Abstract
Exosomes possess a significant role in intercellular communications. In the nervous system, various neural cells release exosomes that not only own a role in intercellular communications but also eliminate the waste of cells, maintain the myelin sheath, facilitate neurogenesis, and specifically assist in normal cognitive function. In neurological conditions including Parkinson's disease (PD), Alzheimer's disease (AD), traumatic brain injury (TBI), and stroke, exosomal cargo like miRNAs take part in the sequela of conditions and serve as a diagnostic tool of neurological disorders, too. Exosomes are not only a diagnostic tool but also their inhibition or administration from various sources like mesenchymal stem cells and serum, which have shown a worthy potential to treat multiple neurological disorders. In addition to neurodegenerative manifestations, cognitive deficiencies are an integral part of neurological diseases, and applying exosomes in improving both aspects of these diseases has been promising. This review discusses the status of exosome therapy in improving neurorestorative and cognitive function following neurological disease.
Keywords: cognition, exosome therapy, neurological disorders, neuroregeneration, neurorestoration
Neurological diseases (including Alzheimer's disease, Parkinson's disease, stroke, multiple sclerosis, epilepsy, traumatic brain injury, encephalopathy, etc.) lead to an increase in inflammation, oxidative stress and cell death in the brain, but exosome therapy reverses the process by reducing inflammation and improving neurogenesis, and ultimately leads to cognitive function improvement.

1. INTRODUCTION
Exosomes are nanosized (40–100 nm) membrane microvesicles that exist in almost all biological fluids, and there has been increasing attention to them over recent years. 1 These microvesicles contain lipids, proteins, nucleic acids, mRNAs, non‐coding RNAs (ncRNAs), cytokines, and other bioactive substances. 2 In recent investigations, the characterization of exosomal cargo through release and uptake has had a vital role in identifying their function in the nervous system. 3 Many findings have demonstrated that exosomal cargo is essential in normal CNS communications, neural regeneration, synaptic function, and plasticity. 4 Moreover, exosomes have some valuable characteristics that make them a potent player in treating various neurological conditions through experimental studies. 5 Considering the small size of exosomes, they can credibly avoid macrophage polarization and effortlessly cross the extracellular matrix and biological barriers. 6 As an advantage, surface CD55 and CD59 in exosomes inhibit coagulation factors and phagocytosis by suppressing some extracellular compounds, such as opsonin bound, and eventually distribute broadly in body fluids. 7 , 8
Due to the natural origin of exosomes, they have suitable biocompatibility, little immunogenicity, and benefit from many advantages in contrast to synthetic nanodelivery systems and liposomes. 9 , 10 Moreover, exosomes efficaciously impress target cells and overcome obstacles, such as the blood–brain barrier (BBB), to serve as a therapeutic choice and a potent natural intermediate for drug delivery. 11 , 12 , 13 Various experimental studies have shown the amazing effects of exosome therapy in attenuating different neurological disease signs. 14 , 15 Exosomes have active participation in the nervous system function, and several types of neural cells, such as neurons, microglia, astrocytes, and oligodendrocytes, direct intercellular communications by secreting exosomes. 16 , 17 , 18 Generally, exosomes of neural cells are involved in normal neurodevelopment, neuroregeneration, and synaptic function regulation. 19 , 20
During various neurological disorders, parallel with the change of exosomal content, the cognitive function of affected people is damaged in multiple manners; hence, exosomes are used as a suitable biomarker for diagnosing these diseases besides cognitive assessments. 21 , 22 , 23 On the other hand, the administration of exosomes in various models of neurological diseases and, in some cases, inhibiting their pathologic release, besides improving neurogenesis in the central nervous system, has improved their cognitive performance in different dimensions, such as spatial memory and recognition. 24 , 25 , 26 Given that, following neurological disorders such as stroke and Alzheimer's disease, despite neurodegenerative manifestations, cognitive functions like spatial memory and recognition are affected; therefore, different therapeutic approaches should target their cognitive function along with neurorestoration. 27 , 28 , 29 , 30 , 31 Herein, we present the current state of exosome therapy in improving neurorestoration and cognitive function following neurological disease.
2. EXOSOMES CHARACTERISTICS AND BIOGENESIS
Various types of extracellular vesicles (EVs) have been identified to date, including apoptotic bodies (about 1 μm), ectosomes that consist of microparticles, microvesicles, shedding vesicles (about 100 nm–1 μm), and finally exosomes, that are specified with a 40–100 nm size. 32 Exosomes have an endocytic origin with molecular ingredients, making them highly conserved among most eukaryotic organisms. 33 Multiple characterization measures are needed to specify exosomes. International Society for Extracellular Vesicles (ISEV) presents that exosomes harvested from various biological fluids should be evaluated and confirmed by two types of proteins, like endosomal transmembrane proteins and recovered cytosolic proteins of EVs. 34 Multiple evaluation procedures are used for measuring exosomes, including dynamic light scattering (DLS), nanoparticle tracking analyses (NTA), transmission electron microscopy (TEM), resistive pulse sensing (RPS), ELISA, flow cytometry, microfluidics and electrochemical detection. 35
Biogenesis supplies the exosomes with nucleic acids, lipids, and proteins. 36 Exosomes embrace multiple nucleic acids like DNAs, mRNAs and non‐coding RNAs like miRNAs. 37 As a cargo content, lipids equip exosomes with cholesterol, sphingomyelin, desaturated lipids, and phosphatidylserine. 38 Also, exosomes contain membrane or cytoplasmic proteins that consist of MVBs tetraspin proteins (such as CD9, CD81, CD63), major histocompatibility complex class I and II (MHC‐I, MHC‐II) antigens, enzymes, fusion proteins (PDL1, CTLA4, Alix), cytokines (IL2, IL6, IL10, TNF) growth factors (like TGF), and chaperones (Figure 1). 39 , 40 , 41 , 42
FIGURE 1.

Exosome structure and composition. Exosomes are released to extracellular space following MVB fusion with the plasma membrane. Exosomes are nanosized compositions generally including nucleic acids, proteins, membrane tetraspins and proteins, Antigens, Growth factors, and lipids dependent on their origin and activation state. CTLA4, Cytotoxic lymphocyte antigen 4; IL, Interleukin; MHC, Major histocompatibility; MVB, Multi Vesicular Body; PDL1, Programmed Death‐Ligand 1; TGF, Transforming growth factor.
Exosomes are formed from cytoplasmic membrane invagination that creates early sorting endosomes (ESE) and endosomes. The endosome buds inwardly and captures specific cytoplasmic molecules that lead to the creation of intraluminal vesicles (ILVs) (Figure 2). 36 The ESE is a relatively “large vesicle” and the primitive membrane site, created by the merger of endocytic vesicles containing the tubular extension, which is developed by RAB5, RAB4, RAB7, RAB11, retromer, and caveolae‐1 molecules. 43 The endocytic vesicles are constructed by clathrin‐mediated endocytosis (CME) or clathrin‐independent (CIE) ways. 44 A part of sorted endosomes may revert to the membrane via “Fast recycling” or “Slow recycling” to recuperate vesicles. 45 Eventually, multivesicular bodies (MVBs) or late‐sorting endosomes (LSE), that consist of ILVs, are transferred and unifies with the membrane. 46 In the fusion process of MVBs with the membrane, some Rab (Rab 27a/b, Rab7, 35, 11) and SNARE (Vamp7, YKT6) proteins participate. 47 On the other hand, some MVBs may be transported to lysosomes for degeneration and apoptotic body formation. 48 The biogenesis mode of ILVs is classified into two categories, endosomal sorting complexes required for transport (ESCRTs) dependent and independent pathways. 49 The ESCRT includes ESCRT‐0, ESCRT‐I, ESCRT‐II, and ESCRT‐III subcomplexes. 50 Besides these subcomplexes, “Syndecan–Syntenin–ALIX pathway” related proteins and some accessory proteins like Vps twenty associated 1 (VTA‐1) and AAA ATPase vacuolar protein‐associate sorting (VPS)4 participate in the ESCRT complex. 51 , 52 ESCRT‐0 has two subunits, as ubiquitin‐binding domains (UBDs), including signal transducing adaptor molecule (STAM) and hepatocyte growth factor‐regulated tyrosine kinase substrate (Hrs), that help to identify ubiquitinated proteins of endosomal membrane and its internalization. 53 A Hrs recruited clathrin coat further stabilizes the ESCRT‐0 subdomains and prevents the diffusion of the exosomal cargo. 54 ESCRT‐0 and ESCRT‐I, by clathrin assistance and constructing a subdomain of the endosomal membrane, invaginates the ILV. 55 TSG101, as a portion of ESCRT‐I, activates ESCRT‐II through different stimuli, and consequently, ESCRT‐I and ESCRT‐II complex create buds through confining and stabilizing the bud neck. 56 ESCRT‐II attaches to the VPS28 component of ESCRT‐I via VPS36, continuing with the interaction of ESCRT‐II‐VPS25 and VPS20 subunits that recruit the ESCRT‐III to the endosome membrane. 57 ESCRT‐II triggers the gathering of the ESCRT‐III complex. Subsequently, VPS24 and VPS2 construct ESCRT‐III complex following Snf7 homo‐oligomerization and contribute to finding an ESCRT‐III component containing vesicle‐shaping properties. 58 After splitting the buds, the ESCRT‐III subdomain and AAA ATPaseVPS4 enroll enzymes to deubiquitinate tag from the cargo proteins before launching them into the ILVs. 59
FIGURE 2.

Mechanism of exosome biogenesis and secretion. Exosomes in the form of ILVs bud inwardly to create early endosomes and MVBs. The membrane invaginate by clathrinid‐mediated or independent endocytosis. Following endosome sorting, a portion of it recycles and goes back to the membrane quickly or slowly to recuperate vesicles (fast or slow recycling). The other section extends into multivesicular bodies (MVB) through the participation of some proteins, including Rabs (4, 5, 7), which pursue intracellular cargo transport and form the ILVs. The biogenesis of ILVs has two pathways: The ESCRT dependent and ESCRT‐independent. The ESCRT‐dependent pathway involves the ESCRT complex‐related portions and the “Syndecan–Syntenin–ALIX pathway.” The ESCRT independent pathway consists of some membranous lipids and tetraspanins, forming a domain as a base for cargo transition. Next to the MVB formation that contains numerous ILVs, it fuses with the lysosome or membrane. Some RAB proteins, such as RAB27a/b, RAB11, RAB7, RAB35, and some SNAREs, including Vamp7 and YKT6, participate in the fusion process of MVB.
The ESCRT‐independent pathway is mediated via some mechanisms that include (1) Tetraspanin‐enriched microdomains (TEMs) that serve as cargo transporters upon interacting with intermediates such as CD83, CD9, 60 (2) Overexpression of PLD2 that work as an effector to control the MVBs budding and exosomal development, 61 (3) Activity of Rab11, and sphingomyelinase (nSMase, a ceramide‐producing enzyme). 62
3. BLOOD–BRAIN BARRIER PERMEABILITY TO EXOSOMES
Barriers comprising the blood–brain barrier (BBB) and the choroid plexus protect the brain from attacking the peripheral agents and are considered the chief barriers of the brain regarding surface and length. 63 Despite the importance of BBB in protecting the brain from exogenous threats and maintaining its homeostasis, the penetration of pharmacological agents such as exosomes through drug delivery approaches is vital in treating neuropsychological conditions. 64 In the next part, following the explanation of BBB structure, we will clarify the mechanism of multiple agents, like exosomes, crossing from the BBB.
3.1. Structure of BBB
BBB comprises vessels assembled with endothelial cells (ECs), pericytes, astrocytes, and neuronal terminations. 65 End feet of astrocytes are expanded along the basal lamina and form a narrow barrier. Pericytes fill the perivascular space between the astrocyte's end‐feet and capillary wall and operate vasculature tone, angiogenesis, repair, and constancy. 66 Eventually, neuronal terminations, as part of the structure, reach all the cells that form the BBB. The tight junctions (TJ) and the adherens junctions (AJ) are the main subcellular responsible for BBB structural integrity. TJs seal the ECs to create a stable tubular construction and contain three major transmembrane proteins: (1) claudin, solely posited in TJ with four trans‐membrane segments containing two intracytoplasmic termini and two extracellular loops, (2) occludin, a protein with four transmembrane segments and three intracytoplasmic regions, and (3) junctional adhesion molecules (JAMs), as vital players in the conservation of TJ integrity and the junctional compound owing to their function in the maintenance of the cells together. Also, zonula occludens (ZOs) proteins play the role of scaffolding that provides a structural basis for TJs via the assembling of multiprotein complexes at the cytoplasmic surface of TJs (Figure 3). 67 The astrocyte's end feet connect closely to ECs in a netlike manner and participate in creation, maintenance, and integrity of BBB. 68
FIGURE 3.

Structure of the BBB and mechanisms of exosomes cross from the BBB. BBB consists of endothelial cells (ECs), pericytes, astrocytes, and neuronal terminations. Tight Junctions (TJ), the major player of BBB structural integrity, include some major proteins such as claudin, occludin, Junctional adhesion molecules (JAMs), Zonula Occulin (ZO), and E‐cadherin. Exosomes can transport across the BBB with carrier‐mediated transport (CMT) through carriers such as GLUT 1 and LAT1/2. Another transport method is receptor‐mediated transport (RMT) through receptors such as TFR, IR, and RAGE. Also, Adsorptive‐mediated transport (AMT) is a receptor‐independent endocytosis that induces inward membrane curvature through oligomerization.
3.2. Mechanisms of exosome transmission from the BBB
Exosomes enter the host cell through variant manners, the most important of which include: (1) triggering a signaling cascade with conjunction to the G‐coupled receptor of the cell surface such as TFR, IR, and RAGE, and transporting exosomes (Receptor‐Mediated Transport/RMT); (2) fusion with the cell and releasing the cargo to the cytoplasm of the cell to induce the target cell signaling, that is mediated via oligomerization of caveolins and absorbing exosomes (adsorptive‐mediated transport/AMT); and (3) transcytosis with conjunction to the carriers of the recipient cell such as GLUT 1 and LAT1/2, that leads to endocytosis and storage of exosomes in the MVB (carrier mediated transport/CMT) (Figure 3). 69 , 70 , 71 Exosomes must accede to ECs to pass through them and cross the BBB to enter the brain. During this mutual interaction, physical contact (fusion) and transcytosis facilitate the transmission of the exosome or its cargo through the BBB. 72 Exosomes attach to ECs during fusion and release the cargo into the host cell's cytosol. Following cellular entry and transcytosis, depending on the size and density, exosomes degrade or are directed to endosomes and transferred to the abluminal surface of the ECs. 73 Also, modification of the exosome surface facilitates their delivery and cross from the BBB to enter the brain and reach the target location. Hijacking RMT is a common strategy for transporting therapeutic agents and passing over the BBB. 74 This strategy is efficient for exosome delivery by labeling target membrane peptides. T7 peptide, a TfR‐binding peptide, and the HAIYPRH sequence have been used as target peptides for exosome delivery (T7‐Exo). Joining the T7 peptide to Lamp2b and intravenous injection of this conjugation could attack the intracranial glioblastoma compared to unmodified exosomes. 75
In several experimental studies, neurotropic virus‐derived peptides, such as RVG, induce brain targeting of exosomes. In a study, RVG expression at the exosomal membrane promoted its fusion with Lamp2b, an exosomal membrane protein, and improved brain delivery of siRNA‐loaded exosomes. 76 Since the exact mechanism of the BBB crossing pathway of exosomes has not been shown, modified exosomes have promising and efficient delivery of siRNA to brain neural cells in experimental models.
4. EXOSOMES IMPROVE NEUROGENESIS IN THE BRAIN
Brain‐derived exosomes (BDEs) released by all cell types in the CNS contain cargo from the lineages that generate them. In physiological conditions, they are attractive as a vital mediator of cellular communications and waste control between neural cells, glial cells, and the brain's connective tissue. 77 Hippocampal neurons secrete exosomes that participate in cargo transfer into other neurons and facilitate activity‐dependent translation. 78 In the denervated hippocampal niche like fimbria–fornix transection (FFT) condition, the proliferation and neurogenesis of neural stem cells (NSCs) improve in the subgranular zone (SGZ). 79 RNA sequencing analysis from the extracted hippocampal exosomes in the FFT rat model demonstrated that miR‐3559‐3P and miR‐6324 increase after FFT. Transfection of these miRNAs' mimics inhibits NSCs' proliferation but promotes differentiation.
On the other hand, inhibitors of miR‐3559‐3p and miR‐6324 promote NSCs proliferation and inhibit their differentiation to neurons. 80 Considering the critical role of exosomes in neural communications of the brain, recent findings implicate the influential role of exosome therapy in the neurogenic niches of the brain, including the hippocampus, so that it promotes the generation of granule cells from neuroblasts in the dentate gyrus and subsequently improves cognitive function 81 (Figure 4). By administration of exosomes derived from human‐induced pluripotent stem cells (hiPSCs) into the rodents brain, exosomes incorporate into the soma of cells in the DG of the hippocampus, which leads to higher proliferation of neural stem cells (Ki‐67+ cells) and high density of DCX+/ BrdU+ cells in the DG. 82 In the next section, we will discuss the effect of exosome delivery on improving cognitive function and neurorestoration in different neurological disorders.
FIGURE 4.

Importance of exosomes in the improvement of hippocampal neurogenesis. Recent findings implicate the effective role of exosome delivery in the neurogenic niches of the brain, including the hippocampus, so it promotes the generation of granular cells from neuroblasts in the dentate gyrus. Exosomes that may home in arteries, such as the inter‐hippocampal artery, after administering, enter the central nervous system and neurogenic niches that finally pass through the blood–brain barrier and improve cognitive function by enhancing neurogenesis.
5. EXOSOME THERAPY ENHANCES NEURORESTORATION, CONCURRING WITH COGNITIVE FUNCTION IMPROVEMENT IN NEUROLOGICAL DISORDERS
Previous findings have confirmed that stem cells interpose their therapeutic consequence in neurological conditions by paracrine mechanisms. Among the paracrine products of stem cells, exosomes are specially used to treat various neurological diseases by carrying the paracrine factors to the degenerated site and promoting regeneration. The effective therapeutic potential of derived exosomes from different cell lines like neural stem cells, mesenchymal stem cells, and embryonic stem cells have been observed in neurological conditions by promoting cognitive function, neurological recovery, neurogenesis, regeneration, synaptic plasticity, immunomodulation, and tissue repair (Table 1).
TABLE 1.
The role of exosome therapy in various neurological conditions.
| Neurological condition | Origin of exosome | Mechanism of neurorestoration | Effect on cognition | Task | Indexes | References |
|---|---|---|---|---|---|---|
| Alzheimer | HUMSCs in 3D cells culture | ↓ Aβ | ↑ Spatial learning and memory | MWM | ↓ Escape latency | 30 |
| ↑ α‐secretase | ↑ Distance & Time in target Quadrant | |||||
| ↓ β‐secretase | ||||||
| Engineered exosomes containing miR‐29 | ↓ BACE1 | ↑ Spatial memory | Barnes maze | ↓ Escape latency | 74 | |
| ↓ BCL2‐like 11 | ↑ Target seeking & Goal hole exploration | |||||
| Exosomes isolated from hippocampal NSC (NSC‐exo) | ↓ Amyloid beta oligomers (Aβo) | ↑ Recognition | NORT | ↑ Spent time with the novel object | 75 | |
| ↑ Long‐term potentiation (LTP) | ||||||
| Human brain microvascular endothelial cells (HBMVECs) derived exosomes | ↑ P‐gp | ↑ Spatial memory | MWM | ↑ Time in the target quadrant | 76 | |
| ↓ AΒ | ↓ Escape latency | |||||
| Mesenchymal stem cell‐derived exosomes (MSC‐exos) | ↑ Uptake of 18F‐ FDG | ↑ Recognition | NORT | ↑ Frequency of tendency to the central area | 78 | |
| ↓ AΒ | ↑ Recognition | OFT | ↑ Rearing & Traveled distance in the central part | |||
| ↑ Sphingosine kinase/sphingosine‐1‐phosphate signaling pathway | NORT | ↑ Preference index of the NORT | ||||
| ↓ Hyperactivity of hippocampal microglia and astrocytes | ↑ Spatial memory and Recognition | MWM | ↑ Swimming time in the target quadrant | 82 | ||
| ↓ IL‐1β, IL‐6, TNF‐α | NORT | ↑ Discrimination index in the NORT | ||||
| ↑ AΒ1‐42, p‐Tau BDNF | ||||||
| ↑ PSA‐NCAM and DCX in the sub‐ventricular zone (SVZ) | ↑ Spatial memory | MWM | ↑ Time in the target quadrant | 83 | ||
| ↓ Escape latency | ||||||
| Exosomes derived from human umbilical cord mesenchymal stem cells | ↓ AΒ accumulation and Neuroinflammation in Microglial activity | ↑ Spatial memory | MWM | ↑ Time in the target quadrant | 79 | |
| ↓ Escape latency | ||||||
| Exosomes derived from hypoxia‐preconditioned mesenchymal cells | ↓ Synaptic dysfunction | ↑ Spatial memory | MWM | ↑ Distance & Spent time in the target quadrant | 80 | |
| ↑ IL‐4 and IL‐10 | ||||||
| ↓ TNF‐α and IL‐1β STAT3 | ||||||
| ↓ NF‐κB | ||||||
| Exosomes harvested from curcumin‐primed cells (Exo‐cur) | ↑ AKT/GSK‐3β pathway | ↑ Spatial memory | MWM | ↓ Escape latency | 84 | |
| ↓ Phosphorylation of the Tau | ↑ Number of crossing | |||||
| ↑ Target quadrant occupancy indexes | ||||||
| Plasma exosomes loaded with Quercetin (Exo‐Que) | ↓ Cyclin‐dependent kinase 5 (CDK5)‐mediated phosphorylation of Tau | ↑ Spatial memory | MWM | ↓ Escape latency | 85 | |
| ↑ Number of crossings | ||||||
| RVG‐tagged MSC‐Exo | ↓ AΒ levels and plaque deposition | ↑ Spatial memory | MWM | ↓ Escape latency | 86 | |
| ↑ Spent time in the target quadrant | ||||||
| Exosomes derived from the biomimetic silibinin‐loaded macrophage | ↓ AΒ aggregation | ↑ Spatial memory | MWM | ↓ Escape latency | 87 | |
| ↓ Activity of astrocytes | ↑ Spent time in the target quadrant & Crossing time | |||||
| Parkinson's Disease (PD) | EVs derived from human teeth stem cells | ↑ Tyrosine hydroxylase (TH) | ↑ Spatial learning & memory | MWM | ↑ Spent time in the target quadrant | 93 |
| ↓ Escape latency | ||||||
| Microglia‐derived exosomes containing α‐synuclein | ↑ Protein aggregation | No significant effect on spontaneous alternation, as an index of spatial memory, of injured animals in the Y‐Maze | Y‐maze | Spontaneous alternation | 94 | |
| Multiple Sclerosis | Intravenous administration EVs | ↓ Brain atrophy | ↑ Passive avoidance memory | Passive avoidance | ↓ Latency to enter the dark compartment | 99 |
| ↑ Neural stem cells proliferation in the SVZ | ||||||
| ↓ Inflammatory cytokines | ||||||
| MSCs harvested exosomes | ↑ Numbers of newly generated neurons | ↑ Social Recognition | Social behavior test | ↑ Spent time in the column that stranger | 100 | |
| ↓ TLR2/IRAK1/NFκB pathway | ||||||
| Stroke | Exosomes derived from CCR2 receptor‐overexpressing HUC‐MSCs | ↑ Bind to CCL2 | ↑ Spatial memory | MWM | ↓ Escape latency | 102 |
| ↑ Remyelination & Oligodendrogenesis | ↑ Spent time in the target quadrant | |||||
| ↑ Macrophage polarization | ||||||
| NSC EV | ↑ M2 cells | ↑ Non‐episodic memory | Tail suspension | ↓ Immobile state | 103, 104 | |
| ↓ Th 17 | NORT | ↑ Spent time with the novel object | ||||
| Transmission of exosomal microRNA‐124 from neurons to microglia | ↑ CX3CL1/CX3CR1 pathway | ↑ Spatial memory | MWM | ↓ Escape latency | 105 | |
| Exosomes derived from human umbilical cord blood derived CD133+ cells (CD133+Exo) | ↑ Synaptogenesis | ↑ Spatial memory and Recognition | NORT | ↑ Discrimination index | 106 | |
| ↑ White matter remodeling | MWM | ↓ Escape latency | ||||
| Serum exosomes | ↑ The ratio of Bcl‐2 / Bax | ↑ Recognition | OFT | ↓ Traveled distance | 107 | |
| ↓ Apoptotic cells | ↓ Spent time in the central part of the OF | |||||
| ↓ Cleaved caspase‐3 | ||||||
| Serum exosomes of young rats | ↑ CD46, as a C3b/C4b inactivator | ↑ Spatial learning and Long‐term memory | MWM | ↓ Latency | 108 | |
| ↓ Iba1 | ↑ Traveled distance to find the platform | |||||
| Extracellular vesicles from adipose‐derived stem cells (ADSC‐EVs) | ↑ Polarization of microglia type2 (M2) | ↑ Spatial working memory | T‐Maze Step through test | ↑ The rate of alternation | 111 | |
| ↑ Repair and Proliferation of endothelial cells | ↑ Time spent in the dark zone & number of dark zone entrance | |||||
| BMSC‐Exosomes | ↓ NLRP3 inflammasome & pyroptosis relevant proteins in neurons | ↑ Spatial memory | MWM | ↓ Escape latency | 112 | |
| ↑ Spent time in the target quadrant | ||||||
| EVs derived from adipose tissue stem cells (hAT‐MSC) | ↑ PTEN/Akt pathway | ↑ Spatial memory | MWM | ↓ Latency | 14 | |
| ↑ Angiogenesis | ↑ Working memory | NORT | ↑ Time on the novel object | 114 | ||
| ↑ Regulating protein transduction | ↑ Short & Long‐term memory | Y‐Maze | ↑ Spontaneous alternation | |||
| ↑ Anxiety‐like Behaviors | EPM | ↑ Spent time in the open arm | ||||
| OFT | ||||||
| Zeb2/Axin2‐supplemented exosomes | ↑ Neurogenesis | ↑ Spatial memory | MWM | ↓ latency to find the platform | 31 | |
| Encephalopathy | Human amniotic fluid‐derived exosomes (hAFEXOs) | ↑ HIF‐1α | ↑ Spatial memory | MWM | ↓ Escape latency | 121 |
| ↑ VEGF | ↑ Spent time in the target quadrant | |||||
| Epilepsy | MSCs derived exosomes | Nrf2‐NF‐KB signaling pathway | ↑ Spatial memory | MWM | ↓ Escape latency | 125 |
| ↓ Activity of A1 astrocytes | ↑ Spent time in the target quadrant | |||||
| MSC‐EVs are enriched in antioxidant miRNAs | ↑ Nrf2 system & Renovation of hippocampal neurons | ↑ Spatial memory | MWM | ↓ Escape latency | 126 | |
| ↑ Antioxidant activity | ↑ Spent time in the target quadrant | |||||
| Intranasal administration of A1‐exosomes derived from human bone marrow MSCs | ↓ Neuron loss & Inflammation | ↑ Recognition | OLT | ↑ Exploring the novel place object | 23 | |
| ↑ Neurogenesis | NORT | ↑ Affinity for novel object | ||||
| IL‐1‐Exo that isolated from IL‐1‐treated MSCs | ↓ Astrogliosis & inflammatory reaction of astrocytes in the lipopolysaccharide (LPS) | ↑ Spatial memory | MWM | ↑ Spent time in the target quadrant | 128 | |
| ↓ Escape latency | ||||||
| Traumatic brain injury (TBI) | Exosomes derived from multi pluripotent MSCs | ↓ Inflammation | ↑ Spatial memory | MWM | ↓ Escape latency | 132 |
| ↑ Neurogenesis & Endogenous angiogenesis | ↑ Spent time in the target quadrant | |||||
| Human umbilical cord mesenchymal stem cells (HUCMSCs) derived exosomes | ↓ Inflammatory cytokine production | ↑ Spatial memory | MWM | ↓ Escape latency | 133 | |
| ↓ NF‐κB signaling pathway | ↑ Spent time in the target quadrant | |||||
| ↓ Neuronal apoptosis | ||||||
| ↑ Cortical neural regeneration | ||||||
| Exosomes derived from MSCs cultured under 2D conventional and 3D collagen scaffolds conditions | ↓ Neuroinflammation | ↑ Spatial memory | MWM | ↓ Escape latency | 134 | |
| ↑ Generation of newborn endothelial cells & newborn mature neurons | ↑ Spent time in the target quadrant | |||||
| miR‐17‐92 cluster‐enriched exosomes derived from MSCs | ↑ Angiogenesis & Neurogenesis | ↑ Spatial learning and memory | MWM | ↑ Spent time in the target quadrant | 109 | |
| ↓ Neuroinflammation | ↑ Cross time | |||||
| Human MSC derived exosomes (MSCexo) | ↑ Hippocampal neural cell loss & Neurogenesis & Angiogenesis | ↑ Spatial memory | MWM | ↓ Escape latency | 110 | |
| ↓ Inflammation | ↑ Spent time in the target quadrant | |||||
| Microglial exosomes | ↑ MiR‐124‐3p Rela/ApoE signaling Pathway | ↑ Recognitive | NORT | ↑ Spent time with the novel object | 113 | |
| ↑ Spatial memory | MWM | ↑ Spent time in the target quadrant | ||||
| Exosome that derived from cortical astrocytes and transfected with shRNA, Bcl‐2, Bax | ↓ Hippocampal apoptosis | ↑ Spatial memory | MWM | ↑ Spent time in the target quadrant | 14 | |
| ↑ Hippocampal EPSP amplitude | ||||||
| Diabetes‐induced cognitive impairment | Intracranial injection of MSCs derived exosomes | ↓ Synaptic loss & the degeneration of hippocampal neurons | ↑ Spatial learning & memory | MWM | ↑ Spent more time in the target quadrant | 29, 139 |
| Enriched environment enhances exosomal miR‐146a secretion from bone BM‐MSCs | ↓ Expression of IRAK1, NF‐κB | ↑ Spatial learning & memory | MWM | ↑ Spent time in the target quadrant | 140 | |
| ↓ Tumor necrosis factor‐α | ↓ Escape latency | |||||
| Loading miR‐146a in to the BECDEs and implementation of this content into the ventricle of diabetic mice | ↓ PrPc levels | ↑ Short‐term memory | NORT | ↑ Discriminatory index | 142 | |
| Y‐Maze | ↑ Spontaneous alternation | |||||
| BECDEs | ↓ Evolving neurovascular dysfunction | ↑ Recognition | Odor recognition tests | ↑ Discriminatory index | 129 | |
| ↑ Spatial memory | MWM | ↑ Spent time in the target quadrant |
5.1. Alzheimer's disease (AD)
The function of exosomes and their content, including miRNAs and mRNAs, alters in AD. 83 As a neurological condition, AD downregulates the expression level of some mRNAs, like Chi3l1, since it up‐regulates the expression level of some mRNAs, like Rhog. 84 The misexpression of amyloid precursor protein (APP) is crucial in driving neuropathological cascades, leading to AD, and isolated exosomes from an animal's brain with AD promote APP's overexpression in neuronal N2a cells. Besides, harvested exosomes from N2a cells with abnormal APP expression dysregulate APP expression in host normal N2a cells, mediated by the low expression of exosomal miR‐185‐5p. 85 Generally, exosomal miRNAs are effective biomarkers for predicting AD a few years before the onset of cognitive malfunctions. 84 As exosomes and their cargo help to diagnose this disease, in recent years, exosome therapy to treat AD and improve the cognitive function of sufferers has been considered by researchers. 86 3D cell culture‐derived exosomes from human umbilical cord mesenchymal stem cells (hUMSCs) up‐regulate the expression of α‐secretase and down‐regulate the β‐secretase and diminish the production of Aβ in both pathological cell lines and transgenic mice model of AD through their special cargo. 25 Injection of engineered exosomes containing miR‐29 to the CA1 region of the AD rats downregulates the expression of BACE1 (β‐site amyloid precursor protein cleaving enzyme 1) and BIM [Bcl − 2 interacting mediator of cell death (BCL2‐like 11)], and show a significant premiere exploration for the goal sector (GS) of the Barnes maze. 87
Exosomes isolated from hippocampal neural stem cells (NSC‐Exo) protect the hippocampus from amyloid beta oligomers (Aβο)‐induced suppression of long‐term potentiation (LTP) and improve the cognitive function of animals, confirmed by Novel Object Recognition (NORT) test. In this study, impaired animals subjected to Aβο and treated with NSC‐Exo significantly spent more time exploring the novel object through the choice phase of the NORT. 88 Human brain microvascular endothelial cells (HBMVECs)‐derived exosomes, containing P‐glycoprotein (P‐GP), enhance the clearance of Aβ by distinguishing capture between Aβ and P‐GP, and considering the results of Morris Water Maze (MWM) task, improve the spatial memory of animals given escape latency and spent time in the target quadrant of the maze (Figure 5). 89 Although mesenchymal stem cell‐derived exosomes (MSC‐exos) are an emerging and admirable therapeutic approach for AD. 90 MSC‐exosomes enhance the uptake of [18F]FDG in the brain and improve the function of AD animals in the NORT test, like NSC‐Exo. 91 Exosomes derived from human umbilical cord mesenchymal stem cells (hucMSC‐exosomes) ameliorate Aβ accumulation and neuroinflammation in AD animals by modulating microglial activity and also considering the Morris Water Maze (MWM) task; these exosomes improve the function of animals in the escape latency and spent time in the target quadrant of the maze. 92 Exosomes derived from hypoxia‐preconditioned MSCs have a similar outcome on the function of APP/PS1 mice in the MWM by alleviating synaptic dysfunction, up‐regulating anti‐inflammatory cytokines (IL‐4 and ‐10), down‐regulating proinflammatory cytokines (TNF‐α and IL‐1β), and reducing the activity of STAT3 and NF‐κB in the brain. 93 Bone marrow mesenchymal stem cells‐derived exosomes (BMSC‐Exo) improve cognitive function via decreasing Aβ accumulation and animating the sphingosine kinase/sphingosine‐1‐phosphate pathway. 94 Administration of these exosomes to the lateral ventricle in the streptozotocin (STZ) model of AD inhibits the hyperactivity of hippocampal microglia and astrocytes, downregulates the expression level of IL‐1β, IL‐6, TNF‐α, Aβ1‐42, p‐Tau and up‐regulate the expression of BDNF as an essential synapse‐related protein. This neural restoration outcome positively correlates with increased frequency of tendency to the central area of the open field test (OFT), increased rearing, and traveled distance in the central part of the OFT and the preference index of the NORT. 95 In a study by Edvin and colleagues, they investigated the effect of MSC‐Exo on the cognitive recovery and neurogenesis in the rodent model of AD, which was established by bilateral injection of beta‐amyloid 1–42 aggregates into the DG of the hippocampus. MSC‐Exo therapy improved the expression of PSA‐NCAM and DCX in AD animals' sub‐ventricular zone (SVZ). Also, they had more swimming time in the target quadrant of MWM and a better discrimination index in the NORT. 96 Exosomes harvested from curcumin‐primed cells (Exo‐cur) prevent neural death through in vitro and in vivo conditions and activate the AKT/GSK‐3β pathway that ameliorates the cognitive dysfunction of animals by inhibiting phosphorylation of the Tau. In the probe trial of MWM, after removing the platform, Exo‐cur treated animals had higher target quadrant occupancy and increased numbers of crossing the platform place compared to AD animals. 97 Also, plasma exosomes loaded with Quercetin (Exo‐Que) relieve the cognitive symptoms of okadaic acid (OA)‐induced AD by inhibiting cyclin‐dependent kinase 5 (CDK5)‐mediated phosphorylation of Tau. AD animals that were treated with Exo‐Que had improvement in the escape latency, the number of crossings, and target quadrant occupancy indexes of MWM, 98 as well as, RVG‐tagged MSC‐Exo improves the targeting of exosomes to the hippocampus and cerebral cortex after intravenous administration, therefore sharply decrease Aβ levels and plaque deposition parallel with a decrease in the escape latency time, and increase of platform location crossing in the MWM. 99 Exosomes derived from the biomimetic silibinin‐loaded macrophage have the same effect on the function of an animal in the MWM through inhibition of Aβ aggregation and activity of astrocytes. 100
FIGURE 5.
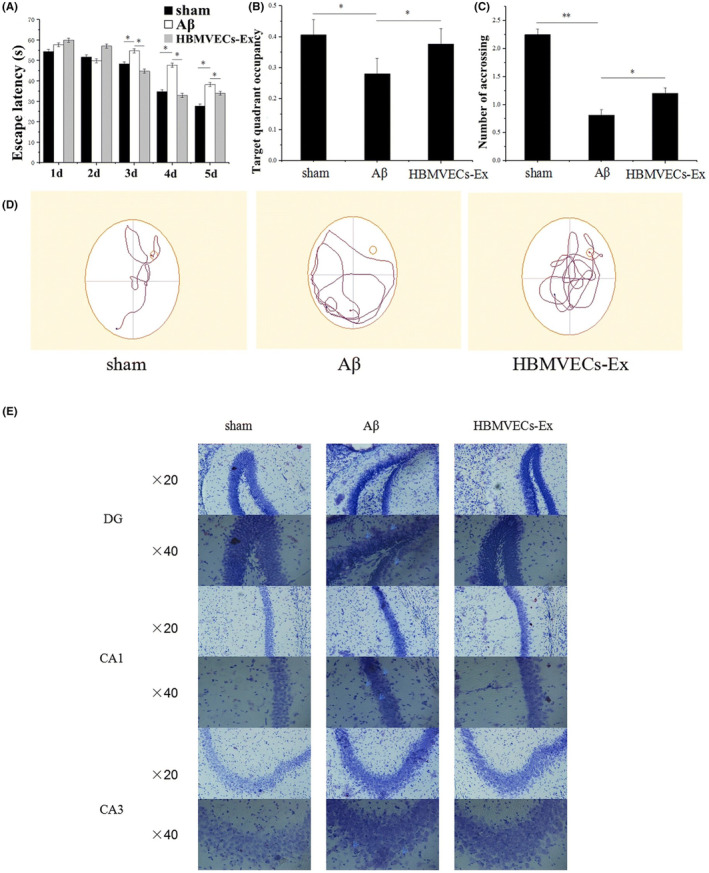
Brain microvascular endothelial cell‐derived exosomes (HBMVECs‐Ex) potently improve cognitive function by enhancing the clearance of Aβ in mouse model of AD. (A‐D) Decrease of escape latency, increase of target quadrant occupancy, and increase of platform crossing number in the MWM after treating animals with HBMVECs‐Exs. (E) Representative Nissl staining of different hippocampal regions. Reproduced with permission.
5.2. Parkinson's disease (PD)
Exosomes' content alters after PD and is a promising potential target for biomarker development in PD. 101 A positive correlation exists between the exosomal content like plasma L1CAM, exosomal Linc‐POU3F3 levels, and nonmotor symptoms of PD patients like mood and attention/memory. 102 In PD, the plasma exosomal prion concentration negatively correlates with the cognitive function level assessed with the Montreal Cognitive Assessment (MoCA). 103 Meanwhile, exosomes serve as a delivery vehicle via loading small interfering RNAs or proteins, allowing site‐specific targeting through the PD treatment. 104
Upon transport of lung‐derived exosomes, the localized transcription factors of exosomes regulate the gene expression at the substantia nigra, and superior frontal gyrus regions of PD. 105 EVs derived from the human teeth stem cells successfully normalize the expression of tyrosine hydroxylase (TH) in the substantia nigra (SN) and striatum of 6‐OHDA‐induced PD in rats and reverse the impairment of spatial learning/memory performance, such that the escape latency to find the platform in MWM decrease, and spent time in the target quadrant increase after the treatment. 106 Microglia as a primary phagocyte of the nervous system, and their released exosomes can influence α‐synuclein pathology, but injecting microglia‐derived exosomes containing α‐synuclein, despite inducing protein aggregation in the recipient neurons, had no significant effect on spontaneous alternation, as an index of spatial memory, of injured animals in the Y‐Maze. 107
5.3. Multiple Sclerosis
Multiple sclerosis (MS) is a major demyelinating disease that exhibits demyelination in the CNS concomitantly with neurological deficits and cognitive impairments. 108 Common treatments for MS consist of immunosuppressors and preventives of brain immune infiltrations. 109 However, these therapeutic approaches do little to enhance myelin renovation besides harmful side effects. Instead, as naturally occurring small vesicles, exosome therapy promotes remyelination and neurorestoration in MS by delivering mRNA and other exosomal cargo. 110
Serum‐derived exosomes that contain mir‐219 and are generated by young rats significantly reduce oxidative stress and increase the hippocampus's oligodendrocyte precursor cell levels and myelin content in the lysolecithin‐induced in vitro MS Model. 111 Intravenous administration of EVs minimizes brain atrophy, increases neural stem cell proliferation in the SVZ, and decreases inflammatory cytokines levels in the serum of mice infected with Theiler's murine encephalomyelitis virus (TMEV), a progressive model of MS. Also, EVs attenuate motor deficits and improve passive avoidance memory (learning to avoid a noninvasive foot shock) of infected animals by encouraging remyelination and diminishing brain atrophy. 112 In Experimental autoimmune encephalomyelitis (EAE) and cuprizone (CPZ) diet models of MS, MSCs harvested exosomes cross the BBB and significantly increase the numbers of newly generated neurons, inhibit the TLR2/IRAK1/NFκB pathway, and improve the function of animals in the social behavior test, such that after the MSC‐Exo treatment, animals of CPZ model spent more time in the column that stranger (unfamiliar) animal is in there. 113
5.4. Stroke
Exosomes from different cell lines reduce inflammation, enhance neurogenesis, and improve cognitive function after stroke. 14 In a study by Yang and colleagues, exosomes derived from CCR2 receptor‐overexpressing HUC‐MSCs, significantly bind to CCL2 and have a dramatically beneficial effect on the remyelination, oligodendrogenesis, macrophage polarization, and cognitive function of post‐stroke cognitive impairment (PSCI) model (Figure 6). 114 Neural stem cell extracellular vesicles (NSC EV) treatment in a rodent model of MCAO positively improves motor function and strength through beam walk and hanging wire tests and increases the spent time with the novel object in NORT, indicating the improvement of the episodic memory formation. 115 Furthermore, NSC EVs improve non‐spatial memory in the murine thromboembolic stroke model evaluated by NORT, where the discrimination index showed that MCAO animals have a significant cognitive deficiency. Still, NSC EV‐treated animals had significantly better cognitive function than the injured group and, as a result, spent more time with the novel object compared to the familiar object in the NORT. 115 One of the reasons for these improvements may be due to the CX3CL1/CX3CR1 pathway, which triggers a cascade and attenuates early brain injury (EBI) after subarachnoid hemorrhage (SAH) via promoting the transmission of exosomal microRNA‐124 from neurons to microglia, which in turn improves the escape latency of animals during the MWM as a spatial memory test. 116
FIGURE 6.

Beneficial effects of exosome therapy on the neurorestoration and cognitive function after the stroke. (A) Exosomes derived from CCR2 receptor‐overexpressing HUC‐MSCs improve the intensity of myelin binding protein (MBP), indicating the integrity of myelin, and also increase the number of BrdU+/NG2+ cells, indicating the oligodendrocyte proliferation around the ischemic area of animals with middle cerebral artery occlusion (MCAO). (B) These Exosomes have increased mean escape latency and the spent time in the target quadrant of MWM, in animals with MCAO. Treated animals had better motor function than untreated animals based on mNSS values. Reproduced with permission.
There is a significant correlation between measures of cognitive tests, such as discrimination index in novel odor recognition test or escape latency in MWM with left ventricular ejection fraction, in type 2 diabetes mellitus (T2DM) stroke rodents that are treated with derived exosomes from human umbilical cord blood CD133+ cells (CD133 + Exo). 117 Treatment with healthy serum exosomes protects the BBB by reversing autophagy‐induced reduction of TJs and remarkably decreases apoptotic cells in the striatum, increases the ratio of Bcl‐2 to Bax, and inhibits cleaved caspase‐3, which coincides with lower traveled distance and spent time in the central part of the OF. 118 Also, serum exosomes of young rats are rich in CD46, as a C3b/C4b inactivator, and systemic administration of these exosomes from young rats into aged ischemic ones decreases the expression of Iba1 and improves spatial learning and long‐term memory, so in treated animals, the latency and traveled distance to find the MWM platform reduce significantly. 119 In recent years, MSCs‐based secretomes have been potentially appealing approaches in treating ischemic stroke. 120 , 121 Extracellular vesicles from adipose‐derived stem cells (ADSC‐EVs) promote the polarization of microglia type2 (M2), resulting in increased repair and proliferation of endothelial cells in the peri‐ischemia of transient middle cerebral artery occlusion (tMCAO) animals. Also, ADSC‐EVs treatment after cerebral ischemia increases the alternation rate in the T‐Maze, the time spent in the dark zone of the step‐through test, and the number of dark zone entrances. 122
BMSC‐Exosomes improve animals' spatial memory after the MCAO, followed by reperfusion, by downregulating the expression of the NLRP3 inflammasome and pyroptosis‐relevant proteins in neurons. 123 After induction of subarachnoid hemorrhage in rats, BMSC‐exosomes alleviate early brain injury (EBI) through miRNA129‐5p‐HMGB1 Pathway, 124 and transfer of miR‐21‐5p enriched exosomes from MSCs, besides significant amelioration of EBI, alleviate neural apoptosis, and improve spatial memory of injured animals via the PTEN/Akt pathway. 125 EVs derived from adipose tissue stem cells (hAT‐MSC) of the healthy individual, referred to as liposuction, reverse the destructive effect of cerebral stroke on motor function, working memory, short‐term memory, long‐term memory, and anxiety‐like behaviors. EVs‐delivered animals spent more time with the novel object in the NORT, had more spontaneous alternation in the Y‐Maze, and spent more time in the open arm of the EPM. 126 Furthermore, Zeb2/Axin2‐supplemented exosomes, harvested from BMSCs transfected plasmid, improve the endogenous neurogenesis and neurobehavioral recovery of ischemic animals, especially in spatial memory manner with decreasing the latency to find the platform in the probe trial of MWM test. 26
5.5. Encephalopathy
Exosomes can play a role in treating encephalopathies and their diagnosis as a biomarker. 23 , 127 Chronic traumatic encephalopathy (CTE), a neurological condition with prior exposure to repetitive head impacts, is associated with tauopathy and higher exosomal Tau in serum and correlates with worse performance in psychomotor and memory tests. 128 After the occurrence of CTE, inhibition of brain‐derived exosomes release, enhanced glucose uptake, altered cytokine production trends, and significantly reversed cognitive impairment, such that treated mice spent less time finding the platform during the MWM and spent more time with the novel object in the NORT. 129 Also, in the Gut–microbiota–brain axis that relates the intestinal microbiota and sepsis‐associated encephalopathy (SAE), intestinal epithelial cell (IEC)‐derived exosomes induce M1 polarization and secretion of pro‐inflammation factors like IL‐1β M1 that impairs the cognitive function of rodents; however, GW4869 inhibits the secretion of exosomes and decrease the distance and latency time to find the platform in MWM test. 130 Hypoxic encephalopathy triggers a kind of CNS dysfunction with high mortality and morbidity in neonates and even life‐lasting paralysis. 131 Brain‐derived extracellular vesicles (BEVs) ameliorate the neurotoxicity in oxygen–glucose deprivation (OGD) brain slices based on a dose‐time dependent procedure. 132 Human amniotic fluid‐derived exosomes significantly augment the expression level of hypoxia‐inducible factor 1 α (HIF‐1α) and vascular endothelial growth factor (VEGF) that coincide with a decrease in the escape latency time and increase in the target quadrant occupancy of MWM test. 133
5.6. Epilepsy
Epilepsy, a neurological disorder, leads to an abnormal electrical discharge of neurons. Emerging findings have confirmed that exosomes could be released following epilepsy and serve as a biomarker for diagnosis. 134 , 135 Since inflammatory pathways and astrocyte malfunction are involved in the evolution of epilepsy, suppressing inflammatory pathways and astrocytes is a promising strategy in epilepsy treatment. 136 It has been shown that MSCs‐derived exosomes attenuate the activity of A1 astrocytes in the animal model of temporal lobe epilepsy (TLE) by regulating the Nrf2‐NF‐KB signaling pathway. 137 MSC‐EVs are enriched in antioxidant miRNAs and, following seizure damage, improve the spatial memory of animals through the MWM test via renovation of hippocampal neurons and remarkable antioxidant activity associated with the Nrf2 defense system. 138 Intranasal administration of NSC‐EVs after seizure induction reduces the expression of inflammatory cytokines, including IFN‐γ, TNF‐⍺ and IL‐1β. 82 Also, intranasal administration of A1‐exosomes derived from human bone marrow MSCs, incorporated into the hippocampal neurons and confine the progress of status epilepticus SE‐induced dysfunction into chronic hippocampal impairment, such that treated animals represent a greater tendency for exploring the novel place object (NPO) in object location test (OLT), and more affinity for novel object area (NOA) in NORT. 139 IL‐1‐Exo isolated from IL‐1‐treated MSCs significantly inhibits the astrogliosis and inflammatory reaction of astrocytes in the lipopolysaccharide (LPS)‐induced model of SE, and simultaneously decreases the escape latency time, increases the spent time in the target quadrant, and the number of platform crossings in the MWM. 140
5.7. Traumatic brain injury (TBI)
TBI is closely associated with neuroinflammation, neuropathological protein accumulation, and ectopic release and spread of brain‐derived exosomes (BDE). After TBI, the rate of phosphorylated Tau elevates remarkably in exosomes. TBI‐isolated exosomes enforce toxicity in neural cultures, aggravate LTP impairment, and exacerbate cognitive and motor deficits after TBI. 141 Current studies have confirmed that cell‐based therapies in experimental and clinical research can substantially improve motor and cognitive recovery by reinforcing neurogenesis and neurite growth following TBI. Moreover, many studies have shown that exosomal miRNAs are leading therapeutic candidates for improving neurorestoration after the TBI. 142 Inhibition of BDE release after repetitive mild traumatic brain injury (TBI) increases glucose uptake, decreases neuropathological protein accumulation, and reverses cognitive deficiency in injured mice. GW4869 (a nMase inhibitor), as an inhibitor of BDE, changes the production outline of cytokine and enhances microglial proliferation, parallel with a significant decrease in escape latency (MWM) and a significant increase in the spent time with the novel object in the NORT. 129 Exosomes derived from multi‐pluripotent MSCs, improve sensory‐motor and cognitive recovery in rats after TBI by reducing inflammation and promoting neurogenesis or endogenous angiogenesis. 143 In a study by Zhang and colleagues, human umbilical cord mesenchymal stem cells (HUCMSCs) derived exosomes significantly decreased inflammatory cytokine production by repressing the NF‐κB signaling pathway, inhibited neuronal apoptosis, promoted cortical neural regeneration, and improved cognitive function of animals after the induction of TBI in rats. 144
After the TBI, systemic administration of exosomes derived from MSCs cultured under 2D conventional and 3D collagen scaffold conditions reduces neuroinflammation, increases newborn endothelial cells' generation in the boundary zone, and remarkably increases newborn mature neurons in the hippocampus. These changes led to the improvement of animal spatial memory performance in the modified MWM, such that with each training trial, the platform was randomly relocated within the target quadrant to act as a probe trial. 145 Also, miR‐17‐92 cluster‐enriched exosomes derived from MSCs improve motor function based on the modified neurological severity scores (mNSS) test, and especially the spatial learning and memory based on the MWM test, so that exosome‐delivered animals spent more time in the target quadrant and passed the platform of the maze more times. 146
Human MSC‐derived exosomes (MSCexo) significantly decrease hippocampal neural cell loss, enhance neurogenesis and angiogenesis, ameliorate inflammation, and improve spatial memory after the TBI in a dose–response and window–response manner, measured by MWM. In this study, treated animals spent more time in the target quadrant and had less latency in finding the platform. 147 Furthermore, MSCexo enhances long‐term memory processing and recall, spatial memory, prioritization, and identification of color in the modified operant conditioning model. 148 Intra‐nasal delivery of exosomes derived from adipose‐derived stem cells (hASCexo) decreases cortical damage after the TBI and improves animals' cognitive function in the reversal trial of 8 arms radial arm water maze. 149
Like MSCs‐derived exosomes and GW4869, microglial exosomes with up‐regulated miR‐124‐3p improve the cognitive function of rmTBI animals in the NORT and MWM indicators via Rela/ApoE signaling pathway that promotes the breaking of β‐amyloid proteolytic and thereby inhibit β‐amyloid malformations. 150 Exosomes that derived from cortical astrocytes and transfected with plasmids expressing short hairpin RNA (shRNA), B‐cell lymphoma‐2 (Bcl‐2), and Bcl‐2‐associated X‐protein (Bax), significantly attenuated hippocampal apoptosis, increased hippocampal EPSP amplitude and increased spent time in the target quadrant of MWM. 151
5.8. Other diseases
5.8.1. Psychiatric disorders induced cognitive impairment
Exosome applications are not restricted to neurological conditions; in recent years, they have also been used in experimental research to treat psychiatric disorders, which are accompanied by cognitive deficits. 152 , 153 , 154 It has been shown that exosomes isolated from human umbilical cord MSC considerably enhance neural stem cell differentiation, improve rodent recognition memory, and mitigate stress‐related symptoms generated by intrahippocampal injection of streptozotocin (STZ). 155
Guoa et al. found that exosome therapy enhanced the sucrose preference index and increased activity levels in depressed animals. Exosome treatment increased hippocampus neurogenesis and raised the expression of miR‐26a and superoxide dismutase (SOD) while simultaneously decreasing the expression of TNF‐α and IL‐1β. 156 Intranasal delivery of MSC‐derived EVs in the phencyclidine (PCP) model of schizophrenia leads to amelioration of schizophrenia‐like behaviors, including improved social interaction and sensorimotor gating as measured by the three chambers social interaction test and the paradigm of prepulse inhibition of the acoustic startle response. This is achieved by significantly increasing the number of GABA‐producing neurons in the prefrontal cortex, a severely affected area in schizophrenia. 157
5.8.2. Diabetes‐induced cognitive impairment
Cognitive impairment due to diabetes is a global problem. Many studies have confirmed that cognitive dysfunctions are much more prevalent in diabetic patients than in non‐diabetic people. 158 Understanding the exosomal cargo can help to diagnose and provide practical treatments for diabetic patients. 159 Intracranial injection of MSCs‐derived exosomes reverses diabetes‐induced cognitive disorder. 160 These exosomes improve synaptic loss, and the degeneration of hippocampal neurons in STZ‐diabetic mice, and exosome‐delivered animals spent more time in the target quadrant of the MWM test. 24 Also, an enriched environment enhances exosomal miR‐146a secretion from bone BM‐MSCs, decreases scape latency, and increases the time spent in the target quadrant of MWM after diabetes‐induced cognitive impairment in rats. 161 In type 2 diabetes mellitus (T2DM) mice, brain endothelial cell‐derived exosomes (BECDEs) increase miR126 expression in the brain and serum and significantly improve their cognitive function. 162 Loading miR‐146a into the BECDEs and implementing this content into the ventricle of diabetic mice decrease PrPc levels and restore short‐term memory confirmed by NORT and Y‐Maze so that treated animals significantly have a better discriminatory index and spontaneous alternation. 163 Besides, reduced evolving neurovascular dysfunction and MRI analysis exhibited that BECDEs treatment in type 2 diabetes mellitus (T2DM) animals show significant elevation of relaxation time constant T2 and cerebral blood flow (CBF) in the brain white matter and amplification of relaxation time constant T1 and reduction of BBB permeability in gray matter. BECDEs significantly increased T1 and CBF in the hippocampus of T2DM animals. Besides, CEC‐Exo reduced diabetes‐induced cognitive deficits, and treated animals spent more time in the target quadrant of MWM and recognized different odors in the odor recognition tests. 164
5.8.3. High fat diet (HFD) induced cognitive impairment
HFD, correlated with cognitive impairments and neurological deficits, increases the risk of neurological disorders like Alzheimer's disease later in life. 165 , 166 HFD inhibits CREB phosphorylation in the hippocampus and downregulates the expression of CREB target genes (Bdnf, nNOS, Sirt1, Egr3, and RelA genes). Still, intranasal administration of NSC‐derived exosomes epigenetically restores the transcription of these genes by the recruitment of CREB and improves recognition and spatial memory of animals analyzed with NORT and object place recognition (OPR) test. 167
5.8.4. Hypothermic circulatory arrest induced cognitive impairment
The neurologic deficit remains a significant complication after cardiovascular surgeries with deep hypothermic circulatory arrest (DHCA). 168 Exosomes derived from gene‐modified MSCs, protect the brain against prolonged DHCA through overexpression of microRNA‐214 (miR‐214) and enhance spatial memory of injured animals, so as the latency time of MWM decreases and platform location crossing increases in treated animals. 169
6. CONCLUSION
Exosomes are vital in cells' physiological functions and interactions. The use of exosome therapy in neurological diseases such as PD, AD, TBI, MS, stroke, epilepsy, encephalopathies, and other neurological conditions which affect cognitive function is developing (Figure 7). Implementing exosomes from multiple cell lines and sources has been shown to evoke neurorestorative effects in neurological models that accompany cognitive function recovery, too. Generally, compared to cell‐based therapy, exosome therapy has the advantage of low immunogenicity and simple cross from the BBB, enabling them to be a potent drug delivery vehicle that improves neurorestoration and cognitive function in neurological conditions. However, using these worthy cellular secretomes in humans remains challenging because of their safety and efficient application in the clinic, so many concerns still need to be solved, such as exosome optimal dose, timing, and other challenging problems.
FIGURE 7.

An overview of the beneficial effects of exosome therapy in neurological diseases. Exosomes have the advantage of low immunogenicity and simple cross from the BBB, enabling them to be a potent drug delivery vehicle that improves neurorestoration and cognitive function in neurological conditions by improving neurogenesis and decreasing neuroinflammation.
AUTHOR CONTRIBUTIONS
FGH, GM, HSZ, and SF designed the project and wrote the manuscript. SF and HS performed the animal modeling and experimental procedures. FF and RRGH helped with the data collection. SF and HS analyzed the data. FGH and GM provided funding acquisition. All authors have read and approved the final manuscript.
FUNDING INFORMATION
This study was supported by a grant (67159) from Drug Applied Research Center, Tabriz University of Medical Sciences (Tabriz, Iran).
CONFLICT OF INTEREST STATEMENT
The authors declared no Conflict of interest.
ACKNOWLEDGMENTS
This article is in line with the Ph.D. thesis of solmaz Fallahi, “The effect of bone marrow mesenchymal stem cell exosomes on cognitive function and hippocampal neurogenesis of methamphetamine‐treated male mice.” (IR.TBZMED.VCR.REC.1400.227).
Fallahi S, Zangbar HS, Farajdokht F, Rahbarghazi R, Mohaddes G, Ghiasi F. Exosomes as a therapeutic tool to promote neurorestoration and cognitive function in neurological conditions: Achieve two ends with a single effort. CNS Neurosci Ther. 2024;30:e14752. doi: 10.1111/cns.14752
Contributor Information
Gisou Mohaddes, Email: gmohaddes@chsu.edu.
Fariba Ghiasi, Email: ghiasir@tbzmed.ac.ir.
DATA AVAILABILITY STATEMENT
All data generated or analyzed in this study are available from the corresponding author on reasonable request.
REFERENCES
- 1. Liang Y, Duan L, Lu J, Xia J. Engineering exosomes for targeted drug delivery. Theranostics. 2021;11(7):3183‐3195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Logozzi M, Di Raimo R, Mizzoni D, Fais S. What we know on the potential use of exosomes for nanodelivery. Semin Cancer Biol. 2021;86:13‐25. [DOI] [PubMed] [Google Scholar]
- 3. Tian Y, Fu C, Wn Y, Lu Y, Liu X, Zhang Y. Central nervous system cell‐derived exosomes in neurodegenerative diseases. Oxidative Med Cell Longev. 2021;2021:9965564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Xia X, Wang Y, Qin Y, Zhao S, Zheng JC. Exosome: A novel neurotransmission modulator or non‐canonical neurotransmitter? Ageing Res Rev. 2022;74:101558. [DOI] [PubMed] [Google Scholar]
- 5. Fan Y, Chen Z, Zhang M. Role of exosomes in the pathogenesis, diagnosis, and treatment of central nervous system diseases. J Transl Med. 2022;20(1):291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Elliott RO, He M. Unlocking the power of exosomes for crossing biological barriers in drug delivery. Pharmaceutics. 2021;13(1):122. doi: 10.3390/pharmaceutics13010122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Clayton A, Harris C L, Court J, Mason MD, Morgan B P. Antigen‐presenting cell exosomes are protected from complement‐mediated lysis by expression of CD55 and CD59. Eur J Immunol. 2003;33(2):522‐531. [DOI] [PubMed] [Google Scholar]
- 8. Hussain MW, Jahangirc S, Ghosh B, et al. Exosomes for regulation of immune responses and immunotherapy. Journal of Nanotheranostics. 2022;3(1):55‐85. [Google Scholar]
- 9. Qambrani A, Rehman FU, Tanziela T, et al. Biocompatible exosomes nanodrug cargo for cancer cell bioimaging and drug delivery. Biomed Mater. 2021;16(2):025026. [DOI] [PubMed] [Google Scholar]
- 10. Akuma P, Okagu OD, Udenigwe CC. Naturally occurring exosome vesicles as potential delivery vehicle for bioactive compounds. Frontiers in Sustainable Food Systems. 2019;3:3. [Google Scholar]
- 11. Jakubec M, Maple‐Grødem J, Akbari S, Nesse S, Halskau Ø, Mork‐Jansson AE. Plasma‐derived exosome‐like vesicles are enriched in lyso‐phospholipids and pass the blood‐brain barrier. PLoS One. 2020;15(9):e0232442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Liu X, Xia T, Fang Y, et al. Overcoming the blood–brain barrier by using a multistage exosome delivery system to inhibit central nervous system lymphoma. Nanomedicine. 2022;41:102523. [DOI] [PubMed] [Google Scholar]
- 13. Banks WA, Sharma P, Bullock KM, Hansen KM, Ludwig N, Whiteside TL. Transport of extracellular vesicles across the blood‐brain barrier: brain pharmacokinetics and effects of inflammation. Int J Mol Sci. 2020;21(12):4407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zhang ZG, Buller B, Chopp M. Exosomes—beyond stem cells for restorative therapy in stroke and neurological injury. Nat Rev Neurol. 2019;15(4):193‐203. [DOI] [PubMed] [Google Scholar]
- 15. Sun B, Peng J, Wang S, et al. Applications of stem cell‐derived exosomes in tissue engineering and neurological diseases. Rev Neurosci. 2018;29(5):531‐546. [DOI] [PubMed] [Google Scholar]
- 16. Fitzner D, Schnaars M, van Rossum D, et al. Selective transfer of exosomes from oligodendrocytes to microglia by macropinocytosis. J Cell Sci. 2011;124(3):447‐458. [DOI] [PubMed] [Google Scholar]
- 17. Fröhlich D, Kuo WP, Fruhbeis C, et al. Multifaceted effects of oligodendroglial exosomes on neurons: impact on neuronal firing rate, signal transduction and gene regulation. Philos Trans R Soc Lond Ser B Biol Sci. 2014;369(1652):20130510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Oyarce K, Cepeda MY, Lagos R, et al. Neuroprotective and neurotoxic effects of glial‐derived exosomes. Front Cell Neurosci. 2022;16:920686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bahram Sangani N, Gomes AR, Curfs LMG, Reutelingsperger CP. The role of extracellular vesicles during CNS development. Prog Neurobiol. 2021;205:102124. [DOI] [PubMed] [Google Scholar]
- 20. Mittelbrunn M, Vicente Manzanares M, Sánchez‐Madrid F. Organizing polarized delivery of exosomes at synapses. Traffic. 2015;16(4):327‐337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Shi J, Han P, Kuniyoshi SM. Cognitive impairment in neurological diseases: lessons from apolipoprotein E. J Alzheimers Dis. 2014;38(1):1‐9. [DOI] [PubMed] [Google Scholar]
- 22. Gayen M, Bhomia M, Balakathiresan N, Knollmann‐Ritschel B. Exosomal MicroRNAs released by activated astrocytes as potential neuroinflammatory biomarkers. Int J Mol Sci. 2020;21(7):2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Goetzl L, Merabova N, Darbinian N, et al. Diagnostic potential of neural exosome cargo as biomarkers for acute brain injury. Ann Clin Transl Neurol. 2018;5(1):4‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Nakano M, Nagaishi K, Konari N, et al. Bone marrow‐derived mesenchymal stem cells improve diabetes‐induced cognitive impairment by exosome transfer into damaged neurons and astrocytes. Sci Rep. 2016;6(1):24805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yang L, Zhai Y, Hao Y, Zhu Z, Cheng G. The regulatory functionality of exosomes derived from hUMSCs in 3D culture for Alzheimer's disease therapy. Small. 2020;16(3):1906273. [DOI] [PubMed] [Google Scholar]
- 26. Wei R, Zhang L, Hu W, Shang X, He Y, Zhang W. Zeb2/Axin2‐enriched BMSC‐derived exosomes promote post‐stroke functional recovery by enhancing neurogenesis and neural plasticity. J Mol Neurosci. 2022;72(1):69‐81. [DOI] [PubMed] [Google Scholar]
- 27. Kalaria RN, Akinyemi R, Ihara M. Stroke injury, cognitive impairment and vascular dementia. Biochim Biophys Acta. 2016;1862(5):915‐925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yang Y, Fuh J, Mok VCT. Vascular contribution to cognition in stroke and Alzheimer's disease. Brain Science Advances. 2018;4(1):39‐48. [Google Scholar]
- 29. Zhou LYY, Wright TE, Clarkson AN. Prefrontal cortex stroke induces delayed impairment in spatial memory. Behav Brain Res. 2016;296:373‐378. [DOI] [PubMed] [Google Scholar]
- 30. Cuartero MI, de la Parra J, Pérez‐Ruiz A, et al. Abolition of aberrant neurogenesis ameliorates cognitive impairment after stroke in mice. J Clin Invest. 2019;129(4):1536‐1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Luo CX, Jiang J, Zhou QG, et al. Voluntary exercise‐induced neurogenesis in the postischemic dentate gyrus is associated with spatial memory recovery from stroke. J Neurosci Res. 2007;85(8):1637‐1646. [DOI] [PubMed] [Google Scholar]
- 32. Hood JL, Wickline SA. A systematic approach to exosome‐based translational nanomedicine. WIREs Nanomed Nanobiotechnol. 2012;4(4):458‐467. [DOI] [PubMed] [Google Scholar]
- 33. Luarte A, Bátiz LF, Wyneken U, Lafourcade C. Potential therapies by stem cell‐derived exosomes in CNS diseases: focusing on the neurogenic niche. Stem Cells Int. 2016;2016:1‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zhang Y, Bi J, Huang J, Tang Y, du S, Li P. Exosome: a review of its classification, isolation techniques, storage, diagnostic and targeted therapy applications. Int J Nanomedicine. 2020;15:6917‐6934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Tiwari S, Kumar V, Randhawa S, Verma SK. Preparation and characterization of extracellular vesicles. Am J Reprod Immunol. 2021;85(2):e13367. [DOI] [PubMed] [Google Scholar]
- 36. Pant S, Hilton H, Burczynski ME. The multifaceted exosome: biogenesis, role in normal and aberrant cellular function, and frontiers for pharmacological and biomarker opportunities. Biochem Pharmacol. 2012;83(11):1484‐1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Amiri A, Bagherifar R, Ansari Dezfouli E, Kiaie SH, Jafari R, Ramezani R. Exosomes as bio‐inspired nanocarriers for RNA delivery: preparation and applications. J Transl Med. 2022;20(1):125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Donoso‐Quezada J, Ayala‐Mar S, González‐Valdez J. The role of lipids in exosome biology and intercellular communication: function, analytics and applications. Traffic. 2021;22(7):204‐220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kalluri R, LeBleu VS. The biology, function, and biomedical applications of exosomes. Science. 2020;367(6478):eaau6977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Gauvreau ME, Côté MH, Bourgeois‐Daigneault MC, et al. Sorting of MHC class II molecules into exosomes through a ubiquitin‐independent pathway. Traffic. 2009;10(10):1518‐1527. [DOI] [PubMed] [Google Scholar]
- 41. Whiteside TL. The potential of tumor‐derived exosomes for noninvasive cancer monitoring: an update. Expert Rev Mol Diagn. 2018;18(12):1029‐1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Jung HH, Kim JY, Lim JE, Im YH. Cytokine profiling in serum‐derived exosomes isolated by different methods. Sci Rep. 2020;10(1):14069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Yang Y, Liu Y, Chai Y, et al. Exosomes in pathogenesis, diagnosis, and treatment of pulmonary fibrosis. Front Pharmacol. 2022;13:927653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Tian T, Zhu YL, Zhou YY, et al. Exosome uptake through clathrin‐mediated endocytosis and macropinocytosis and mediating miR‐21 delivery. J Biol Chem. 2014;289(32):22258‐22267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Maxfield FR, McGraw TE. Endocytic recycling. Nat Rev Mol Cell Biol. 2004;5(2):121‐132. [DOI] [PubMed] [Google Scholar]
- 46. Colombo M, Raposo G, Théry C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu Rev Cell Dev Biol. 2014;30:255‐289. [DOI] [PubMed] [Google Scholar]
- 47. Xu M, Ji J, Jin D, et al. The biogenesis and secretion of exosomes and multivesicular bodies (MVBs): intercellular shuttles and implications in human diseases. Genes Dis. 2023;10(5):1894‐1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Piper RC, Katzmann DJ. Biogenesis and function of multivesicular bodies. Annu Rev Cell Dev Biol. 2007;23(1):519‐547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Babst M. MVB vesicle formation: ESCRT‐dependent, ESCRT‐independent and everything in between. Curr Opin Cell Biol. 2011;23(4):452‐457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Wegner CS, Rodahl LM, Stenmark H. ESCRT proteins and cell signalling. Traffic. 2011;12(10):1291‐1297. [DOI] [PubMed] [Google Scholar]
- 51. Stoten CL, Carlton JG. ESCRT‐dependent control of membrane remodelling during cell division. Semin Cell Dev Biol. 2018;74:50‐65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Friand V, David G, Zimmermann P. Syntenin and syndecan in the biogenesis of exosomes. Biol Cell. 2015;107(10):331‐341. [DOI] [PubMed] [Google Scholar]
- 53. Kojima K, Amano Y, Yoshino K, Tanaka N, Sugamura K, Takeshita T. ESCRT‐0 protein hepatocyte growth factor‐regulated tyrosine kinase substrate (Hrs) is targeted to endosomes independently of signal‐transducing adaptor molecule (STAM) and the complex formation with STAM promotes its endosomal dissociation. J Biol Chem. 2014;289(48):33296‐33310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Frankel EB, Audhya A. ESCRT‐dependent cargo sorting at multivesicular endosomes. Semin Cell Dev Biol. 2018;74:4‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Wenzel EM, Schultz SW, Schink KO, et al. Concerted ESCRT and clathrin recruitment waves define the timing and morphology of intraluminal vesicle formation. Nat Commun. 2018;9(1):2932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Hoffman HK, Fernandez MV, Groves NS, Freed EO, van Engelenburg SB. Genomic tagging of endogenous human ESCRT‐I complex preserves ESCRT‐mediated membrane‐remodeling functions. J Biol Chem. 2019;294(44):16266‐16281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Teo H, Perisic O, González B, Williams RL. ESCRT‐II, an endosome‐associated complex required for protein sorting: crystal structure and interactions with ESCRT‐III and membranes. Dev Cell. 2004;7(4):559‐569. [DOI] [PubMed] [Google Scholar]
- 58. Henne WM, Buchkovich NJ, Zhao Y, Emr SD. The endosomal sorting complex ESCRT‐II mediates the assembly and architecture of ESCRT‐III helices. Cell. 2012;151(2):356‐371. [DOI] [PubMed] [Google Scholar]
- 59. Shestakova A, Hanono A, Drosner S, et al. Assembly of the AAA ATPase Vps4 on ESCRT‐III. Mol Biol Cell. 2010;21(6):1059‐1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Andreu Z, Yáñez‐Mó M. Tetraspanins in extracellular vesicle formation and function. Front Immunol. 2014;5:c109543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Laulagnier K, Grand D, Dujardin A, et al. PLD2 is enriched on exosomes and its activity is correlated to the release of exosomes. FEBS Lett. 2004;572(1–3):11‐14. [DOI] [PubMed] [Google Scholar]
- 62. Stuffers S, Sem Wegner C, Stenmark H, Brech A. Multivesicular endosome biogenesis in the absence of ESCRTs. Traffic. 2009;10(7):925‐937. [DOI] [PubMed] [Google Scholar]
- 63. Kadry H, Noorani B, Cucullo L. A blood–brain barrier overview on structure, function, impairment, and biomarkers of integrity. Fluids and Barriers of the CNS. 2020;17(1):69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Druzhkova TA, Yakovlev AA. Exosome drug delivery through the blood–brain barrier: experimental approaches and potential applications. Neurochem J. 2018;12(3):195‐204. [Google Scholar]
- 65. Abbott NJ, Patabendige AAK, Dolman DEM, Yusof SR, Begley DJ. Structure and function of the blood–brain barrier. Neurobiol Dis. 2010;37(1):13‐25. [DOI] [PubMed] [Google Scholar]
- 66. Tajes M, Ramos‐Fernández E, Weng‐Jiang X, et al. The blood‐brain barrier: structure, function and therapeutic approaches to cross it. Mol Membr Biol. 2014;31(5):152‐167. [DOI] [PubMed] [Google Scholar]
- 67. Lochhead JJ, Yang J, Ronaldson PT, Davis TP. Structure, function, and regulation of the blood‐brain barrier tight junction in central nervous system disorders. Front Physiol. 2020;11:914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Stamatovic SM, Johnson AM, Keep RF, Andjelkovic AV. Junctional proteins of the blood‐brain barrier: new insights into function and dysfunction. Tissue Barriers. 2016;4(1):e1154641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Ramos‐Zaldívar HM, Polakovicova I, Salas‐Huenuleo E, et al. Extracellular vesicles through the blood–brain barrier: a review. Fluids and Barriers of the CNS. 2022;19(1):60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Lajoie JM, Shusta EV. Targeting receptor‐mediated transport for delivery of biologics across the blood‐brain barrier. Annu Rev Pharmacol Toxicol. 2015;55:613‐631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Matsumoto J, Stewart T, Banks WA, Zhang J. The transport mechanism of extracellular vesicles at the blood‐brain barrier. Curr Pharm Des. 2017;23(40):6206‐6214. [DOI] [PubMed] [Google Scholar]
- 72. Heidarzadeh M, Gürsoy‐Özdemir Y, Kaya M, et al. Exosomal delivery of therapeutic modulators through the blood–brain barrier; promise and pitfalls. Cell Biosci. 2021;11(1):142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Saint‐Pol J, Gosselet F, Duban‐Deweer S, Pottiez G, Karamanos Y. Targeting and crossing the blood‐brain barrier with extracellular vesicles. Cells. 2020;9(4):851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Choi H, Choi Y, Yim HY, Mirzaaghasi A, Yoo JK, Choi C. Biodistribution of exosomes and engineering strategies for targeted delivery of therapeutic exosomes. Tissue Engineering and Regenerative Medicine. 2021;18(4):499‐511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Kim G, Kim M, Lee Y, Byun JW, Hwang DW, Lee M. Systemic delivery of microRNA‐21 antisense oligonucleotides to the brain using T7‐peptide decorated exosomes. J Control Release. 2020;317:273‐281. [DOI] [PubMed] [Google Scholar]
- 76. Alvarez‐Erviti L, Seow Y, Yin HF, Betts C, Lakhal S, Wood MJA. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nat Biotechnol. 2011;29(4):341‐345. [DOI] [PubMed] [Google Scholar]
- 77. Salarpour S, Barani M, Pardakhty A, Khatami M, Pal Singh Chauhan N. The application of exosomes and exosome‐nanoparticle in treating brain disorders. J Mol Liq. 2022;350:118549. [Google Scholar]
- 78. Pastuzyn ED, Day CE, Kearns RB, et al. The neuronal gene arc encodes a repurposed retrotransposon gag protein that mediates intercellular RNA transfer. Cell. 2018;172(1):275‐288.e18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Shi J, Li H, Jin G, et al. Lhx8 promote differentiation of hippocampal neural stem/progenitor cells into cholinergic neurons in vitro. In Vitro Cell Dev Biol Anim. 2012;48(10):603‐609. [DOI] [PubMed] [Google Scholar]
- 80. Cheng X, Li W, Zhao R, et al. The role of hippocampal niche exosomes in rat hippocampal neurogenesis after fimbria‐fornix transection. J Biol Chem. 2021;296:100188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Jovanovic VM, Salti A, Tilleman H, et al. BMP/SMAD pathway promotes neurogenesis of midbrain dopaminergic neurons in vivo and in human induced pluripotent and neural stem cells. J Neurosci. 2018;38(7):1662‐1676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Upadhya R, Madhu LN, Attaluri S, et al. Extracellular vesicles from human iPSC‐derived neural stem cells: miRNA and protein signatures, and anti‐inflammatory and neurogenic properties. J Extracellular Vesicles. 2020;9(1):1809064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Soares Martins T, Marçalo R, da Cruz e Silva CB, et al. Novel exosome biomarker candidates for Alzheimer's disease Unravelled through mass spectrometry analysis. Mol Neurobiol. 2022;59:2838‐2854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Su L, Li R, Zhang Z, Liu J, du J, Wei H. Identification of altered exosomal microRNAs and mRNAs in Alzheimer's disease. Ageing Res Rev. 2022;73:101497. [DOI] [PubMed] [Google Scholar]
- 85. Ding L, Yang X, Xia X, et al. Exosomes mediate APP dysregulation via APP‐miR‐185‐5p Axis. Front Cell Dev Biol. 2022;10:793388. doi: 10.3389/fcell.2022.793388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Shi Y, Bao Q, Chen W, et al. Potential roles of extracellular vesicles as diagnosis biomarkers and therapeutic approaches for cognitive impairment in Alzheimer's disease. J Alzheimers Dis. 2022;87:1‐15. [DOI] [PubMed] [Google Scholar]
- 87. Jahangard Y, Monfared H, Moradi A, Zare M, Mirnajafi‐Zadeh J, Mowla SJ. Therapeutic effects of transplanted exosomes containing miR‐29b to a rat model of Alzheimer's disease. Front Neurosci. 2020;14:564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Micci M‐A, Krishnan B, Bishop E, et al. Hippocampal stem cells promotes synaptic resistance to the dysfunctional impact of amyloid beta oligomers via secreted exosomes. Mol Neurodegener. 2019;14(1):25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Pan J, He R, Huo Q, Shi Y, Zhao L. Brain microvascular endothelial cell derived exosomes potently ameliorate cognitive dysfunction by enhancing the clearance of Aβ through up‐regulation of P‐gp in mouse model of AD. Neurochem Res. 2020;45(9):2161‐2172. [DOI] [PubMed] [Google Scholar]
- 90. Guo M, Yin Z, Chen F, Lei P. Mesenchymal stem cell‐derived exosome: a promising alternative in the therapy of Alzheimer's disease. Alzheimers Res Ther. 2020;12(1):109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Chen Y‐A, Lu CH, Ke CC, et al. Mesenchymal stem cell‐derived exosomes ameliorate Alzheimer's disease pathology and improve cognitive deficits. Biomedicine. 2021;9(6):594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Ding M, Shen Y, Wang P, et al. Exosomes isolated from human umbilical cord mesenchymal stem cells alleviate neuroinflammation and reduce amyloid‐Beta deposition by modulating microglial activation in Alzheimer's disease. Neurochem Res. 2018;43(11):2165‐2177. [DOI] [PubMed] [Google Scholar]
- 93. Cui G‐H, Wu J, Mou FF, et al. Exosomes derived from hypoxia‐preconditioned mesenchymal stromal cells ameliorate cognitive decline by rescuing synaptic dysfunction and regulating inflammatory responses in APP/PS1 mice. FASEB J. 2018;32(2):654‐668. [DOI] [PubMed] [Google Scholar]
- 94. Wang X, Yang G. Bone marrow mesenchymal stem cells‐derived exosomes reduce Aβ deposition and improve cognitive function recovery in mice with Alzheimer's disease by activating sphingosine kinase/sphingosine‐1‐phosphate signaling pathway. Cell Biol Int. 2021;45(4):775‐784. [DOI] [PubMed] [Google Scholar]
- 95. Liu S, Fan M, Xu JX, et al. Exosomes derived from bone‐marrow mesenchymal stem cells alleviate cognitive decline in AD‐like mice by improving BDNF‐related neuropathology. J Neuroinflammation. 2022;19(1):35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Reza‐Zaldivar EE, Hernández‐Sapiéns MA, Gutiérrez‐Mercado YK, et al. Mesenchymal stem cell‐derived exosomes promote neurogenesis and cognitive function recovery in a mouse model of Alzheimer's disease. Neural Regen Res. 2019;14(9):1626‐1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Wang H, Sui H, Zheng Y, et al. Curcumin‐primed exosomes potently ameliorate cognitive function in AD mice by inhibiting hyperphosphorylation of the tau protein through the AKT/GSK‐3β pathway. Nanoscale. 2019;11(15):7481‐7496. [DOI] [PubMed] [Google Scholar]
- 98. Qi Y, Guo L, Jiang Y, Shi Y, Sui H, Zhao L. Brain delivery of quercetin‐loaded exosomes improved cognitive function in AD mice by inhibiting phosphorylated tau‐mediated neurofibrillary tangles. Drug Deliv. 2020;27(1):745‐755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Cui G‐h, Guo HD, Li H, et al. RVG‐modified exosomes derived from mesenchymal stem cells rescue memory deficits by regulating inflammatory responses in a mouse model of Alzheimer's disease. Immun Ageing. 2019;16(1):10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Huo Q, Shi Y, Qi Y, Huang L, Sui H, Zhao L. Biomimetic silibinin‐loaded macrophage‐derived exosomes induce dual inhibition of Aβ aggregation and astrocyte activation to alleviate cognitive impairment in a model of Alzheimer's disease. Mater Sci Eng C. 2021;129:112365. [DOI] [PubMed] [Google Scholar]
- 101. Yu H, Sun T, An J, et al. Potential roles of exosomes in Parkinson's disease: from pathogenesis, diagnosis, and treatment to prognosis. Front Cell Dev Biol. 2020;8:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Zou J, Guo Y, Wei L, Yu F, Yu B, Xu A. Long noncoding RNA POU3F3 and α‐synuclein in plasma L1CAM exosomes combined with β‐glucocerebrosidase activity: potential predictors of Parkinson's disease. Neurotherapeutics. 2020;17(3):1104‐1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Leng B, Sun H, Zhao J, et al. Plasma exosomal prion protein levels are correlated with cognitive decline in PD patients. Neurosci Lett. 2020;723:134866. [DOI] [PubMed] [Google Scholar]
- 104. Yuan L, Li J‐Y. Exosomes in Parkinson's disease: current perspectives and future challenges. ACS Chem Neurosci. 2019;10(2):964‐972. [DOI] [PubMed] [Google Scholar]
- 105. Ahmed SSSJ, Paramasivam P, Kamath M, Sharma A, Rome S, Murugesan R. Genetic exchange of lung‐derived exosome to brain causing neuronal changes on COVID‐19 infection. Mol Neurobiol. 2021;58(10):5356‐5368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Narbute K, Pilipenko V, Pupure J, et al. Time‐dependent memory and gait improvement by intranasally‐administered extracellular vesicles in Parkinson's disease model rats. Cell Mol Neurobiol. 2021;41(3):605‐613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Guo M, Wang J, Zhao Y, et al. Microglial exosomes facilitate α‐synuclein transmission in Parkinson's disease. Brain. 2020;143(5):1476‐1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Dobson R, Giovannoni G. Multiple sclerosis – a review. Eur J Neurol. 2019;26(1):27‐40. [DOI] [PubMed] [Google Scholar]
- 109. Hauser SL, Cree BA. Treatment of multiple sclerosis: a review. Am J Med. 2020;133(12):1380‐1390. e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Ojeda‐Hernández DD, Hernández‐Sapiéns MA, Reza‐Zaldívar EE, et al. Exosomes and biomaterials: in search of a new therapeutic strategy for multiple sclerosis. Lifestyles. 2022;12(9):1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Pusic AD, Kraig RP. Youth and environmental enrichment generate serum exosomes containing miR‐219 that promote CNS myelination. Glia. 2014;62(2):284‐299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Laso‐García F, Ramos‐Cejudo J, Carrillo‐Salinas FJ, et al. Therapeutic potential of extracellular vesicles derived from human mesenchymal stem cells in a model of progressive multiple sclerosis. PLoS One. 2018;13(9):e0202590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Zhang J, Buller BA, Zhang ZG, et al. Exosomes derived from bone marrow mesenchymal stromal cells promote remyelination and reduce neuroinflammation in the demyelinating central nervous system. Exp Neurol. 2022;347:113895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Yang H‐C, Zhang M, Wu R, et al. C‐C chemokine receptor type 2‐overexpressing exosomes alleviated experimental post‐stroke cognitive impairment by enhancing microglia/macrophage M2 polarization. World J Stem Cells. 2020;12(2):152‐167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Webb RL, Kaiser EE, Scoville SL, et al. Human neural stem cell extracellular vesicles improve tissue and functional recovery in the murine thromboembolic stroke model. Transl Stroke Res. 2018;9(5):530‐539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Chen X, Jiang M, Li H, et al. CX3CL1/CX3CR1 axis attenuates early brain injury via promoting the delivery of exosomal microRNA‐124 from neuron to microglia after subarachnoid hemorrhage. J Neuroinflammation. 2020;17(1):209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Venkat P, Chopp M. Exosome treatment for stroke with diabetic comorbidity. Neural Regen Res. 2022;17(2):315‐317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Huang L‐Y, Song JX, Cai H, et al. Healthy serum‐derived exosomes improve neurological outcomes and protect blood‐brain barrier by inhibiting endothelial cell apoptosis and reversing autophagy‐mediated tight junction protein reduction in rat stroke model. Front Cell Neurosci. 2022;16:841544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Zhang H, Lin S, McElroy C, et al. Circulating pro‐inflammatory exosomes worsen stroke outcomes in aging. Circ Res. 2021;129(7):e121‐e140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Cunningham CJ, Redondo‐Castro E, Allan SM. The therapeutic potential of the mesenchymal stem cell secretome in ischaemic stroke. J Cereb Blood Flow Metab. 2018;38(8):1276‐1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Bang OY, Kim EH. Mesenchymal stem cell‐derived extracellular vesicle therapy for stroke: challenges and Progress. Front Neurol. 2019;10:211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Hu X, Pan J, Li Y, et al. Extracellular vesicles from adipose‐derived stem cells promote microglia M2 polarization and neurological recovery in a mouse model of transient middle cerebral artery occlusion. Stem Cell Res Ther. 2022;13(1):21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Liu X, Zhang M, Liu H, et al. Bone marrow mesenchymal stem cell‐derived exosomes attenuate cerebral ischemia‐reperfusion injury‐induced neuroinflammation and pyroptosis by modulating microglia M1/M2 phenotypes. Exp Neurol. 2021;341:113700. [DOI] [PubMed] [Google Scholar]
- 124. Xiong L, Sun L, Zhang Y, Peng J, Yan J, Liu X. Exosomes from bone marrow mesenchymal stem cells can alleviate early brain injury after subarachnoid hemorrhage through miRNA129‐5p‐HMGB1 pathway. Stem Cells Dev. 2020;29(4):212‐221. [DOI] [PubMed] [Google Scholar]
- 125. Gao X, Xiong Y, Li Q, et al. Extracellular vesicle‐mediated transfer of miR‐21‐5p from mesenchymal stromal cells to neurons alleviates early brain injury to improve cognitive function via the PTEN/Akt pathway after subarachnoid hemorrhage. Cell Death Dis. 2020;11(5):363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Rohden F, Teixeira LV, Bernardi LP, et al. Extracellular vesicles from human adipose stem cells are neuroprotective after stroke in rats. bioRxiv. 2020. NOV 19: 2020‐11. [Google Scholar]
- 127. Matei AC, Antounians L, Zani A. Extracellular vesicles as a potential therapy for neonatal conditions: state of the art and challenges in clinical translation. Pharmaceutics. 2019;11(8):404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Stern RA, Tripodis Y, Baugh CM, et al. Preliminary study of plasma Exosomal tau as a potential biomarker for chronic traumatic encephalopathy. J Alzheimers Dis. 2016;51:1099‐1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Hu T, Han Z, Xiong X, et al. Inhibition of exosome release alleviates cognitive impairment after repetitive mild traumatic brain injury. Front Cell Neurosci. 2022;16:832140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Xi S, Wang Y, Wu C, Peng W, Zhu Y, Hu W. Intestinal epithelial cell exosome launches IL‐1β‐mediated neuron injury in sepsis‐associated encephalopathy. Front Cellular Infect Microbiol. 2022;11:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Adstamongkonkul D, Hess DC. Ischemic conditioning and neonatal hypoxic ischemic encephalopathy: a literature review. Cond Med. 2017;1(1):9‐16. [PMC free article] [PubMed] [Google Scholar]
- 132. Nguyen NP, Helmbrecht H, Ye Z, et al. Brain tissue‐derived extracellular vesicle mediated therapy in the neonatal ischemic brain. Int J Mol Sci. 2022;23(2):620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Li P, Lu X, Hu J, et al. Human amniotic fluid derived‐exosomes alleviate hypoxic encephalopathy by enhancing angiogenesis in neonatal mice after hypoxia. Neurosci Lett. 2022;768:136361. [DOI] [PubMed] [Google Scholar]
- 134. Moshé SL, Perucca E, Ryvlin P, Tomson T. Epilepsy: new advances. Lancet. 2015;385(9971):884‐898. [DOI] [PubMed] [Google Scholar]
- 135. Batool A, Hill TD, Nguyen NT, et al. Altered biogenesis and MicroRNA content of hippocampal exosomes following experimental status epilepticus. Front Neurosci. 2019;13:1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Vargas‐Sánchez K, Mogilevskaya M, Rodríguez‐Pérez J, Rubiano MG, Javela JJ, González‐Reyes RE. Astroglial role in the pathophysiology of status epilepticus: an overview. Oncotarget. 2018;9(42):26954‐26976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Xian P, Hei Y, Wang R, et al. Mesenchymal stem cell‐derived exosomes as a nanotherapeutic agent for amelioration of inflammation‐induced astrocyte alterations in mice. Theranostics. 2019;9(20):5956‐5975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Luo Q, Xian P, Wang T, et al. Antioxidant activity of mesenchymal stem cell‐derived extracellular vesicles restores hippocampal neurons following seizure damage. Theranostics. 2021;11(12):5986‐6005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Long Q, Upadhya D, Hattiangady B, et al. Intranasal MSC‐derived A1‐exosomes ease inflammation, and prevent abnormal neurogenesis and memory dysfunction after status epilepticus. Proc Natl Acad Sci. 2017;114(17):E3536‐E3545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Liu K, Cai GL, Zhuang Z, et al. Interleukin‐1β‐treated mesenchymal stem cells inhibit inflammation in hippocampal astrocytes through exosome‐activated Nrf‐2 signaling. Int J Nanomedicine. 2021;16:1423‐1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141. Wang B, Han S. Exosome‐associated tau exacerbates brain functional impairments induced by traumatic brain injury in mice. Mol Cell Neurosci. 2018;88:158‐166. [DOI] [PubMed] [Google Scholar]
- 142. Yang Y, Yang H, Yang Y, Ma Y. Exosomal microRNAs have great potential in the neurorestorative therapy for traumatic brain injury. Exp Neurol. 2022;352:114026. [DOI] [PubMed] [Google Scholar]
- 143. Zhang Y, Chopp M, Meng Y, et al. Effect of exosomes derived from multipluripotent mesenchymal stromal cells on functional recovery and neurovascular plasticity in rats after traumatic brain injury. J Neurosurg. 2015;122(4):856‐867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Zhang Z‐W, Wei P, Zhang GJ, et al. Intravenous infusion of the exosomes derived from human umbilical cord mesenchymal stem cells enhance neurological recovery after traumatic brain injury via suppressing the NF‐κB pathway. Open Life Sciences. 2022;17(1):189‐201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. Zhang Y, Chopp M, Zhang ZG, et al. Systemic administration of cell‐free exosomes generated by human bone marrow derived mesenchymal stem cells cultured under 2D and 3D conditions improves functional recovery in rats after traumatic brain injury. Neurochem Int. 2017;111:69‐81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Zhang Y, Zhang Y, Chopp M, et al. MiR‐17–92 cluster‐enriched exosomes derived from human bone marrow mesenchymal stromal cells improve tissue and functional recovery in rats after traumatic brain injury. J Neurotrauma. 2021;38(11):1535‐1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Zhang Y, Zhang Y, Chopp M, Zhang ZG, Mahmood A, Xiong Y. Mesenchymal stem cell‐derived exosomes improve functional recovery in rats after traumatic brain injury: a dose‐response and therapeutic window study. Neurorehabil Neural Repair. 2020;34(7):616‐626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Williams AM, Dennahy IS, Bhatti UF, et al. Mesenchymal stem cell‐derived exosomes provide neuroprotection and improve Long‐term neurologic outcomes in a swine model of traumatic brain injury and hemorrhagic shock. J Neurotrauma. 2018;36(1):54‐60. [DOI] [PubMed] [Google Scholar]
- 149. Moss LD, Sode D, Patel R, et al. Intranasal delivery of exosomes from human adipose derived stem cells at forty‐eight hours post injury reduces motor and cognitive impairments following traumatic brain injury. Neurochem Int. 2021;150:105173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Ge X, Guo M, Hu T, et al. Increased microglial Exosomal miR‐124‐3p alleviates neurodegeneration and improves cognitive outcome after rmTBI. Mol Ther. 2020;28(2):503‐522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Wang B, Han S. Modified exosomes reduce apoptosis and ameliorate neural deficits induced by traumatic brain injury. ASAIO J. 2019;65(3):285‐292. [DOI] [PubMed] [Google Scholar]
- 152. Harrell CR, Volarevic A, Djonov V, Volarevic V. Mesenchymal stem cell‐derived exosomes as new remedy for the treatment of neurocognitive disorders. Int J Mol Sci. 2021;22(3):1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Banigan MG, Kao PF, Kozubek JA, et al. Differential expression of exosomal microRNAs in prefrontal cortices of schizophrenia and bipolar disorder patients. PLoS One. 2013;8(1):e48814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Barry G. Lamarckian evolution explains human brain evolution and psychiatric disorders. Front Neurosci. 2013;7:224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Niu Y, Wang X, Li M, Niu B. Exosomes from human umbilical cord mesenchymal stem cells attenuates stress‐induced hippocampal dysfunctions. Metab Brain Dis. 2020;35(8):1329‐1340. [DOI] [PubMed] [Google Scholar]
- 156. Guo H, Huang B, Wang Y, Zhang Y, Ma Q, Ren Y. Bone marrow mesenchymal stem cells‐derived exosomes improve injury of hippocampal neurons in rats with depression by upregulating microRNA‐26a expression. Int Immunopharmacol. 2020;82:106285. [DOI] [PubMed] [Google Scholar]
- 157. Tsivion‐Visbord H, Perets N, Sofer T, et al. Mesenchymal stem cells derived extracellular vesicles improve behavioral and biochemical deficits in a phencyclidine model of schizophrenia. Transl Psychiatry. 2020;10(1):305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Biessels GJ, Whitmer RA. Cognitive dysfunction in diabetes: how to implement emerging guidelines. Diabetologia. 2020;63(1):3‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159. Garcia‐Contreras M, Brooks RW, Boccuzzi L, Robbins PD, Ricordi C. Exosomes as biomarkers and therapeutic tools for type 1 diabetes mellitus. Eur Rev Med Pharmacol Sci. 2017;21(12):2940‐2956. [PubMed] [Google Scholar]
- 160. Zhao W, Zhang H, Yan J, Ma X. An experimental study on the treatment of diabetes‐induced cognitive disorder mice model with exosomes deriving from mesenchymal stem cells (MSCs). Pak J Pharm Sci. 2019;32(5):1965‐1970. [PubMed] [Google Scholar]
- 161. Kubota K, Nakano M, Kobayashi E, et al. An enriched environment prevents diabetes‐induced cognitive impairment in rats by enhancing exosomal miR‐146a secretion from endogenous bone marrow‐derived mesenchymal stem cells. PLoS One. 2018;13(9):e0204252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. Chen J, Cui C, Zacharek A, Venkat P, Yu P, Chopp M. Abstract WMP43: neurorestorative therapy of stroke in T2DM mice with exosomes derived from brain endothelial cells. Stroke. 2017;48(suppl_1):AWMP43. [Google Scholar]
- 163. Kalani A, Chaturvedi P, Maldonado C, et al. Dementia‐like pathology in type‐2 diabetes: a novel microRNA mechanism. Mol Cell Neurosci. 2017;80:58‐65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164. Ding G, Li L, Zhang L, et al. MRI metrics of cerebral endothelial cell–derived exosomes for the treatment of cognitive dysfunction induced in aging rats subjected to type 2 diabetes. Diabetes. 2022;71(5):873‐880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Beilharz JE, Maniam J, Morris MJ. Diet‐induced cognitive deficits: the role of fat and sugar, potential mechanisms and nutritional interventions. Nutrients. 2015;7(8):6719‐6738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Wang R, Zhou Z, Wang D, et al. Caloric restriction ameliorates high‐fat diet induced cognitive deficits through attenuating neuroinflammation via the TREM2‐PI3K/AKT signaling pathway. Food Funct. 2021;12(14):6464‐6478. [DOI] [PubMed] [Google Scholar]
- 167. Spinelli M, Natale F, Rinaudo M, et al. Neural stem cell‐derived exosomes revert HFD‐dependent memory impairment via CREB‐BDNF signalling. Int J Mol Sci. 2020;21(23):8994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Bhalala US, Appachi E, Mumtaz MA. Neurologic injury associated with rewarming from hypothermia: is mild hypothermia on bypass better than deep hypothermic circulatory arrest? Front Pediatr. 2016;4:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Shi J, Jiang X, Gao S, et al. Gene‐modified exosomes protect the brain against prolonged deep hypothermic circulatory arrest. Ann Thorac Surg. 2021;111(2):576‐585. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed in this study are available from the corresponding author on reasonable request.


