Abstract
Background
Poria acid (PAC) is a triterpene compound found in Poria cocos, a traditional Chinese medicine (TCM). The current study aims to explore the therapeutic effects and potential mechanisms of PAC on the migration and proliferation of human renal cell carcinoma (RCC) cells as well as tumor growth in animal model.
Methods
Cell viability and proliferative capacity of normal renal cells and RCC cells were investigated by MTT assay. In addition, 786-O cells were divided into four groups and treated with different concentrations of PAC (0, 20, 40, and 60 μM) for 48 h. Cell scratch test and cell invasion assay were performed to evaluate the effects of PAC on the invasion and migration of RCC cells, respectively. The effects of PAC on apoptosis of RCC cells and expression levels of PI3K/Akt/NF-kB signaling pathway-related biomarkers were investigated using TUNEL staining and Western blotting methods, respectively. Effects of PAC on the inhibitory activity of RCC tumor in mice were evaluated in a 786-O CDX model.
Results
The study found that PAC inhibited the viability of RCC cells in a dose-dependent manner, as demonstrated by in vitro cell assays (p < 0.05). However, PAC showed no significant inhibitory effect on normal renal cells (p > 0.05). PAC also significantly inhibited the migration and invasion of RCC via EMT/MMP signaling pathways (p < 0.05). Immunofluorescence and immunoblotting results showed that PAC induced the apoptosis of RCC, which was accompanied by changes in the expression levels of apoptosis-related proteins (p < 0.05). Moreover, PAC significantly downregulated the PI3K/Akt/NF-kB signaling pathway in a concentration-dependent manner (p < 0.05). The effect of PAC on RCC apoptosis was dramatically reversed by 740Y–P (PI3K agonist) (p < 0.05) but significantly enhanced in the presence of LY294002 (PI3K inhibitor) (p < 0.05). The results of in vivo experiment also demonstrated that the antitumor activity of PAC was achieved by affecting the PI3K/Akt/NF-kB signaling pathway.
Conclusions
PAC can effectively suppress the proliferation, invasion and migration of RCC cells, and exhibit anti-tumor effects in RCC model by inhibiting the PI3K/Akt/NF-kB signaling pathway.
Keywords: Poria acid (PAC), Renal carcinoma cell (RCC), Proliferation, Metastasis, PI3K/Akt/NF-κB signaling pathway
1. Introduction
In recent years, the incidence of kidney cancer, a common malignant tumor of the urinary system, has been increasing [1,2]. The early symptoms of renal cancer are often hidden, leading to many being diagnosed after the cancer has already metastasized [3]. While treatments such as surgery, radiotherapy and molecularly targeted therapies have improved the survival status of patients to some extent, the postoperative survival rate remains low [[4], [5], [6]]. Remote metastasis is a major cause of treatment failure and recurrence in patients with renal cancer [6].
Tumor metastasis is a unique property of tumor cells, characterized by the process of tumor cells leaving the primary lesion and continuing to proliferate and grow in secondary tissues or organs. This can occur through various mechanisms such as direct diffusion, lymphatic metastasis, hematogenous metastasis, and hair transplantation [7]. The ability of tumor cells to metastasize is a major challenge in clinical practice, as it makes it difficult to surgically remove malignant tumor lesions and achieve a cure [[7], [8], [9]]. Recent evidence suggests that metastasis and invasion of RCC are closely related to a process called Epithelial-mesenchymal transition (EMT) [[10], [11], [12]]. EMT refers to the phenotypic changes induced in epithelial cells [11], where they lose contact between cells and the cell basement membrane, and acquire a spindle-shaped morphology similar to mesenchymal/myofibroblast cells [11]. Abnormal induction of EMT has been shown to contribute to the spread and progression of cancer [13,14]. Matrix metalloproteinases (MMPs) play a critical role in the process of EMT by degrading many proteins in the extracellular matrix (ECM) [15]. The study of therapeutic strategies for EMT, MMP, and the development of drugs targeting these mechanisms has become a research hotspot in RCC.
At present, Traditional Chinese Medicine (TCM) has shown remarkable effects in preventing and treating the invasion and metastasis of various types of cancers [16]. Poria cocos is a type of dried sclerotia of the Poria cocos. It has different sizes and uncertain shapes, with a dark brown or light brown epidermis and a white or slightly pink endoplasm [17]. Poria cocos contains a variety of chemical components, including polysaccharides, triterpenoids, sterols, volatile oils, proteins, amino acids, nucleotides, and trace elements [17]. Among them, polysaccharides were shown to have antitumor activity and antioxidant activity [18]. In addition, clinical studies have shown that the aqueous extract of Poria cocos sclerotia can inhibit cAMP/PKA pathway, TonEBP/Sgk1 signaling pathway, regulate renal water balance, and also show anti-apoptotic activity on the kidney [19].
Previous research has shown that Poria cocos can inhibit the invasion of ovarian and pancreatic cancer cells by participating in the EMT/MMP signaling pathway [20,21]. Li et al. found that PAC had moderate cytotoxic activity against human colon cancer cell lines and inhibited both DNA topoisomerase I and II [22]. In addition, Ling et al. showed that PAC could reduce the degradation of NF-κB inhibitor α (IκBα), inhibit NF-κB signaling pathway, and decrease the expression of MMP-9, thereby inhibiting the invasive ability of breast cancer cells [23]. Invasion and metastasis of tumor cells is one of the main reasons for RCC progression and difficult to cure. PAC is one of the active ingredients in Poria cocos and has been found to inhibit the invasion and metastasis of gastric cancer cells [24]. However, the antitumor effects of PACs in RCC and their underlying mechanisms remain unknown. Therefore, the aim of this study was to investigate the effect of PAC on proliferation, invasion and metastasis of renal cell carcinoma cells and to elucidate its possible molecular mechanism. Finally, the in vivo antitumor activity of PACs was investigated in Renca-bearing mice.
2. Materials and methods
2.1. Materials and reagents
Poria acid (analytical standard, HPLC ≥97 %) was purchased from Shanghai Yuanye Bio-Technology (Shanghai, China) and dissolved in dimethyl sulfoxide (DMSO) (Catalog No. 20688, Thermo Fisher Scientific, USA) at a concentration of 10 mmol/L. It was then diluted to the required concentration with culture medium at the time of use. The MTT kit (Catalog No. C0009S), EDU kit (Catalog No. C0088S), and crystal violet staining solution (Catalog No. C0121) were obtained from Beyotime Biotechnology (Shanghai, China). The 740Y–P (Catalog No. S7865) and LY294002 (Catalog No. S1105) were purchased from Selleck Chemicals (Houston, USA). All antibodies, including N-cadherin (Catalog No. MA5-15633), E-cadherin (Catalog No. 13–1700), MMP-2 (Catalog No. 35-1300Z), vimentin (Catalog No. MA5-16409), MMP-9 (Catalog No. MA5-15886), TIMP-1 (Catalog No. MS608PABX), β-actin (Catalog No. MA1-140), and HRP-labeled goat anti-rabbit secondary antibodies (Catalog No. 31460), were purchased from Thermo Fisher Scientific, USA. BCL-2 (Catalog No. ab692), BAX (Catalog No. ab270742), Caspase-3 (Catalog No. ab13585), Cleaved Caspase-3 (Catalog No. ab32042), PI3K (Catalog No. ab302958), phospho-PI3K (Catalog No. ab278545), Akt (Catalog No. ab179463), phospho-Akt (Catalog No. ab38449), IκBα (Catalog No. ab76429), phospho-IκBα (Catalog No. ab133462), NF-κB (Catalog No. ab288751), and phospho–NF–κB (Catalog No. ab76302) were purchased from Abcam (Cambridge, UK). All other reagents not mentioned specifically were obtained from Abcam (Cambridge, UK).
2.2. Cell culture
The human renal cancer cells (786-O cells, human renal clear cell adenocarcinoma cells, Catalog No. TCHu186) and HK-2 cells (Human renal tubular epithelial cells, Catalog No. SCSP-511) were obtained from the cell bank of the Chinese Academy of Sciences (CAS) (Shanghai, China). The cells were incubated in a water bath at 37 °C and shaken. They were then cultured in a cell culture medium consisting of 10 % phosphate-buffered saline (PBS), 1 % Dulbecco's Modified Eagle's Medium (DMEM) medium with 1 % penicillin/streptomycin, and centrifuged at 2000 g, resuspended, and subcultured in a CO2 incubator at 37 °C. Mycoplasma contamination testing was performed to confirm the negative infection of mycoplasma contamination. The cell lines used have been authenticated using short tandem repeat profiling.
2.3. Cell viability determination
The MTT assay was performed to examine cell viability. First, the cells were counted and plated into 96-well plates. Different concentrations of PAC (0, 20, 40, 60, 80, and 100 μM), 740Y–P or LY294002 were added for 48 or 72 h of incubation. Then, 10 μL of a 5 mg/mL MTT solution was added to each well for 4 h and then 150 μL DMSO was added. The 96-well plates were then deposited on a shaker with low speed for 10 min to fully dissolve the crystals. The absorbance value was measured at OD490 nm using a microplate reader.
2.4. EDU proliferation assay
The EDU kit was used to determine cell proliferation after transfection. First, 6 × 104 cells were plated in a 24-well plate, and 1 mL EDU solution diluted in culture medium (1000:1 for culture medium: EDU solution) was added to each well. The plate was then incubated at 37 °C. After 2 h, 200 μL of 4 % paraformaldehyde was used to fix the cells for 30 min. According to the instructions of the EDU kit, glycine solution (2 mg/mL) and Appollo staining solution were added to each well in turn. Finally, the nuclei were stained for 30 min with DAPI and observed and imaged with a fluorescence microscope (Olympus BX51).
2.5. Determination of cell invasion
Transwell® inserts were used for invasion assays. The chamber was humidified for 2 h before the experiment. Matrigel was diluted with serum-free medium and 50 μL was added to Transwell® inserts to solidify the gel overnight. Excess gel was gently washed with PBS. A cell suspension of 200 μL was placed in the upper chamber. The lower chamber was filled with cell culture medium containing 10 % fetal bovine serum (FBS) and the setup was incubated. Subsequently, the cells were washed twice with PBS and fixed in 4 % paraformaldehyde for 30 min. Crystal violet staining was performed for 15 min, and images were taken after staining to observe and count the cells. Image J software was utilized to calculate the number of transmembrane cells.
2.6. Cell scratch test
786-O cells were digested with trypsin to prepare cell suspensions. The cells were then seeded in 6-well plates containing DMEM with 10 % FBS. A straight line was drawn with a 10 μL tip perpendicular to the bottom of the plate. The cells were gently washed three times with PBS buffer and cultured in DMEM with 2 % FBS. Various concentrations (0, 20, 40, and 60 μM) of PAC were used for cell treatment. After 24 h, cells were imaged using a light microscope (Olympus, Tokyo, Japan) and the wound area was measured.
2.7. TUNEL staining
1 × 104 786-O cells were deposited in 24-well plates and treated with different concentrations of PAC (0, 20, 40, and 60 μM) for 48 h. After incubation, the cells were fixed with 4 % paraformaldehyde and apoptosis was detected using the TUNEL apoptosis kit. Apoptotic cells were observed as fluorescent green under a laser scanning confocal microscope (Olympus, Tokyo, Japan).
2.8. Western blotting
The Cell Total Protein Extraction Kit (Abcam, Cambridge, UK) was used to extract total protein from cells. The protein concentration was determined using the BCA kit (Beyotime Biotechnology, China). Equivalent proteins in different samples, which were treated with different concentrations of PAC (0, 20, 40, 60, 80, and 100 μM), 740Y–P or LY294002, were isolated by SDS-PAGE electrophoresis and then transferred onto PVDF membranes (Merck Millipore, Billerica, MA, USA). All PVDF membranes were incubated at 4 °C overnight with the primary antibody. After washing the membranes several times with Tris-buffered saline with Tween-20 (TBST), the imprints were incubated with secondary antibodies (1:3000). Signals of protein bands were visualized using ECL developer (Merck Millipore, Billerica, MA, USA). β-Actin was utilized as a normalization endogenous protein. The greyscale of protein bands was detected with Image J software. Image J software setting: Image-Type = 8 bit; Set Measurements choose Area, Integrated density, Mean gray value.
2.9. Tumor model establishment, grouping and administration
Forty female BALB/c nude mice (4 weeks old) were purchased from Shanghai Experimental Animal Center (Shanghai). The 786-O cells were cultured in RPMI 1640 culture medium after recovering. Then the 786-O cells were harvested and then suspend at a final concentration of 1E7 cells/mL. The 786-O cells were cultured in RPMI 1640 culture medium after recovering. Then the 786-O cells cells were harvested and then suspend at a final concentration of 1E7 cells/mL. Then 100 μL cell was injected into the armpit of each animals. When tumors grew to approximately 60 mm3, animals were randomly assigned to four groups based on average body weight and tumor size, receiving PBS, PAC, 740Y–P, and PAC in combination with 740Y–P. Mice in the vehicle control group were intragastrically administered with 400 μL PBS once daily; mice in the PAC and 740Y–P group was intragastrically administered with 60 mg/kg and 100 μmol/kg once daily, respectively; mice in the combined treatment group were intragastrically administered with PAC (20 mg/kg) and 740Y–P (100 μmol/kg) once daily for 14 days after inoculation.
2.10. Statistical analysis
Each experiment was repeated at least three times. All the values are displayed as mean ± standard deviation (SD) and statistical analysis was performed using the SPSS 22.0 software (IBM, Armonk, NY, USA). All measurement data were tested for normal distribution and conformed to normal distribution. A two-tailed Student's t-test was used to compare two groups, while ANOVA was employed to compare multiple groups. A p-value <0.05 was considered statistically significant.
3. Results
3.1. PAC inhibits the viability of RCC in vitro
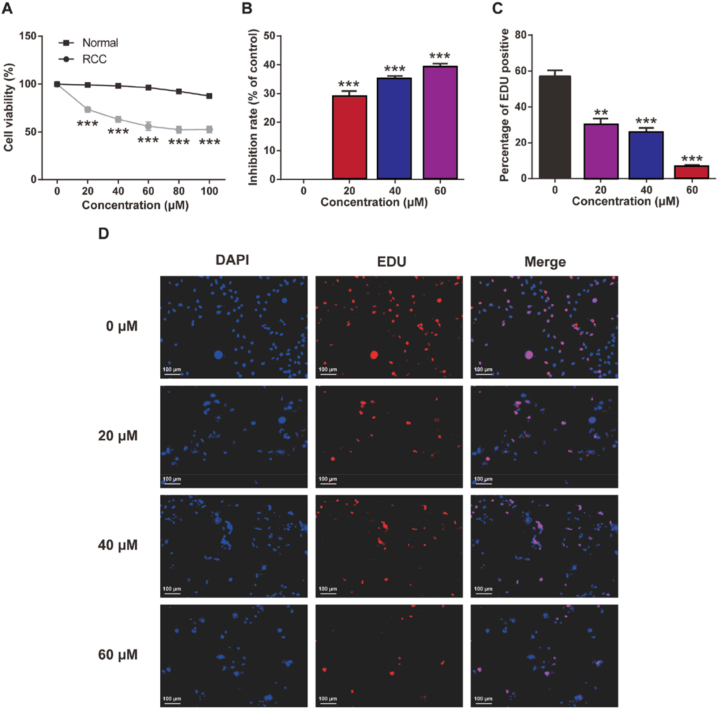
To evaluate the potential anti-cancer activity of PAC in vitro, different concentrations of PAC (0, 20, 40, 60, 80, or 100 μM) were added to renal cancer cell lines (786-O cells) as well as normal HK-2 cells (renal cell lines). The cell viability was tested by MTT assay. The RCC activity was significantly inhibited with the increase of PAC concentration in the dose range of 20–80 μM (p < 0.05) (Fig. 1A–B). In addition, PAC can inhibit the proliferation of RCC in a concentration-dependent manner at the dose range from 20 to 60 μM. Similarly, this conclusion was verified by the results of EDU staining shown in Fig. 1C–D. However, treatment of the PAC at 80 μM or 100 μM also slightly inhibited the viability of normal renal cells but did not show significant change. Therefore, the concentrations of 0, 20, 40, and 60 μM of PAC were selected for further effects on tumor cell invasion and migration to exclude the cytotoxicity of PACs.
Fig. 1.
In vitro cell viability of renal cell carcinoma (RCC) cells after exposure to poria acid (PAC). (A) Cell viability of normal cells and RCC as well as (B) inhibition rate of RCC by Methyl Thiazolyl Tetrazolium (MTT) assay. (C) Percentage of EdU positive cells, and (D) proliferation of RCC (786-O cells) by EdU proliferation assay (scale: 100 μm). Mean ± SD (n = 6). ***p < 0.001 vs. 0 μM PAC group.
3.2. PAC inhibits the migration and invasion of RCC cells
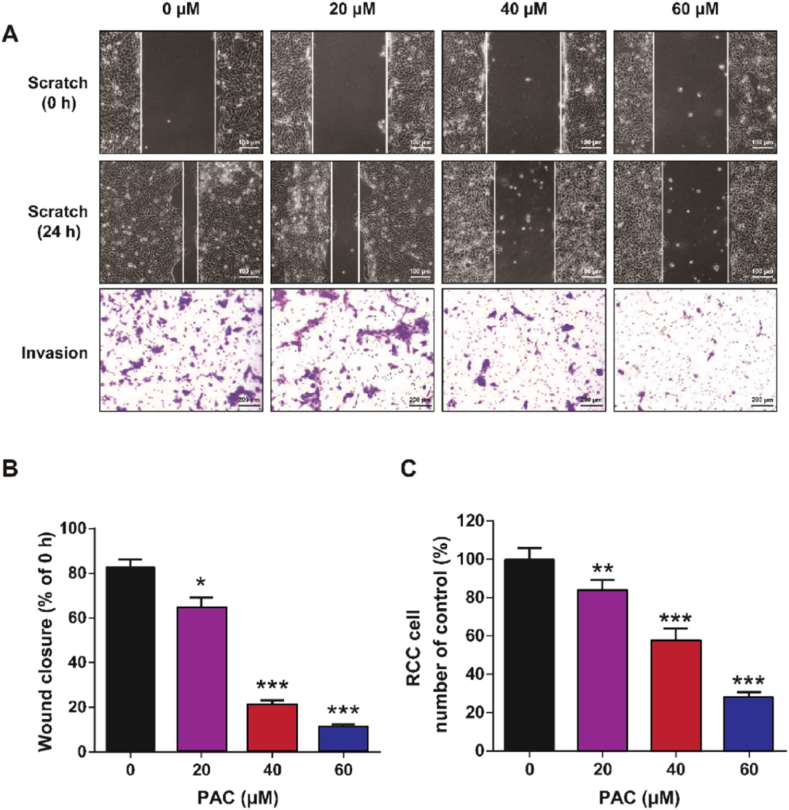
The effects of PAC on the migration and invasion of RCC cells were evaluated by cell scratch assay and Transwell® assay, respectively. As shown in Fig. 2A–B, RCC cells treated with PAC showed a significant decrease in wound healing ability (p < 0.05). Nevertheless, no dose-dependency was evident when the concentration of PAC was more than 40 μM. Consistent with the results of cell scratch assays, Transwell® assays demonstrated that 24-h treatment of PAC could significantly inhibit the invasive ability of RCC cells (Fig. 2A–C). With increasing concentrations of PAC (0, 20, 40, and 60 μM), the number of RCC cells passing through the membrane significantly decreased (p < 0.01). These results collectively suggest that PAC effectively inhibited the migration and invasion of RCC cells in vitro.
Fig. 2.
In vitro cell migrated and invasive capacities of RCC cells after exposure to PAC. (A) Representative images of RCC cell (786-O cells) migration (scale: 100 μm) and cell invasion (scale: 200 μm) and (B) quantitative assessment of cell scratch test and (C) Transwell invasion assay. Mean ± SD (n = 5). *p < 0.05, **p < 0.01 and ***p < 0.001 vs. 0 μM PAC group.
3.3. PAC affects expression levels of the migration- and invasion-associated proteins through EMT/MMP signaling pathways
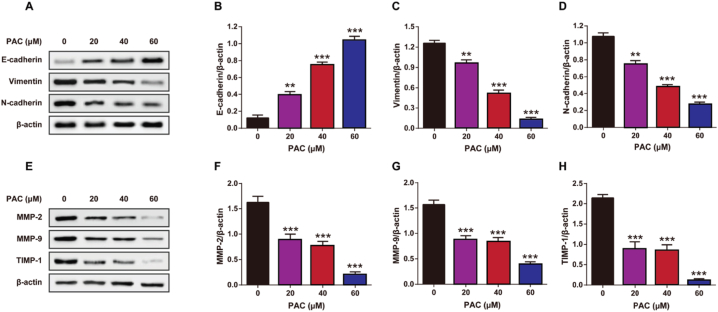
Previous studies have shown that the expression levels of vimentin and N-cadherin are positively correlated with tumor cell proliferation and metastasis, while the expression of E-cadherin is negatively correlated [25]. The results indicate that the expression levels of vimentin and N-cadherin were significantly decreased (p < 0.001) in the PAC-treated group compared to the control group (Fig. 3A–D). On the other hand, the expression of E-cadherin was significantly increased in the PAC-treated group (p < 0.001), suggesting that PAC treatment could effectively reduce biomarkers associated with the migration and invasion of tumor cells. In addition, we performed Western blotting for matrix metalloproteinases (MMP-2/9, TIMP-1) and found that PAC treatment simultaneously led to a decrease in the expression of these proteins (Fig. 3E–H) (p < 0.01). These results further support the idea that PAC may reduce the invasion and migration of RCC cells by inhibiting the EMT/MMP signaling pathway.
Fig. 3.
The impact of PAC on expression of signaling pathways associated with EMT and MMP in RCC. (A) Representative images and quantitative assessment of (B) E-cadherin/β-actin, (C) Vimentin/β-actin, and (D) N-cadherin/β-actin of EMT signaling pathways in RCC by Western blot. (E) Representative images and quantitative analysis of (F) MMP-2/β-actin, (G) MMP-9/β-actin, and (H) TIMP-1/β-actin of MMP signaling pathways in RCC after by Western blot. Mean ± SD (n = 5). *p < 0.05, **p < 0.01 and ***p < 0.001 vs. 0 μM PAC group.
3.4. PAC promotes RCC cells apoptosis
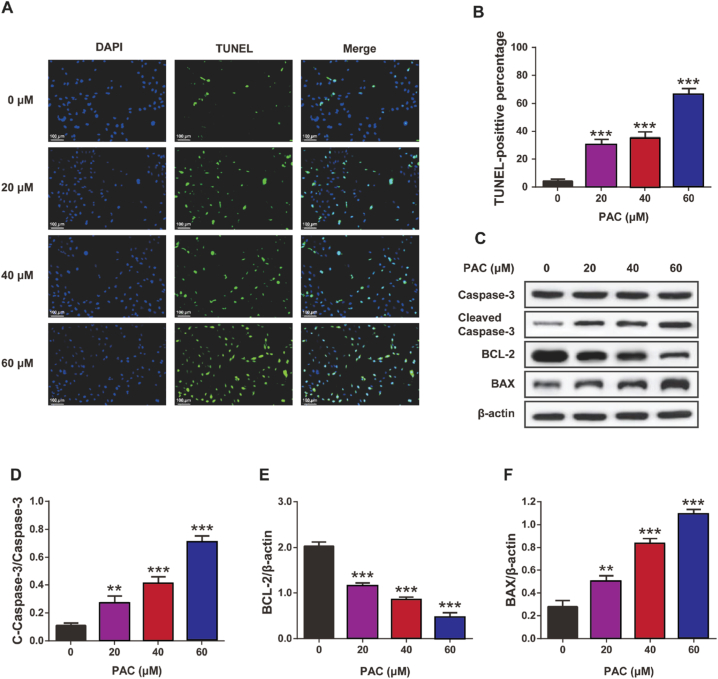
The effect of PAC on the apoptosis of RCC cells was further confirmed by TUNEL staining (Fig. 4A–B). The proportion of apoptosis increased significantly with increasing doses of PAC (p < 0.05). In addition, the results of the Western blot showed that the expression levels of pro-apoptotic proteins, including BAX and cleaved caspase-3, were both significantly increased (p < 0.01), while the expression levels of the anti-apoptotic protein BCL-2 were significantly decreased after PAC treatment (p < 0.001) (Fig. 4C-F). These results suggest that PAC can expedite the apoptosis of RCC cells in vitro in a dose-dependent manner.
Fig. 4.
The impact of PAC on RCC cells apoptosis and related proteins expression. (A) Representative images (scale: 100 μm) and (B) percentage of TUNEL positive cells (786-O cells) by TUNEL staining. (C) Representative images and quantitative assessment of (D) cleaved caspase-3, (E) B-cell lymphoma 2 (BCL-2), and (F) BCL-2 associated X (BAX) in RCC by Western blot. Mean ± SD (n = 5). **p < 0.01 and ***p < 0.001 vs. 0 μM PAC group.
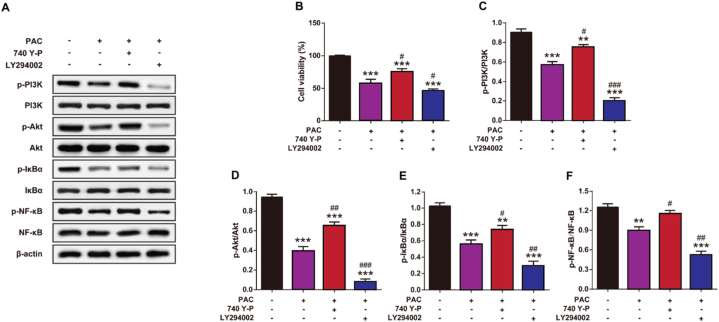
3.5. Inhibition of the PI3K/Akt/NF-κB signaling pathway enhances the pro-apoptotic effects of PAC on RCC
To further investigate the underlying mechanism of PAC-induced cell apoptosis, the expression levels of the PI3K/Akt/NF-κB signaling pathway and the viability of PAC-treated RCC cells in the presence of 740 Y–P (PI3K agonist) or LY294002 (PI3K inhibitor) were examined by Western blot analysis and MTT assay (Fig. 5A–B). PAC significantly inhibited the viability of RCC cells (p < 0.001). However, treatment with 740 Y–P, a PI3K agonist, significantly reversed the PAC-induced decrease in cell viability (p < 0.05). In contrast, LY294002, a PI3K inhibitor, significantly enhanced the effect of PACs on cell viability (p < 0.05). Further results of Western blot analysis showed that PAC treatment dramatically decreased the phosphorylation levels of the PI3K/Akt/NF-κB signaling pathway-related proteins (p < 0.01) (Fig. 5C-F). Interestingly, enhanced phosphorylation levels of the PI3K/Akt/NF-κB signaling pathway were significantly reversed by the presence of 740 Y–P (p < 0.001). In addition, LY294002 significantly enhanced PAC-induced phosphorylation of Akt, PI3K, NF-κB, and IκBα (p < 0.05). These results collectively suggest that PAC-induced RCC cell apoptosis may be relevant to the PI3K/Akt/NF-κB signaling pathway.
Fig. 5.
The impact of PAC on the RCC pathway involving phosphoinositide 3-kinase (PI3K)/protein kinase B (Akt)/nuclear factor (NF) -κB pathway in RCC. (A) Representative images, (B) cell viability of RCC by MTT assay and quantitative assessment of (C) p-PI3K/PI3K, (D) p-Akt/Akt, (E) p-IκBα/IκBα and (F) p–NF–κB/NF-κB in RCC cells with or without the presence of 740 Y–P and LY294002. (B) Cell viability of RCC. Mean ± SD (n = 5). *p < 0.05, **p < 0.01 and ***p < 0.001 vs. 0 μM PAC group; #p < 0.05, ##p < 0.01 and ###p < 0.001 vs. the single PAC treated group.
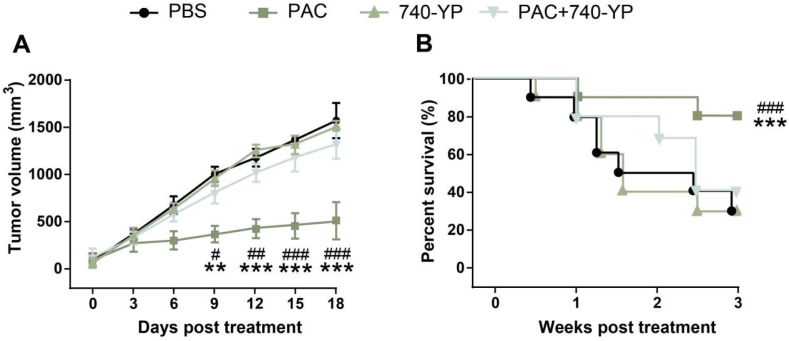
3.6. PAC exhibit anti-RCC activity vi inhibiting PI3K/Akt/NF-κB signaling pathway in RCC CDX animal model
As shown in Fig. 6A, PAC alone can significantly inhibit the growth of renal cancer in mice with an tumor growth inhibition (TGI) rate of 81.4 %, which was significant lower than that of PBS (p < 0.001). Moreover, the anti-tumor activity of PAC was reversed after adding the 740 Y–P, a PI3K agonist, and showed no significant difference with PBS group (p > 0.05, Fig. 6B). Similar trend are also presented in the survival rate of animals in different groups (Fig. 6B). Above results collectively demonstrated that the PAC exhibit anti-RCC activity vi inhibiting PI3K/Akt/NF-κB signaling pathway in RCC CDX animal model.
Fig. 6.
Therapeutic effects of PAC alone or combined with 740 Y–P in the Renca-bearing mice. The (A) tumor size and (B) survival individual of Renca-bearing mice were investigated. **, ***p < 0.01, 0.001 vs PBS control; #, ##, ###p < 0.05, 0.01, 0.001 vs combined group. All results were showed as means ± SD (n = 5).
4. Discussion
In recent years, RCC has become one of the common tumors of the urinary system with high morbidity and mortality [26]. The cause of RCC onset is still unknown, and the main clinical treatment plan is surgery, as there is currently no effective treatment available for advanced and metastatic tumors [26]. Therefore, medical experts are actively searching for new treatment strategies for renal cancer. While Western medicine has made significant advancements in the therapeutic effect of malignant tumors, its high toxicity and cost limit its widespread use. In contrast, TCM has shown advantages in the prevention and treatment of renal cell carcinoma invasion and metastasis [27]. TCM offers the potential for safer, more efficient, and less toxic natural products that can reduce side effects on the liver, kidney, and gastrointestinal tract, ultimately improving the quality of life for patients. TCM has several advantages in the treatment of tumors, including its significant inhibitory effect on tumors, low likelihood of drug resistance development, and minimal damage to the body with fewer side effects [28]. Therefore, studying the anti-tumor effect of TCM holds broad clinical application value.
Invasion and metastasis are the primary causes of cancer-related deaths [24]. Tumor cells proliferate and invade surrounding tissues while spreading through the bloodstream, resulting in the typical characteristics of tissue invasion and metastasis [21]. Recent studies have shown that PAC can affect the invasion and metastasis of RCC cells through the Wnt signaling pathway [29]. In cervical cancer, the same phenomenon was found in the polysaccharide component extracted from PAC [30]. In the present study, it was first found that PAC inhibited the activity of RCC cells by MTT assay. Further EDU staining results showed that PAC could significantly reduce the number of clonal populations of kidney cancer cells and inhibit the proliferation of kidney cancer cells. This finding preliminarily indicated that PAC had a therapeutic effect on kidney cancer. Inhibition of the adhesion of tumor cells is a key step to control invasion and metastasis. In addition, some scholars have proposed that inhibition of cell motility may be an effective way to inhibit tumor metastasis [31]. The Transwell® assay showed that PAC could significantly inhibit the invasive ability of renal carcinoma cells, indicating that PAC could significantly inhibit the invasion of RCC cells at the initial stage of migration. To further verify the inhibitory effects of PAC on the invasion and metastasis of RCC cells, the cell scratch assay was performed, and the result demonstrated that the PAC could effectively inhibit the migration of RCC cells in vitro.
Tumor invasion and metastasis are characterized by the passage of cancer cells through the extracellular matrix (ECM) and basement membrane [32]. Cancer cells secrete many proteolytic enzymes, such as those of the MMP family to penetrate ECMs [32]. Among them, MMP-2 and MMP-9 are considered to be the main ECM-degrading proteases [33]. Current experimental results showed that PAC could down-regulate the expression levels of MMP-2 and MMP-9, which are consistent with the results that MMP-2/9 could be used as a key effector of ECM remodeling as well as a potential target for anti-tumor therapy [20,34]. Tissue inhibitors of metalloproteinases family members (TIMPs) are natural inhibitors that promote cancer metalloproteinases, of which TIMP-1 was earlier found to be a representative of the TIMP family due to its multifunctionality [35]. PAC was also found to upregulate the TIMP-1 expression. The proteolytic activity of MMPs is strictly regulated by the typical anti-proteolytic function of TIMPs, while the absence of TIMPs leads to enhanced matrix proteolytic hydrolysis, and the increase of TIMPs levels leads to the accumulation of ECM. It is believed that the increased expression of TIMPs tries to balance the degradation activity of ECM, but fails to reflect it [36]. In cancer, protease inhibition is thought to prevent aggressive features of malignancy [37]. The imbalance of MMP/TIMP can promote tumor proliferation, invasion and metastasis, so it can also be used as a therapeutic target for tumor invasion and metastasis by regulating MMP/TIMP imbalance [38]. The process of PAC inhibiting invasion and migration of RCC cells is likely to be achieved by regulating the homeostasis of MMP/TIMP.
Apoptosis is one of the main types of cell death, and the induction of tumor cell apoptosis is a crucial mechanism targeted by many anti-cancer drugs [39]. In the context of RCC, the effect of PAC on the apoptosis of RCC cells was examined using a TUNEL assay. The results showed a significant enhancement of apoptosis in RCC cells induced by PAC. Furthermore, Western blot analysis revealed that PAC induced apoptosis in RCC cells through the activation of caspase-3 and the modulation of the Bcl-2 protein family. The proteins of the Bcl-2 family can be divided into two groups: Bcl-2, which promotes apoptosis, and BAX, which inhibits apoptosis. The release of mitochondrial cytochrome C and activation of mitochondria-dependent caspase upregulated BAX and downregulated Bcl-2 to induce apoptosis.
Previous literature has shown that the PI3K/AKT/NF-κB signaling pathway plays a crucial role in inducing tumor cell proliferation, migration and invasion in many cancers by regulating EMT and MMPs [40]. NF-κB is not only a downstream target of PI3K/AKT signaling, but also an upstream regulator of EMT and MMPs gene transcription, which is closely related to tumor cell proliferation, invasion and metastasis [41]. Persistent activation of NF-κB in a variety of tumor cells can induce the expression of different anti-apoptotic proteins and lead to chemotherapy and radiotherapy tolerance [42]. In addition, studies have shown that PAC can suppress the invasion and metastasis of gastric cancer cells by altering the expression of EMT-related proteins [24]. In this study, we found that PAC significantly decreased the expression of proteins related to the PI3K/Akt/NF-κB signaling pathway, including p-PI3K, p-Akt, p-IκBα, and p–NF–κB, in RCC cells. This suggests that PAC may inhibit the proliferation, migration and invasion of renal cancer cells through the PI3K/Akt/NF-κB signaling pathway. In addition, the activation of PI3K further causes downstream AKT phosphorylation and activation. To further investigate the role of the PI3K/Akt/NF-κB signaling pathway in PAC-induced apoptosis, the PI3K agonist 740 Y–P and PI3K inhibitor LY294002 were used. The PI3K agonist (740 Y–P) reversed PAC-induced RCC cell apoptosis and upregulated the expression levels of, p-Akt, p–NF–κB, p-IκBα and PI3K, while the presence of the PI3K inhibitor (LY294002) further promoted PAC-induced apoptosis and downregulated the phosphorylation levels of the PI3K/Akt/NF-κB signaling pathway. Further studies in the RCC CDX animal model also demonstrated that the PAC exhibit anti-RCC activity via inhibiting PI3K/Akt/NF-κB signaling pathway. These findings collectively suggest that PAC may inhibit the proliferation, invasion and migration of RCC cells and promote their apoptosis by inhibiting the phosphorylation of related proteins in the PI3K/Akt/NF-κB signaling pathway.
This study has the following limitations: 1) This study applies only to a single RCC cell line (786-O cells), which may not represent the diversity of RCC cases in the clinical setting; 2) The xenograft mouse model used in this study does not fully replicate the development of human RCC; 3) The long-term potential toxicity of PAC for normal cell or animal are still unknown, and further studies are needed to understand the long-term effects and potential toxicity of PAC in the therapeutic setting; 4) As a preliminary study, the efficacy and safety of PAC in humans still need to be clinically verified subsequently.
5. Conclusions
In summary, this study found that PAC can inhibit the proliferation, migration and invasion of RCC cells, and promote their apoptosis. The degree of inhibition is more significant with the increase in drug concentration. In addition, the tumor suppressor effect of PAC may be associated with the inhibition of phosphorylation of related proteins in the PI3K/Akt/NF-κB pathway. This study provides experimental basis and new ideas for PAC treatment of RCC. Furthermore, PACs are likely to be key antitumor components in Poria cocos. This will lay a huge experimental foundation for further development and utilization of Poria cocos as anticancer agents. Because it is a preliminary in vitro and in vivo study, further attempts to clarify the potential effects of PAC in a broader range of RCC subtypes or in combination with other treatments, or modify the structure of PACs to improve their antitumor activity and bioavailability are needed in the future. Meanwhile, other signaling pathways involved in PAC against RCC still need to be clarified.
Ethics approval
The use of experimental animals for this study was approved by the Ethics Committee of The Hebei General Hospital with the approval number of EC-HBGH-12-3319.
Funding
This research was supported by Hebei Natural Science Foundation (H2020307020).
Data availability statement
Data included in article/supp. Material/referenced in article.
CRediT authorship contribution statement
Haotian Yang: Investigation, Formal analysis, Data curation, Conceptualization. Yue Zhao: Methodology, Investigation. Bingnan Ren: Resources, Methodology. Yin Wu: Software, Resources. Zhihong Qiu: Writing – original draft, Validation, Software. Yan Cheng: Writing – review & editing, Writing – original draft. Bo Qiu: Supervision, Project administration.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
Not applicable.
References
- 1.Craven R.A., Vasudev N.S., Banks R.E. Proteomics and the search for biomarkers for renal cancer. Clin. Biochem. 2013;46:456–465. doi: 10.1016/j.clinbiochem.2012.11.029. [DOI] [PubMed] [Google Scholar]
- 2.Moch H., Srigley J., Delahunt B., Montironi R., Egevad L., Tan P.H. Biomarkers in renal cancer. Virchows Arch. 2014;464:359–365. doi: 10.1007/s00428-014-1546-1. [DOI] [PubMed] [Google Scholar]
- 3.Blick C., Ritchie A.W.S., Eisen T., Stewart G.D. Improving outcomes in high-risk, nonmetastatic renal cancer: new data and ongoing trials. Nat. Rev. Urol. 2017;14:753–759. doi: 10.1038/nrurol.2017.123. [DOI] [PubMed] [Google Scholar]
- 4.Feng T., Zhao J., Wei D., Guo P., Yang X., Li Q., et al. Immunogenomic Analyses of the Prognostic Predictive model for patients with renal cancer. Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.762120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gaillard V., Tricard T., Garnon J., Cazzato R.L., Dalili D., Gangi A., et al. Repeat ablative therapy in hereditary or multifocal renal cancer: Functional and oncological outcomes. Urol. Oncol. 2020;38:797.e15–797.e20. doi: 10.1016/j.urolonc.2020.07.020. [DOI] [PubMed] [Google Scholar]
- 6.Kuusk T., Albiges L., Escudier B., Grivas N., Haanen J., Powles T., et al. Antiangiogenic therapy combined with immune checkpoint blockade in renal cancer. Angiogenesis. 2017;20:205–215. doi: 10.1007/s10456-017-9550-0. [DOI] [PubMed] [Google Scholar]
- 7.Valastyan S., Weinberg R.A. Tumor metastasis: molecular insights and evolving paradigms. Cell. 2011;147:275–292. doi: 10.1016/j.cell.2011.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fu B.M. Tumor metastasis in the Microcirculation. Adv. Exp. Med. Biol. 2018;1097:201–218. doi: 10.1007/978-3-319-96445-4_11. [DOI] [PubMed] [Google Scholar]
- 9.Lin Y., Xu J., Lan H. Tumor-associated macrophages in tumor metastasis: biological roles and clinical therapeutic applications. J. Hematol. Oncol. 2019;12:76. doi: 10.1186/s13045-019-0760-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bakir B., Chiarella A.M., Pitarresi J.R., Rustgi A.K. EMT, MET, Plasticity, and tumor metastasis. Trends Cell Biol. 2020;30:764–776. doi: 10.1016/j.tcb.2020.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pastushenko I., Blanpain C. EMT transition States during tumor progression and metastasis. Trends Cell Biol. 2019;29:212–226. doi: 10.1016/j.tcb.2018.12.001. [DOI] [PubMed] [Google Scholar]
- 12.Su Y., Zhou L., Yu Q., Lu J., Liu W. Long Non-Coding RNA LOC648987 promotes proliferation and metastasis of renal cell carcinoma by regulating epithelial-mesenchymal transition. Technol. Cancer Res. Treat. 2021;20 doi: 10.1177/1533033821997834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.He H., Magi-Galluzzi C. Epithelial-to-mesenchymal transition in renal neoplasms. Adv. Anat. Pathol. 2014;21:174–180. doi: 10.1097/PAP.0000000000000018. [DOI] [PubMed] [Google Scholar]
- 14.Xu H., Xu W.H., Ren F., Wang J., Wang H.K., Cao D.L., et al. Prognostic value of epithelial-mesenchymal transition markers in clear cell renal cell carcinoma. Aging. 2020;12:866–883. doi: 10.18632/aging.102660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nisticò P., Bissell M.J., Radisky D.C. Epithelial-mesenchymal transition: general principles and pathological relevance with special emphasis on the role of matrix metalloproteinases. Cold Spring Harbor Perspect. Biol. 2012;4 doi: 10.1101/cshperspect.a011908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xiang Y., Guo Z., Zhu P., Chen J., Huang Y. Traditional Chinese medicine as a cancer treatment: modern perspectives of ancient but advanced science. Cancer Med. 2019;8:1958–1975. doi: 10.1002/cam4.2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li X., He Y., Zeng P., Liu Y., Zhang M., Hao C., et al. Molecular basis for Poria cocos mushroom polysaccharide used as an antitumour drug in China. J. Cell Mol. Med. 2019;23:4–20. doi: 10.1111/jcmm.13564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang N., Zhang Y., Wang X., Huang X., Fei Y., Yu Y., Shou D. Antioxidant property of water-soluble polysaccharides from Poria cocos Wolf using different extraction methods. Int. J. Biol. Macromol. 2016 Feb;83:103–110. doi: 10.1016/j.ijbiomac.2015.11.032. [DOI] [PubMed] [Google Scholar]
- 19.Lee S.M., Lee Y.J., Yoon J.J., Kang D.G., Lee H.S. Effect of Poria cocos on hypertonic stress-induced water channel expression and apoptosis in renal collecting duct cells. J. Ethnopharmacol. 2012 May 7;141(1):368–376. doi: 10.1016/j.jep.2012.02.048. [DOI] [PubMed] [Google Scholar]
- 20.Jiang H., Li H. Prognostic values of tumoral MMP2 and MMP9 overexpression in breast cancer: a systematic review and meta-analysis. BMC Cancer. 2021;21:149. doi: 10.1186/s12885-021-07860-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li X., Wang J. Mechanical tumor microenvironment and transduction: cytoskeleton mediates cancer cell invasion and metastasis. Int. J. Biol. Sci. 2020;16:2014–2028. doi: 10.7150/ijbs.44943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li G., Xu M.L., Lee C.S., Woo M.H., Chang H.W., Son J.K. Cytotoxicity and DNA topoisomerases inhibitory activity of constituents from the sclerotium of Poria cocos. Arch Pharm. Res. (Seoul) 2004 Aug;27(8):829–833. doi: 10.1007/BF02980174. [DOI] [PubMed] [Google Scholar]
- 23.Ling H., Zhang Y., Ng K.Y., Chew E.H. Pachymic acid impairs breast cancer cell invasion by suppressing nuclear factor-κB-dependent matrix metalloproteinase-9 expression. Breast Cancer Res. Treat. 2011 Apr;126(3):609–620. doi: 10.1007/s10549-010-0929-5. [DOI] [PubMed] [Google Scholar]
- 24.Wang H., Luo Y., Chu Z., Ni T., Ou S., Dai X., et al. Poria acid, triterpenoids extracted from Poria cocos, inhibits the invasion and metastasis of gastric cancer cells. Molecules. 2022;27:3629. doi: 10.3390/molecules27113629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Abdul S., Majid A., Wang J., Liu Q., Sun M.Z., Liu S. Bidirectional interaction of lncRNA AFAP1-AS1 and CRKL accelerates the proliferative and metastatic abilities of hepatocarcinoma cells. J. Adv. Res. 2020;24:121–130. doi: 10.1016/j.jare.2020.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bahadoram S., Davoodi M., Hassanzadeh S., Bahadoram M., Barahman M., Mafakher L. Renal cell carcinoma: an overview of the epidemiology, diagnosis, and treatment. G. Ital. Nefrol. 2022;39:2022. vol3. [PubMed] [Google Scholar]
- 27.Zheng J., Xu W., Liu W., Tang H., Lu J., Yu K., et al. Traditional Chinese medicine Bu-Shen-Jian-Pi-Fang attenuates glycolysis and immune escape in clear cell renal cell carcinoma: results based on network pharmacology. Biosci. Rep. 2021;41 doi: 10.1042/BSR20204421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu Y. Renal fibrosis: new insights into the pathogenesis and therapeutics. Kidney Int. 2006;69:213–217. doi: 10.1038/sj.ki.5000054. [DOI] [PubMed] [Google Scholar]
- 29.Ukiya M., Akihisa T., Tokuda H., Hirano M., Oshikubo M., Nobukuni Y., et al. Inhibition of tumor-promoting effects by poricoic acids G and H and other lanostane-type triterpenes and cytotoxic activity of poricoic acids A and G from Poria cocos. Journal of Natural Products. 2002;65:462–465. doi: 10.1021/np0103721. [DOI] [PubMed] [Google Scholar]
- 30.Zhang D.D., Li H.J., Zhang H.R., Ye X.C. Poria cocos water-soluble polysaccharide modulates anxiety-like behavior induced by sleep deprivation by regulating the gut dysbiosis, metabolic disorders and TNF-α/NF-κB signaling pathway. Food Funct. 2022;13:6648–6664. doi: 10.1039/d2fo00811d. [DOI] [PubMed] [Google Scholar]
- 31.Palmer T.D., Ashby W.J., Lewis J.D., Zijlstra A. Targeting tumor cell motility to prevent metastasis. Adv. Drug Deliv. Rev. 2011;63:568–581. doi: 10.1016/j.addr.2011.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brassart-Pasco S., Brézillon S., Brassart B., Ramont L., Oudart J.B., Monboisse J.C. Tumor microenvironment: extracellular matrix alterations influence tumor progression. Front. Oncol. 2020;10:397. doi: 10.3389/fonc.2020.00397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Conlon G.A., Murray G.I. Recent advances in understanding the roles of matrix metalloproteinases in tumour invasion and metastasis. J. Pathol. 2019;247:629–640. doi: 10.1002/path.5225. [DOI] [PubMed] [Google Scholar]
- 34.Liu H., Zeng Z., Wang S., Li T., Mastriani E., Li Q.H., et al. Main components of pomegranate, ellagic acid and luteolin, inhibit metastasis of ovarian cancer by down-regulating MMP2 and MMP9. Cancer Biol. Ther. 2017;18:990–999. doi: 10.1080/15384047.2017.1394542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Eckfeld C., Häußler D., Schoeps B., Hermann C.D., Krüger A. Functional disparities within the TIMP family in cancer: hints from molecular divergence. Cancer Metastasis Rev. 2019;38:469–481. doi: 10.1007/s10555-019-09812-6. [DOI] [PubMed] [Google Scholar]
- 36.Arpino V., Brock M., Gill S.E. The role of TIMPs in regulation of extracellular matrix proteolysis. Matrix Biol. 2015;44–46:247–254. doi: 10.1016/j.matbio.2015.03.005. [DOI] [PubMed] [Google Scholar]
- 37.Jiang Y., Goldberg I.D., Shi Y.E. Complex roles of tissue inhibitors of metalloproteinases in cancer. Oncogene. 2002;21:2245–2252. doi: 10.1038/sj.onc.1205291. [DOI] [PubMed] [Google Scholar]
- 38.Wang Z.D., Huang C., Li Z.F., Yang J., Li B.H., Liang R.R., et al. Chrysanthemum indicum ethanolic extract inhibits invasion of hepatocellular carcinoma via regulation of MMP/TIMP balance as therapeutic target. Oncol. Rep. 2010;23:413–421. [PubMed] [Google Scholar]
- 39.Jiang Q.L., Zhang S., Tian M., Zhang S.Y., Xie T., Chen D.Y., et al. Plant lectins, from ancient sugar-binding proteins to emerging anti-cancer drugs in apoptosis and autophagy. Cell Prolif. 2015;48:17–28. doi: 10.1111/cpr.12155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Prasad P., Vasas A., Hohmann J., Bishayee A., Sinha D. Cirsiliol suppressed epithelial to mesenchymal transition in B16F10 malignant melanoma cells through alteration of the PI3K/Akt/NF-κB signaling pathway. Int. J. Mol. Sci. 2019;20:608. doi: 10.3390/ijms20030608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yang H.L., Thiyagarajan V., Shen P.C., Mathew D.C., Lin K.Y., Liao J.W., et al. Anti-EMT properties of CoQ0 attributed to PI3K/AKT/NFKB/MMP-9 signaling pathway through ROS-mediated apoptosis. J. Exp. Clin. Cancer Res. 2019;38:186. doi: 10.1186/s13046-019-1196-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mortezaee K., Najafi M., Farhood B., Ahmadi A., Shabeeb D., Musa A.E. NF-κB targeting for overcoming tumor resistance and normal tissues toxicity. J. Cell. Physiol. 2019;234:17187–17204. doi: 10.1002/jcp.28504. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data included in article/supp. Material/referenced in article.