Abstract
The generality of a model for predicting tumor control probability from in vitro clonogenic survival considering of cancer stem-like cells, the so-called integrated microdosimetric-kinetic model, is presented by comparing the model to public data on stereotactic body radiation therapy for non-small cell lung cancer cells.
Introduction
Radiation therapy allows for the eradication of solid tumors in patients, and the curative effects are usually evaluated using tumor control probability (TCP). From a radiobiology standpoint, fractionation regimens are theoretically determined using a mathematical model to predict the relationship between the absorbed dose and clonogenic survival.1 However, the clinically implemented LQ model cannot reproduce patient TCP using the parameters (α [Gy–1] and β [Gy–2]) provided by the in vitro clonogenic surviving fraction. These parameters are often obtained empirically by fitting patient TCP data.
To solve this problem, we developed an integrated microdosimetric-kinetic (IMK) model that considers the existence of cancer stem-like cells (CSCs).2,3 Using this model, we successfully reproduced the clinical TCP of patients with lung cancer after stereotactic body radiation therapy (SBRT) using the parameters obtained after fitting to experimental in vitro survival.4 Based on our previous outcomes from a translational study of in vitro and clinical curative effects, explicit consideration of the CSC fraction plays a key role. The previous patient data were obtained from a single institution. Therefore, a generality assessment of the IMK model by comparing it with public data are essential.
In this study, we systematically collected TCP data on SBRT in patients with non-small cell lung cancer (NSCLC) published in medical journals. When analyzing previously published clinical TCP, the fractionation scheme and prescribed dose were converted into a useful indicator for SBRT, that is, the single fractionation equivalent dose (SFED).4,5 By a comparison between the collected TCP and IMK model estimation, we demonstrated the importance of considering heterogeneous cell populations, particularly the existence of CSCs, when estimating the TCP for lung cancer.
Methods and Materials
We reviewed 55 reports identified through a literature search of the MEDLINE database through PubMed from July to August 2022. The search term used were “NSCLC SBRT,” and “NSCLC SABR,” and inclusion criteria were “original article,” “article in English,” “accessibility to the full article,” and “clinical trial.” Noted that we simply searched for “clinical trial” to exclude retrospective studies because it may include published data. Among 55 studies, 13 studies6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18 were identified using further inclusion criteria, which were “including fractionation scheme,” “including tumor control probability or local control rate associated with a fractionation scheme,” and “only primary NSCLC outcomes available.”
The fractionation schemes reported in the previous reports6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18 were converted to the SFED using model parameters derived from the A549 cell line.4 The SFED represents a single fraction dose equivalent to the biologic effect (ie, the cell-killing effect) induced by any dose fractionation scheme (Fig. 1). The cell survival curve after single (acute) or multiple fractionations (eg, 10 Gy/fraction in Fig. 1) was calculated using the IMK model, as in our previous report.4 We focused on the relationship between the fractionation scheme and local control; thus, patient and tumor characteristics were not considered.
Figure 1.
The schematic of SFED concept. SFED is defined as the dose in a single dose equivalent to the cell survival exhibited by the desired fraction. Abbreviations: IMK = integrated microdosimetric-kinetic; SFED = single fractionation equivalent dose.
Results and Discussions
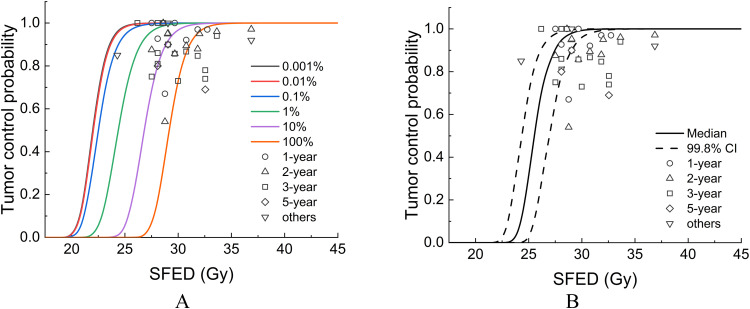
Table 1 summarizes the TCP data associated with various fractionation schemes collected through the literature search. The number of fractions was 1 to 10, and the fractionated dose was 5 to 34 Gy, which was converted to SFED using the IMK model in detail, 24 to 36 Gy. Figure 2 shows the relationship between SFED and TCP, in which the TCP curves were depicted by the IMK model assuming various CSC fractions within the tumor (Fig. 2A) and a constant CSC fraction of 7.98% (Fig. 2B), which was derived from the A549 cell line.4 In a previous study, the IMK model successfully reproduced 3-, 5-, and 8-year TCPs with identical cellular parameters. Similarly, the IMK model successfully reproduced the public TCP data regardless of the observation period (Fig. 2). This may be because the TCP can be determined by the number of surviving tumor cells with clonogenicity (regrowth ability). Liu et al fitted pooled TCP for SBRT in NSCLC using 6 radiobiological models.19 Among these, the model that considered regrowth was in better agreement with the TCP for various observation periods. Although it is an important parameter for converting radiobiological models to the TCP model, regrowth associated with CSCs should be considered for higher accuracy prediction in future studies. Indeed, cell injection of the CSC fraction forms tumors with a higher efficiency than injection of the noncancer stem cell fraction in animal experiments.20,21
Table 1.
Summary of the TCP with various fractionation scheme
| Authors | Fractionation scheme | TCP | Patient no. | SFED (Gy) |
|---|---|---|---|---|
| Bezjak et al,6 2019 | 10 Gy/5 fr | 1-y 100%, 2-y 87.5%, 3-y 75% | 8 | 27.51 |
| 11 Gy/5 fr | 1-y 100%, 2-y 100%, 3-y 100% | 7 | 28.60 | |
| 11.5 Gy/5 fr | 1-y 100%, 2-y 85.7%, 3-y 85.7% | 14 | 29.68 | |
| 12 Gy/5 fr | 1-y 92.1%, 2-y 89.4%, 3-y 86.7% | 38 | 30.76 | |
| 10.5 Gy/5 fr | 1-y 97%, 2-y 87.9%, 3-y 84.7% | 33 | 31.83 | |
| Timmerman et al,7 2018 | 18 Gy/3 fr | 2-y 96% | 33 | 33.63 |
| Martin et al,8 2016 | 18 Gy/3 fr | 3-y 94% | 22 | 33.63 |
| Caivano et al,9 2018 | 15 Gy/3 fr | 1-y 67%, 2-y 54% | 22 | 28.76 |
| Miyakawa et al,10 2017 | 12 Gy/4 fr | 3-y 100%, 5-y 80% | 6 | 28.08 |
| 12.5 Gy/4 fr | 3-y 90%, 5-y 90% | 51 | 29.04 | |
| 17.3 Gy/ 4 fr | 3-y 78%, 5-y 69% | 14 | 32.55 | |
| Baba et al,11 2010 | 12 Gy/4 fr | 3-y 81% | 85 | 28.08 |
| 17.3 Gy/4 fr | 3-y 74% | 37 | 32.55 | |
| Chang et al,12 2008 | 12.5 Gy/4 fr | Median follow-up 17 mo (range, 6-40 mo) 100% | 27 | 29.04 |
| Shen et al,13 2015 | 20 Gy/3 fr | Median follow-up 35 mo 86% | 50 | 36.87 |
| Singh et al,14 2019 | 30 Gy/1 fr | 2-y 94.9% | 48 | 29.01 |
| 20 Gy/3 fr | 2-y 97.1% | 40 | 36.87 | |
| Shibamoto et al,15 2012 | 11 Gy/4 fr | 3-y 100% | 4 | 26.18 |
| 12 Gy/4 fr | 3-y 86% | 124 | 28.08 | |
| 13 Gy/4 fr | 3-y 73% | 52 | 29.99 | |
| Matsuo et al,16 2022 | 12.5 Gy/4 fr | 2-y 95.2% | 48 | 29.04 |
| Videtic et al,17 2019 | 34 Gy/1 fr | 1-y 97% | 39 | 32.74 |
| 12 Gy/4 fr | 1-y 92.7% | 45 | 28.08 | |
| Vahdat et al,18 2010 | 14-20 Gy/3 fr | 2-y 95% | 20 | 32.01 |
The fractionation scheme is presented as fractionated dose (Gy)/number of fractions.
Abbreviations: fr = fraction; SFED = single fractionation equivalent dose; TCP = tumor control probability.
Figure 2.
Tumor control probability (TCP) depicted by the TCP model considering cancer stem-like cells (CSC). The circles, triangles, squares, diamonds, and inverted triangles represent 1-, 2-, 3-, and 5-year, and others (ie, nonspecified) TCP, respectively. Various fractionation schemes were converted to single fractionation equivalent dose. The TCP curve depicted by the TCP model with (A) arbitrarily CSC fractions (ie, 0.001% with dark gray, 0.01% with red, 0.1% with blue, 1% with green, 10% with purple, and 100% with orange solid line) and (B) constant CSC fractions (ie, 7.983%). Abbreviation: SFED = single fractionation equivalent dose.
In our previous study, we obtained the CSC fraction from a CSC marker-positive cell population using flow cytometry. A representative lung cell line, A549, was used as a reference representing TCP in patients with NSCLC, and its CSC fraction, which was measured by aldehyde dehydrogenase activity at 7.98% ± 0.36%. This percentage may vary depending on the CSC marker used, the cell line type, and the tissue type.22 In addition, heterogeneous cell populations are known to change dynamically depending on the microenvironment in the human body (ie, tumor microenvironment).23,24 Additionally, marker-dependent changes in the intratumoral CSC fraction have been reported.25 Consequently, the accurate estimate of the CSC fraction has not been established in clinical. Although biopsy specimens are often not available for lung cancers treated with SBRT, using intratumoral hypoxia imaging techniques may be possible to indirectly estimate the CSC fraction because hypoxia is the tumor microenvironment maintain the CSC property.26,27 Other limitations in this study, we did not specify the dose prescription (ie, isocenter or D95), tumor localization, and respective tumor volumes. These factors can influence the relationship between dose, CSC fraction and TCP, but were not considered in the model of this study.
The conventional LQ model has been modified for predicting the therapeutic effects of SBRT due to the unreliability of the high-dose range estimation.28,29 Meanwhile, the model parameters often directly derived from published TCP data by fitting approach or just showing a hypothesis30,31; thus, a large gap remains in the relationship between cell survival fraction and TCP. In this study, we presented the generality of the all-in-one IMK model considering the CSC for TCP predicting based on the model parameters derived from a cell line. The predicted TCP curve with CSC fractions of 0.001%–100% covered most of the pooled TCP between 1 and 100%. Although there is room for verification of the validity of the CSC fraction, introducing the concept of CSC into TCP estimation shows the possibility of high-precision clinical outcome prediction. Therefore, future investigations of the stemness plasticity during radiation therapy and accurate CSC fraction estimation in clinical are important for linking the local control and the CSC fraction of tumors.
Disclosures
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors would like to thank Hiroyuki Date (Faculty of Health Sciences, Hokkaido University, deceased in August, 2022) for useful discussions.
Footnotes
Sources of support: This work had no specific funding.
All data generated and analyzed during this study are included in this article.
References
- 1.Qiu B, Li QW, Ai XL, et al. Investigating the loco-regional control of simultaneous integrated boost intensity-modulated radiotherapy with different radiation fraction sizes for locally advanced non-small-cell lung cancer: Clinical outcomes and the application of an extended LQ/TCP model. Radiat Oncol. 2020;15:124. doi: 10.1186/s13014-020-01555-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Saga R, Matsuya Y, Takahashi R, et al. Analysis of the high-dose-range radioresistance of prostate cancer cells, including cancer stem cells, based on a stochastic model. J Radiat Res. 2019;60:298–307. doi: 10.1093/jrr/rrz011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fukui R, Saga R, Matsuya Y, et al. Tumor radioresistance caused by radiation-induced changes of stem-like cell content and sub-lethal damage repair capability. Sci Rep. 2022;12:1056. doi: 10.1038/s41598-022-05172-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Saga R, Matsuya Y, Sato H, et al. Translational study for stereotactic body radiotherapy against non-small cell lung cancer, including oligometastases, considering cancer stem-like cells enable predicting clinical outcome from in vitro data. Radiother Oncol. 2023;181:109444. doi: 10.1016/j.radonc.2022.109444. [DOI] [PubMed] [Google Scholar]
- 5.Park C, Papiez L, Zhang S, Story M, Timmerman RD. Universal survival curve and single fraction equivalent dose: Useful tools in understanding potency of ablative radiotherapy. Int J Radiat Oncol Biol Phys. 2008;70:847–852. doi: 10.1016/j.ijrobp.2007.10.059. [DOI] [PubMed] [Google Scholar]
- 6.Bezjak A, Paulus R, Gaspar LE, et al. Safety and efficacy of a five-fraction stereotactic body radiotherapy schedule for centrally located non-small cell lung cancer: NRG Oncology/RTOG0813 trial. J Clin Oncol. 2019;37:1316–1325. doi: 10.1200/JCO.18.00622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Timmerman RD, Paulus R, Pass HI, et al. Stereotactic body radiation therapy for operable early-stage lung cancer: Findings from the NRG Oncology RTOG 0618 Trial. JAMA Oncol. 2018;4:1263–1266. doi: 10.1001/jamaoncol.2018.1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Martin AN, Aso S, Cacicedo J, et al. Phase II trial of SBRT for stage I NSCLC: Survival, local control, and lung function at 36 months. J Thorac Oncol. 2016;11:1101–1111. doi: 10.1016/j.jtho.2016.03.021. [DOI] [PubMed] [Google Scholar]
- 9.Caivano D, Valeriani M, Matteis SD, et al. Re-irradiation in lung disease by SBRT: A retrospective, single institutional study. Radiat Oncol. 2018;13:87. doi: 10.1186/s13014-018-1041-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miyakawa A, Shibamoto Y, Baba F, et al. Stereotactic body radiotherapy for stage I non-small-cell lung cancer using higher doses for larger tumors: Results of the second study. Radiat Oncol. 2017;12:152. doi: 10.1186/s13014-017-0888-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baba F, Shibamoto Y, Ogino HR., et al. Clinical outcomes of stereotactic body radiotherapy for stage I non-small cell lung cancer using different doses depending on tumor size. Radiat Oncol. 2010;5:81. doi: 10.1186/1748-717X-5-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chang JY, Balter PA, Dong L, et al. Stereotactic body radiation therapy in centrally and superiorly located stage I or isolated recurrent non-small-cell lung cancer. Int J Radiat Oncol Biol Phys. 2008;72:967–971. doi: 10.1016/j.ijrobp.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shen ZT, Wu XH, Li B, Zhu XX. Clinical outcomes of CyberKnife stereotactic body radiotherapy for peripheral stage I non-small cell lung cancer. Med Oncol. 2015;32:55. doi: 10.1007/s12032-015-0506-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Singh AK, Suescun JAG, Stephans KL, et al. One versus three fractions of stereotactic body radiation therapy for peripheral stage I to II non-small cell lung cancer: A randomized, multi-institution, phase 2 trial. Int J Radiat Oncol Biol Phys. 2019;105:752–759. doi: 10.1016/j.ijrobp.2019.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shibamoto Y, Hashizume C, Baba F, et al. Stereotactic body radiotherapy using a radiobiology-based regimen for stage I nonsmall cell lung cancer: A multicenter study. Cancer. 2012;118:2078–2084. doi: 10.1002/cncr.26470. [DOI] [PubMed] [Google Scholar]
- 16.Matsuo Y, Hiraoka M, Karasawa K, et al. Multi-institutional phase II study on the safety and efficacy of dynamic tumor tracking-stereotactic body radiotherapy for lung tumors. Radiother Oncol. 2022;172:18–22. doi: 10.1016/j.radonc.2022.04.028. [DOI] [PubMed] [Google Scholar]
- 17.Videtic GM, Paulus R, Singh AK, et al. Long-term follow-up on NRG oncology RTOG 0915 (NCCTG N0927): A randomized phase 2 study comparing 2 stereotactic body radiation therapy schedules for medically inoperable patients with stage I peripheral non-small cell lung cancer. Int J Radiat Oncol Biol Phys. 2019;103:1077–1084. doi: 10.1016/j.ijrobp.2018.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vahdat S, Oermann EK, Collins SP, et al. CyberKnife radiosurgery for inoperable stage IA non-small cell lung cancer: 18F-fluorodeoxyglucose positron emission tomography/computed tomography serial tumor response assessment. J Hematol Oncol. 2010;3:6. doi: 10.1186/1756-8722-3-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu F, Tai A, Lee P, et al. Tumor control probability modeling for stereotactic body radiation therapy of early-stage lung cancer using multiple bio-physical models. Radiother Oncol. 2017;122:286–294. doi: 10.1016/j.radonc.2016.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Todoroki K, Ogasawara S, Akiba J, et al. CD44v3+/CD24− cells possess cancer stem cell-like properties in human oral squamous cell carcinoma. Int J Oncol. 2016;48:99–109. doi: 10.3892/ijo.2015.3261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Andriani F, Bertolini G, Facchinetti F, et al. Conversion to stem-cell state in response to microenvironmental cues is regulated by balance between epithelial and mesenchymal features in lung cancer cells. Mol Oncol. 2016;10:253–271. doi: 10.1016/j.molonc.2015.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leon G, MacDonagh L, Finn SP, Cuffe S, Barr MP. Cancer stem cells in drug resistant lung cancer: Targeting cell surface markers and signaling pathways. Pharmaco Ther. 2016;158:71–90. doi: 10.1016/j.pharmthera.2015.12.001. [DOI] [PubMed] [Google Scholar]
- 23.Prasetyanti PR, Medema JP. Intra-tumor heterogeneity from a cancer stem cell perspective. Mol Cancer. 2017;16:41. doi: 10.1186/s12943-017-0600-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sahoo S, Ashraf B, Duddu AS, Biddle A, Jolly MK. Interconnected high-dimensional landscapes of epithelial-mesenchymal plasticity and stemness in cancer. Clin Exp Metastasis. 2022;39:279–290. doi: 10.1007/s10585-021-10139-2. [DOI] [PubMed] [Google Scholar]
- 25.Liu S, Cong Y, Wang D, et al. Breast cancer stem cells transition between epithelial and mesenchymal states reflective of their normal counterparts. Stem Cell Rep. 2013;2:78–91. doi: 10.1016/j.stemcr.2013.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grimes DR, Warren DR, Warren S. Hypoxia imaging and radiotherapy: Bridging the resolution gap. Br J Radiol. 2017;90 doi: 10.1259/bjr.20160939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McKeown SR. Defining normoxia, physoxia and hypoxia in tumours: Implications for treatment response. Br J Radiol. 2014;87 doi: 10.1259/bjr.20130676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McKenna FW, Ahmad S. Fitting techniques of cell survival curves in high-dose region for use in stereotactic body radiation therapy. Phys Med Biol. 2009;54:1593–1608. doi: 10.1088/0031-9155/54/6/013. [DOI] [PubMed] [Google Scholar]
- 29.Wang JZ, Huang Z, Lo SS, Yuh WTC, Mayr NA. A generalized linear-quadratic model for radiosurgery, stereotactic body radiation therapy, and high-dose rate brachytherapy. Sci Trans Med. 2010;2:39ra48. doi: 10.1126/scitranslmed.3000864. [DOI] [PubMed] [Google Scholar]
- 30.Zaider M. Tumor control probability in radiation treatment. Med Phys. 2011;38:574–583. doi: 10.1118/1.3521406. [DOI] [PubMed] [Google Scholar]
- 31.Dhawan A, Kohandel M, Hill R, Sivaloganathan S. Tumour control probability in cancer stem cells hypothesis. PLoS One. 2014;9:e96093. doi: 10.1371/journal.pone.0096093. [DOI] [PMC free article] [PubMed] [Google Scholar]