Abstract
Lactic acid was formerly regarded as a byproduct of metabolism. However, extensive investigations into the intricacies of cancer development have revealed its significant contributions to tumor growth, migration, and invasion. Post-translational modifications involving lactate have been widely observed in histone and non-histone proteins, and these modifications play a crucial role in regulating gene expression by covalently attaching lactoyl groups to lysine residues in proteins. This discovery has greatly enhanced our comprehension of lactic acid’s involvement in disease pathogenesis. In this article, we provide a comprehensive review of the intricate relationship between lactate and tumor immunity, the occurrence of lactylation in malignant tumors, and the exploitation of targeted lactate-lactylation in tumor immunotherapy. Additionally, we discuss future research directions, aiming to offer novel insights that could inform the investigation, diagnosis, and treatment of related diseases.
Keywords: lactate, lactylation, metabolic reprogramming, tumor immunotherapy, microenvironment
1. Introduction
During the process of glycolysis, pyruvate molecules are converted into lactate through the action of cytoplasmic lactate dehydrogenase (LDH), rather than directly entering the tricarboxylic acid (TCA) cycle (1). In 1923, Otto Heinrich Warburg made the observation that cancer cells exhibit a proclivity for producing significant amounts of lactate via glycolysis, irrespective of the presence of oxygen ( Figure 1 ). This observation came to be known as the Warburg effect (2). Subsequent investigations have revealed that lactate serves as a signaling molecule, exerting notable influences on immune cell function, immune response modulation, cell metabolism regulation, and immune surveillance (3–5). The tumor microenvironment (TME) constitutes a multifaceted network comprising tumor cells, stromal cells, blood vessels, endothelial cells, growth factors, nutrients, and cell metabolites (6). Expanding upon the postulated Warburg effect hypothesis, researchers have observed that the release of lactate from tumor cells contributes to the acidification of the TME. This acidic microenvironment promotes tumor angiogenesis, triggers metastasis development, induces drug resistance, and facilitates immune evasion (7). Recent studies have additionally indicated that cancer cells can utilize lactate as an energy source (8, 9). Consequently, therapeutic strategies targeting metabolic processes, including lactate synthesis, have emerged as potential innovative approaches for the treatment of cancer patients (10).
Figure 1.
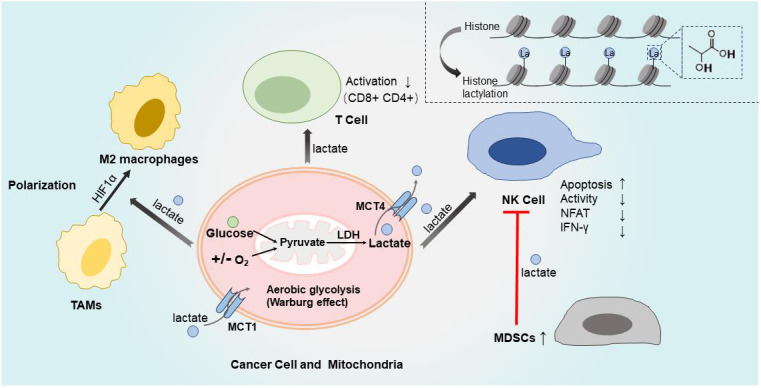
Lactate and tumor immune microenvironment. Cancer cells produced significant amounts of lactate via glycolysis, irrespective of the presence of oxygen, which is called Warburg effect. During the process of glycolysis, pyruvate conversion to lactate through the action of LDH. Lactate are export by MCT4 from cytoplasm to extracellular fluid, MCT1 import lactate to cytoplasm. Then different kinds of immune cells are influenced by the TME of high levels of lactate, with suppression of anti-tumor immune responses. The activation of effector CD8+ and CD4+ T cells is suppressed when the pH value decreased because lactate increased. High levels of lactate directly impede the activity of NK cells and induce apoptosis. Additionally, lactate inhibits the activation of NFAT in NK cells, resulting in reduced production of IFN-γ. Moreover, lactate indirectly suppresses NK cells by increasing the population of MDSCs.HIF1α promotes tumor growth and facilitates TAMs transformation into M2-like phenotype, which is induced by lactic acid derived from tumors. The top right corner represents the process of histone lactylation modification. LDH, lactate dehydrogenase; MCT1/4, monocarboxylate transporter 1/4; MDSCs, myelid derived suppressor cells; TAMs, transformation of tumor-associated macrophages; NFAT, nuclear factor-activated T cells; TME, tumor microenvironment; IFN-γ, Interferon γ.
Lactylation, alternatively known as lysine lactylation (Kla), is a post-translational modification (PTM) that involves the covalent attachment of lactic acid moieties to protein lysine residues, thereby exerting influence on gene expression regulations. The elucidation of lactylation has significantly broadened our comprehension of lactate’s role in biological systems. Consequently, the presence of lactylated histone and non-histone proteins holds paramount importance in the modulation of gene transcription (11). As a prevalent PTM, lactate-induced protein lactylation not only contributes to normal physiological processes (12), such as the regulation of immune homeostasis during cardiac repair (13), but also plays a significant role in the etiology and progression of various diseases, particularly cancer (14, 15). Evidence suggests that lactylation of tumor cells, tumor stem cells, and tumor-infiltrating immune cells in the TME can actively contribute to cancer progression through downstream modulation of gene expression, thus emerging as a promising therapeutic target in cancer treatment (16). However, our understanding of the intricate regulatory mechanisms involving lactate-induced lactylation in malignant tumors and the clinical potential of therapeutic interventions targeting this pathway remains incomplete.
Here, we have summarized recent literature in this area to gain a more encompassing understanding on the current research landscape, delineate potential avenues for future investigation, overcome the constraints of current cancer treatments, and present novel avenues for therapeutic strategies targeting lactate-induced lactylation.
2. Lactate and tumor immune microenvironment
The ability of cancer cells to undergo metabolic reprogramming and avoid detection by the immune system is regarded as an emerging hallmark of cancer (17). As previously mentioned, the Warburg effect is a pivotal aspect of energy metabolism in cancer cells, where they preferentially rely on glycolysis to sustain biosynthetic processes (18). This results in the production of high levels of lactate, actively maintaining an acidic TME that suppresses anti-tumor immune responses (19). Consequently, lactate plays a crucial role in bridging metabolic reprogramming with immune evasion mechanisms (20). Remarkably, lactate has intricate effects on both tumor cells and immune cells that infiltrate the tumor within the TME ( Figure 1 and Table 1 ).
Table 1.
Lactate-Lactylation in Malignancy and treatment.
| Malignancy | Objects | Intervention | Comments | Ref |
|---|---|---|---|---|
| Lactate and tumor immune microenvironment | ||||
| Neuroblastoma | Cell lines | 100% O2 or N2 | Warburg effect contribute to cellular lactic acid production. | (19) |
| Melanoma | Cell lines, mouse | MCT1 inhibitor | Treg cell specific deletion of MCT1 not only results in decreased tumor growth but synergy with checkpoint blockade immunotherapy. | (21) |
| Melanoma | Cell lines; mouse; human samples | LDH-A low; Lactate treatment | Increased lactic acid inhibits tumor immunosurveillance and promoting tumor growth. | (22) |
| Pancreatic cancer | Cell lines, mouse | LDH-A-deficient; Lactate treatment | Lactate inhibits NK cell function via direct inhibition of cytolytic function as well as indirectly by increasing the numbers of MDSCs. | (23) |
| Lung carcinoma; lung carcinoma; colon carcinoma | Cell lines, mouse | Hypoxia; HIF1a -/- | Lactic acid has a critical function in signaling, mediated by HIF1a, through inducing the M2-like polarization. | (24) |
| Breast cancer | Cell lines, mouse | Gpr132-KO; oxamic acid | Lactate activated M2-like macrophage, facilitates cancer cell adhesion, migration, and invasion. | (25) |
| Lysine lactylation in malignancy | ||||
| Non-small cell lung cancer | Cell lines, human samples | Lactate stock solution | Lactate modulates cellular metabolism through histone lactylation-mediated gene expression. | (26) |
| Hepatocellular carcinoma | Cell lines; human samples | Lactylome profiling; lactate treatment | Lactylation at K28 facilitates the proliferation and metastasis of hepatocellular carcinoma cells. | (27) |
| Glioblastoma | Cell lines, mouse, human samples | Bioinformatics analysis; Xenograft | NF-κB pathway promoted Warburg Effect, induced the lactylation of H3 histone associating with poor progression of glioblastoma. | (28) |
| Clear cell renal cell carcinoma | Cell lines; mouse; human samples | Xenograft; oxamate | PDGFRβ signaling is shown to stimulate histone lactylation, thereby forming an oncogenic positive feedback loop in ccRCC. | (29) |
| Prostate cancer | Cell lines; tissue microarray | Lactate treatment; silencing of KIAA1199 | Lactate is transcriptional enhancer of KIAA1199. Silencing of KIAA1199 inhibited angiogenesis and VM in pca. | (30) |
| Pancreatic ductal adenocarcino-ma | Cell lines, mouse; human tissue | NUSAP1 treatment | NUSAP1 plays a critical role in metastasis of PDAC by regulating lactate dehydrogenase A mediated glycolysis. | (31) |
| Melanoma | Cell lines, mouse, human tissues | lactylation inhibitors | Histone lactylation contributes to tumorigenesis by facilitating YTHDF2 expressio-n. | (32) |
| Melanoma | Cell lines, mouse, human tissues | Xenograft; ALKBH3; lactylation inhibitors(oxamate and 2-DG) | Histone lactylation increases the expression of ALKBH3 thereby accelerating tumor. | (33) |
| Colorectal cancer | Cell lines, mouse, human tissues | Xenograft; glycolytic inhibitors (oxamate and 2-DG); LDH-A; Bevacizumab | CRC patients resistant to bevacizumab presented with elevated levels of lactylation. | (34) |
| Colon cancer | Cell lines, mouse, human tissues | Xenograft; target to lactylation of MRE11 | Inhibition of CBP or LDH downregulated lactylation of MRE11 and enhanced chemosensitivity of tumor cells. | (35) |
| Gastric cancer | Cells lines, mouse, human tissues | Xenograft; copper stress; deacetylation enzyme | Elevated METTL lactylation improves the therapeutic efficacy of the copper ionophore elesclomol. | (36) |
| Neuroblastoma | Cells lines | Deacetylation enzyme (SIRT2) | As an efficient inhibition for multiple histone lactylation sites of histones in neuroblastoma cells. | (37) |
| Acute myeloid leukemia | Cell lines, human blood | Upregulated glycolysis (STAT5) | The accumulation of lactate driven by facilitated histone lactylation on PD-L1 promoter and ultimately induced PD-L1 expression. | (38) |
| Bladder cancer | Cell lines, mouse, human tissues | Overexpression of circXRN2 (transfect plasmids) | CircXRN2 suppresses tumor progression driven by H3K18 lactylation. | (39) |
| Lactate-Lactylation in Malignancy treatment | ||||
| MCT1-targeted treatment | ||||
| Advanced solid tumors or lymphoma | Human (Phase I trial) |
MCT1 inhibitor | AZD3965 is tolerated, the dose-limiting toxicities were on target and dose-dependent. A Phase 2 dose of 10 mg was established. | (40) |
| PD-1 & MCT1/4 | ||||
| MYC-amplified tumors and liver tumors | Cell lines, mouse, human and human tissues | MCT1; highly glycolytic; Anti-PD-1 mAb RMP1-14 or nivolumab | Treg cells actively absorbed LA through MCT1, enhancing the expression of PD-1, and dampening expression of PD-1 by effector T cells. | (41) |
| Melanoma | Cell lines, mouse, human tissues | m6A demethylases; anti–PD 1 pembrolizumab and nivolumab | Alkbh5 modulates Mct4/Slc16a3 expression, lactate content and the composition of tumor-infiltrating Treg and myeloid derived suppressor cells. | (42) |
| Hepatocellular carcinoma | Mouse; human tissues | MCT4 inhibition; anti–PD 1 toripalimab | Inhibition of MCT4 can heighten activity of CD8+ T cells and reduce acidification in tumor microenvironment. | (43) |
| Colorectal carcinoma | Cell lines, mouse; human blood | MCT4 inhibition; anti-PD-L1 antibody | Combination of MCT4 and ICB increased intratumoral pH, delayed tumor growth, and prolonged survival in vivo. | (44) |
| PD-1 & LDH-A | ||||
| Non-small cell lung cancer | Mouse | Oxamate; anti–PD 1 pembrolizumab | Preclinical findings: LDH inhibitor oxamate treatment enhanced the therapeutic effects of pembrolizumab. | (45) |
| Melanoma | Cell Lines; mouse | Deletion of LDH-A; Anti-PD-1 antibody (clone 29F.1A12) | Deficiency of LDH-A increased infiltration of NK cells and CD8+ cytotoxic T cells, improving the efficacy of anti-PD-1 therapy. | (46) |
| Cancer vaccines | ||||
| Melanoma and colon adenocarcinoma | Cell lines, mouse, Human blood | Glucose or sodium lactate; CD8+ T cellvaccine | HDAC inhibition induced by lactate enhanced CD8+ T cell exhaustion efficiently inhibit tumor growth. | (47) |
| Lymphoma | Cell lines, mouse | Lactic acid; irradiation | Lactic acid could augment the immunogenicity of whole UV-irradiated tumor cell vaccines. | (48) |
| CAR-T therapy | ||||
| Glioblastoma | Cell lines, mouse | Oxamate,LDH-A inhibitor; CAR-T cells | Oxamate promoted immune activation of tumor-infiltrating CAR-T cells. | (49) |
YTHDF2, YTH N6-methyladenosine RNA-binding protein 2; CRC, colorectal cancer; LDH, lactate dehydrogenase; HR,homologous recombination; PD-1/PD-L1, Programmed cell death protein 1/programmed cell death-ligand; MCT1/4, monocarboxylate transporter 1/4; LA,lactic acid; ROS, Reactive Oxygen Species; NF-κ, nuclear factor kappa-B;ICB, immune checkpoint blockade; DC, dendritic cell; MDSC, Myeloid-derived suppressor cells; CAR-T,chimeric antigen receptor T cell; NSCLC, non-small-cell lung cancer; ccRCC, clear cell renal cell carcinoma; VHL, Inactive von Hippel-Lindau; PDGFRβ, platelet-derived growth factor receptor β; HIF1α,hypoxia-inducible factor 1α. NUSAP1, Nucleolar and spindle associated protein 1; PDAC, pancreatic ductal adenocarcinoma; Gpr132,G protein-coupled receptor 132;KO/-/-,knock out.
Excessive lactate within the TME can hinder the effectiveness of anti-tumor immunity by interfering with the function of various immune cells that infiltrate the tumor (50). Watson MJ, et al., and Angelin, Alessia et al. (21, 51) have confirmed that the activation of effector CD8+ and CD4+ T cells is commonly suppressed when the pH of the TME falls within the range of 6.0 to 6.5, resulting in diminished cytotoxicity and cytokine production. Lactic acid plays a crucial role in enhancing the growth and performance of tumor-infiltrating regulatory T cells (Tregs). Kouidhi S, et al. (52) and Wu H, et al. (53) have demonstrated that the reversal of the acidic TME through the application of proton pump inhibitors can restore the inhibition of anti-tumor immunity and enhance immunotherapy, thereby corroborating these findings. Moreover, a number of studies have indicated that a high concentration of lactate can impede the activity of natural killer (NK) cells and induce apoptosis in these cells (54–56). Mechanistically, Brand A, et al. (22) revealed that lactic acid impedes the activation of nuclear factor-activated T cells (NFAT) in NK cells, resulting in reduced production of IFN-γ. Husain Z, et al. (23) discovered that lactate not only directly impairs the functionality of NK cells, but also indirectly suppresses these cells by increasing the population of myeloid-derived suppressor cells (MDSCs) ( Figure 1 ).
In a recent study by Colegio OR et al. (24), it was discovered that lactic acid derived from tumors plays a crucial role in inducing the transformation of tumor-associated macrophages (TAMs) into an M2-like phenotype. This process is facilitated by the activation of hypoxia-inducing factor 1α (HIF1α), which subsequently promotes tumor growth within the context of the TME ( Figure 1 ). Significantly, the regulation of extracellular signals also plays a crucial role in several intracellular signaling pathways, a mechanism that holds particular importance within TME (57). Consistent with this, Chen P. et al. (25, 58) demonstrated that lactate induces the polarization of M2 macrophages through the upregulation of vascular endothelial growth factor (VEGF) and arginase-1 (ARG1) via the extracellular signal-regulated kinase/transcription 3 (ERK/STAT3) signaling pathway.
3. Lysine lactylation in malignancy
As a ubiquitous biological process, lactylation has been proven to be associated with the growth of numerous cancers. Recent investigations have not just delved into its crucial role in ocular melanoma, colorectal cancer, gastric cancer, acute myeloid leukemia, and bladder cancer (details below), but also investigated its implications in non-small cell lung cancer (26), hepatocellular carcinoma (27), glioma (28), clear cell renal cell carcinoma (29), prostate cancer (30), and pancreatic ductal adenocarcinoma (31) ( Table 1 ). In a recent investigation involving 82 cases of ocular melanoma and 28 cases of normal tissues, researchers observed elevated levels of lactylation in tumor tissues compared to normal tissues, particularly at the histone H3K18 site. This process was found to hinder the proliferation and migration of tumor cells (32). Mechanistically, lactylation of H3K18 affects the development of ocular melanoma by regulating the reader protein YTHDF2, which is responsible for RNA m6A modifications. Notably, increased expression of YTHDF2 is associated with a negative prognosis for patients (59). Additional research has unveiled that histone lactylation increases the expression of ALKBH3 in ocular melanoma patients at high risk. This modification influences the formation of the tumor suppressor protein PML condensate by reducing N1-methyladenosine (m1A) methylation on SP100A, thereby accelerating tumor progression (33). Thus, strategies targeting ALKBH3 may offer substantial potential for melanoma treatment.
Chemotherapeutics, including platinum drugs and targeted agents such as bevacizumab, play essential roles in the management of advanced and metastatic colorectal cancer (CRC) (60, 61). Nevertheless, the widespread issue of drug resistance cannot be overlooked (62–64). Notably, CRC patients who are resistant to bevacizumab therapy exhibit significantly elevated glycolytic signaling and histone H3K18la (histone H3 lysine-18 lactylation) levels. These observations may provide insight into a potential underlying cause for patient resistance to this agent (34). In a separate study, investigators explored organoid models and xenotransplantation models (PDXs) of CRC patients, revealing that the Warburg effect can enhance homologous recombination (HR) and therefore contribute to chemotherapy resistance in cancer cells. Additionally, they observed that the inhibition of HR and reversal of drug resistance can be achieved by using cell-penetrating peptides that block the lactylation of MRE11, which encodes a nuclear protein involved in HR and DNA double-strand break (DSB) repair. Consequently, this approach increases the sensitivity of cancer cells to cisplatin and polyADP ribose polymerase inhibitors (PARPi) (35). This finding exposes the critical regulatory role of MRE11 lactylation in HR and offers a novel perspective on the relationship between tumor cell metabolism and DSB. Furthermore, it suggests a potential therapeutic strategy for overcoming chemotherapy resistance in CRC patients (65).
Elevated lactate and copper concentrations have been observed in gastric cancer (GC) (36). The researchers discovered that the m6A modification on ferredoxin 1 (FDX1) mRNA, mediated by an atypical methyltransferase called METTL16, plays a crucial role in copper-induced apoptosis. To further clarify, FDX1 encodes a reductase responsible for reducing Cu2+ to its more toxic form, Cu1+. They found that under conditions of copper stress, the lactylation of METTL16 at the K229 site is enhanced but inhibited by SIRT2 (37). Interestingly, the elevated levels of lactylation induced by METTL16 can enhance the therapeutic effectiveness of the copper ionophore elesclomol (66). When elesclomol is combined with the SIRT2 inhibitor AGK2, it induces copper-induced apoptosis in gastric tumors both in vitro and in vivo (36). This combination therapy offers a promising treatment strategy for GC.
In acute myeloid leukemia (AML), the upregulation of glycolysis by STAT5 results in the accumulation of lactate (38). This, in turn, promotes the translocation of E3 binding protein (E3BP) and histone lactylation to the nucleus, ultimately enhancing the transcription of PD-L1 in leukemia cells. The inhibition of PD-1/PD-L1 using immune checkpoint inhibitors (ICIs) can restore the activity of CD8+ T cells when co-cultured with AML cells that express high levels of STAT5. This suggests that PD-1/PD-L1 based immunotherapy may be beneficial for AML patients with STAT5-induced glycolysis and lactate accumulation (45, 67, 68).
A comprehensive investigation has been conducted to gain a deeper understanding of the underlying mechanism by which circXRN2 regulates tumor growth in bladder cancer (39). The findings revealed that circXRN2 has the capacity to bind with LATS1 protein, thus protecting it from undergoing speckle-type POZ protein-mediated ubiquitination and subsequent degradation. This interplay triggers activation of the Hippo signaling pathway, consequently restraining H3K18 lactylation and ultimately impeding the progression of bladder cancer. Importantly, these groundbreaking observations shed light on a potentially robust target for therapeutic intervention in the clinical management of bladder cancer.
4. Targeted lactate-lactylation in tumor immunotherapy
4.1. Targeted lactate-lactylation in combination with immune checkpoint inhibitor therapy
ICIs, as a revolutionary breakthrough in tumor immunotherapy, have demonstrated remarkable efficacy and long-lasting therapeutic responses in a subset of tumor patients (69–71). Currently FDA-approved ICIs encompass diverse formulations targeting programmed cell death 1 (PD-1), programmed cell death ligand 1 (PD-L1), and cytotoxic T lymphocyte-associated antigen-4 (CTLA-4) (72).
However, up to 85% of tumor patients exhibit poor response to ICIs. This can be attributed to individual genetic variations and the unique metabolic landscape of the TME (73, 74). Notably, the TME serves as one of the key contributing factors to this phenomenon (75, 76). In line with this notion, synergistic effects have been observed when combining mTOR inhibitors with glycolysis inhibitors across various cancer types including lymphoma, leukemia, and colorectal cancer (77, 78). Therefore, exploring metabolic modulators within the TME as adjuvants for combination therapy involving ICIs holds great promise ( Table 1 ).
Kumagai et al. (41) recently reported that in highly glycolytic TME conditions, such as MYC-amplified tumors and liver tumors, Tregs uptake lactic acid via monocarboxylate transporter 1 (MCT1), which enhances nuclear translocation of NFAT1 and promotes PD-1 expression. Consequently, targeting PD-1 activation alone may lead to treatment failure due to the activation of PD-1+ Treg cells. This observation highlights the potential role of lactic acid as an effective checkpoint in regulating Treg function under low glucose conditions, and further supports the theoretical basis for synergistic effects attained by combining ICIs with strategies that target lactic acid metabolism.
It has been previously observed by other researchers that inhibiting or eliminating the m6A demethylase ALK-BH5 during anti-PD-1 therapy in mouse models of melanoma and colorectal cancer leads to a notable decrease in lactate levels within the TME. Simultaneously, it also reduces the recruitment of Treg cells and myeloid-derived suppressor cells (MDSCs). These observations emphasize the potential of ALK-BH5 inhibitors as a novel approach to tackling resistance to tumor ICIs (42, 79).
A recent study has shown that inhibiting the high-affinity lactate transporter MCT4, either genetically or pharmacologically (43, 80), greatly enhances the therapeutic efficacy of anti-PD-1 therapy. This improvement was observed in a mouse model of hepatocellular carcinoma (HCC), resulting in prolonged survival. This effect can potentially be attributed to the heightened activity of CD8+ T cells, a reduction in tumor microenvironment acidification, and the increased secretion of chemokine ligands (81). These outcomes were induced by the ROS/NF-κB signaling pathway. Furthermore, the research team discovered higher levels of MCT4 expression in HCC patients who did not respond well to toripalimab neoadjuvant therapy. Similarly, the combination treatment of MCT4 inhibitors and anti-PD-L1 therapy exhibited beneficial effects in 3D colorectal cancer sphere models. However, this positive outcome was not observed when combining MCT1 inhibitor AZD3965 (44) with anti-PD-L1 therapy. Notably, AZD 3965 is currently undergoing a dose-escalation Phase I trial for the treatment of advanced solid tumors and lymphomas (NCT 01791595) (40).
In addition, extensive research has focused on therapeutic strategies targeting LDH. It has been reported that targeting LDH to reduce the production of lactic acid can turn tumors into “hot” tumors, characterized by a high degree of T cell infiltration and a better response towards ICIs therapies (45, 82). Qiao, T et al. (45) demonstrated in a humanized mouse model of non-small cell lung cancer (NSCLC) that the LDH inhibitor oxamate may enhance the therapeutic effect of pembrolizumab by a mechanism mainly associated with an increase in activated CD8 + T cells in tumors. Consistent with this, other researchers have found that mice with lactate dehydrogenase A (LDH-A) deficient B16-F10 melanoma have a better response to anti-PD-1 treatment, which is manifested by increased infiltration of NK cells and CD8 + cytotoxic T cells (46). Interestingly, although it is also a glycolytic pathway inhibitor, it is different from proton pump inhibitors (83) because LDH-A is not a key enzyme in normal cell metabolism, selective targeting of LDH-A has minimal theoretical side effects, making it a new target with more promising prospects and development value (45).
4.2. Effects of lactate and lactic acid in cancer vaccines
As an active immunotherapy, tumor vaccines utilize tumor-specific antigens (TSAs) or tumor-associated antigens (TAAs) to stimulate the body’s specific immune response, which has emerged as a prominent area of research in tumor immunotherapy (84). However, the intricate immune evasion mechanisms employed by tumor tissues pose challenges for achieving desired efficacy with tumor vaccines (85), and inadequate immunogenicity remains a key concern in current clinical applications (86).
Numerous researchers have explored the impact of lactate and lactic acid on the effectiveness of tumor vaccines. Feng et al. (47) compared the therapeutic effects of PC7A nano-tumor vaccine in lactate solution (1.68 g/kg, pH 7.4) and glucose solution (5 g/kg, pH 7.4) using an MC38 mouse tumor model, revealing significantly improved anti-tumor efficacy in the lactate group. Conversely, decreased anti-tumor efficacy was observed in the glucose group. Notably, subcutaneous injection of sodium lactate did not elevate tumor acidity; instead, it solely augmented the lactate concentration. This suggests that the lactate’s positive effect on anti-tumor immunity is not necessarily tied to pH alterations but may potentially be attributed to the enhanced exhaustion of CD8+ T cells mediated by lactate-induced HDAC inhibition. These findings suggest that lactate may enhance the effectiveness of T cell-based immunotherapies such as tumor vaccines. Another study demonstrated that lactic acid can augment the immunogenicity of whole UV-irradiated tumor cell vaccines by promoting dendritic cell (DC) maturation and aggregation within mouse xenograft models while enhancing phagocytosis (48). Given DCs’ crucial role in anti-tumor immunity, it is speculated that lactic acid-stimulated tumor vaccines may be more effective at inducing immune responses (87). Additionally, increased numbers of IFN-γ-expressing CD4+T and CD8+T cells were detected within spleen and lymph nodes from experimental mice, indicating potential dominance of cellular immunity mediated by CD8+T cells during this process—consistent with previous studies’ conclusions (88). Furthermore, the injection of lactic acid-stimulated tumor vaccines significantly reduces the number of CD11b+Gr1+MDSCs in tumor tissues, which plays a crucial role in immune evasion, tumor occurrence, and development (89). The aforementioned studies collectively indicate that lactate and lactic acid may exhibit different effects on tumor cells and infiltrating immune cells in vitro compared to in vivo experiments. However, at high concentrations, they can induce tumor cell apoptosis and enhance the efficacy of tumor vaccines (48).
4.3. Lactate-lactylation in CAR-T therapy
In recent years, chimeric antigen receptor T cell (CAR-T) therapy has emerged as a promising immunotherapy for various hematological tumors due to its remarkable effectiveness (90, 91). Nevertheless, the therapeutic outcome of CAR-T therapy in solid tumors remains unsatisfactory due to limitations imposed by the immunosuppressive TME and other factors (92, 93).
Numerous researchers have attempted to investigate the impact of lactate-lactylation targeted strategies on the efficacy of tumor vaccines. Sun et al. conducted a study exploring combined treatment with an LDH-A inhibitor and CAR-T therapy in a mouse model of glioblastoma multiforme (GBM) (49). Their findings demonstrated that LDH-A inhibitor Oxamate effectively reduced CAR-Treg cell levels and adenosine production within the TME by decreasing histone H3K18 lactylation levels. This reduction downregulated CD39, CD73, CCR8 gene promoter activity while reprogramming glucose metabolism in tumor stem cells. Ultimately, it promoted immune activation within the TME and showcased potential for improving GBM patient prognosis when combined with CAR-T therapy (94, 95). Additionally, some scholars have proposed that lactate may exert an immunoprotective role against anti-tumor immunity. The addition of lactate during the ex vivo expansion of T cells could potentially enhance the efficacy of CAR-T therapy (47), further highlighting the complex effects of lactate on both tumors and immune cells.
5. Conclusion and perspective
When confronted with environmental changes, tumor cells undergo metabolic reprogramming to adapt to the new environment (96). Lactate, as a byproduct of glycolysis, can lactylate both histone and non-histone proteins under the influence of specific enzymes (11). Although lactate was once regarded as a mere “metabolic waste” of glycolysis, numerous studies have gradually unraveled the Warburg effect, confirming its integral role in the TME. It is involved in tumor angiogenesis and mediates immune suppression among other processes (7), making it a potential target for cancer therapy. Further exploration of lactate’s potential role in tumorigenesis and the immune microenvironment is expected to yield fascinating discoveries.
Based on these findings, targeting lactate-lactylation and its associated metabolic pathways has emerged as a novel research avenue for cancer therapy. One strategy involves interfering with tumor cell metabolism by inhibiting lactate production and transport to reduce lactate accumulation and immunosuppression within the TME. Another strategy focuses on developing targeted drugs that affect lactate-lactylation to interfere with its effects on tumors and immune cells. Currently, notable progress has been achieved in studies targeting MCT4 (43, 44) and LDH (45, 49), but inhibitors targeting glycolysis are still at the preclinical stage involving animal model experiments without sufficient clinical translation. Despite the potential of targeting Lactate-Lactylation, there exist several challenges and limitations that hinder its clinical translation. For instance, shared enzymes exist between lactylation and acetylation, posing the risk of complications during treatment. Moreover, the risk lies in the expression of MCT1 in normal tissues, particularly the retina and heart. There have been reports of reversible vision loss and elevations in cardiac troponin levels in patients undergoing MCT1-targeted therapies, which are indicators of retinal effects and myocardial injury, respectively (40). It is imperative to carefully consider the balance between potential benefits and risks when pursuing targeted lactate therapy and explore strategies to mitigate these side effects. Still, inhibitors with more specificity targeting MCT and LDH remains limited. On top of that, current strategies and clinical trials do not prioritize the consideration of pH value, an aspect that could significantly impact therapeutic outcomes.
Although current research has gradually illuminated the role of lactate-lactylation in the TME, there are still intriguing avenues to explore. Firstly, certain studies have indicated that the immunoprotective effect of lactate may be underestimated. In contrast to lactic acid, lactate might exert an immunoprotective role against tumor immunity, primarily due to the confounding influence of proton-induced immunosuppression within the acidic TME. This discovery offers a novel perspective for further investigation (47). Additionally, investigations into the impact of lactic acid and lactate on tumor cells and immune infiltrates within TME can sometimes be influenced by experimental conditions both in vitro and in vivo (48). Consequently, comprehending the effects of lactic acid and lactate on TME and tumor immunotherapy is likely intricate; thus necessitating additional reliable experimental studies to clarify their potential implications on TME while reassessing specific roles played by lactic acid and lactate.
Currently, the bulk of investigations on lactylation focus on its downstream. To fully understand the complex conditions that lead to lactylation, more researches are needed. Besides, the specific reader of lactylation remains unclear, and the study concerning inhibitors for lactylation epigenetic tools are limited. Notably, lactylation and acetylation share certain enzymes, indicating a potential competitive relationship. Thus, it becomes imperative to discern its complex interplay with other PTMs such as acetylation, methylation, ubiquitination, SUMOylation etc., within organisms; thereby further investigating whether lactylation exerts broader impacts on physiological and pathological processes within organisms.
To summarize, Lactate-Lactylation plays a pivotal role in tumor metabolic reprogramming as well as tumor immunity. Enhancing our understanding of the intricate involvement of lactate-lactylation in TME will facilitate better understanding of tumorigenesis and development biological processes. Consequently, this will pave the way for the exploration of novel therapeutic targets aimed at improving the prognosis of cancer patients.
Author contributions
JZ: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. JZ: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. JL: Data curation, Formal analysis, Resources, Visualization, Software, Writing – original draft, Writing – review & editing. GZ: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. MH: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. WG: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Software, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. JY: Methodology, Conceptualization, Formal analysis, Project administration, Validation, Investigation, Visualization, Writing – original draft, Writing – review & editing. GF: Data curation, Methodology, Supervision, Conceptualization, Project administration, Validation, Funding acquisition, Resources, Visualization, Software, Writing – original draft, Writing – review & editing.
Funding Statement
The author(s) declare financial support was received for the research, authorship, and/or publication of this article. The project is supported by the National Natural Science Foundation of China (82001489), Shenzhen Natural Science Foundation (JCYJ 20220530141613031), Shenzhen Nanshan District Science and Technology Plan Project (NSZD2023010, NSZD2023041, NS044), Huazhong University of Science and Technology Union Shenzhen Hospital Foundation.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- 1. Yoshida GJ. Metabolic reprogramming: the emerging concept and associated therapeutic strategies. J Exp Clin Cancer Res. (2015) 34:111. doi: 10.1186/s13046-015-0221-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Liberti MV, Locasale JW. The warburg effect: how does it benefit cancer cells? Trends Biochem Sci. (2016) 41:211–8. doi: 10.1016/j.tibs.2015.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Pucino V, Cucchi D, Mauro C. Lactate transporters as therapeutic targets in cancer and inflammatory diseases. Expert Opin Ther Targets. (2018) 22:735–43. doi: 10.1080/14728222.2018.1511706 [DOI] [PubMed] [Google Scholar]
- 4. Böttcher M, Baur R, Stoll A, Mackensen A, Mougiakakos D. Linking immunoevasion and metabolic reprogramming in B-cell-derived lymphomas. Front Oncol. (2020) 10:594782. doi: 10.3389/fonc.2020.594782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zhang D, Tang Z, Huang H, Zhou G, Cui C, Weng Y, et al. Metabolic regulation of gene expression by histone lactylation. Nature. (2019) 574:575–80. doi: 10.1038/s41586-019-1678-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jin M, Cao W, Chen B, Xiong M, Cao G. Tumor-derived lactate creates a favorable niche for tumor via supplying energy source for tumor and modulating the tumor microenvironment. Front Cell Dev Biol. (2022) 10:808859. doi: 10.3389/fcell.2022.808859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pérez-Tomás R, Pérez-Guillén I. Lactate in the tumor microenvironment: an essential molecule in cancer progression and treatment. Cancers (Basel). (2020) 12(11):3244. doi: 10.3390/cancers12113244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Faubert B, Li KY, Cai L, Hensley CT, Kim J, Zacharias LG, et al. Lactate metabolism in human lung tumors. Cell. (2017) 171:358–371.e9. doi: 10.1016/j.cell.2017.09.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hensley CT, Faubert B, Yuan Q, Lev-Cohain N, Jin E, Kim J, et al. Metabolic heterogeneity in human lung tumors. Cell. (2016) 164:681–94. doi: 10.1016/j.cell.2015.12.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gao Y, Zhou H, Liu G, Wu J, Yuan Y, Shang A. Tumor microenvironment: lactic acid promotes tumor development. J Immunol Res. (2022) p:3119375. doi: 10.1155/2022/3119375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Xin Q, Wang H, Li Q, Liu S, Qu K, Liu C, et al. Lactylation: a passing fad or the future of posttranslational modification. Inflammation. (2022) 45:1419–29. doi: 10.1007/s10753-022-01637-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Xie Y, Hu H, Liu M, Zhou T, Cheng X, Huang W, et al. The role and mechanism of histone lactylation in health and diseases. Front Genet. (2022) 13:949252. doi: 10.3389/fgene.2022.949252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wang N, Wang W, Wang X, Mang G, Chen J, Yan X, et al. Histone lactylation boosts reparative gene activation post-myocardial infarction. Circ Res. (2022) 131(11):893–908. doi: 10.1161/CIRCRESAHA.122.320488 [DOI] [PubMed] [Google Scholar]
- 14. Wang T, Ye Z, Li Z, Jing DS, Fan GX, Liu MQ, et al. Lactate-induced protein lactylation: A bridge between epigenetics and metabolic reprogramming in cancer. Cell Prolif. (2023) 56(10):e13478. doi: 10.1111/cpr.13478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lv X, Lv Y, Dai X. Lactate, histone lactylation and cancer hallmarks. Expert Rev Mol Med. (2023) 25:e7. doi: 10.1017/erm.2022.42 [DOI] [PubMed] [Google Scholar]
- 16. Qu J, Li P, Sun Z. Histone lactylation regulates cancer progression by reshaping the tumor microenvironment. Front Immunol. (2023) 14:1284344. doi: 10.3389/fimmu.2023.1284344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. (2011) 144:646–74. doi: 10.1016/j.cell.2011.02.013 [DOI] [PubMed] [Google Scholar]
- 18. Vander Heiden MG, DeBerardinis RJ. Understanding the intersections between metabolism and cancer biology. Cell. (2017) 168:657–69. doi: 10.1016/j.cell.2016.12.039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mazzio EA, Boukli N, Rivera N, Soliman KF. Pericellular pH homeostasis is a primary function of the Warburg effect: inversion of metabolic systems to control lactate steady state in tumor cells. Cancer Sci. (2012) 103(3):422–32. doi: 10.1111/j.1349-7006.2012.02206.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chen L, Huang L, Gu Y, Cang W, Sun P, Xiang Y. Lactate-lactylation hands between metabolic reprogramming and immunosuppression. Int J Mol Sci. (2022) 23(19):11943. doi: 10.3390/ijms231911943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Watson MJ, Vignali PDA, Mullett SJ, Overacre-Delgoffe AE, Peralta RM, Grebinoski S, et al. Metabolic support of tumour-infiltrating regulatory T cells by lactic acid. Nature. (2021) 591:645–51. doi: 10.1038/s41586-020-03045-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Brand A, Singer K, Koehl GE, Kolitzus M, Schoenhammer G, Thiel A, et al. LDHA-associated lactic acid production blunts tumor immunosurveillance by T and NK cells. Cell Metab. (2016) 24(5):657–71. doi: 10.1016/j.cmet.2016.08.011 [DOI] [PubMed] [Google Scholar]
- 23. Husain Z, Seth P, Sukhatme VP. Tumor-derived lactate and myeloid-derived suppressor cells: Linking metabolism to cancer immunology. Oncoimmunology. (2013) 2(11):e26383. doi: 10.4161/onci.26383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Colegio OR, Chu NQ, Szabo AL, Chu T, Rhebergen AM, Jairam V, et al. Functional polarization of tumour-associated macrophages by tumour-derived lactic acid. Nature. (2014) 513(7519):559–63. doi: 10.1038/nature13490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chen P, Zuo H, Xiong H, Kolar MJ, Chu Q, Saghatelian A, et al. Gpr132 sensing of lactate mediates tumor-macrophage interplay to promote breast cancer metastasis. Proc Natl Acad Sci U.S.A. (2017) 114(3):580–5. doi: 10.1073/pnas.1614035114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Jiang J, Huang D, Jiang Y, Hou J, Tian M, Li J, et al. Lactate modulates cellular metabolism through histone lactylation-mediated gene expression in non-small cell lung cancer. Front Oncol. (2021) 11:647559. doi: 10.3389/fonc.2021.647559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Yang Z, Yan C, Ma J, Peng P, Ren X, Cai S, et al. Lactylome analysis suggests lactylation-dependent mechanisms of metabolic adaptation in hepatocellular carcinoma. Nat Metab. (2023) 5(1):61–79. doi: 10.1038/s42255-022-00710-w [DOI] [PubMed] [Google Scholar]
- 28. Li L, Li Z, Meng X, Wang X, Song D, Liu Y, et al. Histone lactylation-derived LINC01127 promotes the self-renewal of glioblastoma stem cells via the cis-regulating the MAP4K4 to activate JNK pathway. Cancer Lett. (2023) 579:216467. doi: 10.1016/j.canlet.2023.216467 [DOI] [PubMed] [Google Scholar]
- 29. Yang J, Luo L, Zhao C, Li X, Wang Z, Zeng Z, et al. A positive feedback loop between inactive VHL-triggered histone lactylation and PDGFRβ Signaling drives clear cell renal cell carcinoma progression. Int J Biol Sci. (2022) 18(8):3470–83. doi: 10.7150/ijbs.73398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Luo Y, Yang Z, Yu Y, Zhang P. HIF1α lactylation enhances KIAA1199 transcription to promote angiogenesis and vasculogenic mimicry in prostate cancer. Int J Biol Macromol. (2022) 222(Pt B):2225–43. doi: 10.1016/j.ijbiomac.2022.10.014 [DOI] [PubMed] [Google Scholar]
- 31. Chen M, Cen K, Song Y, Zhang X, Liou YC, Liu P, et al. NUSAP1-LDHA-Glycolysis-Lactate feedforward loop promotes Warburg effect and metastasis in pancreatic ductal adenocarcinoma. Cancer Lett. (2023) 567:216285. doi: 10.1016/j.canlet.2023.216285 [DOI] [PubMed] [Google Scholar]
- 32. Yu J, Chai P, Xie M, Ge S, Ruan J, Fan X, et al. Histone lactylation drives oncogenesis by facilitating m(6)A reader protein YTHDF2 expression in ocular melanoma. Genome Biol. (2021) 22(1):85. doi: 10.1186/s13059-021-02308-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gu X, Zhuang A, Yu J, Yang L, Ge S, Ruan J, et al. Histone lactylation-boosted ALKBH3 potentiates tumor progression and diminished promyelocytic leukemia protein nuclear condensates by m1A demethylation of SP100A. Nucleic Acids Res. (2023). doi: 10.1093/nar/gkad1193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Li W, Zhou C, Yu L, Hou Z, Liu H, Kong L, et al. Tumor-derived lactate promotes resistance to bevacizumab treatment by facilitating autophagy enhancer protein RUBCNL expression through histone H3 lysine 18 lactylation (H3K18la) in colorectal cancer. Autophagy. (2024) 20(1):114–30. doi: 10.1080/15548627.2023.2249762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Chen Y, Wu J, Zhai L, Zhang T, Yin H, Gao H, et al. Metabolic regulation of homologous recombination repair by MRE11 lactylation. Cell. (2024) 87(2):294–311.e21. doi: 10.1016/j.cell.2023.11.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sun L, Zhang Y, Yang B, Sun S, Zhang P, Luo Z, et al. Lactylation of METTL16 promotes cuproptosis via m(6)A-modification on FDX1 mRNA in gastric cancer. Nat Commun. (2023) 14(1):6523. doi: 10.1038/s41467-023-42025-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zu H, Li C, Dai C, Pan Y, Ding C, Sun H, et al. SIRT2 functions as a histone delactylase and inhibits the proliferation and migration of neuroblastoma cells. Cell Discovery. (2022) 8(1):54. doi: 10.1038/s41421-022-00398-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Huang ZW, Zhang XN, Zhang L, Liu LL, Zhang JW, Sun YX, et al. STAT5 promotes PD-L1 expression by facilitating histone lactylation to drive immunosuppression in acute myeloid leukemia. Signal Transduct Target Ther. (2023) 8(1):391. doi: 10.1038/s41392-023-01605-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Xie B, Lin J, Chen X, Zhou X, Zhang Y, Fan M, et al. CircXRN2 suppresses tumor progression driven by histone lactylation through activating the Hippo pathway in human bladder cancer. Mol Cancer. (2023) 22(1):151. doi: 10.1186/s12943-023-01856-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Halford S, Veal GJ, Wedge SR, Payne GS, Bacon CM, Sloan P, et al. A phase I dose-escalation study of AZD3965, an oral monocarboxylate transporter 1 inhibitor, in patients with advanced cancer. Clin Cancer Res. (2023) 29(8):1429–39. doi: 10.1158/1078-0432.CCR-22-2263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kumagai S, Koyama S, Itahashi K, Tanegashima T, Lin YT, Togashi Y, et al. Lactic acid promotes PD-1 expression in regulatory T cells in highly glycolytic tumor microenvironments. Cancer Cell. (2022) 40(2):201–218.e9. doi: 10.1016/j.ccell.2022.01.001 [DOI] [PubMed] [Google Scholar]
- 42. Li N, Kang Y, Wang L, Huff S, Tang R, Hui H, Agrawal K, et al. ALKBH5 regulates anti-PD-1 therapy response by modulating lactate and suppressive immune cell accumulation in tumor microenvironment. Proc Natl Acad Sci U.S.A. (2020) 117(33):20159–70. doi: 10.1073/pnas.1918986117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Fang Y, Liu W, Tang Z, Ji X, Zhou Y, Song S, et al. Monocarboxylate transporter 4 inhibition potentiates hepatocellular carcinoma immunotherapy through enhancing T cell infiltration and immune attack. Hepatology. (2023) 77(1):109–23. doi: 10.1002/hep.32348 [DOI] [PubMed] [Google Scholar]
- 44. Babl N, Decking SM, Voll F, Althammer M, Sala-Hojman A, Ferretti R, et al. MCT4 blockade increases the efficacy of immune checkpoint blockade. J Immunother Cancer. (2023) 11(10):e007349. doi: 10.1136/jitc-2023-007349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Qiao T, Xiong Y, Feng Y, Guo W, Zhou Y, Zhao J, et al. Inhibition of LDH-A by oxamate enhances the efficacy of anti-PD-1 treatment in an NSCLC humanized mouse model. Front Oncol. (2021) 11:632364. doi: 10.3389/fonc.2021.632364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Daneshmandi S, Wegiel B, Seth P. Blockade of lactate dehydrogenase-A (LDH-A) improves efficacy of anti-programmed cell death-1 (PD-1) therapy in melanoma. Cancers (Basel). (2019) 11(4):450. doi: 10.3390/cancers11040450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Feng Q, Liu Z, Yu X, Huang T, Chen J, Wang J, et al. Lactate increases stemness of CD8 + T cells to augment anti-tumor immunity. Nat Commun. (2022) 13(1):4981. doi: 10.1038/s41467-022-32521-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Yu J, Shao B, Luo M, Du W, Nie W, Yang J, et al. Irradiated lactic acid-stimulated tumour cells promote the antitumour immunity as a therapeutic vaccine. Cancer Lett. (2020) 469:367–79. doi: 10.1016/j.canlet.2019.11.018 [DOI] [PubMed] [Google Scholar]
- 49. Sun T, Liu B, Li Y, Wu J, Cao Y, Yang S, et al. Oxamate enhances the efficacy of CAR-T therapy against glioblastoma via suppressing ectonucleotidases and CCR8 lactylation. J Exp Clin Cancer Res. (2023) 42(1):253. doi: 10.1186/s13046-023-02815-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ngwa VM, Edwards DN, Philip M, Chen J. Microenvironmental metabolism regulates antitumor immunity. Cancer Res. (2019) 79:4003–8. doi: 10.1158/0008-5472.CAN-19-0617 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Angelin A, Gil-de-Gómez L, Dahiya S, Jiao J, Guo L, Levine MH, et al. Foxp3 reprograms T cell metabolism to function in low-glucose, high-lactate environments. Cell Metab. (2017) 25:1282–1293.e7. doi: 10.1016/j.cmet.2016.12.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Kouidhi S, Elgaaied AB, Chouaib S. Impact of metabolism on T-cell differentiation and function and cross talk with tumor microenvironment. Front Immunol. (2017) 8:270. doi: 10.3389/fimmu.2017.00270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Wu H, Estrella V, Beatty M, Abrahams D, El-Kenawi A, Russell S, et al. T-cells produce acidic niches in lymph nodes to suppress their own effector functions. Nat Commun. (2020) 11:4113. doi: 10.1038/s41467-020-17756-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Habif G, Crinier A, André P, Vivier E, Narni-Mancinelli E. Targeting natural killer cells in solid tumors. Cell Mol Immunol. (2019) 16(5):415–22. doi: 10.1038/s41423-019-0224-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Terrén I, Orrantia A, Vitallé J, Zenarruzabeitia O, Borrego F. NK cell metabolism and tumor microenvironment. Front Immunol. (2019) 10:2278. doi: 10.3389/fimmu.2019.02278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Renner K, Singer K, Koehl GE, Geissler EK, Peter K, Siska PJ, et al. Metabolic hallmarks of tumor and immune cells in the tumor microenvironment. Front Immunol. (2017) 8:248. doi: 10.3389/fimmu.2017.00248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Hashmi F, Liu M, Shen S, Qiao LY. EXPRESS: Phospholipase C gamma mediates endogenous brain-derived neurotrophic factor - regulated calcitonin gene-related peptide expression in colitis - induced visceral pain. Mol Pain. (2016) 12:1744806916657088. doi: 10.1177/1744806916657088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Yang K, Xu J, Fan M, Tu F, Wang X, Ha T, et al. Lactate suppresses macrophage pro-inflammatory response to LPS stimulation by inhibition of YAP and NF-κB activation via GPR81-mediated signaling. Front Immunol. (2020) 11:587913. doi: 10.3389/fimmu.2020.587913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Zhang Y, Peng Q, Zheng J, Yang Y, Zhang X, Ma A, et al. The function and mechanism of lactate and lactylation in tumor metabolism and microenvironment. Genes Dis. (2023) 10(5):2029–37. doi: 10.1016/j.gendis.2022.10.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Biller LH, Schrag D. Diagnosis and treatment of metastatic colorectal cancer: A review. JAMA. (2021) 325(7):669–85. doi: 10.1001/jama.2021.0106 [DOI] [PubMed] [Google Scholar]
- 61. Ohishi T, Kaneko MK, Yoshida Y, Takashima A, Kato Y, Kawada M. Current targeted therapy for metastatic colorectal cancer. Int J Mol Sci. (2023) 24(2):1702. doi: 10.3390/ijms24021702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Ranasinghe R, Mathai ML, Zulli A. Cisplatin for cancer therapy and overcoming chemoresistance. Heliyon. (2022) 8(9):e10608. doi: 10.1016/j.heliyon.2022.e10608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Garcia J, Hurwitz HI, Sandler AB, Miles D, Coleman RL, Deurloo R, et al. Bevacizumab (Avastin®) in cancer treatment: A review of 15 years of clinical experience and future outlook. Cancer Treat Rev. (2020) 86:102017. doi: 10.1016/j.ctrv.2020.102017 [DOI] [PubMed] [Google Scholar]
- 64. Kitamura T, Suzuki M, Nishimatsu H, Kurosaki T, Enomoto Y, Fukuhara H, et al. Final report on low-dose estramustine phosphate (EMP) monotherapy and very low-dose EMP therapy combined with LH-RH agonist for previously untreated advanced prostate cancer. Aktuelle Urol. (2010) 41 Suppl 1:S34–40. doi: 10.1055/s-0029-1224657 [DOI] [PubMed] [Google Scholar]
- 65. Trenner A, Sartori AA. Harnessing DNA double-strand break repair for cancer treatment. Front Oncol. (2019) 9:1388. doi: 10.3389/fonc.2019.01388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Zheng P, Zhou C, Lu L, Liu B, Ding Y. Elesclomol: a copper ionophore targeting mitochondrial metabolism for cancer therapy. J Exp Clin Cancer Res. (2022) 41(1):271. doi: 10.1186/s13046-022-02485-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Yi M, Jiao D, Xu H, Liu Q, Zhao W, Han X, et al. Biomarkers for predicting efficacy of PD-1/PD-L1 inhibitors. Mol Cancer. (2018) 17(1):129. doi: 10.1186/s12943-018-0864-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Dai MY, Shi YY, Wang AJ, Liu XL, Liu M, Cai HB. High-potency PD-1/PD-L1 degradation induced by Peptide-PROTAC in human cancer cells. Cell Death Dis. (2022) 13(11):924. doi: 10.1038/s41419-022-05375-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Robert C. A decade of immune-checkpoint inhibitors in cancer therapy. Nat Commun. (2020) 11(1):3801. doi: 10.1038/s41467-020-17670-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Bagchi S, Yuan R, Engleman EG. Immune checkpoint inhibitors for the treatment of cancer: clinical impact and mechanisms of response and resistance. Annu Rev Pathol. (2021) 16:223–49. doi: 10.1146/annurev-pathol-042020-042741 [DOI] [PubMed] [Google Scholar]
- 71. Marin-Acevedo JA, Kimbrough EO, Lou Y. Next generation of immune checkpoint inhibitors and beyond. J Hematol Oncol. (2021) 14(1):45. doi: 10.1186/s13045-021-01056-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Twomey JD, Zhang B. Cancer immunotherapy update: FDA-approved checkpoint inhibitors and companion diagnostics. AAPS J. (2021) 23(2):39. doi: 10.1208/s12248-021-00574-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Suzuki M, Liu M, Kurosaki T, Suzuki M, Arai T, Sawabe M, et al. Association of rs6983561 polymorphism at 8q24 with prostate cancer mortality in a Japanese population. Clin Genitourin Cancer. (2011) 9(1):46–52. doi: 10.1016/j.clgc.2011.04.004 [DOI] [PubMed] [Google Scholar]
- 74. Zhou Y, Liu M, Li J, Hashmi F, Mao Z, Zhang N, et al. Impact of V-ets erythroblastosis virus E26 oncogene homolog 1 gene polymorphisms upon susceptibility to autoimmune diseases: A meta-analysis. Med (Baltimore). (2015) 94(22):e923. doi: 10.1097/MD.0000000000000923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Hanahan D. Hallmarks of cancer: new dimensions. Cancer Discovery. (2022) 12(1):31–46. doi: 10.1158/2159-8290.CD-21-1059 [DOI] [PubMed] [Google Scholar]
- 76. Xiao Q, Nobre A, Piñeiro P, Berciano-Guerrero MÁ, Alba E, Cobo M, et al. Genetic and epigenetic biomarkers of immune checkpoint blockade response. J Clin Med. (2020) 9(1):286. doi: 10.3390/jcm9010286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Xing BC, Wang C, Ji FJ, Zhang XB. Synergistically suppressive effects on colorectal cancer cells by combination of mTOR inhibitor and glycolysis inhibitor, Oxamate. Int J Clin Exp Pathol. (2018) 11:4439–45. [PMC free article] [PubMed] [Google Scholar]
- 78. Xu RH, Pelicano H, Zhang H, Giles FJ, Keating MJ, Huang P. Synergistic effect of targeting mTOR by rapamycin and depleting ATP by inhibition of glycolysis in lymphoma and leukemia cells. Leukemia. (2005) 19(12):2153–8. doi: 10.1038/sj.leu.2403968 [DOI] [PubMed] [Google Scholar]
- 79. Fujimura T, Kambayashi Y, Aiba S. Crosstalk between regulatory T cells (Tregs) and myeloid derived suppressor cells (MDSCs) during melanoma growth. Oncoimmunology. (2012) 1(8):1433–4. doi: 10.4161/onci.21176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Liu T, Han S, Yao Y, Zhang G. Role of human monocarboxylate transporter 1 (hMCT1) and 4 (hMCT4) in tumor cells and the tumor microenvironment. Cancer Manag Res. (2023) 15:957–75. doi: 10.2147/CMAR.S421771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Dangaj D, Bruand M, Grimm AJ, Ronet C, Barras D, Duttagupta PA, et al. Cooperation between constitutive and inducible chemokines enables T cell engraftment and immune attack in solid tumors. Cancer Cell. (2019) 35(6):885–900.e10. doi: 10.1016/j.ccell.2019.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Galon J, Bruni D. Approaches to treat immune hot, altered and cold tumours with combination immunotherapies. Nat Rev Drug Discovery. (2019) 18(3):197–218. doi: 10.1038/s41573-018-0007-y [DOI] [PubMed] [Google Scholar]
- 83. Spugnini EP, Fais S. Drug repurposing for anticancer therapies. A lesson from proton pump inhibitors. Expert Opin Ther Pat. (2020) 30(1):15–25. doi: 10.1080/13543776.2020.1704733 [DOI] [PubMed] [Google Scholar]
- 84. Sahin U, Türeci Ö. Personalized vaccines for cancer immunotherapy. Science. (2018) 359(6382):1355–60. doi: 10.1126/science.aar7112 [DOI] [PubMed] [Google Scholar]
- 85. Wang M, Zhao J, Zhang L, Wei F, Lian Y, Wu Y, et al. Role of tumor microenvironment in tumorigenesis. J Cancer. (2017) 8(5):761–73. doi: 10.7150/jca.17648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Morse MA, Gwin WR, 3rd, Mitchell DA. Vaccine therapies for cancer: then and now. Target Oncol. (2021) 16(2):121–52. doi: 10.1007/s11523-020-00788-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. MacNabb BW, Chen X, Tumuluru S, Godfrey J, Kasal DN, Yu J, et al. Dendritic cells can prime anti-tumor CD8(+) T cell responses through major histocompatibility complex cross-dressing. Immunity. (2022) 55(11):982–997.e8. doi: 10.1016/j.immuni.2022.04.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Ercolini AM, Ladle BH, Manning EA, Pfannenstiel LW, Armstrong TD, Machiels JP, et al. Recruitment of latent pools of high-avidity CD8(+) T cells to the antitumor immune response. J Exp Med. (2005) 201(10):1591–602. doi: 10.1084/jem.20042167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Lu X, Horner JW, Paul E, Shang X, Troncoso P, Deng P, et al. Effective combinatorial immunotherapy for castration-resistant prostate cancer. Nature. (2017) 543(7647):728–32. doi: 10.1038/nature21676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Lu J, Jiang G. The journey of CAR-T therapy in hematological Malignancies. Mol Cancer. (2022) 21(1):194. doi: 10.1186/s12943-022-01663-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Weber EW, Maus MV, Mackall CL. The emerging landscape of immune cell therapies. Cell. (2020) 181(1):46–62. doi: 10.1016/j.cell.2020.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Ma S, Li X, Wang X, Cheng L, Li Z, Zhang C, et al. Current progress in CAR-T cell therapy for solid tumors. Int J Biol Sci. (2019) 15(12):2548–60. doi: 10.7150/ijbs.34213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Maalej KM, Merhi M, Inchakalody VP, Mestiri S, Alam M, Maccalli C, et al. CAR-cell therapy in the era of solid tumor treatment: current challenges and emerging therapeutic advances. Mol Cancer. (2023) 22(1):20. doi: 10.1186/s12943-023-01723-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Haradhvala NJ, Leick MB, Maurer K, Gohil SH, Larson RC, Yao N, et al. Distinct cellular dynamics associated with response to CAR-T therapy for refractory B cell lymphoma. Nat Med. (2022) 28(9):1848–59. doi: 10.1038/s41591-022-01959-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Kepp O, Bezu L, Yamazaki T, Di Virgilio F, Smyth MJ, Kroemer G, et al. ATP and cancer immunosurveillance. EMBO J. (2021) 40(13):e108130. doi: 10.15252/embj.2021108130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Faubert B, Solmonson A, DeBerardinis RJ. Metabolic reprogramming and cancer progression. Science. (2020) 368(6487):eaaw5473. doi: 10.1126/science.aaw5473 [DOI] [PMC free article] [PubMed] [Google Scholar]


