all epithelial ovarian malignancies with an incidence of 1.8–4.8 per 100,000 women per year [[5], [6], [7], [8]]. Notably, they occur in younger women, are present at an early stage, and have a favorable prognosis, but symptomatic recurrence may be found as long as 20 years after [5]. In the case of our patient, she was 24 years old and consistent with existing literature.
Most BOTs are serous (53.3 %) but mucinous tumors constitute 42.5 % of all BOTs [5]. Mucinous BOTs are difficult masses to diagnose preoperatively using imaging methods because their macroscopic features may overlap with invasive and benign ovarian tumors. There is no standard tumour marker for mucinous BOTs and CA125 is often negative in patients with borderline tumors [5]. Du Bois et al. in their systematic review found negative (CA125 ≤ 35 U/ml) in 53.8 % of the 1937 patients with borderline tumors and the multicentre prospective International Ovarian Trial Analysis (IOTA) reported negative CA125 levels in 53 % of patients with BOTs [9,10]. In the case we presented, her CA125 was marginally elevated at 54.3u/ml, and the radiological imaging tests including both abdominopelvic ultrasound scan and MRI were less definitive. Risk factors for mucinous BOTs are similar to other ovarian carcinomas with a few cases associated with BRCA 1 and 2 mutations and cytogenesis associated with mutations in KRAS codons 12 and 13 [5]. Our patient did not have any significant risk factor.
A third of patients diagnosed with BOTs are younger than 40 years and frequently are candidates for fertility-sparing surgery [5] as was the case in this client presented. In recent times, surgical therapy has shifted from a radical approach to more conservative treatment especially in younger patients; however, oncologic safety must always be balanced. For patients with early-stage disease, the extra ovarian recurrence only occurs in 2 % of patients compared with 20 % of patients with advanced disease (FIGO stages II-III) [11]. Follow-up is essential using routine ultrasound imaging, with special attention paid to the contralateral ovary in conservatively treated patients [5]. During the initial 2 years, follow-up evaluation is performed every 3 months. Patients are then evaluated biannually for 3–5 years after surgery, and then annually thereafter [5]. Transvaginal and transabdominal ultrasound are currently the optimal techniques for the surveillance of patients treated for BOTs because of their high ability to detect discrete intraovarian abnormalities as well as extraovarian implants when performed by an experienced examiner. Our patient underwent laparotomy due the size of the tumour and duration of her disease. Following discharge, she was followed up every three months and she had thorough transvaginal ultrasound scan performed to examine the contralateral (left) ovary. The management of BOTs.
Multidisciplinary team (MDT) approach to managing BOTs have been shown as beneficial to patients. MDT ensures accurate diagnosis, appropriate and minimally invasive management, and minimal psychological impact of the disease. Further, because the tumour affects mostly reproductive age women, fertility preservation through fertility-sparing surgery could be offered with involvement of reproductive endocrinologists in the management. The team typically would include paediatric oncologists, gynaecological surgeons, pathologists, radiologists, fertility experts, geneticists and clinical psychologists and social worker [22]. The team members share the responsibility to assess the scope of the health problem, planning the management and supporting the patient and family through diagnosis, treatment and aftercare in the patients socio-cultural for improved overall outcome [22].
.Mucinous BOTs are classified as gastrointestinal (or intestinal) type (85 %) which seldom present with peritoneal implants and endocervical-like type (also referred as Müllerian or seromucinous) (15 %), depending on the histological architecture and type of tumour cells [5]. Mucinous tumors with high-grade atypias without invasion are classified as BOTs with intraepithelial carcinoma as in the index case. Borderline tumors with intraepithelial carcinoma and/or microinvasion provide evidence that these tumors form a morphologic spectrum with individual types representing steps in the sequence of mucinous carcinogenesis in the ovary. There were no peritoneal implants, capsular invasions noted intraoperatively, nor microinvasions detected at histology in the case we presented. The patient's 3-monthly follow up is planned to continue for the first two years, to be followed by twice yearly visits till the fifth year post surgery.
5. Conclusion
Our case reported the successful management of a giant mucinous BOT with about 25 l in a 24 year old woman in Ghana surgically. Our successful management of this giant mucinous BOT reiterates the fact that in the absence of precise prognostic marker of malignancy, clinicians should always balance the oncologic safety of the patient against less radical treatment modality.
Strength and weakness
To the best of our knowledge, this is the first case of such a huge Borderline Ovarian Tumour reported in Ghana and the Sub-Saharan African region to inform clinicians on safe surgical management in our context. The inherent limitations of a single case report would necessitate a larger study with multiple client numbers to generate larger data for clinical interventions in our context.
Ethics statement
Ethical approval was not applicable, however, written informed consent from the patient, as well as permission were obtained prior to publication of this case report.
Patient consent
Written informed consent was obtained from the patient for publication and any accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
Authors' contribution
CNO performed the surgery as lead surgeon and conceptualized the idea of the manuscript, drafted first manuscript. PT, ASG, JKAA and CKK all assisted in the patient management and review of draft manuscript. PES wrote part of the manuscript and reviewed the write-up.
All authors have read and approved the final manuscript for publication.
Funding
This work received no funding.
Declaration of competing interest
All authors declared no conflict of interest.
Acknowledgement
The authors express their profound appreciation to the Management and staff in the Gynaecology, Anaesthesia, Physiotherapy and Pathology departments of the University of Ghana Medical Center.
References
- 1.Pramana C., Almarjan L., Mahaputera P., Wicaksono S.A., Respati G., Wahyudi F., Hadi C. A Giant ovarian cystadenoma in a 20-year-old nulliparous woman: a case report. Front. Surg. 2022;May 5(9) doi: 10.3389/fsurg.2022.895025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yeika E.V., Efie D.T., Tolefac P.N., Fomengia J.N. Giant ovarian cyst masquerading as a massive ascites: a case report. BMC. Res. Notes. 2017;Dec 19;10(1):749. doi: 10.1186/s13104-017-3093-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Narayan D., Jain A., Shrivastava A., et al. Giant ovarian cyst presenting as an intraabdominal mass. J Evolution Med Dent Sci. 2022;11(03):428–430. [Google Scholar]
- 4.Boyd C.A., Riall T.S. Unexpected gynecologic findings during abdominal surgery. Curr. Probl. Surg. 2012;Apr;49(4):195–251. doi: 10.1067/j.cpsurg.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fischerova D., Zikan M., Dundr P., Cibula D. Diagnosis, treatment, and follow-up of borderline ovarian tumors. Oncologist. 2012;17(12):1515–1533. doi: 10.1634/theoncologist.2012-0139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Katsube Y., Berg J.W., Silverberg S.G. Epidemiologic pathology of ovarian tumors: a histopathologic review of primary ovarian neoplasms diagnosed in the Denver standard metropolitan statistical area, 1 July-31 December 1969 and 1 July-31 December 1979. Int. J. Gynecol. Pathol. 1982;1:3–16. doi: 10.1097/00004347-198201000-00003. [DOI] [PubMed] [Google Scholar]
- 7.Skirnisdottir I., Garmo H., Wilander E., et al. Borderline ovarian tumors in Sweden 1960–2005: trends in incidence and age at diagnosis compared to ovarian cancer. Int. J. Cancer. 2008;123:1897–1901. doi: 10.1002/ijc.23724. [DOI] [PubMed] [Google Scholar]
- 8.Bjorge T., Engeland A., Hansen S., et al. Trends in the incidence of ovarian cancer and borderline tumours in Norway, 1954–1993. Int. J. Cancer. 1997;71:780–786. doi: 10.1002/(sici)1097-0215(19970529)71:5<780::aid-ijc15>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 9.Du Bois A., Ewald-Riegler N., Du Bois O., et al. Borderline tumors of the ovary: a systematic review. Geburtshilfe Frauenheilkd. 2009;69:807–833. [Google Scholar]
- 10.Kaijser J. Towards an evidence-based approach for diagnosis and management of adnexal masses: findings of the international ovarian tumour analysis (IOTA) studies. Facts Views Vis. Obgyn. 2015;7(1):42–59. [PMC free article] [PubMed] [Google Scholar]
- 11.Seong S.J., Kim D.H., Kim M.K., Song T. Controversies in borderline ovarian tumors. J. Gynecol. Oncol. 2015;Oct;26(4):343–349. doi: 10.3802/jgo.2015.26.4.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sohrabi C., Mathew G., Maria N., Kerwan A., Franchi T., Agha R.A. The SCARE 2023 guideline: updating consensus surgical CAse REport (SCARE) guidelines. Int J Surg Lond Engl. 2023;109(5):1136. doi: 10.1097/JS9.0000000000000373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gershenson D.M. Management of borderline ovarian tumours. Best Pract. Res. Clin. Obstet. Gynaecol. 2017;May 1(41):49–59. doi: 10.1016/j.bpobgyn.2016.09.012. [DOI] [PubMed] [Google Scholar]
- 14.Vine M.F., Ness R.B., Calingaert B., Schildkraut J.M., Berchuck A. Types and duration of symptoms prior to diagnosis of invasive or borderline ovarian tumor. Gynecol. Oncol. 2001;83(3) doi: 10.1006/gyno.2001.6411. [DOI] [PubMed] [Google Scholar]
- 15.Ushijima K., Kawano K., Tsuda N., Nishio S., Terada A., Kato H., Tasaki K., Matsukuma K. Epithelial borderline ovarian tumor: diagnosis and treatment strategy. Obstetrics & Gynecology Science. 2015;May;58(3):183. doi: 10.5468/ogs.2015.58.3.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Borrelli G.M., de Mattos L.A., Andres M.P., Goncalves M.O., Kho R.M., Abrao M.S. Role of imaging tools for the diagnosis of borderline ovarian tumors: a systematic review and meta-analysis. J. Minim. Invasive Gynecol. 2017;24(3):353–363. doi: 10.1016/j.jmig.2016.12.012. [DOI] [PubMed] [Google Scholar]
- 17.Yazbek J., Raju K.S., Ben-Nagi J., Holland T., Hillaby K., Jurkovic D. Accuracy of ultrasound subjective ‘pattern recognition’ for the diagnosis of borderline ovarian tumors. Ultrasound Obstet. Gynecol. 2007;29(5):489–495. doi: 10.1002/uog.4002. [DOI] [PubMed] [Google Scholar]
- 18.Yazbek J., Ameye L., Timmerman D., et al. Use of ultrasound pattern recognition by expert operators to identify borderline ovarian tumors: a study of diagnostic performance and interobserver agreement. Ultrasound Obstet. Gynecol. 2010;35(1):84–88. doi: 10.1002/uog.7334. [DOI] [PubMed] [Google Scholar]
- 19.Yang S., Tang H., Xiao F., Zhu J., Hua T., Tang G. Differentiation of borderline tumors from type I ovarian epithelial cancers on CT and MR imaging. Abdom Radiol (NY). 2020 doi: 10.1007/s00261-020-02467-w. [DOI] [PubMed] [Google Scholar]
- 20.Shin K.H., Kim H.H., Kwon B.S., Suh D.S., Joo J.K., Kim K.H. Clinical usefulness of cancer antigen (CA) 125, human epididymis 4, and CA72-4 levels and risk of ovarian malignancy algorithm values for diagnosing ovarian tumors in korean patients with and without endometriosis. Ann. Lab. Med. 2020;40(1):40–47. doi: 10.3343/alm.2020.40.1.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Valentin L., Ameye L., Testa A., et al. Ultrasound characteristics of different types of adnexal malignancies. Gynecol. Oncol. 2006;102(1):41–48. doi: 10.1016/j.ygyno.2005.11.015. [DOI] [PubMed] [Google Scholar]
- 22.van Heerden J., Tjalma W.A. The multidisciplinary approach to ovarian tumours in children and adolescents. Eur. J. Obstet. Gynecol. Reprod. Biol. 2019;243:103–110. doi: 10.1016/j.ejogrb.2019.10.032. [DOI] [PubMed] [Google Scholar]