Abstract
Purpose
Radiation therapy for early-stage breast cancer is typically delivered in a hypofractionated regimen to the whole breast followed by a tumor bed boost. This results in a treatment course of approximately 4 weeks. In this study, the tumor bed boost was delivered in a single fraction as part of a safety and feasibility study for FDA clearance of the device.
Methods and Materials
Eligible women with early-stage breast cancer underwent lumpectomy followed by radiation therapy. Patients underwent breast immobilization using a system specific to the GammaPod followed by CT simulation, boost treatment planning, and boost treatment delivery all in a single treatment day. Patients then started whole-breast radiation therapy within 1 week of the boost treatment. Patients and treatments were assessed for safety and feasibility. Acute toxicities were recorded.
Results
A single-fraction boost of 8 Gy was delivered to the tumor bed before a course of whole-breast radiation. The GammaPod treatment was successfully delivered to 14 of 17 enrolled patients. Acute toxicities from all radiation therapy, inclusive of the boost and whole-breast radiation, were limited to grade 1 events.
Conclusions
The GammaPod device successfully delivered a single-fraction boost treatment to the tumor bed with no change in expected acute toxicities. The results of this study led to FDA clearance of the device through the Investigational Device Exemption process at the FDA. The GammaPod is in clinical use at 4e institutions nationally and internationally, with additional sites pending in 2023.
Introduction
Breast-conserving therapy (BCT), consisting of surgical lumpectomy followed by whole-breast radiation therapy (RT), is a standard of care for treating early-stage breast cancer. In comparison with mastectomy, BCT has shown similar outcomes with superior cosmesis and reduced psychological and emotional trauma, based on multiple randomized trials.1, 2, 3, 4 Over several decades, multiple sophisticated approaches5, 6, 7, 8, 9 have been developed to deliver radiation to only a portion of the breast. These include intracavitary brachytherapy, various intraoperative (IORT) approaches using either electron beam or low-energy x-rays, as well as external-beam RT using 3-dimensional (3D) conformal RT or intensity modulated RT (IMRT). Brachytherapy approaches have the best dose fall-off and, with modern multilumen advances, can shape the dose to minimize chest wall and skin toxicity. The major downside is the invasive nature of the treatment and the heterogeneity of dose, which can increase treatment morbidity. IORT approaches have the advantage of being 1-day treatments, with the major downside of not knowing the histologic margins before treatment, which can lead to as many as 20% of patients requiring whole-breast radiation and raises concerns about increased local failures resulting from inadequately covering the highest-risk target.8,9 The major advantage of external-beam radiation is its noninvasive nature and ease of delivery. The disadvantage of this approach is the need to treat a significantly larger volume of tissue, which appears to increase the rate of poor cosmesis.10, 11, 12
Faculty members at the University of Maryland School of Medicine invented a breast cancer-specific device for delivering stereotactic RT,13 the GammaPod (Xcision Medical Systems, LLC, Columbia, Maryland), with the support of a National Institutes of Health Small Business Innovation Research grant. Based on stereotactic radiosurgery principles, this device can deliver focused radiation to the target while sparing surrounding normal tissues and structures with rapid dose fall-off and homogeneous target delivery.14, 15, 16, 17, 18, 19, 20 In the study presented here, this device used 36 noncoplanar 60Co sources that rotate around a single isocenter, producing a dose distribution similar to that of the Gamma Knife. Dosimetric studies comparing this device to Food and Drug Administration (FDA)-approved brachytherapy applicators, 3D-conformal techniques and IMRT have demonstrated advantages in reducing the dose to the skin, chest wall, normal breast, heart, and lung with high conformality and a homogeneous dose distribution.15, 16, 17, 18 Compared with brachytherapy, the GammaPod is completely noninvasive, mitigating the need for placing and removal of a catheter. With the patient in the prone position, the device uses a unique breast stereotactic immobilization system that conforms the mobile, pliable breast into a uniform shape (Fig. 1). The breast cup is embedded with a stereotactic fiducial system, and the breast is held in place under negative pressure from simulation to treatment, similar to the frame-based approach in cranial stereotactic radiosurgery. Based on 2 prospective protocols, the breast target motion was determined to be <3 mm.19
Figure 1.
GammaPod workflow demonstrating (left to right): breast cup immobilization device, image loader, treatment plan, and treatment device.
This study reports the first in-human use of the GammaPod. The FDA requested this clinical demonstration of the device under an Investigational Device Exemption before obtaining FDA marketing clearance via the 510k mechanism. The study design was evaluated and approved by the FDA, authorizing use of an approach similar to that in early balloon-based brachytherapy, with a single fraction of radiation delivered with the GammaPod as a boost to the tumor bed, followed by whole-breast radiation. The primary endpoint of this study was to demonstrate feasibility and patient safety. Here we present initial data with a minimum of 18 months of follow-up that led to FDA clearance.
Methods and Materials
This protocol was approved through the Institutional Review Board at the University of Maryland School of Medicine. In this study, a single fraction 8-Gy tumor bed boost using the GammaPod system was followed by a hypofractionated (40 Gy in 15 fractions or 42.56 Gy in 16 fractions) course of whole-breast radiation. The single fraction boost replaced the commonly used 4-5 fraction (10 Gy) boost that follows the whole breast portion. Using the linear-quadratic model with α/β = 4.0 Gy for local tumor control, the EQD2 of an 8-Gy single-fraction dose is 16 Gy delivered in 8 fractions.21,22 Overall treatment time has been hypothesized to affect outcome in subclinical breast cancer with a time factor of 0.60 Gy/d.23 This would theoretically make a single-fraction boost more effective; however, no correction for overall time was implemented in the design of this study. Patients were considered eligible if they were women, ≥60 years old, and had a diagnosis of an invasive or noninvasive <4 cm breast cancer that had been treated surgically with a lumpectomy, with negative surgical margins. Before enrollment, the recommended RT plan must have included whole-breast RT with a tumor bed boost. To make the group more homogenous, patients were excluded if regional nodal radiation had been recommended. Because of device design, patients had to weigh <150 kg and be <6’6” tall. After meeting these criteria, participants were evaluated by the radiation oncologist on the day of CT simulation for whole-breast radiation to ensure that: (1) the surgical cavity was clearly visible; (2) the tumor bed was ≥5 mm from the skin surface; and (3) the tumor bed accounted for <25% of the whole breast. Patients were then approached for consent to participate in the study.
Procedure
Each patient underwent breast cup fitting by 1 of 2 physicians (Elizabeth Nichols, MD or Steven Feigenberg, MD). The cup has 3 rigid outer cup sizes that vary in the diameter of the base: small, medium, and large (Fig. 1). The breast cup was visually aligned so that it would collocate within the predrilled peg locations on the image loader. Each outer cup has 9 to 10 sets of inner cups with varying volumes to customize fitting for each patient. The rigid outer cup has an incorporated fiducial system that establishes the stereotactic coordinate system. The perforated inner cup is connected to a silicone flange that locks into the outer cup, forming the breast immobilization system. The area between the inner and outer cups is subjected to negative pressure by a vacuum pump and, once the system is secured to the breast, is continuously monitored until treatment is completed. This system has been described in greater detail previously.15, 16, 17, 18, 19 Patient body habitus was also evaluated, and patients were required to be able to walk short distances, stand for short periods of time, and lie comfortably in the prone position. Patient breast size and shape were also evaluated to ensure that they fit in the immobilization cups.
Once the breast cup was fitted and the vacuum seal applied, each patient was placed on the device-specific image loader, which consisted of a prone table and lifting mechanism, in the upright position, and the breast immobilization system was aligned in the loader through predrilled coordinate peg locations. The patient was then lowered onto the simulation table, into the prone position, via the image loader. The simulation table was released from the loader, and a CT was obtained with 1-mm slice thickness extending from at least 3 cm above the immobilization system to 3 cm below. After simulation, each patient was removed from the CT table via the image loader with the breast cup in place and under continuous negative pressure until treatment.
After the CT scan, images were transferred to the treatment planning system, which was specifically designed by Xcision Medical Systems for the GammaPod. The system uses inverse planning principles to optimize a dynamic path of the focal spot to paint the prescribed dose distribution, in contrast to the sphere-packing approach used for GammaKnife-based radiosurgery.15 One of 2 physicians contoured the tumor bed (defined as the surgical cavity including surgical clips and any surgical changes) plus a 5-mm uniform isotropic expansion. An additional 5-mm uniform expansion in all directions was added to this clinical target volume (CTV) to generate the planning target volume (PTV). The PTV was truncated at 5 mm from the skin surface or chest wall (defined as the pectoral muscles, ribs, and intercostals). A 5-mm margin was used in this study to make the target equivalent to that of a LINAC-based boost plan. Subsequent studies use a 3-PTV margin due to the breast immobilization device. After target delineation, a GammaPod treatment plan was optimized based on the dose prescriptions and normal tissue or organ-at-risk dose constraints. The predefined limits used in this study were: (1) no rib should receive >75% of the prescription dose; and (2) the maximum dose to heart and lung should be ≤2.5 Gy. If the dose was above the acceptable defined dose, additional contours of an avoidance structure were generated to assist the treatment planning system in pushing dose away from the organ at risk to obtain an acceptable plan. The GammaPod planning system does not currently use a conformity index as one of the optimization parameters.
Treatment delivery was not performed if the seal of the breast cup was lost or pressure fell <100 mm Hg following CT simulation. If pressure dropped before or after CT simulation but before treatment delivery, the immobilization cup was reapplied, and CT simulation was repeated. See Fig. 1 for a schematic of the GammaPod workflow.
At the conclusion of the treatment, patients were questioned about the comfort of the cup and procedure by a clinical research coordinator, and their skin was checked for signs of mechanical irritation from the breast immobilization system. Photographs were obtained. Standard whole-breast RT was administered and started within 7 days of completion of the boost treatment. Whole-breast treatments were devised using 3D techniques with field-within-field design to minimize the volume of the breast receiving 105% of the prescription dose when indicated.
Response evaluation
Patients were seen on the day of the boost treatment and weekly during the course of their whole-breast radiation. Toxicity was recorded using the NCI Common Terminology Criteria for Adverse Events (CTCAE) version 4.0 at each of these time points. Patients were then seen in follow-up at 1, 6, and 12 months after completion of therapy, with all toxicities reported and attributed (or not attributed) to treatment. Further follow-up was performed per physician-patient discussion/preferences.
Statistical design
The primary objective of this study was to demonstrate the feasibility and safety of delivering a tumor bed boost dose using the GammaPod stereotactic system for patients undergoing BCT. The primary feasibility endpoint was an estimate of reproducibility of the radiation technique. Secondary safety endpoints included the number and types of serious adverse device-related events and the incidence of acute radiation. Acute toxicity was assessed using CTCAE. Toxicities were defined as acute when they occurred within 1 month of protocol therapy. Sample size for the trial was based on a primary endpoint of quality of the radiation dose distribution.
The primary feasibility endpoint was the ability of the device to deliver an acceptable dose plan for a given patient. The trial was designed as a Simon's 2-stage design with a “good” success rate (P1) defined as 85% and a “poor” rate (P0) as 55%. Power was set at 80%, and the significance level at α = 0.05. Early study stoppage was planned if, after evaluating the device on 8 patients in the first stage, the dose distribution was acceptable for ≤5 patients. However, the trial proceeded past this point based on the recommendation of the Internal Review Board (IRB), and having met this threshold, a total of 17 patients were enrolled. Based on this expanded number, the device would meet the primary endpoint for acceptance if the dose distribution was acceptable in 12 or more patients.
Results
Between March 2016, and August 2017, 17 patients signed consent for participation in the study. The median age was 65 years (range, 60-74 years), 14 (82%) had invasive ductal carcinoma, and 3 (18%) had ductal carcinoma in situ. The majority had favorable features, including being estrogen receptor (ER) positive (82%), Her2/neu negative (65%), with negative pathologic axillary lymph nodes (100%), and small tumor size (94% <2 cm). Table 1 includes patient characteristics.
Table 1.
Patient characteristics
| Follow-up, median (range) | 32 (6-46 months) |
| Age, median (range) | 65 (60-74 years) |
| Tumor laterality, n (%) | |
| Left | 8 (47%) |
| Right | 9 (53%) |
| ER status, n (%) | |
| Positive | 14 (82%) |
| Negative | 3 (18%) |
| Her2 neu status, n (%) | |
| Positive | 1 (6%) |
| Negative | 11 (65%) |
| Unknown/not tested | 5 (29%) |
| Tumor size, n (%) | |
| <1 cm | 7 (41%) |
| 1-2 cm | 9 (53%) |
| >2 cm | 1 (6%) |
| Bra cup size, n (%) | |
| B | 4 (24%) |
| C | 5 (29%) |
| D | 6 (35%) |
| DD | 2 (12%) |
| Histology, n (%) | |
| Ductal carcinoma in situ | 3 (18%) |
| Invasive ductal carcinoma | 14 (82%) |
A GammaPod treatment was successfully completed in 14 of the 17 patients enrolled, corresponding to a success rate of 82% with a lower 1-sided 95% confidence limit of 60%. Three patients could not be treated. One was ineligible after the breast cup immobilization device was fitted because a portion of the tumor bed fell outside the treatment parameters. In another patient, the negative pressure seal of the immobilization device could not be maintained after several attempts at application. In the third patient, the vacuum pump failed after the cup was fitted but before treatment. In this small study there was no correlation between the breast cup size and feasibility. Further experience and ongoing equipment improvements have yielded very low rates of procedure failure (<2%).
For the 14 treated patients, the median treatment time was 24.45 minutes (range, 16.4-42.47 minutes). Increasing treatment times were seen over time due to 60Co decay. Subsequent experience outside of this study has demonstrated longer treatment times are associated with larger targets and targets closer to the chest wall; experience has also shown the overall procedure time from breast cup placement to removal to be an average of 1 hour. At the end of the treatment, patients were administered a questionnaire and asked to compare this treatment experience with those of undergoing a mammogram, MR imaging, and/or a biopsy. The uniform response (100%) was “It [the GammaPod] was easier.” This questionnaire was designed uniquely for the GammaPod treatment to ascertain specific answers regarding the procedure and experience.
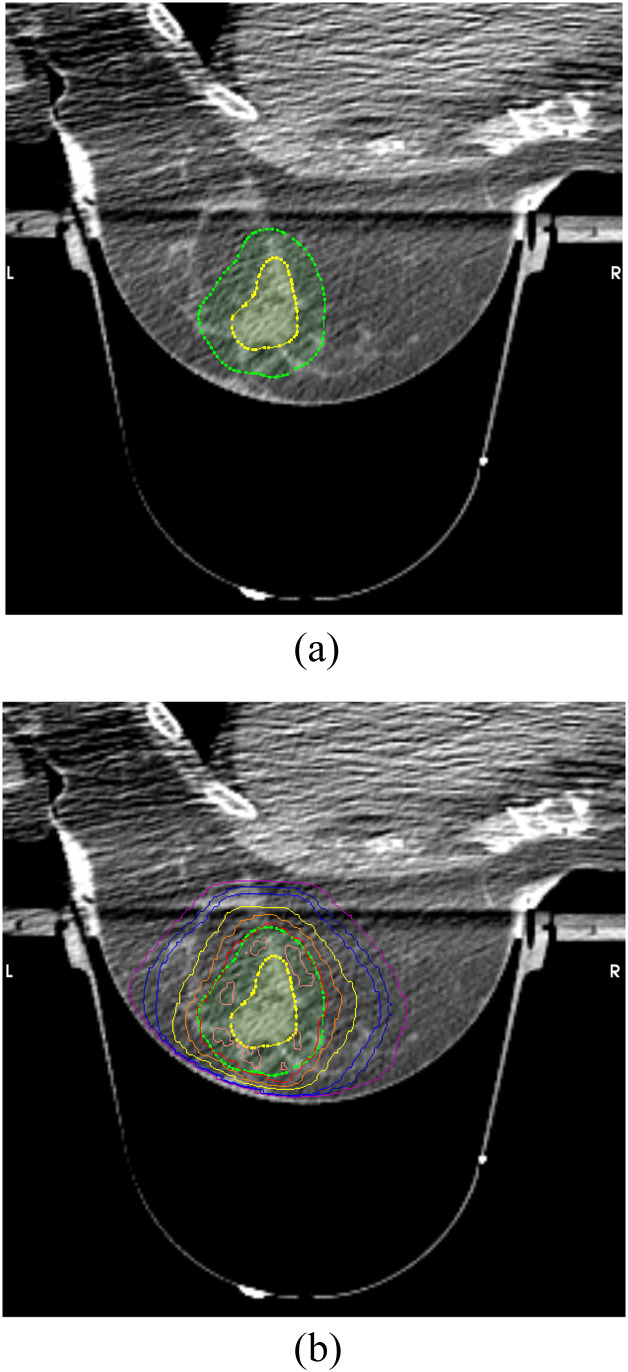
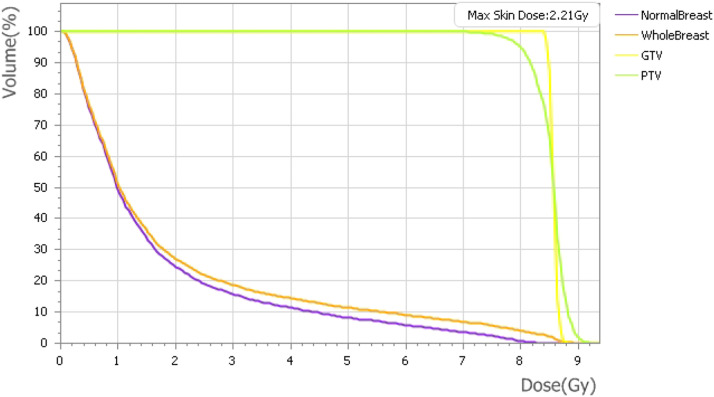
Treatment volumes and maximum doses to organs at risk are included in Table 2. Figure 2 shows a representative plan and the rapid dose fall-off from the prescription isodose volume. Doses to the skin, heart, and lung varied depending on tumor bed location. Figure 3 shows a representative dose–volume histogram.
Table 2.
Treatment parameters
| Median | Range | IQR | |
|---|---|---|---|
| Tumor bed volume (CTV) | 9.67 cm3 | 2.86-29.08 cm3 | 8.355 |
| PTV | 62.54 cm3 | 21.69-153.85 cm3 | 37.38 |
| PTV maximum dose | 8.89 Gy | 8.35-9.36 Gy | 0.4 |
| Skin maximum dose | 2.21 Gy | 1.13-7.43 Gy | 0.87 |
| Heart maximum dose | 0.75 Gy | 0.01-2.29 Gy | 1.035 |
| Lung maximum dose | 0.70 Gy | 0.10-1.98 Gy | 0.885 |
Abbreviations: IQR = interquartile range; CTV = clinical treatment volume, PTV = planning treatment volume.
Figure 2.
Representative treatment plan. Isodose lines from 95% (red) to 10% (cyan) demonstrated.
Figure 3.
Representative dose–volume histogram for a boost plan.
One patient developed small blisters related to the negative pressure used with the immobilization device. It is hypothesized that a small airgap that could not be visualized between the skin and the inner cup resulted in the skin not being supported. The blisters were not painful and resolved spontaneously without intervention. The recommended treatment was conservative, and the patient was assessed during weekly on-treatment visits during their whole breast portion of treatment. At the patient's 1-month follow-up visit, no blisters/scars were visualized.
Whole-breast radiation was delivered to all patients using a hypofractionated approach typically within one week after GammaPod treatment. Two patients were treated using 4256 cGy in 266-cGy fractions, and the remainder received 4005 cGy in 267-cGy fractions. Overall acute treatment toxicities (inclusive of boost and whole-breast treatment) were limited to grade 1 events and included: 10 patients with fatigue, 11 with dermatitis, 3 with hyperpigmentation, 4 with breast pain, and 1 with limb edema and nausea. Seven patients experienced grade 1 toxicity, 4 patients grade 2, 2 patients grade 3, and 2 patients grade 4. Whole breast radiation side effects were managed as per standard of care.
At 12-month follow-up, which was the last protocol-specified time point, complete resolution of all acute side effects initially recorded was observed. At the time of this writing, no local recurrences and no unexpected late toxicities have been reported. Cosmetic outcomes were not recorded in this initial safety and feasibility study.
Discussion
Current standard adjuvant RT for breast cancer includes treatment to the whole breast over 3 to5 weeks followed by a “boost” delivered to the tumor bed over an additional week. Two prospective trials have demonstrated a significant reduction in local failures with the addition of a radiation boost of 10 Gy in 4 fractions at 2.5 Gy per fraction or 16 Gy in 8 fractions at 2 Gy per fraction.24,25 The RTOG 1005 trial evaluated the use of concomitant versus sequential boost in women receiving whole breast radiation therapy with either conventional or hypofractionated treatment and showed no difference in treatment-related adverse events between arms as well as 3-year rates of excellent/good cosmesis of 86 and 84%, respectively.26
The GammaPod represents a breast stereotactic RT system that can deliver a precise treatment to a target in the breast with 2 key advantages over other external-beam delivery platforms: (1) a unique, dynamic dose-painting delivery technique; and (2) a device-specific breast stereotactic immobilization system. In prior publications, we demonstrated dosimetric advantages to the delivery in comparison to 3D conformal radiation, intensity modulated photon and proton therapy, and brachytherapy. Based on the results from the study described here, we were able to demonstrate the feasibility and safety of delivering a tumor bed boost dose using the GammaPod stereotactic system for patients undergoing BCT. This study produced results similar to those reported for targeted IORT (TARGIT trial),8,9,27 which used low-energy photons intraoperatively to deliver radiation to the tumor bed in a single-fraction approach immediately after surgical excision of the tumor. Initial reports of TARGIT followed by whole-breast radiation demonstrated 5-year local recurrence rates <2% with no grade 3 side effects.8 However, this was accompanied by a 12% risk of grade 2 subcutaneous fibrosis, which is comparable to results from large series using conventional boost techniques.9 In the randomized TARGIT-A trial, a subset of patients also received IORT followed by whole breast radiation for high-risk pathologic features; the results of this study are similar to those.8 In Europe, based on short follow-up in the European Organisation for Research and Treatment of Cancer 22881/10882, no differences were noted in local control or cosmesis between IORT and conventional electrons.25 In a similar feasibility study of a novel device, Hamid et al reported a multi-institutional experience of implementing noninvasive breast brachytherapy for a tumor bed boost.28 In that study, the researchers were similarly limited in treating tumors close to the chest wall, requiring >1 cm distance between the applicator edge and the chest wall. Long-term results of fibrosis and other late toxicities were not included in this study but will be the source of future publications.
This clinical trial is an initial step in exploring the potential of the device. It allows for smaller target volumes, which will ultimately allow more hypofractionated regimens to be delivered with potentially improved toxicities. At the time of this writing, 2 additional clinical trials have been activated and are accruing: a subsequent boost study expanding patient eligibility (NCT03562273) and an adjuvant partial-breast irradiation trial (NCT03581136). Additional multi-institutional studies are in development.
Conclusion
In women with early-stage breast cancer, the GammaPod device successfully delivered a single-fraction boost treatment to the tumor bed in 82% of enrolled patients with acceptable rates of acute toxicity. This approach reduced overall treatment time by 3 to 7 fractions, resulting in a positive impact on quality of life. Early adverse events from the whole treatment course were all low-grade (grade 1). Reduction in treatment time also has a potential positive impact on health care dollars spent, which is meaningful in the era of advanced payment models. Additional studies are currently accruing or under development with a goal of enhancing improvements in quality of life and mitigation of toxicity while maintaining local control.
Disclosures
The authors report no conflicts of interest.
Footnotes
Sources of support: Development of the device before this study was partly funded by a Maryland Industrial Partnerships grant # 4804.28.
Data sharing statement: The entirety of this project was performed while all authors were part of the University of Maryland School of Medicine.
References
- 1.Kemeny MM, Wellisch DK, Schain WS. Psychosocial outcome in a randomized surgical trial for treatment of primary breast cancer. Cancer. 1988;62:1231–1237. doi: 10.1002/1097-0142(19880915)62:6<1231::aid-cncr2820620631>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 2.Clarke M, Collins R, Darby S, et al. Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: An overview of the randomised trials. Lancet. 2005;366 doi: 10.1016/S0140-6736(05)67887-7. 2087-1026. [DOI] [PubMed] [Google Scholar]
- 3.Christian MC, McCabe MS, Korn EL, et al. The National Cancer Institute audit of the National Surgical Adjuvant Breast and Bowel Project Protocol B-06. N Engl J Med. 1995;333:1469–1474. doi: 10.1056/NEJM199511303332206. [DOI] [PubMed] [Google Scholar]
- 4.Fisher B, Bauer M, Margolese R, et al. Five-year results of a randomized clinical trial comparing total mastectomy and segmental mastectomy with or without radiation in the treatment of breast cancer. N Engl J Med. 1985;312:665–673. doi: 10.1056/NEJM198503143121101. [DOI] [PubMed] [Google Scholar]
- 5.Formenti SC, Truong MT, Goldberg JD, et al. Prone accelerated partial breast irradiation after breast-conserving surgery: Preliminary clinical results and dose-volume histogram analysis. Int J Radiat Oncol Biol Phys. 2004;60:493–504. doi: 10.1016/j.ijrobp.2004.04.036. [DOI] [PubMed] [Google Scholar]
- 6.Beitsch PD, Wilkinson JB, Vicini FA, et al. Tumor bed control with balloon-based accelerated partial breast irradiation: Incidence of true recurrences versus elsewhere failures in the American Society of Breast Surgery MammoSite (®) Registry Trial. Ann Surg Oncol. 2012;19:3165–3170. doi: 10.1245/s10434-012-2489-x. [DOI] [PubMed] [Google Scholar]
- 7.NSABP B-39, RTOG 0413: A randomized phase III study of conventional whole breast irradiation versus partial breast irradiation for women with stage 0, I, or II breast cancer. Clin Adv Hematol Oncol. 2006;4:719–721. [PubMed] [Google Scholar]
- 8.Vaidya JS, Joseph DJ, Tobias JS, et al. Targeted intraoperative radiotherapy versus whole breast radiotherapy for breast cancer (TARGIT-A trial): An international, prospective, randomized, non-inferiority phase 3 trial. Lancet. 2010;376:91–102. doi: 10.1016/S0140-6736(10)60837-9. [DOI] [PubMed] [Google Scholar]
- 9.Veronesi U, Orecchia R, Maisonneuve P, et al. Intraoperative radiotherapy versus external radiotherapy for early breast cancer (ELIOT): A randomized controlled equivalence trial. Lancet Oncol. 2013;14:1269–1277. doi: 10.1016/S1470-2045(13)70497-2. [DOI] [PubMed] [Google Scholar]
- 10.Hepel JT, Tokita M, MacAusland SG, et al. Toxicity of three-dimensional conformal radiotherapy for accelerated partial breast irradiation. Int J Radiat Oncol Biol Phys. 2009;75:1290–1296. doi: 10.1016/j.ijrobp.2009.01.009. [DOI] [PubMed] [Google Scholar]
- 11.Jagsi R, Ben-David MA, Moran JM, et al. Unacceptable cosmesis in a protocol investigating intensity-modulated radiotherapy with active breathing control for accelerated partial-breast irradiation. Int J Radiat Oncol Biol Phys. 2010;75:71–78. doi: 10.1016/j.ijrobp.2009.01.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Olivotto IA, Whelan TJ, Parpia S, et al. Interim cosmetic and toxicity results from RAPID: A randomized trial of accelerated partial breast irradiation using three-dimensional conformal external beam radiation therapy. J Clin Oncol. 2013;31:4038–4045. doi: 10.1200/JCO.2013.50.5511. [DOI] [PubMed] [Google Scholar]
- 13.Yu XC, Regine WF, inventors. Method and equipment for image-guided stereotactic radiosurgery of breast cancer. US Patent 8788017. July 22, 2014.
- 14.Mutaf Y. Dosimetric advantage of SBRT in partial breast irradiation using a novel breast specific stereotactic radiosurgery system. A comparative analysis. Paper presented at the European Society for Radiotherapy and Oncology; May 8-12; London, UK; 2011. [Google Scholar]
- 15.Nichols EM, Mutaf YD, Feigenberg SJ, et al. In: Advances in Medical Physics 2014. Godfrey DJ, Das SK, Wolbarst AB, editors. Medical Physics Publishing; 2014. Stereotactic radiotherapy for breast cancer: GammaPod.www.medicalphysics.org eBook. Accessible at. [Google Scholar]
- 16.Snider JW, Mutaf Y, Nichols E, et al. Dosimetric improvements with a novel breast stereotactic radiotherapy device for delivery of preoperative partial-breast irradiation. Oncology. 2017;92:21–30. doi: 10.1159/000449388. [DOI] [PubMed] [Google Scholar]
- 17.Snider JW, 3rd, Mutaf Y, Nichols E, et al. Projected improvements in accelerated partial breast irradiation using a novel breast stereotactic radiotherapy device: A dosimetric analysis. Technol Cancer Res Treat. 2017;16:1031–1037. doi: 10.1177/1533034617718961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Becker S, Sabouri P, Niu Y, et al. Commissioning and acceptance guide for the GammaPod. Phys Med Biol. 2019;64 doi: 10.1088/1361-6560/ab41bd. [DOI] [PubMed] [Google Scholar]
- 19.Becker SJ, Niu Y, Mutaf Y, et al. Development and validation of a comprehensive patient-specific quality assurance program for a novel stereotactic radiation delivery system for breast lesions. J Appl Clin Med Phys. 2019;20:138–148. doi: 10.1002/acm2.12778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Snider JW, Nichols EM, Mutaf YD, et al. Reproducibility of a novel, vacuum-assisted immobilization for breast stereotactic radiotherapy. J Appl Clin Med Phys. 2021;22:8–15. doi: 10.1002/acm2.13127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Qi XS, White J, Li XA. Is alpha/beta for breast cancer really low? Radiother Oncol. 2011;100:282–288. doi: 10.1016/j.radonc.2011.01.010. [DOI] [PubMed] [Google Scholar]
- 22.Haviland JS, Owen JR, Dewar JA, et al. The UK Standardisation of Breast Radiotherapy (START) trials of radiotherapy hypofractionation for treatment of early breast cancer: 10-year follow-up results of two randomised controlled trials. Lancet Oncol. 2013;14:1086–1094. doi: 10.1016/S1470-2045(13)70386-3. [DOI] [PubMed] [Google Scholar]
- 23.Haviland JS, Bentzen SM, Bliss JM, et al. Prolongation of overall treatment time as a cause of treatment failure in early breast cancer: An analysis of the UK START (Standardisation of Breast Radiotherapy) trials of radiotherapy fractionation. Radiother Oncol. 2016;121:420–423. doi: 10.1016/j.radonc.2016.08.027. [DOI] [PubMed] [Google Scholar]
- 24.Romestaing P, Lehingue Y, Carrie C, et al. Role of a 10-Gy boost in the conservative treatment of early breast cancer: Results of a randomized clinical trial in Lyon, France. J Clin Oncol. 1997;15:963–968. doi: 10.1200/JCO.1997.15.3.963. [DOI] [PubMed] [Google Scholar]
- 25.Poortmans PM, Collette L, Bartelink H, et al. The addition of a boost dose on the primary tumour bed after lumpectomy in breast conserving treatment for breast cancer. A summary of the results of EORTC 22881-10882 “boost versus no boost” trial. Cancer Radiother. 2008;12:565–570. doi: 10.1016/j.canrad.2008.07.014. [DOI] [PubMed] [Google Scholar]
- 26.Vicini FA, Winter K, Freedman G, et al. NRG RTOG 1005: A phase III trial of hypofractionated whole breast irradiation with concurrent boost versus. conventional whole breast irradiation plus sequential boost following lumpectomy for high risk early-stage breast cancer. Int J Radiat Oncol Biol Phys. 2022;114:S1. [Google Scholar]
- 27.Vaidya JS, Baum M, Tobias JS, et al. Long-term results of targeted intraoperative radiotherapy (TARGIT) boost during breast-conserving surgery. Int J Radiat Oncol Biol Phys. 2011;81:1091–1097. doi: 10.1016/j.ijrobp.2010.07.1996. [DOI] [PubMed] [Google Scholar]
- 28.Hamid S, Rocchio K, Arthur D, et al. A multi-institutional study of feasibility, implementation, and early clinical results with noninvasive breast brachytherapy for tumor bed boost. Int J Radiat Oncol Biol Phys. 2012;83:1374–1380. doi: 10.1016/j.ijrobp.2011.10.016. [DOI] [PubMed] [Google Scholar]