Abstract
Purpose
Soft tissue sarcomas (STS) are historically radioresistant, with surgery being an integral component of their treatment. With their low α/β, STS may be more responsive to hypofractionated radiation therapy (RT), which is often limited by long-term toxicity risk to surrounding normal tissue. An isotoxic approach using a hypofractionated accelerated radiation dose-painting (HARD) regimen allows for dosing based on clinical risk while sparing adjacent organs at risk.
Methods and Materials
We retrospectively identified patients from 2019 to 2022 with unresected STS who received HARD with dose-painting to high, intermediate, and low-risk regions of 3.0 Gy, 2.5 Gy, and 2.0 to 2.3 Gy, respectively, in 20 to 22 fractions. Clinical endpoints included local control, locoregional control, progression free survival, overall survival, and toxicity outcomes.
Results
Twenty-seven consecutive patients were identified and had a median age of 68 years and tumor size of 7.0 cm (range, 1.2-21.0 cm). Tumors were most often high-grade (70%), stage IV (70%), located in the extremities (59%), and locally recurrent (52%). With a median follow-up of 33.4 months, there was a 3-year locoregional control rate of 100%. The 3-year overall and progression-free survival were 44.9% and 23.3%, respectively. There were 5 (19%) acute and 2 (7%) late grade 3 toxicities, and there were no grade 4 or 5 toxicities at any point.
Conclusions
The HARD regimen is a safe method of dose-escalating STS, with durable 3-year locoregional control. This approach is a promising alternative for unresected STS, though further follow-up is required to determine long-term control and toxicity.
Introduction
Soft tissue sarcomas (STS) are a rare1 group of malignant tumors that arise from mesenchymal and connective tissue within almost all anatomic locations.2,3 Historically, surgery is an integral component in the treatment of STS. In cases where a patient is metastatic or a poor surgical candidate (eg, comorbidities, high surgical morbidity, or no limb-sparing options), radiation therapy (RT) is often used for local control, allowing early initiation of systemic therapy.4, 5, 6
STS are historically considered radioresistant,7 with local control rates of approximately 50 to 70% with traditional standard fractionation for unresectable STS.4, 5, 6,8 To overcome this radioresistance, dose escalation (≥63-65 Gy) can offer an improved local control but is often limited by increased toxicity rates.9, 10, 11
Because of their low α/β (4-6), STS may be more responsive to hypofractionated radiation therapy (HFRT),12, 13, 14 which is consistent with the higher 5-year local control rates (>80-90%) seen with stereotactic body radiation therapy (SBRT).15, 16, 17, 18, 19, 20, 21 The utility of hypofractionation is often limited by the higher risk of long-term toxicity seen to adjacent normal tissue (α/β = 3), especially for larger unresected masses. However, technological advancements in radiation oncology have improved the precision and tolerability of radiation therapy for all sites and tumor types,22,23 including STS.24 Subsequently, many other tumors with known lower α/β ratios have been treated safely with hypofractionated regimens with excellent disease outcomes and low rates of radiation associated toxicity.25,26 Despite these advancements in other disease sites, there remain limited data for unresected STS.16,27, 28, 29
To mitigate the long-term toxicity of HFRT, we created a novel moderately hypofractionated accelerated radiation dose-painting (HARD) regimen, with risk based isotoxic dose escalation, using intensity modulated radiation therapy (IMRT) with volumetric modulating arc therapy (VMAT) and simultaneous integrated boost (SIB). Our institution replaced the standard definitive RT approach (eg, sequential cone down with 1.8-2 Gy/fraction) with a risk-based dose painting approach of gross tumor volume (GTV, or “high risk”), intermediate risk, and low risk volumes with 3 Gy, 2.5 Gy, and 2 to 2.3 Gy per fraction, respectively. The purpose of this study was to evaluate the safety and efficacy of the HARD regimen for unresected STS patients.
Methods and Materials
Patient details and clinical evaluation
The HARD regimen was created and prospectively collected for unresected soft tissue sarcoma (STS) patients. After obtaining institutional review board approval, a retrospective analysis was performed of patients with STS who were treated with this approach at our institution between November 2019 and November 2022. Clinicopathologic characteristics, treatments, and outcomes were collected via clinical chart review. All STS histologies were included for analysis. Patients were not excluded based upon any tumor size, anatomic site, grade, or stage.4 Clinical staging and histologic grading were performed according to the American Joint Committee on Cancer (AJCC) 8th edition and the Federation Nationale des Center de Lutte Contre Le Cancer (FNCLCC), respectively. Patients were reviewed by a multidisciplinary team, and tumors were determined to be unresectable due to tumor location and involvement with local structures, medical status of patient, or extent of metastatic disease. Both primary and metastatic tumors treated with HARD were included for analyses, but only oligometastatic patients (≤3 lesions) treated within a definitive or consolidative approach were included in this study.
Radiation therapy treatment planning and delivery
A computed tomography (CT) simulation was performed with ≤3 mm slice thickness, with immobilization with a vac-lock, body fixation, or aquaplast. All patients received magnetic resonance imaging (MRI) for treatment planning that was fused to the CT simulation for target delineation. A T1 post fat-saturated image was used to define gross tumor volume (GTV) and a T2 fat-saturated, or STIR image, was used to delineate peritumoral edema.
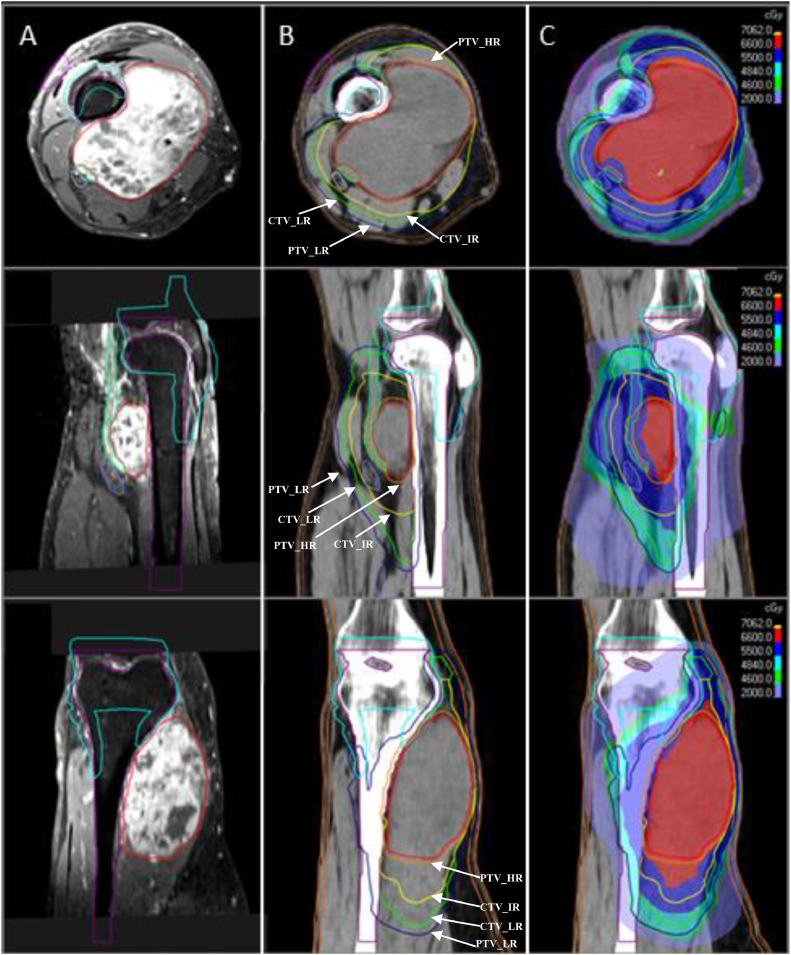
The HARD regimen consisted of high- (HR), intermediate- (IR), and low-risk (LR) regions, which were prescribed to 66 Gy, 55 Gy, and 44-50.6 Gy, in 22 fractions, respectively. The doses were reduced to 20 fractions (60 Gy, 50 Gy, and 40-46 Gy) for patients who had received prior radiation therapy to the site or who had been treated with concurrent systemic therapy. The high-risk planning target volume (PTV_HR) was defined as the GTV expanded with a 3 to 5 mm margin. The intermediate-risk clinical target volume (CTV_IR) was defined as the GTV expanded by a 2 × 1 cm (longitudinal x radial) or 2 cm uniform expansion for muscular or subcutaneous-based tumors, respectively. The low-risk CTV (CTV_LR) was defined as the GTV expanded 3 × 1.5 cm (muscle) or 3 cm uniform expansion (subcutaneous), including edema up to 4 cm from GTV, along with areas at risk of seeding. CTV_LR dose of 2.0 versus. 2.3 Gy per fraction was often determined by the volume's proximity to neighboring organs at risk and whether hypofractionation may have significantly increased the risk of long-term sequela. The CTV was then expanded by 3 to 5 mm, depending on setup and daily imaging, to create the PTV. All patients were planned with intensity-modulated radiation therapy (IMRT) using volumetric modulated arc therapy (VMAT) and daily image guided radiation therapy (IGRT) with daily cone beam CT (CBCT). The GTV and PTV_HR were prescribed to >99% and >95% of the volume (eg, V60-66 Gy > 95-99%), with a minimum dose (0.03 cc) receiving >95% and >90%, respectively. CTV_IR dose was prescribed to >95% volume, and a minimum dose of 95%. PTV_LR was prescribed to >95% volume with a minimum dose of 90 to 95%. Target, organs, descriptions, and dosimetric parameters are detailed in Table 1 and demonstrated on Fig. 1.
Table 1.
HARD regimen treatment planning details and constraints
| Risk regions | Targets | Target descriptions | Target prescriptions |
|
|---|---|---|---|---|
| 66/55/50.6 Gy Regimen | 60/50/46 GyRegimen | |||
| High risk | GTV | Gross tumor volume | 66 Gy > 99% Min. 95% Max. 105-110% |
60 Gy > 99% Min. 95% Max. 105-110% |
| PTV_HR | GTV + 3 – 5 mm | 66 Gy > 95% Min. 90-95% |
60 Gy > 95% Min. 90-95% |
|
| Intermediate risk | CTV_IR | GTV + 2 × 1 cm (muscular) or 2 cm (subcutaneous) | 55 Gy > 99% Min. 90-95% |
50 Gy > 99% Min. 90-95% |
| Low risk | PTV_LR | CTV1 (GTV + 3 × 1.5 cm (muscular) or 3 cm (subcutaneous)) + 3 – 5 mm | 44-50.6 Gy > 95% Min. 95% |
40-46 Gy > 95% Min. 95% |
| Organs at risk | Organ descriptions | Organ constraints |
||
|---|---|---|---|---|
| Recommended | Required | |||
| Bone | Long bone slices contoured the length of the PTV_LR (eg, femur, humerus, radius, ulna) | V40 Gy < 50% | V46 Gy < 50% | |
| Joint | Joint includes joint space, bursa, and proximal 1 cm of articulating bone (eg, elbow, knee) | V40 Gy < 50% | V46 Gy < 50% | |
| Skin strip | 2 cm contiguous strip including the depth of the subcutaneous tissue, contoured the length of the PTV_LR | V10 Gy < 50% | V20 Gy < 50% | |
| Subcutaneous 5 mm | A 5 mm rind representing the superficial skin surface, created by subtracting the external minus external contracted 5 mm | Max ≤ 69.3 Gy | Max ≤ 70.62 Gy | |
| Cord/Cauda | Cord and cauda contoured 3 cm above and below PTV_LR | Max ≤ 40 Gy | Max ≤ 46 Gy V42 Gy < 5 cc |
|
| Nerves | Major neurovascular structures (eg, brachial plexus, lumbosacral plexus, sciatic or femoral neurovascular bundle) | Max ≤ 54 Gy | Max ≤ 58 Gy V54 Gy ≤ 5 cc |
|
| Anorectum | Anus and rectum. If tumor not abutting the rectum, consider generous planning organ at risk to account for daily filling | V54 Gy < 40 cc V40 Gy < 40% |
Max ≤ 70 Gy V58 Gy < 30 cc |
|
| Genitalia | External and internal genitalia (eg, majora, minora, and vagina in females; penile bulb, penis in males) | Max ≤ 69.3 Gy V30 < 35% V20 < 50% |
Max ≤ 70.62 Gy V46 Gy < 50% |
|
| Lungs | Include both lungs if any portion on same axial slices as PTV_LR | V18 Gy < 37% V18 Gy < Lungs – 1500 cc |
V19 Gy < 37% V18 Gy < Lungs – 950 cc |
|
| Heart | Include entire heart if any portion on same axial slices as PTV_LR | Max ≤ 52 Gy V46 Gy < 15 cc Mean < 20 Gy |
Max ≤ 69.7 Gy | |
| Esophagus | Include entire esophagus if any portion on same axial slices as PTV_LR | Max ≤ 48 Gy Mean < 20 Gy |
Max ≤ 58 Gy Mean < 32 Gy |
|
| Small bowel/stomach | Small bowel loops 3 cm above and below PTV_LR; entire stomach contour if any portion on same axial slices as PTV_LR | Max ≤ 44 Gy | Max ≤ 50 Gy V42 Gy < 50 cc |
|
| Colon | Colon loops contour, 3 cm above and below PTV_LR | Max ≤ 50 Gy | Max ≤ 55 Gy V50 Gy < 20 cc |
|
| Kidneys | Include both kidneys if any portion on same axial slices as PTV_LR | V20 < 20% Mean < 12 Gy |
V26 Gy < 200 cc | |
| Liver | Include entire liver if any portion on same axial slices as PTV_LR | V30 Gy < Liver – 700 cc | V32 Gy < Liver – 700 cc | |
| Bladder | Include entire bladder if any portion on same axial slices as PTV_LR | V40 Gy < 50% V60 Gy < 3% |
V46 Gy < 50% | |
Abbreviations: CTV_IR = intermediate risk clinical target volume; GTV = gross tumor volume; OAR = organs at risk; PTV_HR = high risk planning target volume; PTV_LR = low risk planning treatment volume.
Please see preceding text for full definitions. All PTVs are subtracted off OAR. Subcutaneous tumor description in parentheses. Max: maximum dose to 0.03 cc of the target
Figure 1.
Volumes and dosimetry for definitive hypofractionated accelerated radiation dose-painting (HARD) for an unresected STS in the lower extremity. (A) Treatment planning MRI: T1post fat-saturated image is used for delineation of the gross tumor volume (GTV, red) and organs-at-risk (OARs), including long bone (purple), joint (cyan), skin strip (violet), subcutaneous 5 mm (brown), neurovascular bundle (light purple), and sciatic nerve (turquoise). Note the GTV excludes the abutting neurovascular structures that are not involved. (B) CT simulation scan: target volumes are depicted, and image includes the PTV_HR (orange), CTV_IR (yellow), CTV_LR (green), and PTV_LR (navy). Note that the GTV, CTV_IR, and CTV_LR respect anatomic boundaries, with a 3 to 5 mm expansion to create the PTV_HR and PTV_LR, excluding 3 to 5 mm from the skin surface. (C) Treatment plan: to meet OAR dosimetric constraints, the PTV_HR overlap with adjacent organs at risk were purposely treated to 95% of the prescribed dose (2.85 Gy/fraction), mitigating long-term toxicity risk. Note the avoidance of the neurovascular structures, joint, and bone by the 6600 cGy isodose line.
Follow-up and outcomes assessment
Patients were followed with imaging (CT and/or MRI) typically starting 4 to 6 weeks after completion of radiation therapy, then every 3 to 4 months for the first 2 years, then every 4 to 6 months for years 3 to 5, then annually thereafter. Imaging and follow up could have been sooner if it was clinically indicated. Toxicity was defined according to version 5.0 of the Common Terminology Criteria for Adverse Events (CTCAE v5).30 Toxicity was evaluated as the highest-grade specific toxicity experienced by each patient. Acute toxicity was defined as having occurred during or within 90 days after the first fraction. All toxicity was prospectively evaluated upon each clinic encounter and recorded in the patient's electronic medical record.
Statistical analysis
All statistical tests were performed with SPSS version 29 software (IBM). Follow-up was defined from the date of current diagnosis to the last contact or death. The reverse Kaplan-Meier method was used to estimate median follow up. Clinical outcomes were estimated from current diagnosis to last follow-up, progression, or death. Disease progression was determined by either (1) histologic confirmation or (2) growth on multiple imaging studies with consensus among the multidisciplinary sarcoma team and/or changes in treatment plan. Growth of the treated site was compared with first baseline image after completion of radiation therapy, to avoid false positive events due to pseudoprogression. Local control (LC) was defined as freedom from progression within the PTV_LR treatment volume. Regional control (RC) was defined as progression outside of the PTV_LR 100% isodose line but within the PTV_LR 50% isodose line. Progression-free survival (PFS) was defined as freedom from any disease progression or death. The date of disease progression was defined as the date of histologic tissue confirmation or imaging that was consistent with progression based on multidisciplinary consensus. Overall survival (OS) was defined as freedom from death of any cause. The Kaplan-Meier method was used to estimate the time to events and analyzed via log-rank. Univariate Cox analysis (UVA) was performed to evaluate associations between all collected clinical variables with clinical outcomes. Chi-square analysis was used to assess predictors of grade 3 toxicity. The predetermined threshold for statistical significance was P < .05. Results are reported with 95% confidence intervals (95% CI) where available.
Results
Patient, tumor, and treatment characteristics
A total of 27 consecutive patients were evaluated, with median age of 68 years (26-94 years) and tumor size of 7 cm (1.2-21 cm), which were most often tumors in the extremity (59%), stage IV disease (70%), grade 3 (70%), Karnofsky performance scale (KPS) ≥80 (81%), undifferentiated pleomorphic sarcoma histology (UPS; 22%), and locally recurrent disease (52%) (Table 2). RT was often 20 fractions (60/50/40-46 Gy) (n = 18, 67%), either due to concurrent systemic therapy (n = 8) or prior RT to the site (n = 10).
Table 2.
Patient, tumor, and treatment characteristics
| Characteristic | n or median (% or range) |
|---|---|
| Cohort size | 27 |
| Age at treatment, years | 68 (26-94) |
| Sex | |
| Female | 14 (52%) |
| Male | 13 (48%) |
| KPS | |
| 100 | 7 (26%) |
| 90 | 11 (41%) |
| 80 | 4 (15%) |
| 70 | 5 (19%) |
| <70 | 0 |
| Histology | |
| UPS | 6 (22%) |
| Spindle cell neoplasm, NOS | 4 (15%) |
| Leiomyosarcoma | 3 (11%) |
| Synovial sarcoma | 3 (11%) |
| Undifferentiated spindle cell sarcoma | 2 (7%) |
| Myxofibrosarcoma | 2 (7%) |
| Myxoid sarcoma | 1 (4%) |
| Atypical spindle cell lipomatous tumor | 1 (4%) |
| Fibroblastic sarcoma | 1 (4%) |
| Extraskeletal Ewing sarcoma | 1 (4%) |
| Extraskeletal osteosarcoma | 1 (4%) |
| PEComa | 1 (4%) |
| Angiosarcoma | 1 (4%) |
| Histology Grouped | |
| UPS/Spindle cell sarcoma | 12 (44%) |
| Leiomyosarcoma | 3 (11%) |
| Synovial sarcoma | 3 (11%) |
| Liposarcoma | 1 (4%) |
| Angiosarcoma | 1 (4%) |
| Other | 7 (26%) |
| Location | |
| Extremity | 16 (59%) |
| Head and neck | 1 (4%) |
| Trunk | 4 (15%) |
| Lung | 4 (15%) |
| Abdomen/pelvis | 2 (7%) |
| Clinical tumor size, cm | 7.0 (1.2-21.0) |
| Clinical tumor size group* | |
| ≤5 cm | 10 (37%) |
| >5 cm to 10 cm | 7 (26%) |
| >10 cm to 15 cm | 5 (19%) |
| ≥15 cm | 5 (19%) |
| Tumor grade | |
| 1 | 3 (11%) |
| 2 | 5 (19%) |
| 3 | 19 (70%) |
| Clinical prognostic stage group | |
| IA | 0 |
| IB | 1 (4%) |
| II | 2 (7%) |
| IIIA | 4 (15%) |
| IIIB | 1 (4%) |
| IV | 19 (70%) |
| Distantly metastatic | |
| Yes | 19 (70%) |
| No | 8 (30%) |
| Lesion type treated | |
| Untreated primary | 10 (37%) |
| Locally recurrent disease | 4 (15%) |
| Prior resection | 10 (37%) |
| Prior resection plus RT | 3 (11%) |
| Metastasis | |
| Systemic therapy timing related to RT | |
| None | 9 (33%) |
| Neoadjuvant | 7 (26%) |
| Neoadjuvant + concurrent | 3 (11%) |
| Neoadjuvant + concurrent + adjuvant | 1 (4%) |
| Neoadjuvant + adjuvant | 2 (7%) |
| Concurrent | 3 (11%) |
| Concurrent + adjuvant | 1 (4%) |
| Adjuvant | 1 (4%) |
| Concurrent systemic therapy regimen | |
| Ifosfamide | 6 (15%) |
| Paclitaxel | 1 (4%) |
| Pazopanib | 1 (4%) |
| RT Regimen | |
| 66/55/50.6 Regimen | 9 (33%) |
| 60/50/46 Regimen | 18 (67%) |
Abbreviations: KPS = Karnofsky performance status scale; NOS = not otherwise specified; PEComa = malignant perivascular epithelioid cell neoplasm; RT = radiation therapy; UPS = undifferentiated pleomorphic sarcoma.
Clinical tumor size group represents the tumors of interest divided into categories based upon T staging criteria.
Tumor control and survival
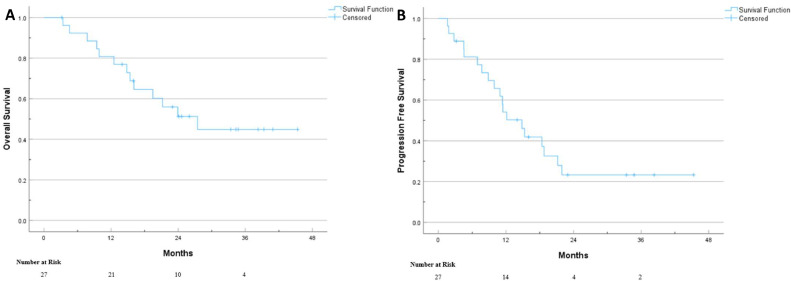
The median follow-up for all patients was 33.4 months (95% CI, 20.3-46.5 months). There were no instances of local or regional progression in the overall cohort. 3-year OS and PFS were 44.9% (Fig. 2A) and 23.3% (Fig. 2B), respectively. On UVA, both treated tumor size >15 cm and locally recurrent disease were significant predictors of OS and PFS (Table 3). Additionally, tumor size ≤5 cm was associated with improved PFS.
Figure 2.
Kaplan Meier survival curves for OS (A) and PFS (B) for STS patients treated definitively with hypofractionated accelerated radiation dose-painting (HARD).
Table 3.
Univariate analysis for overall survival and progression-free survival
| Overall Survival |
Progression-free survival |
|||
|---|---|---|---|---|
| Variable | HR (95% CI) | P | HR (95% CI) | P |
| Age at treatment | 0.98 (0.94, 1.02) | 0.313 | 0.99 (0.96, 1.02) | .528 |
| Karnofsky Performance Status | ||||
| 90-100 | 1.00 (Reference) | - | 1.00 (Reference) | - |
| 70-80 | 2.46 (0.82, 7.43) | 0.110 | 0.86 (0.33, 2.27) | .764 |
| Histology | ||||
| UPS/spindle cell sarcoma | 1.00 (Reference) | 0.303 | 1.00 (Reference) | .432 |
| Leiomyosarcoma | 1.82 (0.17, 20.1) | 0.625 | 1.82 (0.34, 9.62) | .483 |
| Synovial sarcoma | 2.79 (4.86, 0.28) | 0.279 | 0.84 (0.08, 8.40) | .880 |
| Liposarcoma | - | - | - | - |
| Angiosarcoma | 3.91 (0.45, 33.7) | 0.215 | 0.86 (0.23, 3.23) | .825 |
| Other | 0.29 (0.02, 4.69) | 0.381 | 0.29 (0.06, 1.44) | .130 |
| Tumor size | ||||
| ≤5 cm | 1.00 (Reference) | 0.110 | 1.00 (Reference) | .021 |
| >5 cm to 10 cm | 2.07 (0.41, 10.3) | 0.378 | 2.02 (0.60, 6.81) | .256 |
| >10 cm to 15 cm | 3.72 (0.74, 18.6) | 0.110 | 2.02 (0.56, 7.22) | .282 |
| ≥15 cm | 6.09 (0.50, 18.5) | 0.019 | 14.5 (2.69, 78.6) | .002 |
| Distantly metastatic | ||||
| No | 1.00 (Reference) | - | 1.00 (Reference) | - |
| Yes | 3.03 (0.67, 13.7) | 0.151 | 2.31 (0.76, 6.99) | .139 |
| Tumor grade | ||||
| 1 | 1.00 (Reference) | 0.625 | 1.00 (Reference) | - |
| 2 | 0.99 (0.09, 11.1) | 0.994 | 2.70 (0.28, 26.2) | .392 |
| 3 | 1.89 (0.24, 14.8) | 0.544 | 3.75 (0.49, 28.6) | .202 |
| Location | ||||
| Extremity | 1.00 (Reference) | 0.406 | 1.00 (Reference) | .843 |
| Head and neck | 0.22 (0.02, 2.68) | 0.236 | 0.70 (0.07, 6.88) | .759 |
| Trunk | 0.23 (0.03, 2.10) | 0.193 | 0.62 (0.08, 5.01) | .651 |
| Lung | 0.79 (0.08, 7.86) | 0.837 | 0.92 (0.09, 9.00) | .940 |
| Abdomen/pelvis | - | - | 1.49 (0.13, 16.8/) | .746 |
| Lesion type | ||||
| Untreated primary | 1.00 (Reference) | 0.98 | 1.00 (Reference) | .061 |
| Locally recurrent disease | 0.29 (0.09, 0.92) | 0.036 | 0.31 (0.12, 0.84) | .021 |
| Metastasis | 0.36 (0.04, 2.99) | 0.346 | 0.37 (0.08, 1.78) | .212 |
| RT regimen | ||||
| 66/55/50.6 Gy | 1.00 (Reference) | - | 1.00 (Reference) | - |
| 60/50/46 Gy | 0.30 (0.07, 1.35) | 0.117 | 0.67 (0.25, 1.95) | .494 |
| Any systemic therapy | ||||
| No | 1.00 (Reference) | - | 1.00 (Reference) | - |
| Yes | 1.29 (0.40, 4.20) | 0.671 | 1.36 (0.52, 3.58) | .536 |
Abbreviations: CI = confidence interval; HR = hazard ratio; NOS = not otherwise specified; RT = radiation therapy; UPS = undifferentiated pleomorphic sarcoma.
Toxicity outcomes
A total of 5 patients (19%) experienced an acute grade 3 toxicity, and 2 patients (7%) experienced late grade 3 toxicity (Table 4). There were no grade 4 or 5 toxicities related to HARD at any time. Radiation dermatitis was the most common acute toxicity, with 17 (63%) patients experiencing grade ≤2 toxicity and 5 (19%) experiencing grade 3 toxicities. On UVA, tumor size, tumor location, RT regimen, smoking history, and utilization of concurrent systemic failed to predict grade 3 acute toxicity.
Table 4.
Acute and Late Toxicity
| Acute |
Late |
|||||
|---|---|---|---|---|---|---|
| None | Grades 1-2 | grade 3 | None | Grades 1-2 | grade 3 | |
| Highest grade toxicity, any* | 0 | 22 (81%) | 5 (19%) | 24 (89%) | 3 (11%) | 2 (7%) |
| Fatigue | 21 (78%) | 6 (22%) | 0 | 27 (100%) | 0 | 0 |
| GI, any* | 16 (59%) | 11 (41%) | 0 | 27 (100%) | 0 | 0 |
| Nausea | 22 (81%) | 5 (19%) | 0 | 27 (100%) | 0 | 0 |
| Dysphagia | 25 (93%) | 2 (7%) | 0 | 27 (100%) | 0 | 0 |
| Odynophagia | 23 (85%) | 4 (15%) | 0 | 27 (100%) | 0 | 0 |
| Diarrhea | 24 (89%) | 3 (11%) | 0 | 27 (100%) | 0 | 0 |
| Constipation | 27 (100%) | 0 | 0 | 27 (100%) | 0 | 0 |
| Integumentary, any* | 5 (19%) | 17 (63%) | 5 (19%) | 26 (96%) | 1 (4%) | 2 (7%) |
| Dermatitis | 5 (19%) | 17 (63%) | 5 (19%) | 27 (100%) | 0 | 0 |
| Wound infection | 27 (100%) | 0 | 0 | 26 (96%) | 1 (4%) | 0 |
| Poor wound healing | 27 (100%) | 0 | 0 | 27 (100%) | 0 | 2 (7%) |
| GU, any* | 26 (96%) | 1 (4%) | 0 | 27 (100%) | 0 | 0 |
| Dysuria | 26 (96%) | 1 (4%) | 0 | 27 (100%) | 0 | 0 |
| Urinary frequency | 26 (96%) | 1 (4%) | 0 | 27 (100%) | 0 | 0 |
| Urinary urgency | 26 (96%) | 1 (4%) | 0 | 27 (100%) | 0 | 0 |
| Hematuria | 27 (100%) | 0 | 0 | 27 (100%) | 0 | 0 |
| Respiratory, any* | 25 (93%) | 2 (7%) | 0 | 27 (100%) | 0 | 0 |
| Cough | 25 (93%) | 2 (7%) | 0 | 27 (100%) | 0 | 0 |
| Dyspnea | 25 (93%) | 2 (7%) | 0 | 27 (100%) | 0 | 0 |
| MSK, any* | 21 (78%) | 6 (22%) | 0 | 24 (89%) | 3 (11%) | 0 |
| Chest pain, noncardiac | 25 (93%) | 2 (7%) | 0 | 27 (100%) | 0 | 0 |
| Bone pain | 26 (96%) | 1 (4%) | 0 | 27 (100%) | 0 | 0 |
| Decreased ROM | 21 (78%) | 6 (22%) | 0 | 26 (96%) | 2 (7%) | 0 |
| Edema | 26 (96%) | 1 (4%) | 0 | 27 (100%) | 0 | 0 |
| Paresthesia | 26 (96%) | 1 (4%) | 0 | 27 (100%) | 0 | 0 |
| Muscle weakness | 25 (93%) | 2 (7%) | 0 | 26 (96%) | 1 (4%) | 0 |
Abbreviations: GI = gastrointestinal; GU = genitourinary; MSK = musculoskeletal; ROM = range of motion.
Toxicities designated as “any” only report the highest-grade toxicity per patient.
Discussion
Our study demonstrates that the novel definitive HARD regimen is a safe and effective method for patients with unresected STS. Achieving durable LC in unresected STS with RT has been limited by its radioresistant nature,4, 5, 6, 7, 8 with photon-based 5-year LC of 28 to 73%.31 Many attempts have been made to improve the LC for unresectable STS treated with RT, including concurrent systemic therapy,32 heavy ion therapy,33 combined hyperthermia,34 and hypofractionation.35 However, our study demonstrated impressive outcomes using our novel HARD photon-based regimen, with no instances of local or regional progression observed in this historically radioresistant cohort.
Though highly heterogeneous, STS is thought to be generally radioresistant, with an estimated α/β of 2 to 6 Gy.7,12 To overcome their innate radiobiology, both dose escalation (≥63-65 Gy)9, 10, 11 and higher radiation doses per fraction36 may improve STS local control, as seen with SBRT for metastatic lesions.15, 16, 17, 18, 19, 20, 21 The utility of dose escalation and hypofractionation is limited by the risk of long-term toxicity (eg, bone fracture, fibrosis, joint stiffness). This can be mitigated by a fractionated approach and the use of hypofractionation with isotoxic dose-painting. HFRT has been explored extensively in the neoadjuvant setting, safety of 5,37, 38, 39, 40 8,41,42 10,35,43,44 and 15 fraction45 regimens before surgery. However, few studies have explored the role of hypofractionated RT for unresectable disease.
The available literature investigating moderately hypofractionated RT (2.4-4 Gy per fraction) for unresected STS is limited to small retrospective reviews with heterogeneous RT dose and fractionation regimens. In 2010, Soyfer et al reported their experience of treating metastatic STS (n = 15) with a hypofractionated RT (39 Gy/13 fractions) and demonstrated a 80% LC rate (12 of 15 patients), without grade 2 to 5 toxicity in 25 weeks of followup.46 In addition, Boyce-Fappiano et al showed the utility of hypofractionated RT, most commonly 15 fractions to 52.5 Gy/45 Gy (GTV/PTV), which provided a 1-year/2-year LC of 73%/47% with 49% grade 1 to 2 toxicity, and no grade 3 to 5 toxicity.35 The higher LC with HARD (3-year = 100%) may be due to the higher equivalent dose in 2 Gy per fraction (EQD2) delivered, where prior studies showed improved LC (5-year LC: 60% vs 22%) with dose escalation to ≥ 63 Gy.11 Assuming an α/β of 4 Gy for STS, prior photon based hypofractionation studies35,46 treated with a lower EQD2(45.5-66 Gy) than our current study (EQD2: 70-77 Gy), and reported LC rates of approximately 70% at 1 year. Similar dosing to the HARD regimen has only been observed with heavy ions (70.4 Gy/16 fractions28,47 and 60 Gy/20 fractions48,49 with protons/carbons, which had a LC comparable with our findings (2-year LC = 77-96%).
Although dose escalation can improve LC, doses ≥68 Gy have been associated with a higher rate of major complications (26% vs 8%).11 The present study demonstrates that the HARD regimen's isotoxic approach can allow photon-based planning that may mitigate toxicity. Overall, the HARD regimen is well-tolerated, with a relatively low rate of acute grade 3 toxicity (19%), and no instances of acute grade 4 or 5 toxicity (Table 4). Radiation dermatitis was the most prevalent acute toxicity, with grades 1 to 2 toxicity observed in 63% of patients and grade 3 observed in 19% of patients, comparable to previously reported toxicity after definitive RT for unresectable STS (grade 3 or higher toxicity ∼ 6-18%).31 The 2 patients who experienced late grade 3 poor wound healing both had multiple prior surgical resections at that site, and one patient also had prior RT with an overlapping field.
This study has a few important limitations, including its retrospective nature, which may lead to selection biases and confounding by indication in the cohort composition. Importantly, there were no local or regional disease progression events to estimate the effect size of the HARD regimen. Conversely, there was large proportion of patients with oligometastatic and recurrent disease that may have impacted OS and PFS, but the study is likely underpowered to show a significant association between these historic prognostic predictors and outcome. HARD had a low long-term grade 3 toxicity rate (7%) at 33 months, but longer follow up and a larger cohort size is required to confirm these findings.
Conclusion
The definitive HARD regimen is associated with excellent locoregional control with a favorable toxicity profile in patients with unresectable STS tumors. The use of isotoxic dose-painting offers radiobiological treatment advantages and condensed treatment times, and it mitigates long-term toxicity with photon-based therapy. Future prospective studies are needed to validate these findings and compare the efficacy of this approach to standard definitive treatment.
Disclosures
No author has any conflicts of interest to declare.
Footnotes
Sources of support: There is no funding to declare for the manuscript.
Data sharing statement: Research data are stored in an institutional repository and will be shared upon request to the corresponding author.
References
- 1.Burningham Z, Hashibe M, Spector L, Schiffman JD. The epidemiology of sarcoma. Clin Sarcoma Res. 2012;2:14. doi: 10.1186/2045-3329-2-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Clark MA, Fisher C, Judson I, Thomas JM. Soft-tissue sarcomas in adults. N Engl J Med. 2005;353:701–711. doi: 10.1056/NEJMra041866. [DOI] [PubMed] [Google Scholar]
- 3.Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72:7–33. doi: 10.3322/caac.21708. [DOI] [PubMed] [Google Scholar]
- 4.von Mehren M, Kane JM, Bui MM, et al. NCCN Guidelines insights: Soft tissue sarcoma, Version 1.2021. J Natl Compr Canc Netw. 2020;18:1604–1612. doi: 10.6004/jnccn.2020.0058. [DOI] [PubMed] [Google Scholar]
- 5.Haas RL, Delaney TF, O'Sullivan B, et al. Radiotherapy for management of extremity soft tissue sarcomas: Why, when, and where? Int J Radiat Oncol Biol Phys. 2012;84:572–580. doi: 10.1016/j.ijrobp.2012.01.062. [DOI] [PubMed] [Google Scholar]
- 6.Nussbaum DP, Rushing CN, Lane WO, et al. Preoperative or postoperative radiotherapy versus surgery alone for retroperitoneal sarcoma: A case-control, propensity score-matched analysis of a nationwide clinical oncology database. Lancet Oncol. 2016;17:966–975. doi: 10.1016/S1470-2045(16)30050-X. [DOI] [PubMed] [Google Scholar]
- 7.Yang G, Yuan Z, Ahmed K, et al. Genomic identification of sarcoma radiosensitivity and the clinical implications for radiation dose personalization. Transl Oncol. 2021;14 doi: 10.1016/j.tranon.2021.101165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Salerno KE, Alektiar KM, Baldini EH, et al. Radiation therapy for treatment of soft tissue sarcoma in adults: Executive summary of an ASTRO clinical practice guideline. Pract Radiat Oncol. 2021;11:339–351. doi: 10.1016/j.prro.2021.04.005. [DOI] [PubMed] [Google Scholar]
- 9.Tepper JE, Suit HD. Radiation therapy alone for sarcoma of soft tissue. Cancer. 1985;56:475–479. doi: 10.1002/1097-0142(19850801)56:3<475::aid-cncr2820560311>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 10.Slater JD, McNeese MD, Peters LJ. Radiation therapy for unresectable soft tissue sarcomas. Int J Radiat Oncol Biol Phys. 1986;12:1729–1734. doi: 10.1016/0360-3016(86)90312-3. [DOI] [PubMed] [Google Scholar]
- 11.Kepka L, DeLaney TF, Suit HD, Goldberg SI. Results of radiation therapy for unresected soft-tissue sarcomas. Int J Radiat Oncol Biol Phys. 2005;63:852–859. doi: 10.1016/j.ijrobp.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 12.Haas RL, Floot BGJ, Scholten AN, et al. Cellular radiosensitivity of soft tissue sarcoma. Radiat Res. 2021;196:23–30. doi: 10.1667/RADE-20-00226.1. [DOI] [PubMed] [Google Scholar]
- 13.van Leeuwen CM, Oei AL, Crezee J, et al. The alfa and beta of tumours: A review of parameters of the linear-quadratic model, derived from clinical radiotherapy studies. Radiat Oncol. 2018;13:96. doi: 10.1186/s13014-018-1040-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thames HD, Suit HD. Tumor radioresponsiveness versus fractionation sensitivity. Int J Radiat Oncol Biol Phys. 1986;12:687–691. doi: 10.1016/0360-3016(86)90081-7. [DOI] [PubMed] [Google Scholar]
- 15.Savina M, Le Cesne A, Blay JY, et al. Patterns of care and outcomes of patients with METAstatic soft tissue SARComa in a real-life setting: The METASARC observational study. BMC Med. 2017;15:78. doi: 10.1186/s12916-017-0831-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Falk AT, Moureau-Zabotto L, Ouali M, et al. Effect on survival of local ablative treatment of metastases from sarcomas: A study of the French sarcoma group. Clin Oncol (R Coll Radiol) 2015;27:48–55. doi: 10.1016/j.clon.2014.09.010. [DOI] [PubMed] [Google Scholar]
- 17.Dhakal S, Corbin KS, Milano MT, et al. Stereotactic body radiotherapy for pulmonary metastases from soft-tissue sarcomas: Excellent local lesion control and improved patient survival. Int J Radiat Oncol Biol Phys. 2012;82:940–945. doi: 10.1016/j.ijrobp.2010.11.052. [DOI] [PubMed] [Google Scholar]
- 18.Mehta N, Selch M, Wang PC, et al. Safety and efficacy of stereotactic body radiation therapy in the treatment of pulmonary metastases from high grade sarcoma. Sarcoma. 2013;2013 doi: 10.1155/2013/360214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Navarria P, Ascolese AM, Cozzi L, et al. Stereotactic body radiation therapy for lung metastases from soft tissue sarcoma. Eur J Cancer. 2015;51:668–674. doi: 10.1016/j.ejca.2015.01.061. [DOI] [PubMed] [Google Scholar]
- 20.Baumann BC, Nagda SN, Kolker JD, et al. Efficacy and safety of stereotactic body radiation therapy for the treatment of pulmonary metastases from sarcoma: A potential alternative to resection. J Surg Oncol. 2016;114:65–69. doi: 10.1002/jso.24268. [DOI] [PubMed] [Google Scholar]
- 21.Matsushita H, Jingu K, Umezawa R, et al. Stereotactic radiotherapy for oligometastases in lymph nodes-A Review. Technol Cancer Res Treat. 2018;17 doi: 10.1177/1533033818803597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ahmad SS, Duke S, Jena R, Williams MV, Burnet NG. Advances in radiotherapy. BMJ. 2012;345:e7765. doi: 10.1136/bmj.e7765. [DOI] [PubMed] [Google Scholar]
- 23.Ma L, Wang L, Tseng CL, Sahgal A. Emerging technologies in stereotactic body radiotherapy. Chin Clin Oncol. 2017;6:S12. doi: 10.21037/cco.2017.06.19. [DOI] [PubMed] [Google Scholar]
- 24.Wang D, Zhang Q, Eisenberg BL, et al. Significant reduction of late toxicities in patients with extremity sarcoma treated with image-guided radiation therapy to a reduced target volume: Results of radiation therapy oncology group RTOG-0630 trial. J Clin Oncol. 2015;33:2231–2238. doi: 10.1200/JCO.2014.58.5828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Whelan TJ, Pignol JP, Levine MN, et al. Long-term results of hypofractionated radiation therapy for breast cancer. N Engl J Med. 2010;362:513–520. doi: 10.1056/NEJMoa0906260. [DOI] [PubMed] [Google Scholar]
- 26.Widmark A, Gunnlaugsson A, Beckman L, et al. Ultra-hypofractionated versus conventionally fractionated radiotherapy for prostate cancer: 5-year outcomes of the HYPO-RT-PC randomised, non-inferiority, phase 3 trial. Lancet. 2019;394:385–395. doi: 10.1016/S0140-6736(19)31131-6. [DOI] [PubMed] [Google Scholar]
- 27.Kamada T, Tsujii H, Tsuji H, et al. Efficacy and safety of carbon ion radiotherapy in bone and soft tissue sarcomas. J Clin Oncol. 2002;20:4466–4471. doi: 10.1200/JCO.2002.10.050. [DOI] [PubMed] [Google Scholar]
- 28.Imai R, Kamada T, Araki N. Working Group for Carbon Ion Radiotherapy for Bone and Soft Tissue Sarcomas. Carbon ion radiotherapy for unresectable localized axial soft tissue sarcoma. Cancer Med. 2018;7:4308–4314. doi: 10.1002/cam4.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Serizawa I, Kagei K, Kamada T, et al. Carbon ion radiotherapy for unresectable retroperitoneal sarcomas. Int J Radiat Oncol Biol Phys. 2009;75:1105–1110. doi: 10.1016/j.ijrobp.2008.12.019. [DOI] [PubMed] [Google Scholar]
- 30.Services USDoHaH. Common Terminology Criteria for Adverse Events (CTCAE). 2017. Version 5.0. https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/ctcae_v5_quick_reference_5x7.pdf.
- 31.Allignet B, Sunyach MP, Geets X, Waissi W. Is there a place for definitive radiotherapy in the treatment of unresectable soft-tissue sarcoma? A systematic review. Acta Oncol. 2022;61:720–729. doi: 10.1080/0284186X.2022.2066983. [DOI] [PubMed] [Google Scholar]
- 32.Eckert F, Matuschek C, Mueller AC, et al. Definitive radiotherapy and single-agent radiosensitizing ifosfamide in patients with localized, irresectable soft tissue sarcoma: A retrospective analysis. Radiat Oncol. 2010;5:55. doi: 10.1186/1748-717X-5-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cuccia F, Fiore MR, Barcellini A, et al. Outcome and toxicity of carbon ion radiotherapy for axial bone and soft tissue sarcomas. Anticancer Res. 2020;40:2853–2859. doi: 10.21873/anticanres.14260. [DOI] [PubMed] [Google Scholar]
- 34.Spalek MJ, Borkowska AM, Telejko M, et al. The feasibility study of hypofractionated radiotherapy with regional hyperthermia in soft tissue sarcomas. Cancers (Basel) 2021;13:1332. doi: 10.3390/cancers13061332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Boyce-Fappiano D, Damron EP, Farooqi A, et al. Hypofractionated radiation therapy for unresectable or metastatic sarcoma lesions. Adv Radiat Oncol. 2022;7 doi: 10.1016/j.adro.2022.100913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Joiner M, van der Kogel AJ. Fifth edition. CRC Press/Taylor & Francis Group; 2018. Basic clinical radiobiology. [Google Scholar]
- 37.Bedi M, Singh R, Charlson JA, et al. Is 5 the new 25? Long-term oncologic outcomes from a phase II, prospective, 5-fraction preoperative radiation therapy trial in patients with localized soft tissue sarcoma. Adv Radiat Oncol. 2022;7 doi: 10.1016/j.adro.2021.100850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Roohani S, Ehret F, Kobus M, et al. Preoperative hypofractionated radiotherapy for soft tissue sarcomas: A systematic review. Radiat Oncol. 2022;17:159. doi: 10.1186/s13014-022-02072-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mayo ZS, Parsai S, Asha W, et al. Early outcomes of ultra-hypofractionated preoperative radiation therapy for soft tissue sarcoma followed by immediate surgical resection. Radiother Oncol. 2023;180 doi: 10.1016/j.radonc.2022.109439. [DOI] [PubMed] [Google Scholar]
- 40.Kubicek GJ, LaCouture T, Kaden M, et al. Preoperative radiosurgery for soft tissue sarcoma. Am J Clin Oncol. 2018;41:86–89. doi: 10.1097/COC.0000000000000236. [DOI] [PubMed] [Google Scholar]
- 41.Ryan CW, Montag AG, Hosenpud JR, et al. Histologic response of dose-intense chemotherapy with preoperative hypofractionated radiotherapy for patients with high-risk soft tissue sarcomas. Cancer. 2008;112:2432–2439. doi: 10.1002/cncr.23478. [DOI] [PubMed] [Google Scholar]
- 42.Pennington JD, Eilber FC, Eilber FR, et al. Long-term outcomes with ifosfamide-based hypofractionated preoperative chemoradiotherapy for extremity soft tissue sarcomas. Am J Clin Oncol. 2018;41:1154–1161. doi: 10.1097/COC.0000000000000443. [DOI] [PubMed] [Google Scholar]
- 43.Pisters PW, Patel SR, Prieto VG, et al. Phase I trial of preoperative doxorubicin-based concurrent chemoradiation and surgical resection for localized extremity and body wall soft tissue sarcomas. J Clin Oncol. 2004;22:3375–3380. doi: 10.1200/JCO.2004.01.040. [DOI] [PubMed] [Google Scholar]
- 44.Temple WJ, Temple CL, Arthur K, Schachar NS, Paterson AH, Crabtree TS. Prospective cohort study of neoadjuvant treatment in conservative surgery of soft tissue sarcomas. Ann Surg Oncol. 1997;4:586–590. doi: 10.1007/BF02305541. [DOI] [PubMed] [Google Scholar]
- 45.Guadagnolo BA, Bassett RL, Mitra D, et al. Hypofractionated, 3-week, preoperative radiotherapy for patients with soft tissue sarcomas (HYPORT-STS): A single-centre, open-label, single-arm, phase 2 trial. Lancet Oncol. 2022;23:1547–1557. doi: 10.1016/S1470-2045(22)00638-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Soyfer V, Corn BW, Kollender Y, Tempelhoff H, Meller I, Merimsky O. Radiation therapy for palliation of sarcoma metastases: A unique and uniform hypofractionation experience. Sarcoma. 2010;2010 doi: 10.1155/2010/927972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Imai R, Kamada T, Araki N. Working Group For Bone and Soft-Tissue Sarcomas. Clinical efficacy of carbon ion radiotherapy for unresectable chondrosarcomas. Anticancer Res. 2017;37:6959–6964. doi: 10.21873/anticanres.12162. [DOI] [PubMed] [Google Scholar]
- 48.Uhl M, Mattke M, Welzel T, et al. High control rate in patients with chondrosarcoma of the skull base after carbon ion therapy: First report of long-term results. Cancer. 2014;120:1579–1585. doi: 10.1002/cncr.28606. [DOI] [PubMed] [Google Scholar]
- 49.Mattke M, Vogt K, Bougatf N, et al. High control rates of proton- and carbon-ion-beam treatment with intensity-modulated active raster scanning in 101 patients with skull base chondrosarcoma at the Heidelberg Ion Beam Therapy Center. Cancer. 2018;124:2036–2044. doi: 10.1002/cncr.31298. [DOI] [PubMed] [Google Scholar]