Abstract
Validation of potential therapeutic targets in cancer requires functional live assays that recapitulate the biology, anatomy and physiology of human tumours. We present a methodology for maintaining mouse and patient tumour samples ex vivo for in vitro drug-screening as well as for the guidance of patient-specific chemotherapies. The harvested tumour biopsy, excised from mice or patients, is integrated into a support tissue that includes extended stroma and vasculature. The methodology is more representative than tissue culture assays, faster than patient-derived xenograft models, easy to implement, amenable to high-throughput assays and does not carry the ethical issues or expense associated with animal studies. Our physiologically-relevant model can be successfully used for high-throughput drug screening.
Keywords: Cancer, chemotherapy, high-throughput assay, tumour explant, vascular bed, vascularization
1. Introduction
Methodologies for testing and applying existing and emerging treatments on a patient-by-patient basis would help to prioritize treatment strategies based on the potential for response or the likelihood of toxicities. By testing patient tumor samples at the time of resection, it should be possible to formulate patient-specific treatment strategies in case of progression through initial therapies. In some cancers, such as pancreatic cancer, treatment decisions must be made in the time between surgery and progression–typically weeks or months.
Many approaches have been developed to recreate tumors in culture to study cancer biology or identify promising drugs. Cancer cell lines or circulating cancer cells (CTCs) grown in 2-D cultures or as 3D spheroids are valuable tools for identifying targetable pathways, but inconsistently predict clinical response– likely due to the omission of important antagonistic or synergistic interactions between tumor components. Recent efforts to grow patient-derived xenograft (PDX) tumors in mice for subsequent drug testing are promising, but this approach necessitates that the original human tumor be passaged through mice. In addition, in our 10-year experience in an ongoing project at the Massachussetts General Hospital (MGH), the PDX take rate is less than 60%, and it requires ~145 days to produce a primary PDX from a PDAC patient sample. For personalized medicine, many treatment decisions need to be made more rapidly than this.
Currently-available organoid cultures have similar limitations in speed and reliability, and like PDX systems, can only provide useful information in specific applications (1). Tissue slice (2) cultures retain cell subpopulations, histoarchitecture, and phenotypic heterogeneity of the primary tumour. However, they still lack an extended, supporting vasculature and often use nonrepresentative matrices such as matrigel for support. The inconsistent ability of organoid and PDX assay systems to predict clinical success has generated substantial interest in human-based tissue-mimetic constructs for disease modeling and drug testing, also referred to as “organs-on-a-chip” (3, 4). Clinical oncologists need such tools to improve the reliability of drug screening and implementation of personalized medicine (5, 6).
The vasculature and its associated stroma control much of tumour biology, including metabolism, invasion, and metastasis. To date, there are a few vascularized models of human tumours that use microfluidic models (7) and bioreactors (8). Although the microfluidic model achieved tumour vascularization and early tumour intravasation steps were identified, the tumour spheroid consisted of a single cancer cell population and lacked other components of the tumour microenvironment. Bioreactors have been successful in the culture of tumour explants under dynamic flow but are difficult to adapt for high-throughput drug screening and are not amenable for longitudinal microscopic imaging; furthermore, the explant blood vessels are isolated, not integrated into a surrounding network formed in vitro.
Tumor growth and response to treatment are shaped by the interactions with host tissue-derived stroma. To recreate the correct micro-anatomy – and thus, a truly biomimetic tumor – the cancer and the host tissue have to co-evolve naturally. Only in this way will the correct mixture and organization of host macrophages, fibroblasts, stromal matrix, blood vessels and cancer cells be produced. Therefore, it may be more appropriate to use intact ex vivo tumor tissue rather than synthetic tumors created from isolated cancer cells. These considerations are of particular relevance to pancreatic cancer, a tumor known to have a very substantial stromal component that mediates tumor progression.
We have established such a method by implanting tumour explants into a supporting self-assembled tissue bed that contains stromal cells, matrix, and vasculature (9). The resulting vascularized tumour explants (VTEs) maintain their anatomy and viability and can be used for studying tumour biology, for drug screening, or for testing personalized therapies. We will describe the following steps for implementing this methodology: generation of a vascularised network (Subheadings 2.1 and 3.1) (Fig. 1), preparation of mouse and patient-derived tumour explants (Subheadings 2.2, 3.2 and 3.3) (Fig. 3), imaging and analysis (Subheadings 2.3, 3.4 - 3.6) (Fig. 2). Using this methodology, the harvested patient biopsy (10) or mouse tumour explant integrates with the stromal bed and vasculature, providing the correct extracellular matrix (collagen I, IV, fibronectin), associated stromal cells, and a lumenized vessel network.
Fig. 1: In vitro vascularization.
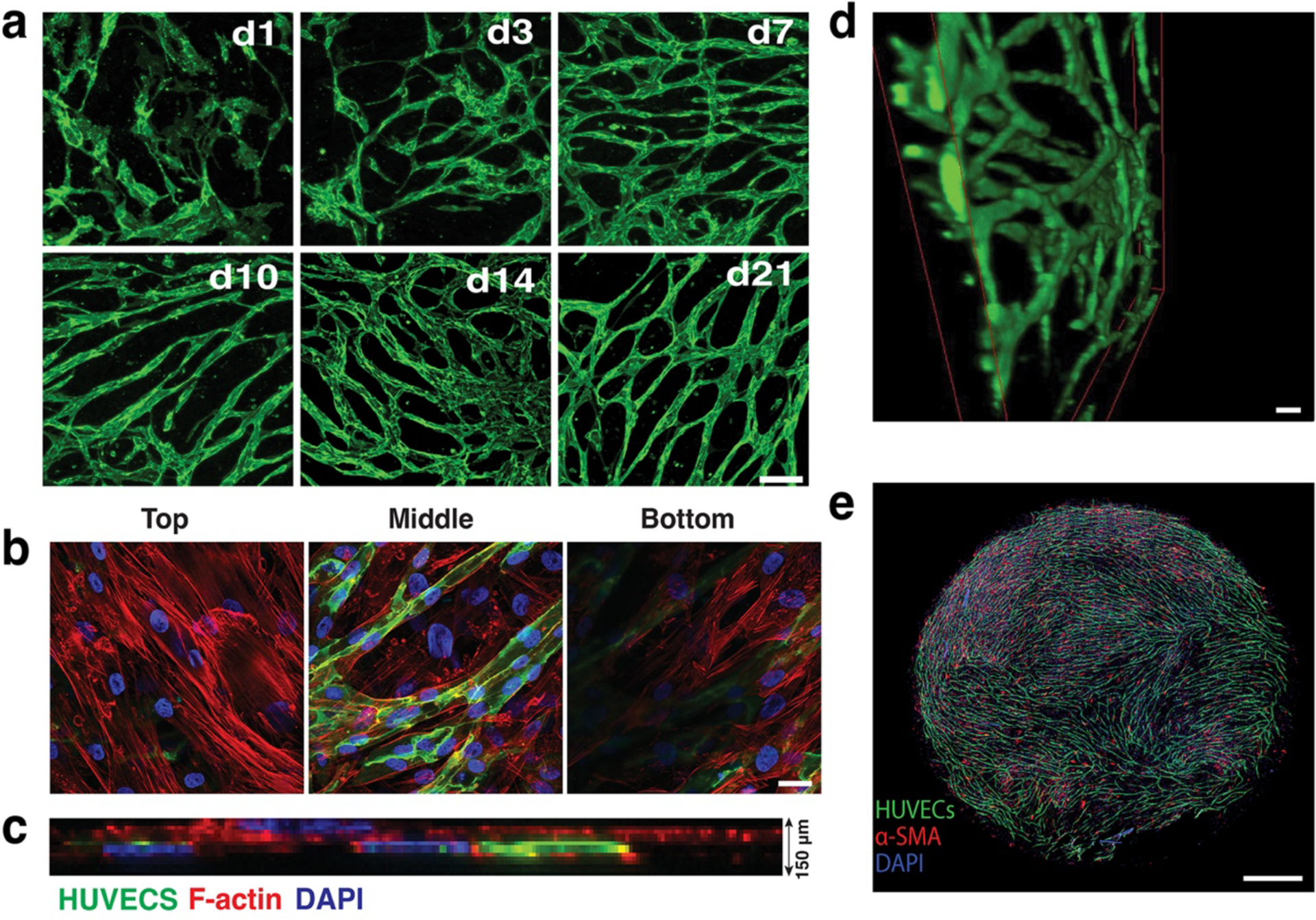
a) Co-culturing HUVECs (green) with SMCs (not shown here for clarity) leads to the formation of an interconnected vessel network by day 21. Scale bar, 200 μm.
b) Co-cultures (shown at Day 7 in culture) form a multi-layered tissue with SMCs surrounding the HUVECs (green) are seen underneath as well as above the vessel network. F-actin of both cell populations is stained with Phalloidin-568 (red). Blue depicts nuclei stained with DAPI. Scale bar, 20 μm.
c) The thickness of the multi-layered tissue (HUVECs, SMCs and matrix) is approximately 150 μm (a side view of a confocal stack is shown).
d) 3-D reconstruction of part of an in vitro network. Scale bar, 50 μm.
e) The complete network fully covers a well of a 96-well plate (Scale bar, 800 μm).
Reproduced with permission from (9).
Fig. 3: VTE cultures.
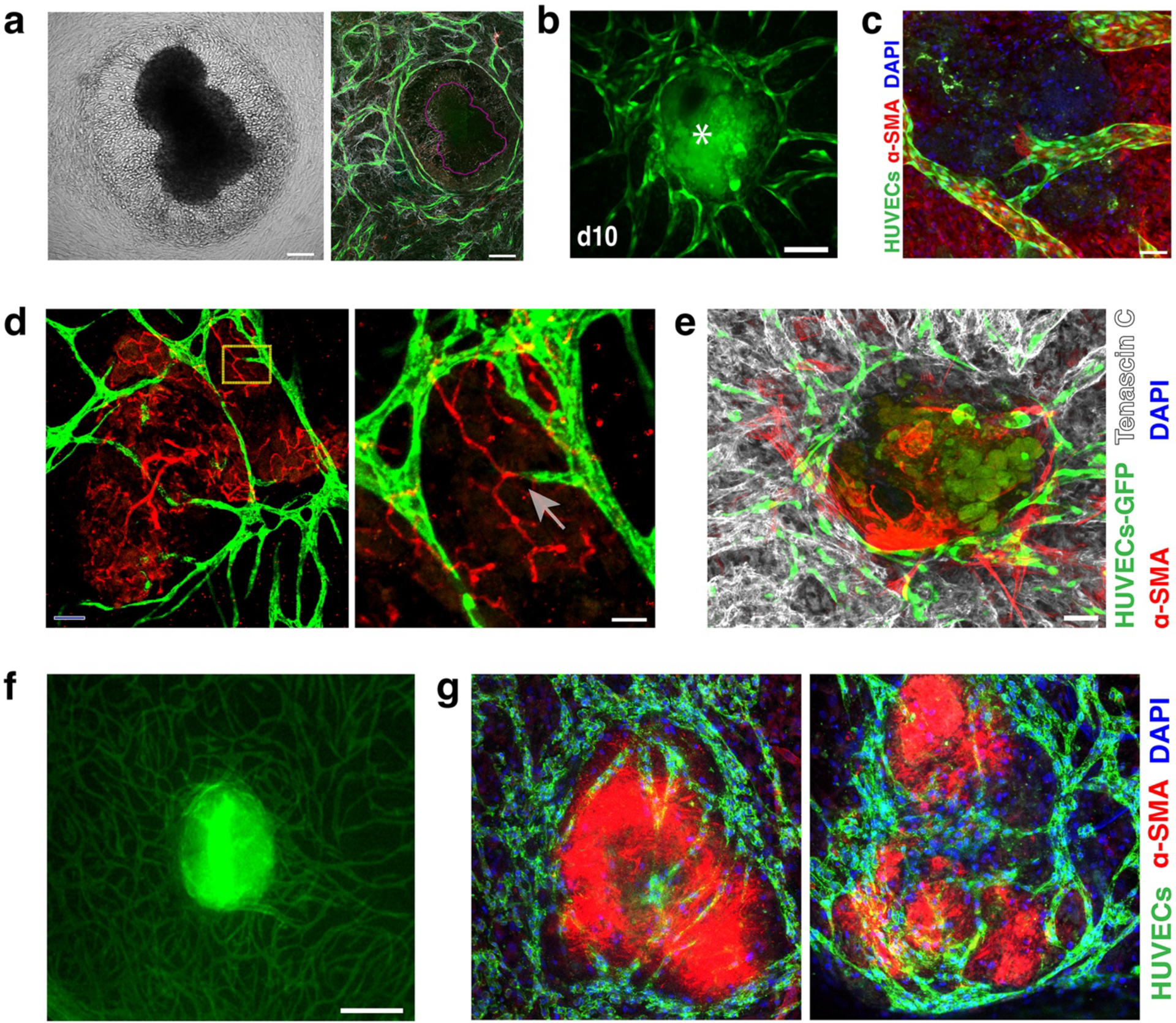
a) Brightfield image showing the disruption of Mu89 tumors when placed on the EC-SMC co-culture at Day 0. The corresponding mosaic confocal image of the same tumour (purple outline) shows that the formed vessels (green) create a ‘ring’ around the Mu89 tumor explant.
b) Vascularization of PANC-1 VTEs (asterisk), showing the vessels (green) surrounding and penetrating the tumor explants (due to auto-fluorescence, the tumour explants are also shown in green) over 10 days. Scale bar, 150 μm.
c) High-resolution confocal image showing a vessel (green) with a clearly formed lumen extending into a tumor explant. Scale bar, 50 μm. Nuclei stained with DAPI (blue).
d) Vessels (green) connect with the mouse explant vessels (red). Scale bar, 50 μm. Zoomed-in yellow area shows two vessels connecting (arrow); scale bar, 25 μm.
e) Tenascin-C (grey) was strongly expressed by Mu89 VTEs ex-vivo. Scale bar, 100 μm.
f) A patient-derived pancreatic VTE on Day 7 in culture was highly vascularized in vitro. Vessels (green); Scale bar, 150 μm.
g) Two different patient-derived VTEs (which appear red due to high levels of αSMA) show significant vessel infiltration within 7 days in culture. Vessels (green), nuclei (blue, DAPI). Scale bar, 150 μm.
Fig. 2: Co-cultures define their own microenvironment.
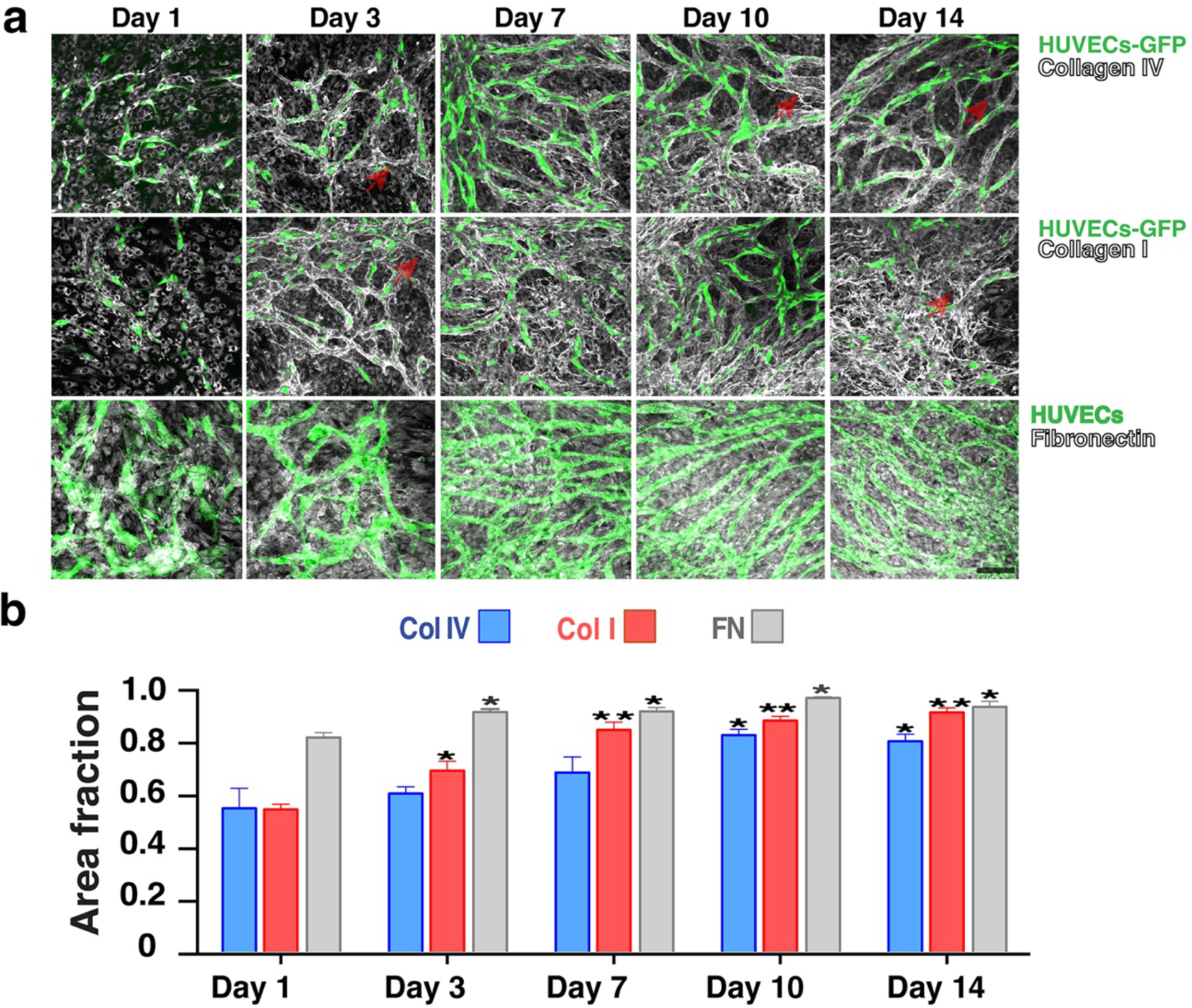
a) Top row: Collagen IV (grey) is strongly present around the vessel network (green) at Day 3 and by Day 10 it has completely covered the vessel network. Middle row: Collagen I (grey) also shows a similar peri-endothelial localization pattern in co-culture. Bottom row: Fibronectin (grey) is significantly produced by the vessel network. Scale bar, 50 μm.
b) Collagen I, IV and fibronectin abundance levels increase over time in the developing vessel network (assessed by the fractional area of staining in the immunofluorescent images). Error bars are standard errors from n = 3.
Reproduced with permission from (9).
2. Materials
2.1. Cellular Components
Reagents
Human umbilical vein endothelial cells (HUVECs) (see Note 1).
Endothelial growth medium (EGM; Lonza).
Tissue grade gelatin solution (2 %, Sigma), dilute to 0.1 % to coat tissue culture flasks or dishes.
Trypsin-EDTA: 0.025 % (wt/vol) (Invitrogen).
Human vascular endothelial growth factor (VEGF; R&D Systems).
Pulmonary artery smooth muscle cells (SMCs)(Lonza).
Smooth muscle growth medium (SmGM-2; Lonza).
Endothelial cell growth medium-2 (EGM-2;Lonza).
2.2. Mouse tumour explants
Human melanoma (Mu89) tumours grown orthotopically in 8-week-old severe combined immunodeficient (SCID) females (see Note 2).
Human breast (BT474) tumours grown in the mammary fat pad of female nude mice (see Note 2) (11).
Human pancreatic (PANC-1) tumors were grown orthotopically in 8-week-old severe combined immunodeficient mice (SCID) females (see Note 2) (12).
Patient-derived tumour explants were prepared from freshly resected human pancreatic ductal adenocarcinoma that had not been treated (chemotherapy or chemoradiation) prior to surgery (see Note 3).
2.3. In Situ Immunofluorescence staining and confocal imaging analysis
Reagents
Washing solution (1X PBS).
Fixation solution (paraformaldehyde, 3 % w/w in 1X~ PBS).
Blocking solution (5 % donkey serum/0.1 % Triton in 1Å~ PBS).
Primary antibodies.
Fluorescently conjugated secondary antibodies.
DAPI nuclear stain (Invitrogen, 1:200 dilution).
Fluorescently conjugated phalloidin (AlexaFluor 568, stain for the actin cytoskeleton; Thermofisher; 1:200 dilution).
Equipment
Phase contrast/epi-fluorescence microscope (Olympus IX70, 4X and 10X air lens, PRIOR automated stage, OpenLab software).
Confocal microscope (Olympus IX81 inverted confocal microscope, 20X air lens and 60X oil lens).
Image acquisition software (Fluoview).
Image analysis software (ImageJ, Adobe Photoshop).
On-stage cell culture incubator system (Tokai)
3. Methods
3.1. Co-culturing Endothelial cells (ECs) and SMCs
3.2. Mu89, BT474, PANC-1 Vascularized Tumour Explant (VTE) culture
When tumours reach 4–5 mm diameter resect them and cut them into explants of size between 0.2 – 0.5 mm for addition into the co-culture wells.
Incorporate the tumour explants at Day 3 of vasculature network formation (one explant per well) (See Note 5) (Fig. 3a–e).
Monitor the VTEs (i.e. tumour explants with the vascular network) for up to 10 days with the culture media being changed every third day.
3.3. Patient-derived pancreatic adenocarcinoma Vascularized Tumour Explant (VTE) culture
Following surgery, collect tumour pieces from the Pathology Department and cut them into explants of size between 0.2 – 0.5 mm for addition into the co-culture wells, as with the mouse samples described in 3.2 above.
Incorporate the tumour explants at Day 3 of vasculature network formation (one explant per well) (See Note 5) (Fig. 3f–g).
Monitor the patient-derived VTEs (i.e. tumour explants with the vascular network) for up to 10 days with the culture media being changed every third day.
3.4. Immunofluorescence staining of ECs-SMCs co-cultures
Wash co-cultures with Phosphate Buffered Saline (PBS) to remove any cell media.
Fix with 4% formaldehyde for 20 min
Wash 3X with PBS and subsequently
Permeabilize with 0.5% Triton in PBS for 10 min at 4°C.
Rinse the co-cultures 3X with PBS-Glycine.
Block with 5% donkey serum/0.1% Triton in PBS for 60 min.
Stain co-cultures with CD31 (Ready to use, DAKO), α-SMA (1:500, Sigma), Collagen I (1:500, AbCAM) overnight at 4°C (Fig. 2a).
Use the same procedure to detect Collagen IV (1:10, Millipore) and Fibronectin (1:500, AbCAM) (Fig. 2a).
Apply the appropriate fluorescently labelled secondary antibodies for 60 min and wash three times with saline prior to confocal microscopy.
To label actin filaments apply Phalloidin-AlexaFluor 568 (Thermofisher) for 30 min at room temperature.
To label cell nuclei, apply DAPI nuclear stain for ~ 5 min. Wash 3X with PBS.
Acquire images with confocal microscopy with 1 – 5 μm slice thickness.
Process the confocal projections with the ImageJ analysis software.
3.5. Immunofluorescence staining of VTE co-cultures
Wash co-cultures with Phosphate Buffered Saline (PBS) to remove any cell media.
Fix with 4% formaldehyde for 20 min
Wash 3X with PBS and subsequently
Permeabilize with 0.5% Triton in PBS for 10 min at 4°C.
Rinse the co-cultures 3X with PBS-Glycine.
Block with 5% donkey serum/0.1% Triton in PBS for 60 min.
Stain VTEs with CD31 (1:100, Millipore), Tenascin-C (1:250, R&D systems; see Note 6) and α-SMA (1:500, Sigma)
Apply appropriate fluorescently labeled secondary antibodies for 60 min and wash three times with saline.
Stain cell nuclei with DAPI nuclear stain (Invitrogen, 1:200) and wash three more times with saline prior to confocal microscopy (Fig. 3a–g).
3.6. Extracellular matrix quantification in EC-SMC co-cultures
For all analyses, use at least triplicate co-cultures with two images randomly selected from each repeat, i.e. six images in total.
Use the ImageJ software to manually threshold the corresponding images to include the collagen I, IV and fibronectin-positive signals.
Convert the images to binary and then quantify the stained area as a fraction of the total (Fig. 2b).
4. Notes
Human umbilical vein endothelial cells (HUVECs) were acquired from the Center for Excellence in Vascular Biology, Brigham & Women’s Hospital, Harvard Medical School, Boston, MA. These can also be obtained commercially (e.g. Lonza, Sigma, Thermofisher etc.)
The Massachusetts General Hospital Subcommittee on Research Animal Care approved all mouse experiments.
The use of human tumour tissue was approved by the Partners human research committee.
To ensure efficient mixing of ECs and SMCs in a 96-well plate, rotate the plate in a figure of eight motion.
When adding the tumour explants to the culture together with the ECs and SMCs, the explants contact the plastic and tend to disperse, as cells from the tumour migrate away on the plastic. When this happens in the co-cultures, cells from the explant are able to displace the vascular bed (Fig. 3a). For this reason the tumour explants are added on Day 3; the explants retain their morphology and the vasculature migrates to surround the tumours over 10 days in culture (Fig. 3b–g).
Tenascin-C is an extracellular matrix molecule that is highly associated with melanoma and in fact was expressed in Mu89 explants (Fig. 3e).
Acknowledgements
We acknowledge support from the National Institutes of Health (R01HL106584). We also thank Julia Kahn for her help with tumour implantations.
References
- 1.Huang L, Holtzinger A, Jagan I, BeGora M, Lohse I, Ngai N, et al. Ductal pancreatic cancer modeling and drug screening using human pluripotent stem cell- and patient-derived tumor organoids. Nat Med. 2015;21(11):1364–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Merz F, Gaunitz F, Dehghani F, Renner C, Meixensberger J, Gutenberg A, et al. Organotypic slice cultures of human glioblastoma reveal different susceptibilities to treatments. Neuro Oncol. 2013;15(6):670–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Neuzi P, Giselbrecht S, Lange K, Huang TJ, Manz A. Revisiting lab-on-a-chip technology for drug discovery. Nat Rev Drug Discov. 2012;11(8):620–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van der Meer AD, van den Berg A. Organs-on-chips: breaking the in vitro impasse. Integr Biol (Camb). 2012;4(5):461–70. [DOI] [PubMed] [Google Scholar]
- 5.Bersini S, Jeon JS, Dubini G, Arrigoni C, Chung S, Charest JL, et al. A microfluidic 3D in vitro model for specificity of breast cancer metastasis to bone. Biomaterials. 2014;35(8):2454–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jeon JS, Bersini S, Gilardi M, Dubini G, Charest JL, Moretti M, et al. Human 3D vascularized organotypic microfluidic assays to study breast cancer cell extravasation. Proc Natl Acad Sci U S A. 2015;112(1):214–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ehsan SM, Welch-Reardon KM, Waterman ML, Hughes CC, George SC. A three-dimensional in vitro model of tumor cell intravasation. Integr Biol (Camb). 2014;6(6):603–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ferrarini M, Steimberg N, Ponzoni M, Belloni D, Berenzi A, Girlanda S, et al. Ex-vivo dynamic 3-D culture of human tissues in the RCCS bioreactor allows the study of Multiple Myeloma biology and response to therapy. PLoS One. 2013;8(8):e71613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bazou D, Maimon N, Gruionu G, Munn LL. Self-assembly of vascularized tissue to support tumor explants in vitro. Integr Biol (Camb). 2016;8(12):1301–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bazou D, Maimon N, Gruionu G, Grahovac J, Seano G, Liu H, et al. Vascular beds maintain pancreatic tumour explants for ex vivo drug screening. J Tissue Eng Regen Med. 2018;12(1):e318–e22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jain RK, Munn LL, Fukumura D. Mammary fat pad tumor preparation in mice. Cold Spring Harb Protoc. 2012;2012(10):1115–6. [DOI] [PubMed] [Google Scholar]
- 12.Jain RK, Munn LL, Fukumura D. Pancreatic tumor preparation in mice. Cold Spring Harb Protoc. 2012;2012(12). [DOI] [PubMed] [Google Scholar]


