Abstract
Sodium–glucose cotransporter 2 inhibitors, efficacious antidiabetic agents that have cardiovascular and renal benefits, can promote pancreatic β-cell regeneration in type 2 diabetic mice. However, the underlying mechanism remains unclear. In this study, we aimed to use multiomics to identify the mediators involved in β-cell regeneration induced by dapagliflozin. We showed that dapagliflozin lowered blood glucose level, upregulated plasma insulin level, and increased islet area in db/db mice. Dapagliflozin reshaped gut microbiota and modulated microbiotic and plasmatic metabolites related to tryptophan metabolism, especially l-tryptophan, in the diabetic mice. Notably, l-tryptophan upregulated the mRNA level of glucagon-like peptide 1 (GLP-1) production–related gene (Gcg and Pcsk1) expression and promoted GLP-1 secretion in cultured mouse intestinal L cells, and it increased the supernatant insulin level in primary human islets, which was eliminated by GPR142 antagonist. Transplant of fecal microbiota from dapagliflozin-treated mice, supplementation of l-tryptophan, or treatment with dapagliflozin upregulated l-tryptophan, GLP-1, and insulin or C-peptide levels and promoted β-cell regeneration in db/db mice. Addition of exendin 9-39, a GLP-1 receptor (GLP-1R) antagonist, or pancreatic Glp1r knockout diminished these beneficial effects. In summary, treatment with dapagliflozin in type 2 diabetic mice promotes β-cell regeneration by upregulating GLP-1 production, which is mediated via gut microbiota and tryptophan metabolism.
Article Highlights
Sodium–glucose cotransporter 2 inhibitors, novel and efficacious antidiabetic agents, can preserve β-cell mass in type 2 diabetic animals, but the mechanism remains unclear.
We find that dapagliflozin reshapes gut microbiota, improves microbiotic and plasmatic metabolites related to tryptophan metabolism, and increases glucagon-like peptide 1 (GLP-1) production mediated via tryptophan metabolism. GLP-1−GLP-1 receptor signaling participates in the dapagliflozin-induced β-cell regeneration.
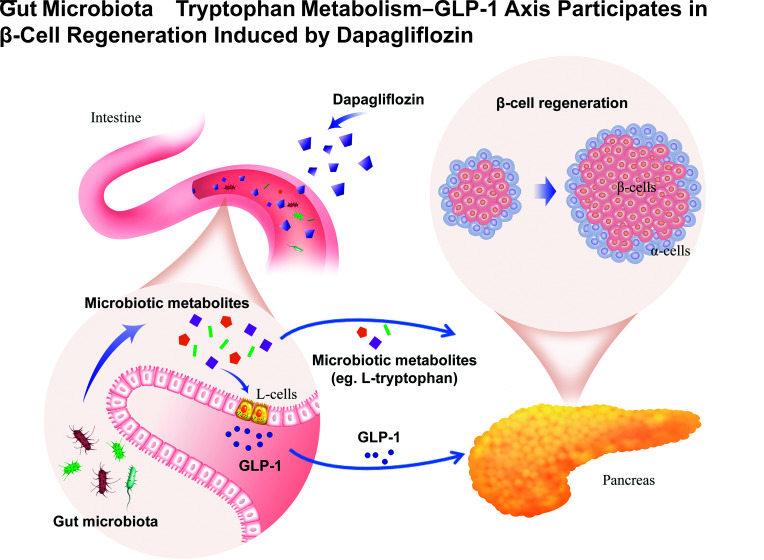
Our study reveals that the gut microbiota–tryptophan metabolism–GLP-1 axis is a novel mechanism of β-cell regeneration induced by dapagliflozin and provides experimental evidence for its β-cell protection in treating type 2 diabetes.
Graphical Abstract
Introduction
Diabetes has become a major global health concern. It is estimated that by 2045, >600 million people worldwide will have type 2 diabetes (1). Sodium–glucose cotransporter 2 (SGLT2) inhibitors improve glycemic control in an insulin-independent fashion by reducing renal glucose reabsorption (2). SGLT2 inhibitors have other benefits, including cardiovascular and renal protection (3,4).
Clinical studies have shown that SGLT2 inhibitors improve β-cell function in patients with type 2 diabetes (5,6). Animal studies have demonstrated that SGLT2 inhibitors preserve β-cell mass and islet morphology (7–10). Since β-cells do not express SGLT2, the protective effect of SGLT2 inhibitors on β-cells should be due to the indirect mechanisms (11). So far, the specific mediators remain unclear.
Herein, we profiled plasmatic nontargeted metabolomics to screen the humoral mediators involved in β-cell regeneration induced by dapagliflozin. We also performed shotgun metagenomic sequencing of cecal contents and analyzed the cecal metabolomics. Next, mouse intestinal L-cell line and primary human islets were incubated with metabolites, including l-tryptophan, with or without GPR142 antagonist to detect the effect of metabolites on L cells and islets, respectively. To confirm the involvement of the gut microbiota–tryptophan–glucagon-like peptide 1 (GLP-1) axis in dapagliflozin-induced β-cell regeneration, we performed fecal microbiota transplant (FMT), l-tryptophan supplementation, and dapagliflozin treatment with or without GLP-1 receptor (GLP-1R) blockage in db/db mice. Our study provides a novel insight into the mechanism of β-cell protection induced by SGLT2 inhibitors in type 2 diabetes.
Research Design and Methods
Animals and Intervention
Seven-week-old male db/db and db/m mice were used in the experiments. Mice were treated with dapagliflozin (1 mg/kg; AstraZeneca Pharmaceutical Co. Ltd., London, U.K.) or vehicle (water) for 5 weeks. After treatment, 12 db/db mice and 12 db/m mice were used for plasmatic nontargeted metabolomics, cecal shotgun metagenomics, and cecal metabolomics, while 20 db/db mice were used to collect cecum fecal samples.
Fecal microbiota from dapagliflozin- or vehicle-treated mice were intragastrically administered with or without GLP-1R antagonist exendin 9-39 (50 nmol/kg/day; Bachem, Bubendorf, Switzerland) treatment for 5 weeks in another batch of db/db mice (n = 28). Glycemic control was maintained by using insulin implants (LinBit; Linshin, Scarborough, ON, Canada).
To investigate the involvement of l-tryptophan and GLP-1R, db/db mice were given vehicle, l-tryptophan (200 mg/kg), or l-tryptophan combined with exendin 9-39 for 5 weeks. Also, insulin implants were used for glycemic control. Besides, db/db mice were treated with vehicle, dapagliflozin (1 mg/kg), exendin 9-39, or dapagliflozin combined with exendin 9-39 for 5 weeks. Age-matched db/m mice were included as normal control. Six-week-old male pancreas-specific Glp1r knockout (Glp1rpan−/−) mice and Pdx1-Cre or Glp1r-flox littermates were induced to type 2 diabetes (Supplementary Fig. 1) and were treated with dapagliflozin (1 mg/kg) or vehicle for 6 weeks.
Glucose Measurement, and Blood Sample and Intestinal Tissue Collection
Oral glucose tolerance test was performed after starvation. Blood samples were added with aprotinin, heparin sodium, and a dipeptidyl peptidase 4 inhibitor for hormone detection. Intestinal tissues were homogenized in radioimmunoprecipitation assay lysis buffer, and supernatants were collected for GLP-1 detection.
Immunofluorescence Quantification
Immunofluorescent images were captured under an automatic digital slide scanner (Pannoramic MIDI; 3DHISTECH, Budapest, Hungary). The total area of positive-staining cells was analyzed using Fiji software (National Institutes of Health, Bethesda, MD).
Nontargeted Metabolomic Analysis
Plasma and cecal contents were subjected to metabonomic analysis. The statistics function prcomp within R (https://www.r-project.org) was used for unsupervised principal component analysis. According to variable importance in projection (VIP) ≥1 and P < 0.05, the differential metabolites among the three groups were screened. VIP values were extracted from the orthogonal partial least squares-discriminant analysis results and generated using the R package MetaboAnalystR. The Kyoto Encyclopedia of Genes and Genomes database was used to annotate identified metabolites.
Shotgun Metagenomic Sequencing and Analysis
Total DNA of cecal contents was extracted. Sequencing libraries were generated using NEBNext Ultra DNA Library Prep Kit for Illumina (New England Biolabs, Ipswich, MA). Libraries were analyzed for size distribution using an Agilent 2100 Bioanalyzer (Agilent Technologies, Palo Alto, CA) and quantified using real-time PCR. The library preparations were sequenced on a NovaSeq platform (Illumina, San Diego, CA), and paired-end reads were generated. After sequencing, the sequences of bacteria, fungi, archaea, and viruses were extracted from the nonredundant database. DIAMOND software was used to blast unigenes to the functional database.
L-Cell Intervention
Mouse intestinal L-cell line STC1 cells (CRL-3254; ATCC) were incubated with different concentrations of l-tryptophan, indoleacetate, and N-acetylserotonin; cultured with l-tryptophan (10 mmol/L) with or without GPR142 antagonist CLP-3094 (10 μmol/L); or incubated with dapagliflozin (25 μmol/L) or vehicle with or without palmitate (0.5 mmol/L). Cells and supernatants were collected for mRNA and hormone detection, respectively.
Primary Human Islet Intervention
Human islets (Supplementary Table 1) were incubated with l-tryptophan (1 mmol/L) and/or CLP-3094 (1 μmol/L) with or without palmitate (0.5 mmol/L) for 24 h. Culture supernatants were collected for insulin detection.
Real-Time Quantitative Reverse Transcription-PCR
Total RNA was extracted by TRIzol reagent. Quantitative reverse transcription-PCR was performed on a QuantStudio 5 Real-Time PCR System (Thermo Fisher Scientific, Waltham, MA). The primer sequences are listed in Supplementary Table 2.
Hormone Measurements
ELISA kits were used to detect hormone concentration in blood samples, intestinal tissue lysates, or cell supernatants according to the manufacturer’s instructions.
Statistical Analysis
Data are expressed as the mean ± SEM or median (interquartile range). P < 0.05 was considered statistically significant. The statistical analysis was performed using GraphPad Prism 9.0 (GraphPad Software Inc., San Diego, CA). More details about the methodology are provided in the Supplementary Material.
Data and Resource Availability
The data sets generated and analyzed during the current study are available from the corresponding authors upon reasonable request.
Results
Dapagliflozin Promotes β-Cell Regeneration and Alters Tryptophan Metabolism of Plasmatic Metabolites in Diabetic db/db Mice
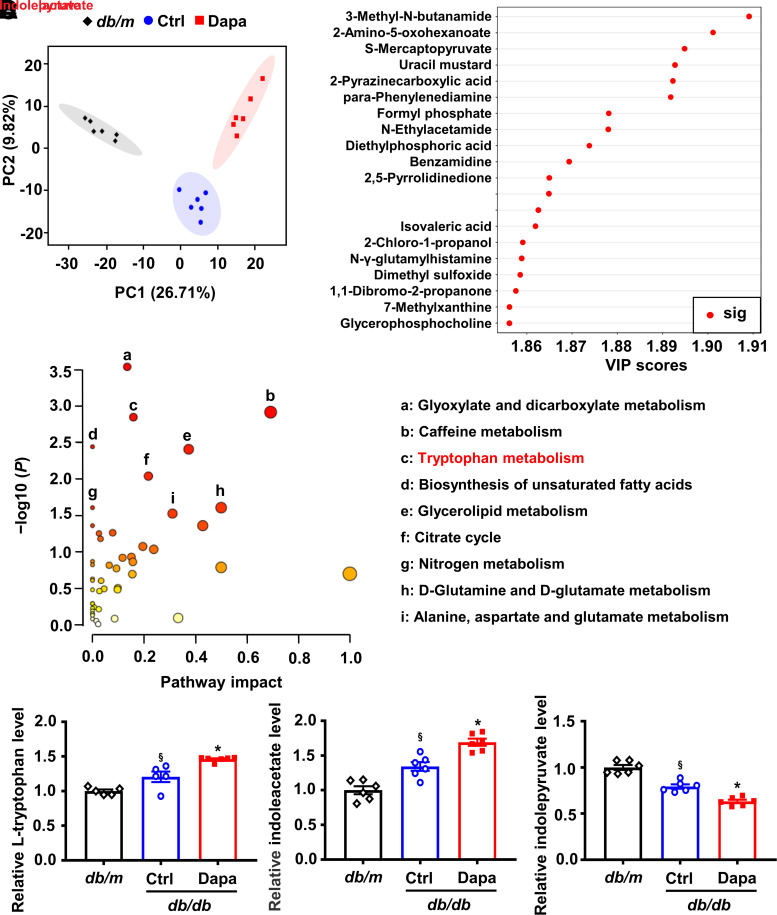
Dapagliflozin significantly reduced blood glucose level and increased plasma insulin level and islet areas in db/db mice (Supplementary Fig. 2). To identify the related humoral factors involved in β-cell regeneration, we profiled the plasmatic metabolome and found significant changes in the principal component analysis score plot (Fig. 1A). Metabolites in the top 20 of VIP scores and metabolic pathway analysis of altered metabolites indicated that tryptophan metabolism was enriched (Fig. 1B and C). We further analyzed the levels of l-tryptophan and its related metabolites. Results showed that dapagliflozin modulated l-tryptophan–related metabolites, especially upregulated l-tryptophan and indoleacetate, and downregulated indolepyruvate (Fig. 1D and Supplementary Figs. 3 and 4).
Figure 1.
Alteration of the plasma metabolomic profile induced by dapagliflozin (Dapa) in db/db mice. A: Principal component (PC) analysis plots of plasma metabolomic profiles. B: Differential plasmatic metabolites in the top 20 of VIP scores. C: Metabolic pathway analysis of differential plasmatic metabolites. The circles that are closer to the top-right diagonal region are significantly changed, and they are more likely to have significant impacts on the pathways. D: Relative levels of l-tryptophan, indoleacetate, and indolepyruvate in plasma. n = 5 − 6 mice/group. Data are mean ± SEM. Statistical analysis was performed by one-way ANOVA followed by Dunnett T3 multiple comparisons test in D. §P < 0.05 (vs. db/m); *P < 0.05 (vs. control [Ctrl]). 2,5-pyrrolidinedione, 3,3-dichloro-4-(dichloromethylene)-2,5-pyrrolidinedione; 3-methyl-N-butanamide, 3-methyl-N-(2-naphthyl)butanamide; N-γ-glutamylhistamine, N(α)-γ-l-glutamylhistamine; sig, significant; S-mercaptopyruvate, S-(4-bromophenyl)-mercaptopyruvate.
Dapagliflozin Modulates Tryptophan Metabolism by Gut Microbiota in db/db Mice
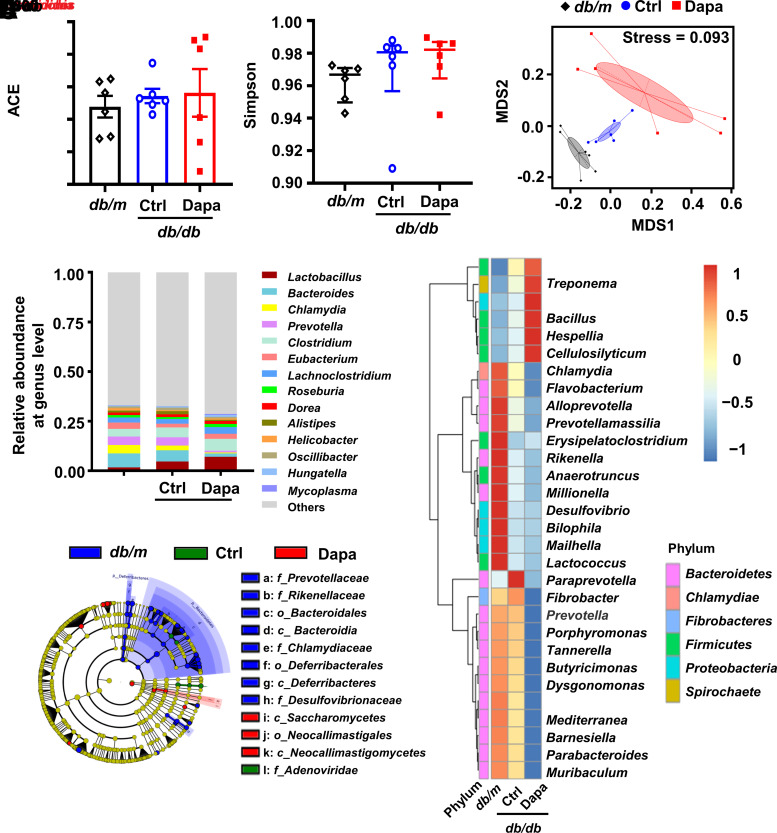
l-Tryptophan is an essential amino acid in mammals and mainly derived from diet (12). Gut microbiota have a crucial impact on intestinal tryptophan availability and metabolism (13). SGLT2 inhibitors modulate the composition of gut microbiota (14). Hence, we inferred that the dapagliflozin-mediated changes of plasmatic tryptophan–related metabolites were closely related to gut microbiota. We collected cecal contents and performed shotgun metagenomic sequencing. α-Diversity was not altered, while high β-diversity indicated by nonmetric multidimensional scaling was observed among three groups (Fig. 2A−C). Dapagliflozin altered the composition of gut microbiota at the genus level, especially those related to tryptophan metabolism, including Escherichia, Clostridium, and Bacteroides (Fig. 2D and E). A higher abundance of Escherichia and Clostridium was observed in db/db mice versus db/m mice and further increased after dapagliflozin treatment (Fig. 2D and E). Escherichia produces tryptophan, and Clostridium degrades l-tryptophan into indole-3-acetamide and indoleacetate (15,16). Hence, we inferred that elevated l-tryptophan, indole-3-acetamide, and indoleacetate might be associated with an increase in Escherichia and Clostridium (Figs. 1D and 2E and Supplementary Fig. 3). Bacteroides, which metabolizes l-tryptophan into indolelactate (17), showed opposite changes to Escherichia and Clostridium. Consistently, plasma indolelactate level was lower in db/db mice than in db/m mice and was further decreased by dapagliflozin. Similarly, the linear discriminant analysis effect size method revealed that Bacteroides was enriched in the db/m group and that Clostridium was enriched in the dapagliflozin-treated db/db group (Fig. 2F and Supplementary Fig. 5).
Figure 2.
The composition of gut microbial communities after treatment with dapagliflozin (Dapa) in db/db mice. A and B: Abundance-based coverage estimator (ACE) and Simpson index to describe the α-diversity of gut microbial communities. C: Nonmetric multidimensional scaling (MDS) to describe the β-diversity of gut microbial communities. D: The top 14 maximum abundances of gut microbiota at the genus level. E: The top 30 differential gut microbiota at the genus level. F: Cladogram representation of differentially abundant taxonomic clades with linear discriminant analysis score >4. Significantly distinct taxonomic nodes are colored, and the branch areas are shaded according to the effect size of the taxa. n = 6 mice/group. Data are the mean ± SEM or median (interquartile range). Statistical analysis was performed by one-way ANOVA followed by Tukey multiple comparisons test in A or by Kruskal-Wallis test followed by Dunn multiple comparisons test in B. Ctrl, control.
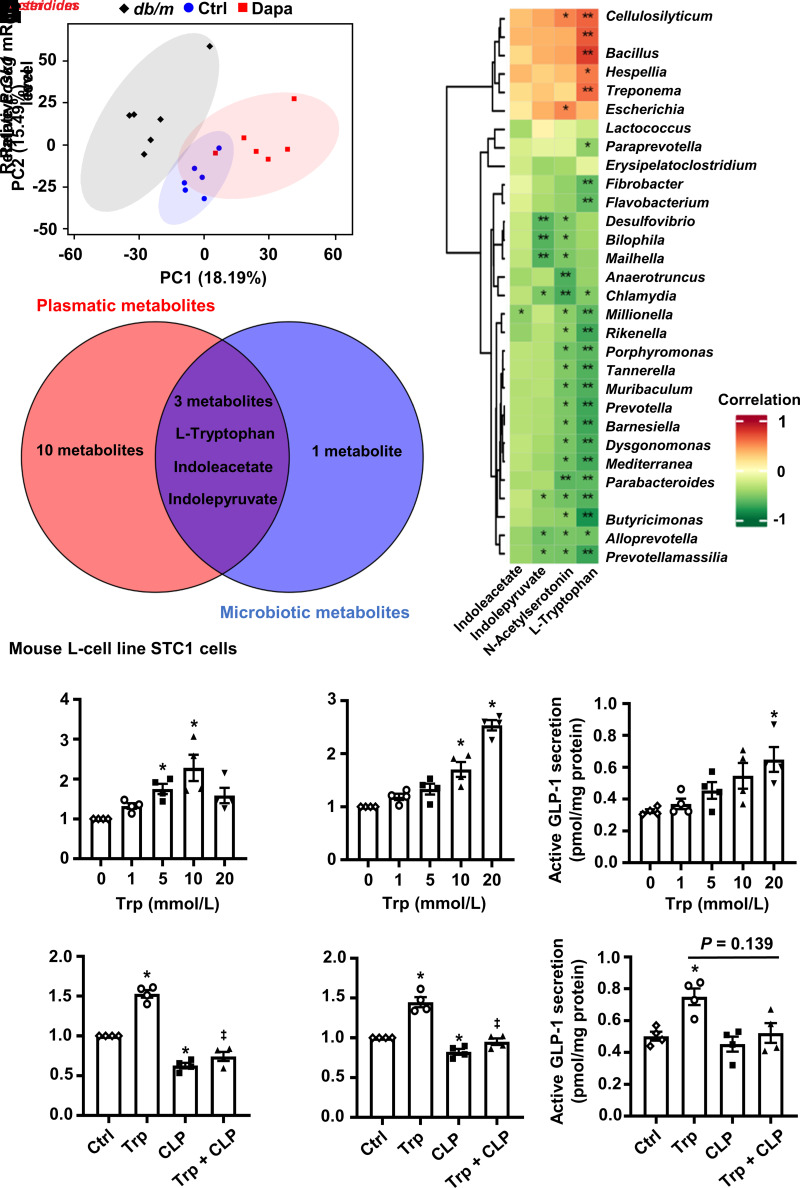
Next, nontargeted metabolomic analysis of cecal contents was performed. Dapagliflozin changed the microbiotic metabolite profile (Fig. 3A). Notably, metabolites related to tryptophan metabolism, especially l-tryptophan, were modulated (Supplementary Fig. 6A). The tryptophan metabolism pathway was also enriched after treatment with dapagliflozin (Supplementary Fig. 6B). In the top 30 differential gut microbiota, l-tryptophan positively correlated with 5 gut microbiota and negatively correlated with 18 gut microbiota, including Bacteroides and Clostridium (Fig. 3B). These results indicate that dapagliflozin modulates tryptophan metabolism through gut microbiota in db/db mice. By using cross-analysis, l-tryptophan, indoleacetate, and indolepyruvate were screened, but only l-tryptophan was modulated by dapagliflozin in the two metabolomic analyses (Figs. 1D and 3C and Supplementary Fig. 6A). Moreover, plasma l-tryptophan level measured by ELISA showed similar results with metabolomics (Supplementary Fig. 6C). l-Tryptophan has been reported to increase β-cell mass and promote insulin secretion in rodents (18,19). We found that l-tryptophan increased supernatant insulin level in primary human islets, which was eliminated after antagonizing GPR142 (Supplementary Fig. 7), a G-protein–coupled receptor involved in glucose-stimulated insulin secretion (20). Hence, we inferred that l-tryptophan might participate in dapagliflozin-induced β-cell regeneration.
Figure 3.
The relationship among the gut microbiota, microbiotic metabolites, and GLP-1 production. A: Principal component (PC) analysis plots of microbial metabolomic profiles. B: Spearman correlation analysis between the top 30 differential gut microbiota and differential microbiotic metabolites related to l-tryptophan (Trp) metabolism. C: The number of overlapping metabolites between microbiotic and plasmatic metabolites. D and E: Relative Gcg and Pcsk1 mRNA levels in the intestinal L-cell line STC1 cells incubated with different concentrations of Trp. F: GLP-1 secretion in STC1 cells cultured with different concentrations of Trp. G–I: Relative Gcg and Pcsk1 mRNA levels and GLP-1 secretion in STC1 cells incubated with Trp (10 mmol/L) or vehicle with or without CLP-3094 (CLP) (10 μmol/L). n = 4. Data are mean ± SEM. Statistical analysis was performed by one-way ANOVA followed by Dunnett multiple comparisons test in D and E, one-way ANOVA followed by Tukey multiple comparisons test in G and H, or one-way ANOVA followed by Dunnett T3 multiple comparisons test in F and I. *P < 0.05 (vs. control [Ctrl]); ‡P < 0.05 (vs. Trp).
Microbiotic and Plasmatic Metabolite l-Tryptophan Upregulates GLP-1 Production–Related Gene Expression and Promotes GLP-1 Secretion
Whether β-cell regeneration induced by l-tryptophan is due to its direct or indirect action remains to be clarified. We speculated that a high concentration of microbiotic metabolites might affect the metabolism of the whole body by modulating the function of local intestinal cells. Intestinal L cells secrete GLP-1, which helps to stimulate glucose-dependent insulin secretion and promote β-cell regeneration (21,22). We found that l-tryptophan upregulated Gcg (encoding proglucagon) and Pcsk1 (encoding prohormone convertase 1/3, an important enzyme for processing proglucagon into GLP-1 [23]) mRNA levels and promoted GLP-1 secretion in L cells (Fig. 3D−F), and these effects were reversed by GPR142 antagonist (Fig. 3G−I). Consistently, dapagliflozin not only increased microbiotic metabolite l-tryptophan level but also upregulated plasma and intestinal active GLP-1 levels in db/db mice (Supplementary Fig. 6A, D, and E). Other microbiotic metabolites, including indoleacetate and N-acetylserotonin, did not promote GLP-1 production–related gene expression and GLP-1 secretion (Supplementary Fig. 8A–F). Besides, dapagliflozin had no direct effect on L cells (Supplementary Fig. 8G and H). Hence, we inferred that dapagliflozin might promote β-cell regeneration via reshaping gut microbiota, altering tryptophan metabolism, and thereby upregulating GLP-1–GLP-1R signaling.
Gut Microbiota Reshaped by Dapagliflozin Promotes β-Cell Regeneration in a GLP-1−GLP-1R Signaling–Dependent Manner in db/db Mice
To determine whether and how gut microbiota reshaped by dapagliflozin affected β-cell regeneration, we performed FMT from dapagliflozin- or vehicle-treated mice with or without exendin 9-39. Glycemic control was maintained at the level similar to dapagliflozin treatment by using insulin implants (Supplementary Fig. 9A and B). No significant difference in body weight was observed (Supplementary Fig. 9C). Plasma l-tryptophan and plasma and intestinal GLP-1 levels were elevated in db/db mice after receiving fecal microbiota from dapagliflozin-treated mice (Supplementary Fig. 9D–F). Notably, FMT from the dapagliflozin group significantly increased postload plasma C-peptide level, which was abolished by exendin 9-39 (Supplementary Fig. 9G). FMT had no effects on plasma glucagon level (Supplementary Fig. 9H).
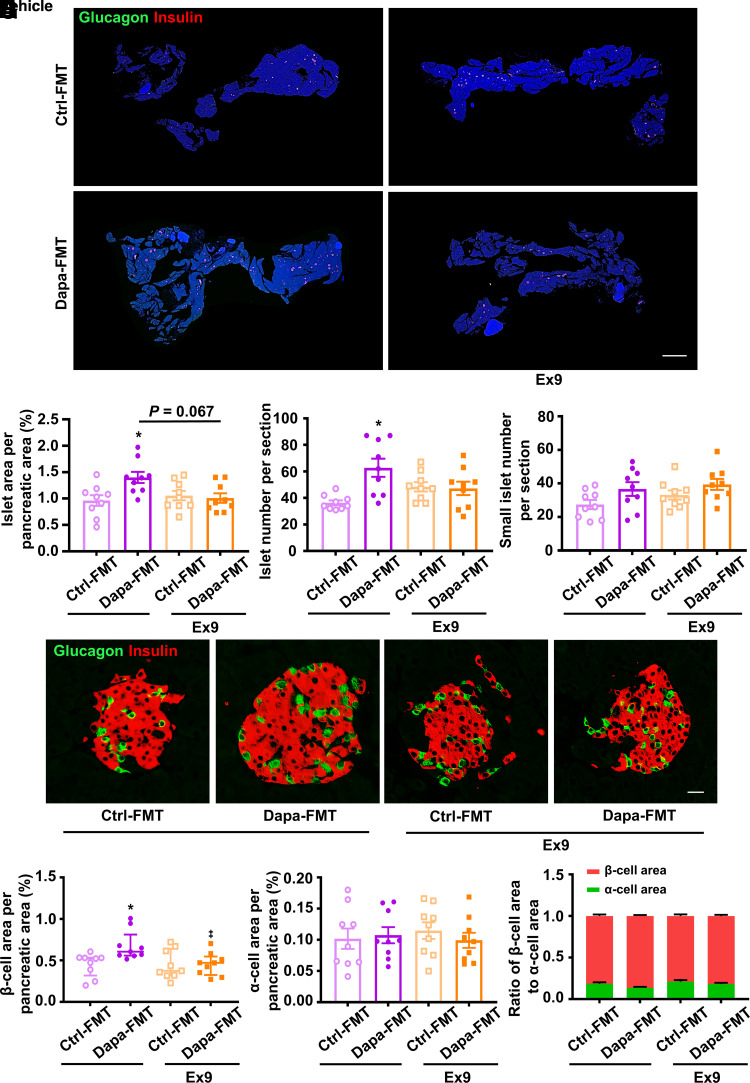
Islet area, islet number, and β-cell area were boosted in db/db mice receiving fecal microbiota from dapagliflozin-treated mice, and addition of exendin 9-39 attenuated these beneficial effects (Fig. 4A−C, E, and F). Small islet number (an indicator of islet neogenesis), α-cell area, and ratio of β-cell area to α-cell area remained unchanged among the four groups (Fig. 4D, G, and H). Subsequently, we estimated β-cell proliferation by BrdU tracing or proliferating cell nuclear antigen (PCNA) immunofluorescent staining. Gut microbiota from dapagliflozin-treated mice significantly increased numbers of BrdU+insulin+ cells or PCNA+insulin+ cells, while this effect was eliminated by exendin 9-39 (Supplementary Fig. 10A–D). These results indicate that gut microbiota reshaped by dapagliflozin promotes β-cell regeneration through GLP-1−GLP-1R signaling.
Figure 4.
Histological analysis in the pancreatic tissues of db/db mice receiving FMT from dapagliflozin (Dapa)- or vehicle-treated mice with or without exendin 9-39 (Ex9) for 5 weeks. A: Representative photographs of the entire pancreata immunostained for glucagon and insulin. Scale bar = 2,000 μm. B: Quantification of the islet area per pancreatic area. C: Quantification of the islet number per section. D: Quantification of the small islet number per section. An islet with a cell number less than eight is defined as a small islet. E: Representative photographs of an islet immunostained for glucagon and insulin. Scale bar = 20 μm. F: Quantification of the β-cell area per pancreatic area. G: Quantification of the α-cell area per pancreatic area. H: The ratio of β-cell area to α-cell area. n = 3 sections/mouse and 3 mice/group. Data are mean ± SEM or median (interquartile range). Statistical analysis was performed by one-way ANOVA followed by Dunnett T3 multiple comparisons test in B and C, by Kruskal-Wallis test followed by Dunn multiple comparisons test in F, or by one-way ANOVA or two-way ANOVA followed by Tukey multiple comparisons test as appropriate in D, G, and H. *P < 0.05 (vs. control [Ctrl]-FMT + vehicle).
l-Tryptophan Promotes β-Cell Regeneration in a GLP-1−GLP-1R Signaling–Dependent Manner in db/db Mice
To investigate whether l-tryptophan had direct beneficial effects on islet regeneration in diabetic mice and whether GLP-1−GLP-1R signaling was involved in this process, db/db mice were treated with vehicle, l-tryptophan, or l-tryptophan combined with exendin 9-39. Glycemic control was kept at the level similar to dapagliflozin administration in db/db mice by using insulin implants (Supplementary Fig. 11A and B). There was no significant difference in body weight (Supplementary Fig. 11C). Plasma l-tryptophan increased to a level comparable with dapagliflozin treatment (Supplementary Fig. 11D). Plasma and intestinal GLP-1 levels and postload plasma C-peptide level were significantly increased by l-tryptophan, and exendin 9-39 eliminated these effects (Supplementary Fig. 11E–G). No significant difference was observed in plasma glucagon level (Supplementary Fig. 11H).
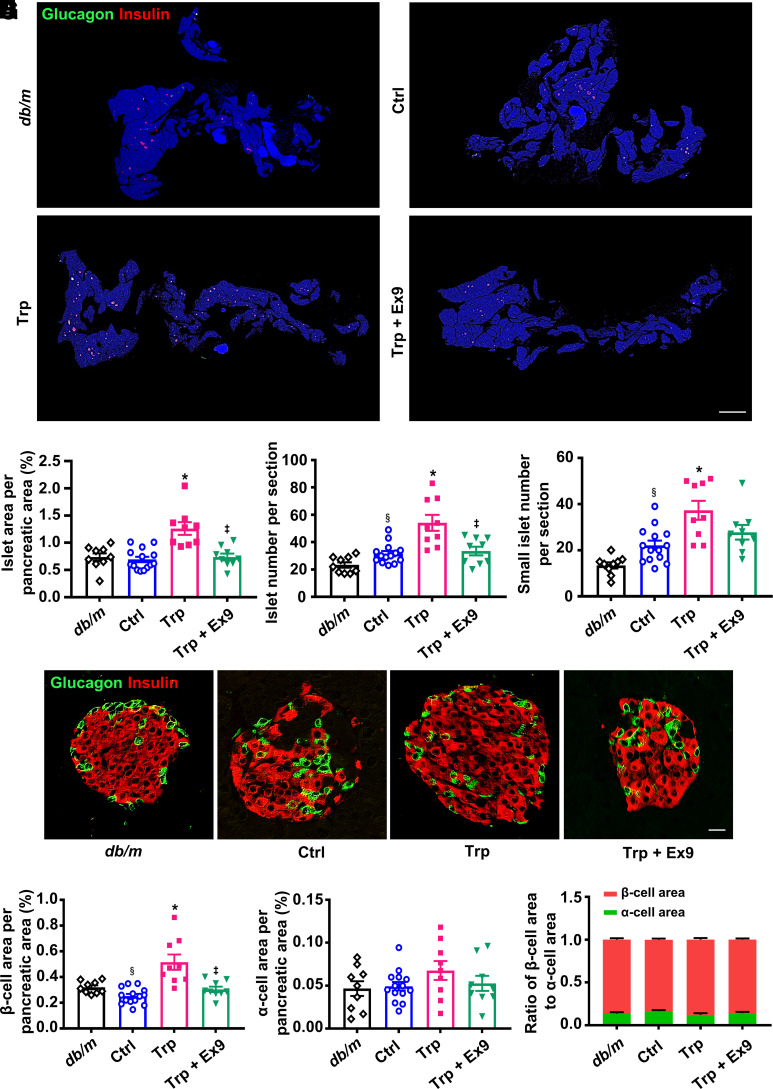
Islet area, islet number, small islet number, and β-cell area were enlarged by l-tryptophan treatment, and these effects were attenuated by exendin 9-39 (Fig. 5A−F). No significant change was shown in α-cell area and ratio of β-cell area to α-cell area (Fig. 5G and H). l-Tryptophan remarkably increased the number of BrdU+insulin+ cells or PCNA+insulin+ cells, while exendin 9-39 antagonized this effect (Supplementary Fig. 12A–D). These results suggest that l-tryptophan promotes β-cell regeneration through GLP-1−GLP-1R signaling in type 2 diabetic mice.
Figure 5.
Histological analysis in the pancreatic tissues of db/db mice treated with vehicle, l-tryptophan (Trp), or Trp combined with exendin 9-39 (Ex9) for 5 weeks. Age-matched db/m mice treated with vehicle were included as normal control (Ctrl). A: Representative photographs of the entire pancreata immunostained for glucagon and insulin. Scale bar = 2,000 μm. B: Quantification of the islet area per pancreatic area. C: Quantification of the islet number per section. D: Quantification of the small islet number per section. An islet with a cell number less than eight is defined as a small islet. E: Representative photographs of an islet immunostained for glucagon and insulin. Scale bar = 20 μm. F: Quantification of the β-cell area per pancreatic area. G: Quantification of the α-cell area per pancreatic area. H: Ratio of β-cell area to α-cell area. n = 3 sections/mouse and 3 mice/group. Data are mean ± SEM. Statistical analysis was performed by one-way ANOVA followed by Dunnett T3 multiple comparisons test in B–D and F, or by one-way ANOVA or two-way ANOVA followed by Tukey multiple comparisons test as appropriate in G and H. §P < 0.05 (vs. db/m); *P < 0.05 (vs. Ctrl); ‡P < 0.05 (vs. Trp).
Systemic or Pancreatic GLP-1R Plays a Small Role in Glucose-Lowering Effect Induced by Dapagliflozin in Type 2 Diabetic Mice
To explore the role of GLP-1−GLP-1R signaling in the beneficial effects of dapagliflozin, db/db mice were treated with dapagliflozin with or without exendin 9-39. Since db/db mice treated with exendin 9-39 alone died soon afterward due to the extreme hyperglycemia, data in this group were excluded. Compared with the baseline and control group, dapagliflozin significantly lowered fasting, random, and postload blood glucose levels, and merely postload glucose level was partially antagonized by exendin 9-39 (Supplementary Fig. 13A–D). Body weight showed no significant difference among the three groups in db/db mice (Supplementary Fig. 13E). Dapagliflozin remarkably increased plasma insulin level, while the combination with exendin 9-39 did not show such effect (Supplementary Fig. 13F). There was no difference in plasma glucagon level among the groups (Supplementary Fig. 13G).
Diabetic Glp1rpan−/− mice and Flox/Cre littermates were used to investigate the role of GLP-1R in pancreata. Fasting, random, and postload blood glucose levels were significantly decreased by dapagliflozin treatment, although the glucose level after dapagliflozin treatment was slightly higher in Glp1rpan−/− mice than Flox/cre littermates (Supplementary Fig. 13H–K). Dapagliflozin had little effect on body weight (Supplementary Fig. 13L). There was no significant difference in plasma insulin and glucagon levels between the dapagliflozin and control group in Glp1rpan−/− mice and Flox/cre littermates (Supplementary Fig. 13M and N). These results indicate that pancreatic GLP-1R might only play a minor role in a dapagliflozin-induced glucose-lowering effect.
Blockage of GLP-1R by Exendin 9-39 Eliminates β-Cell Regeneration Induced by Dapagliflozin in db/db Mice
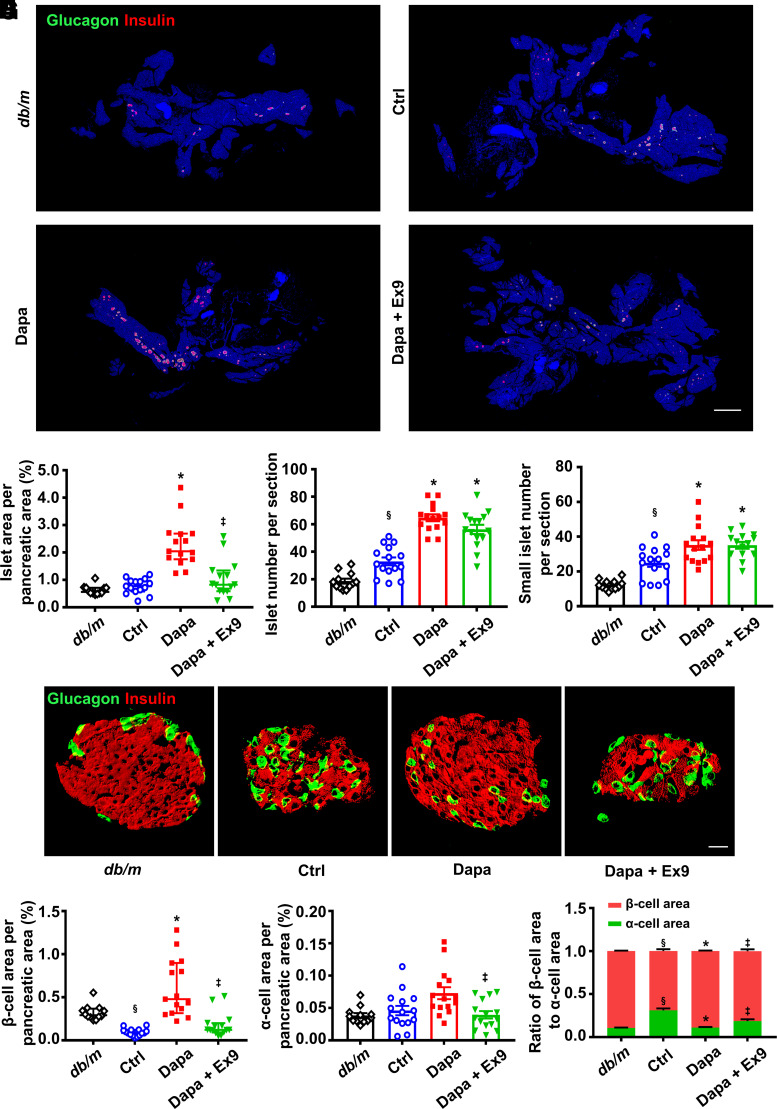
Treatment with dapagliflozin in db/db mice induced a twofold increase in islet area, and this increment was reversed by exendin 9-39 (Fig. 6A and B). Dapagliflozin significantly increased islet number and small islet number, and addition of exendin 9-39 did not alter these effects (Fig. 6C and D). Subsequently, we analyzed the ratio of islets with different size. Compared with db/m mice, the proportion of islets with large size (area >20,000 μm2) remarkably decreased, while the proportion of islets with small size (area <5,000 μm2) significantly increased in db/db mice. Dapagliflozin reversed these alterations to the normal level, while the effects were abolished by exendin 9-39 (Supplementary Fig. 14).
Figure 6.
Histological analysis in the pancreatic tissues of db/db mice treated with vehicle, dapagliflozin (Dapa), or Dapa with exendin 9-39 (Ex9) for 5 weeks. Age-matched db/m mice treated with vehicle served as normal control (Ctrl). A: Representative photographs of the entire pancreata immunostained for glucagon and insulin. Scale bar = 2,000 μm. B: Quantification of the islet area per pancreatic area. C: Quantification of the islet number per section. D: Quantification of the small islet number per section. An islet with a cell number less than eight is defined as a small islet. E: Representative photographs of an islet immunostained for glucagon and insulin. Scale bar = 20 μm. F: Quantification of the β-cell area per pancreatic area. G: Quantification of the α-cell area per pancreatic area. H: Ratio of β-cell area to α-cell area. n = 3–4 sections/mouse and 4 mice/group. Data are mean ± SEM or median (interquartile range). Statistical analysis was performed by one-way ANOVA followed by Dunnett T3 multiple comparisons test in C, D, and G, two-way ANOVA followed by Tukey multiple comparisons test in H, or Kruskal-Wallis test followed by Dunn multiple comparisons test in B and F. §P < 0.05 (vs. db/m); *P < 0.05 (vs. Ctrl); ‡P < 0.05 (vs. Dapa).
Dapagliflozin induced a marked expansion in β-cell area in db/db mice, which was eliminated by exendin 9-39 (Fig. 6E and F). Dapagliflozin did not affect α-cell area (Fig. 6E and G). The proportion of β-cells to α-cells was upregulated by dapagliflozin treatment, and this effect was diminished by exendin 9-39 (Fig. 6H). Proliferative β-cells as indicated by BrdU+insulin+ cells or PCNA+insulin+ cells were augmented by dapagliflozin, while GLP-1R blockage antagonized this effect (Supplementary Fig. 15A–D). These results indicate that GLP-1R is involved in dapagliflozin-induced β-cell regeneration in db/db mice.
Glp1rpan−/− Eliminates β-Cell Regeneration Induced by Dapagliflozin in High-Fat Diet + Streptozotocin–Induced Diabetic Mice
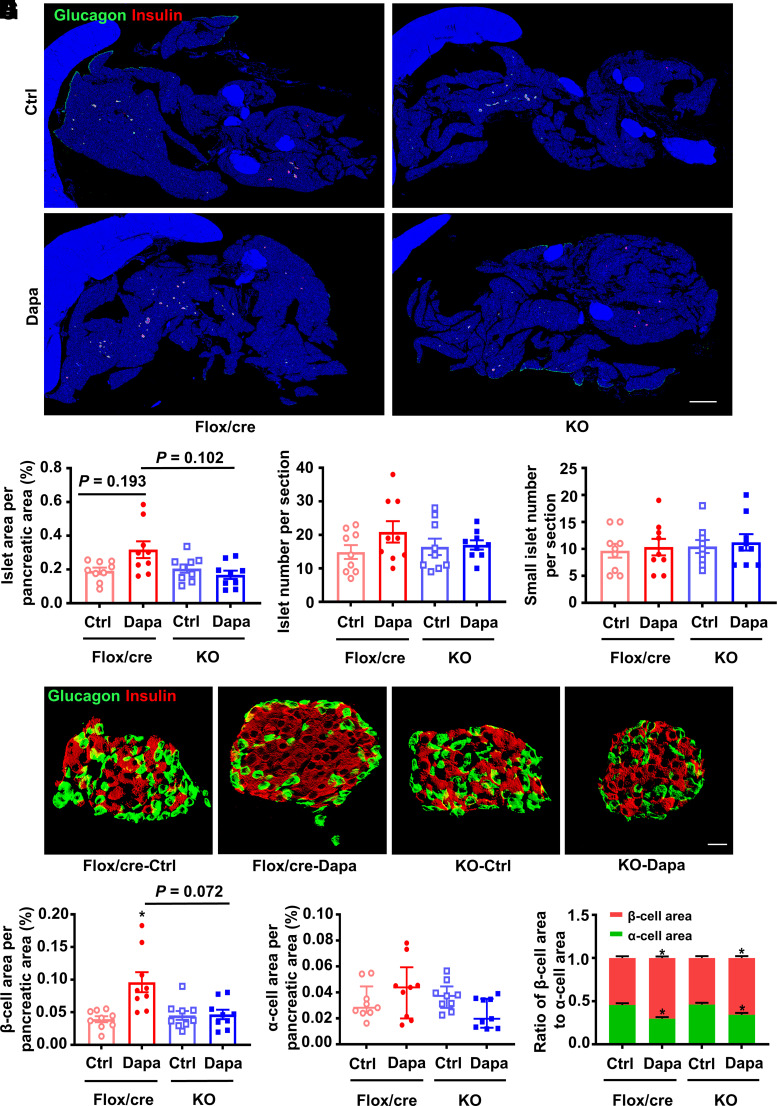
Treatment with dapagliflozin had a tendency to increase islet area in high-fat diet + streptozotocin–induced diabetic Flox/Cre littermates but not in diabetic Glp1rpan−/− mice. Thus, compared with dapagliflozin-treated Flox/Cre littermates, islet area seemed less in dapagliflozin-treated Glp1rpan−/− mice (Fig. 7A and B). Dapagliflozin did not affect islet number and small islet number in Glp1rpan−/− mice and Flox/Cre littermates (Fig. 7C and D). Dapagliflozin significantly increased β-cell area in Flox/Cre littermates but had no such effect in Glp1rpan−/− mice (Fig. 7E and F). There was no significant difference in α-cell area among the four groups (Fig. 7G). The ratio of β-cells to α-cells was increased by dapagliflozin in diabetic Glp1rpan−/− mice and Flox/Cre littermates (Fig. 7H). To exclude the effect of gene manipulation on β-cell regeneration, age-matched wild-type, Pdx1-Cre or Glp1r-flox mice were used. No difference in glucose metabolism and β-cell mass was observed among wild-type, Pdx1-Cre and Glp1r-flox mice (Supplementary Fig. 16). These results indicate that pancreatic GLP-1R participates in dapagliflozin-induced β-cell regeneration.
Figure 7.
Histological analysis in the pancreatic tissues of diabetic Glp1rpan−/− (KO) mice and Flox/Cre littermates treated with dapagliflozin (Dapa) or vehicle control (Ctrl) for 6 weeks. Diabetes was induced by high-fat diet and streptozotocin before the treatment. A: Representative photographs of the entire pancreata immunostained for glucagon and insulin. Scale bar = 2,000 μm. B: Quantification of the islet area per pancreatic area. C: Quantification of the islet number per section. D: Quantification of the small islet number per section. An islet with a cell number less than eight is defined as a small islet. E: Representative photographs of an islet immunostained for glucagon and insulin. Scale bar = 20 μm. F: Quantification of the β-cell area per pancreatic area. G: Quantification of the α-cell area per pancreatic area. H: The ratio of β-cell area to α-cell area. n = 3 sections/mouse and 3 mice/group. Data are mean ± SEM or median (interquartile range). Statistical analysis was performed by one-way ANOVA or two-way ANOVA followed by Tukey multiple comparisons test as appropriate in C, D, and H, one-way ANOVA followed by Dunnett T3 multiple comparisons test in B and F, or Kruskal-Wallis test followed by Dunn multiple comparisons test in G. *P < 0.05 (vs. Ctrl in the same genotype).
Discussion
SGLT2 inhibitors, safe and efficacious antidiabetic agents, exert organ protective effects, including improvement of β-cell function and mass, but the mechanism has remained unclear. By using integrated multiomics analyses, including plasmatic metabolomics, cecal shotgun metagenomics, and cecal metabolomics, we found that dapagliflozin modulated microbiotic and plasmatic metabolites related to tryptophan metabolism, especially l-tryptophan. l-Tryptophan promoted GLP-1 and insulin secretion in mouse intestinal L-cell line and primary human islets, respectively. FMT from dapagliflozin-treated mice, l-tryptophan supplementation, and dapagliflozin treatment all upregulated l-tryptophan, GLP-1, and insulin or C-peptide levels and increased islet area and β-cell area in diabetic mice. Addition of exendin 9-39, a GLP-1R antagonist, or Glp1rpan−/− diminished the beneficial effects of dapagliflozin on β-cell regeneration. This study provides clear evidence that increased GLP-1 production mediated via tryptophan metabolism is indeed an important mechanism for β-cell regeneration induced by dapagliflozin.
Multiple lines of evidence have demonstrated that dapagliflozin improves β-cell function and promotes β-cell regeneration (6,7), but the mechanisms have remained unclear. Some researchers explained the protective effects of SGLT2 inhibitors on β-cells as an indirect action, which might be secondary to the improvement of glucolipotoxicity (24). Notably, although glycemic control showed a similar level, β-cell mass increased after treatment with dapagliflozin alone or in combination with insulin glargine but not with insulin glargine alone, which indicates that dapagliflozin might have other mechanisms of β-cell protection except for the glucose-lowering effect (25). However, the specific humoral factors remain unknown. Our study showed that dapagliflozin significantly modulated plasmatic metabolites related to tryptophan metabolism, suggesting its potential role in the dapagliflozin-induced protective effects.
Gut microbiota and their metabolites are closely associated with type 2 diabetes and considered as important contributors for metabolic disorders (26,27). Antidiabetic agents, such as metformin, have been shown to ameliorate type 2 diabetes by modulating gut microbiota (28,29). However, the effects of dapagliflozin on gut microbiota are still controversial. Although dapagliflozin had different metabolic actions in patients with type 2 diabetes, it could not alter the fecal microbiome (30). On the contrary, empagliflozin ameliorates diabetic nephropathy via reducing lipopolysaccharide-producing bacteria and increasing short-chain fatty acid–producing bacteria in type 2 diabetic mice (31). Luseogliflozin prevents sarcopenic obesity, the central pathological factor in type 2 diabetes, by increasing the abundance of intestinal bacteria and improving amino acid metabolism (14). Our results show that dapagliflozin reshaped gut microbiota, including Escherichia, Bacteroides, and Clostridium, which were closely related to tryptophan metabolism. Furthermore, our FMT experiment revealed that gut microbiota reshaped by dapagliflozin increased plasma l-tryptophan level and promoted β-cell regeneration. Collectively, these observations indicate that gut microbiota and their metabolites are involved in the improvement of glycometabolism induced by SGLT2 inhibitors.
Abnormal tryptophan metabolism is associated with glucose dysmetabolism (32,33). A clinical study that involved 9,180 participants of diverse racial and ethnic backgrounds from five cohorts revealed that l-tryptophan and l-tryptophan–related metabolites were associated with type 2 diabetes risk (34). In addition, indoleamine 2,3-dioxygenase activity was increased in obesity, which shifted tryptophan metabolism from indole derivative production toward kynurenine production (35). In obesity or type 2 diabetes, the capacity of microbiota to metabolize l-tryptophan into derivatives decreased, which were able to activate the aryl hydrocarbon receptor (36). Based on our cross-analysis of microbiotic and plasmatic metabolomics, we focused on l-tryptophan and found that l-tryptophan level was higher in type 2 diabetes than in nondiabetes and that l-tryptophan level was further increased after dapagliflozin treatment or FMT. Notably, l-tryptophan enhanced insulin secretion in primary human islets by activating GPR142, suggesting a protective effect on β-cells. Furthermore, l-tryptophan promoted β-cell regeneration partially mediated by increasing β-cell proliferation in db/db mice. Hence, we inferred that upregulated plasmatic l-tryptophan by dapagliflozin might exert protective effects on β-cells. In the early stage of the disease, the body tries to alleviate diabetes by upregulating l-tryptophan, just like insulin and some beneficial cytokines (e.g., fibroblast growth factor 21 [FGF21]). However, the compensatory effects of relatively low-level l-tryptophan was insufficient. Notably, exogenous insulin injection, FGF21 treatment, or some antidiabetic agents that increase FGF21 level (e.g., GLP-1 and glucagon receptor antagonism) could still decrease blood glucose efficiently and could even promote β-cell regeneration in type 2 diabetic mice (37,38). These results indicate that some cytokines and metabolomics that are elevated in individuals with diabetes are good rather than bad factors and can be used as potential targets for diabetes treatment.
Microbiotic metabolites, with high concentrations in local intestine, can modulate cell phenotype and function in intestinal cells. For instance, l-tryptophan metabolites enhance intestinal epithelial barrier function and regulatory immune responses at mucosal surfaces (39). Short-chain fatty acids promote GLP-1 secretion from intestinal L cells (40). Similar to previous studies in humans and rodents (18,41,42), we found that l-tryptophan increased GLP-1 production in cultured L cells and upregulated plasma and intestinal GLP-1 levels in db/db mice. Considering that GLP-1 promotes β-cell regeneration, we supposed that a tryptophan metabolism−GLP-1 axis might participate in SGLT2 inhibitor–induced β-cell regeneration. Interestingly, change in GLP-1 level was inconsistent with that in l-tryptophan level in type 2 diabetic mice. This might be explained by the fact that GLP-1 secretion could be affected by various factors, such as fasting glucagon level and insulin resistance, not merely l-tryptophan (43,44). After dapagliflozin treatment, elevated l-tryptophan stimulated GLP-1 secretion, which became a vital factor in stimulating β-cell regeneration in type 2 diabetic mice. Although GLP-1 has little effect in promoting β-cell regeneration in adult human pancreas (45), GLP-1 synergized with DYRK1A inhibitors could potentiate human β-cell proliferation (46). Therefore, GLP-1 might synergistically promote human β-cell regeneration with l-tryptophan or other factors regulated by dapagliflozin, which deserves further investigation.
We used GLP-1R antagonist exendin 9-39 or Glp1rpan−/− to determine the role of systemic or pancreatic GLP-1R in β-cell regeneration induced by dapagliflozin, FMT, or l-tryptophan. We found that blockage of GLP-1R only showed a very limited impact on the glucose-lowering effect of dapagliflozin in type 2 diabetic mice. This is consistent with the mechanism for the glucose-lowering action of dapagliflozin, primarily relying on inhibition of glucose reabsorption in the renal tubule but not β-cell regeneration. By contrast, dapagliflozin-, FMT-, or l-tryptophan–induced increase of insulin secretion and β-cell regeneration was attenuated by GLP-1R blockage, indicating that β-cell function and β-cell regeneration depend on GLP-1R. By the way, the effect of Glp1r knockout on histological end points was milder than that observed in db/db mice treated with exendin 9-39. We supposed that there were two possible reasons: 1) The effect of constitutive Glp1r knockout might be compensated by other receptors and signaling pathways during embryonic development, while addition of exendin 9-39 to adult db/db mice was a pharmacological intervention without the compensation, and 2) Glp1r knockout was pancreas specific rather than systemic, while the exendin 9-39 intervention affected the whole body. Consistently, l-tryptophan level was associated with the indicators of islet regeneration, including islet number and small islet number, but not blood glucose level. These results indicate that islet regeneration induced by dapagliflozin is independent of the glucose-lowering effect.
To our knowledge, this study is the first to identify the involvement of l-tryptophan–related metabolites and GLP-1 in β-cell protection induced by SGLT2 inhibitors. However, there are some limitations and some unanswered questions. First, we did not repeat experiments with other SGLT2 inhibitors to determine whether the gut microbiota−tryptophan metabolism−GLP-1–mediated β-cell protection was a class effect. Second, we cannot exclude the contribution of other metabolites to the stimulation of GLP-1 production and β-cell regeneration. Nevertheless, this study reveals a novel mechanism of β-cell regeneration induced by SGLT2 inhibitors and provides experimental evidence for the application of SGLT2 inhibitors to improve β-cell function in type 2 diabetes.
This article contains supplementary material online at https://doi.org/10.2337/figshare.25375459.
Article Information
Acknowledgments. The authors are grateful to AstraZeneca Pharmaceutical Co. Ltd. for providing dapagliflozin powder and to Prof. Shusen Wang (Organ Transplant Center, Tianjin First Central Hospital) for gifting primary human islets.
Funding. This work was supported by National Clinical Key Specialty Construction Program, P.R. China (2023) special funding; National Natural Science Foundation of China grants 82270843, 82170875, 82371588, 82100885, 82200908, and 82271611; Beijing Municipal Natural Science Foundation grants 7232198 and 7222216; Clinical Medicine Plus X-Young Scholars Project of Peking University grant PKU2023LCXQ025; the Talent Project of Clinical Key Project of Peking University Third Hospital; and Tianjin Municipal Human Resources and Social Security Bureau grant XB202011.
The funding sources had no role in study design, collection, analysis, and interpretation of data; writing of the report; and decision to submit the manuscript for publication.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. Y.J. and J.Y. prepared the manuscript. Y.J., J.Y., L.X., T.W., X.C., Z.J., X.L., and F.L. performed the experiments. Y.J., J.Y., L.X., T.H., and R.W. contributed to the study design. Y.J., L.X., D.W., S.L., Y.L., and J.H. performed the statistical analyses. J.Y., K.Y., Z.Z., T.H., and R.W. interpreted the results. Z.Z., T.H., and R.W. contributed to the conception. All authors reviewed and edited the manuscript and approved the final version. R.W. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Funding Statement
This work was supported by National Clinical Key Specialty Construction Program, P.R. China (2023) special funding; National Natural Science Foundation of China grants 82270843, 82170875, 82371588, 82100885, 82200908, and 82271611; Beijing Municipal Natural Science Foundation grants 7232198 and 7222216; Clinical Medicine Plus X-Young Scholars Project of Peking University grant PKU2023LCXQ025; the Talent Project of Clinical Key Project of Peking University Third Hospital; and Tianjin Municipal Human Resources and Social Security Bureau grant XB202011.
References
- 1. Cosentino F, Grant PJ, Aboyans V, et al.; ESC Scientific Document Group . 2019 ESC guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur Heart J 2020;41:255–323 [DOI] [PubMed] [Google Scholar]
- 2. Koepsell H. The Na+-D-glucose cotransporters SGLT1 and SGLT2 are targets for the treatment of diabetes and cancer. Pharmacol Ther 2017;170:148–165 [DOI] [PubMed] [Google Scholar]
- 3. Wiviott SD, Raz I, Bonaca MP, et al.; DECLARE–TIMI 58 Investigators . Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N Engl J Med 2019;380:347–357 [DOI] [PubMed] [Google Scholar]
- 4. Perkovic V, Jardine MJ, Neal B, et al.; CREDENCE Trial Investigators . Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N Engl J Med 2019;380:2295–2306 [DOI] [PubMed] [Google Scholar]
- 5. Al Jobori H, Daniele G, Adams J, et al. Empagliflozin treatment is associated with improved β-cell function in type 2 diabetes mellitus. J Clin Endocrinol Metab 2018;103:1402–1407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Luo M, Kong X, Wang H, et al. Effect of dapagliflozin on glycemic variability in patients with type 2 diabetes under insulin glargine combined with other oral hypoglycemic drugs. J Diabetes Res 2020;2020:6666403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kanno A, Asahara SI, Kawamura M, et al. Early administration of dapagliflozin preserves pancreatic β-cell mass through a legacy effect in a mouse model of type 2 diabetes. J Diabetes Investig 2019;10:577–590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Koike M, Saito H, Kohno G, Takubo M, Watanabe K, Ishihara H.. Effects of GLP-1RA and SGLT2i, alone or in combination, on mouse models of type 2 diabetes representing different disease stages. Int J Mol Sci 2021;22:11463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wei R, Cui X, Feng J, et al. Dapagliflozin promotes β cell regeneration by inducing pancreatic endocrine cell phenotype conversion in type 2 diabetic mice. Metabolism 2020;111:154324. [DOI] [PubMed] [Google Scholar]
- 10. Tanday N, Irwin N, Flatt PR, Moffett RC.. Dapagliflozin exerts positive effects on β cells, decreases glucagon and does not alter β- to α-cell transdifferentiation in mouse models of diabetes and insulin resistance. Biochem Pharmacol 2020;177:114009. [DOI] [PubMed] [Google Scholar]
- 11. Saponaro C, Mühlemann M, Acosta-Montalvo A, et al. Interindividual heterogeneity of SGLT2 expression and function in human pancreatic islets. Diabetes 2020;69:902–914 [DOI] [PubMed] [Google Scholar]
- 12. Kałużna-Czaplińska J, Gątarek P, Chirumbolo S, Chartrand MS, Bjørklund G.. How important is tryptophan in human health? Crit Rev Food Sci Nutr 2019;59:72–88 [DOI] [PubMed] [Google Scholar]
- 13. Gao K, Mu CL, Farzi A, Zhu WY.. Tryptophan metabolism: a link between the gut microbiota and brain. Adv Nutr 2020;11:709–723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hata S, Okamura T, Kobayashi A, et al. Gut microbiota changes by an SGLT2 inhibitor, luseogliflozin, alters metabolites compared with those in a low carbohydrate diet in db/db mice. Nutrients 2022;14:3531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Liu S, Xu JZ, Zhang WG.. Advances and prospects in metabolic engineering of Escherichia coli for l-tryptophan production. World J Microbiol Biotechnol 2022;38:22. [DOI] [PubMed] [Google Scholar]
- 16. Russell WR, Duncan SH, Scobbie L, et al. Major phenylpropanoid-derived metabolites in the human gut can arise from microbial fermentation of protein. Mol Nutr Food Res 2013;57:523–535 [DOI] [PubMed] [Google Scholar]
- 17. Roager HM, Licht TR.. Microbial tryptophan catabolites in health and disease. Nat Commun 2018;9:3294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chen Q, Gao F, Gao Y, et al. Intestinal ACE2 regulates glucose metabolism in diet-induced obese mice through a novel gut-islet axis mediated by tryptophan. Obesity (Silver Spring) 2023;31:1311–1325 [DOI] [PubMed] [Google Scholar]
- 19. Lindström P, Sehlin J.. Aromatic amino acids and pancreatic islet function: a comparison of l-tryptophan and l-5-hydroxytryptophan. Mol Cell Endocrinol 1986;48:121–126 [DOI] [PubMed] [Google Scholar]
- 20. Al-Amily IM, Dunér P, Groop L, Salehi A.. The functional impact of G protein-coupled receptor 142 (Gpr142) on pancreatic β-cell in rodent. Pflugers Arch 2019;471:633–645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Müller TD, Finan B, Bloom SR, et al. Glucagon-like peptide 1 (GLP-1). Mol Metab 2019;30:72–130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wei T, Cui X, Jiang Y, et al. Glucagon acting at the GLP-1 receptor contributes to β-cell regeneration induced by glucagon receptor antagonism in diabetic mice. Diabetes 2023;72:599–610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sandoval DA, D’Alessio DA.. Physiology of proglucagon peptides: role of glucagon and GLP-1 in health and disease. Physiol Rev 2015;95:513–548 [DOI] [PubMed] [Google Scholar]
- 24. Takasu T, Takakura S.. Protective effect of ipragliflozin on pancreatic islet cells in obese type 2 diabetic db/db Mice. Biol Pharm Bull 2018;41:761–769 [DOI] [PubMed] [Google Scholar]
- 25. Omori K, Nakamura A, Miyoshi H, et al. Effects of dapagliflozin and/or insulin glargine on β cell mass and hepatic steatosis in db/db mice. Metabolism 2019;98:27–36 [DOI] [PubMed] [Google Scholar]
- 26. Qin J, Li Y, Cai Z, et al. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature 2012;490:55–60 [DOI] [PubMed] [Google Scholar]
- 27. Yang G, Wei J, Liu P, et al. Role of the gut microbiota in type 2 diabetes and related diseases. Metabolism 2021;117:154712. [DOI] [PubMed] [Google Scholar]
- 28. Sun L, Xie C, Wang G, et al. Gut microbiota and intestinal FXR mediate the clinical benefits of metformin. Nat Med 2018;24:1919–1929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bauer PV, Duca FA, Waise TMZ, et al. Metformin alters upper small intestinal microbiota that impact a glucose-SGLT1-sensing glucoregulatory pathway. Cell Metab 2018;27:101–117.e5 [DOI] [PubMed] [Google Scholar]
- 30. van Bommel EJM, Herrema H, Davids M, Kramer MHH, Nieuwdorp M, van Raalte DH.. Effects of 12-week treatment with dapagliflozin and gliclazide on faecal microbiome: results of a double-blind randomized trial in patients with type 2 diabetes. Diabetes Metab 2020;46:164–168 [DOI] [PubMed] [Google Scholar]
- 31. Deng L, Yang Y, Xu G.. Empagliflozin ameliorates type 2 diabetes mellitus-related diabetic nephropathy via altering the gut microbiota. Biochim Biophys Acta Mol Cell Biol Lipids 2022;1867:159234. [DOI] [PubMed] [Google Scholar]
- 32. Yu E, Papandreou C, Ruiz-Canela M, et al. Association of tryptophan metabolites with incident type 2 diabetes in the PREDIMED trial: a case-cohort study. Clin Chem 2018;64:1211–1220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. de Mello VD, Paananen J, Lindström J, et al. Indolepropionic acid and novel lipid metabolites are associated with a lower risk of type 2 diabetes in the Finnish Diabetes Prevention Study. Sci Rep 2017;7:46337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Qi Q, Li J, Yu B, et al. Host and gut microbial tryptophan metabolism and type 2 diabetes: an integrative analysis of host genetics, diet, gut microbiome and circulating metabolites in cohort studies. Gut 2022;71:1095–1105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Laurans L, Venteclef N, Haddad Y, et al. Genetic deficiency of indoleamine 2,3-dioxygenase promotes gut microbiota-mediated metabolic health. Nat Med 2018;24:1113–1120 [DOI] [PubMed] [Google Scholar]
- 36. Natividad JM, Agus A, Planchais J, et al. Impaired aryl hydrocarbon receptor ligand production by the gut microbiota is a key factor in metabolic syndrome. Cell Metab 2018;28:737–749.e4 [DOI] [PubMed] [Google Scholar]
- 37. Cui X, Feng J, Wei T, et al. Pancreatic α cell glucagon-liver FGF21 axis regulates β cell regeneration in a mouse model of type 2 diabetes. Diabetologia 2023;66:535–550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Liu J, Yang K, Yang J, et al. Liver-derived fibroblast growth factor 21 mediates effects of glucagon-like peptide-1 in attenuating hepatic glucose output. EBioMedicine 2019;41:73–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Gasaly N, de Vos P, Hermoso MA.. Impact of bacterial metabolites on gut barrier function and host immunity: a focus on bacterial metabolism and its relevance for intestinal inflammation. Front Immunol 2021;12:658354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zhang XY, Chen J, Yi K, et al. Phlorizin ameliorates obesity-associated endotoxemia and insulin resistance in high-fat diet-fed mice by targeting the gut microbiota and intestinal barrier integrity. Gut Microbes 2020;12:1–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Steinert RE, Luscombe-Marsh ND, Little TJ, et al. Effects of intraduodenal infusion of l-tryptophan on ad libitum eating, antropyloroduodenal motility, glycemia, insulinemia, and gut peptide secretion in healthy men. J Clin Endocrinol Metab 2014;99:3275–3284 [DOI] [PubMed] [Google Scholar]
- 42. Acar I, Cetinkaya A, Lay I, Ileri-Gurel E.. The role of calcium sensing receptors in GLP-1 and PYY secretion after acute intraduodenal administration of l-tryptophan in rats. Nutr Neurosci 2020;23:481–489 [DOI] [PubMed] [Google Scholar]
- 43. Nauck MA, Vardarli I, Deacon CF, Holst JJ, Meier JJ.. Secretion of glucagon-like peptide-1 (GLP-1) in type 2 diabetes: what is up, what is down? Diabetologia 2011;54:10–18 [DOI] [PubMed] [Google Scholar]
- 44. Rask E, Olsson T, Söderberg S, et al.; Northern Sweden Monitoring of Trends and Determinants in Cardiovascular Disease (MONICA) . Impaired incretin response after a mixed meal is associated with insulin resistance in nondiabetic men. Diabetes Care 2001;24:1640–1645 [DOI] [PubMed] [Google Scholar]
- 45. Dai C, Hang Y, Shostak A, et al. Age-dependent human β cell proliferation induced by glucagon-like peptide 1 and calcineurin signaling. J Clin Invest 2017;127:3835–3844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ackeifi C, Wang P, Karakose E, et al. GLP-1 receptor agonists synergize with DYRK1A inhibitors to potentiate functional human β cell regeneration. Sci Transl Med 2020;12:eaaw9996. [DOI] [PMC free article] [PubMed] [Google Scholar]