Abstract
Background
Inflammatory bowel disease (IBD) affects over 3 million Americans and has a relapsing and remitting course with up to 30% of patients experiencing exacerbations each year despite the availability of immune targeted therapies. An urgent need exists to develop adjunctive treatment approaches to better manage IBD symptoms and disease activity. Circadian disruption is associated with increased disease activity and may be an important modifiable treatment target for IBD. Morning light treatment, which advances and stabilizes circadian timing, may have the potential to improve IBD symptoms and disease activity, but no studies have explored these potential therapeutic benefits in IBD. Therefore, in this study, we aim to test the effectiveness of morning light treatment for patients with IBD.
Methods
We will recruit sixty-eight individuals with biopsy-proven IBD and clinical symptoms and randomize them to 4-weeks of morning light treatment or 4-weeks of treatment as usual (TAU), with equivalent study contact. Patient-reported outcomes (IBD-related quality of life, mood, sleep), clinician-rated disease severity, and a biomarker of gastrointestinal inflammation (fecal calprotectin) will be assessed before and after treatment. Our primary objective will be to test the effect of morning light treatment versus TAU on IBD-related quality of life and our secondary objectives will be to test the effects on clinician-rated disease activity, depression, and sleep quality. We will also explore the effect of morning light treatment versus TAU on a biomarker of gastrointestinal inflammation (fecal calprotectin), and the potential moderating effects of steroid use, restless leg syndrome, and biological sex.
Discussion
Morning light treatment may be an acceptable, feasible, and effective adjunctive treatment for individuals with active IBD suffering from impaired health-related quality of life.
Trial registration
The study protocol was registered on ClinicalTrials.gov as NCT06094608 on October 23, 2023, before recruitment began on February 1, 2024.
Keywords: Crohn’s disease, Ulcerative colitis, Sleep, Light treatment
Background
Inflammatory bowel disease (IBD) is a chronic immune-mediated condition of the gastrointestinal tract that includes two subtypes, ulcerative colitis (UC) and Crohn’s disease (CD). IBD affects over 3 million Americans and IBD-related healthcare use is rising with an estimated cost to the U.S. health system of over $25.4 billion annually [1, 2]. Patients with IBD suffer from relapsing and remitting flares with gastrointestinal symptoms such as diarrhea, rectal bleeding, and abdominal pain as well as extraintestinal symptoms such as sleep and mood disturbances [3]. In fact, an estimated 56% of IBD patients report poor sleep quality [4] and mood disorders such as depression affects at least 20–30% of patients with IBD [5–8].
Immune targeted medications such as biologics are effective at controlling IBD symptoms, reducing the likelihood of IBD flares, and preventing IBD-related complications such as surgery [9–11]. However, despite the availability of effective medical treatments, patients with IBD continue to suffer from high rates of suboptimal disease control and symptomatic flares, and persistent extraintestinal manifestations such as fatigue, depression, poor sleep, and reduced health-related quality of life [12–17]. Thus, novel adjunctive treatments are needed to better manage gastrointestinal and extraintestinal symptoms of IBD and improve health-related quality of life for patients with IBD.
There is evidence that circadian disruption (disruption of the “body clock”) is present in IBD and is associated with worse disease activity and symptoms. A summary of the literature examining the influence of circadian factors on IBD-related outcomes in tissue, rodent, and human models is summarized in Table 1. This body of research suggests that circadian disruption may be an important modifiable treatment target in patients with IBD. As light is the strongest environmental signal affecting circadian timing [18], morning light treatment in combination with a regularly timed sleep schedule is a key approach to reducing circadian disruption in humans. Morning light treatment can phase advance (shift earlier) circadian timing, and when administered around habitual wake time will usually phase advance circadian timing by ~ 1 h [19, 20]. In addition to reducing circadian disruption, meta-analyses have confirmed that morning light treatment improves mood (reduces nonseasonal depression) with medium effect sizes similar to those observed with pharmacological antidepressants [21, 22]. Similarly, a meta-analysis indicates that morning light treatment improves sleep with medium effect sizes [23]. Importantly, while light treatment is associated with some side effects (headache, eyestrain, nausea, agitation [24]), these often spontaneously remit [24, 25], and patients rarely discontinue treatment due to side effects [25]. Furthermore, light devices (with UV filter) are considered safe with no changes in ophthalmologic exams observed after 6 years of daily use (in fall and winter months) [26]. Thus, morning light treatment, a treatment that can reduce circadian disruption, has potential to enhance usual treatment and improve symptoms and disease activity in IBD with minimal side effects. Furthermore, light treatment devices are commercially available and light treatment can be self-administered at home, allowing easy access and dissemination once efficacy is established.
Table 1.
A summary of the literature exploring circadian factors in IBD-related models
| Model | Reference | Circadian Factor | Summary of Findings |
|---|---|---|---|
| Tissue | Palmieri et al. 2015; [60] Weintraub et al., 2020; [61] Liu et al. 2017 [62]; Mosna et al. 2021[63] | Clock gene expression in colonic mucosa, leukocytes | Altered expression in IBD and in inflamed mucosa, associated with increased endoscopic disease activity, inflammation |
| Rodent | Eum et al. 2023; [64] | Circadian disruption (constant light exposure) | Increased intestinal epithelial permeability, altered expression of tight junction proteins |
| Tran et al. 2021; [65] Voigt et al. 2014; [66] Liu et al. 2021; [67] Preuss et al. 2008 [68] | Circadian disruption (shifting light/dark cycles) | Increased intestinal epithelial permeability; altered gut microbiota; reduced resistance to colonic injury | |
| Kyoko et al. 2014; [69] Stokes et al. 2017; [70] Liu et al. 202167 | Clock gene mutation | Altered expression of tight junction proteins, altered resistance to colonic injury; altered intestinal regeneration | |
| Human | Burgess et al. 2010; [71] Conley et al. 2020 [72] | Melatonin rhythms | ~ 25–73% of IBD patients had disrupted melatonin rhythms |
| Chakradeo et al. 2018; [73] Swanson et al. 2021 [74] | Variability in sleep timing | Observed more in IBD patients vs. controls (whether inactive or active disease), associated with more severe IBD disease history, more intestinal permeability, more pro-inflammatory gut microbiota | |
| Chakradeo et al. 2018; [73] Chrobak et al. 2018 [75] | Later chronotype/more eveningness | Associated with reduced IBD-related quality of life, increased fatigue |
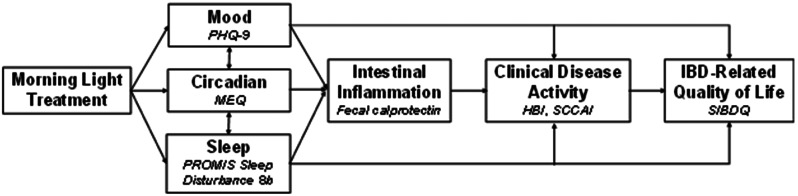
Our research group has previously tested a wearable commercially available light device (Re-timer®) to administer morning light treatment to people with chronic pain conditions and found good acceptability, feasibility, and significant improvements in mood, sleep, and pain outcomes [27, 28]. However, there are no studies testing the effects of morning light treatment on IBD disease activity, symptoms, or health-related quality of life. Given the known impact of morning light treatment on reducing circadian disruption and improving sleep and mood, we hypothesize that morning light treatment has potential to improve IBD-related quality of life directly, and potentially also clinical disease activity by reducing intestinal inflammation (see conceptual model in Fig. 1).
Fig. 1.
A conceptual model illustrating that morning light treatment, which is known to improve mood and sleep and to reduce circadian disruption, may also reduce intestinal inflammation and clinical disease activity, all with potential to ultimately improve IBD-related quality of life. The measure used to assess each domain is shown in italics: Patient Health Questionnaire (PHQ-9), Morningness-Eveningness Questionnaire (MEQ), PROMIS Sleep Disturbance 8b, Harvey Bradshaw Index (HBI), Simple Clinical Colitis Activity Index (SCCAI), and Short Inflammatory Bowel Disease Questionnaire (SIBDQ)
To test this, we will conduct a single-center randomized controlled trial for patients with IBD to test the effect of 4 weeks of morning light treatment on IBD-related quality of life (primary outcome), mood (depression), sleep, and clinical disease activity. In addition, we will explore the effect of morning light treatment on gastrointestinal inflammation and identify any possible moderating effects of sex, corticosteroid use, or restless leg syndrome (which occurs in 20–30% of IBD patients) on quality of life [29–31]. We hypothesize that participants who receive morning light treatment will report greater improvement in clinician-rated disease activity and patient reported outcomes than participants receiving treatment as usual (TAU). The study findings will provide novel preliminary data on the acceptability, feasibility, and efficacy of morning light treatment in IBD to inform a future large-scale confirmatory trial.
Methods/Design
Study design
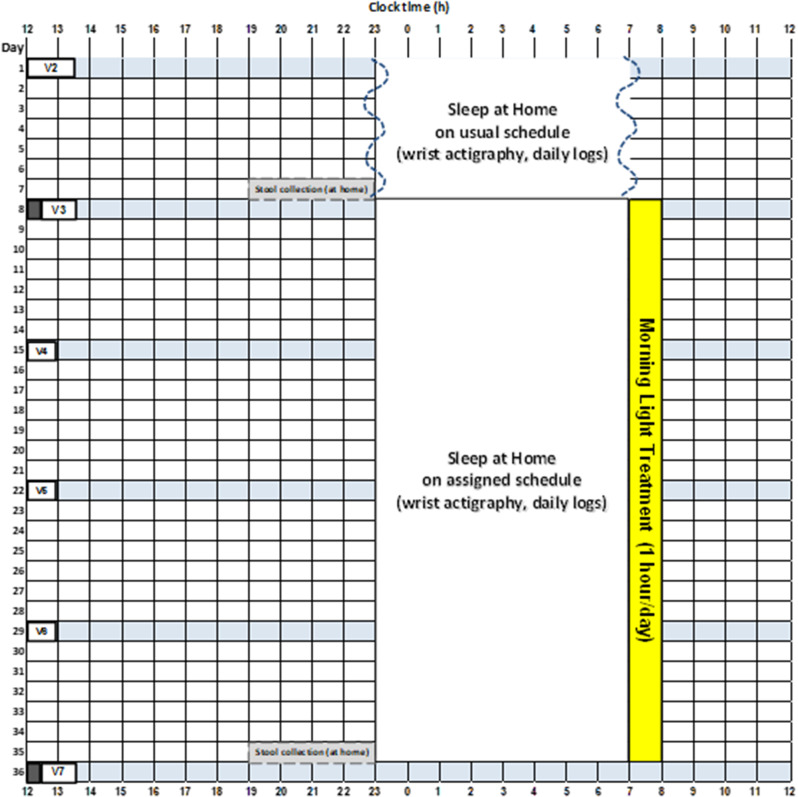
The Inflammatory Bowel Disease (IBD) Sleep Study is a prospective single-center clinical trial with a two-arm parallel groups, randomized design comparing 4 weeks of morning light treatment versus treatment as usual (TAU) in patients with IBD. The TAU group is presented to participants as a study of sleep timing to minimize group differences in treatment expectations. A sample diagram of the study protocol is shown in Fig. 2. There are weekly study visits during the 5-week protocol, with outcome variables assessed after a week of sleep monitoring at pre-treatment (visit 3) and then 4 weeks later at post-treatment (visit 7).
Fig. 2.
A diagram of the 5-week study protocol for a participant with an average sleep schedule of 11pm to 7am who is assigned to the morning light treatment. During visit 2 (V2), participants receive a wrist actigraphy monitor and are instructed on how to complete daily logs while sleeping ad lib at home. A week later at visit 3 (pre-treatment, V3) participants bring in a stool sample collected at home the day before, outcome measures are collected (including the clinical disease activity assessment represented by the dark rectangle), and participants are randomized to morning light treatment or treatment as usual (TAU). During weekly visits (V4, V5, V6) participants’ adherence to the light treatment and sleep schedule are checked (if assigned to morning light treatment) and daily logs and side effects are reviewed (both groups). A week later during visit 7 (post-treatment, V7) participants bring in a stool sample collected at home the day before, and post-treatment outcome measures are collected again. The TAU study protocol looks similar to this morning light treatment protocol except the ad lib sleep during the first week of the study continues throughout the 5 week study protocol. The anticipated duration of each study visit is shown
Setting
Study participants will be recruited from the University of Michigan Health System. The University of Michigan Health System is comprised of hospitals, health centers, and clinics owned and operated by the University of Michigan in Southeast Michigan and conducts approximately 1.6 million ambulatory care visits annually. The IBD team at the University of Michigan comprises one of the largest IBD-specialized centers in the United States and cares for over 8,000 patients with IBD each year. With IRB approval, potentially eligible participants will be identified through a review of electronic medical records. They will be emailed a study flier, with a follow up phone call a few days later. The study is also advertised on the University of Michigan Research Participant Registry (umhealthresearch.org). All study visits occur at the Sleep and Circadian Research Laboratory in the Department of Psychiatry at the University of Michigan.
Study population
We aim to enroll 68 individuals with a biopsy-proven diagnosis of IBD, aged 18 years or older, who meet criteria for impaired IBD-related quality of life and report recent abdominal pain or bowel symptoms (Table 2). Impaired IBD-related quality of life will be reflected by a Short IBD questionnaire (SIBDQ) score < 60. We have chosen inclusion/exclusion criteria to permit as many people with symptomatic IBD as possible to participate safely, while maximizing generalizability of findings (Table 2). For example, prescribed hypnotics, over-the-counter sleep aids, and antidepressants will be permitted as their use is common in IBD, but these permitted medications will need to be stable for 30 days before and during the study. Changes in medications to treat IBD during follow-up will be permitted as this is typical in clinical practice (although changes in corticosteroids will be cause for exclusion due to impact on sleep quality which could confound study results, see Table 2). Participants will be able to continue psychological therapy, physical therapy, and exercise if the treatment was started 30 days prior to enrollment and continues during the study period. Participants must be physically able to travel for the study visits and have no experience with light treatment in the past year. Participation will be scheduled ≥ 1 month from night work, travel outside the eastern time zone, other research participation, and will be scheduled during a period with minimal special events (e.g., weddings, concerts, exams).
Table 2.
Inclusion and exclusion criteria
| Inclusion criteria |
|---|
| 1. Age ≥ 18 years old |
| 2. Biopsy-proven IBD |
| 3. Impaired quality of life: Short IBD Questionnaire (SIBDQ) < 60 |
| 4. Symptoms: at least “some of the time” abdominal pain or bowel symptoms on SIBDQ |
| 5. Fluent in English |
| 6. Physically able to travel to study visits, within 1.5 h drive of study location |
| Exclusion criteria |
| Health: |
| 1. Current ileostomy, colostomy, ileoanal pouch, ileorectal anastomosis, or short bowel syndrome |
| 2. Other significant chronic physical disease (e.g., uncontrolled diabetes, advanced liver disease, cancer, kidney failure, uncontrolled cardiovascular disease, seizures, light-triggered migraines). |
| 3. Has a pacemaker or defibrillator (potential interference from Fitbit device) |
| 4. Retinal pathology, cataracts, glaucoma, colorblindness |
| 5. Lifetime history of psychotic or bipolar disorders |
| 6. Suicidality in past 6 months |
| 7. Alcohol or substance use disorder in past 3 months (cannabis use ≤ 1/week ok) |
| 8. High risk for or diagnosed with obstructive sleep apnea and/or narcolepsy |
| 9. Severe hearing problem, cognitive impairment, not fluent in English |
| 10. Pregnant, trying to get pregnant, or breastfeeding |
| 11. Has a child or pet at home that disturbs sleep often |
| 12. Pending medical leave application at work, pending legal case/litigation |
| 13. Working shiftwork that affects sleep |
| 14. Recent (< 1 month) travel outside Eastern Time Zone |
| 15. Participating in another research study |
| Medications: |
| 17. Taking photosensitizing medications to blue/green light (including methotrexate, sulfasalazine, promethazine, prochlorperazine) |
| 18. Taking melatonin |
| 19. Unstable non-IBD medication use 30 days prior to or during study |
| 20. Change of ≥ 10 mg/day of steroid medications use 30 days prior to or during study |
| Light treatment: |
| 21. Average wake time is before 5:00am |
| 22. Unable to fit 1 h of light treatment into morning routine |
| 23. Previous use of light treatment device in the past year |
Procedures and assessments
Potential participants will be pre-screened for major inclusion and exclusion criteria using a combination of an online survey and telephone interview. Biopsy-proven IBD will be verified in each participant’s medical record. If eligible, participants will then complete visit 1 (eligibility visit) which includes obtaining written consent and collection of self-report measures related to clinical history, demographics, and health information to determine further eligibility. Medications will be extracted from the medical record and reviewed with each participant for accuracy. Height and weight will also be collected along with vision tests for colorblindness and visual acuity with corrective lens in place. Lastly, participants will be breathalyzed and complete a urine drug screen (see schedule of assessments in Table 3).
Table 3.
Schedule of measure administration
| Screening | Study visits | |||||||
|---|---|---|---|---|---|---|---|---|
| Measures | Survey, phone interview | Visit 1 | Visit 2 | Visit 3 | Visit 4 | Visit 5 | Visit 6 | Visit 7 |
| Screening and baseline measures | ||||||||
| Written consent, eligibility confirmation | X | |||||||
| Demographics | X | X | ||||||
| Vision screening | X | |||||||
| Height and weight | X | |||||||
| Breathalyzer | X | X | X | X | X | X | X | |
| Urine drug test | X | |||||||
| Medical history, medications | X | X | ||||||
| Restless legs (CHQ, sIRLS) | X | |||||||
| Clinician-administered measures | ||||||||
| HBI for CD | X | X | ||||||
| SCCAI for UC | X | X | ||||||
| Self-report measures | ||||||||
| SIBDQ (primary outcome) | X | X | X | |||||
| PHQ-9 | X | X | ||||||
| PROMIS Sleep Disturbance 8b | X | X | ||||||
| Morningness-Eveningness Questionnaire | X | X | ||||||
| Daily logs (sleep & events) | X | X | X | X | X | X | ||
| Treatment Expectation | X | |||||||
| Treatment Satisfaction | X | |||||||
| Objective measures | ||||||||
| Wrist actigraphy | X | X | X | X | X | X | ||
| Fecal calprotectin | X | X | ||||||
| Safety measures | ||||||||
| BDI | X | X | X | X | X | X | ||
| C-SSRS (past 6 months) | X | |||||||
| C-SSRS (since last visit) | X | X | X | X | X | X | ||
| SAFTEE | X | X | X | X | ||||
Eligible participants will be enrolled in the study at visit 2, during which some questionnaires to characterize the sample will be completed (Table 3). Participants will be given a Fitbit Charge 5™ to wear on their nondominant wrist to track their sleep and activity throughout the study protocol, along with daily logs to report their subjective experience of sleep and alcohol, drug, and medication use. One week later, at visit 3, pre-treatment outcome assessments will be administered, and participants will be randomized to the morning light treatment or TAU group (see below for more details). Treatment expectations will also be assessed at visit 3. Morning light treatment (or TAU) will then begin the morning after visit 3. Thereafter, there will be weekly visits (visits 4, 5, 6) to review each participant’s adherence to instructions (sleep timing and use of the Re-timer® in the morning light treatment group) and to systematically assess side effects (both groups). At visit 7, post-treatment outcome assessments will be administered, and study satisfaction will be assessed. Participants will be required to abstain from alcohol use within 24 h of visits, and to ensure compliance participants will be breathalyzed at the beginning of each visit.
Randomization and blinding
Participants will be randomized at visit 3 to either 4 weeks of morning light treatment or 4 weeks of TAU in a 1:1 ratio using a minimization approach to reduce imbalances in important covariates including SIBDQ score (≤ 45 vs. > 45 to reflect moderately and severely impaired IBD–related quality of life), age 18–45 years vs. > 45 years, and sex. We will also stratify randomization by CD vs. UC and aim to enroll equal numbers of CD vs. UC and male vs. female participants. Study research assistants assessing outcomes and the clinicians assessing disease activity at study visits 3 and 7 will be blinded to treatment assignment and will be instructed not to discuss any aspect of the treatment with participants. These blinded staff will also wear badges instructing participants to not discuss the study treatment with them. Otherwise, unblinded study staff will meet with participants weekly to download data from the wrist monitor and Re-timer® device (in the light treatment group), provide feedback on adherence, and assess treatment side effects.
Intervention: morning light treatment
The morning light treatment will be self-administered at home using the commercially available wearable Re-timer® light therapy glasses which emit a green light and is designed to optimize therapeutic wavelength (~ 500 nm, 230 µW/m2, 500 lx) by being close to the peak sensitivity of the circadian photoreceptors (~ 480 nm) [32, 33]. The Re-timer® device can be worn over glasses and does not interfere with ambulation, vision, reading, or computer work. Participants are instructed to start the light treatment the morning after visit 3, immediately following their assigned wake time. Their assigned wake time was their average final wake time as determined during the baseline week, or up to one hour earlier than their average wake time, to allow time for light treatment [34]. If the participant’s assigned wake time is advanced from their average wake time, then their bedtime is also shifted earlier to avoid sleep deprivation. The earliest start time for light treatment is 6am. The Re-timer® device automatically turns off after 1 h. Participants complete the 1 h light treatment sessions for 28 consecutive days at the same time each day. Participants are also instructed not to sleep or meditate (closing eyes) within four hours after their assigned light treatment start time, to avoid creating a dark pulse that can counteract the effect of the light.
Adherence is assessed via light and actigraphy data measured by a monitor (30 s epochs, Actiwatch Spectrum Plus, Respironics, Bend, OR) attached to the Re-timer®, which is reviewed with participants at the weekly study visits by unblinded staff. Research staff will contact all participants via phone call or text 10 min after their assigned light treatment start time each morning to ensure that they have started the light treatment. Participants are also given an alarm clock set to their assigned wake time to promote adherence.
Comparator: treatment as usual
Participants assigned to TAU will be instructed at visit 3 to follow their usual ad lib sleep schedule, as they did during the baseline week. Research staff will contact these participants via phone call or text each day to encourage them to complete their daily logs. This contact, together with the weekly study visits, will ensure equivalent study contact and attention between the morning light treatment group and the TAU group.
Outcome measures
Study outcomes will be assessed in both groups at pre-treatment (visit 3) and post-treatment (visit 7). The study assessment schedule is shown in Table 3.
Self-reported outcomes
The primary outcome measure is IBD-related quality of life and this will be assessed using the Short IBD Questionnaire (SIBDQ) [35]. The SIBDQ consists of 10 items and measures the impact of IBD on social, emotional, systemic, and bowel well-being. The SIBDQ score ranges from 10 to 70 with higher scores representing better IBD-related quality of life. The SIBDQ is reliable and responsive to clinically meaningful change [36, 37]. Secondary outcome measures include the Patient Health Questionnaire-9 (PHQ-9), which is a reliable measure of the severity of depressive symptoms [38]. The PHQ-9 consists of 9 items and higher scores reflect higher levels of depressive symptoms. Sleep quality will be assessed using the PROMIS Sleep Disturbance Short-Form 8b, which is a reliable measure of sleep quality [39]. Higher scores on the PROMIS Sleep Disturbance Short-Form 8b reflect worse sleep quality.
Clinician-rated outcomes
Clinician-rated disease activity will be assessed with the Harvey Bradshaw Index (HBI) for participants with Crohn’s disease or the Simple Clinical Colitis Activity Index (SCCAI) for participants with ulcerative colitis or IBD-unclassified [40, 41]. The HBI addresses general well-being, abdominal pain, liquidity of stool, presence of an abdominal mass, and complications, and must be completed in-person to conduct an abdominal examination. The SCCAI addresses bowel frequency, defecation urgency, blood in stool, general well-being, and extracolonic disease characteristics and can be completed in-person or remotely via video communication services.
Exploratory outcome
We will assess for gastrointestinal inflammation by measuring fecal calprotectin. Fecal calprotectin is an inflammatory biomarker found in stool that is produced by neutrophils that infiltrate the colon and is more sensitive and specific for intestinal inflammation than systemic biomarkers derived from blood [42, 43]. Research participants will collect 1–5 gram stool samples at home the day before visit 3 (pre-treatment) and visit 7 (post-treatment). and deliver them to the study team during the study visits. The University of Michigan Central Labs will assay the samples using the commercially available INova QUANTA Flash Calprotectin Chemiluminescent Immunoassay. Participants will also complete Crohn’s disease and ulcerative colitis specific patient reported outcomes (CD-PRO and UC-PRO) that assess bowel symptoms, abdominal symptoms, systemic symptoms, daily life impact, emotional impact, and use of coping strategies [44, 45]. The CD-PRO and UC-PRO were developed in accordance with U.S. Food and Drug Administration Guidelines for use in clinical trials.
Additional measures
At visit 2, participants will complete the Cambridge-Hopkins questionnaire [46] to screen for restless leg syndrome (RLS) which is present in 20–30% of patients with IBD [29–31]. For those that screen positive, the severity of RLS will be determined with the validated patient-report International Restless Leg Syndrome study group severity rating scale [47]. This scale assesses symptoms over the past 7 days and the total score ranges from 0 (no symptoms) to 40 (very severe symptoms). Patients who do not screen positive will be assigned a severity score of 0. The severity score will be used as a moderator in the statistical analysis (see below).
Participants will also complete several questionnaires at pre-treatment (visit 3) and post-treatment (visit 7). The Morningness-Eveningness Questionnaire (MEQ) [48] will be used as a proxy marker of circadian timing. The MEQ score correlates well with the gold standard circadian phase marker, the dim light melatonin onset, and significantly increases towards more morningness after 13–28 days of a morning light treatment [49]. This will allow us to confirm an expected phase advance or earlier shift in circadian timing in response to morning light treatment. Fatigue will be assessed with the PROMIS Fatigue Scale, where higher scores reflect more fatigue [50, 51]. Anxiety will be assessed with the PROMIS Anxiety Scale to measure anxiety, where higher scores reflect more anxiety [52, 53]. Finally, participants will also complete a brief questionnaire about their study expectations at visit 3 after they are informed of the condition they have been randomized to, and a study satisfaction questionnaire at visit 7.
Participants will wear the Fitbit Charge 5™ on their non-dominant wrist to monitor activity and sleep. The Fitbit Charge 5™ uses accelerometry and cardiac autonomic signals to estimate sleep and has been compared to gold-standard polysomnography, demonstrating differentiation of sleep and wake states superior to US Food and Drug Administration cleared actigraphy [54]. We will export data from the Fitbit Charge 5™ in 30 s intervals using Fitabase software. To augment the sleep data collected by the Fitbit device, sleep diaries will be completed, and participants will text a research email account to identify when they are trying to fall asleep (bedtime) and at their final wake time. Bedtime and rise time data through the time stamped text messages will allow for confirmation of correct sleep onset and offset and calculation of time in bed (TIB), total sleep time (TST), wake after sleep onset (WASO), and sleep efficiency (SE). These objective sleep parameters will be primarily used to verify participant adherence to the morning light treatment but will also be examined for group differences. Participants will also track their daily use of alcohol, caffeine, nicotine, medications, exercise, psychotherapy, and food timing on a daily event log.
Safety measures
At each weekly visit after the start of treatment (visits 4–7), participants will complete a self-reported measure of physical and emotional symptoms they have experienced in the past week using the Systematic Assessment for Treatment Emergent Events (SAFTEE) as used in a previous light treatment study [55]. Unblinded research staff assess the severity of symptoms and their relevance to the study and assigned group, to ensure no significant negative effects are associated with the study participation. If severity meets a pre-determined threshold based on SAFTEE, the unblinded research staff alerts an unblinded study physician to further assess the participant’s safety and risk for an adverse event. In addition, at visits 4–7, suicidality will be assessed using the Columbia Suicide Severity Rating Scale (C-SSRS) [56], and mood worsening will examined with the Beck Depression Inventory [57].
Statistical power and analysis
We plan to enroll 68 individuals with a biopsy-proven diagnosis of IBD, aged 18 years or older, who meet criteria for impaired IBD-related quality of life and report recent abdominal pain or bowel symptoms, with the aim of a final sample of 50 study participants, assuming 25% attrition during the 5-week study period. A 9-point change in SIBDQ score is considered clinically meaningful and corresponds to a standardized effect size of 1.0 SD [35, 58, 59]. This proposed sample of 50 participants (25 participants in the morning light treatment group and 25 participants in the TAU group) provides ≥ 80% power to detect a clinically meaningful change in our primary outcome measure (SIBDQ) with a two-tailed alpha of 0.05.
We will conduct an intention-to-treat and completer analyses. We will report descriptive statistics of the data by each assessment time (pre-treatment, post-treatment) using appropriate summary statistics. We will analyze both primary and secondary outcomes using a mixed-effects longitudinal data model with participants as random intercepts to account for between participant variability. Predictors will include a post-randomization time indicator, intervention group indicator, and the interaction of time by group. We will examine the extent and pattern of key missing outcomes data and if missingness is greater than 15%, we will assess for baseline characteristics predictive of missingness post-randomization and will include those baseline characteristics as covariates in the final model. While clinician-rated disease activity will be examined as a continuous variable, we will also examine the proportion of participants who show clinical response and/or clinical remission as a binary variable using a generalized linear mixed model with logit link. Clinical response is defined as a decrease in SCCAI score ≥ 3 or a decrease in HBI score ≥ 3. Clinical remission is defined as an SCCAI score of ≤ 2 or an HBI score of < 5.40,41
We will explore steroid use, severity of RLS, and biological sex as potential moderators of any treatment effects. We will also perform subgroup analyses to explore differences in responses to morning light treatment between participants with CD versus UC/IBD-unclassified to inform future studies.
Discussion
This protocol manuscript describes a single center randomized controlled trial of morning light treatment vs. TAU in IBD. This study will provide insight into the effect of morning light treatment on IBD-related quality of life, depression, sleep quality, and clinical disease activity for patients with IBD to inform its role as an adjunctive therapy for patients with active symptoms. Despite the availability of effective IBD-targeted pharmacological therapies, many patients with IBD continue to suffer from inadequate disease control and impaired health-related quality of life. Many patients also identify preferences for non-pharmacological treatment options, but few evidence-based non-pharmacologic strategies exist for IBD. Therefore, morning light treatment has the potential to play an important role in IBD treatment if found to be efficacious. Based on published literature and our prior work in chronic pain populations, morning light treatment has the potential to improve not only mood and sleep in IBD, but also improve gastrointestinal symptoms and possibly even intestinal inflammation. Even if morning light treatment were to only improve mood and sleep in patients with IBD, this treatment would assist at least 50% of IBD patients who suffer from such symptoms [4–8].
The strength of this study includes the randomized trial design and control group, blinding of study staff directly involved in assessing study outcomes, and a well-described conceptual model of the potential mechanistic relationship between morning light treatment and study outcomes. The anticipated study limitations include exclusion of patients with an ileostomy, colostomy, ileoanal pouch, ileorectal anastomosis, or short bowel syndroe which will limit generalizability of study findings to such patients who have undergone these major surgical procedures. Further, the study period is limited to five weeks and so long-term effects of morning light treatment in IBD will not be evaluated. However, we will plan to test the long-term effects of morning light treatment in IBD in future work.
This is the first NIH-funded randomized controlled trial of morning light treatment in IBD and has the potential to improve our understanding of the impact of morning light treatment on patients with IBD and fill a gap in adjunctive non-pharmacological IBD treatment. This study will also increase our understanding of the mechanistic relationships between sleep, mood, clinical disease activity, and IBD symptoms. If found to be efficacious, morning light treatment can be further examined in a larger clinical trial with the possibility of remote study visits to increase ease of participation to patients and a longer follow up period.
Acknowledgements
We would like to thank the Michigan Institute for Clinical and Health Research at the University of Michigan, supported by NIH grant UM1TR004404.
Abbreviations
- IBD
Inflammatory bowel disease
- TAU
Treatment as usual
- CD
Crohn’s disease
- UC
Ulcerative colitis
- PHQ-9
Patient Health Questionnaire
- HBI
Harvey Bradshaw Index
- SCCAI
Simple Clinical Colitis Activity Index
- SIBDQ
Short Inflammatory Bowel Disease Questionnaire
- CD-PRO
Crohn’s disease-patient reported outcomes
- UC-PRO
Ulcerative colitis-patient reported outcomes
- RLS
Restless leg syndrome
- TIB
Time in bed
- TST
Total sleep time
- WASO
Wake after sleep onset
- SE
Sleep efficiency
- SAFTEE
Systematic Assessment for Treatment Emergent Events
Author contributions
SCM, CG, HJB, HMK contributed to the conceptualization of the study, methodology, drafting of the manuscript, and critical revisions. CG and HJB also contributed to supervision. All authors including MR, ZF, PP, JB, SB, KDC contributed to investigation, methodology, writing the original draft, and critical revisions. All authors approved the final version of the manuscript. This manuscript has not been previously published and is not under consideration in the same or substantially similar form in any other peer-reviewed media. All authors listed have contributed sufficiently to the project to be included as authors, all contributors agreed to submit this paper for publication and have reviewed this final version, and all those who are qualified to be authors are listed in the author byline.
Funding
Research reported in this publication was supported by the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health under Award Number R01 DK136520. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Kelly Cushing-Damm is supported by K08DK133640 and The University of Michigan Department of Internal Medicine. Shirley Cohen-Mekelburg is supported by K23DK136928. Jeffrey Berinstein is supported by K23DK134764. Shrinivas Bishu is supported by K08DK123403.
Data availability
No datasets were generated or analysed during the current study.
Declarations
Ethics approval and consent to participate
The study protocol follows the Standard Protocol Items: Recommendations for interventional trials (SPIRIT) 2013 and has been approved by the institutional review board (IRB) of the University of Michigan Medical School (IRB# HUM00234262). The study started recruitment in February 2024, and the study protocol was registered on ClinicalTrials.gov as NCT06094608 on October 23, 2023, before recruitment began on February 1, 2024. All human subjects will be required to sign an informed consent document prior to study participation.
Consent for publication
Not applicable.
Competing interests
HJB is a consultant for Natrol, LLC. All other authors report no financial or non-financial interests to disclose.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Dahlhamer JM, Zammitti EP, Ward BW, Wheaton AG, Croft JB. Prevalence of inflammatory bowel disease among adults aged >/=18 years - United States, 2015. MMWR Morb Mortal Wkly Rep. 2016;65(42):1166–9. doi: 10.15585/mmwr.mm6542a3. [DOI] [PubMed] [Google Scholar]
- 2.Singh S, Qian AS, Nguyen NH, et al. Trends in U.S. Health Care spending on inflammatory Bowel diseases, 1996–2016. Inflamm Bowel Dis. 2022;28(3):364–72. doi: 10.1093/ibd/izab074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chang JT. Pathophysiology of Inflammatory Bowel diseases. N Engl J Med. 2020;383(27):2652–64. doi: 10.1056/NEJMra2002697. [DOI] [PubMed] [Google Scholar]
- 4.Barnes A, Mountifield R, Baker J, et al. A systematic review and meta-analysis of the prevalence of poor sleep in inflammatory bowel disease. Sleep Advances: J Sleep Res Soc. 2022;3(1):zpac025. doi: 10.1093/sleepadvances/zpac025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.van Gennep S, Evers SW, Rietdijk ST, et al. High Disease Burden drives indirect costs in Employed Inflammatory Bowel Disease patients: the WORK-IBD Study. Inflamm Bowel Dis. 2021;27(3):352–63. doi: 10.1093/ibd/izaa082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van Gennep S, de Boer NKH, Gielen ME, et al. Impaired quality of Working Life in Inflammatory Bowel Disease patients. Dig Dis Sci. 2021;66(9):2916–24. doi: 10.1007/s10620-020-06647-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kamp KJ, Clark-Snustad K, Barahimi M, Lee S. Relationship between endoscopic and clinical disease activity with fatigue in inflammatory bowel disease. Gastroenterol Nurs. 2022;45(1):21–8. doi: 10.1097/SGA.0000000000000600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mikocka-Walus A, Knowles SR, Keefer L, Graff L. Controversies revisited: a systematic review of the Comorbidity of Depression and anxiety with inflammatory Bowel diseases. Inflamm Bowel Dis. 2016;22(3):752–62. doi: 10.1097/MIB.0000000000000620. [DOI] [PubMed] [Google Scholar]
- 9.Lichtenstein GR, Loftus EV, Isaacs KL, Regueiro MD, Gerson LB, Sands BE. ACG Clinical Guideline: management of Crohn’s disease in adults. Am J Gastroenterol. 2018;113(4):481–517. doi: 10.1038/ajg.2018.27. [DOI] [PubMed] [Google Scholar]
- 10.Feuerstein JD, Ho EY, Shmidt E, et al. AGA Clinical Practice guidelines on the Medical management of moderate to severe luminal and Perianal Fistulizing Crohn’s Disease. Gastroenterology. 2021;160(7):2496–508. doi: 10.1053/j.gastro.2021.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lamb CA, Kennedy NA, Raine T, et al. British Society of Gastroenterology consensus guidelines on the management of inflammatory bowel disease in adults. Gut. 2019;68(Suppl 3):s1–106. doi: 10.1136/gutjnl-2019-318484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hou JK, Turkeltaub JA, McCarty Iii TR, El-Serag HB. Assessment of disease specific knowledge and health-related quality of life among United States military veterans with inflammatory bowel disease. World J Gastroenterol. 2015;21(19):6001–7. doi: 10.3748/wjg.v21.i19.6001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Williet N, Sarter H, Gower-Rousseau C, et al. Patient-reported outcomes in a French Nationwide Survey of Inflammatory Bowel Disease patients. J Crohns Colitis. 2017;11(2):165–74. doi: 10.1093/ecco-jcc/jjw145. [DOI] [PubMed] [Google Scholar]
- 14.Targownik LE, Tennakoon A, Leung S, Lix LM, Singh H, Bernstein CN. Temporal trends in initiation of Therapy with Tumor necrosis factor antagonists for patients with inflammatory bowel disease: a Population-based analysis. Clin Gastroenterol Hepatol. 2017;15(7):1061–e10701061. doi: 10.1016/j.cgh.2017.01.035. [DOI] [PubMed] [Google Scholar]
- 15.Targownik LE, Bernstein CN, Benchimol EI et al. Trends in Corticosteroid Use during the era of Biologic Therapy: a Population-based analysis. Am J Gastroenterol. 2021. [DOI] [PubMed]
- 16.Verdon C, Reinglas J, Coulombe J, et al. No change in Surgical and Hospitalization trends despite higher exposure to Anti-tumor Necrosis factor in inflammatory bowel disease in the Québec Provincial Database from 1996 to 2015. Inflamm Bowel Dis. 2021;27(5):655–61. doi: 10.1093/ibd/izaa166. [DOI] [PubMed] [Google Scholar]
- 17.Agrawal M, Cohen-Mekelburg S, Kayal M, et al. Disability in inflammatory bowel disease patients is associated with race, ethnicity and socio-economic factors. Aliment Pharmacol Ther. 2019;49(5):564–71. doi: 10.1111/apt.15107. [DOI] [PubMed] [Google Scholar]
- 18.Duffy JF, Wright KP. Entrainment of the human circadian system by light. J Biol Rhythms. 2005;20(4):326–38. doi: 10.1177/0748730405277983. [DOI] [PubMed] [Google Scholar]
- 19.Burgess HJ, Fogg LF, Young MA, Eastman CI. Bright light therapy for winter depression - is phase advancing beneficial? Chronobiol Int. 2004;21:759–75. doi: 10.1081/CBI-200025979. [DOI] [PubMed] [Google Scholar]
- 20.Eastman CI, Young MA, Fogg LF, Liu L, Meaden PM. Bright light treatment of winter depression: a placebo-controlled trial. Arch Gen Psychiatry. 1998;55:883–9. doi: 10.1001/archpsyc.55.10.883. [DOI] [PubMed] [Google Scholar]
- 21.Mårtensson B, Pettersson A, Berglund L, Ekselius L. Bright white light therapy in depression: a critical review of the evidence. J Affect Disord. 2015;182:1–7. doi: 10.1016/j.jad.2015.04.013. [DOI] [PubMed] [Google Scholar]
- 22.Al-Karawi D, Jubair L. Bright light therapy for nonseasonal depression: Meta-analysis of clinical trials. J Affect Disord. 2016;198:64–71. doi: 10.1016/j.jad.2016.03.016. [DOI] [PubMed] [Google Scholar]
- 23.van Maanen A, Meijer AM, Van der Heijden KB, Oort FJ. The effects of light therapy on sleep problems: a systematic review and meta-analysis. Sleep Med Rev. 2016;29:52–62. doi: 10.1016/j.smrv.2015.08.009. [DOI] [PubMed] [Google Scholar]
- 24.Pail G, Huf W, Pjrek E, et al. Bright-light therapy in the treatment of mood disorders. Neuropsychobiology. 2011;64(3):152–62. doi: 10.1159/000328950. [DOI] [PubMed] [Google Scholar]
- 25.Terman M, Terman JS. Light therapy for seasonal and nonseasonal depression: efficacy, protocol, safety, and side effects. CNS Spectr. 2005;10:647–63. doi: 10.1017/S1092852900019611. [DOI] [PubMed] [Google Scholar]
- 26.Gallin PF, Terman M, Reme CE, Rafferty B, Terman JS, Burde RM. Ophthalmologic examination of patients with seasonal affective disorder, before and after bright light therapy. Am J Ophthalmol. 1995;119:202–10. doi: 10.1016/S0002-9394(14)73874-7. [DOI] [PubMed] [Google Scholar]
- 27.Burgess HJ, Bahl S, Wilensky K, et al. A 4-week morning light treatment with stable sleep timing for individuals with fibromyalgia: a randomized controlled trial. Pain Med. 2023;24(7):787–95. doi: 10.1093/pm/pnad007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Burgess HJ, Rizvydeen M, Kimura M, et al. An Open Trial of Morning Bright Light Treatment among US Military Veterans with Chronic Low Back Pain: a pilot study. Pain Med. 2019;20(4):770–8. doi: 10.1093/pm/pny174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Weinstock LB, Bosworth BP, Scherl EJ, et al. Crohn’s disease is associated with restless legs syndrome. Inflamm Bowel Dis. 2010;16(2):275–9. doi: 10.1002/ibd.20992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hoek PD, Smits MG, de Roos NM, Rijsman RM, Witteman BJ. Increased prevalence of restless legs syndrome in patients with Crohn’s disease. Eur J Gastroenterol Hepatol. 2015;27(8):951–5. doi: 10.1097/MEG.0000000000000386. [DOI] [PubMed] [Google Scholar]
- 31.Takahara I, Takeshima F, Ichikawa T, et al. Prevalence of restless legs syndrome in patients with inflammatory bowel disease. Dig Dis Sci. 2017;62(3):761–7. doi: 10.1007/s10620-016-4420-y. [DOI] [PubMed] [Google Scholar]
- 32.LeGates TA, Fernandez DC, Hattar S. Light as a central modulator of circadian rhythms, sleep and affect. Nat Rev Neurosci. 2014;15(7):443–54. doi: 10.1038/nrn3743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gooley JJ, Lu J, Chou TC, Scammell TE, Saper CB. Melanopsin in cells of origin of the retinohypothalamic tract. Nat Neurosci. 2001;4(12):1165. doi: 10.1038/nn768. [DOI] [PubMed] [Google Scholar]
- 34.Eastman CI, Young MA, Fogg LF, Liu L, Meaden PM. Bright light treatment of winter depression: a placebo-controlled trial. Arch Gen Psychiatry. 1998;55(10):883–9. doi: 10.1001/archpsyc.55.10.883. [DOI] [PubMed] [Google Scholar]
- 35.Irvine EJ, Zhou Q, Thompson AK. The short inflammatory bowel Disease Questionnaire: a quality of life instrument for community physicians managing inflammatory bowel disease. CCRPT investigators. Canadian Crohn’s Relapse Prevention Trial. Am J Gastroenterol. 1996;91(8):1571–8. [PubMed] [Google Scholar]
- 36.Jowett SL, Seal CJ, Barton JR, Welfare MR. The short inflammatory bowel disease questionnaire is reliable and responsive to clinically important change in ulcerative colitis. Am J Gastroenterol. 2001;96(10):2921–8. doi: 10.1111/j.1572-0241.2001.04682.x. [DOI] [PubMed] [Google Scholar]
- 37.Han SW, Gregory W, Nylander D, et al. The SIBDQ: further validation in ulcerative colitis patients. Am J Gastroenterol. 2000;95(1):145–51. doi: 10.1111/j.1572-0241.2000.01676.x. [DOI] [PubMed] [Google Scholar]
- 38.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606–13. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yu L, Buysse DJ, Germain A, et al. Development of short forms from the PROMIS™ sleep disturbance and sleep-related impairment item banks. Behav Sleep Med. 2011;10(1):6–24. doi: 10.1080/15402002.2012.636266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Walmsley RS, Ayres RC, Pounder RE, Allan RN. A simple clinical colitis activity index. Gut. 1998;43(1):29–32. doi: 10.1136/gut.43.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Harvey RF, Bradshaw JM. A simple index of Crohn’s-disease activity. Lancet. 1980;1(8167):514. doi: 10.1016/S0140-6736(80)92767-1. [DOI] [PubMed] [Google Scholar]
- 42.Rodrigues BL, Mazzaro MC, Nagasako CK, Ayrizono MLS, Fagundes JJ, Leal RF. Assessment of disease activity in inflammatory bowel diseases: non-invasive biomarkers and endoscopic scores. World J Gastrointest Endoscopy. 2020;12(12):504–20. doi: 10.4253/wjge.v12.i12.504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Alghoul Z, Yang C, Merlin D. The current status of molecular biomarkers for inflammatory bowel disease. Biomedicines 2022;10(7). [DOI] [PMC free article] [PubMed]
- 44.Higgins PDR, Harding G, Leidy NK, et al. Development and validation of the Crohn’s disease patient-reported outcomes signs and symptoms (CD-PRO/SS) diary. J Patient Rep Outcomes. 2017;2(1):24. doi: 10.1186/s41687-018-0044-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Higgins PDR, Harding G, Revicki DA, et al. Development and validation of the Ulcerative Colitis patient-reported outcomes signs and symptoms (UC-pro/SS) diary. J Patient Rep Outcomes. 2017;2(1):26. doi: 10.1186/s41687-018-0049-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Allen RP, Burchell BJ, MacDonald B, Hening WA, Earley CJ. Validation of the self-completed Cambridge-Hopkins questionnaire (CH-RLSq) for ascertainment of restless legs syndrome (RLS) in a population survey. Sleep Med. 2009;10(10):1097–100. doi: 10.1016/j.sleep.2008.10.007. [DOI] [PubMed] [Google Scholar]
- 47.Sharon D, Allen RP, Martinez-Martin P, et al. Validation of the self-administered version of the international restless legs syndrome study group severity rating scale - the sIRLS. Sleep Med. 2019;54:94–100. doi: 10.1016/j.sleep.2018.10.014. [DOI] [PubMed] [Google Scholar]
- 48.Horne JA, Ostberg O. A self-assessment questionnaire to determine morningness-eveningness in human circadian rhythms. Int J Chronobiology. 1976;4(2):97–110. [PubMed] [Google Scholar]
- 49.Burgess HJ, Kikyo F, Valdespino-Hayden Z et al. Do the morningness-eveningness questionnaire and Munich ChronoType Questionnaire Change after Morning Light Treatment? Sleep Sci Pract 2018;2. [DOI] [PMC free article] [PubMed]
- 50.National Institutes of Health. PROMIS domain framework/definitions. 2007 Retrieved from http://www.nihpromis.org/measures/domainframework.
- 51.Feagan BG, Sandborn WJ, Sands BE, et al. Qualitative and psychometric evaluation of the PROMIS®-Fatigue SF-7a scale to assess fatigue in patients with moderately to severely active inflammatory bowel disease. J Patient Rep Outcomes. 2023;7(1):115. doi: 10.1186/s41687-023-00645-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cella D, Riley W, Stone A, et al. The patient-reported outcomes Measurement Information System (PROMIS) developed and tested its first wave of adult self-reported health outcome item banks: 2005–2008. J Clin Epidemiol. 2010;63(11):1179–94. doi: 10.1016/j.jclinepi.2010.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.IsHak WW, Pan D, Steiner AJ, et al. Patient-reported outcomes of quality of Life, Functioning, and GI/Psychiatric Symptom Severity in patients with inflammatory bowel disease (IBD) Inflamm Bowel Dis. 2017;23(5):798–803. doi: 10.1097/MIB.0000000000001060. [DOI] [PubMed] [Google Scholar]
- 54.Lujan MR, Perez-Pozuelo I, Grandner MA. Past, Present, and Future of Multisensory Wearable Technology to monitor sleep and circadian rhythms. Front Digit Health. 2021;3:721919. doi: 10.3389/fdgth.2021.721919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dauphinais DR, Rosenthal JZ, Terman M, DiFebo HM, Tuggle C, Rosenthal NE. Controlled trial of safety and efficacy of bright light therapy vs. negative air ions in patients with bipolar depression. Psychiatry Res. 2012;196(1):57–61. doi: 10.1016/j.psychres.2012.01.015. [DOI] [PubMed] [Google Scholar]
- 56.Posner K, Brown GK, Stanley B, et al. The Columbia-suicide severity rating scale: initial validity and internal consistency findings from three multisite studies with adolescents and adults. Am J Psychiatry. 2011;168(12):1266–77. doi: 10.1176/appi.ajp.2011.10111704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–71. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- 58.Travis S, Feagan BG, Peyrin-Biroulet L, et al. Effect of Adalimumab on Clinical outcomes and Health-related quality of life among patients with Ulcerative Colitis in a clinical practice setting: results from InspirADA. J Crohns Colitis. 2017;11(11):1317–25. doi: 10.1093/ecco-jcc/jjx093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lichtiger S, Binion DG, Wolf DC, et al. The CHOICE trial: adalimumab demonstrates safety, fistula healing, improved quality of life and increased work productivity in patients with Crohn’s disease who failed prior infliximab therapy. Aliment Pharmacol Ther. 2010;32(10):1228–39. doi: 10.1111/j.1365-2036.2010.04466.x. [DOI] [PubMed] [Google Scholar]
- 60.Palmieri O, Mazzoccoli G, Bossa F, et al. Systematic analysis of circadian genes using genome-wide cDNA microarrays in the inflammatory bowel disease transcriptome. Chronobiol Int. 2015;32(7):903–16. doi: 10.3109/07420528.2015.1050726. [DOI] [PubMed] [Google Scholar]
- 61.Weintraub Y, Cohen S, Chapnik N, et al. Clock gene disruption is an initial manifestation of Inflammatory Bowel diseases. Clin Gastroenterol Hepatol. 2020;18(1):115–e122111. doi: 10.1016/j.cgh.2019.04.013. [DOI] [PubMed] [Google Scholar]
- 62.Liu X, Yu R, Zhu L, Hou X, Zou K. Bidirectional regulation of circadian disturbance and inflammation in inflammatory bowel disease. Inflamm Bowel Dis. 2017;23(10):1741–51. doi: 10.1097/MIB.0000000000001265. [DOI] [PubMed] [Google Scholar]
- 63.Mosna K, Janega P, Sedlak J, Babal P. Complex changes of circadian proteins expression in inflammatory bowel disease. Bratisl Lek Listy. 2021;122(4):235–41. doi: 10.4149/BLL_2021_038. [DOI] [PubMed] [Google Scholar]
- 64.Eum SY, Schurhoff N, Teglas T, Wolff G, Toborek M. Circadian disruption alters gut barrier integrity via a ß-catenin-MMP-related pathway. Mol Cell Biochem. 2023;478(3):581–95. doi: 10.1007/s11010-022-04536-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tran L, Jochum SB, Shaikh M, et al. Circadian misalignment by environmental light/dark shifting causes circadian disruption in colon. PLoS ONE. 2021;16(6):e0251604. doi: 10.1371/journal.pone.0251604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Voigt RM, Forsyth CB, Green SJ, et al. Circadian disorganization alters intestinal microbiota. PLoS ONE. 2014;9(5):e97500. doi: 10.1371/journal.pone.0097500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Liu JL, Wang CY, Cheng TY, et al. Circadian clock disruption suppresses PDL1(+) Intraepithelial B cells in Experimental Colitis and Colitis-Associated Colorectal Cancer. Cell Mol Gastroenterol Hepatol. 2021;12(1):251–76. doi: 10.1016/j.jcmgh.2021.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Preuss F, Tang Y, Laposky AD, Arble D, Keshavarzian A, Turek FW. Adverse effects of chronic circadian desynchronization in animals in a challenging environment. Am J Physiol Regul Integr Comp Physiol. 2008;295(6):R2034–2040. doi: 10.1152/ajpregu.00118.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kyoko OO, Kono H, Ishimaru K, et al. Expressions of tight junction proteins Occludin and Claudin-1 are under the circadian control in the mouse large intestine: implications in intestinal permeability and susceptibility to colitis. PLoS ONE. 2014;9(5):e98016. doi: 10.1371/journal.pone.0098016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Stokes K, Cooke A, Chang H, Weaver DR, Breault DT, Karpowicz P. The circadian clock gene BMAL1 coordinates intestinal regeneration. Cell Mol Gastroenterol Hepatol. 2017;4(1):95–114. doi: 10.1016/j.jcmgh.2017.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Burgess HJ, Swanson GR, Keshavarzian A. Endogenous melatonin profiles in asymptomatic inflammatory bowel disease. Scand J Gastroenterol. 2010;45(6):759–61. doi: 10.3109/00365521003749818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Conley S, Proctor DD, Lehner V, Jeon S, Redeker NS. The feasibility of measuring sleep and circadian characteristics in adults with inflammatory bowel disease. West J Nurs Res. 2020;43(1):53–9. doi: 10.1177/0193945920933926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chakradeo PS, Keshavarzian A, Singh S, et al. Chronotype, social jet lag, sleep debt and food timing in inflammatory bowel disease. Sleep Med. 2018;52:188–95. doi: 10.1016/j.sleep.2018.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Swanson GR, Kochman N, Amin J, et al. Disrupted Circadian Rest-Activity cycles in inflammatory bowel Disease are Associated with Aggressive Disease phenotype, subclinical inflammation, and Dysbiosis. Front Med. 2021;8:770491. doi: 10.3389/fmed.2021.770491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chrobak AA, Nowakowski J, Zwolińska-Wcisło M, et al. Associations between chronotype, sleep disturbances and seasonality with fatigue and inflammatory bowel disease symptoms. Chronobiol Int. 2018;35(8):1142–52. doi: 10.1080/07420528.2018.1463236. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No datasets were generated or analysed during the current study.