ABSTRACT
Background
We report 2023/2024 season interim influenza vaccine effectiveness for three studies, namely, primary care in Great Britain, hospital settings in Scotland and hospital settings in England.
Methods
A test negative design was used to estimate vaccine effectiveness.
Results
Estimated vaccine effectiveness against all influenzas ranged from 63% (95% confidence interval 46 to 75%) to 65% (41 to 79%) among children aged 2–17, from 36% (20 to 49%) to 55% (43 to 65%) among adults 18–64 and from 40% (29 to 50%) to 55% (32 to 70%) among adults aged 65 and over.
Conclusions
During a period of co‐circulation of influenza A(H1N1)pdm09 and A(H3N2) in the United Kingdom, evidence for effectiveness of the influenza vaccine in both children and adults was found.
Keywords: effectiveness, hospitalisation, influenza, vaccine
1. Introduction
In the United Kingdom, seasonal influenza vaccination is offered freely to those aged ≥ 65, aged 16–64 within clinical groups and at increased risk of severe influenza‐related outcomes and children aged 2–15 [1]. In Scotland, this offer was extended to healthy adults aged 50–64 [2]. The 2023/2024 season northern hemisphere influenza vaccine included an updated A(H1N1)pdm09 strain: a cell culture–propagated A/Wisconsin/67/2022 or egg‐propagated A/Victoria/4897/2022 (H1N1)pdm09–like virus, while the A(H3N2) and B components remained the same as for 2022/2023 [3].
Throughout the United Kingdom, indicators of influenza activity suggested low levels of circulation prior to December 2023 [4, 5, 6]. Influenza activity rose throughout December, reaching a modest peak by late December 2023, before dropping slightly early‐ to mid‐January 2024. However, by late January 2024, influenza activity was increasing again. To January 2024, the United Kingdom has primarily seen cocirculation of influenza A(H1N1)pdm09 and A(H3N2).
We report interim vaccine effectiveness (VE) in primary and secondary care settings within the United Kingdom.
2. Methods
We brought together primary care sentinel swabbing data from England, Scotland and Wales (GB‐PC) and conducted two separate data‐linkage studies on hospitalised patients in England (EN‐H) and Scotland (SC‐H). Study characteristics are summarised in Table 1. Methods for all three studies have been described elsewhere [8, 9, 10, 11]; a protocol for GB‐PC is provided in the supporting information.
TABLE 1.
Summary of methods and characteristics of three 2023/2024 season influenza vaccine effectiveness studies in Great Britain.
| Study characteristics | Study | ||
|---|---|---|---|
| GB–Primary Care (GB‐PC) | England–Hospital (EN‐H) | Scotland–Hospital (SC‐H) | |
| Study period | 4 Sep 2023 to 19 Jan 2024 | 2 Oct 2023 to 15 Jan 2024 | 2 Oct 2023 to 28 Jan 2024 |
| Setting | Primary care | Hospital | Hospital |
| Location | England, Scotland, Wales | England | Scotland |
| Study design | Test negative design | Test negative design | Test negative design |
| Data source | Physicians and laboratory, in some sites data linkage to electronic health records | Data linkage of sentinel laboratory surveillance (Respiratory DataMart), the Immunisations System (IIS), and the Emergency Care DataSet (ECDS) | EAVE‐II national patient‐level dataset, Electronic Communication of Surveillance in SC (ECOSS; all virology testing national database), Rapid Preliminary Inpatient Data (RAPID; Scottish hospital admissions data), National Records of Scotland (NRS; death certification), National Clinical Data Store (NCDS; vaccination events in SC) |
| Age groups of study population | All ages | All ages | All ages |
| Case definition for patient recruitment | Patients consulting within primary care with ARI symptoms | Patients with an influenza swab test 14 days before to 2 days after hospitalisation via emergency care | Patients with EU ARI a symptoms and clinician's judgement that there is an infection b and limited to emergency care where the influenza test occurs 14 days before admission or within 48 hours after admission |
| Selection of patients | At practitioner's/clinician's judgement | At practitioner's/clinician's judgement | At practitioner's/clinician's judgement |
| Vaccine types in the study among controls aged 2–17 | LAIV 96%, QIVc 4% | LAIV 88%, QIVc 12% | LAIV 85%, QIVc 15% |
| Vaccine types in the study among controls aged 18–64 | QIVc 94%, aQIV 4%, QIVe 2%, QIVr 1% | QIVc 86%, aQIV 6%, QIVe 6%, QIVr 3% | QIVc 97%, aQIV 3% |
| Vaccine types in the study among controls aged 65 and above | aQIV 98%, QIVc 2%, QIVr 1% | aQIV 96%, QIVc 3%, QIVr 1% | aQIV 99%, QIVc 1% |
| Vaccination definition | ≥14 days post vaccination | ≥14 days post vaccination | ≥14 days post vaccination |
| Variables of adjustment | Age group, region, clinical risk status, sex, calendar time as week (spline) | Age group, region, clinical risk status, calendar time as week (spline) | Age (spline), sex, number of QCOVID c clinical risk groups (0, 1, 2, 3, 4, ≥ 5) c , time (days, spline), setting (community or hospital) and deprivation quintile (SIMD) |
Abbreviations: aQIV, adjuvanted QIV; ARI, acute respiratory infection; GB, Great Britain; GP, general practitioner; LAIV, quadrivalent live attenuated influenza vaccine; QIVc, cell‐grown quadrivalent influenza vaccine; QIVe, egg‐grown quadrivalent influenza vaccine; QIVr, recombinant quadrivalent influenza vaccine; SIMD, Scottish Index of Multiple Deprivation; TND, test‐negative design.
The EU‐ARI definition is sudden onset of symptoms AND ≥ 1 of cough, sore throat, shortness of breath or coryza AND a clinician's judgement that the illness is due to an infection.
Varies according to SARS‐CoV‐2/influenza testing practices by Health Board.
The QCOVID risk groups are defined as the number of generic comorbidity conditions of a patient, and are used as a measure of comorbidity. The list of conditions is found in the study of Clift et al. [7].
All studies used the test negative design, applying logistic regression with adjustments as outlined in Table 1 to estimate the odds ratio (OR) of vaccination in influenza positive cases and influenza negative controls. VE is reported as a percentage: 100 × (1 − OR). For the GB‐PC study, patients presented to primary care with acute respiratory infection (ARI), swabs known to be taken beyond 7 days of onset were not used to estimate VE (see supporting information for full detail). Symptom onset date was not available within hospital records. In EN‐H, influenza A subtype VE analyses were restricted to data from labs that carried out subtyping. In SC‐H, potential vaccine contaminants and influenza co‐detections were removed from all analyses. SARS‐CoV‐2–positive controls were excluded in the GB‐PC and EN‐H studies [12].
Note that for Scotland and England, the primary source of vaccination histories is immunisation databases; these capture vaccinations offered freely on the National Health Service well but may miss vaccines administered privately, for example, via workplaces, likely leading to a small amount of exposure misclassification among working age adults. In Wales the primary source of vaccination histories was through questionnaire administered by the GP at the point of swabbing.
A sample of influenza virus positive primary care specimens was further characterised, as described in the supporting information.
3. Results
The GB‐PC study included 1193 cases and 12,098 controls, including 6343 samples from England, 6108 from Scotland and 840 from Wales; the number of specimens positive for influenza A(H1N1)pdm09 was 461, 475 A(H3N2), 215 influenza A (untyped) and 46 influenza B, with 4 dual infections. The EN‐H study included 1359 cases and 22,539 controls; 770 influenza A samples were untyped, 161 were influenza A(H1N1)pdm09, 395 were influenza A(H3N2) (note that more laboratories subtyped influenza A/H3 than A/H1) and 32 were influenza B, including 5 dual infections. The SC‐H study included 1977 cases and 34,476 controls; 1567 influenza A samples were untyped, 172 were influenza A(H1N1)pdm09, 188 were influenza A(H3N2) and 50 were influenza B.
We note from Table 1 the distribution of influenza vaccine types among controls. Those aged 2–17 primarily received live attenuated influenza vaccine (LAIV) via nasal spray, those aged 18–64 mostly received quadrivalent cell‐based vaccine (QIVc) and those aged ≥ 65 mostly received adjuvanted egg‐based vaccine (aQIV). Use of other vaccine types—standard‐dose quadrivalent egg‐based vaccines (QIVe) and recombinant quadrivalent vaccines (QIVr)—was limited.
VE for all studies is summarised in Figure 1. There were insufficient influenza B samples in all studies to estimate VE against influenza B.
FIGURE 1.
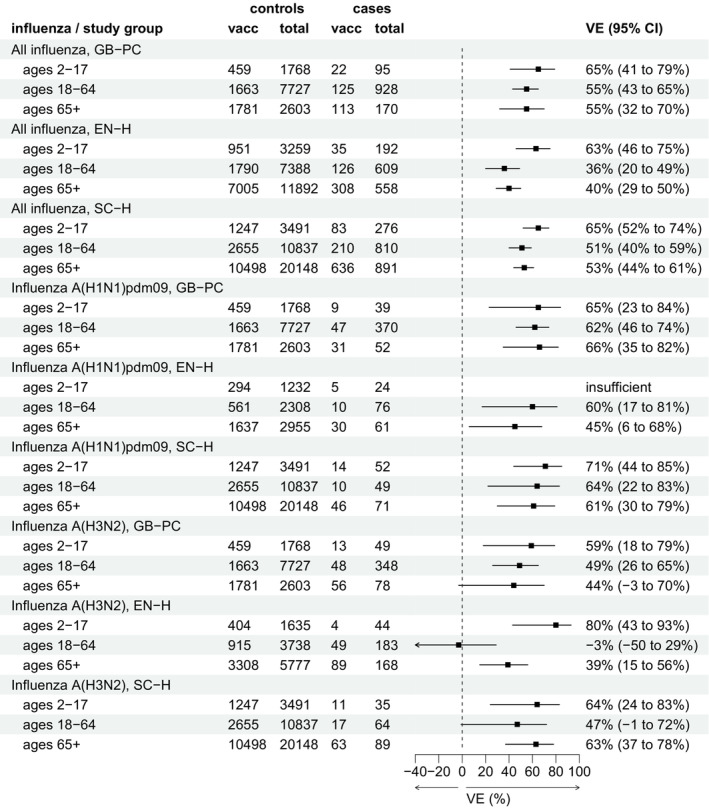
Interim vaccine effectiveness (VE) against all laboratory‐confirmed influenza (A and B), A(H1N1)pdm09 and A(H3N2), by each of three studies in Great Britain and by age group, influenza season 2023/2024.
For all influenza cases (both influenza A and B) across all settings, VE ranged from 63% (95% confidence interval [CI]: 46%–75%) to 65% (95% CI: 41%–79%) among children aged 2–17, from 36% (95% CI: 20%–49%) to 55% (95% CI 43%–65%) among adults 18–64 and from 40% (95% CI: 29%–50%) to 55% (95% CI: 32%–70%) among adults aged 65 and over.
In all settings, VE against influenza A(H1N1)pdm09 ranged from 65% (95% CI: 23%–84%) to 71% (95% CI: 44%–85%) among children aged 2–17, from 60% (95% CI: 17%–81%) to 64% (95% CI: 22%–83%) among adults aged 18–64 and from 45% (95% CI: 6%–68%) to 66% (95% CI: 35%–82%) among adults aged 65 and above.
VE against influenza A(H3N2) ranged from 59% (95% CI: 18%–79%) to 80% (95% CI: 43%–93%) among children aged 2–17, from −3% (95% CI: −50% to 29%) to 49% (95% CI: 26%–65%) among adults aged 18–64 and from 39% (95% CI: 15%–56%) to 63% (95% CI: 37%–78%) among adults aged 65 and over. Except for one result (SC‐H, ages 65+), VE point estimates against influenza A(H3N2) were lower than those against influenza A(H1N1)pdm09.
Where influenza viruses from positive specimens in the GB‐PC study were further characterised, all A(H3N2) viruses belonged in genetic subclade 3C.2a1b.2a.2 in the 2a.3a.1 subgroup, while A(H1N1)pdm09 viruses were split between subgroups 6B.1A.5a.2a (70%) and 6B.1A.5a.2a.1 (30%).
4. Discussion
During a period of co‐circulation of influenza A(H1N1)pdm09 and A(H3N2) in the United Kingdom, we found evidence of moderate VE in both children and adults. Our results concur with those reported for Canada during the early 2023/2024 season, who reported moderate VE during an influenza A(H1N1)pdm09–dominated period [13].
During the 2022/2023 season, estimates of interim VE against influenza A(H1N1)pdm09 in Europe, including some corresponding results for EN‐H and SC‐H, were typically lower than those we report for the 2023/2024 season, especially among those aged 65 and above [10]. Our results suggest that VE may have been improved by the update to the A(H1N1)pdm09 component of the northern hemisphere vaccine for 2023/2024.
The 2023/2024 A(H3N2) northern hemisphere vaccine strain belongs to subgroup 2a of genetic subclade 3C.2a1b.2a.2. Experiments to evaluate the vaccine's ability to recognise subgroup 2a.3a.1 viruses similar to those circulating have reported mixed findings [14]. Our 2023/2024 VE estimates against A(H3N2) are higher than those reported for 2022/2023 in SC‐H as well as among children and adults aged 65 and above in EN‐H [8], suggesting that the differences in subgroup of the vaccine and circulating viruses have not resulted in reduced VE. However, no effectiveness was demonstrated in the 2023/2024 EN‐H for those aged 18–64 years. Healthy adults aged 50–64 were influenza vaccine eligible in 2022/2023, but in 2023/2024, this was withdrawn in England and Wales while continuing in Scotland; evidence for residual protection leading to reduced VE will be investigated at the end of the season.
Interim estimates are inevitably restricted by sample size. End‐of‐season final estimates will further explore VE by vaccine type and possibly provide VE against influenza B, depending on circulation during the rest of the season. VE estimates were often lower in EN‐H; hospitalisation definitions differ to SC‐H and further exploration of this is planned. In a hospital setting, testing is among patients presenting with ARI symptoms, but a limitation is that this may not be the main reason for hospitalisation. Unlike previous seasons, Scotland successfully collected individual‐level vaccination data for children from all health boards, resulting for the first time in a national hospital study population.
Estimates of interim 2023/2024 season influenza VE within the United Kingdom are encouraging, especially in that reasonably robust effectiveness against hospitalisation with both influenza A(H1N1)pdm09 and A(H3N2) was demonstrated among those aged 65. These findings further reinforce the importance of the annual UK influenza vaccination programme.
Author Contributions
Heather Whitaker: Formal analysis; Writing – original draft; Methodology; Conceptualization. Beth Findlay: Writing – original draft; Formal analysis; Methodology; Conceptualization. Jana Zitha: Data curation; Writing – review and editing; Project administration. Rosalind Goudie: Data curation; Methodology; Writing – review and editing. Katie Hassell: Data curation; Writing – review and editing; Methodology. Josie Evans: Writing – review and editing; Supervision; Conceptualization; Data curation. Panoraia Kalapotharakou: Writing – review and editing; Data curation. Utkarsh Agrawal: Methodology; Writing – review and editing. Beatrix Kele: Data curation; Writing – review and editing; Formal analysis; Investigation. Mark Hamilton: Data curation; Writing – review and editing. Catherine Moore: Writing – review and editing; Supervision; Project administration; Investigation. Rachel Byford: Data curation; Writing – review and editing. Julia Stowe: Methodology; Data curation; Writing – review and editing. Chris Robertson: Methodology; Writing – review and editing; Supervision; Conceptualization. Anastasia Couzens: Investigation; Writing – review and editing. Gavin Jamie: Data curation; Writing – review and editing. Katja Hoschler: Supervision; Writing – review and editing; Investigation. Kathleen Pheasant: Writing – review and editing; Investigation. Elizabeth Button: Project administration; Writing – review and editing; Resources. Catherine Quinot: Data curation; Writing ‐ review and editing. Tim Jones: Writing – review and editing; Investigation. Sneha Anand: Project administration; Writing – review and editing; Resources. Conall Watson: Supervision; Methodology; Writing – review and editing; Conceptualization. Nick Andrews: Methodology; Writing – review and editing; Supervision; Conceptualization. Simon de Lusignan: Supervision; Writing – review and editing; Methodology; Project administration. Maria Zambon: Supervision; Writing – review and editing; Methodology; Investigation; Project administration. Christopher Williams: Writing – review and editing; Supervision; Project administration; Conceptualization. Simon Cottrell: Methodology; Writing – review and editing; Supervision; Data curation; Project administration. Kimberly Marsh: Writing – review and editing; Methodology; Supervision; Project administration. Jim McMenamin: Project administration; Supervision; Methodology; Conceptualization; Writing – review and editing. Jamie Lopez Bernal: Conceptualization; Methodology; Writing – original draft; Project administration; Supervision.
Ethics Statement
UK public health agencies have permission to process patient confidential information for national surveillance of communicable diseases under: Regulation 3 of the Health Service Regulation 2002 for England, the Public Health (Scotland) Act 2008 and the NHS Scotland Act 1978 for Scotland and the Public Health Wales National Health Service Trust (Establishment) Order 2009 for Wales. As such, specific ethical approval was not necessary.
Conflicts of Interest
SdeL, within his academic role, is Director of the RCGP RSC. He has received grants through his University from AstraZeneca, GSK, Moderna, Sanofi and Seqirus for vaccine related research and been members of advisory boards for AstraZeneca, GSK, Sanofi and Seqirus. HW and CW's department has received cost‐recovery payment from CSL Seqirus for analysis undertaken for regulatory review.
Peer Review
The peer review history for this article is available at https://www.webofscience.com/api/gateway/wos/peer‐review/10.1111/irv.13284.
Supporting information
Data S1. Supporting information
Funding: This study was funded by UK Health Security Agency, Public Health Scotland and Public Health Wales.
Data Availability Statement
Data supporting this study cannot be made available due to ethical and legal reasons.
References
- 1. UK Health Security Agency , “The Green Book; Chapter 19: Influenza. UKHSA Gateway Number 2020296,” accessed 15 Feb 2024, https://www.gov.uk/government/publications/influenza‐the‐green‐book‐chapter‐19.
- 2. Smith G., McMahon A., and Strath A., “Seasonal Flu Immunisation Programme 2023/24: Confirmation of Adult Cohorts. 18 April 2023,” Letter, The Scottish Government, accessed February 15, 2024, https://www.sehd.scot.nhs.uk/cmo/CMO(2023)05.pdf.
- 3. World Health Organization (WHO) , “Recommended Composition of Influenza Virus Vaccines for Use in the 2023–2024 Northern Hemisphere Influenza Season,” 24 February 2023, Meeting report, Geneva: WHO, accessed February 6, 2023, https://www.who.int/publications/m/item/recommended‐composition‐of‐influenza‐virus‐vaccines‐for‐use‐in‐the‐2023‐2024‐northern‐hemisphere‐influenza‐season.
- 4. Public Health Wales , “Public Health Wales Influenza Surveillance Report: Week 4 2024,” accessed February 6, 2024, https://phw.nhs.wales/topics/immunisation‐and‐vaccines/fluvaccine/weekly‐influenza‐and‐acute‐respiratory‐infection‐report/.
- 5. Public Health Scotland , “Viral Respiratory Diseases (Including Influenza and COVID‐19) in Scotland Surveillance Report: 01 February 2024,” accessed February 6, 2024, https://www.publichealthscotland.scot/publications/viral‐respiratory‐diseases‐including‐influenza‐and‐covid‐19‐in‐scotland‐surveillance‐report/viral‐respiratory‐diseases‐including‐influenza‐and‐covid‐19‐in‐scotland‐surveillance‐report‐1‐february‐2024/.
- 6. UK Health Security Agency , “National Influenza and COVID‐19 Surveillance Report. Week 5 Report (up to Week 4 2024 Data), 1 February 2024,” accessed February 6, 2024, https://www.gov.uk/government/statistics/national‐flu‐and‐covid‐19‐surveillance‐reports‐2023‐to‐2024‐season.
- 7. Clift A. K., Coupland C. A. C., Keogh R. H., et al., “Living Risk Prediction Algorithm (QCOVID) for Risk of Hospital Admission and Mortality from Coronavirus 19 in Adults: National Derivation and Validation Cohort Study,” BMJ 371 (2020): m3731, 10.1136/bmj.m3731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pebody R. G., Whitaker H., Ellis J., et al., “End of Season Influenza Vaccine Effectiveness in Primary Care in Adults and Children in the United Kingdom in 2018/19,” Vaccine 38, no. 3 (2020): 489–497, 10.1016/j.vaccine.2019.10.071. [DOI] [PubMed] [Google Scholar]
- 9. Whitaker H., Hassell K., Hoschler K., et al., “Influenza Vaccination During the 2021/22 Season: A Data‐Linkage Test‐Negative Case‐Control Study of Effectiveness Against Influenza Requiring Emergency Care in England and Serological Analysis of Primary Care Patients,” Vaccine 42 (2024): 1656–1664, 10.1016/j.vaccine.2024.02.006. [DOI] [PubMed] [Google Scholar]
- 10. Kissling E., Maurel M., Emborg H. D., et al., “Interim 2022/23 Influenza Vaccine Effectiveness: Six European Studies, October 2022 to January 2023,” Eurosurveillance 28, no. 21 (2023): pii=2300116, 10.2807/1560-7917.ES.2023.28.21.2300116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Public Health Scotland , “Community Acute Respiratory Infection (CARI) Surveillance in Primary Care,” 2023, https://publichealthscotland.scot/media/23330/cari‐surveillance‐in‐primary‐care‐english‐november2023.pdf.
- 12. Doll M. K., Pettigrew S. M., Ma J., and Verma A., “Effects of Confounding Bias in Coronavirus Disease 2019 (COVID‐19) and Influenza Vaccine Effectiveness Test‐Negative Designs Due to Correlated Influenza and COVID‐19 Vaccination Behaviors,” Clinical Infectious Diseases 75, no. 1 (2022): e564–e571, 10.1093/cid/ciac234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Smolarchuk C., Ickert C., Zelyas N., Kwong J. C., and Buchan S. A., “Early Influenza Vaccine Effectiveness Estimates Using Routinely Collected Data, Alberta, Canada, 2023/24 Season,” Eurosurveillance 29, no. 2 (2024): 2300709, 10.2807/1560-7917.ES.2024.29.2.2300709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. World Health Organization (WHO) , “Recommended Composition of Influenza Virus Vaccines for Use in the 2024 Southern Hemisphere Influenza Season,” 29 September 2023, Meeting report, Geneva: WHO, accessed February 15, 2023, https://www.who.int/publications/m/item/recommended‐composition‐of‐influenza‐virus‐vaccines‐for‐use‐in‐the‐2024‐southern‐hemisphere‐influenza‐season.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Supporting information
Data Availability Statement
Data supporting this study cannot be made available due to ethical and legal reasons.


