Abstract
Background
There is a lack of comprehensive profile assessment on complete blood count (CBC)-derived systemic-inflammatory indices, and their correlations with clinical outcome in patients with anterior circulation acute ischemic stroke (AIS) who achieved successful recanalization by endovascular thrombectomy (EVT).
Methods
Patients with anterior circulation AIS caused by large vessel occlusion (AIS-LVO) were retrospectively screened from December 2018 to December 2022. Systemic-inflammatory indices including ratios of neutrophil-to-lymphocyte (NLR), monocyte-to-lymphocyte (MLR), platelet-to-lymphocyte (PLR), and platelet-to-neutrophil (PNR), systemic immune-inflammation index (SII), systemic inflammation response index (SIRI), and aggregate inflammation systemic index (AISI) on admission and the first day post-EVT were calculated. Their correlations with symptomatic intracranial hemorrhage (sICH) and unfavorable 90-day functional outcome (modified Rankin Scale score of 3–6) were analyzed.
Results
A total of 482 patients [65 (IQR, 56–72) years; 33 % female] were enrolled, of which 231 (47.9 %) had unfavorable 90-day outcome and 50 (10.4 %) developed sICH. Day 1 neutrophil and monocyte counts, NLR, MLR, PLR, SII, SIRI, and AISI were increased, while lymphocyte and PNR were decreased compared to their admission levels. In multivariate analyses, neutrophil count, NLR, SII, and AISI on day 1 were independently associated with 90-day functional outcome. Moreover, day 1 neutrophil count, NLR, MLR, PLR, PNR, SII, and SIRI were independently linked to the occurrence of sICH. No admission variables were identified as independent risk factors for patient outcomes.
Conclusion
CBC-derived systemic-inflammatory indices measured on the first day after successful EVT are predictive of 90-day functional outcome and the sICH occurrence in patients with anterior circulation AIS-LVO.
Keywords: Acute ischemic stroke, Endovascular thrombectomy, Inflammatory indice, Outcome, Symptomatic intracranial hemorrhage
Highlights
-
•
Day 1 systemic-inflammatory indices were independently associated with 90-day outcome.
-
•
Day 1 systemic-inflammatory indices were independently associated with sICH.
-
•
No admission systemic-inflammatory indices were independently associated with AIS outcome.
1. Introduction
Acute ischemic stroke (AIS) is a major cause of morbidity and mortality worldwide, imposing a substantial socioeconomic burden to the society [1]. Endovascular thrombectomy (EVT) is a well-established treatment for anterior circulation AIS caused by large vessel occlusion (AIS-LVO) [[2], [3], [4]]. With the advancements in interventional devices and recanalization techniques, the success rate of EVT now stands at 80%–90 %. However, postoperative reperfusion injury continues to be a prevalent concern leading to early complications [e.g., symptomatic intracranial hemorrhage (sICH)] and neurological deterioration, which can ultimately affect long-term clinical outcomes [[5], [6], [7]]. Additionally, the invasive nature of EVT may cause vascular wall injury (VWI), which can result in peri-interventional hemorrhagic and ischemic complications, ultimately reducing treatment efficacy and clinical outcomes [[8], [9], [10]]. Randomized controlled trials have reported device- or procedure-related complications ranging from 4 % to 29 %, contributing to peri-interventional morbidity and mortality [8]. Mereuta et al. [9] demonstrated that 12 % of extracted AIS thrombi exhibited histological markers of VWI, and these markers were associated with a higher number of maneuver passes and poorer revascularization outcome. Early outcome prediction utilizing blood-based biomarkers for EVT-treated AIS patients has the potential to guide the timely initiation of adjuvant therapies [[10], [11], [12]]. However, none of the biomarkers reported so far have shown adequate reliability to be recommended for clinical use. This lack of reliability may be attributed to the inherent heterogeneity of the disease (e.g., various etiologies), variations in populations (e.g., diverse genetic backgrounds), and the heterogeneity observed in futile recanalization.
Inflammatory and thrombotic responses to acute cerebral ischemia and subsequent recanalization, known as thromboinflammation, are a key contributor to the occurrence and development of both early and long-term adverse outcomes [[13], [14], [15]]. Neutrophils and monocytes serve as mediators of innate immune and inflammatory responses, while lymphocytes are involved in adaptive immune responses [[15], [16], [17], [18]]. These immune cells have been extensively studied in the context of AIS. It is generally understood that neutrophils and monocytes have a deleterious effect, while lymphocytes primarily exert a protective role in AIS prognosis [[15], [16], [17], [18]]. Additionally, platelets have been shown to regulate hemostasis, thrombosis, and inflammation [19]. The activation and adhesion of platelets on damaged endothelium, along with platelet interaction with leukocytes, are important pathophysiological processes in thromboinflammation, resulting in cerebrovascular damage [13,14,19]. Complete blood count (CBC)-derived inflammatory indices, based on two or more circulating cell subtypes, are increasingly being studied as they reflect the systemic inflammatory burden. These indices have been proven effective in predicting the severity and prognosis of conditions such as cardiovascular disease [20,21], cancer [22], and COVID-19 [23]. Current studies have also found that neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), platelet-to-neutrophil ratio (PNR), monocyte × NLR (systemic inflammation response index, SIRI), and platelet × NLR (systemic immune inflammation index, SII) are associated with clinical outcomes of patients with AIS [[24], [25], [26], [27], [28], [29], [30], [31], [32]]. However, the majority of these studies are based on admission CBC data, which may not fully consider the progression of thromboinflammation after AIS, as well as the potential impact of interventional factors and subsequent reperfusion injury. Moreover, most studies only focused on the predictive role of one or a few indicators (primarily NLR), without comprehensive analyses of multiple indicators. To fill this critical knowledge gap, we conducted a retrospective study on patients with anterior circulation AIS-LVO who underwent successful recanalization by EVT. Our study was designed to comprehensively determine (1) the concentrations of CBC-derived systemic-inflammatory indices on admission and the first day after successful EVT, and (2) to investigate the correlations between their levels and the occurrence of sICH and 90-day functional outcome.
2. Material and methods
2.1. Study design and participants
This retrospective study screened consecutive AIS patients at a comprehensive stroke center between December 2018 and December 2022. The inclusion criteria were as follows: (1) aged ≥18 years; (2) anterior circulation AIS-LVO, namely occlusion of the internal carotid artery (ICA) or the proximal segments of the middle cerebral artery (MCA) [4]; (3) successful recanalization achieved by EVT with or without intravenous thrombolysis (IVT), as determined by the modified Thrombolysis in Cerebral Infarction score (mTICI) of 2b–3. The exclusion criteria were [33]: (1) pre-stroke disability [a premorbid modified Rankin scale (mRS) score of ≥3]; (2) severe heart, hepatic or renal failure; (3) hematological, rheumatic, or autoimmune diseases; (4) concurrent/recent infectious diseases, or ongoing anti-inflammatory or immunomodulatory therapies; (5) AIS with active cancer; and (6) missing clinical or follow up data. This study was approved by the Ethics Committee of the Xuanwu Hospital, Capital Medical University (ID-CRB-61/2023). It was performed according to the Principles of Declaration of Helsinki. The requirement for informed consent was waived because of its retrospective nature and minimal risk to patients. This study was reported in accordance with STrengthening the Reporting of OBservational studies in Epidemiology (STROBE) guidelines [34].
2.2. Clinical, imaging, and outcome data collection
We retrospectively collected (1) age and sex; (2) hypertension, diabetes mellitus, dyslipidemia, coronary heart disease, atrial fibrillation, smoking and drinking habits, prior ischemic stroke and myocardial infarction, and current medications; (3) time parameters, anesthesia techniques, number of passes, and final mTICI score; (4) location of vessel occlusion; (5) stroke severity (assessed by admission National Institutes of Health Stroke Scale; NIHSS); (6) stroke etiology (defined by Trial of Org 10172 in Acute Stroke Treatment; TOAST); (7) ischemic lesions (assessed by Alberta Stroke Program Early CT Score; ASPECTS). The primary outcome of the study was 90-day unfavorable outcome, defined as a mRS score of 3–6. The secondary outcome was sICH, defined as an increase in NIHSS score of ≥4 points due to intracranial hemorrhage within the first 24–36 h after EVT according to the ECASS-II (European-Australian Acute Stroke Study II) definition [35]. All follow-up assessments were conducted by certified neurologists who were blinded to the clinical data.
2.3. CBC-derived systemic-inflammatory indices
Peripheral venous blood was routinely collected for CBC assessment upon admission and within the following 24 h (day 1) after successful recanalization. In our stroke center, blood samples on day 1 were drawn at 6–8 a.m. and 5–7 p.m. In cases where multiple laboratory data were obtained during these time period, the highest value was recorded [[29], [30], [31], [32]]. Absolute counts of neutrophil, lymphocyte, monocyte, and platelet were collected, which were then used to calculate NLR, monocyte-to-lymphocyte ratio (MLR), PLR, PNR, SII, SIRI, and aggregate inflammation systemic index [AISI; (neutrophil × monocyte × platelet)/lymphocyte].
2.4. Statistics analysis
Statistical analysis was performed using the SPSS 26.0.0 software (IBM Corporation, Armonk, NY, USA). Quantitative variables were reported as mean (standard deviation; SD) or median (interquartile range; IQR) based on the Kolmogorov-Smirnov test. Intergroup differences were analyzed using Student's t-test or Mann-Whitney U test, as appropriate. Categorical data were presented as count (percentage), and intergroup differences were analyzed by Chi-square test (or Yates's correction for continuity or Fisher's exact test, as appropriate). The association of measured variables with 90-day functional outcome or the occurrence of sICH were analyzed using multivariate logistic regression analysis that adjust for potential confounding variables (p < 0.05 in univariate logistic regression analysis). Bonferroni correction were further performed to control the familywise error rate in multiple comparisons. To determine the sensitivity and specificity of predictive capacity of the measured variables, receiver operating characteristic (ROC) analysis and area under curve (AUC) were performed. A p value < 0.05 was considered statistically significant.
3. Results
3.1. Baseline characteristics of the study population
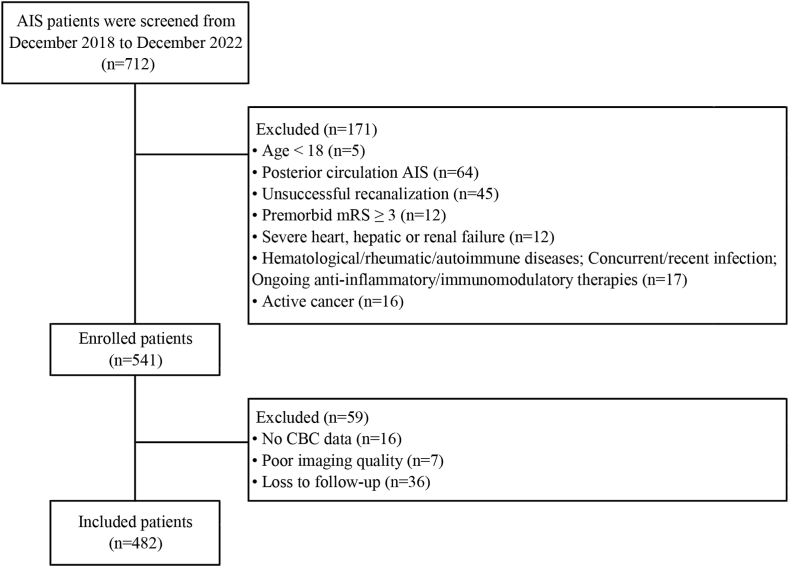
A total of 712 consecutive patients with anterior circulation AIS-LVO, underwent EVT and achieved successful recanalization were screened, and 482 patients [65 (IQR, 56–72) years; 33 % female] were included in the final analyses (Fig. 1). The median NIHSS and ASPECTS scores on admission were 15 (IQR, 12–18) and 8 (IQR, 7–9), respectively. Three hundred and eight (63.9 %) patients underwent EVT alone, and 174 (36.1 %) patients received IVT before EVT. A total of 278 (57.7 %) patients achieved complete recanalization (mTICI score of 2c-3). The median time from recanalization to day 1 blood testing was 375 (IQR, 208–540) minutes. The detailed demographic and clinical characteristics of the study population are summarized in Table 1.
Fig. 1.
Flow chart of patient selection.
Table 1.
Baseline characteristics of the study population.
| Characteristic | AIS Patients (n = 482) | 90-day functional outcome |
Symptomatic ICH |
||||
|---|---|---|---|---|---|---|---|
| mRS 0–2 (n = 251) | mRS 3–6 (n = 231) | p | Yes (n = 50) | No (n = 432) | p | ||
| Age, year, median (IQR) | 65 (56–72) | 62 (52–69) | 69 (60–75) | <0.001a | 67.5 (61–73) | 64 (56–72) | 0.102a |
| Gender, male/female, n | 323/159 | 184/67 | 139/92 | 0.002b | 34/16 | 289/143 | 0.875b |
| Vascular Risk factors, n (%) | |||||||
| Hypertension | 312 (64.7) | 156 (62.2) | 156 (67.5) | 0.217b | 37 (74) | 275 (63.7) | 0.147b |
| Diabetes mellitus | 120 (24.9) | 46 (18.3) | 74 (32) | 0.001b | 13 (26) | 107 (24.8) | 0.849b |
| Dyslipidemia | 98 (20.3) | 48 (19.1) | 50 (21.6) | 0.492b | 4 (8) | 94 (21.8) | 0.022b |
| Coronary artery disease | 105 (21.8) | 36 (14.3) | 69 (29.9) | <0.001b | 23 (46) | 82 (19) | <0.001b |
| Atrial fibrillation | 120 (24.9) | 54 (21.5) | 66 (28.6) | 0.073b | 18 (36) | 102 (23.6) | 0.055b |
| Smoking | 196 (40.7) | 113 (45) | 83 (35.9) | 0.042b | 16 (32) | 180 (41.7) | 0.188b |
| Drinking | 170 (35.3) | 105 (41.8) | 65 (28.1) | 0.002b | 11 (22) | 159 (36.8) | 0.038b |
| Prior stoke, n (%) | 82 (17) | 29 (11.6) | 53 (22.9) | 0.001b | 8 (16) | 74 (17.1) | 0.84b |
| Prior myocardial infarction, n (%) | 13 (2.7) | 5 (2) | 8 (3.5) | 0.319b | 2 (4) | 11 (2.5) | 0.889c |
| Admission NIHSS, median (IQR) | 15 (12–18) | 13 (10–17) | 17 (13–20) | <0.001a | 14 (12–18) | 15 (12–18.5) | 0.944a |
| Admission ASPECTS, median (IQR) | 8 (7–9) | 9 (7–9) | 8 (7–9) | 0.003a | 8 (6–9) | 8 (7–9) | 0.023a |
| Stroke location, n (%) | |||||||
| Internal carotid artery | 197 (40.9) | 94 (37.5) | 103 (44.6) | 0.169b | 23 (46) | 174 (40.3) | 0.707b |
| Middle cerebral artery-M1 | 228 (47.3) | 129 (51.4) | 99 (42.9) | 21 (42) | 207 (47.9) | ||
| Middle cerebral artery-M2 | 57 (11.8) | 28 (11.2) | 29 (12.6) | 6 (12) | 51 (11.8) | ||
| Stroke cause (TOAST), n (%) | |||||||
| Cardioembolic | 289 (60) | 140 (55.8) | 149 (64.5) | 0.148b | 37 (74) | 252 (58.3) | 0.061b |
| Large artery atherosclerosis | 174 (36.1) | 100 (39.8) | 74 (32) | 13 (26) | 161 (37.3) | ||
| Others | 19 (3.9) | 11 (4.4) | 8 (3.5) | 0 (0) | 19 (4.4) | ||
| Time parameters, min, median (IQR) | |||||||
| onset-to-door | 263 (154–386) | 279 (165–420) | 245 (151–363) | 0.094a | 218.5 (131–360) | 269.5 (160.5–390) | 0.133a |
| groin puncture-to-recanalization | 39 (28–59) | 36 (25–50) | 40 (30–66) | 0.015a | 51.5 (31–80) | 38 (27–55) | 0.002a |
| onset-to-recanalization | 438 (336–594) | 457 (341–600) | 429.5 (325–568) | 0.177a | 400.5 (300–558) | 442 (339–595) | 0.266a |
| door-to-recanalization | 174 (141–221) | 166.5 (135–215) | 175 (146–222) | 0.223a | 191 (146–231) | 173 (141–220) | 0.215a |
| recanalization-to-blood test | 375 (208–540) | 369 (204–547) | 377 (230–534) | 0.993a | 315 (203–512) | 376 (213.5–541) | 0.267a |
| General anesthesia, n (%) | 84 (17.4) | 35 (13.9) | 49 (21.2) | 0.036b | 22 (44) | 62 (14.4) | <0.001b |
| Number of passes, median (IQR) | 1 (1–2) | 1 (1–2) | 2 (1–2) | 0.074a | 2 (1–2) | 1 (1–2) | 0.194a |
| mTICI, 2b/2c-3, n | 204/278 | 86/165 | 118/113 | <0.001b | 33 (66) | 245 (56.7) | 0.208b |
| Prior IVT, n (%) | 174 (36.1) | 83 (33.1) | 91 (39.4) | 0.149b | 27 (54) | 147 (34) | 0.005b |
| Medications, n (%) | |||||||
| Antiplatelet | 89 (18.5) | 35 (13.9) | 54 (23.4) | 0.008b | 10 (20) | 79 (18.3) | 0.768b |
| Anticoagulation | 49 (10.2) | 26 (10.4) | 23 (10) | 0.884b | 9 (18) | 40 (9.3) | 0.053b |
| sICH, n (%) | 50 (10.4) | 6 (2.4) | 44 (19) | <0.001b | – | – | – |
Note.
Abbreviations: AIS, acute ischemic stroke; ASPECTS: Alberta Stroke Program Early CT Score; mRS, modified Rankin scale; mTICI, modified Treatment in Cerebral Infarction; NIHSS, National Institutes of Health Stroke Scale; TOAST, Trial of Org 10172 in Acute Stroke Treatment; ICH, intracerebral hemorrhage; IVT, intravenous thrombolysis; IQR, interquartile range.
Analyzed by Mann-Whitney U test.
Analyzed by Chi-square test.
Analyzed by Yates's correction for continuity.
3.2. Longitudinal changes of neutrophil, monocyte, lymphocyte, and platelet counts, and their derived systemic-inflammatory indices
As shown in Table 2, the counts of neutrophils and monocytes were higher, while lymphocyte count was lower on the first day after successful EVT compared to their pre-operative levels. However, there was no significant statistical difference in platelet count. Furthermore, the post-procedure NLR, MLR, PLR, SII, SIRI, and AISI on day 1 were found to be significantly higher than their admission levels, except for PNR which showed a decrease after successful EVT.
Table 2.
Longitudinal changes of neutrophil, monocyte, lymphocyte, and platelet counts, and their derived systemic-inflammatory indices in AIS patients.
| AIS patients (n = 482) |
|||
|---|---|---|---|
| Admission | Day 1 | pa | |
| Neutrophil, × 109/L, IQR | 6.49 (4.84–8.75) | 7.89 (6.32–10.12) | <0.001 |
| Monocyte, × 109/L, IQR | 0.4 (0.3–0.52) | 0.42 (0.29–0.56) | 0.021 |
| Lymphocyte, × 109/L, IQR | 1.26 (0.91–1.77) | 1 (0.69–1.39) | <0.001 |
| Platelet, × 109/L, IQR | 203.5 (168.8–245) | 205 (169–243) | 0.631 |
| NLR, IQR | 5.15 (3.04–8.15) | 7.7 (5.03–12.08) | <0.001 |
| MLR, IQR | 0.3 (0.22–0.41) | 0.41 (0.27–0.59) | <0.001 |
| PLR, IQR | 156.1 (108.7–227.8) | 201.8 (140.6–291.1) | <0.001 |
| PNR, IQR | 31.31 (22.91–42.66) | 25.43 (19.36–34.27) | <0.001 |
| SII, IQR | 1040 (556.7–1748) | 1538 (943.1–2649) | <0.001 |
| SIRI, IQR | 1.87 (1.12–3.43) | 3.11 (1.87–5.47) | <0.001 |
| AISI, IQR | 367.6 (214–698.7) | 616.7 (354.2–1167) | <0.001 |
Analyzed by Wilcoxon signed-rank test. Abbreviations: IQR, interquartile range.
3.3. CBC-derived systemic-inflammatory indices and 90-day functional outcome
Of the 482 patients, 231 (47.9 %) had unfavorable 90-day functional outcome. The favorable group had a lower proportion of female and younger individuals than the unfavorable group. Significant differences were also found between the two groups in the prevalence of diabetes mellitus and coronary artery disease, prior history of stroke, current antiplatelet use, current smoking, and drinking. Compared to the unfavorable group, patients in the favorable group exhibited lower admission NIHSS scores, higher admission ASPECTS scores, shorter puncture-to-recanalization time, lower proportion of general anesthesia, higher complete recanalization rate, and lower incidence of sICH(Table 1 and Table S1). Univariate analyses showed that patients with unfavorable outcome had higher levels of admission NLR, as well as day 1 neutrophil count, NLR, MLR, PLR, SII, SIRI, and AISI. In contrast, they had lower levels of day 1 PNR compared to those with favorable outcome (Table 3). After adjusting for confounding variables, higher neutrophil count, NLR, SII, and AISI on day 1 post-EVT remained independently associated with 90-day unfavorable functional outcome (Table 3). In ROC analyses, the AUCs of day 1 neutrophil count, NLR, SII, and AISI to discriminate 90-day functional outcome were 0.608, 0.662, 0.633, and 0.581, respectively (Table 5). Their optimal cut-off values were 7.02, 9.34, 1617.42, and 822.28, respectively (Table 5).
Table 3.
Univariate and multivariate logistics regression analyses of CBC-derived systemic-inflammatory indices associated with 90-day functional outcome.
| 90-day functional outcome |
|||||||
|---|---|---|---|---|---|---|---|
| mRS 0–2 (n = 251) | mRS 3–6 (n = 231) | OR (95%CI) | P | aOR (95%CI)a | P | aPb | |
| Admission, median (IQR) | |||||||
| Neutrophil, × 109/L | 6.33(4.99–8.28) | 6.76(4.62–8.99) | 1.046(0.988–1.107) | 0.126 | 1.091(1.005–1.184) | 0.037 | 0.407 |
| Monocyte, × 109/L | 0.41(0.32–0.52) | 0.39(0.28–0.52) | 0.911(0.619–1.340) | 0.635 | 0.976(0.624–1.528) | 0.915 | Ns |
| Lymphocyte, × 109/L | 1.36(1–1.87) | 1.18(0.86–1.65) | 0.836(0.680–1.029) | 0.091 | 0.862(0.679–1.096) | 0.225 | Ns |
| Platelet, × 109/L | 201(173–243.5) | 205(165–247.5) | 1.000(0.997–1.003) | 0.929 | 1.003(0.999–1.006) | 0.121 | Ns |
| NLR | 4.99(3.02–7.48) | 5.7(3.07–8.99) | 1.033(1.002–1.065) | 0.04 | 1.031(0.997–1.065) | 0.074 | 0.815 |
| MLR | 0.29(0.22–0.4) | 0.32(0.21–0.44) | 1.064(0.789–1.434) | 0.685 | 1.041(0.725–1.495) | 0.828 | Ns |
| PLR | 149.2(108.5–212.4) | 167.2(111.5–243.2) | 1.001(1.000–1.002) | 0.188 | 1.001(0.999–1.003) | 0.22 | Ns |
| PNR | 31.05(23.85–42.82) | 31.41(21.62–41.95) | 0.995(0.987–1.003) | 0.242 | 0.996(0.987–1.005) | 0.423 | Ns |
| SII | 960.2(543.6–1628) | 1165(590.7–1827) | 1.000(1.000–1.000) | 0.112 | 1.000(1.000–1.000) | 0.066 | 0.727 |
| SIRI | 1.78(1.14–3.31) | 1.97(1.11–3.54) | 1.008(0.980–1.036) | 0.595 | 1.007(0.975–1.041) | 0.654 | Ns |
| AISI | 361(216.2–633.5) | 393.3(205–769.2) | 1.000(1.000–1.000) | 0.878 | 1.000(1.000–1.000) | 0.656 | Ns |
| Day 1, median (IQR) | |||||||
| Neutrophil, × 109/L | 7.36(5.7–9.03) | 8.43(6.94–10.83) | 1.141(1.072–1.215) | <0.001 | 1.128(1.040–1.223) | 0.004 | 0.045 |
| Monocyte, × 109/L | 0.44(0.32–0.55) | 0.41(0.27–0.59) | 0.665(0.297–1.491) | 0.323 | 1.268(0.419–3.833) | 0.674 | Ns |
| Lymphocyte, × 109/L | 1.16(0.79–1.5) | 0.88(0.61–1.23) | 0.806(0.602–1.081) | 0.15 | 0.889(0.728–1.086) | 0.251 | Ns |
| Platelet, × 109/L | 207(173.5–243) | 202(161–243) | 0.999(0.996–1.001) | 0.35 | 1.002(0.999–1.006) | 0.173 | Ns |
| NLR | 6.65(4.26–9.42) | 10.05(5.93–15.06) | 1.086(1.053–1.119) | <0.001 | 1.061(1.022–1.102) | 0.002 | 0.024 |
| MLR | 0.38(0.27–0.55) | 0.48(0.29–0.66) | 2.861(1.537–5.326) | 0.001 | 2.382(1.010–5.617) | 0.047 | 0.517 |
| PLR | 180(136.4–251) | 219.2(152.9–328.6) | 1.003(1.002–1.004) | <0.001 | 1.002(1.001–1.004) | 0.005 | 0.058 |
| PNR | 28.66(21.78–36.73) | 22.68(18.03–30.7) | 0.981(0.968–0.995) | 0.008 | 0.994(0.983–1.006) | 0.324 | Ns |
| SII | 1339(865.9–1998) | 1878(1154–3019) | 1.000(1.000–1.000) | <0.001 | 1.000(1.000–1.000) | 0.001 | 0.009 |
| SIRI | 2.64(1.83–4.64) | 3.92(2.02–6.24) | 1.105(1.048–1.165) | <0.001 | 1.092(1.018–1.172) | 0.014 | 0.157 |
| AISI | 537.1(347.6–909.4) | 829.5(370.6–1296) | 1.000(1.000–1.001) | 0.001 | 1.001(1.000–1.001) | 0.003 | 0.035 |
Note.
Abbreviations: OR, odds ratio; aOR, adjusted odds ratio; aP, adjusted p value; CI, confidence interval; IQR, interquartile range; ns, not significant.
adjustment for age, sex, diabetes mellitus, coronary heart disease, drinking, prior stroke, antiplatelet use, admission NIHSS and ASPECTS scores, groin puncture-to-recanalization time, anesthesia type, the number of passes, mTICI, and sICH.
Bonferroni correction.
Table 5.
ROC curve analysis of CBC-derived systemic-inflammatory indices in predicting AIS outcomes.
| 90-day functional outcome |
||||||
|---|---|---|---|---|---|---|
| AUC | 95%CI | p | Cut-off | Sensitivity | Specificity | |
| Day 1 | ||||||
| Neutrophil | 0.608 | 0.558–0.659 | <0.001 | 7.02 | 0.74 | 0.454 |
| NLR | 0.662 | 0.613–0.710 | <0.001 | 9.34 | 0.554 | 0.745 |
| SII | 0.633 | 0.583–0.683 | <0.001 | 1617.42 | 0.606 | 0.641 |
| AISI | 0.581 | 0.529–0.632 | 0.002 | 822.28 | 0.502 | 0.709 |
|
Symptomatic ICH |
||||||
|---|---|---|---|---|---|---|
| AUC | 95%CI | p | Cut-off | Sensitivity | Specificity | |
| Day 1 | ||||||
| Neutrophil | 0.668 | 0.595–0.74 | <0.001 | 8.06 | 0.72 | 0.553 |
| NLR | 0.758 | 0.691–0.826 | <0.001 | 7.51 | 0.92 | 0.535 |
| MLR | 0.584 | 0.481–0.686 | 0.052 | 0.44 | 0.66 | 0.565 |
| PLR | 0.661 | 0.579–0.743 | <0.001 | 234.42 | 0.64 | 0.662 |
| PNR | 0.701 | 0.628–0.775 | <0.001 | 21.79 | 0.681 | 0.64 |
| SII | 0.707 | 0.639–0.776 | <0.001 | 1871.83 | 0.7 | 0.65 |
| SIRI | 0.622 | 0.529–0.715 | 0.005 | 5.06 | 0.52 | 0.736 |
Abbreviations: AUC, area under curve; CI, confidence interval; ICH, intracerebral hemorrhage.
3.4. CBC-derived systemic-inflammatory indices and sICH
A total of 50 patients experienced sICH (10.4 %). These patients had a lower prevalence of dyslipidemia and drinking habit, but a higher prevalence of coronary artery disease. They also had lower admission ASPECTS scores, longer puncture-to-recanalization time, and a higher proportion of general anesthesia and prior IVT compared to those without sICH (Table 1 and Table S1). In univariate analyses (Table 4), elevated levels of post-procedure neutrophil count, NLR, MLR, PLR, SII, and SIRI were associated with an increased risk of sICH. Conversely, lower levels of post-procedure lymphocyte count, platelet count, and PNR were linked to a higher risk of sICH. After adjustment, the occurrence of sICH was significantly associated with the following parameters measured on day 1: neutrophil count, NLR, MLR, PLR, PNR, SII, and SIRI(Table 4). In ROC analyses, the AUC values for day 1 neutrophil count, NLR, MLR (p = 0.052), PLR, PNR, SII, and SIRI in discriminating the development of sICH were 0.668, 0.758, 0.584, 0.661, 0.701, 0.707, and 0.622, respectively (Table 5). Their optimal cut-off values were 8.06, 7.51, 0.44, 234.42, 21.79, 1871.83, and 5.06, respectively (Table 5).
Table 4.
Univariate and multivariate logistics regression analyses of CBC-derived systemic-inflammatory indices associated with sICH.
| Symptomatic ICH |
|||||||
|---|---|---|---|---|---|---|---|
| Yes (n = 50) | No (n = 432) | OR (95%CI) | P | aOR (95%CI)a | P | aPb | |
| Admission, median (IQR) | |||||||
| Neutrophil, × 109/L | 7.21(4.57–8.76) | 6.41(4.89–8.75) | 1.060(0.978–1.149) | 0.155 | 1.053(0.953–1.165) | 0.311 | Ns |
| Monocyte, × 109/L | 0.42(0.29–0.52) | 0.4(0.3–0.52) | 0.900(0.418–1.940) | 0.788 | 0.969(0.376–2.495) | 0.948 | Ns |
| Lymphocyte, × 109/L | 1.09(0.8–1.61) | 1.29(0.95–1.82) | 0.677(0.432–1.061) | 0.089 | 0.673(0.412–1.098) | 0.113 | Ns |
| Platelet, × 109/L | 199.5(158–244) | 205(170.5–245) | 0.997(0.992–1.002) | 0.186 | 0.999(0.994–1.005) | 0.832 | Ns |
| NLR | 6.72(4.02–10.57) | 5.02(2.93–7.7) | 1.032(0.999–1.067) | 0.056 | 1.032(0.994–1.071) | 0.096 | Ns |
| MLR | 0.34(0.23–0.45) | 0.3(0.21–0.4) | 1.303(0.947–1.792) | 0.104 | 1.315(0.911–1.899) | 0.144 | ns |
| PLR | 179.8(123.9–253.2) | 154.7(106.4–225.1) | 1.001(1.000–1.002) | 0.075 | 1.001(1.000–1.003) | 0.124 | ns |
| PNR | 32.1(20.24–41.79) | 31.31(22.99–42.82) | 0.987(0.968–1.007) | 0.197 | 0.990(0.970–1.011) | 0.348 | ns |
| SII | 1378(632.1–1929) | 1003(540.6–1722.9) | 1.000(1.000–1.000) | 0.329 | 1.000(1.000–1.000) | 0.225 | ns |
| SIRI | 2.29(1.24–3.58) | 1.86(1.1–3.38) | 1.023(0.993–1.055) | 0.138 | 1.025(0.990–1.060) | 0.16 | ns |
| AISI | 409.2(256.3–697.3) | 365.7(210.6–699.3) | 1.000(1.000–1.000) | 0.464 | 1.000(1.000–1.000) | 0.252 | ns |
| Day 1, median (IQR) | |||||||
| Neutrophil, × 109/L | 9.3(7.62–11.78) | 7.7(6.005–9.925) | 1.175(1.080–1.278) | <0.001 | 1.201(1.084–1.331) | <0.001 | 0.003 |
| Monocyte, × 109/L | 0.36(0.16–0.56) | 0.43(0.31–0.56) | 0.333(0.078–1.413) | 0.136 | 0.547(0.119–2.511) | 0.438 | ns |
| Lymphocyte, × 109/L | 0.69(0.44–0.97) | 1.06(0.72–1.41) | 0.305(0.149–0.622) | 0.001 | 0.338(0.159–0.717) | 0.005 | 0.056 |
| Platelet, × 109/L | 185(141–230) | 206(171–245) | 0.993(0.988–0.998) | 0.01 | 0.996(0.990–1.002) | 0.185 | ns |
| NLR | 12.67(9.65–21.24) | 7.11(4.7–11.38) | 1.092(1.059–1.127) | <0.001 | 1.107(1.064–1.151) | <0.001 | <0.001 |
| MLR | 0.54(0.22–0.83) | 0.4(0.28–0.58) | 4.042(1.925–8.489) | <0.001 | 4.898(1.964–12.22) | 0.001 | 0.006 |
| PLR | 257(196.7–400) | 196.7(138.7–280) | 1.003(1.001–1.004) | 0.001 | 1.004(1.002–1.005) | <0.001 | 0.002 |
| PNR | 19.99(14.62–25.57) | 25.91(19.87–34.97) | 0.920(0.886–0.955) | <0.001 | 0.927(0.890–0.967) | <0.001 | 0.002 |
| SII | 2639(1509–3534) | 1463(901.1–2449) | 1.000(1.000–1.000) | <0.001 | 1.000(1.000–1.000) | 0.001 | 0.005 |
| SIRI | 5.18(2.29–8.31) | 2.98(1.85–5.29) | 1.094(1.038–1.152) | 0.001 | 1.102(1.039–1.169) | 0.001 | 0.01 |
| AISI | 784.8(510.2–1462) | 592.1(350.7–1125) | 1.000(1.000–1.000) | 0.136 | 1.000(1.000–1.000) | 0.239 | ns |
Note.
Abbreviations: OR, odds ratio; aOR, adjusted odds ratio; aP, adjusted p value; CI, confidence interval; ICH, intracerebral hemorrhage; IQR, interquartile range; mRS, modified Rankin scale; ns, not significant.
adjustment for dyslipidemia, coronary heart disease, anticoagulants use, admission NIHSS and ASPECTS scores, groin puncture-to-recanalization time, prior IVT, anesthesia type, and the number of passes.
Bonferroni correction test.
4. Discussion
The thromboinflammatory response has been observed in all phases of AIS [[13], [14], [15]]. Importantly, this process can also occur in patients who initially achieve successful mechanical recanalization, potentially leading to secondary tissue damage and exacerbating stroke outcomes [14,36,37]. Previous study has also demonstrated that patients undergoing EVT are more likely to experience significant dynamic changes in a variety of immune indices compared to those receiving IVT alone [29]. In this retrospective cohort study of 482 consecutive patients who underwent successful EVT for anterior circulation AIS-LVO, we comprehensively analyzed the correlations between a range of CBC-derived systemic-inflammatory indices and patient outcomes. The main findings were as follows: (1) day 1 neutrophil and monocyte counts, and the values of NLR, MLR, PLR, SII, SIRI, AISI were increased, while lymphocyte count and PNR value were decreased compared to their admission levels; (2) day 1 neutrophil count, NLR, SII, and AISI were independently associated with functional outcome at 90 days after EVT; (3) day 1 neutrophil count, NLR, MLR, PLR, PNR, SII, and SIRI were independently associated with the occurrence of sICH; and (4) no admission single-cell counts and CBC-derived systemic-inflammatory indices were identified as independent risk factors for the clinical outcomes of patients with AIS-LVO.
We focused on the admission and day 1 CBC-derived systemic-inflammatory indices for several important reasons. First, the pathophysiological changes, particularly the thromboinflammatory response, occurring within the first day post-reperfusion have a substantial impact on patient prognosis [12,16,18,38]. Second, early neurological deterioration or improvement within the first day post-reperfusion is closely related to the functional outcome and mortality of AIS-LVO patients [39,40], even though predicting clinical progression within the first day remains challenging [41]. Third, early prediction of outcome is conducive to more effective and timely clinical treatment [10,11]. Last, CBC tests are cost-effective and routinely performed at these two time-points in clinical practice, and have been commonly used as the time points of assessments in previous studies [[29], [30], [31], [32]].
Neutrophils are the primary cell type that adhere to the activated endothelium and transmigrate into the brain parenchyma within the first few hours, but reaching their peak levels on days 1–3. Neutrophils exert their function through multiple mechanisms including phagocytosis, degranulation (e.g., elastase, myeloperoxidase, and matrix metalloproteinase), production of reactive oxygen and nitrogen species, and cytokines, and formation of neutrophil extracellular traps. These pro-inflammatory and destructive mediators promote cellular injury, damage the extracellular matrix and the brain-blood barrier, eventually leading to secondary brain injuries such as hemorrhagic transformation and cerebral edema [16,17]. Recent study demonstrates that neutrophil recruitment persists even after successful mechanical recanalization. Neutrophils stimulate pro-thrombotic pathways and obstruct capillaries in the microvascular bed following reperfusion, contributing to the no-reflow phenomenon and enlarged infarct size [42]. Monocyte-derived macrophages, upon activation by danger signals, infiltrate the core as well as peri-infarct areas within 24 h, reaching their peak levels at 3 days after AIS. These infiltrated monocytes/macrophages primarily contribute to the accumulation of inflammatory mediators, further aggravating brain injury [16,43]. Lymphocytes are the primary neuroprotective immunomodulators by suppressing various inflammatory pathways and reducing the activation and recruitment of resident and invading immune cells [38,44]. However, the specific biological functions of lymphocytes vary depending on their phenotypes. For instance, CD4+, CD8+, and γδ T cells have deleterious effects, whereas regulatory T cells release protective anti-inflammatory cytokines (e.g., IL-10) [18]. Lymphopenia is a prevalent condition in AIS and is associated with stroke severity and clinical outcomes [38]. Lymphopenia is linked to stroke-induced immunosuppression, which can result in infections (e.g., stroke-associated pneumonia) and peripheral organ injuries, ultimately exacerbating clinical outcomes of AIS patients [45]. Early studies investigating the immune profiling of patients with AIS have identified profound peripheral neutrophilia, monocytosis, and lymphopenia as early as 1 day after AIS [18,31,38]. Recent studies also reported that blood samples taken from the core of the occluded vessel compartment exhibit a significant increase in neutrophils but slight increases in lymphocytes and monocytes [46,47]. This increase in neutrophils is associated with unfavorable short-term outcome, indicating an acute local neutrophil-dominant inflammation [47]. NLR and MLR are the most studied inflammatory indicators to reflect the balance of innate and adaptive immune response, as well as the overall burden of systemic inflammation. In the present study, we observed that levels of neutrophil and NLR on day 1 were correlated with sICH and worse 90-day functional outcome, which were consistent with previous studies [[29], [30], [31], [32]]. Furthermore, Li et al. [48] found that an elevated NLR within the first 3 days following successful EVT was linked to an increased risk of mortality at 1 month. In addition, our findings indicated that day 1 MLR served as an independent predictor for sICH occurrence. These results suggest that the detrimental effects of neutrophils and monocytes, along with the lack of cerebral protective effects by lymphocytes, may contribute to the unfavorable outcome of AIS patients.
Following AIS, platelets undergo activation and release a variety of inflammatory mediators [14]. They also form heterologous aggregates with leukocytes, skewing leukocytes toward a pro-inflammatory and pro-thrombotic state [49]. AIS patients typically show a decrease in circulating platelet count, along with an increase in platelet distribution width, mean platelet volume, as well as platelet-monocyte and platelet-neutrophil aggregations. These changes are associated with disease severity, reperfusion injury, and prognosis of AIS patients [50]. A recent study also observed locally reduced platelet count, platelet activation, platelet-derived chemokine release, and platelet-neutrophil interactions, suggesting a role of platelets in mediating neutrophil-dominant inflammation within clot intracranial environment [47]. The ratio of platelet to leukocyte subsets, such as PNR and PLR, are thus useful indicators to comprehensively assess the severity of both thrombosis and inflammation. In the present study, we demonstrated that there was an increase in PLR and a decrease in PNR on the first day after successful recanalization. Furthermore, we identified that elevated PLR and reduced PNR on day 1 were independently linked to a higher risk of sICH. These findings suggest that abnormal activation and excessive consumption of platelets and the dysregulated immune system, characterized by neutrophilia and lymphopenia during post-AIS thromboinflammation, may contribute to hemorrhagic transformation in EVT-treated patients.
SII, SIRI, and AISI are innovative markers of the thromboinflammatory response initially utilized in cancer research [21]. Our study demonstrated that day 1 SII and AISI were independent predictors of 90-day functional outcome, while day 1 SII and SIRI served as independent predictors of sICH occurrence. These markers are derived from the well-established NLR, may partially explain their predictive capacities [37]. Given that these markers offer a comprehensive reflection of the host's inflammatory, immune, and thrombotic status, and have shown superior predictive ability compared to single-cell counts and variables based on two cell subtypes [21]. Future prospective clinical trials with large sample sizes are warranted to validate their predictive accuracy and elucidate potential mechanisms of action.
An important finding of our study is that, in contrast to previous studies [[24], [25], [26], [27],[51], [52], [53]], admission variables in our cohort did not have any independent predictive value for patient outcomes. These discrepancies may be attributed to diverse study populations, variations in the time of blood sample collection, or differences in data processing methods (e.g., continuous or dichotomous). Of particular importance may be the impact of EVT treatment on altering outcomes. Other possible reasons include the VWI caused by interventional therapy [[8], [9], [10]], the development and progression of thromboinflammation, and the subsequent reperfusion injury [14,36], which could not be reflected by the admission indices. Wu et al. [37] conducted a meta-analysis involving 52 studies, which found that the post-treatment NLR has a stronger predictive ability for the poor prognosis of AIS patients after reperfusion therapy compared to admission NLR. Further confirmation of our conclusion is needed.
This study has several limitations. First, this was retrospective and single-center study with a moderate sample size. Second, we have not established an independent validation cohort, which is able to enhance the credibility of our results. Third, all participants enrolled were Chinese patients (97.9 % of Han ethnicity), whether these findings are reproducible in other races/ethnicities or countries remains to be investigated. Fourth, although we adjusted for multiple potential confounders during the data analyses, possible residual confounding influences (e.g., blood loss or heparinization during the EVT procedure) cannot be completely excluded. Furthermore, although the treatment strategy and equipment used were consistent, the relative prolonged duration (December 2018 and December 2022) may introduce additional confounding variables. Fifth, relying solely on admission and day 1 indices may not be sufficient to reflect the complex underlying mechanisms, and may yield limited results. Therefore, future studies should consider dynamically monitoring these indices throughout the whole treatment process. Finally, the correlations between CBC-derived systemic-inflammatory indices and outcome did not necessarily imply a causal-effect relationship, which requires further investigations.
5. Conclusion
In conclusion, we demonstrated that several day 1 CBC-derived systemic-inflammatory indices could independently predict the occurrence of sICH and unfavorable 90-day functional outcome for anterior circulation AIS-LVO patients who achieved successful recanalization. These results suggested that targeted immunomodulatory treatment as adjuvant therapy for high-risk patients identified by these markers may improve the prognosis of patients with AIS-LVO.
Ethics Statement
This study was approved by the Ethics Committee of the Xuanwu Hospital, Capital Medical University (ID-CRB-61/2023), and was performed according to the Principles of Declaration of Helsinki. The requirement for informed consent was waived because of its retrospective nature and minimal risk to patients.
Funding
This study was supported by grants from the National Natural Science Foundation of China (grant 82001317 and 82171303) and the Beijing Hospitals Authority Youth Programme (grant QML20230801).
Data Availability Statement
The raw data that support the findings of this study are available from the corresponding author upon reasonable request.
CRediT authorship contribution statement
Wenbo Cao: Writing – review & editing, Data curation. Yiming Song: Writing – review & editing, Data curation. Xuesong Bai: Data curation. Bin Yang: Visualization, Formal analysis. Long Li: Visualization, Formal analysis. Xinyu Wang: Data curation. Yuxin Wang: Data curation. Wenxuan Chang: Data curation. Yanfei Chen: Formal analysis. Yabing Wang: Formal analysis. Jian Chen: Formal analysis. Peng Gao: Visualization, Data curation. Liqun Jiao: Writing – review & editing, Writing – original draft, Supervision, Project administration, Methodology, Funding acquisition, Conceptualization. Xin Xu: Writing – review & editing, Writing – original draft, Supervision, Project administration, Methodology, Funding acquisition, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors thank the study participants and their families, as well as the clinical staff for their support and contributions to this study. Additionally, the authors thank Prof. Jing-fei Dong (Bloodworks Research Institute; Division of Hematology, Department of Medicine, University of Washington, School of Medicine, Seattle, WA, USA) and Dr. Adam A Dmytriw (Neurointerventional Program, Departments of Medical Imaging & Clinical Neurological Sciences, London Health Sciences Centre, London, Ontario, Canada; Neuroendovascular Program, Massachusetts General Hospital, Boston, MA, USA) for their insightful review and comments on the manuscript.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2024.e31122.
Contributor Information
Liqun Jiao, Email: liqunjiao@sina.cn.
Xin Xu, Email: xuxindoc@hotmail.com.
Appendix A. Supplementary data
The following is the Supplementary data to this article.
References
- 1.Fan J., Li X., Yu X., Liu Z., Jiang Y., Fang Y., et al. Global burden, risk factor analysis, and prediction study of ischemic stroke, 1990-2030. Neurology. 2023;101:e137–e150. doi: 10.1212/WNL.0000000000207387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Smith E.E., Zerna C., Solomon N., Matsouaka R., Mac Grory B., Saver J.L., et al. Outcomes after endovascular thrombectomy with or without alteplase in routine clinical practice. JAMA Neurol. 2022;79:768–776. doi: 10.1001/jamaneurol.2022.1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Phipps M.S., Cronin C.A. Management of acute ischemic stroke. Bmj. 2020;368 doi: 10.1136/bmj.l6983. [DOI] [PubMed] [Google Scholar]
- 4.Powers W.J., Rabinstein A.A., Ackerson T., Adeoye O.M., Bambakidis N.C., Becker K., et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 Update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for Healthcare Professionals from the American heart association/American stroke association. Stroke. 2019;50:e344–e418. doi: 10.1161/STR.0000000000000211. [DOI] [PubMed] [Google Scholar]
- 5.van der Ende N.A.M., Kremers F.C.C., van der Steen W., Venema E., Kappelhof M., Majoie C., et al. Symptomatic intracranial hemorrhage after endovascular stroke treatment: External validation of prediction Models. Stroke. 2023;54:476–487. doi: 10.1161/STROKEAHA.122.040065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Seners P., Baron J.C. Revisiting 'progressive stroke': incidence, predictors, pathophysiology, and management of unexplained early neurological deterioration following acute ischemic stroke. Journal of neurology. 2018;265:216–225. doi: 10.1007/s00415-017-8490-3. [DOI] [PubMed] [Google Scholar]
- 7.Wang L., Xiong Y. Advances in futile reperfusion following endovascular treatment in acute ischemic stroke due to large vessel occlusion. Eur. Neurol. 2023;86:95–106. doi: 10.1159/000528922. [DOI] [PubMed] [Google Scholar]
- 8.Pilgram-Pastor S.M., Piechowiak E.I., Dobrocky T., Kaesmacher J., Den Hollander J., Gralla J., et al. Stroke thrombectomy complication management. J. Neurointerventional Surg. 2021;13:912–917. doi: 10.1136/neurintsurg-2021-017349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mereuta O.M., Abbasi M., Fitzgerald S., Dai D., Kadirvel R., Hanel R.A., et al. Histological evaluation of acute ischemic stroke thrombi may indicate the occurrence of vessel wall injury during mechanical thrombectomy. J. Neurointerventional Surg. 2022;14:356–361. doi: 10.1136/neurintsurg-2021-017310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Krishnan R., Mays W., Elijovich L. Complications of mechanical thrombectomy in acute ischemic stroke. Neurology. 2021;97:S115–S125. doi: 10.1212/WNL.0000000000012803. [DOI] [PubMed] [Google Scholar]
- 11.Li Q., Zhao L., Chan C.L., Zhang Y., Tong S.W., Zhang X., et al. Multi-level biomarkers for early Diagnosis of Ischaemic stroke: a systematic review and meta-analysis. Int. J. Mol. Sci. 2023;24 doi: 10.3390/ijms241813821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Correia M., Silva I., Gabriel D., Simren J., Carneiro A., Ribeiro S., et al. Early plasma biomarker dynamic profiles are associated with acute ischemic stroke outcomes. Eur. J. Neurol. 2022;29:1630–1642. doi: 10.1111/ene.15273. [DOI] [PubMed] [Google Scholar]
- 13.De Meyer S.F., Langhauser F., Haupeltshofer S., Kleinschnitz C., Casas A.I. Thromboinflammation in brain ischemia: recent Updates and future Perspectives. Stroke. 2022;53:1487–1499. doi: 10.1161/STROKEAHA.122.038733. [DOI] [PubMed] [Google Scholar]
- 14.Denorme F., Ajanel A., Campbell R.A. Immunothrombosis in neurovascular disease. Research and practice in thrombosis and haemostasis. 2024;8 doi: 10.1016/j.rpth.2023.102298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Szepanowski R.D., Haupeltshofer S., Vonhof S.E., Frank B., Kleinschnitz C., Casas A.I. Thromboinflammatory challenges in stroke pathophysiology. Semin. Immunopathol. 2023;45:389–410. doi: 10.1007/s00281-023-00994-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Carmona-Mora P., Knepp B., Jickling G.C., Zhan X., Hakoupian M., Hull H., et al. Monocyte, neutrophil, and whole blood transcriptome dynamics following ischemic stroke. BMC Med. 2023;21:65. doi: 10.1186/s12916-023-02766-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Simats A., Liesz A. Systemic inflammation after stroke: implications for post-stroke comorbidities. EMBO Mol. Med. 2022;14 doi: 10.15252/emmm.202216269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu R., Song P., Gu X., Liang W., Sun W., Hua Q., et al. Comprehensive Landscape of immune infiltration and Aberrant pathway activation in ischemic stroke. Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.766724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sharma S., Tyagi T., Antoniak S. Platelet in thrombo-inflammation: Unraveling new therapeutic targets. Front. Immunol. 2022;13 doi: 10.3389/fimmu.2022.1039843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fan W., Wei C., Liu Y., Sun Q., Tian Y., Wang X., et al. The prognostic value of hematologic inflammatory markers in patients with acute coronary Syndrome undergoing Percutaneous coronary intervention. Clinical and applied thrombosis/hemostasis : official journal of the International Academy of Clinical and Applied Thrombosis/Hemostasis. 2022;28 doi: 10.1177/10760296221146183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ye Z., Hu T., Wang J., Xiao R., Liao X., Liu M., et al. Systemic immune-inflammation index as a potential biomarker of cardiovascular diseases: a systematic review and meta-analysis. Frontiers in cardiovascular medicine. 2022;9 doi: 10.3389/fcvm.2022.933913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jarmuzek P., Kozlowska K., Defort P., Kot M., Zembron-Lacny A. Prognostic values of systemic inflammatory Immunological markers in Glioblastoma: a systematic review and meta-analysis. Cancers. 2023;15 doi: 10.3390/cancers15133339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gambichler T., Schuleit N., Susok L., Becker J.C., Scheel C.H., Torres-Reyes C., et al. Prognostic performance of inflammatory biomarkers based on complete blood counts in COVID-19 patients. Viruses. 2023;15 doi: 10.3390/v15091920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goyal N., Tsivgoulis G., Chang J.J., Malhotra K., Pandhi A., Ishfaq M.F., et al. Admission neutrophil-to-lymphocyte ratio as a prognostic biomarker of outcomes in large vessel occlusion strokes. Stroke. 2018;49:1985–1987. doi: 10.1161/STROKEAHA.118.021477. [DOI] [PubMed] [Google Scholar]
- 25.Pikija S., Sztriha L.K., Killer-Oberpfalzer M., Weymayr F., Hecker C., Ramesmayer C., et al. Neutrophil to lymphocyte ratio predicts intracranial hemorrhage after endovascular thrombectomy in acute ischemic stroke. J. Neuroinflammation. 2018;15:319. doi: 10.1186/s12974-018-1359-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ma J., Guo W., Xu J., Li S., Ren C., Wu L., et al. Association of platelet-to-lymphocyte ratio and neutrophil-to-lymphocyte ratio with outcomes in stroke patients achieving successful recanalization by endovascular thrombectomy. Front. Neurol. 2022;13 doi: 10.3389/fneur.2022.1039060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yi H.J., Sung J.H., Lee D.H. Systemic inflammation response index and systemic immune-inflammation index are associated with clinical outcomes in patients treated with mechanical thrombectomy for large artery occlusion. World neurosurgery. 2021;153:e282–e289. doi: 10.1016/j.wneu.2021.06.113. [DOI] [PubMed] [Google Scholar]
- 28.Wang L., Cheng Q., Peng M., Lv D., Zi W., Xu G., et al. The relationship between the platelet to leukocyte ratio and mechanical thrombectomy outcomes in acute ischemic stroke patients. Neurol. Res. 2020;42:890–896. doi: 10.1080/01616412.2020.1790868. [DOI] [PubMed] [Google Scholar]
- 29.Chen S., Cheng J., Ye Q., Ye Z., Zhang Y., Liu Y., et al. Day 1 neutrophil-to-lymphocyte ratio (NLR) predicts stroke outcome after intravenous thrombolysis and mechanical thrombectomy. Front. Neurol. 2022;13 doi: 10.3389/fneur.2022.941251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lux D., Alakbarzade V., Bridge L., Clark C.N., Clarke B., Zhang L., et al. The association of neutrophil-lymphocyte ratio and lymphocyte-monocyte ratio with 3-month clinical outcome after mechanical thrombectomy following stroke. J. Neuroinflammation. 2020;17:60. doi: 10.1186/s12974-020-01739-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Semerano A., Laredo C., Zhao Y., Rudilosso S., Renu A., Llull L., et al. Leukocytes, Collateral circulation, and reperfusion in ischemic stroke patients treated with mechanical thrombectomy. Stroke. 2019;50:3456–3464. doi: 10.1161/STROKEAHA.119.026743. [DOI] [PubMed] [Google Scholar]
- 32.Che F., Zhao X., Ding Y., Wang A., Cheng Z., Tong Y., et al. Association of early Longitudinal changes in the neutrophil-to-lymphocyte ratio with adverse clinical outcomes in acute ischemic stroke patients after endovascular treatment. World neurosurgery. 2024;182:e579–e596. doi: 10.1016/j.wneu.2023.11.151. [DOI] [PubMed] [Google Scholar]
- 33.Wang X., Wang X., Ma J., Jia M., Wu L., Li W., et al. Association between the time of day at stroke onset and functional outcome of acute ischemic stroke patients treated with endovascular therapy. J. Cerebr. Blood Flow Metabol. : official journal of the International Society of Cerebral Blood Flow and Metabolism. 2022;42:2191–2200. doi: 10.1177/0271678X221111852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vandenbroucke J.P., von Elm E., Altman D.G., Gotzsche P.C., Mulrow C.D., Pocock S.J., et al. Strengthening the reporting of Observational studies in Epidemiology (STROBE): explanation and elaboration. Int. J. Surg. 2014;12:1500–1524. doi: 10.1016/j.ijsu.2014.07.014. [DOI] [PubMed] [Google Scholar]
- 35.Hacke W., Kaste M., Bluhmki E., Brozman M., Davalos A., Guidetti D., et al. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N. Engl. J. Med. 2008;359:1317–1329. doi: 10.1056/NEJMoa0804656. [DOI] [PubMed] [Google Scholar]
- 36.Zhou Y., He Y., Yan S., Chen L., Zhang R., Xu J., et al. Reperfusion injury is associated with poor outcome in patients with recanalization after thrombectomy. Stroke. 2023;54:96–104. doi: 10.1161/STROKEAHA.122.039337. [DOI] [PubMed] [Google Scholar]
- 37.Wu B., Liu F., Sun G., Wang S. Prognostic role of dynamic neutrophil-to-lymphocyte ratio in acute ischemic stroke after reperfusion therapy: a meta-analysis. Front. Neurol. 2023;14 doi: 10.3389/fneur.2023.1118563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xiao J., Qiu Q.W., Qin C., Tao R., Qiao S.Y., Chen M., et al. Dynamic changes of peripheral blood lymphocyte subsets in acute ischemic stroke and prognostic value. Brain and behavior. 2021;11 doi: 10.1002/brb3.1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bhole R., Nouer S.S., Tolley E.A., Turk A., Siddiqui A.H., Alexandrov A.V., et al. Predictors of early neurologic deterioration (END) following stroke thrombectomy. J. Neurointerventional Surg. 2023;15:584–588. doi: 10.1136/neurintsurg-2022-018844. [DOI] [PubMed] [Google Scholar]
- 40.Kobeissi H., Ghozy S., Bilgin C., Kadirvel R., Kallmes D.F. Early neurological improvement as a predictor of outcomes after endovascular thrombectomy for stroke: a systematic review and meta-analysis. J. Neurointerventional Surg. 2023;15:547–551. doi: 10.1136/neurintsurg-2022-019008. [DOI] [PubMed] [Google Scholar]
- 41.Saver J.L., Altman H. Relationship between neurologic deficit severity and final functional outcome shifts and strengthens during first hours after onset. Stroke. 2012;43:1537–1541. doi: 10.1161/STROKEAHA.111.636928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.El Amki M., Gluck C., Binder N., Middleham W., Wyss M.T., Weiss T., et al. Neutrophils obstructing brain capillaries are a major cause of No-reflow in ischemic stroke. Cell Rep. 2020;33 doi: 10.1016/j.celrep.2020.108260. [DOI] [PubMed] [Google Scholar]
- 43.Bai M., Sun R., Cao B., Feng J., Wang J. Monocyte-related cytokines/chemokines in cerebral ischemic stroke. CNS Neurosci. Ther. 2023 doi: 10.1111/cns.14368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gee J.M., Kalil A., Shea C., Becker K.J. Lymphocytes: potential mediators of postischemic injury and neuroprotection. Stroke. 2007;38:783–788. doi: 10.1161/01.STR.0000248425.59176.7b. [DOI] [PubMed] [Google Scholar]
- 45.Faura J., Bustamante A., Miro-Mur F., Montaner J. Stroke-induced immunosuppression: implications for the prevention and prediction of post-stroke infections. J. Neuroinflammation. 2021;18:127. doi: 10.1186/s12974-021-02177-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kollikowski A.M., Schuhmann M.K., Nieswandt B., Mullges W., Stoll G., Pham M. Local leukocyte invasion during Hyperacute Human ischemic stroke. Ann. Neurol. 2020;87:466–479. doi: 10.1002/ana.25665. [DOI] [PubMed] [Google Scholar]
- 47.Kollikowski A.M., Pham M., Marz A.G., Papp L., Nieswandt B., Stoll G., et al. Platelet activation and chemokine release are related to local neutrophil-dominant inflammation during Hyperacute Human stroke. Translational stroke research. 2022;13:364–369. doi: 10.1007/s12975-021-00938-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li S., Hu L., Wang J., Zou F., Han B., Wang Y., et al. Prolonged increased neutrophil-to-lymphocyte ratio is associated with mortality after successful revascularization for treatment of acute ischemic stroke. BMC Neurol. 2022;22:326. doi: 10.1186/s12883-022-02847-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Finsterbusch M., Schrottmaier W.C., Kral-Pointner J.B., Salzmann M., Assinger A. Measuring and interpreting platelet-leukocyte aggregates. Platelets. 2018;29:677–685. doi: 10.1080/09537104.2018.1430358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chen Y., Xiao Y., Lin Z., Xiao X., He C., Bihl J.C., et al. The role of circulating platelets Microparticles and platelet parameters in acute ischemic stroke patients. J. Stroke Cerebrovasc. Dis. : the official journal of National Stroke Association. 2015;24:2313–2320. doi: 10.1016/j.jstrokecerebrovasdis.2015.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kim S.Y., Yi H.J., Shin D.S., Kim B.T. Prognostic significance of platelet-to-lymphocyte and platelet-to-neutrophil ratios in patients with mechanical thrombectomy for acute ischemic stroke. Journal of cerebrovascular and endovascular neurosurgery. 2022;24:221–231. doi: 10.7461/jcen.2022.E2021.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lattanzi S., Norata D., Divani A.A., Di Napoli M., Broggi S., Rocchi C., et al. Systemic inflammatory response index and futile recanalization in patients with ischemic stroke undergoing endovascular treatment. Brain Sci. 2021;11 doi: 10.3390/brainsci11091164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yang Y., Cui T., Bai X., Wang A., Zhang X., Wan J., et al. Association between systemic immune-inflammation index and symptomatic intracranial hemorrhage in acute ischemic stroke patients undergoing endovascular treatment. Curr. Neurovascular Res. 2022;19:83–91. doi: 10.2174/1567202619666220406102429. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The raw data that support the findings of this study are available from the corresponding author upon reasonable request.