Abstract
Aims
This study aimed at evaluating the age, sex, and country-income patterns in aortic aneurysm disease burden, analysing trends in mortality and years of life lost (YLLs), as well as their causal drivers and risk factors, using the 2017 Global Burden of Diseases, Injuries, and Risk Factors Study (GBD 2017).
Methods and results
We described the temporal, global, and regional (195 countries) patterns of aortic aneurysm (thoracic and abdominal) mortality, YLLs, their drivers [sociodemographic index (SDI), healthcare access and quality index (HAQ index)] and risk factors using the GBD 1990–2017. Correlation and mixed multilevel modelling between aortic aneurysm mortality, YLLs, HAQ index and other variables were applied. From 1990 to 2017, a global declining trend in age-standardized aortic aneurysm mortality was found [2.88 deaths/100 000 (95% uncertainty intervals, UI 2.79 to 3.03) in 1990 and 2.19 deaths/100 000 (95% UI 2.09 to 2.28) in 2017]. Among high-income countries (HICs) a consistent declining Spearman’s correlation between age-standardised aortic aneurysm mortality, SDI (HICs; 1990 rho: 0.57, P ≤ 0.001; 2017 rho: 0.41, P = 0.001) and HAQ index was observed (HICs; 1990 rho: 0.50, P <0.001; 2016 rho: 0.35, P = 0.006); in comparison with low- and middle-income countries where correlation trends were weak and mixed. At a global level, higher HAQ index was related with lower aortic aneurysm mortality and YLLs [mortality, coef: −0.05, 95% confidence interval (CI): −0.06, −0.04; YLLs, coef: −0.94, 95% CI: −1.17, −0.71].
Conclusions
Age-standardized aortic aneurysm mortality declined globally between 1990 and 2017. Globally, age-standardized aortic aneurysm mortality and YLLs were related to changes in SDI and HAQ index levels, while country-level income-related variations were also observed.
Keywords: Aortic aneurysm, Low- and middle-income countries, Mortality, Years of life lost
Introduction
Aortic aneurysm is defined as a bulge is a vascular condition characterized by aorta’s segmental dilatation. it can be developed in any week part of the aorta, starting from the part in the chest (thoracic aneurysm) or in the abdomen (abdominal aneurysm).1 Most of the aortic aneurysms are developed silently without any signs,2 a fact that makes the diagnosis and management of the disease difficult. For example, based on data published in 2003, it was estimated that 1.5 million Americans had undiagnosed abdominal aortic aneurysm.3 Aortic aneurysms carry the risk of rupture, associated with subsequent high risk for sudden death and low chances <20% of survival.4 Rupture risk is substantially increased once the size of the aneurysm exceeds 5 cm, and at this stage, physicians along with the patient have to decide the appropriate treatment and management option, either surgery or conservative therapy.1
While ischaemic heart disease and stroke, the leading causes of cardiovascular burden globally,5 draw increasing attention from international policymakers and stakeholders, there is limited global epidemiological information on the burden of aortic aneurysm disease, even though this is almost equally lethal as various cancer types, e.g. brain neoplasms.6 Aortic aneurysms are typically described in the middle-aged population, with higher prevalence among those over 65 years.3 Currently, the global population is ageing rapidly with the old (65þ years old) and oldest old (90þ years old) now being the fastest growing segment in regions including Europe, Asia, and the USA.7 This is accompanied by a parallel increase in aortic aneurysm mortality. For example, 15000 deaths per year in males over 65 years old were estimated in the USA.3 Apart from advanced age, other risk factors that have been related with aortic aneurysm expansion and consequent rupture are smoking, abnormal arterial pressure and cholesterol levels, as well as previous family history of aneurysm.3
Knowledge on aortic aneurysm epidemiology is mostly based on abdominal aortic aneurysm, showing a respective occurence that varies between males (2 to 13%) and females (6%) at the age of 65 and beyond.8 Available data show that back in the 20th century, aortic disease incidence and mortality increased mostly among high-income countries (HICs).9 Since then, ultrasound screening as well as preventive and health education strategies were applied, and a relative decline in the 21th century was reported among developed nations.10 However, the epidemiology of aortic aneurysm has not been well characterized in low- and middle-income countries (LMICs). In 2013, a cross-national study evaluated abdominal aneurysm mortality among 19 member states of the World Health Organization,11 showing that abdominal aneurysm mortality is not declining “globally” (meaning across the analysed sample of countries) and with a high country variability. Studies from high-income settings focusing on aortic aneurysm and vascular disease epidemiology do exist,12 but the majority of these studies were small, used a clinical approach and, for instance, results could not be generalized to the population level. The Aneurysm Global Epidemiology Study, conducted some years ago, only included 19 countries, in their majority high-income ones HICs.11 Thus, global information on the epidemiology of aortic aneurysm, including LMICs, is limited.13
This study aimed at evaluating the age, sex, and country-income patterns in aortic aneurysm disease burden, analysing temporal levels and trends in aortic aneurysm mortality and years of life lost (YLLs), causal drivers of changes in aortic aneurysm mortality and YLLs, and the risk factors of these findings, using 2017 Global Burden of Diseases, Injuries, and Risk Factors Study (GBD 2017).14,16 To contextualize these patterns, and the effect of causal drivers and risk factors, we also performed secondary analyses including Spearman correlation and mixed-effect multilevel regression analysis of aortic aneurysm mortality and YLLs with societal indicators of sociodemographic index (SDI), country-level income differences (high-, middle-, and low income) and the healthcare access and quality (HAQ) index.
Methods
GBD measures, aortic aneurysm mortality, and years of life lost
We used the results of GBD 1990–2017 to evaluate temporal and regional trends in age-standardized and age-specific : aneurysm mortality and YLLs rates and YLLs and to assess age, sex, and country-income temporal patterns of causal drivers and risk factors on a global, regional, and national scale.14,16 Additional details of methods used to estimate mortality rates and YLLs, including all other analytic approaches for assessment of relative morbidity and mortality from individual diseases and injuries, are available in current GBD 2017 publications.5
Aortic aneurysm is among the 10 most common global causes of cardiovascular disease (CVD)-related mortality. In the GBD, death due to aortic aneurysm was defined based on the International Classification of Disease (ICD) coding system [(ICD)-9 and (ICD)-10 codes].16 The GBD study 2017 is organized by a geographical hierarchy of seven super regions consisting of 21 sub-regions, with 195 countries and territories nested within those sub-regions and this level of hierarchy was used for the local estimation of aortic aneurysm. The full GBD cause hierarchy, including corresponding ICD-9 and ICD-10 codes,17 is detailed in GBD 2017 publications16 on cause-specific mortality, with cause-specific methods detailed in the corresponding appendices.
GBD estimates aorticaortic aneurysm-specific mortality using standardized modelling processes—most commonly, the Cause of Death Ensemble model (CoD), which uses covariate selection and out-of-sample validity analyses and generates estimates for each location-year, age group, and sex. The GBD cause of death database includes seven types of data sources. These are vital registration data, verbal autopsy and cancer registries, as well as police records, sibling history, surveillance, and survey/census data. Information from countries/territories with complete registration systems was considered to be of high quality. For countries having incomplete vital registration systems, vital statistics for causes of death were supplemented with other data types to provide cause-specific estimates. As reported by the GBD 2017 study, only vital registration data have been used modelling the cause of death for aortic aneurysm. The exact methodology of the vital registration data use can be found in the GBD 2017 publications.16 Only the countries that used the ICD-9 and ICD-10 were included in the GBD data input. Specifically, these were 441–441.9 for ICD-9 and I71-I71.9 for ICD-10. Aortic aneurysm data based on ICD-8 coding were excluded for discontinuity with the rest of time series as well as data of Oman for the same reason.16 Additional details, including model specifications and data availability for each cause-specific model, can be found in the GBD 2017 mortality and causes of death publications.16 Oman for the same reason” please add: Following the aforementioned procedure, aortic aneurysm deaths by location, age, year and sex calculated and then were used in conjuction with the relative reference lifetables for the estimation of the aortic aneurysm YLLs.
Socioeconomic, healthcare access and quality indicators as causal drivers; risk factors estimation and uncertainty levels
To further assess the effects of socioeconomic factors and health system performance on global/regional health metrics, we employed the SDI and the HAQ index, respectively. SDI is a composite indicator based upon the country-level income per capita, the average educational attainment among individuals over age 15, and the total fertility rate among women under 25. The SDI ranges from 0 to 1.18 Each country’s income level [high (HICs)-, middle (MICs)-, low (LICs)-] was based on the World Bank’s classification.19
HAQ index is a composite metric developed following GBD 2016 that is based on comparative mortality rates for healthcare sensitive diseases, standardized to risk exposure level, and is meant to quantify the overall performance of health systems. The HAQ index ranges from 0 to 100.20,21
The GBD 2017 comparative risk assessment (CRA) framework classified all the risk factors and and the respective risk factor clusters into one of three categories: behavioural, environmental/occupational, or metabolic. Data on risk factor exposure levels were identified, and modelled using approaches similar to non-fatal models. Quantitative relative risk was estimated for each risk-outcome pair, and population-attributable fraction statistics were calculated using standard GBD CRA methods.18 Risk factors were expressed as summary exposure values (SEVs) which reflect the measure of a population’s exposure to a risk factor taking into account the extent of exposure by risk level as well as the severity of that risk’s contribution to disease burden. SEV score varies from 0 to 1. A ‘0’ SEV score means that no excess risk for a population exists while a score ‘1’ reflects the highest risk level. SEV is expressed on a scale from 0 to 100% to reflect the risk-weighted prevalence.We focused our risk factor analysis (see secondary analysis below) on specific aortic aneurysm risk factors,11 based on the literature. These were high low-density lipoprotein (LDL)-cholesterol, high fasting plasma glucose and smoking habits, as defined by the latest GBD 2017methodology.
It is reported earlier. GBD estimations, we report 95% uncertainty intervals (UIs) derived from 1000 draws from the posterior distribution of each step in the estimation process. In difference with confidence intervals (CIs), UIs capture uncertainty from various modelling steps, and also from sources such as model estimation and model specification, rather than from sampling error alone. Uncertainty related with mortality and YLLs estimation reflects sample sizes of data sources, adjustment and standardization methods applied to to the respective data, parameter uncertainty in in the modelling estimations, and also uncertainty within the cause-specific mortality models.16
Primary analysis and trends estimation
Aortic aneurysm mortality as annualized rates of change (ARC) was used to compare trends of mortality across countries between 1990 and 2017. ARCs%, computed as natural log-transformed (final estimates/initial estimates)/(# of years), were used to compare trends. Additionally, global, regional (by GBD super regions), and World Bank income level age-standardized mortality rates (per 100 000) between 1990 and 2017 were applied, as univariate trend analysis through time.
Secondary analysis and patterns assessment
Correlation analysis
Associations between age-standardized aortic aneurysm mortality and SDI and HAQ index were tested by the Spearman’s rho coefficients. The associations were tested for the years 1990, 1995, 2000, 2005, 2010, 2015, and 2017 among all 195 countries, by sex, and by country-income level (low-, middle-, and high). All P-values are based on two-sided tests. A P-value <0.05 was considered as significant. We followed previously reported criteria21 to classify a correlation as weak (<0.3), moderate (0.4–0.6), and strong (>0.7) (coefficients are presented as absolute values).
Linear regression mixed model analysis
Mixed-effect multilevel regression models were carried out to assess whether age-standardised and age-specific aortic aneurysm mortality and YLLs rates (outcomes) were related with HAQ index (independent variable) between 1990 and 2017, after adjustment for various confounders. Specifically, the mixed-effect multilevel model22 was conducted to assess the effect of HAQ index on aortic aneurysm mortality and YLLs (both sexes estimates), adjusting for the effect of SDI × HAQ index, smoking habits, high LDL-cholesterol, and high fasting plasma glucose. The interaction of SDI with HAQ index (SDI × HAQ index) was used based on the literature.23 The analysis was repeated also by age group (50–69 years old and 70þ years old) and by country-income level. Maximum likelihood estimation was used in the multilevel analysis, where years were considered the first level of analysis while the countries were considered as the 2nd level where the observations of the repeated measures were aggregated. Corresponding p values and 95 CIs are reported. Correlation analyses and linear mixed model analysis were performed with the base R software and the package glmmTMB.
Role of the funding source
The funder of the study had no role in the study design, data collection, data analysis, data interpretation, or the writing of the report. All authors had full access to the data in the study and had final responsibility for the decision to submit for publication.
Results
Primary analysis
Trends in aortic aneurysm age-standardized mortality and YLLs, for both sexes, by males and females and by country-income level, between 1990 and 2017
Age-standardized estimates of aortic aneurysm-related globally and across across LMICs, for both sexes in 1990 and 2017, as well as the percent change in mortality over the period 1990–2017, are shown in Supplementary material online, Table S1 and Figure S1. A series of countries and territories had positive age-standardized annual percent change in the aortic aneurysm rate of death. Among them are the regions of central and eastern Europe, central Asia, north Africa and middle East, Latin America, and the rest of Asia. Among these regions, the countries with the highest positive annual percent of mortality change (ARC% between 1990 and 2017) due to aortic aneurysm were among others, Uzbekistan (ARC= 2.90%) and Georgia (ARC= 4.17%), while those with the most negative annual percent of mortality change were Australia (ARC = −3.34%) and Rwanda (ARC= −3.31%).
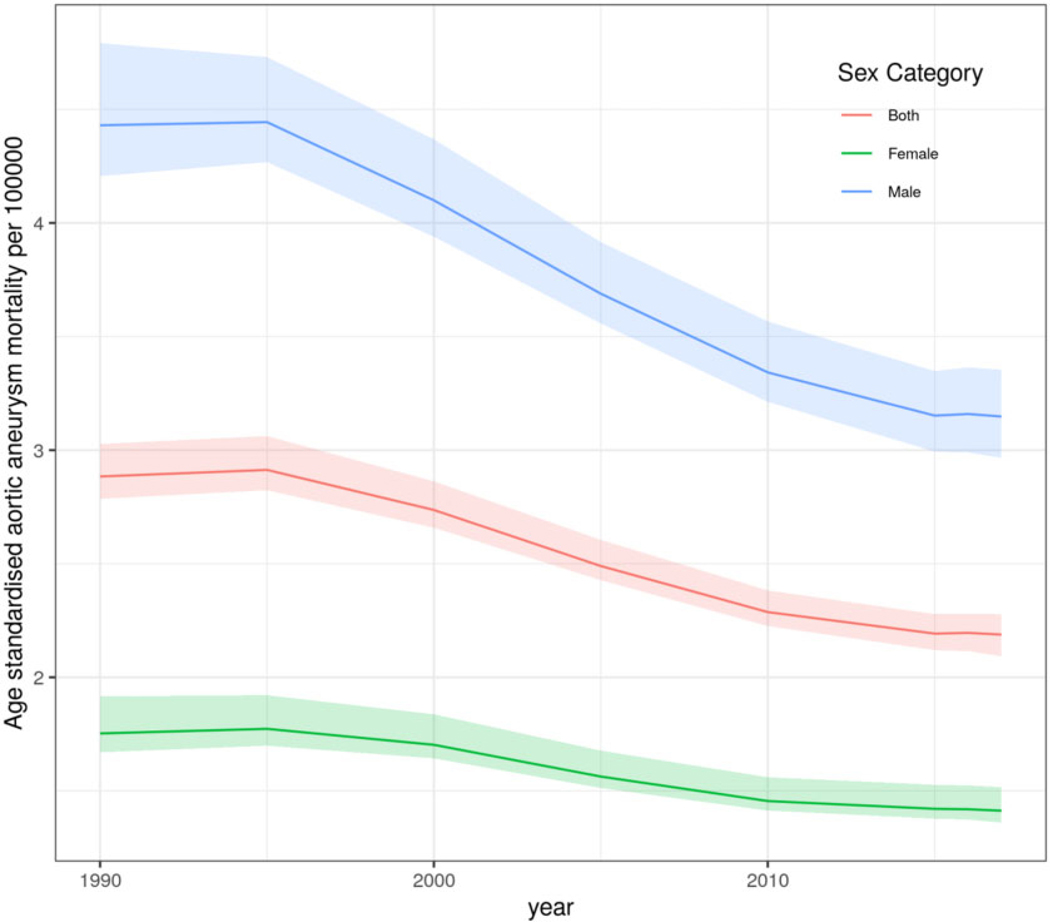
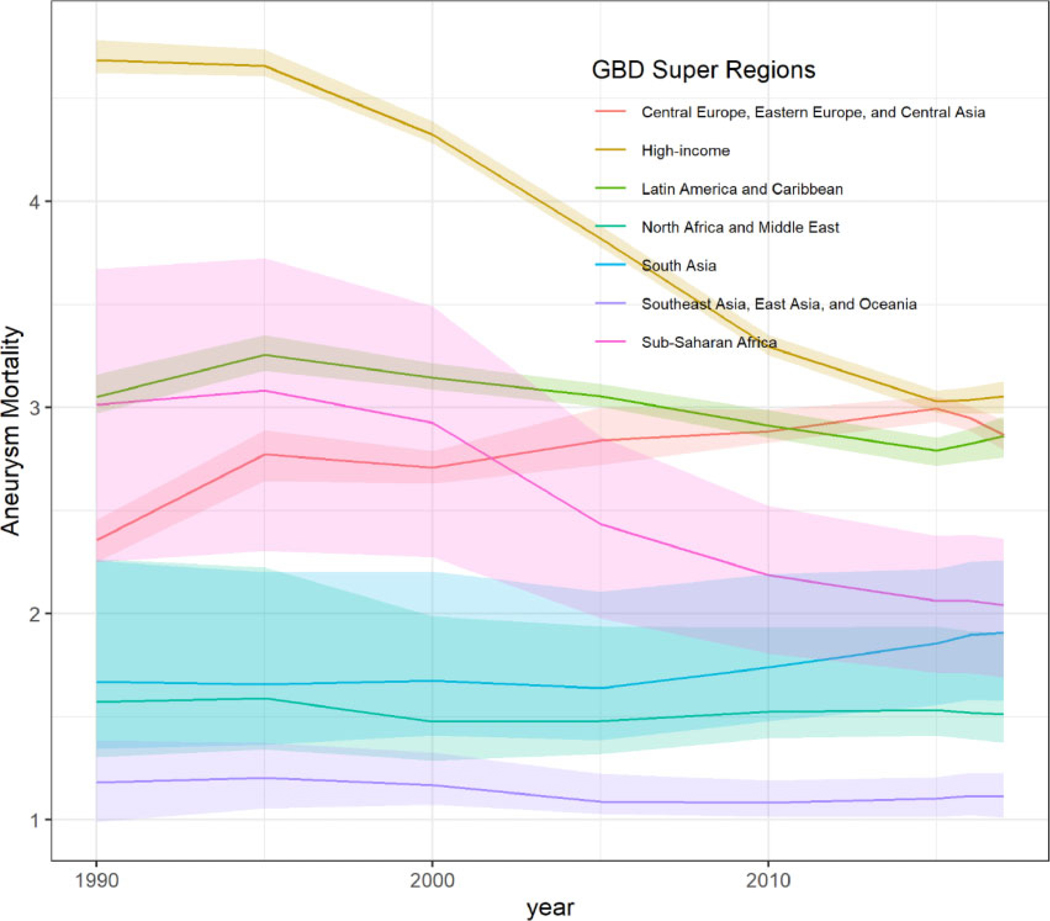
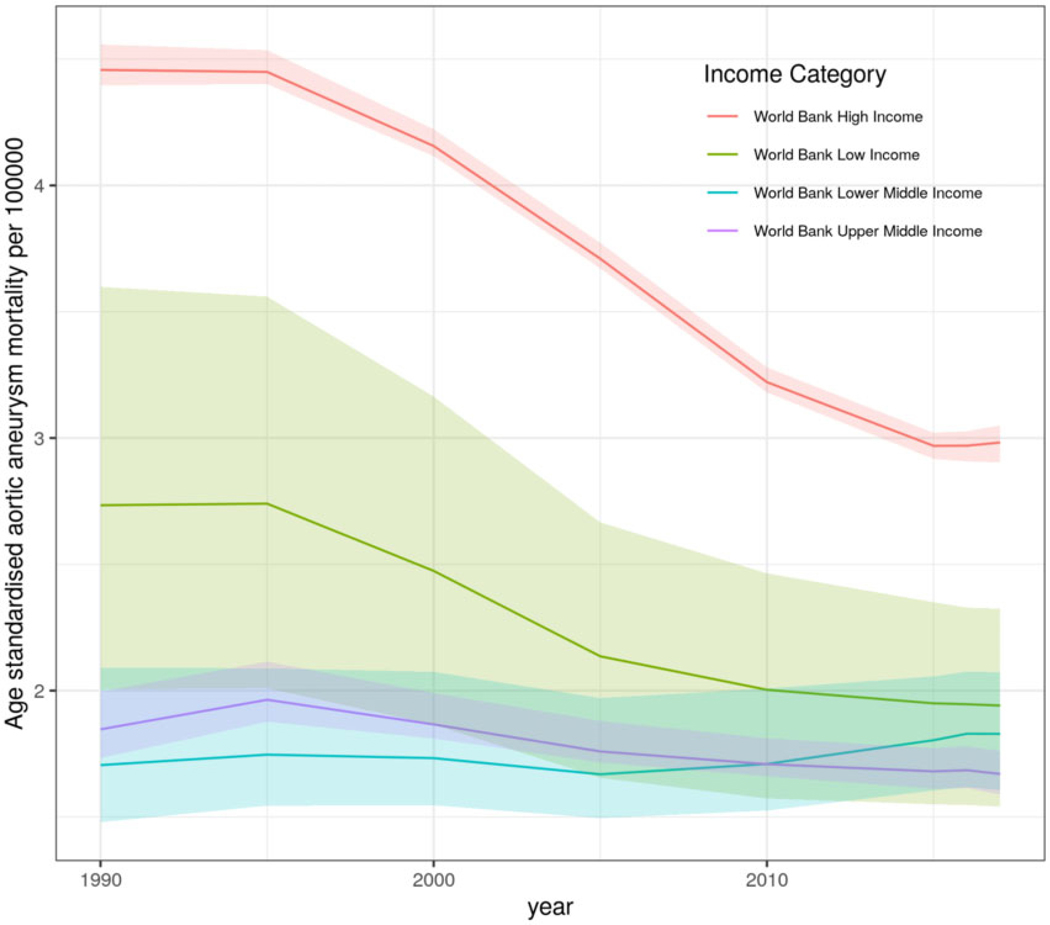
Globally, age-standardized aortic aneurysm mortality resulted in 2.88 deaths/100 000 (95% UI 2.79 to 3.03) in 1990 and 2.19 deaths/100 000 (95% UI 2.09 to 2.28) in 2017. For females, global age-standardized aortic aneurysm mortality in 1990 was 1.75 deaths/100 000 (95% UI 1.67 to 1.92), and in 2017, this was 1.41 deaths/100 000 (95% UI 1.36 to 1.52), while for males, global age-standardized aortic aneurysm mortality was 4.43 deaths/100 000 (95% UI 4.21 to 4.79) in 1990 and 3.15 deaths/100 000 (95% UI 2.97 to 3.35) in 2017 (Figure 1). Time trend pattern analysis by GBD super-regions showed that Central and Eastern Europe and Central Asia had increased overall age-standardized aortic aneurysm mortality rates between 1990 and 2017 compared to the rest of GBD super-regions. Similarly, the super-region of sub-Saharan Africa showed a plateau in aortic aneurysm mortality between 1990 and 2000 and then a decreasing trend until 2017 (Figure 2). Aortic aneurysm mortality patterns by country-income level showed that HICs were following a profound decline in age-standardized aortic aneurysm mortality rate between 1990 and 2017. However, MICs and LICs followed a slight decline in age-standardized aortic aneurysm mortality rate which plateaued after 2010. Comparative analysis showed that the age-standardized trajectory in HICs significantly differs from that in LMICs for the period 1990–2010 (P for all < 0.0007) (Figure 3).
Figure 1.
Global age-standardized aortic aneurysm mortality rates for both sexes, and by males and females between 1990 and 2017.
Figure 2.
Age-standardized aortic aneurysm mortality rates for both sexes, by GBD super-regions, between 1990 and 2017.
Figure 3.
Age-standardized aortic aneurysm mortality rates for both sexes, by country-income levels, between 1990 and 2017. HICs, high-income countries; LICs, low-income countries; MICs, middle-income countries. Comparisons between HIC vs. LICs and MICs for the period 1990–2010 were significant (P < 0.0007).
Worldwide, among the 17 leading cardiovascular causes of age-standardized YLLs rates, aortic aneurysm declined from 9th in 1990 (51.09 YLLs/100 000, 95% UI: 49.01–54.52) to 10th in 2017 (38.18 YLLs/100 000, 95% UI: 36.21–40.00). Even though aortic aneurysm was in the 10th rank, this was related with higher YLLs burden than other CVDs such as endocarditis, myocarditis, peripheral artery disease (data shown in full detail in the GBD visualization tool).
Secondary analysis
Correlation patterns between 1990 and 2017, in aortic aneurysm age-standardized mortality, SDI and HAQ index, by country-income level
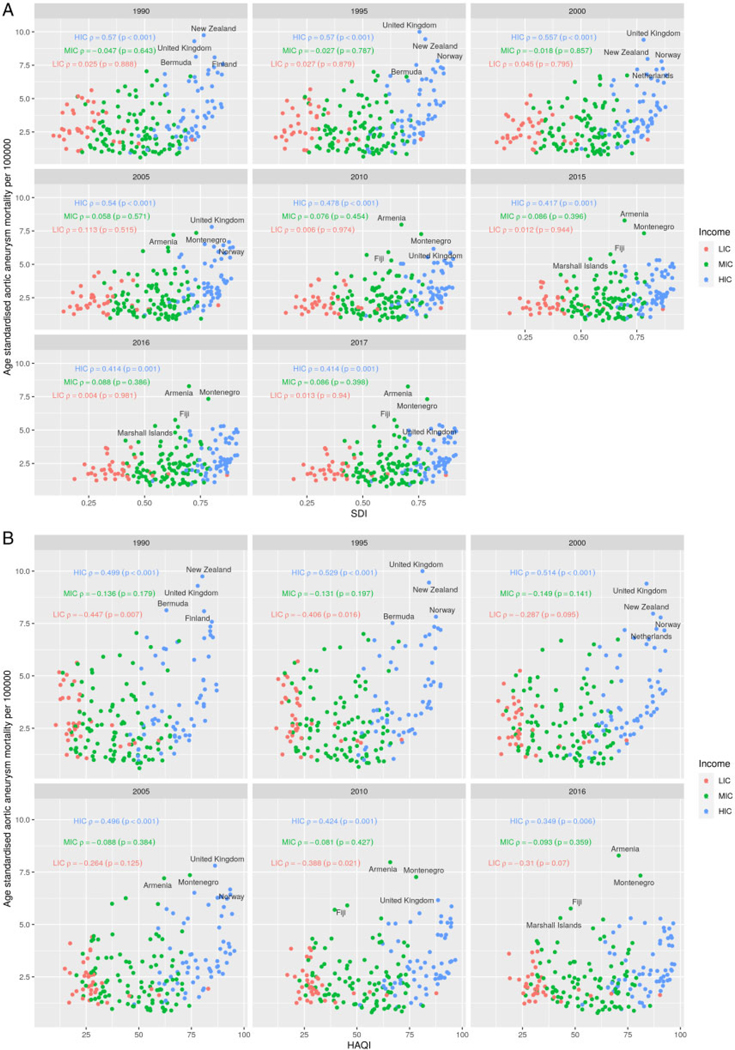
Figure 4 presents countries’ temporal correlation coefficients between age-standardized aortic aneurysm mortality rates and SDI for LICs, MICs and HICs, between 1990 and 2017. A consistent (but weak) correlation between aortic aneurysm mortality and SDI was observed (1990 Rho: 0.23, P = 0.001; 2017 rho: 0.33, P ≤ 0.001). When focusing on country-income level, a consistent and suppressed correlation trend was shown, mostly for HICs (1990 rho: 0.57, P ≤ 0.001; 2017 rho: 0.41, P = 0.001). MICs and LICs showed a correlation trend which was weak (rho< 0.3). When the univariate correlation analysis focused on the HAQ index, a consistent and suppressed positive correlation trend was shown across HICs (1990 Rho: 0.50, P < 0.001; 2016 rho: 0.35, P = 0.006), while among LICs and MICs reversed correlation trends were reported (although not consistently significant) (Figure 4A and B). Stratified analysis between age-standardized aneurysm mortality, SDI and HAQ index, by males and females, showed similar trends as the above-mentioned analysis among the different country-income levels (Supplementary material online, Figures S2 and S3).
Figure 4.
Correlation between age-standardized aortic aneurysm mortality, sociodemographic index (A) and healthcare access and quality index (B) by country’s income level. SDI: Sociodemographic index; HAQindex: Healthcare access and quality index.
Causal drivers and risk factors in relation with aortic aneurysm mortality and YLLs, by country-income level and by age-specific groups, between 1990 and 2017
In order to further explore the relation between the aforementioned drivers, other factors and aortic aneurysm mortality and YLLs rates, go beyond univariate correlation analysis, mixed-effect multilevel regression analysis was conducted, adjusting for various factors. Specifically, in Table 1, after adjusting for all confounders, age-standardised aortic aneurysm mortality and YLLs were inversely related with HAQ index (mortality, coef: −0.05, S.E: 0.01, 95% CI: −0.06, −0.04; YLLs, coef: −0.94, S.E: 0.12, 95% CI: −1.17, −0.71). The simultaneous effect of SDI and HAQ index revealed a synergistic effect with both age-standardised aortic aneurysm mortality and YLLs (mortality, coef: 0.04, S.E: 0.01, 95% CI: 0.03, 0.05; YLLs, coef: 0.72, S.E: 0.11, 95% CI: 0.50, 0.94), showing the magnitude of the effect of sociodemographic level on the aforementioned relationships. In addition, high LDL-cholesterol, smoking habits and high fasting plasma glucose were positively related with age-standardisedage-adjusted aortic aneurysm mortality and YLLs.
Table 1.
Mixed-effect multilevel regression to assess the relationship between aortic aneurysm burden (age-standardisedage-adjusted mortality and YLLs), HAQ index and SDI, for both sexes
| Fixed effects | Coef. (S.E) | 95% CI | Coef. (S.E) | 95% CI |
|---|---|---|---|---|
| Mortality | Years of life lost (YLLs) | |||
| HAQ index | −0.05 (0.01)** | −0.06, −0.04 | −0.94 (0.12)** | −1.17, −0.71 |
| SDI x HAQ index | 0.04(0.01)** | 0.03, 0.05 | 0.72(0.11)** | 0.50, 0.94 |
| High LDL-c | 0.07 (0.02)* | 0.02, 0.11 | 1.60(0.41)** | 0.80, 2.39 |
| Smoking | 0.18 (0.02)** | 0.14, 0.22 | 3.70 (0.37)** | 2.97, 4.43 |
| High FPG | 0.06 (0.03)$ | 0.001, 0.11 | 1.61 (0.53)* | 0.57, 2.65 |
HAQ index, healthcare access and quality index; High FPG, high fasting plasma glucose are expressed as age-standardised summary exposure values (0–100) summary exposure values (0–100); High LDL-c, high low-density lipoprotein-cholesterol; SDI, sociodemographic index.
P <0.05,
P <0.01,
P <0.001.
Table 2 presents the relationship between aortic aneurysm (age-adjusted mortality and YLLs: rates) and various factors, by country income-levels, applying mixed-effect multilevel regression analysis. It was shown that the inverse effect of HAQ index on age-standardised aortic aneurysm YLLs was almost double among LICs, in comparison with HICs and MICs. A similar but synergistic country-income level trend was shown for the interaction of SDI and HAQ index on aortic aneurysm age-standardized YLLs - A relation that was confirmed when SDI was inserted in the model as tertiles (data not shown in text). Risk factors were positively related with increased aortic aneurysm YLLs among HICs and MICs but no relation was observed among LICs. In addition to the above-mentioned, HAQ index was inversely related with lower aortic aneurysm age-standardized mortality rates among all country-income levels, between 1990 and 2017, while its interaction with SDI was synergistically related with aortic aneurysm mortality among high-, middle-, and low-income countries.
Table 2.
Mixed-effect multilevel regression to assess the relationship between aortic aneurysm burden (age-standardised mortality and YLLs) HAQ index and SDI, by country income-levels (low-, medium-, high-)
| Fixed effects | Coef. (S.E) | 95% CI | Coef. (S.E) | 95% CI | Coef. (S.E) | 95% CI |
|---|---|---|---|---|---|---|
| Mortality | ||||||
| HICs | MICs | LICs | ||||
| HAQ index | −0.09 (0.02)** | −0.14, −0.04 | −0.02 (0.01)** | −0.04, −0.01 | −0.10(0.01)** | −0.12, −0.08 |
| SDI × HAQ index | 0.07 (0.02)** | 0.03,0.11 | 0.03 (0.01)** | 0.01, 0.04 | 0.08 (0.01)** | 0.06, 0.11 |
| High LDL-c | 0.07 (0.04)$ | 0.001, 0.14 | −0.07 (0.03)$ | −0.14, −0.01 | 0.06 (0.06) | −0.06, 0.19 |
| Smoking | 0.28 (0.04)** | 0.19, 0.36 | 0.12 (0.02)** | 0.08, 0.17 | 0.04 (0.05) | −0.06, 0.15 |
| High FPG | 0.01 (0.08) | −0.15,0.17 | 0.10(0.03)** | 0.04, 0.15 | 0.03 (0.08) | −0.12, 0.19 |
| Years of life lost (YLLs) | ||||||
| HICs | MICs | LICs | ||||
| Fixed effects | Coef. (S.E) | 95% CI | Coef. (S.E) | 95% CI | Coef. (S.E) | 95% CI |
| HAQ index | −1.43 (0.45)** | −2.31,−0.55 | −0.56(0.16)** | −0.87, −0.26 | −2.05 (0.23)** | −2.51, −1.60 |
| SDI × HAQ index | 0.90 (0.37)$ | 0.17, 1.62 | 0.55 (0.16)** | 0.23, 0.87 | 1.52 (0.28)** | 0.98, 2.06 |
| High LDL-c | 1.78 (0.66)* | 0.49, 3.07 | −1.22 (0.64) | −2.47, 0.04 | 1.82 (1.35) | −0.83, 4.47 |
| Smoking | 5.68 (0.73)** | 4.24, 7.11 | 2.53 (0.47)** | 1.61,3.44 | 0.60 (1.14) | −1.64, 2.84 |
| High FPG | 1.09 (1.47) | −1.80, 3.97 | 1.93 (0.57)** | 0.81, 3.05 | 1.39 (1.65) | −1.84, 4.63 |
Separation by country-income level have been based on World Banking categorization. For more detail see Methods section.
HAQ index, healthcare access and quality index; HICs, high-income countries; High FPG, high fasting plasma glucose are expressed as age-standardised summary exposure values (0–100) summary exposure values (0–100); High LDL-c, high low-density lipoprotein cholesterol; LICs, low-income countries; MICs, middle-income countries; SDI, sociodemographic index.
P <0.05,
P <0.01,
P <0.001.
Stratified mixed-effect regression analysis was also applied for those within the age groups of 50–69 years and ≥70 years. In Table 3, the magnitude (inverse) effect of HAQ index on specific-age aortic aneurysm mortality and YLLs: rates was higher among older adults (70+ years old) compared to the age group of 50–69 years. Similarly, SDI × HAQ index, high LDL-cholesterol, and smoking habits showed higher coefficients in the group of older adults. High fasting plasma glucose was not related with aortic aneurysm mortality and YLLs among those aged ≥70 years.
Table 3.
Mixed-effect multilevel regression to assess the relationship between aortic aneurysm mortality and YLLs, HAQ index and SDI, by age group
| Fixed effects | Coef. (S.E) | 95% CI | Coef. (S.E) | 95% CI |
|---|---|---|---|---|
| Mortality | ||||
| Age group 50–69 years old | Age group 70+ years old | |||
| HAQ index | −0.08 (0.01)** | −0.10, −0.05 | −0.49 (0.05)** | −0.59, −0.38 |
| SDI × HAQ index | 0.04(0.01)** | 0.02, 0.06 | 0.51 (0.05)** | 0.41, 0.62 |
| High LDL-c | 0.12(0.02)** | 0.09, 0.15 | 0.20 (0.08)* | 0.05, 0.35 |
| Smoking | 0.14(0.01)** | 0.11, 0.16 | 0.69 (0.08)** | 0.53, 0.86 |
| High FPG | 0.08 (0.02)** | 0.04, 0.12 | 0.07 (0.04) | −0.01, 0.14 |
| Years of Life Lost (YLLs) | ||||
| Age group 50–69 years old | Age group 70+ years old | |||
| Fixed effects | Coef. (S.E) | 95% CI | Coef. (S.E) | 95% CI |
| HAQ index | −2.14(0.33)** | −2.79, −1.48 | −6.00 (0.76)** | −7.50, −4.51 |
| SDI x HAQ index | 1.15(0.32)** | 0.51, 1.79 | 5.70 (0.73)** | 4.26, 7.13 |
| High LDL-c | 3.03 (0.44)** | 2.17, 3.89 | 3.76 (1.01)** | 1.77, 5.75 |
| Smoking | 3.72 (0.39)** | 2.96, 4.49 | 11.11 (1.14)** | 8.88, 13.35 |
| High FPG | 2.20 (0.52)** | 1.18, 3.21 | 0.66 (0.51) | −0.35,1.66 |
HAQ index, healthcare access and quality index; High FPG, high fasting plasma glucose are expressed as age-specific summary exposure values (0–100); High LDL-c, high low-density lipoprotein cholesterol; SDI, sociodemographic index.
P <0.01,
P <0.001.
In Table 4, further stratification by country-income levels were applied to capture differences in income driven trends, between 1990 and 2017, for the 50–69 and 70+ age groups. For the population 50–69 years old, HAQ index showed similar patterns as those previously reported in Table 2, however with no significance among HICs. The case was different for the age group of older adults where a consistent relation was observed among all country-income levels. The magnitude of the effect of the HAQ index on aortic aneurysm mortality and YLLs was greatest for the population 70+ years old, across HICs and LICs.
Table 4.
Mixed-effect multilevel regression to assess the relationship between aortic aneurysm burden and HAQ index, by age groups and country-income
| Fixed effects | Coef. (S.E) | 95% CI | Coef. (S.E) | 95% CI | Coef. (S.E) | 95% CI | Coef. (S.E) | 95% CI | Coef. (S.E) | 95% CI | Coef. (S.E) | 95% CI |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mortality | ||||||||||||
| 50–69 years old | 70+ years old | |||||||||||
| HIC | MIC | LIC | HIC | MIC | LIC | |||||||
| HAQ index | −0.06 (0.05) | −0.15, 0.03 | −0.04(0.02)* | −0.07, −0.01 | −0.22 (0.02)** | −0.26, −0.17 | −0.87 (0.23)** | −1.31,−0.43 | −0.25 (0.07)** | −0.39, −0.11 | −0.80 (0.10)** | −1.01,−0.60 |
| Years of Life Lost (YLLs) | ||||||||||||
| 50–69 years old | 70+ years old | |||||||||||
| HIC | MIC | LIC | HIC | MIC | LIC | |||||||
| HAQ index | −1.94 (1.23) | −4.34,0.47 | −1.20(0.44)* | −2.06, −0.34 | −5.83 (0.62)** | −7.05, −4.61 | −11.54(3.15)** | −17.71,−5.37 | −3.15 (0.93)** | −4.98,−1.32 | −11.53 (1.48)** | −14.44,−8.62 |
HAQ index, healthcare access and quality index.
Table is mutually adjusted for the same covariates as the above-mentioned ones.
P<0.01,
P< 0.001.
Discussion
The first main result of our analysis was, in terms of global, regional, and national trends between 1990 and 2017, an increase in a gest and ardized ARC% of aortic aneurysm mortality of almost 29% of the sample. The majority of them were LICs and MICs, located in America. Second, global age-standardized mortality trajectories for the entire sample as well as for males and females declined between 1990 and 2017. Third, trend analysis by country-income levels showed that, except for HIC regions that showed a declining trajectory between 1990 and 2017, there were declining trajectories in age-standardized mortality rates among LMICs, but those stagnated after 2010. Fourth, our secondary analysis showed a consistent but suppressed correlation between age-adjusted aortic aneurysm mortality, SDI and HAQ index, among HICs, for 1990 to 2017. Fifth, our multi-level mixed modelling analysis showed that countries’ HAQ index system is the most consistent and protective driver against aortic aneurysm mortality and YLLs across all country-income levels. The magnitude of the association between HAQ index system with aortic aneurysm mortality and YLLs was particularly pronounced among LICs which was almost equal to that of HICs. Sixth, when mixed model analysis was stratified by specific age groups, the population beyond 70 s seems to acquire a survival benefit of 6 years (reflecting the decline in YLLs), if a better healthcare system is established in regional area of residence, which is doubled among LICs and HICs. Finally, smoking habits and high LDL-cholesterol were the risk factors that were significantly associated with increased aortic aneurysm mortality and YLLs among HICs and MICs but not among LICs.
Our main findings signal to diversity of areas that merit discussion. In the following paragraphs, we will address aortic aneurysms’ mortality and YLLs trends (globally, regionally, by sex, age groups, and country-income levels), their univariate and adjusted relation with social and healthcare drivers as well as with metabolic and behavioural risk factors. We address these topics due to the scarcity of information on the epidemiology of aortic aneurysm as well as on its silent and multi-factorial and multi-dynamic nature.
Our results regarding gender and global trends are in line with the literature, with males reporting higher aortic aneurysm rates than females.24 Also the applied geographical analysis showed that various countries located in central Europe, Asia, northern Africa and middle East and Latin America, had a positive age-standardized ARC% of aortic aneurysm mortality rate. This is a regional pattern of agest and ardized ARC% mortality rate which follows previous and current reported trends.15,25 Our results are in line with the Aneurysm Global Epidemiology study and others that found that abdominal aortic aneurysm mortality is declining in most developed countries, however with much regional variations.11,26 Based on our analysis, Georgia was the country with the highest positive age-standardized ARC% mortality due to aortic aneurysm in these 27 years, while Australia was the one with the highest declining ARC%. Due to limited age-specific national and global data on both abdominal and thoracic aortic aneurysm mortality, adequate comparisons among the results and estimations from previous studies were limited. Analysis of trajectories by GBD-super-regions showed a sharp declining trend in age-standardized aortic aneurysm mortality after 2000, among sub-Saharan populations. This may be attributed to the chronic disease and especially CVD screening strategies planned in early 2000s in this region.27 In addition, we found that, since 1990, age-standardized aortic aneurysm mortality trajectories continue to increase in central, eastern Europe and central Asia. This finding may be related to changes in diagnostic strategies and adaptations in registration of death certificates,28 but could also be due to specific risk factors. This, together with the fact that there is lack of aortic aneurysm diagnosis and screening, low healthcare budget allocations, and limited aortic aneurysm surgical repair options in some settings, calls for changes and enhancements in vascular health preventive strategies and aortic aneurysm treatment/management in specific regions of Europe29 and Asia.30
Within the past years, there has been increased attention within the scientific community on the complex pathophysiological and clinical mechanisms of aortic aneurysm.31 However, until today, there are limited data and research on global, regional, and temporal population-based social and healthcare drivers that could be related with aortic aneurysm mortality and disease burden. Sociodemographic drivers and quality/availability of healthcare services have been reported to be strongly related with various chronic diseases and mostly with CVD established diseases.32,33 Our analysis showed a declining trajectory of age-standardized mortality in high-income regions, but with mixed patterns among low- and middle-income regions. This could be attributed to the well-known relation between high-income regions, SDI, government health expenditure and targeted health screening and education strategies (e.g. among smokers), as has been described in the literature.34 Trends in age-standardized mortality among LMICs appeared to be stagnated after 2010. A fact that could be explained from the degree of effectiveness of cardiovascular health promotion strategies, risk factors intervention and availability of cardiovascular medicines among LMICs.35,36 To further analyse the effect of some of these drivers, SDI and HAQ index univariate spearman’s correlation analysis between SDI, HAQ index and aortic aneurysm mortality was applied. Our analysis showed that higher SDI and HAQ index levels were consistently related to suppressed trends in age-standardized aortic aneurysm mortality among high-income regions (however results were mixed and not-consistent among the rest of the regions). As has been reported recently, low income and low educational levels are related with increased risk of abdominal aortic aneurysm rupture.37 These data can benefit first the enhancement of vascular health education at the individual level and second can help regional stakeholders to better organize aortic aneurysm and vascular health preventive policies, interventions38 and healthcare system planning especially among low- and middle-income regions.
When linear multilevel regression analysis adjusted for all confounders was applied, HAQ was inversely related with age-standardized aortic aneurysm mortality and YLLs. A result that was consistent across all regions and country-income levels. It was shown that among low-income regions, higher HAQ could be related with a 2-year survival benefit from aortic aneurysm. At the same time, the interaction of SDI and HAQ index showed positive interrelation with age-adjusted aortic aneurysm mortality and YLLs, illustrating the strong role of sociodemographic determinants. To the best of our knowledge, these findings are reported for the first time on a global and by country-income level scale. It is well-known that open and endovascular surgery options are used as interventional options for aortic aneurysm with high risk of rupture.40 However, this treatment encompasses a high cost and often is applied among advanced healthcare and HICs settings.37,41 Among LICs and MICs, this could pose a major public health concern due to the low availability of health resources and the increase in ageing population in these settings.7 If HICs with advanced health care systems are failing to diagnose this salient and lethal disease, while fatality remains high, there may exist a disadvantaged situation in many LMICs.
Following literature in clinical cardiology, it is known that aortic aneurysm cases are increasing particularly for the population over 50 years old,3 however epidemiological information especially among low- and middle-income regions is scarce. For this reason, the aforementioned multilevel regression analysis was stratified for those age 50–69 years old and 70+ years in the entire sample and stratified by county-income level. The survival benefit of better HAQ (as reflected by HAQ index) was more pronounced in the group of 70+. Similar results were shown when analysis was stratified by country-income level, where a survival benefit of almost 12 YLLs could be gained among 70+ populations residing in low- and high-income regions. To this end, cardiovascular-related healthcare services could be redeveloped, having as a common goal early detection and clinical management of aortic aneurysm disease in the community.42
It is well known that mostly behavioural and metabolic risk factors are associated with aortic aneurysm diagnosis, development and rupture,11 and for this reason, our applied mixed model analysis was controlled for several of these risk factors. The results of the multilevel analysis on age- and specific age-standardized aortic aneurysm mortality and YLLs were in line with previous clinical and epidemiological studies in the field.43 Smoking and LDL-cholesterol were the strongest risk factors for aortic aneurysm burden especially for high-income regions and for the populations 70þ years old. Apart from that, in our analysis, we also observed a risk factor variation specifically in LICs, where the aforementioned factors were not significant. This difference could also be explained by the proportion of population remaining undiagnosed for aneurysm and/or lack of information on vascular disease risk factors among LICs.44 Information on cardiovascular risk factors are particularly scarce among LMICs.45 Furthermore, the effect of smoking and high LDL-cholesterol on aortic aneurysm burden reveal deficiencies in the establishment or implementation of targeted cardiovascular health policies indicating that behavioural health policies encompassing smoking cessation and nutrition education need enhancement at the community level.
All the above-mentioned indicate the necessity for early and sustainable vascular disease prevention, population cardiovascular health education, and enhancement of cardiovascular and other chronic disease screening strategies with a specific focus on MICs and LICs. The findings of our work have a triple set of policy implications. Frist, it will help LMICs to plan, develop, and implement aortic aneurysm management, screening and intervention programmes in order to address and reduce further the disease burden. Second, it will help high-income regions to enhance even more their current preventive strategies and healthcare services focusing on vascular health.
Strengths and limitations
This is the first study of its kind using the GBD 1990–2017 data analysing the mortality related to aortic aneurysm, YLLs and its risk factors, globally. All limitations of the GBD 2017 publications are also applicable to this study,16 mostly the challenges of capturing sources of uncertainty, lags in data availability, variation in coding practices and other biases, and limitations of existing analytical tools, which may not fully capture temporal trends in aortic aneurysm mortality and disease burden. For 1990–2017, available data sources for the GBD population included, among others surveys, censuses adjusted and unadjusted, and WHO vital registration systems. Additional limitations that could alter the presented findings are: (i) the difficulty to distinguish between aortic dissection (which often develop secondary aneurysms) and thoracic as well as abdominal aortic aneurysms; (ii) the sudden nature of aortic aneurysm rupture could make the cause of death to be categorized as general cardiac death. Another limitation is that the data analysis could not adjust for other factors influencing aortic aneurysm population mortality and YLLs (due to the GBD study’s population based nature), such as variance in genetic factors, adherence to specific medication (e.g. antihypertensives), changes in the treatment and different type of aneurysm surgery repair that could also alter the presented estimations.
Conclusion
Globally, a declining trend on age-standardized aortic aneurysm death rates between 1990 and 2017 was observed, with males showing higher rates of aortic aneurysm mortality than females. Age standardised aortic aneurysm mortality is declining among high-income territories, while a more trend was observed in low- and middle-income areas after 2010. HAQ index was among the main drivers of aortic aneurysm mortality and YLLs between 1990 and 2017 globally, by country-income level and by age group (50–69 and 70+ years old). Smoking and LDL-cholesterol were the risk factors which showed the most consistent effects on aortic aneurysm mortality and YLLs. These estimates provide useful insights to guide policymakers, especially in LMICs, promoting vascular health.
Supplementary Material
Acknowledgments
We would like to thank all the persons that have contributed to the synthesis of the GBD 2017 study. This research work is dedicated to the memory of Athanasios S. Tyrovolas.
Funding
GBD is supported by the Bill & Melinda Gates Foundation. S.T. was supported by the Foundation for Education and European Culture, the Miguel Servet programme (reference CP18/00006), and the Fondos Europeos de Desarrollo Regional.
Footnotes
Supplementary material
Supplementary material is available at European Journal of Preventive Cardiology online.
Ethical approval
The GBD study’s protocol has been approved by the research ethics board at the University of Washington (UW) and also complies with the Declaration of Helsinki. The GBD shall be conducted in full compliance with UW policies and procedures, as well as applicable federal, state, and local laws.
Conflict of interest: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf and declare no support from any organization for the submitted work; no financial relationships with any organizations that might have an interest in the submitted work in the previous 3 years; no other relationships or activities that could appear to have influenced the submitted work. The authors report no relationships that could be construed as a conflict of interest.
Data sharing and transparency
Data of the GBD study are publicly available at www.healthdata.org. The corresponding author (Tyrovolas Stefanos) affirm that the manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained. The GBD study is compliant with the Guidelines for Accurate and Transparent Health Estimates Reporting (GATHER).
References
- 1.Isselbacher Eric M. Thoracic and abdominal aortic aneurysms. Circulation 2005; 111:816–828. [DOI] [PubMed] [Google Scholar]
- 2.Eckstein H-H, Maegdefessel L. Linking obesity with abdominal aortic aneurysm-development. Eur Heart J 2020;41:2469–2471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.O’Gara PT. Aortic aneurysm. Circulation 2003;107:e43–e45. [DOI] [PubMed] [Google Scholar]
- 4.Heikkinen M, Salenius J-P, Auvinen O. Ruptured abdominal aortic aneurysm in a well-defined geographic area. J Vasc Surg 2002;36:291–296. [DOI] [PubMed] [Google Scholar]
- 5.GBD 2017 DALYs and HALE Collaborators. Global, regional, and national disability-adjusted life-years (DALYs) for 359 diseases and injuries and healthy life expectancy (HALE) for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018;392: 1859–1922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.GBD 2016 Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017;390:1211–1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mills A. Health care systems in low- and middle-income countries. N Engl J Med. 2014;370:552–557. [DOI] [PubMed] [Google Scholar]
- 8.Patient education: abdominal aortic aneurysm (Beyond the Basics)—UpToDate,https://www.uptodate.com/contents/abdominal-aortic-aneurysm-beyond-thebasics (21 March 2020).
- 9.Filipovic M, Goldacre MJ, Roberts SE, Yeates D, Duncan ME, Cook-Mozaffari P. Trends in mortality and hospital admission rates for abdominal aortic aneurysm in England and Wales, 1979–1999. Br J Surg 2005;92:968–975. [DOI] [PubMed] [Google Scholar]
- 10.Ashton HA, Buxton MJ, Day NE, Kim LG, Marteau TM, Scott RAP, Thompson SG, Walker NM; Multicentre Aneurysm Screening Study Group. The Multicentre Aneurysm Screening Study (MASS) into the effect of abdominal aortic aneurysm screening on mortality in men: a randomised controlled trial. Lancet 2002;360: 1531–1539. [DOI] [PubMed] [Google Scholar]
- 11.Sidloff D, Stather P, Dattani N, Bown M, Thompson J, Sayers R, Choke E. Aneurysm global epidemiology study. Circulation 2014;129:747–753. [DOI] [PubMed] [Google Scholar]
- 12.Lopez-de-Andrés A, Jiménez-Trujillo I, Jiménez-García R, Hernández-Barrera V, de Miguel-Yanes JM, Méndez-Bailón M, Perez-Farinos N, Salinero-Fort MÁ, Carrasco-Garrido P. National trends in incidence and outcomes of abdominal aortic aneurysm among elderly type 2 diabetic and non-diabetic patients in Spain (2003–2012). Cardiovasc Diabetol 2015;14:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yerly P, Madeleine G, Riesen W, Bovet P. Low prevalence of abdominal aortic aneurysm in the Seychelles population aged 50 to 65 years. Cardiovasc J Afr. 2013;24:17–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.GBD 2017 Mortality Collaborators. Global, regional, and national age-sex-specific mortality and life expectancy, 1950–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018;392:1684–1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roth GA, Johnson C, Abajobir A, Abd-Allah F, Abera SF, Abyu G, Ahmed M,Aksut B, Alam T, Alam K, Alla F, Alvis-Guzman N, Amrock S, Ansari H, Ärnlöv J, Asayesh H, Atey TM, Avila-Burgos L, Awasthi A, Banerjee A, Barac A, Bärnighausen T, Barregard L, Bedi N, Belay Ketema E, Bennett D, Berhe G, Bhutta Z, Bitew S, Carapetis J, Carrero JJ, Malta DC, Castañeda-Orjuela CA, Castillo-Rivas J, Catalá-López F, Choi J-Y, Christensen H, Cirillo M, Cooper L, Criqui M, Cundiff D, Damasceno A, Dandona L, Dandona R, Davletov K, Dharmaratne S, Dorairaj P, Dubey M, Ehrenkranz R, El Sayed Zaki M, Faraon EJA, Esteghamati A, Farid T, Farvid M, Feigin V, Ding EL, Fowkes G, Gebrehiwot T, Gillum R, Gold A, Gona P, Gupta R, Habtewold TD, Hafezi-Nejad N, Hailu T, Hailu GB, Hankey G, Hassen HY, Abate KH, Havmoeller R, Hay SI, Horino M, Hotez PJ, Jacobsen K, James S, Javanbakht M, Jeemon P, John D, Jonas J, Kalkonde Y, Karimkhani C, Kasaeian A, Khader Y, Khan A, Khang Y-H, Khera S, Khoja AT, Khubchandani J, Kim D, Kolte D, Kosen S, Krohn KJ, Kumar GA, Kwan GF, Lal DK, Larsson A, Linn S, Lopez A, Lotufo PA, El Razek HMA, Malekzadeh R, Mazidi M, Meier T, Meles KG, Mensah G, Meretoja A, Mezgebe H, Miller T, Mirrakhimov E, Mohammed S, Moran AE, Musa KI, Narula J, Neal B, Ngalesoni F, Nguyen G, Obermeyer CM, Owolabi M, Patton G, Pedro J, Qato D, Qorbani M, Rahimi K, Rai RK, Rawaf S, Ribeiro A, Safiri S, Salomon JA, Santos I, Santric Milicevic M, Sartorius B, Schutte A, Sepanlou S, Shaikh MA, Shin M-J, Shishehbor M, Shore H, Silva DAS, Sobngwi E, Stranges S, Swaminathan S, Tabarés-Seisdedos R, Tadele Atnafu N, Tesfay F, Thakur JS, Thrift A, Topor-Madry R, Truelsen T, Tyrovolas S, Ukwaja KN, Uthman O, Vasankari T, Vlassov V, Vollset SE, Wakayo T, Watkins D, Weintraub R, Werdecker A, Westerman R, Wiysonge CS, Wolfe C, Workicho A, Xu G, Yano Y, Yip P, Yonemoto N, Younis M, Yu C, Vos T, Naghavi M, Murray C. Global, regional, and national burden of cardiovascular diseases for 10 causes, 1990 to 2015. J Am Coll Cardiol 2017;70:1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.GBD 2017 Causes of Death Collaborators. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018;392:1736–1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.IHME_GBD_2017_ICD_CAUSE_MAPS_INFO_SHEET_Y2018M11D08.pdf, http://ghdx.healthdata.org/sites/default/files/record-attached-files/IHME_GBD_2017_ICD_CAUSE_MAPS_INFO_SHEET_Y2018M11D08.PDF (21 March 2020).
- 18.GBD 2017 Risk Factor Collaborators. Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018;392: 1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.New country classifications by income level: 2018–2019. World Bank Blogs, https://blogs.worldbank.org/opendata/new-country-classifications-income-level-2018-2019 (19 June 2019).
- 20.GBD 2015 Healthcare Access and Quality Collaborators. Healthcare access andquality index based on mortality from causes amenable to personal health care in 195 countries and territories, 1990–2015: a novel analysis from the Global Burden Of Disease Study 2015. Lancet 2017;390:231–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.GBD 2016. Healthcare Access and Quality Collaborators. Measuring performance on the Healthcare Access and Quality Index for 195 countries and territories and selected subnational locations: a systematic analysis from the Global Burden of Disease Study 2016.. Lancet 2018;391:2236–2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tyrovolas S, Moneta V, Giné Vázquez I, Koyanagi A, Abduljabbar AS, Haro JM.Mental disorders, musculoskeletal disorders and income-driven patterns: evidence from the Global Burden Of Disease Study 2017. J Clin Med 2020;9:2189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Caballero FF, Soulis G, Engchuan W, Sánchez-Niubó A, Arndt H, Ayuso-MateosJL, Haro JM, Chatterji S, Panagiotakos DB. Advanced analytical methodologies for measuring healthy ageing and its determinants, using factor analysis and machine learning techniques: the Athlos Project. Sci Rep 2017;7:43955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Filc D, Davidovich N, Novack L, Balicer RD. Is socioeconomic status associated with utilization of health care services in a single-payer universal health care system? Int J Equity Health 2014;13:115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Howard DP, Banerjee A, Fairhead JF, Vanda A, Silver LE, Rothwell PM; Oxford Vascular Study. Population-based study of incidence of acute abdominal aortic aneurysms with projected impact of screening strategy. J Am Heart Assoc 2015;4: e001926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roth GA, Mensah GA, Johnson CO, Addolorato G, Ammirati E, Baddour LM, Barengo NC, Beaton AZ, Benjamin EJ, Benziger CP, Bonny A, Brauer M, Brodmann M, Cahill TJ, Carapetis J, Catapano AL, Chugh SS, Cooper LT, Coresh J, Criqui M, DeCleene N, Eagle KA, Emmons-Bell S, Feigin VL, Fernández-Solà J, Fowkes G, Gakidou E, Grundy SM, He FJ, Howard G, Hu F, Inker L, Karthikeyan G, Kassebaum N, Koroshetz W, Lavie C, Lloyd-Jones D, Lu HS, Mirijello A, Temesgen AM, Mokdad A, Moran AE, Muntner P, Narula J, Neal B, Ntsekhe M, Moraes de Oliveira G, Otto C, Owolabi M, Pratt M, Rajagopalan S, Reitsma M, Ribeiro ALP, Rigotti N, Rodgers A, Sable C, Shakil S, Sliwa-Hahnle K, Stark B, Sundström J, Timpel P, Tleyjeh IM, Valgimigli M, Vos T, Whelton PK, Yacoub M, Zuhlke L, Murray C, Fuster V; GBD-NHLBI-JACC Global Burden of Cardiovascular Diseases Writing Group. Global burden of cardiovascular diseases and risk factors, 1990–2019: update from the GBD 2019 study. J Am Coll Cardiol 2020;76:2982–3021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sampson UK, Norman PE, Fowkes FG, Aboyans V, Song Y, Harrell FE Jr, Forouzanfar MH, Naghavi M, Denenberg JO, McDermott MM, Criqui MH, Mensah GA, Ezzati M, Murray C. Estimation of global and regional incidence and prevalence of abdominal aortic aneurysms 1990 to 2010. Glob Heart 2014;9: 159–170. [DOI] [PubMed] [Google Scholar]
- 28.Noncommunicable diseases: a strategy for the African Region. WHO | Regional Office for Africa. https://www.afro.who.int/publications/noncommunicable-diseases-strategy-african-region (21 March 2020).
- 29.Levi F, Lucchini F, Negri E, La Vecchia C. Trends in mortality from cardiovascularand cerebrovascular diseases in Europe and other areas of the world. Heart 2002;88:119–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reile R, Võrno T, Kals J, Ilves P, Kiivet R-A. The cost-effectiveness of abdominal aortic aneurysm screening in Estonia. Value Health Reg Issues 2020;22:1–6. [DOI] [PubMed] [Google Scholar]
- 31.Chan WK, Yong E, Hong Q, Zhang L, Lingam P, Tan GWL, Chandrasekar S, LoZJ. Systematic review and meta-analysis of the prevalence of abdominal aortic aneurysm in Asian populations. J Vasc Surg 2020;39: e83–e90. [DOI] [PubMed] [Google Scholar]
- 32.Davis FM, Daugherty A, Lu HS.Updates of recent aortic aneurysm research. Arterioscler Thromb Vasc Biol 2019;39:e83–e90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tyrovolas S, Tountas Y, Polychronopoulos E, Panagiotakos D. A parametricmodel of the role of nutritional services within the health care system, in relation to cardiovascular disease risk among older individuals. Int J Cardiol 2012;155: 110–114. [DOI] [PubMed] [Google Scholar]
- 34.Schultz WM, Kelli HM, Lisko JC, Varghese T, Shen J, Sandesara P, Quyyumi AA, Taylor HA, Gulati M, Harold JG, Mieres JH, Ferdinand KC, Mensah GA, Sperling LS. Socioeconomic status and cardiovascular outcomes. Circulation 2018;137: 2166–2178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.WHO | The determinants of health expenditure. WHO, http://www.who.int/health_financing/documents/cov-report_e_11-deter-he/en/ (21 March 2020). [Google Scholar]
- 36.Owolabi M, Miranda JJ, Yaria J, Ovbiagele B. Controlling cardiovascular diseases in low and middle income countries by placing proof in pragmatism. BMJ Global Health 2016;1:e000105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Agyemang C, van den Born B-J. Limited access to CVD medicines in low-income and middle-income countries: poverty is at the heart of the matter. Lancet Global Health 2018;6:e234–e235. [DOI] [PubMed] [Google Scholar]
- 38.Zommorodi S, Leander K, Roy J, Steuer J, Hultgren R. Understanding abdomen alaortic aneurysm epidemiology: socioeconomic position affects outcome. J Epidemiol Community Health 2018;72:904–910. [DOI] [PubMed] [Google Scholar]
- 39.Bernabe-Ortiz A, Sal Y Rosas VG, Ponce-Lucero V, Cárdenas MK, Carrillo-Larco RM, Diez-Canseco F, Pesantes MA, Sacksteder KA, Gilman RH, Miranda JJ. Effect of salt substitution on community-wide blood pressure and hypertension incidence. Nat Med 2020;26:374–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kontopodis N, Pantidis D, Dedes A, Daskalakis N, Ioannou CV.The—not so—solid 5.5 cm threshold for abdominal aortic aneurysm repair: facts, misinterpretations, and future directions. Front Surg 2016;3:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Forbes TL, DeRose G, Kribs S, Harris KA. A cost-effectiveness analysis of standard versus endovascular abdominal aortic aneurysm repair. Can J Surg 2002;45:420–424. [PMC free article] [PubMed] [Google Scholar]
- 42.Norman PE, Jamrozik K, Lawrence-Brown MM, Le MT, Spencer CA, Tuohy RJ, Parsons RW, Dickinson JA. Population based randomised controlled trial on impact of screening on mortality from abdominal aortic aneurysm. BMJ 2004;329:1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li X, Zhao G, Zhang J, Duan Z, Xin S. Prevalence and trends of the abdominal aortic aneurysms epidemic in general population—a meta-analysis. PLoS One 2013;8:e81260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bovet P, Chiolero A, Paccaud F, Banatvala N. Screening for cardiovascular disease risk and subsequent management in low and middle income countries: challenges and opportunities. Public Health Rev 2015;36:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Anand S, Bradshaw C, Prabhakaran D. Prevention and management of CVD in LMICs: why do ethnicity, culture, and context matter? BMC Med. 2020;18:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data of the GBD study are publicly available at www.healthdata.org. The corresponding author (Tyrovolas Stefanos) affirm that the manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained. The GBD study is compliant with the Guidelines for Accurate and Transparent Health Estimates Reporting (GATHER).