Abstract
Background and Objectives
GGC repeat expansions in the NOTCH2NLC gene are associated with a broad spectrum of progressive neurologic disorders, notably, neuronal intranuclear inclusion disease (NIID). We aimed to investigate the population-wide prevalence and clinical manifestations of NOTCH2NLC-related disorders in Koreans.
Methods
We conducted a study using 2 different cohorts from the Korean population. Patients with available brain MRI scans from Seoul National University Hospital (SNUH) were thoroughly reviewed, and NIID-suspected patients presenting the zigzag edging signs underwent genetic evaluation for NOTCH2NLC repeats by Cas9-mediated nanopore sequencing. In addition, we analyzed whole-genome sequencing data from 3,887 individuals in the Korea Biobank cohort to estimate the distribution of the repeat counts in Koreans and to identify putative patients with expanded alleles and neurologic phenotypes.
Results
In the SNUH cohort, among 90 adult-onset leukoencephalopathy patients with unknown etiologies, we found 20 patients with zigzag edging signs. Except for 2 diagnosed with fragile X-associated tremor/ataxia syndrome and 2 with unavailable samples, all 16 patients (17.8%) were diagnosed with NIID (repeat range: 87–217). By analyzing the Korea Biobank cohort, we estimated the distribution of repeat counts and threshold (>64) for Koreans, identifying 6 potential patients with NIID. Furthermore, long-read sequencing enabled the elucidation of transmission and epigenetic patterns of NOTCH2NLC repeats within a family affected by pediatric-onset NIID.
Discussion
This study presents the population-wide distribution of NOTCH2NLC repeats and the estimated prevalence of NIID in Koreans, providing valuable insights into the association between repeat counts and disease manifestations in diverse neurologic disorders.
Introduction
Neuronal intranuclear inclusion disease (NIID) is a complex neurodegenerative condition characterized by the widespread distribution of eosinophilic hyaline intranuclear inclusions within various somatic cells, including neuronal cells. Despite being first reported in the 1960s, the limited number of reported cases until the mid-2010s was due to the diagnostic challenges posed by the need for postmortem brain biopsies.1 However, with the development of an antemortem diagnostic modality using skin biopsies,2,3 more patients have been diagnosed. Furthermore, brain MRI characteristics have been identified as valuable clues for diagnosing NIID. Notably, most patients with NIID exhibit a distinct pattern called the “zigzag edging sign,” characterized by confluent white matter hyperintensities along the corticomedullary junction on diffusion-weighted imaging (DWI).4
In recent years, there has been a significant breakthrough in understanding the genetic basis of the neurologic disorder. Specifically, GGC repeat expansions located in the 5′ untranslated region of the notch 2 N-terminal-like C (NOTCH2NLC) gene have been identified as the genetic cause of NIID. Since the discovery of repeat expansions, a range of other disorders have also been associated with NOTCH2NLC repeat expansions, such as oculopharyngodistal myopathy, multiple system atrophy, retinopathy, dementia, Parkinson disease, essential tremor, and amyotrophic lateral sclerosis.5-16 This discovery has not only broadened the clinical spectrum of NOTCH2NLC-related disorders but also expedited the diagnostic evaluation of patients with NIID. While this genetic abnormality has been rarely reported in Europeans,17,18 it may contribute to a substantial portion of undiagnosed adult-onset leukoencephalopathies among East Asians.19-21
Although NOTCH2NLC repeat expansions have been widely studied in other East Asian populations, there has been a lack of investigation into this genetic abnormality in Koreans. In addition, the population-wide burden of NOTCH2NLC-related diseases remains poorly understood. In this study, we aimed to address this knowledge gap using 2 different approaches, encompassing both patient and population cohorts.
Methods
SNUH Cohort
Participants were retrospectively enrolled from the Seoul National University Hospital (SNUH), who meet the eligible criteria as follows: (1) Korean patients who visited the Department of Neurology at SNUH between September 2019 and March 2023; (2) patients suspected to have adult-onset leukoencephalopathy, exhibiting confluent white matter hyperintensities in both hemispheres based on brain MRI studies; and (3) patients presenting the zigzag edging sign on DWI, which were thoroughly reviewed by 2 independent neurologists. Only patients who satisfied all these criteria were selected for further evaluation. Through a comprehensive retrospective review of medical records, we excluded patients suspected to have white matter changes secondary to other medical conditions, such as cerebral infarction, hemorrhage, brain tumor, and viral infection.
Korea Biobank Cohort
A total of 3,887 Korean individuals, including patients diagnosed with rare diseases and their relatives, were obtained from the Korea Biobank cohort. Of these, 1,672 exhibited 19 distinct phenotypes with the most frequently observed being neurologic and neurodevelopmental disorders (665, 39.8%), followed by cardiovascular disorders (219, 13.1%) and tumor syndromes (210, 12.6%) (eTable 1). The remaining 2,215 reported no health problems. Ancestry prediction was determined through principal component analysis of whole-genome data alongside reference data from the 1K Genomes Project via Somalier (eFigure 1).22 Kinship was assessed with Somalier by comparing a selected array of informative genetic markers between pairs of individuals, and we refined the cohort to ensure all sample pairs were second-degree relatives or more distantly related, by excluding any with a relatedness coefficient above 0.125. Among these fairly unrelated individuals, 596 (21.8%) were patients with rare diseases, while 2,141 had not received any rare disease diagnosis. All data collected were deidentified in compliance with relevant guidelines and regulations.
Whole-Genome Sequencing Analysis
Paired 150-bp sequencing reads, generated from the NovaSeq 6000 platform (Illumina, San Diego, CA) for WGS, were aligned to the human reference genome GRCh38. We used ExpansionHunter (v5.0.0)23 to identify short tandem repeats (STRs), specifically GGC repeat expansions within the 5′ untranslated region of the NOTCH2NLC gene (NM_001364012). We filtered the candidate STRs based on the allele depth and visually inspected the reads using REViewer (v0.2.7)24 to eliminate potential false positives. To compare GGC repeat expansion lengths among various ethnic groups, we used data sourced from the 100,000 Genomes Project cohort. The quantification of GGC repeat expansion length was performed following the methodology previously described.25
Cas9-Mediated Nanopore Long-Read Sequencing
Genomic DNA was extracted from whole blood samples using the Qiagen Puregene blood kit (Qiagen, Maryland; cat. 158023). Cas9-mediated target enrichment was conducted as described previously.14 Briefly, 5 µg of DNA was subjected to the Cas9 sequencing kit protocol (cat. SQK-CS9109; Oxford Nanopore Technologies, United Kingdom). Prepared libraries were loaded onto R9.4 flow cells (FLO-MIN107) and sequenced by the GridION platform (Oxford Nanopore Technology). Base calling and FASTQ conversion were performed using MinKNOW software (v5.3.6). FASTQ files were aligned to the human reference genome GRCh38 using minimap2 (v2.24-r1122).26 We used the software Straglr27 to estimate the NOTCH2NLC repeat counts. The detection of methylated base modifications was performed using Nanopolish.28 The methylation log-likelihood was computed from the index files and subsequently transformed into binary methylated/unmethylated calls. The percentage of methylation was determined by calculating the proportion of methylated reads within the NOTCH2NLC promoter region (chr1:149,390,103–149,391,842, 1.74 kb).
Repeat-Primed PCR, Skin Biopsy, and PacBio HiFi Long-Read Sequencing
Fourteen patients (except for P06 and P08 with no additional samples) underwent repeat-primed PCR (RP-PCR) to cross-validate the results following the previous study.14 The following primers were used for the experiments:
5′-FAM-GGCATTTGCGCCTGTGCTTCGGACCGT-3′,
5′-CAGGAAACAGCTATGACCTCCTCCGCCGCCGCCGCC-3′, and
5′-CAGGAAACAGCTATGACC-3′.
Two patients (P02, P04) underwent a skin biopsy on their upper arms. The specimen was fixed and embedded with 4% formalin solution and paraffin, respectively. The paraffin block was cut into 4-mm section slides and stained with hematoxylin and eosin. Immunohistochemical staining was performed using the anti-ubiquitin antibody (ab7780, Abcam, United Kingdom) and the SQSTM1/P62 antibody (Santa Cruz Biotechnology, CA; sc-28359). One affected patient (KBB1S) underwent PacBio HiFi long-read WGS on the Sequel II platform (Pacific Biosciences) following the protocol described previously (target coverage: 10X).29 Sequencing and subsequent data processing were conducted by DNA Link (Seoul, Korea).
Statistical Analysis
Statistical analyses were performed using the software R (version 4.1.2). A p value <0.05 was considered significant. All data are presented as n (%) or median (range). Categorical variables were compared using the Fisher exact test, and continuous variables were compared using the Mann-Whitney U test. Methylation frequencies were compared using the Wilcoxon rank-sum test.
Standard Protocol Approvals, Registrations, and Patient Consents
The study protocol was approved by the Institutional Review Board of Seoul National University Hospital (2210-004-1364), and the study was conducted in accordance with relevant guidelines and regulations. Informed consent was duly obtained from all participants.
Data Availability
The SNUH cohort data are not publicly available to ensure the protection of participant anonymity. The Korea Biobank cohort data are available at coda.nih.go.kr with the accession number (CODA_D22004).
Results
Identification of Patients With NIID in the SNUH Cohort
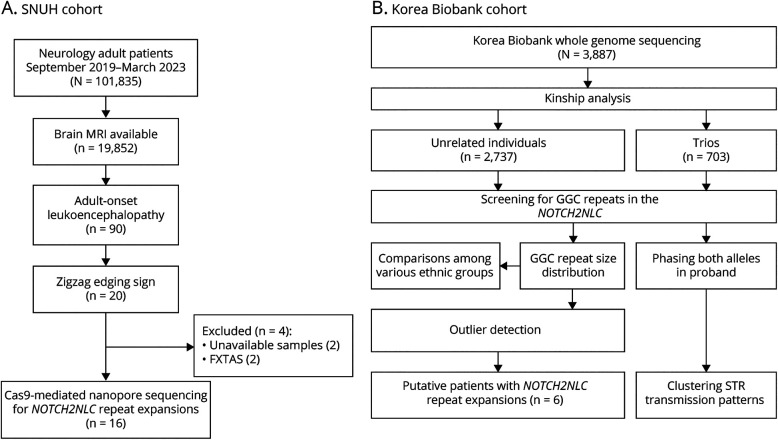
Among the 19,852 patients who underwent brain MRI studies during the study period, we identified 90 adult-onset leukoencephalopathy patients with suggestive image findings, requiring differential diagnosis for NIID. Of these patients, 20 exhibited the zigzag edging sign on DWI (Figure 1A). Thirteen patients underwent FMR1 genetic testing, of whom 2 were positive for FMR1 premutation indicative of fragile X-associated tremor/ataxia syndrome (FXTAS). After excluding these 2 patients with FXTAS and 2 patients for whom additional DNA samples were not available, we performed Cas9-mediated nanopore long-read sequencing on the remaining 16 patients. Of interest, all of these patients (16/90, 17.8%) were found to have heterozygous NOTCH2NLC GGC repeat expansions, with a range of 87–172 repeats (eFigure 2). We cross-validated the presence of repeat expansions in 14 patients using RP-PCR (eFigure 3), and intranuclear inclusions were also confirmed from 2 patients (P02, P04), who underwent skin biopsies (eFigure 4). Female patients (10/16, 62.5%) were predominant, and the median age at onset was 57 years (range: 34–76). Among the 16 patients, 4 (25.0%) had a family history of neurologic disorders in their first-degree relatives or siblings. Table 1 provides the detailed clinical characteristics of these patients.
Figure 1. Study Cohorts and Overall Workflow.
(A) We screened the brain MRI scans of 90 undiagnosed patients with adult-onset leukoencephalopathy who showed the zigzag edging signs on DWI (SNUH cohort). As a result, 20 patients were identified, and Cas9-mediated nanopore sequencing was performed for 16 patients after excluding 2 for whom samples were unavailable and 2 patients with FXTAS. (B) We included 3,887 individuals from the Korea Biobank cohort who underwent whole-genome sequencing (Korea Biobank cohort). We investigated the NOTCH2NLC GGC repeats and identified 6 putative patients with repeat expansions. DWI = diffusion-weighted imaging.
Table 1.
Clinical Characteristics of Adult-Onset Leukoencephalopathy Patients With NOTCH2NLC GGC Repeat Expansions Identified in the SNUH Cohort
| ID | Sex/onset age, y | Expanded repeat counts | Initial symptom | EE | HA | Fever | CI | VD | Tremor | Abnormal gait | Pism | Dementia | PN/MW | UI/FI |
| P01 | M/34 | 87 | CI | + | + | − | + | + | − | − | − | − | + | − |
| P02 | F/76 | 103 | CI | + | + | + | + | + | − | + | − | − | − | + |
| P03a | F/50 | 90 | Tremor | + | + | + | + | + | + | − | − | − | − | − |
| P04 | F/55 | 106 | Dysarthria | + | + | + | + | − | − | − | − | − | − | − |
| P05 | M/65 | 94 | Aphasia, apraxia | + | + | + | + | + | − | − | − | − | − | − |
| P06 | F/58 | 163 | Dementia | + | + | + | + | + | − | + | + | + | − | + |
| P07 | M/59 | 98 | Nausea, HA, CI | + | + | + | + | + | + | + | − | − | − | − |
| P08 | F/57 | 172 | Tremor | − | − | − | − | + | + | − | + | − | − | − |
| P09a | F/51 | 135 | Tremor | − | − | − | − | − | + | + | − | − | − | − |
| P10a | M/68 | 160 | De | − | − | − | + | − | − | + | − | + | − | + |
| P11 | F/60 | 155 | Tremor | − | + | + | + | − | + | − | − | − | − | − |
| P12 | M/62 | 125 | HA | − | + | − | + | − | − | − | − | − | − | − |
| P13 | M/52 | 166 | Neck, lumbar pain | − | − | − | − | − | − | + | − | − | + | − |
| P14 | F/57 | 142 | Ataxia | − | − | − | + | − | − | + | − | − | − | − |
| P15 | F/58 | 115 | VD | − | − | − | + | + | − | − | − | − | − | − |
| P16a | F/55 | 126 | CI, apraxia | − | − | − | + | − | + | + | + | − | + | − |
Abbreviations: CI = cognitive impairment; EE = encephalitis-like episode; HA = headache; Pism = parkinsonism; PN/MW = peripheral neuropathy/muscle weakness; UI/FI = urinary/fecal incontinence; VD = visual disturbance.
Positive cases are expressed in bold.
Family history of neurologic disorders in their first-degree relatives or siblings.
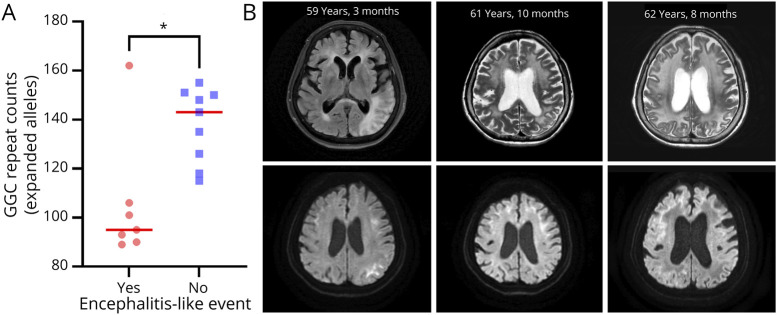
Mitochondrial encephalomyopathy, lactic acidosis, and stroke-like episode (MELAS)-like or encephalitis-like episodes are major devastating conditions observed in patients with NIID.30 We observed that 7 of 16 patients (43.8%) with NIID had these encephalitis-like episodes with distinguishable clinical features. These episodes were significantly accompanied by headache (100%, p = 0.0032), fever (83.3%, p = 0.0087), and visual disturbance (83.3%, p = 0.041). Of interest, the patients with NIID displaying encephalitis-like episodes tended to have significantly shorter expanded repeats than those without encephalitis-like episodes (p = 0.016; Figure 2A). In patients displaying encephalitis-like episodes, various changes were noticed in their brain MRI findings, particularly with the timing of the encephalitis-like episodes (Figure 2B). The zig-zag edging signs tended to exhibit a more extensive and pronounced distribution during the events, even manifesting as widespread high-signal intensities that extended to cortical areas in T2-weighted imaging. Collectively, our results show that NOTCH2NLC repeat expansions are a frequent genetic cause of adult-onset leukoencephalopathy in Koreans who exhibit the typical zigzag edging signs.
Figure 2. Association Between NOTCH2NLC Repeats and Clinical Manifestations of Encephalitis-Like Episodes.
(A) A negative association between NOTCH2NLC repeats and the occurrence of encephalitis-like episodes. The SNUH cohort of NIID patients with encephalitis-like episodes had significantly shorter NOTCH2NLC repeats than those without these events (Mann-Whitney U test, p = 0.016). (B) Changes in brain MRI findings over time in patient P04. The left and right images were acquired during encephalitis-like episodes, while the middle images were obtained during a stable stage. Two images on the left (upper, T2-FLAIR; lower, DWI) show high-signal intensities (SIs) with diffusion restriction in the left parieto-occipital lobe and multifocal confluent high SIs in both the periventricular and subcortical white matter areas. The middle and right images were taken 2 and 3 years later, respectively (upper, T2-weighted; lower, DWI). In this series of images, it is evident that leukoencephalopathy deteriorates over time, and the regions exhibiting diffusion restriction vary at each time point. DWI = diffusion-weighted imaging.
Distribution of NOTCH2NLC Repeat Counts in 2,737 Unrelated Koreans
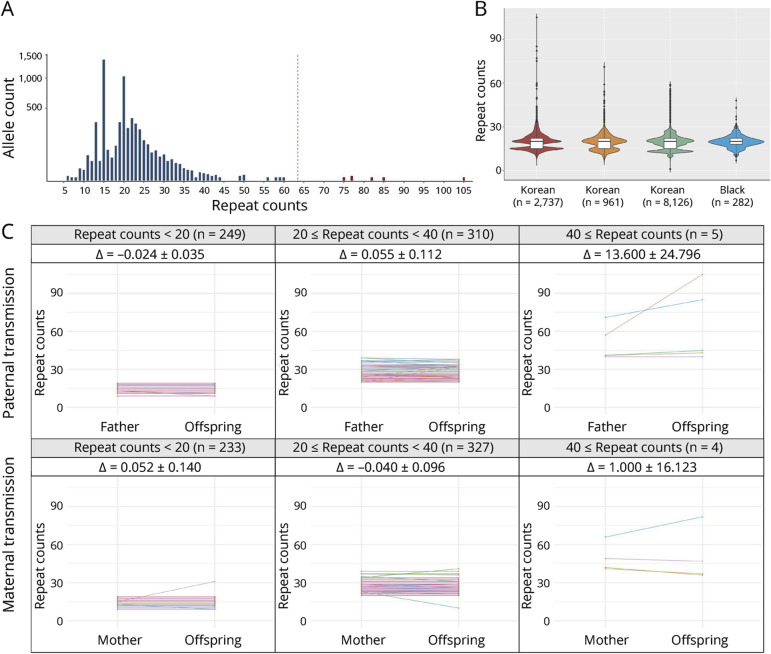
To determine the prevalence of expanded alleles in the Korean population, we investigated the GGC repeat counts in the Korea Biobank cohort of 2,737 unrelated individuals (Figure 1B). After removing alleles with an allele depth of less than 3.0, 5,446 alleles were used for further analysis. The most common repeat size was 15, found in 25.5% (1,389/5,446) of the total alleles, followed by 20 repeats occurring in 18.9% (1,031/5,446). The repeat count ranged between 6 and 105 (95% confidence interval: 13–32; Figure 3A). We identified 6 extreme outliers (Z scores >6) above the threshold (>64 repeats), based on the expected range of a normal distribution. After excluding these outliers, the distribution of repeat counts in the Korean population was 6–60, with a median of 20 repeats. We also identified 9 individuals with intermediate length GGC repeats in the range of 49–60 (eTable 2). Among these, 2 individuals (KBB9, KBB10) had neurologic symptoms which were potentially associated with NOTCH2NLC-related disorders, while others have no associated symptoms.
Figure 3. Exploring NOTCH2NLC GGC Repeat Expansions in the Korea Biobank Cohort.
(A) Distribution of the sizes of GGC repeats in 2,737 unrelated controls (5,446 alleles). The red dashed line represents the obtained cutoff value of 63.48. The 6 outliers are colored in red. The y-axis displays the allele count value on a square root transformed scale. (B) Size comparison of the GGC repeats among various ethnicities: 2,737 Korean, 961 Asian, 8,126 White, and 282 Black populations. (C) Clustering of STR transmission patterns within the NOTCH2NLC region, which has been categorized based on the number of STRs present in the parents. The magnitude of change (∆) within the interval is indicated by the 95% confidence interval.
We also compared this distribution with other populations using the 100,000 Genomes Project data, and GGC repeats did not significantly vary among Asian, White, and Black populations.25,31 When we juxtaposed our findings with the aforementioned data, the distribution showed a high degree of concordance across Korean, Asian, and White populations (Figure 3B, eTable 3). The distribution in the Black population displayed a subtle deviation from other populations, suggesting possible variations in their genetic architecture, while comparatively low numbers of the African population (n = 282) might also affect the distribution.
By phasing the parental origin, we analyzed the changes in GGC repeat counts among 703 trios. We observed that length changes tended to be more frequent in longer alleles than in shorter alleles (Figure 3C). However, no evidence of bias in allele transmission was observed depending on parental sex. Finally, we scrutinized the trios that stood as outliers, which had 82, 85, and 105 repeats. The repeat counts in the offspring expanded more during transmission compared with that in the corresponding parent (66, 71, and 57 repeats, respectively).
Putative NOTCH2NLC-Related Patients Identified in the Korea Biobank Cohort
A total of 3,887 individuals were assessed in the Korea Biobank cohort, and 6 individuals (Table 2) were suspected of being putative patients based on the expected distribution of NOTCH2NLC repeats (Figure 3A). Two were pediatric patients, while the remaining 4 were adults with reported neurologic disorders, including Charcot-Marie-Tooth (CMT) disease, adult-onset leukodystrophy, and/or retinitis pigmentosa. Except for the patient with a metabolic disorder (KBB4), the neurologic or ophthalmologic symptoms in the other patients could be attributed to NOTCH2NLC-related disorders.5-11 Although detailed clinical information was not available for the other patients, one pediatric-onset patient (KBB1P) was enrolled from our institution, allowing us to conduct a more comprehensive investigation.
Table 2.
Putative Patients Associated With the Expanded NOTCH2NLC Alleles Identified in the Korea Biobank Cohort
| Patient | Sex | Age |
NOTCH2NLC GGC repeatsa |
Clinical information | Phenotypic association with NOTCH2NLC-related disorders |
| KBB1 | Male | 17 | 105 (109)b | Neurology and neurodevelopmental disorders Motor and sensory disorders of the peripheral nervous system Hereditary neuropathy (including Charcot-Marie-Tooth disease) |
Yes |
| KBB2 | Female | 48 | 85 | Neurology and neurodevelopmental disorders Motor and sensory disorders of the peripheral nervous system Charcot-Marie-Tooth disease |
Yes |
| KBB3 | Male | 3 | 82 | Metabolic disorders Specific metabolic abnormalities |
Unknown |
| KBB4 | Male | 54 | 77 | Ophthalmologic disorders Posterior segment abnormalities Retinal disorders, retinitis pigmentosa |
Yes |
| KBB5 | Female | 64 | 77 | Neurology and neurodevelopmental disorders Motor and sensory disorders of the peripheral nervous system Charcot-Marie-Tooth disease |
Yes |
| KBB6 | Male | 68 | 75 | Neurology and neurodevelopmental disorders Adult-onset leukodystrophy |
Yes |
The repeat counts were estimated using short-read whole-genome sequencing.
The calculated repeat counts using nanopore long-read sequencing in the parenthesis.
Characterization of Transmission and Epigenomic Patterns in the KBB1 Family
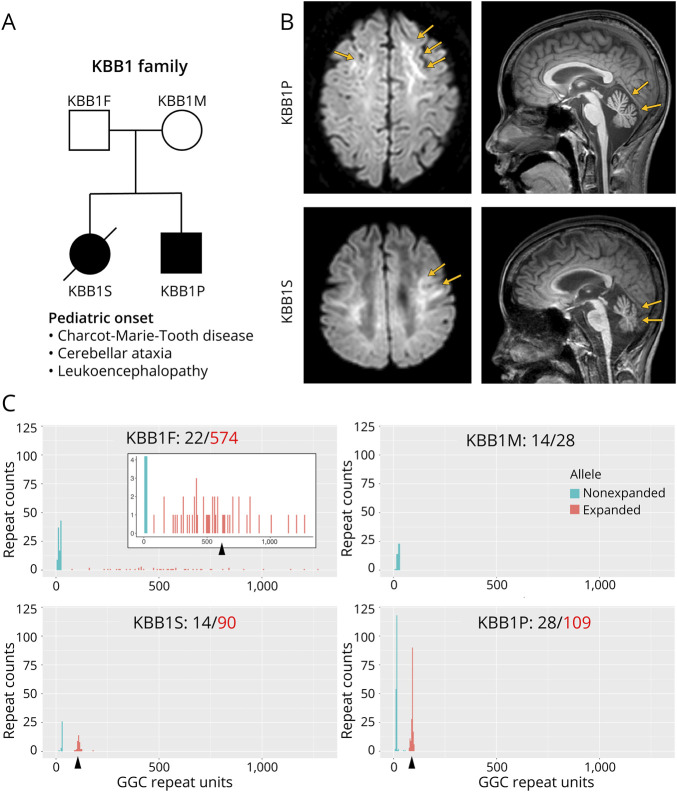
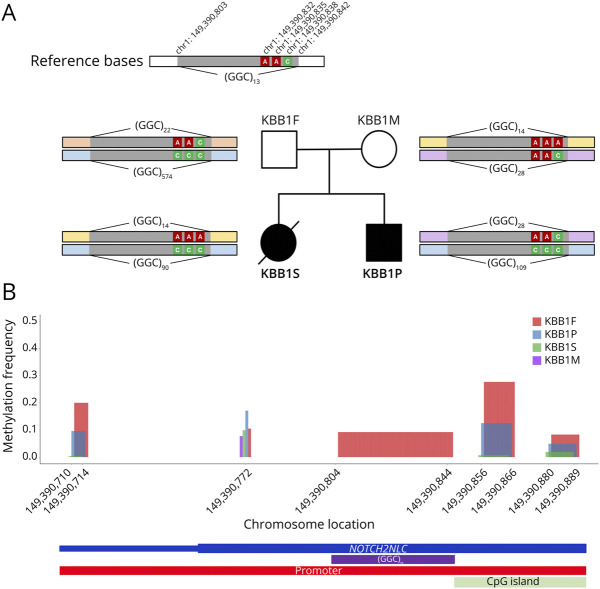
The patient (KBB1P) was the second child in the KBB1 family. NOTCH2NLC repeat counts, estimated by ExpansionHunter using short-read WGS data, were as follows in the KBB1 family: 29/105 (KBB1P), 22/57 (KBB1F), and 15/29 (KBB1M). Of interest, we found that the first child (KBB1S) was also affected, and both patients exhibited progressive neurologic symptoms resembling CMT disease and cerebellar ataxia, with symptom onset at early school age (Figure 4A). The first child experienced more severe manifestations and tragically died in the late 10s, with no definitive cause identified. Brain MRI findings of these 2 patients unveiled distinctive features, including the presence of zigzag edging signs and cerebellar atrophy, consistent with the characteristic imaging patterns associated with NOTCH2NLC-related disorders (Figure 4B).
Figure 4. A Korean Family With NOTCH2NLC-Related Disorders Identified in the Korea Biobank Cohort.
(A) Pedigree information of the KBB1 family. Both the first child (KBB1S) and the second child (KBB1P) exhibited cerebellar ataxia and Charcot-Marie-Tooth disease. The first child had more severe symptoms with rapid progression and died at the age of late 10s. (B) Brain MRI of both affected patients showed the zigzag edging sign and cerebellar atrophy. (C) Nanopore long-read sequencing for the family members. Both affected patients had NOTCH2NLC repeat expansions within the disease-associated range (KBB1S: 90; KBB1P: 109), while the father was found to possess an extremely long allele (574) that differed from the estimation by ExpansionHunter with the short-read data (57).
To validate our findings, we conducted Cas9-mediated nanopore long-read sequencing using DNA samples from all 4 members of the KBB1 family, including preserved DNA from the deceased first child (Figure 4C). The sequencing results yielded repeat lengths of 22/574 for the father (KBB1F), 14/28 for the mother (KBB1M), 14/90 for the first child (KBB1S), and 28/109 for the second child (KBB1P). In addition, PacBio HiFi long-read sequencing further ascertained the presence of repeat expansion in KBB1S (eFigure 5). These results confirmed the utility of short-read STR analysis as a preliminary screening tool for the NOTCH2NLC repeat expansions. However, it is noteworthy that the asymptomatic father indeed possessed an exceptionally long allele (574 repeats), significantly deviating from the estimation provided by ExpansionHunter in the short-read data (57 repeats). This discrepancy underscores the limitations of short-read WGS in precisely estimating lengthy repeat counts.
To investigate the origin of disease-associated repeats, we conducted allele-phasing using 3 single-nucleotide variants (rs1278726608, rs1325353797, and rs1396053493) located within the NOTCH2NLC gene (Figure 5A). This analysis determined that the expanded allele in the father (574 repeats) was passed on to the offspring, albeit with a reduction in repeat numbers (KBB1P: 109; KBB1S: 90 repeats). A previous study has reported similar instances of repeat contraction and demethylation during paternal transmission of unusually long NOTCH2NLC repeats.32,33 Particularly, the previous study demonstrated that the contraction of expanded GGC repeat expansion in sperm could be a possible mechanism for the paternal-biased origin in some sporadic or recessive inherited NIID individuals.32
Figure 5. Patterns of Allele Transmissions and Methylation Within the NOTCH2NLC Promoter Region in the KBB1 Family.
(A) Investigation of allele transmission based on 3 single-nucleotide variants. The allele-phasing analysis reveals that the long-expanded allele (pale blue) in the asymptomatic father was transmitted to the offspring with contraction. (B) Methylation frequencies within the NOTCH2NLC promoter region (chr1:149,390,710-149,390,901) among the KBB1 family members. The asymptomatic father (KBB1F, red) exhibits significantly higher methylation frequencies (hypermethylation) than other family members.
Therefore, we hypothesize that the asymptomatic status of the father may be attributed to hypermethylation in the promoter regions of the NOTCH2NLC gene, which would suppress the expression of these disease-associated repeats. To validate our hypothesis, we conducted an in-depth examination of the methylation profiles within the NOTCH2NLC promoter region for the 4 family members, using Nanopolish (Figure 5B). Intriguingly, our analysis unveiled noteworthy variations in methylation levels within the promoter region among the KBB1 family members (eTable 4) and patients in the SNUH cohort (eTable 5). The asymptomatic father exhibited a significantly elevated methylation frequency (mean: 13.5%) compared with their affected offspring, KBB1P (mean: 4.2%), and KBB1S (mean: 0.6%) as well as other patients. Furthermore, the second child (KBB1P) displayed significantly higher methylation levels (p = 0.027) than the first child (KBB1S), implying that not only repeat counts but also methylation levels in the expanded allele might be associated with the disease's severity. Specifically, the more pronounced demethylation in KBB1S likely resulted in increased transcription of the disease-associated repeat expansions, manifesting as more severe neurologic symptoms.
Discussion
NIID has been regarded as a rare disease entity within the spectrum of adult-onset leukoencephalopathy. However, the identification of genetic loci within the NOTCH2NLC gene has led to an increased recognition of patients with various neurologic disorders in East Asian populations.5-16 Notably, a recent study reported that the NOTCH2NLC-related disorder is the second most common cause of genetic leukoencephalopathy, accounting for 19% in a Chinese cohort.20 By contrast, the occurrence of NOTCH2NLC GGC expansion has been rarely observed in Europeans, indicating a distinct genetic etiology that varies across ethnic groups.17,18
Despite extensive studies on NOTCH2NLC repeat expansions in other East Asian populations, the burden of NOTCH2NLC-related diseases remains poorly investigated in Koreans. In this study, we conducted large-scale investigations using 2 distinct cohorts. For the SNUH cohort, we used long-read nanopore sequencing to screen highly suspicious cases based on brain imaging findings and identified 16 patients with NOTCH2NLC repeat expansions, accounting for 17.8% of patients with adult-onset leukoencephalopathy. In addition, we characterized the distribution of repeat counts using the Korean population data (Korea Biobank cohort) and identified 6 putative patients whose clinical features were largely consistent with those of NOTCH2NLC-related disorders. Our findings provide compelling evidence that NOTCH2NLC repeat expansions represent a frequent cause of adult-onset leukoencephalopathy in Koreans as reported in other East Asian populations. However, the reported prevalence of NOTCH2NLC-related disorders in the Korean Biobank cohort, which is up to 0.26% (7 of 2,737; Table 2, eTable 2), might represent an overestimation compared with the general Korean population.
In the SNUH cohort, we screened patients with NIID using the pathognomonic brain MRI findings of the zigzag edging sign, which was previously reported to have high sensitivity (88.2%) and specificity (98.4%).19 Strikingly, all 16 patients with the zigzag edging signs had expanded alleles. However, 2 patients with FXTAS also showed the typical zigzag edging pattern on DWI. This result implied that FXTAS should be ruled out in patients with the zigzag edging sign because the brain MRI findings are indistinguishable between NIID and FXTAS.34 It is noteworthy that when the zigzag edging sign is presented, it is more likely to be NIID (88.9%) rather than FXTAS (11.1%) in Koreans.
We found a higher frequency (7/16, 43.8%) of encephalitis-like episodes in our cohort than in the largest NIID cohort (58/247, 23.5%).12 We observed that encephalitis-like episodes were frequently accompanied by headache, fever, and visual disturbances, which have also been observed in previous studies.30,35-37 Noticeably, we observed that patients with NIID experiencing encephalitis-like episodes tended to have significantly shorter repeat counts in the expanded alleles than the others (p = 0.016, Figure 2A). Despite the marginal p value (p = 0.06), this negative association between the number of GGC repeats and encephalitis-like episodes was also observed in a Chinese cohort (n = 247).12 Recent studies have established fly and mouse models for NOTCH2NLC repeat expansions.38,39 In these animal models, neurotoxicity caused by polyglycines and mitochondrial dysfunction have been suggested as the major disease-associated mechanisms. In our study, approximately half of the patients exhibited MELAS-like or encephalitis-like episodes, and recurrent episodes largely affected disease progression and prognosis.30 Further research is required to determine the molecular mechanisms responsible for the formation of MELAS-like lesions in NIID. This investigation could potentially provide insights into viable treatment options for symptom prevention or management, including the exploration of antioxidants or anti-inflammatory agents.
The first and second children in the KBB1 family remained undiagnosed for over a decade, highlighting the diagnostic challenge in such cases. Fortunately, the detection of NOTCH2NLC repeat expansions by ExpansionHunter provided a valuable diagnostic clue. Notably, our patients exhibited clear neurologic symptoms that manifested before the age of 10 years, possibly constituting one of the earliest-onset cases reported globally.40 Despite exhibiting neurologic symptoms at an early age, the lengths of their repeat expansions did not significantly differ from those observed in other adult patients. This observation raises the possibility that additional genetic or environmental factors may contribute to disease prognosis. The asymptomatic father in the KBB1 family implies that the presence of an exceptionally long repeat more than 300 may not invariably lead to disease manifestation.33 Our epigenetic analysis further corroborated previous findings that hypermethylation of the NOTCH2NLC promoter region can silence the expression of disease-associated repeats, resulting in an unaffected status in their father.32 Specifically, it has been identified that hypermethylation in the asymptomatic carrier led to decreased mRNA levels in muscle biopsies.33 Furthermore, a previous study reported that the DNA methylation status of the NOTCH2NLC promoter region is negatively correlated with the age at onset, the number of multi-systemic involvements, and positively correlated with GGC repeats.41 Our findings further support the previously documented phenomenon of large repeat contractions leading to shorter disease-associated repeat expansions, a process that may occur during spermatogenesis in male carriers. Future studies with repeat count estimation combined with comprehensive methylation profiling in a larger cohort may yield deeper insight into the underlying mechanism modulating disease severity of NIID. While sex differences in the transmission of NOTCH2NLC repeats were considered, our study did not yield significant variations between paternal and maternal transmissions (Figure 3C). This finding underscores the need for larger cohort studies to delve deeper into this aspect, shedding more light on the intricate mechanisms governing the transmission and effect of NOTCH2NLC repeats.
Although NOTCH2NLC-related disorders have been shown to be prevalent in the Korean population, our observations did not reveal significant differences in the distribution of repeat counts across various ethnic groups, based on estimations using short-read sequencing. However, our findings do include a few instances of intermediate to long repeats in the NOTCH2NLC genes, unique to the Korean population (Figure 3B). Given the discrepancy in repeat counts observed in KBB1F between long-read (577 repeats) and short-read (57 repeats) sequencing, it is plausible that expanded alleles might have been underestimated in short-read data sets. It is also plausible that some low-frequency variants, specific to the East Asian population, may serve as founder mutations without significantly affecting the repeat count distribution.17,18 In addition, population-specific genetic modifiers, such as GGA sequence interruptions observed in Southeast Asia, could exacerbate the manifestation and severity of NIID.42 Furthermore, as indicated in our familial case study, epigenetic mechanisms may also contribute to shaping the landscape of NIID across diverse populations.32,33,41 Collectively, these genetic and epigenetic differences may explain why NOTCH2NLC-related disorders are more prevalent and diverse in East Asians than in other populations. However, further research and collaborative efforts are required to comprehensively understand the full spectrum of genetic factors affecting the manifestations of NIID across various ethnic groups.
Until now, NIID has been scarcely investigated within the Korean population. Our aim was to screen for patients with NIID in a conservative manner: 2 independent neurologists thoroughly reviewed not only the MRI images to identify representative features of NIID, but also the medical records to exclude medical conditions that could cause or mimic leukodystrophy. Consequently, a significant number of patients were excluded from the initial cohort, resulting in a relatively small final sample size for NIID screening in this study (n = 16). Given the diverse clinical conditions associated with NOTCH2NLC repeat expansions, it is possible that some patients with atypical or subtle imaging features or clinical presentations might have been overlooked. Future studies should aim to screen a broader range of imaging findings and clinical presentations suspected of being associated with NIID in the Korean population.
In conclusion, this study showed that NOTCH2NLC repeat expansions are a major genetic cause of adult-onset leukoencephalopathy in Koreans, particularly when the typical zigzag edging sign is observed. In addition, our study provided the population-wide distribution of the NOTCH2NLC repeat counts and underscored the utility of short-read WGS in screening disease-associated repeat counts within the NOTCH2NLC gene, while long-read sequencing platforms are indispensable for precise repeat count estimation. Furthermore, our familial case expands the disease spectrum of NOTCH2NLC-related disorders to include pediatric-onset patients. Our results provide compelling evidence for the role of differential methylation in the promoter regions of the NOTCH2NLC gene, coupled with contraction during paternal transmission, contributing to disease severity. These results emphasize the necessity of considering not only repeat count estimation but also epigenomic profiling for a comprehensive characterization of NOTCH2NLC-related disorders.
Acknowledgment
The authors thank the participating patients who contributed to this study. This research was made possible through access to data and findings in the National Genomic Research Library via the Genomics England Research Environment.
Glossary
- DWI
diffusion-weighted imaging
- MELAS
mitochondrial encephalomyopathy, lactic acidosis, and stroke
- NIID
neuronal intranuclear inclusion disease
- SNUH
Seoul National University Hospital
- STRs
short tandem repeats
Appendix. Authors
| Name | Location | Contribution |
| Seungbok Lee, MD, PhD | Department of Genomic Medicine, Seoul National University Hospital; Department of Pediatrics, Seoul National University College of Medicine, Seoul National University Children’s Hospital, Korea | Drafting/revision of the manuscript for content, including medical writing for content; study concept or design; analysis or interpretation of data |
| Jihoon G. Yoon, MD, PhD | Department of Genomic Medicine, Seoul National University Hospital, Korea | Drafting/revision of the manuscript for content, including medical writing for content; study concept or design; analysis or interpretation of data |
| Juhyeon Hong, BS | Department of Biomedical Sciences, Korea University College of Medicine, Korea | Drafting/revision of the manuscript for content, including medical writing for content; analysis or interpretation of data |
| Taekeun Kim, BS | Department of Biomedical Sciences, Korea University College of Medicine, Korea | Drafting/revision of the manuscript for content, including medical writing for content; analysis or interpretation of data |
| Narae Kim, PhD | Department of Neurology, Seoul National University Hospital, Korea | Major role in the acquisition of data |
| Jana Vandrovcova, PhD | Department of Neuromuscular Diseases, Institute of Neurology, University College London, United Kingdom | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data |
| Wai Yan Yau, MD | Perron Institute for Neurological and Translational Science, the University of Western Australia, Nedlands, Australia | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data |
| Jaeso Cho, MD | Department of Genomic Medicine, Seoul National University Hospital; Department of Pediatrics, Seoul National University College of Medicine, Seoul National University Children’s Hospital, Korea | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data |
| Sheehyun Kim, MD | Department of Genomic Medicine, Seoul National University Hospital, Korea | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data |
| Man Jin Kim, MD, PhD | Department of Genomic Medicine; Department of Laboratory Medicine, Seoul National University Hospital, Korea | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data |
| Soo Yeon Kim, MD, PhD | Department of Genomic Medicine, Seoul National University Hospital; Department of Pediatrics, Seoul National University College of Medicine, Seoul National University Children’s Hospital, Korea | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data |
| Soon-Tae Lee, MD, PhD | Department of Neurology, Seoul National University Hospital, Korea | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data |
| Kon Chu, MD, PhD | Department of Neurology, Seoul National University Hospital, Korea | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data |
| Sang Kun Lee, MD, PhD | Department of Neurology, Seoul National University Hospital, Korea | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data |
| Han-Joon Kim, MD, PhD | Department of Neurology, Seoul National University Hospital, Korea | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data |
| Jungmin Choi, PhD | Department of Biomedical Sciences, Korea University College of Medicine, Seoul | Drafting/revision of the manuscript for content, including medical writing for content; study concept or design; analysis or interpretation of data |
| Jangsup Moon, MD, PhD | Department of Genomic Medicine; Department of Neurology, Seoul National University Hospital, Korea | Drafting/revision of the manuscript for content, including medical writing for content; major role in the acquisition of data; study concept or design |
| Jong-Hee Chae, MD, PhD | Department of Genomic Medicine, Seoul National University Hospital; Department of Pediatrics, Seoul National University College of Medicine, Seoul National University Children’s Hospital, Korea | Drafting/revision of the manuscript for content, including medical writing for content; study concept or design; analysis or interpretation of data |
Study Funding
This study (CODA_S2200009-01) was provided with bioresources from CODA in the National Biobank of Korea, the Agency for Disease Control and Prevention, Republic of Korea. This research was made possible through access to the data and findings generated by the 100,000 Genomes Project. The 100,000 Genomes Project is managed by Genomics England Limited (a wholly owned company of the Department of Health and Social Care). The 100,000 Genomes Project is funded by the National Institute for Health Research and NHS England. The Wellcome Trust, Cancer Research UK, and the Medical Research Council have also funded research infrastructure. The 100,000 Genomes Project uses data provided by patients and collected by the National Health Service as part of their care and support. This study was supported by the Institute of Information & communications Technology Planning & Evaluation (IITP) grant funded by the Korean government (MSIT, grant number: 2022-0-00333) and was also supported by funds from the Research of Korea Centers for Disease Control and Prevention (2020-ER6904-01) and the Seoul National University Hospital Research Fund (0420220470). This research was supported by the Bio & Medical Technology Development Program of the National Research Foundation (NRF-2020M3E5D7085175) funded by the Ministry of Health and Welfare, Ministry of Science and ICT, Ministry of Trade Industry and Energy, Korea Disease Control and Prevention Agency (The National Project of Bio Big Data).
Disclosure
J. Choi received support from a Korea University Grant (K1925121). J. Moon was supported by the Korean Society of Medical Genetics and Genomics (KSMG-AR-202203). All other authors report no disclosures relevant to the manuscript. Go to Neurology.org/NG for full disclosures.
References
- 1.Lindenberg R, Rubinstein LJ, Herman MM, Haydon GB. A light and electron microscopy study of an unusual widespread nuclear inclusion body disease. A possible residuum of an old herpesvirus infection. Acta Neuropathol. 1968;10(1):54-73. doi: 10.1007/BF00690510 [DOI] [PubMed] [Google Scholar]
- 2.Sone J, Tanaka F, Koike H, et al. Skin biopsy is useful for the antemortem diagnosis of neuronal intranuclear inclusion disease. Neurology. 2011;76(16):1372-1376. doi: 10.1212/WNL.0b013e3182166e13 [DOI] [PubMed] [Google Scholar]
- 3.Sone J, Mori K, Inagaki T, et al. Clinicopathological features of adult-onset neuronal intranuclear inclusion disease. Brain. 2016;139(Pt 12):3170-3186. doi: 10.1093/brain/aww249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen L, Chen A, Lei S, He L, Zhou M. Teaching NeuroImages: the zigzag edging sign of adult-onset neuronal intranuclear inclusion disease. Neurology. 2019;92(19):e2295-e2296. doi: 10.1212/WNL.0000000000007464 [DOI] [PubMed] [Google Scholar]
- 5.Ishiura H, Shibata S, Yoshimura J, et al. Noncoding CGG repeat expansions in neuronal intranuclear inclusion disease, oculopharyngodistal myopathy and an overlapping disease. Nat Genet. 2019;51(8):1222-1232. doi: 10.1038/s41588-019-0458-z [DOI] [PubMed] [Google Scholar]
- 6.Fang P, Yu Y, Yao S, et al. Repeat expansion scanning of the NOTCH2NLC gene in patients with multiple system atrophy. Ann Clin Transl Neurol. 2020;7(4):517-526. doi: 10.1002/acn3.51021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jiao B, Zhou L, Zhou Y, et al. Identification of expanded repeats in NOTCH2NLC in neurodegenerative dementias. Neurobiol Aging. 2020;89:142 e1-e42 e7. doi: 10.1016/j.neurobiolaging.2020.01.010 [DOI] [PubMed] [Google Scholar]
- 8.Ma D, Tan YJ, Ng ASL, et al. Association of NOTCH2NLC repeat expansions with Parkinson disease. JAMA Neurol. 2020;77(12):1559-1563. doi: 10.1001/jamaneurol.2020.3023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sun QY, Xu Q, Tian Y, et al. Expansion of GGC repeat in the human-specific NOTCH2NLC gene is associated with essential tremor. Brain. 2020;143(1):222-233. doi: 10.1093/brain/awz372 [DOI] [PubMed] [Google Scholar]
- 10.Yuan Y, Liu Z, Hou X, et al. Identification of GGC repeat expansion in the NOTCH2NLC gene in amyotrophic lateral sclerosis. Neurology. 2020;95(24):e3394-e3405. doi: 10.1212/WNL.0000000000010945 [DOI] [PubMed] [Google Scholar]
- 11.Nakamura N, Tsunoda K, Mitsutake A, et al. Clinical characteristics of neuronal intranuclear inclusion disease-related retinopathy with CGG repeat expansions in the NOTCH2NLC gene. Invest Ophthalmol Vis Sci. 2020;61(11):27. doi: 10.1167/iovs.61.11.27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tian Y, Zhou L, Gao J, et al. Clinical features of NOTCH2NLC-related neuronal intranuclear inclusion disease. J Neurol Neurosurg Psychiatry. 2022;93(12):1289-1298. doi: 10.1136/jnnp-2022-329772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ando M, Higuchi Y, Yuan JH, et al. Clinical phenotypic diversity of NOTCH2NLC-related disease in the largest case series of inherited peripheral neuropathy in Japan. J Neurol Neurosurg Psychiatry. 2023;94(8):622-630. doi: 10.1136/jnnp-2022-330769 [DOI] [PubMed] [Google Scholar]
- 14.Sone J, Mitsuhashi S, Fujita A, et al. Long-read sequencing identifies GGC repeat expansions in NOTCH2NLC associated with neuronal intranuclear inclusion disease. Nat Genet. 2019;51(8):1215-1221. doi: 10.1038/s41588-019-0459-y [DOI] [PubMed] [Google Scholar]
- 15.Tian Y, Wang JL, Huang W, et al. Expansion of human-specific GGC repeat in neuronal intranuclear inclusion disease-related disorders. Am J Hum Genet. 2019;105(1):166-176. doi: 10.1016/j.ajhg.2019.05.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Okubo M, Doi H, Fukai R, et al. GGC repeat expansion of NOTCH2NLC in adult patients with leukoencephalopathy. Ann Neurol. 2019;86(6):962-968. doi: 10.1002/ana.25586 [DOI] [PubMed] [Google Scholar]
- 17.Yau WY, Sullivan R, Chen Z, et al. GGC Repeat expansion in NOTCH2NLC is rare in European leukoencephalopathy. Ann Neurol. 2020;88(3):641-642. doi: 10.1002/ana.25818 [DOI] [PubMed] [Google Scholar]
- 18.Chen Z, Yan Yau W, Jaunmuktane Z, et al. Neuronal intranuclear inclusion disease is genetically heterogeneous. Ann Clin Transl Neurol. 2020;7(9):1716-1725. doi: 10.1002/acn3.51151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu YH, Chou YT, Chang FP, et al. Neuronal intranuclear inclusion disease in patients with adult-onset non-vascular leukoencephalopathy. Brain. 2022;145(9):3010-3021. doi: 10.1093/brain/awac135 [DOI] [PubMed] [Google Scholar]
- 20.Wu C, Wang M, Wang X, et al. The genetic and phenotypic spectra of adult genetic leukoencephalopathies in a cohort of 309 patients. Brain. 2023;146(6):2364-2376. doi: 10.1093/brain/awac426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chun MY, Park HK, Kim GH, et al. Adult-onset neuronal intranuclear inclusion disease: first Korean case confirmed by skin biopsy. J Clin Neurol. 2020;16(4):720-722. doi: 10.3988/jcn.2020.16.4.720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pedersen BS, Bhetariya PJ, Brown J, et al. Somalier: rapid relatedness estimation for cancer and germline studies using efficient genome sketches. Genome Med. 2020;12(1):62. doi: 10.1186/s13073-020-00761-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dolzhenko E, Deshpande V, Schlesinger F, et al. ExpansionHunter: a sequence-graph-based tool to analyze variation in short tandem repeat regions. Bioinformatics. 2019;35(22):4754-4756. doi: 10.1093/bioinformatics/btz431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dolzhenko E, Weisburd B, Ibanez K, et al. REViewer: haplotype-resolved visualization of read alignments in and around tandem repeats. Genome Med. 2022;14(1):84. doi: 10.1186/s13073-022-01085-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yau WY, Vandrovcova J, Sullivan R, et al. Low prevalence of NOTCH2NLC GGC repeat expansion in white patients with movement disorders. Mov Disord. 2021;36(1):251-255. doi: 10.1002/mds.28302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li H. Minimap2: pairwise alignment for nucleotide sequences. Bioinformatics. 2018;34(18):3094-3100. doi: 10.1093/bioinformatics/bty191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chiu R, Rajan-Babu IS, Friedman JM, Birol I. Straglr: discovering and genotyping tandem repeat expansions using whole genome long-read sequences. Genome Biol. 2021;22(1):224. doi: 10.1186/s13059-021-02447-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Loman NJ, Quick J, Simpson JT. A complete bacterial genome assembled de novo using only nanopore sequencing data. Nat Methods. 2015;12(8):733-735. doi: 10.1038/nmeth.3444 [DOI] [PubMed] [Google Scholar]
- 29.Cohen ASA, Farrow EG, Abdelmoity AT, et al. Genomic answers for children: dynamic analyses of >1000 pediatric rare disease genomes. Genet Med. 2022;24(6):1336-1348. doi: 10.1016/j.gim.2022.02.007 [DOI] [PubMed] [Google Scholar]
- 30.Xie F, Hu X, Liu P, Zhang D. A case report of neuronal intranuclear inclusion disease presenting with recurrent migraine-like attacks and cerebral edema: a mimicker of MELAS. Front Neurol. 2022;13:837844. doi: 10.3389/fneur.2022.837844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Caulfield M, Davies J, Dennys M, et al. The National Genomics Research and Healthcare Knowledgebase, 5 ed; 2017. Genomics England. [Google Scholar]
- 32.Fukuda H, Yamaguchi D, Nyquist K, et al. Father-to-offspring transmission of extremely long NOTCH2NLC repeat expansions with contractions: genetic and epigenetic profiling with long-read sequencing. Clin Epigenetics. 2021;13(1):204. doi: 10.1186/s13148-021-01192-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Deng J, Zhou B, Yu J, et al. Genetic origin of sporadic cases and RNA toxicity in neuronal intranuclear inclusion disease. J Med Genet. 2022;59(5):462-469. doi: 10.1136/jmedgenet-2020-107649 [DOI] [PubMed] [Google Scholar]
- 34.Padilha IG, Nunes RH, Scortegagna FA, et al. MR imaging features of adult-onset neuronal intranuclear inclusion disease may be indistinguishable from fragile X-associated tremor/ataxia syndrome. AJNR Am J Neuroradiol. 2018;39(9):E100-E101. doi: 10.3174/ajnr.A5729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ishihara T, Okamoto T, Saida K, et al. Neuronal intranuclear inclusion disease presenting with an MELAS-like episode in chronic polyneuropathy. Neurol Genet. 2020;6(6):e531. doi: 10.1212/NXG.0000000000000531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huang Y, Jin G, Zhan QL, Tian Y, Shen L. Adult-onset neuronal intranuclear inclusion disease, with both stroke-like onset and encephalitic attacks: a case report. BMC Neurol. 2021;21(1):142. doi: 10.1186/s12883-021-02164-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhou Q, Tian M, Yang H, Luo YB. Adult-onset neuronal intranuclear inclusion disease with mitochondrial encephalomyopathy, lactic acidosis, and stroke-like (MELAS-like) episode: a case report and review of literature. Brain Sci. 2022;12(10):1377. doi: 10.3390/brainsci12101377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Boivin M, Deng J, Pfister V, et al. Translation of GGC repeat expansions into a toxic polyglycine protein in NIID defines a novel class of human genetic disorders: the polyG diseases. Neuron. 2021;109(11):1825-1835.e5. doi: 10.1016/j.neuron.2021.03.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yu J, Liufu T, Zheng Y, et al. CGG repeat expansion in NOTCH2NLC causes mitochondrial dysfunction and progressive neurodegeneration in Drosophila model. Proc Natl Acad Sci U S A. 2022;119(41):e2208649119. doi: 10.1073/pnas.2208649119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Miyamoto Y, Okazaki T, Watanabe K, et al. First detailed case report of a pediatric patient with neuronal intranuclear inclusion disease diagnosed by NOTCH2NLC genetic testing. Brain Dev. 2023;45(1):70-76. doi: 10.1016/j.braindev.2022.09.002 [DOI] [PubMed] [Google Scholar]
- 41.Cao Y, Tian W, Wu J, Song X, Cao L, Luan X. DNA hypermethylation of NOTCH2NLC in neuronal intranuclear inclusion disease: a case-control study. J Neurol. 2022;269(11):6049-6057. doi: 10.1007/s00415-022-11272-y [DOI] [PubMed] [Google Scholar]
- 42.Chen Z, Xu Z, Cheng Q, et al. Phenotypic bases of NOTCH2NLC GGC expansion positive neuronal intranuclear inclusion disease in a Southeast Asian cohort. Clin Genet. 2020;98(3):274-281. doi: 10.1111/cge.13802 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The SNUH cohort data are not publicly available to ensure the protection of participant anonymity. The Korea Biobank cohort data are available at coda.nih.go.kr with the accession number (CODA_D22004).