Abstract
Antibody-drug conjugates and bicycle toxin conjugates represent a tremendous advance in drug delivery technology and have shown great promise in the treatment of urothelial cancer. Previously approved systemic therapies, including chemotherapy and immunotherapy, are often impractical due to comorbidities, and outcomes for patients with advanced disease remain poor, even when receiving systemic therapy. In this setting, antibody-drug and bicycle toxin conjugates have emerged as novel treatments, dramatically altering the therapeutic landscape. These drugs harness unique designs consisting of antibody or bicycle peptide, linker, and cytotoxic payload with more targeted delivery than conventional chemotherapy, thus eliminating malignant cells while reducing systemic toxicities. Potential targets investigated in urothelial cancer include Nectin-4, TROP2, HER2, and EphA2. Initial clinical trials demonstrated efficacy in treatment of refractory advanced urothelial cancer, as well as improvement in quality of life. These initial studies led to FDA approval of two antibody-drug conjugates, enfortumab vedotin and sacituzumab govitecan. Moreover, antibody-drug and bicycle toxin conjugates are being studied in ongoing clinical trials in frontline treatment of advanced disease as well as for localized cancer. These studies highlight the potential for additional future therapies with novel targets, novel antibodies, cytotoxic and immunomodulatory payloads, and unique structural designs enhancing efficacy and safety. There is increasing evidence that combinations with other cancer therapies, especially immunotherapy, improve treatment outcomes. The combination of enfortumab vedotin and pembrolizumab was recently approved for first-line treatment of advanced urothelial carcinoma. Despite the great promise of these novel drugs, robust predictive biomarkers are needed to determine the patients who would maximally benefit. This review surveys the rationale and current state of the evidence for these new drugs and describes future directions actively being explored.
Keywords: antibody-drug conjugates, ASG-15ME, bicycle toxin conjugates, bladder cancer, disitamab vedotin, enfortumab vedotin, sacituzumab govitecan, trastuzumab deruxtecan, trastuzumab emtansine, urothelial cancer
Plain language summary
Review of recent advances in novel treatments of urothelial cancer
Two new types of drugs, called antibody-drug conjugates (ADCs) and bicycle toxin conjugates (BTCs) have shown great promise in treating urothelial cancer. Both types of drugs consist of a structure targeting a specific protein on bladder cancer cells, linked to a drug that can kill cells. This allows for effective treatment of cancer with potentially less toxicity due to the targeted nature of these treatments. We discuss the potential targets in urothelial cancer and the drugs in these classes that could treat each target. Two of these drugs, enfortumab vedotin and sacituzumab govitecan, are in clinical use for cancers that have spread, while the others are in clinical trials. Moreover, the combination of enfortumab vedotin and pembrolizumab, an immunotherapy drug, has excellent results and was recently approved for first-line treatment of urothelial cancer that has spread. Additional studies are looking into these treatments for cancers that have not spread. In the future, management of side effects, determination of which patients benefit, and overcoming when the drugs become no longer effective will be important.
Introduction
Urothelial cancer is a common malignancy in the United States and worldwide with more than 80,000 new cases diagnosed and more than 17,000 deaths yearly in the United States. Urothelial cancer can present as bladder cancer, upper tract urothelial cancer, or urethral cancer, as well as nonmuscle invasive bladder cancer (NMIBC), muscle-invasive bladder cancer (MIBC), or locally advanced/metastatic urothelial cancer (la/mUC). 1 For the more advanced stages of this disease, prognosis is poor, with 5-year survival rates of less than 5% in patients with distant metastases. 2 The management of urothelial cancer varies greatly with disease stage. Those with NMIBC are typically treated with local therapies. Patients with MIBC are optimally managed with either radical nephroureterectomy, radical cystectomy with pelvic lymph node dissection, or chemoradiation in carefully selected patients. For advanced and metastatic disease, systemic treatment is administered primarily with palliative intent. 1
Platinum-based therapies have traditionally been the first-line choice for management of la/mUC. 1 However, many patients are not eligible for cisplatin, and about half of patients are disqualified due to poor renal function and comorbidities. 3 Real-world studies show significant underutilization of first-line systemic treatment for la/mUC, disproportionately high use of carboplatin, and high attrition rate even after first-line therapy use. This is likely due to the numerous toxicities experienced with first-line therapy and the level of comorbidities and poor performance status with advanced disease. 4 Even when patients receive systemic treatment, outcomes for la/mUC remain poor, with median overall survival (OS) of less than 2 years. 5 Therefore, more efficacious treatments are needed. In the past years, several new and promising therapies have emerged, including immune checkpoint inhibitors (ICIs), FGFR inhibitors, antibody-drug conjugates (ADCs), and bicycle toxin conjugates (BTCs). 2
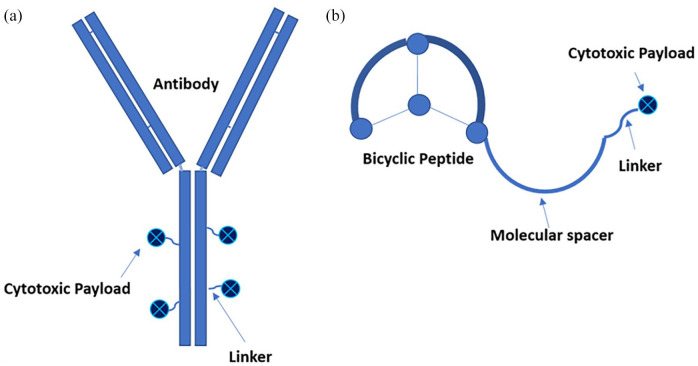
ADCs are targeted therapies composed of an antibody directed toward a specific protein on the surface of a malignant cell, which has been conjugated to a cytotoxic payload drug via a linker [Figure 1(a)]. Binding of the ADC to tumor cells results in internalization of the ADC, uptake into the lysosome, and release of the cytotoxic drug inside the cells, which induces cell killing. For some ADCs, the linker can be hydrolyzed while the antibody resides on the cell surface, thereby releasing the drug into the tumor microenvironment and killing adjacent tumor cells.6–8 Some ADCs may also additionally utilize the ‘bystander effect’, by which the cytotoxic payload diffuses through the tumor cell membrane after internalization and targets neighboring cells, which may or may not exhibit expression of the target antigen. 9 The benefits of this therapeutic strategy manifest in several ways, primarily by ensuring that the drug reaches the intended target. Additionally, the use of precise targeting limits systemic effects of the drug by allowing lower concentrations to be used and also by restricting activity principally to the tumor tissue itself. 10 The antibody component of the ADC may also play an anticancer role independent of its cytotoxic payload by disrupting the targeted antigen’s function, promoting its degradation, and activating an immune response. 8 ADCs have already established their success in treating a variety of cancers, with 11 ADCs currently FDA approved and numerous others in various stages of clinical development. 11 The diseases and mechanisms of these ADCs are summarized in Table 1.
Figure 1.
Structures of antibody-drug conjugates and bicycle toxin conjugates. (a) ADCs are composed of an antibody engineered to target an antigen preferentially expressed on tumor cells. This is connected via a cleavable or non-cleavable linker to a cytotoxic payload with antitumor effect. Additional tumor death may be instituted by a bystander effect. The typical ratio of payload to antibody 3:1 or 4:1. (b) BTCs are composed of a peptide constrained by three cysteine residues that target a tumor antigen, thereby forming a bicycle structure. This is connected to cytotoxic payload via a molecular spacer to reduce steric hindrance by the bicycle and a cleavable linker, which is cleaved extracellularly. The peptide to payload ratio is 1:1.
ADC, antibody-drug conjugate; BTC, bicycle toxin conjugate.
Table 1.
ADCs and BTCs across multiple cancers.
| ADC/BTC | Disease | Antibody/peptide | Target | Linker type | Payload | Payload mode of action | Classic bystander effect proposed |
|---|---|---|---|---|---|---|---|
| Gemtuzumab ozogamicin (Mylotarg) | Acute Myeloid Leukemia | Human IgG4 kappa antibody | CD33 | Acyl hydrazone; hydrolysable | Calicheamicin | Produces site-specific double-strand breaks | Yes 7 |
| Brentuximab vedotin (Adcetris) | Hodgkin’s Lymphoma, Anaplastic Large Cell Lymphoma | Chimeric monoclonal antibody | CD30 | Dipeptide valine-citrulline; Protease-cleavable | MMAE | Disrupts microtubule assembly | Yes12,13 |
| Trastuzumab emtansine (Kadcyla) | Breast cancer | Humanized monoclonal IgG1 antibody | HER2 | Maleimidomethyl cyclohexane-1-carboxylate linker, non-cleavable | Emtansine (DM1) | Microtubule inhibitor | No 14 |
| Inotuzumab ozogamicin (Besponsa) | B cell Acute Lymphoblastic Leukemia | Humanized monoclonal IgG4 antibody | CD22 | Acid-labile 4-acetyl butyrate linker | Calicheamicin | Produces site-specific double-strand breaks | Not discussed in literature |
| Polatuzumab vedotin (Polivy) | Diffuse Large B Cell Lymphoma | Humanized monoclonal IgG1 antibody | CD79b | Valine-citrulline peptide linker, protease-cleavable | MMAE | Disrupts microtubule assembly | Yes 15 |
| Enfortumab vedotin—(Padcev) | Urothelial cancer | Fully human IgG1-kappa antibody | Nectin-4 | Maleimidocaproyl valine-citrulline linker, protease-cleavable | MMAE | Disrupts microtubule assembly | Yes 16 |
| Trastuzumab deruxtecan (Enhertu) | Breast cancer | Humanized monoclonal IgG1 – kappa antibody | HER2 | Peptide linker, enzymatically cleavable | DXd | Inhibits topoisomerase I | Yes 17 |
| Sacituzumab govitecan (Trodelvy) | Urothelial Cancer, breast cancer, pancreatic cancer | Humanized monoclonal IgG1 kappa antibody | TROP2 | Maleimide group, hydrolysable | SN-38 | Inhibits topoisomerase I | Yes 17 |
| Loncastuximab Tesirine (Zynlonta) | Diffuse Large B Cell Lymphoma, B cell acute lymphoblastic leukemia | Humanized monoclonal IgG1 antibody | CD19 | Valine-alanine linker, cathepsin-cleavable | SG3199 | DNA crosslinking | Yes18,19 |
| Tisotumab vedotin (Tivdak) | Cervical cancer | Human monoclonal IgG1 antibody | TF | Valine-citrulline peptide linker, protease-cleavable | MMAE | Disrupts microtubule assembly | Yes20,21 |
| Mirvetuximab soravtansine (Elahere) | Ovarian cancer | Humanized monoclonal IgG1 antibody | FOLR1 | Cleavable linker | DM4 | Disrupts microtubule assembly | Yes 22 |
| BT5528 | Urothelial cancer | Bicyclic peptide | EphA2 | Valine-citrulline cleavable linker | MMAE | Disrupts microtubule assembly | Yes 23 |
| BT8009 | Urothelial cancer | Bicyclic peptide | Nectin-4 | Valine-citrulline cleavable linker | MMAE | Disrupts microtubule assembly | Yes 24 |
ADC, antibody-drug conjugate; BTC, bicycle toxin conjugate; HER2, Human epidermal growth factor receptor 2; MMAE: monomethyl auristatin E.
Another novel class of drugs with a targeting strategy and structure similar to that of the ADCs is BTCs [Figure 1(b)]. Like ADCs, these are composed of an entity with high affinity for a specific target on tumor cells that has been conjugated to a cytotoxic payload. The uniqueness of BTCs lies in their targeting, which is composed of a highly constrained, synthetic bicyclic peptide whose structure is optimized to bind with high affinity to a target protein. The bicyclic peptide is conjugated via a spacer and a cleavable linker to the payload drug, which when delivered to the target, induces tumor cell death. 25 Several advantages of BTCs over ADCs have been proposed. First, they are significantly smaller than antibody-based conjugates and, therefore, may have more rapid distribution to tissues and greater tumor penetrance. Moreover, they do not require internalization, potentially allowing for more uptake of payload by supportive adjacent stromal cells, resulting in more rapid response. 24 Additionally, the peptide formulation of these molecules results in a short duration of systemic exposure, with half-lives measured in hours as opposed to days for ADCs, and allows for renal elimination, limiting exposure to the toxic payload.24,25 Conversely, shorter half-life may require more frequent dosing. Furthermore, a bicycle-to-toxin ratio of 1:1, compared with a drug-to-antibody ratio of 3–4:1 for most ADCs, allows for BTCs to potentially prevent more delivery of the payload than necessary for eliciting cell death 24
This systematic review will survey the current state of the evidence for the use of ADCs and BTCs in the treatment of advanced and localized urothelial cancer as well as ongoing studies of not yet approved agents.
Methods
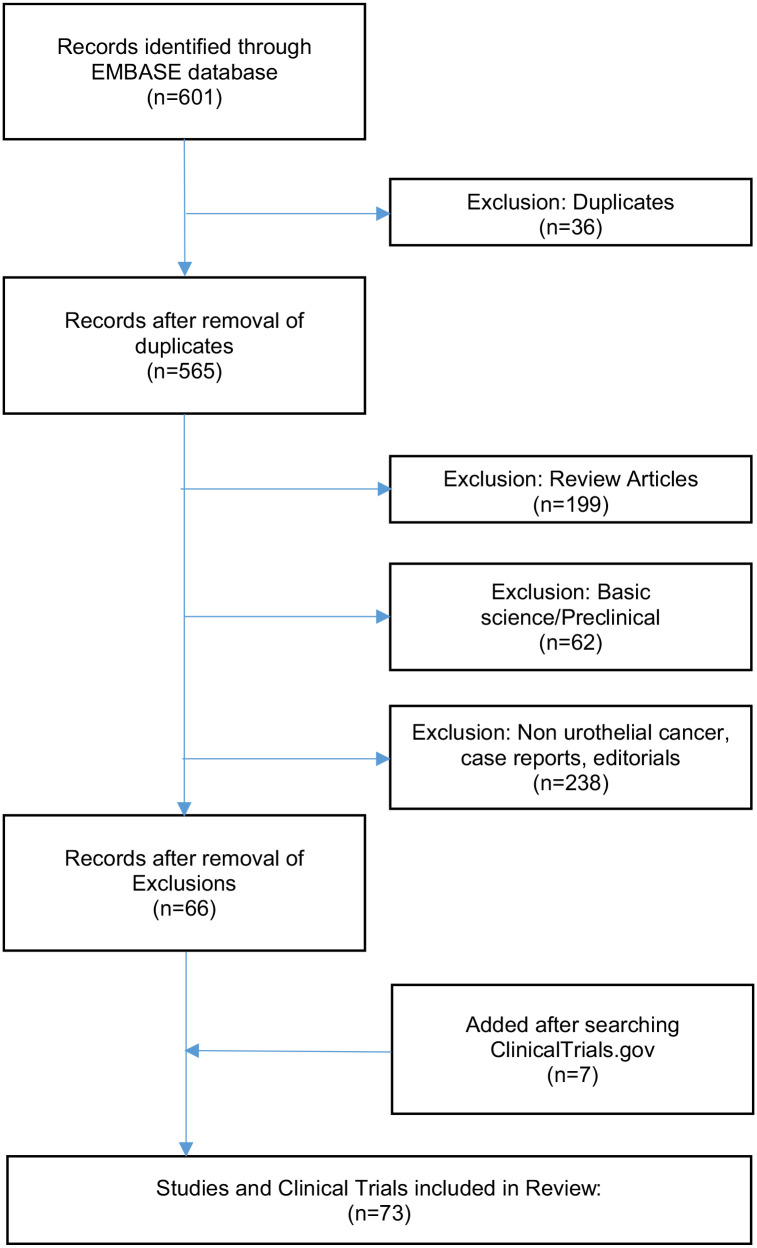
A systematic literature review was performed by two authors, CD and JRB, in accordance with PRISMA guidelines. 26 A search was conducted of the EMBASE database on 3 July 2023 using the terms ‘enfortumab vedotin’, ‘sacituzumab govitecan’, ‘disitamab vedotin’, ‘trastuzumab emtansine’, ‘trastuzumab deruxtecan’, and ‘ASG-15ME’, all in combination with the terms ‘bladder cancer’ or ‘urothelial cancer’. Review articles, case reports, editorials, and basic science papers were excluded. Clinicaltrials.gov was also searched to ensure full capture of all trials of interest, including currently enrolling studies (Figure 2). Both authors conducting the review collected all data deemed pertinent from the manuscript, working independently, and organized the data by targeted antigen. Risk of bias assessment was performed using the ROBIS tool.
Figure 2.
Consort diagram consisting of inclusion and exclusion of studies reviewed. 601 sources were identified via EMBASE that potentially discussed ADCs and BTCs in urothelial carcinoma. Exclusion criteria included preclinical studies, review articles, and case reports in order to select completed and ongoing clinical trials. A second search of ClinialTrials.gov was necessary to ensure all relevant studies were included.
ADC, antibody-drug conjugate; BTC, bicycle toxin conjugate.
Results
A total of 601 articles were initially identified. Of these, 199 were review articles, 62 were preclinical or basic science papers, 36 were duplicates, and 238 either pertained to a different type of cancer or discussed topics not relevant to the topic of present review. A total of 66 records remained for review. An additional 7 records were identified using ClinicalTrials.gov for a total of 73 records included in this analysis (Figure 2).
Nectin-4 targeting therapies
Nectin-4, also known as PVRL4 or poliovirus receptor-related protein 4, is a member of the nectin family of adhesion molecules. It has been proposed to mediate calcium independent cell-cell adhesion at adherens junctions. 27 The Nectin-4 transcript is present at low levels in various normal tissues such as skin, bladder, salivary glands, esophagus, breast, and stomach but is upregulated in several cancer tissues, with the highest levels of expression identified in bladder cancer specimens. 27 Additionally, the frequency of Nectin-4 protein expression across bladder cancer specimens is quite high, with one study reporting significant levels of Nectin-4 expression in more than 80% of specimens tested. 27 This preferential expression in neoplastic tissue makes it an ideal target for tumor-directed anti-neoplastic agents. Conversely, decreased Nectin-4 expression has been implicated in metastatic progression and resistance to Nectin-4 targeted therapies. 28
Enfortumab vedotin
Enfortumab vedotin (EV) is an antibody-drug conjugate comprising the human anti-Nectin-4 antibody, enfortumab, conjugated to a monomethyl auristatin E (MMAE) payload. 29 MMAE is a highly potent microtubule disrupting agent engineered by creating a synthetic analog of the natural antimitotic agent dolastatin 10, originally isolated from the sea hare Dolabella auricularia. 30 Delivery of MMAE by targeting Nectin-4 on tumor cells induces immunogenic cell death with release of damage-associated molecular patterns. 3 These elements are subsequently taken up by antigen-presenting cells and presented to cytotoxic T cells, which then mount an antigen-specific immune response. 3 This immune response underlies the potential facilitation of a synergistic effect with ICIs. 3
Several studies in different treatment settings have established the efficacy of EV in the treatment of locally advanced or metastatic urothelial cancer in a variety of treatment settings (Table 2). EV-201 was a phase II study of EV in patients previously treated with an anti-PD-1 ICI. Two cohorts were studied; one comprised patients with prior treatment with platinum-based chemotherapy, and one comprised platinum naïve patients. For patients in the cohort treated previously with both platinum-based chemotherapy and immunotherapy, objective response rate (ORR) was 44%, with 12% of patients achieving a complete response. Median time to response was 1.84 months. After a median follow-up of 22.3 months, median OS was 12.4 months (95% CI 9.46–15.57) 31 ; estimated median progression-free survival (PFS) was 5.8 months (95% CI 4.93–7.46). Consistent clinical activity was noted for EV across subgroups and regardless of response to prior therapy with an anti-PD(L)-1 antibody. This study led to accelerated approval by the FDA in 2019 for advanced urothelial cancer that had progressed on platinum and ICI therapy. 32 EV was also effective in patients who had not received prior platinum, with ORR 51%, complete response rate 22%, median PFS of 6.7 months (95% CI 5.0–8.3), and median OS 16.1 months (95% CI 11.3–24.1). 33
Table 2.
Anti-nectin-4 ADC completed trials.
| Name of trial | Target of the ADC/BTC | Phase of trial | Enrollment | Stage of disease | Disease setting | Intervention | ORR % | mOS | mPFS | References |
|---|---|---|---|---|---|---|---|---|---|---|
| EV-103 Cohort H | Nectin-4 | Phase I/II | 22 | MIBC | Cisplatin ineligible | Neoadjuvant EV | Not reported | Not reported | Not reported | Petrylak et al. 34 |
| EV-103 Cohort A | Nectin-4 | Phase I/II | 45 | la/mUC | Untreated; cisplatin ineligible | EV + pembro | 73.30% | 26.1 mos. | 12.3 mos. | Hoimes et al. 3 |
| EV-103 Cohort K | Nectin-4 | Phase III | 149 | la/mUC | Untreated; cisplatin ineligible | EV versus EV + pembro | EV 45.2% EV+ pembro 64.5% |
EV – 21.7 mos. (preliminary) EV + pembro – 22.3 mos. |
EV 8 mos. EV+pembro not yet reported |
O’Donnell et al. 35 |
| EV-201 Cohort 1 | Nectin-4 | Phase II | 125 | la/mUC | Post platinum + anti-PD | EV | 44% | 12.4 mos. | 5.8 mos. (estimated) | O’Donnell et al. 31 |
| EV-201 Cohort 2 | Nectin-4 | Phase II | 89 | la/mUC | Post anti-PD | EV | 51% | 16.1 mos. | 6.7 mos. | McGregor et al. 33 |
| EV-301 | Nectin-4 | Phase III | 608 | la/mUC | Post platinum + anti-PD | EV versus docetaxel or paclitaxel or vinflunine | EV – 40.6% Chemo – 17.9% |
EV – 12.8 mos. Chemo – 8.97 mos. |
EV – 5.55 mos. Chemo – 3.71 mos. |
Rosenberg et al. 36 |
| EV-301 – Japanese subgroup | Nectin-4 | Phase III | 86 | la/mUC | Post platinum + anti-PD | EV versus docetaxel or paclitaxel or vinflunine | EV – 34.4% Chemo – 21.3% |
EV – 15.18 mos. Chemo – 10.55 mos |
EV – 6.47 mos. Chemo – 5.39 mos. |
Matsubara et al. 37 |
| EV-302 | Nectin-4 | Phase III | 886 | la/mUC | Untreated | EV + pembro versus gemcitabine + cisplatin or carboplatin | EV + pembro – 67.7% Chemo – 44.4% |
EV + pembro – 31.5 mos. Chemo – 16.1 mos. |
EV + pembro – 12.5 mos. Chemo – 6.3 mos. |
Powles et al. 38 |
EV, Enfortumab vedotin; mos, months; MIBC, Muscle invasive bladder cancer.
Building upon these findings, EV-301 was a phase III multicenter study that compared EV to standard-of-care chemotherapy in patients who previously received platinum-based chemotherapy and progressed during or following treatment with an anti-PD(L)-1 antibody. A total of 608 patients were randomized 1:1 to either EV or the investigator’s choice of docetaxel, paclitaxel, or vinflunine. Here, EV significantly prolonged OS, which was the primary endpoint of this trial. OS for EV compared to chemo had a hazard ration of 0.70 (95% CI 0.56–0.89); median OS was 12.88 months for EV (95% CI 10.58–15.21) versus 8.97 months for chemo (95% CI 8.05–10.74). Median PFS with EV was 5.55 months versus 3.71 months with chemo (95% CI 0.51–0.75). Overall response rate was 40.6% with EV versus 17.9% with chemo. A total of 4.9% of patients with EV had a complete response; 2.7% of those treated with chemo had a complete response. The benefits of EV over chemo were seen across subgroups, including in those with liver metastases. Rates of treatment-related adverse events were similar between groups, with 93.9% of patients treated with EV experiencing some treatment-related adverse events (TRAEs) compared with 91.8% of those who received chemotherapy. Skin reactions and peripheral neuropathy were the most frequent TRAEs seen for EV.36,39 Following early study termination due to positive results at interim analysis, 37 EV was granted full approval by the FDA in 2021.32,39
EV-301 was a large study performed across multiple countries, and a subgroup analysis was performed on a Japanese subset of 86 participants. This cohort had a median OS of 15.18 months for the EV arm versus 10.55 months for the chemo arm [HR: 0.437 (95% CI 0.209–0.914)]. Median PFS was 6.47 months for EV versus 5.39 months for chemo [HR: 0.464 (95% CI 0.258–0.835)]. A total of 6.7% of those in the EV arm had a complete response versus zero of those receiving chemo. Although this cohort did experience about a 10% higher rate of TRAEs compared with the overall EV-301 population, tolerability was still maintained as evidenced by similar rates of treatment discontinuation, dose reduction, or interruption between this cohort and the overall study population. 37
The efficacy of EV has been established in real-world settings as well. UNITE is a multi-institutional retrospective real-world study of outcomes for patients receiving EV. A total of 304 patients from 16 institutions across the US who received EV for la/mUC were analyzed. Many of these patients would have been excluded from clinical trials due to comorbidities and performance status. Median time from diagnosis of advanced disease to EV treatment initiation was 12 months. EV was used as monotherapy for the majority of these patients. ORR for this group was 52%, with 7% having a complete response. Median PFS was 6.8 months (95% CI 5.6–7.4), and median OS was 14.4 months (95% CI 11.8–16.9). Even among patients with upper tract primary tumors, liver metastases, and multiple comorbidities, high rates of response to EV treatment were seen. Among 28 evaluable patients with FGFR3 alterations, treatment with EV resulted in an ORR of 57%. 40 In a subanalysis of the UNITE cohort, 186 patients who had most recently received an ICI were evaluated. In this subset, efficacy was greater compared with 61 patients who most recently received chemotherapy. The ICI group had an ORR of 58%, median PFS of 6.9 months, and median OS of 15.2 months versus ORR of 37% (p = 0.02), median PFS of 4.8 months (p = 0.02), and median OS of 8.8 months (p = 0.01) for the chemo group. 41
EV has also been investigated in combination with other therapeutic agents. EV-103 was a multi-cohort study designed to evaluate this. Cohorts A and K evaluated the role of EV + pembrolizumab in previously untreated advanced urothelial cancer. In phase I/II Cohort A, 45 cisplatin-ineligible patients with untreated advanced UC received EV + pembrolizumab. Overall response rate was 73.3%, with a complete response in 15.6% of patients. Median PFS was 12.3 months (95% CI 7.98–NE), and median OS was 26.1 months (95% CI 15.74–NE). Median time to response was 2.1 months, and median duration of response was 25.6 months, suggesting a rapid and durable response. The response to treatment in this study was independent of nectin-4 and PD-L1 expression level. 3
EV-103 Cohort K was a phase II trial designed to assess both EV monotherapy and combined with pembrolizumab. A total of 149 patients with previously untreated locally advanced or metastatic UC were randomized 1:1 to either EV as monotherapy or combined with pembrolizumab. In the EV + pembrolizumab arm, ORR was 64.5% (95% CI 52.7–75.1), PFS at 12 months was 55.1%. Median OS was 22.3 months. 10.5% of patients had a complete response. For the EV monotherapy arm, ORR was 45.2% (95% CI 33.5–57.3). 12 month PFS was 35.8%, 12-month OS was 70.7%. 4.1% of patients had a complete response. Notably, response rates in both arms of this study exceed expected rates for standard-of-care treatment.42,35 EV-302 further assessed combined EV and pembrolizumab compared to platinum-based regimens for first-line treatment of la/mUC. 43 Recently published data from this trial were quite encouraging, with PFS of 12.5 months for the EV + pembrolizumab arm versus 6.3 months for the chemotherapy arm (HR: 0.45, 95% CI 0.38–0.45). Median OS was 31.5 months in the EV + pembrolizumab arm versus 16.1 months in the chemotherapy arm (HR: 0.47, 95% CI 0.38–0.58). 38
EV is also actively being investigated for utility in earlier stages of bladder cancer, such as in MIBC and NMIBC, with some trials showing early promising results. EV-103 Cohort H evaluated neoadjuvant EV as monotherapy for patients with MIBC who were ineligible for neoadjuvant cisplatin-based chemotherapy. The enrolled 22 patients received three cycles of neoadjuvant EV prior to radical cystectomy. Of these patients, 36.4% had a pathological complete response, and 50% had pathologic downstaging at the time of cystectomy. These data are very promising and support further evaluation of EV in cisplatin-ineligible MIBC. 34 Several other cohorts, including cohort L with perioperative chemotherapy, are ongoing and will hopefully provide additional data useful for treatment decision making in these patients.
Toxicities of EV are typically manageable with proper recognition, interruptions, and dose modifications. Treatment-related adverse events have been reported in studies, with grade 3 or higher TRAEs reported in 51–63% of patients.37,39 The most common treatment-related adverse events reported are peripheral sensory neuropathy, fatigue, and alopecia. The most common grade 3 or higher adverse events are asymptomatic lipase elevation, fatigue, and rash. 3 Hyperglycemia is also seen in a minority of patients. 39 TRAE rates were 10% higher in the Japanese subpopulation compared with the overall population of EV-301, indicating potential ethnic differences in TRAEs. 37 Rarely, adverse events lead to death.36,39 EV carries a black box warning for severe cutaneous reactions, such as Stevens-Johnson Syndrome and Toxic Epidermal Necrolysis. However, despite the reported AEs, EV appears to be a relatively well-tolerated treatment, as evidenced by the low rates of treatment discontinuation reported. 37
Several of the EV trials evaluated health-related quality of life (QoL) and found EV to be beneficial. In EV-103 Cohort K, QoL was measured and remained stable throughout the study. Improvements were noted in emotional functioning, pain, and sleep disturbance scores. 44 Similar findings were reported in EV-201, with scores for fatigue and pain trending toward improvement for those patients with a reported overall response to treatment. For patients with bone metastases, pain scores with EV treatment were lower than baseline. 45 EV-301 reported pain as the QoL variable with the greatest difference between groups, with 51.6% in the EV group reporting pain improvement compared with 28.8% of those in the chemotherapy group. 46 These pain control improvements should be considered in the context of the higher levels of pain typically reported by patients with la/mUC in comparison to other solid tumors. 45
There are many ongoing and planned future trials evaluating EV in a variety of combinations for treatment of la/mUC (Table 3). In addition to the phase III EV-302 study, 43 EV-ECLIPSE is a phase II trial investigating combined EV plus pembrolizumab in locally advanced or node-positive UC prior to surgery. 47 Another future trial is planned to evaluate this combination in the treatment of la/mUC of variant histology. 48 Other combinations such as EV with Evorpacept (ASPEN-07), 49 Erdafitinib, 50 Cabozantinib, 51 Sacituzumab govitecan (DAD), 52 and Atezolizumab (MORPHEUS) 53 are being actively investigated as well.
Table 3.
Anti-nectin-4 ADC and BTC ongoing/future trials.
| Name of trial | Target of the ADC/BTC | Phase of trial | Stage of disease | Intervention | Primary endpoint | Planned enrollment | References |
|---|---|---|---|---|---|---|---|
| EV-104 NCT05014139 |
Nectin-4 | Phase I | NMIBC | Intravesical EV | Adverse Events; Lab Abnormalities, Dose-Limiting Toxicities | 58 | Kamat et al. 54 |
| EV-103 Cohort J NCT03288545 |
Nectin-4 | Phase I/II | MIBC | EV + Pembro | Pathologic Complete Response | 348 (EV-103 total) | Hoimes et al. 55 |
| EV-103 Cohort L NCT03288545 |
Nectin-4 | Phase I/II | MIBC | EV monotherapy | Pathologic Complete Response | 50 | Hoimes et al. 56 |
| EV-304 NCT04700124 |
Nectin-4 | Phase III | MIBC | EV + Pembro; perioperative | Event Free Survival | 784 | Hoimes et al. 57 |
| VOLGA NCT04960709 |
Nectin-4 | Phase III | MIBC | 1. Durvalumab + Tremelimumab + EV 2. Durvalumab + EV 3. Cystectomy |
Pathologic Complete Response; Event Free Survival; Adverse Events; Lab and vital Signs Abnormalities; Change in Eastern Cooperative Oncology Group Performance Status | 830 | Powles et al. 58 |
| PEVRAD NCT05879653 |
Nectin-4 | Phase II | MIBC | EV + Pembro + Radiation | Bladder Intact – Event Free Survival | 30 | Kobayashi et al. 59 |
| EV-303 NCT03924895 |
Nectin-4 | Phase III | MIBC | Pembro versus EV + Pembro versus RC + PLND alone | Event Free Survival | 857 | Necchi et al. 60 |
| DAD NCT04724018 |
Nectin-4 + TROP2 | Phase I | mUC progressed on platinum + anti-PD(L)1 | EV + SG | Maximum Tolerated Dose; Dose-limiting Toxicity | 24 | McGregor et al. 52 |
| MORPHEUS-UC NCT03869190 |
Nectin-4 | Phase I/II | la/mUC & MIBC | 1. Atezolizumab (A) versus A + EV versus A + Niraparib versus A + Magrolimab versus A + Isatuximab versus A + Linagliptin versus A + Tocilizumab 2. A + EV versus A + Linagliptin |
ORR; Pathologic Complete Response | 645 | Drakaki et al. 53 |
| NCT04878029 | Nectin-4 | Phase I/II | la/mUC | EV + Cabozantinib | Adverse Events | 32 | Bilen et al. 51 |
| NCT05756569 | Nectin-4 | Phase II | la/mUC of Variant Histology | EV + Pembro | Overall Response Rate | 25 | Nazha et al. 48 |
| EV-ECLIPSE NCT05239624 |
Nectin-4 | Phase II | la/node-positive UC | EV + Pembro prior to surgery | Pathologic Complete Response Rate | 23 | Memorial Sloan Kettering Cancer Center 47 |
| ASPEN-07 NCT05524545 |
Nectin-4 | Phase I | la/mUC | EV + Evorpacept | Dose-Limiting Toxicities; Adverse Events | 30 | ALX Oncology Inc. 61 |
| NCT04963153 | Nectin-4 | Phase I | mUC with FGFR2/3 alterations | EV + Erdafitinib | Adverse Events; Maximum Tolerated Dose | 30 | Jain et al. 50 |
| BT8009-100 NCT04561362 |
Nectin-4 | Phase I/II | la/mUC | BT8009 monotherapy and combined with Pembro | Adverse Events; Dose-Limiting Toxicities; Objective Response Rate | 329 | Baldini et al. 62 |
ADC, antibody-drug conjugate; BTC, bicycle toxin conjugate; HER2, Human epidermal growth factor receptor 2; mOS, median overall survival; mos, months; mPFS, median progression-free survival; ORR, objective response rate; SG, Sacituzumab govitecan.
EV may also alter the current treatment paradigm for localized cancers. EV-304 is a multi-institutional phase III study, enrolling approximately 780 patients who are randomized to either perioperative EV combined with pembrolizumab or standard-of-care cisplatin-based neoadjuvant chemotherapy prior to radical cystectomy. 57 EV-303 is a similar study investigating EV combined with pembrolizumab in the perioperative setting for patients ineligible for cisplatin-based chemotherapy.63,60 EV-103 cohorts J 55 and L 56 are investigating EV’s role in MIBC for cisplatin-ineligible patients, either combined with pembrolizumab or as monotherapy.
Several other novel combinations of EV are being investigated as well for the treatment of MIBC. The combination of Durvalumab, Tremelimumab, and EV is being studied in the phase III VOLGA trial for cisplatin-ineligible patients. 58 The PEVRAD trial is planned to evaluate the combination of EV with pembrolizumab followed by radiation for patients with MIBC who are deemed unfit for radical cystectomy. 59 Intravesical instillation of EV in high-risk, BCG-unresponsive NMIBC is being investigated as a potential treatment in the phase I trial EV-104. 54
BT8009
BT8009 is a drug composed of a bicyclic peptide designed for high binding affinity and selectivity for the nectin-4 protein. The peptide is conjugated with the cytotoxin monomethyl auristatin E (MMAE) via a cleavable linker with a peptide toxin ratio of 1:1. 24 It is of low molecular weight (approximately 4–4.5 kDa) and is primarily renally eliminated, with a half-life of 1–2 h. 24 This drug has demonstrated potent anticancer activity in in vivo models 25 and is being investigated as a treatment for several cancers, including urothelial carcinoma.
BT8009-100 is a phase I/II study investigating the clinical utility of the BTC, BT8009, in patients with several different solid tumors, including UC. For EV-naïve patients with la/mUC, results from the first eight treated patients reveal one complete response and three partial responses. This study also has a cohort of la/mUC patients with prior EV therapy. This trial is still ongoing, but initial results show promise for the treatment of urothelial cancer. 62
TROP2 targeting therapies
TROP2 is a cell-surface protein that functions as a transmembrane calcium sensor. It is highly expressed in multiple cancers, including urothelial carcinoma, with several studies reporting expression rates greater than 90%.64,65 High TROP2 expression levels in advanced cancers portend a poor prognosis. In UC, TROP2 expression has been reported as being higher than that of nectin-4, indicating its suitability as a target for an ADC treating bladder cancer 66 (Table 3).
Sacituzumab govitecan
Sacituzumab govitecan (SG) is composed of an antibody targeting TROP2, conjugated to a payload of SN-38. SN-38 is an active metabolite of irinotecan, a topoisomerase 1 (TOPO1) inhibitor. Delivery of SN-38 to tumor cells results in internalization of the ADC, where release of the drug inside the cells leads to cytotoxic effects. The antibody-drug linker can also be cleaved extracellularly, releasing the drug into the tumor microenvironment and killing adjacent tumor cells. 6 SG has been investigated as treatment for UC in multiple trials.
SG has proven efficacy for la/mUC. The phase I/II multicenter IMMU-132-01 study established the utility of SG for la/mUC in patients who had progressed after at least one prior standard therapeutic treatment. Patients in this trial had received a median of two prior therapy lines, including platinum-based chemotherapy and ICIs. ORR was 31%, with 2 complete responses (CR) and 12 partial responses (PR) reported. Median PFS was 7.3 months, and median OS was 18.9 months.67,68
Trophy-U-01 is a follow-up phase II trial with multiple cohorts designed to elucidate the clinical benefits of SG alone and in combination for patients with UC. Trophy-U-01 Cohort 1 demonstrated the efficacy of SG in patients with la/mUC who progressed after platinum-based chemotherapy and ICI therapy. Patients in this cohort had a median of three prior anticancer regimens. A total of 113 patients were enrolled and had an ORR of 27% (95% CI 19.5–36.6) with 5.3% having a CR; median PFS was 5.4 months (95% CI 3.5–7.2), and median OS was 10.9 months (95% CI 9.0–13.8). Efficacy was demonstrated in all subgroups, including in those with liver metastases. 6 Cohort 2 evaluated SG in platinum-ineligible patients who had progressed after prior ICI therapy. The first 38 patients treated demonstrated on ORR of 32% (95% CI 17.5–48.7), median PFS 5.6 months (95% CI 4.1–8.3), and median OS of 13.5 months (95% CI 7.6–15.6). 69
SG combinations have been investigated as well. SG combined with pembrolizumab was evaluated in Trophy-U-01 Cohort 3 for patients who had progressive or recurrent disease after platinum-based chemotherapy. Primary analysis of this cohort included 41 patients enrolled. ORR was 41% (95% CI 26.3–57.9), median PFS was 5.3 months (95% CI 3.4–10.2), and median OS was 12.7 months (95% CI 10.7-NE). 70
Treatment-related toxicities have been reported with SG, with rates of grade 3 or greater TRAEs reported as 61–65% in cohorts of Trophy-U-01.70,71 The most commonly noted TRAEs were neutropenia, leukopenia, anemia, and diarrhea. 71 There was one treatment-related death in Cohort 1 due to sepsis resulting from febrile neutropenia. Reported rates of treatment-related rash, peripheral neuropathy, and hyperglycemia, which are common toxicities of EV, are low. 6 Despite these rates of adverse events, most patients tolerate SG well, with reported treatment discontinuation in 18% of patients in one cohort. 69
Numerous ongoing and future studies continue to investigate the benefit of treatment with SG. The multicenter randomized phase III Tropics-04 trial will randomize patients who have progressed on prior platinum-based chemo and ICI therapy to either SG or physician’s choice of paclitaxel, docetaxel, or vinflunine. 72 The Trophy-U-01 trial has several cohorts studying SG combinations, including with cisplatin and either a PD-L1 inhibitor Avelumab or a PD-1 inhibitor Zimberelimab for treatment naïve la/mUC in cohort 4. 73 Cohorts 5 and 6 will compare SG and Zimberelimab combination to either single-agent immunotherapy or single-agent SG in cisplatin-ineligible treatment naïve la/mUC patients.74,75 The JAVELIN BLADDER MEDLEY trial is investigating the addition of SG to maintenance Avelumab for patients treated with first-line platinum-based chemo. 76 The combination of Ipilimumab and Nivolumab with SG is also being investigated for cisplatin-ineligible patients with la/mUC. Initial results from this trial demonstrated an ORR of 66.6%, with one CR and three PRs. The phase II trial is ongoing.77,78
SG is also being investigated for the treatment of MIBC. The SURE-02 trial is assessing SG in patients with MIBC who are ineligible for cisplatin-based chemotherapy. In this trial, one cohort will receive neoadjuvant SG as monotherapy, and the other cohort will receive SG in combination with pembrolizumab. 79 RAD-SG is another MIBC trial planned to investigate the role of SG and radiation for bladder preservation in patients with MIBC who are either ineligible or unwilling to undergo radical cystectomy. 80 SG is also being evaluated for a neoadjuvant role for variant histology MIBC in another trial. 81
Datopotamab deruxtecan
Datopotamab deruxtecan (DS1062a) is another ADC in development targeting TROP2. Datopotamab is a humanized anti-TROP2 immunoglobulin G1 monoclonal antibody. It is linked to deruxtecan (DXd), a highly potent TOPO1 inhibitor, via a tetrapeptide cleavable linker. 82 This drug is actively being investigated for use in a variety of cancers with the TROPION-PanTumor01 study, with recently reported data demonstrating benefit in the treatment of advanced or metastatic non-small cell lung cancer. 82 Part 2 of this study will include unresectable la/mUC that has been treated with at least one prior line of therapy, including an ICI. This study is currently recruiting. 82
HER2 targeting therapies
Another potential therapeutic target for ADCs in a variety of cancers, including urothelial cancer, is the Human epidermal growth factor receptor 2 (HER2) receptor. HER2 is a member of the epidermal growth factor receptor family. These are transmembrane receptor tyrosine kinases that are involved in cell proliferation and survival via activation of several intracellular signaling cascades, including the MAPK and PI3K/Akt pathways. HER2 levels are well established to have prognostic value in breast and some gastrointestinal cancers. 83 In bladder cancer, HER2 overexpression strongly correlates with tumor progression and a poor prognosis. 84 The success achieved with HER2-targeted ADCs in other cancers 85 has generated interest to study its potential efficacy in UC, with multiple completed and ongoing trials (Tables 4 and 5).
Table 4.
Anti-TROP2 ADC completed trials.
| Name of trial | Target of the ADC/BTC | Phase of trial | Enrollment | Stage of disease | Disease setting | Intervention | ORR % | mOS | mPFS | References |
|---|---|---|---|---|---|---|---|---|---|---|
| IMMU-132-01 | TROP2 | Phase I/II | 45 | la/mUC | Previously treated | SG | 31% | 18.9 months | 7.3 months | Tagawa et al. 68 |
| TROPHY-U-01 Cohort 1 | TROP2 | Phase II | 113 | la/mUC | Previous platinum and ICI | SG | 27% | 10.9 months | 5.4 months | Tagawa et al. 6 |
| TROPHY-U-01 Cohort 2 | TROP2 | Phase II | 38 | mUC | Cisplatin ineligible, prior ICI | SG | 32% | 13.5 months | 5.6 months | Petrylak et al. 69 |
| TROPHY-U-01 Cohort 3 | TROP2 | Phase II | 41 | mUC | Previous platinum | SG + Pembrolizumab | 41% | 12.7 months | 5.3 months | Grivas et al. 70 |
| NCT04863885 | TROP2 | Phase I | 9 | mUC | Cisplatin ineligible | SG + Ipilimumab + Nivolumab | 66.60% | not reported | 8.8 months | Jain et al. 77 |
ADC, antibody-drug conjugate; BTC, bicycle toxin conjugate; ICI, Immune checkpoint inhibitor; SG, Sacituzumab govitecan.
Table 5.
Anti-TROP2 and Anti-HER2 ongoing/future trials.
| Name of trial | Target of the ADC/BTC | Phase of trial | Stage of disease | Intervention | Primary endpoint | Planned enrollment | References |
|---|---|---|---|---|---|---|---|
| SURE-02 NCT05535218 |
TROP2 | Phase II | MIBC in patients ineligible for cisplatin | SG monotherapy and SG + Pembro | Complete Pathological Response | 48 | Necchi et al. 79 |
| RAD-SG NCT05833867 |
TROP2 | Phase I | MIBC ineligible for RC | SG + radiation | Dose-Limiting Toxicity | 20 | Mian et al. 80 |
| TROPHY-U-01 Cohort 4 NCT03547973 |
TROP2 | Phase II | mUC; platinum naïve | SG + Cisplatin | Overall Response Rate | 643 (All Cohorts) | Tagawa et al. 73 |
| TROPHY-U-01 Cohort 5 NCT03547973 |
TROP2 | Phase II | la/mUC treated with gemcitabine + cisplatin | 1. Avelumab monotherapy 2. SG + Zimberelimab 3. Zimberelimab monotherapy |
Progression-Free Survival | 643 (All Cohorts) | Powles et al. 74 |
| TROPHY-U-01 Cohort 6 NCT03547973 |
TROP2 | Phase II | mUC; cisplatin ineligible, treatment naïve | 1. SG monotherapy 2. SG + Zimberelimab 3. Zimberelimab + Domvanalimab 4. Carboplatin + Gemcitabine + Avelumab maintenance |
Overall Response Rate | 643 (All Cohorts) | Duran et al. 75 |
| JAVELIN BLADDER MEDLEY NCT05327530 |
TROP2 | Phase II | la/mUC | 1. Avelumab monotherapy 2. SG + Avelumab 3. Avelumab + M6223 4. Avelumab + NKTR-255 |
Progression-Free Survival; Adverse Events | 252 | Hoffman-Censits et al. 76 |
| Tropics-04 NCT04527991 |
TROP2 | Phase III | la/mUC | SG versus physician’s choice of paclitaxel, docetaxel, or vinflunine | Overall Survival | 696 | Vulsteke et al. 72 |
| NCT05581589 | TROP2 | Phase II | MIBC, non-urothelial | SG prior to RC | Pathologic Complete Response | 18 | University of Washington 81 |
| TROPION-PanTumor-01 NCT03401385 |
TROP2 | Phase I | la/mUC | Datopotamab deruxtecan | Dose-Limiting Toxicities; Adverse Events; | 890 (Total Trial) | Daiichi Sankyo 82 |
| NCT04863885 | TROP2 | Phase I/II | mUC; cisplatin ineligible | SG + Ipilimumab + Nivolumab | Maximum Tolerated Dose; Overall Response Rate | 46 | Jain et al. 77 |
| RC48-G001 NCT04879329 |
HER2 | Phase II | la/mUC; with and without prior systemic therapy | DV + Pembro | ORRs; Adverse Events; Lab & EKG Abnormalities; Pharmacokinetics | 332 | Powles et al. 86 |
DV, Disitamab vedotin; MIBC, Muscle invasive bladder cancer; ORR, objective response rate; RC, Radical cystectomy; SG, Sacituzumab govitecan.
Trastuzumab emtansine
One of the drugs being investigated is Trastuzumab emtansine (T-DM1). This ADC is currently approved for treatment of some HER2-positive breast cancers and also has shown evidence of efficacy in HER2-positive UC. The KAMELEON study was a phase II study of single-agent T-DM1 in metastatic HER2-positive UC. This study was terminated early due to poor recruitment, with only 13 patients enrolled. Among these patients, five exhibited a partial response for an ORR of 38.5%, which would have cleared the ORR threshold of four of 27 patients in the initial design. 87
Disitamab vedotin
Disitamab vedotin (DV) is another HER2 directed ADC under study. It is a humanized anti-HER2 antibody conjugated to MMAE via a cleavable linker. 84 Multiple studies are evaluating the utility of this ADC for treating UC, including the RC48-C005 and RC48-C009 trials. These phase II studies investigated DV in patients with mUC who had received at least one line of systemic chemotherapy. In RC48-C005, 43 patients were enrolled with an ORR of 51.2% (95% CI 35.5–66.7). Median PFS was 6.9 months (95% CI 5.6–8.9), and median OS was 13.9 months (95% CI 9.1–NE). Among patients with liver metastases, response rates were even higher, with an ORR of 65%. Even among the group of patients classified as having a lower level of HER2 positivity, ORR was 40% (95% CI 19.1–63.9). 84 In RC48-C009, 64 patients were enrolled with an ORR of 46.9% (95% CI 34.3–59.8). Median PFS was 4.3 months (95% CI 4.0–6.8), and median OS was 14.8 months (95% CI 8.7–21.0). 88 Similar responses were seen across subgroups in both of these studies, including those with liver metastases and prior anti-PD-1/L1 antibody treatment. 89
The combination of DV with pembrolizumab in advanced UC is being investigated as well. RC48-G001 is an ongoing phase II trial evaluating this combination for HER2-positive unresectable or metastatic UC. Several cohorts will be studied, apportioning patients according to their level of HER2 positivity and prior treatments. DV will be investigated in this trial both as monotherapy and in combination with pembrolizumab. 86 (Table 6)
Table 6.
Anti-HER2, SLITRK6 ADC, and anti-EphA2 BTC completed and ongoing trials.
| Name of trial | Target of the ADC/BTC | Phase of trial | Enrollment | Stage of disease | Disease setting | Intervention | ORR % | mOS | mPFS | References |
|---|---|---|---|---|---|---|---|---|---|---|
| RC48-C005 | HER2 | Phase II | 107 | mUC | Prior chemo | Disitamab vedotin | 51.20% | 13.9 mos. | 6.9 months | Sheng et al. 84 |
| RC48-C009 | HER2 | Phase II | 43 | la/mUC | Prior chemo | Disitamab vedotin | 46.90% | 14.8 mos. | 4.3 months | Sheng et al. 88 |
| KAMELEON | HER2 | Phase II | 13 (terminated early) | mUC | HER2+ | Trastuzumab emtansine | 38.50% | not reported | not reported | de Vries et al. 87 |
| DESTINY-Pantumor-02 | HER2 | Phase II | 41 | mUC | Prior systemic treatment | Trastuzumab deruxtecan | 39% | not yet reported | not yet reported | Meric-Bernstam et al. 90 |
| AGS-15ME | SLITRK6 | Phase I | 51 | mUC | Metastatic | Sirtratumab vedotin | not reported | not reported | 16 weeks | Petrylak et al. 91 |
| BT5528-100 NCT04180371 |
EphA2 | Phase I/II | 288 | mUC | Prior treatment | BT5528 | Not yet reported | Not yet reported | Not yet reported | Bendall et al. 92 |
ADC, antibody-drug conjugate; BTC, bicycle toxin conjugate; HER2, Human epidermal growth factor receptor 2; mOS, median overall survival; mos, months; mPFS, median progression-free survival; ORR, objective response rate.
The trials conducted thus far indicate that DV is a relatively well-tolerated treatment with low levels of grade 3 TRAEs reported 89 and some reporting no grade 4 or higher TRAEs. 84 The most common TRAEs that have been seen are hypoesthesia, leukopenia, LFT elevations, decreased appetite, and asthenia. 89 Peripheral neuropathy has been reported as well in RC48-C005, with a frequency of 14%, and one patient experienced grade 3 or higher neuropathy. 84
Trastuzumab deruxtecan
Trastuzumab deruxtecan (T-DXd) is another ADC targeting HER2 that is composed of an anti-HER2 antibody conjugated to a TOPO1 inhibitor payload. This drug has already received approval for the treatment of HER2-low breast cancer based on the DESTINY-Breast04 trial. 93 The DESTINY-Pantumor-02 trial is investigating this drug for use in multiple solid tumor types, including a cohort for la/mUC that progressed following one or more prior systemic therapies. 94 In the urothelial cancer cohort, 41 patients enrolled. ORR for the entire cohort was 39%, and for strongly HER2 positive tumors, IHC3+, ORR was 56.3%. Grade 3 or higher TRAEs were reported in 58.4% of patients, with notable TRAEs including interstitial lung disease and pneumonitis. 90
Other molecular targets
Several other proteins have been identified as possible ADC targets in urothelial cancer. SLITRK6 is a member of a family of transmembrane proteins found to play important roles in cell adhesion, differentiation, cancer cell migration, and invasion. Studies have shown that SLITRK6 is expressed on a variety of epithelial tumors, with bladder cancer demonstrating high levels of expression. 95
Sirtratumab vedotin (AGS-15ME) is an ADC targeting SLITRK6 with preliminary efficacy in la/mUC treatment. This drug is composed of an anti-SLITRK6 antibody conjugated to an MMAE payload via a cleavable linker. A phase I trial that enrolled 51 patients reported an ORR of 33%, median PFS of 16 weeks, and median duration of response of 15 weeks. 91% of patients had a TRAE, most notably fatigue and ocular toxicity. The rate of Grade 3 or higher TRAEs was 50%. 91
Ephrin A receptor 2 (EphA2) is another protein identified as a potential target in UC. EphA2 is a surface cell receptor that mediates signaling converging on pathways integral to cell growth, proliferation, migration, and invasion. Increased EphA2 expression has been identified as a resistance mechanism to EGFR TKI-based therapy. 92 This protein is targeted by the drug BT5528, which is a bicycle toxin conjugate composed of a bicyclic peptide targeting EphA2, conjugated to MMAE via a cleavable linker. 23 The BT5528-100 trial is investigating the use of this drug in a variety of cancers, with one cohort to be composed of patients with metastatic urothelial cancer who have either failed or are ineligible for appropriate treatment options. This trial will evaluate BT5528 alone and in combination with nivolumab and is actively recruiting. 92
Biomarkers
Despite high expression of the antibody targets of ADCs in UC, evaluation of tumor expression levels of the targets has not shown a definitive predictive value. The FDA review of EV concluded that Nectin-4 expression levels do not appear to identify patients likely to preferentially benefit from EV, and therefore, routine testing for this purpose is unwarranted. 96 Evaluation of TROP2 expression levels and response to treatment in the Trophy-U-01 trial also failed to find any statistically significant difference in outcomes based on TROP2 expression. 86 The data for HER2 give a less clear picture as to the prognostic and predictive value of HER2 overexpression. Some studies have shown that HER2 overexpression is associated with shorter PFS, whereas other studies have shown a more positive prognosis. 83 The Destiny-Pantumor-02 trial suggested that patients with higher levels of HER2 positivity may be associated with a better response to treatment with T-DXd, although patients with low expression may respond as well. 90 While some level of HER2 expression is a prerequisite for treatment with a HER2 ADC, the absolute level of expression may not substantially affect treatment decisions.
Alternatively, other biomarkers may predict response to therapy. In the UNITE study, next-generation DNA sequencing data was available for 170 patients, and occurrence of specific genetic alterations was correlated with outcomes data. In patients with an ERBB2 alteration, treatment with EV had an ORR of 67% versus 44% for those without this alteration. Superior outcomes were also seen in those with TSC1 alterations, with ORR of 68% versus 25% for those without the alteration. Shorter median survival was seen in patients with CDKN2A, CDKN2B, and MTAP alterations. 97
Conclusion
ADCs and BTCs present recent advancements in the therapeutic landscape of UC, especially for locally advanced and metastatic disease refractory to standard agents. The landmark trials discussed in this review established the efficacy of these treatments in advanced disease and proposed consideration earlier in the course of treatment. Two ADCs, EV, and SG, as well as the combination of EV and pembrolizumab, have been approved for treatment of la/mUC. Ongoing studies highlight the potential for additional future therapies with novel antibody targets, cytotoxic payloads, and structures, as well as unique combinations.
Real-world analyses have further demonstrated the efficacy of these treatments in patients who may not qualify for clinical trials. Even among patients with significant comorbidities and poor performance status, in some situations, ADCs have provided clinical benefit.98–100
There are some limitations to the promise of ADCs. Toxicities have been associated with ADCs, for example, peripheral neuropathy and rash with EV or cytopenias and diarrhea with SG. Nonetheless, toxicities are typically manageable and do not result in reduced QoL in studies. The mechanism of action of these drugs is complex and resistance can occur at any point along their pathway, with proposed mechanisms of resistance including altered tumor antigen expression, impaired lysosomal function, drug efflux pump overexpression, and altered downstream signaling pathways. 101 Another limitation of ADCs is the cost of treatments. Significant differences have been noted between the costs of various ADCs. 102 Finally, novel biomarkers are needed to predict which patients benefit from therapy.
ADCs also represent a tremendous advance in drug delivery technology. With novel combinations of molecular targets and payloads, these drugs will be prototypical examples for further drug development, not just for the treatment of cancer but for the treatment of various other illnesses. Optimization of precise drug delivery with limited systemic toxic exposure underlies a core value within medicine, providing maximal benefit to patients while minimizing harm.
Acknowledgments
None.
Appendix
Abbreviations
ADC Antibody-drug conjugate
AGS-15ME Sirtratumab vedotin
BTC Bicycle toxin conjugate
DS1062a Datopotamab deruxtecan
DV Disitamab vedotin
EphA2 Ephrin A receptor 2
EV Enfortumab vedotin
FGFR Fibroblast growth factor receptor
FOLR1 Folate receptor 1
HER2 Human epidermal growth factor receptor 2
La/mUC Locally advanced / metastatic urothelial carcinoma
ICI Immune checkpoint inhibitor
MIBC Muscle invasive bladder cancer
MMAE Monomethyl auristatin E
mOS Median overall survival
mPFS Median progression-free survival
NMIBC Nonmuscle invasive urothelial cancer
ORR Objective response rate
OS Overall survival
Pembro Pembrolizumab
PFS Progression-Free Survival
QoL Quality of life
RC Radical cystectomy
SG Sacituzumab govitecan
T-DM1 Trastuzumab emtansine
T-DXd Trastuzumab deruxtecan
TF Tissue Factor
TOPO1 Topoisomerase I
TRAE Treatment-related adverse event
TROP2 Trophoblast cell-surface antigen 2
UC Urothelial Cancer
Footnotes
ORCID iD: Jason R. Brown  https://orcid.org/0000-0001-6225-7555
https://orcid.org/0000-0001-6225-7555
Contributor Information
Chaim Domb, University Hospitals Seidman Cancer Center, Cleveland, OH, USA.
Jorge A. Garcia, University Hospitals Seidman Cancer Center, Cleveland, OH, USA Case Western Reserve University, Cleveland, OH, USA.
Pedro C. Barata, University Hospitals Seidman Cancer Center, Cleveland, OH, USA Case Western Reserve University, Cleveland, OH, USA.
Prateek Mendiratta, University Hospitals Seidman Cancer Center, Cleveland, OH, USA; Case Western Reserve University, Cleveland, OH, USA.
Santosh Rao, University Hospitals Seidman Cancer Center, Cleveland, OH, USA; Case Western Reserve University, Cleveland, OH, USA.
Jason R. Brown, University Hospitals Seidman Cancer Center, 11100 Euclid Ave., Lakeside 1200, Mailstop LKS 5079, Cleveland, OH 44106, USA; Case Western Reserve University, Cleveland, OH, USA.
Declarations
Ethics approval and consent to participate: Not applicable.
Consent for publication: Not applicable.
Author contributions: Chaim Domb: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Project administration; Resources; Visualization; Writing – original draft; Writing – review & editing.
Jorge A. Garcia: Writing – original draft; Writing – review & editing.
Pedro C. Barata: Writing – original draft; Writing – review & editing.
Prateek Mendiratta: Writing – original draft; Writing – review & editing.
Santosh Rao: Writing – original draft; Writing – review & editing.
Jason R. Brown: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Project administration; Resources; Supervision; Validation; Visualization; Writing – original draft; Writing – review & editing.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
The authors declare that there is no conflict of interest.
Availability of data and materials: Not applicable.
References
- 1. Lenis AT, Lec PM, Chamie K. Bladder cancer. JAMA 2020; 324: 1980–1980. [DOI] [PubMed] [Google Scholar]
- 2. Mar N, Dayyani F. Management of urothelial bladder cancer in clinical practice: real-world answers to difficult questions. J Oncol Pract 2019; 15: 421–428. [DOI] [PubMed] [Google Scholar]
- 3. Hoimes CJ, Flaig TW, Milowsky MI, et al. Enfortumab vedotin plus pembrolizumab in previously untreated advanced urothelial Cancer. J Clin Oncol 2023; 41: 22–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Swami U, Grivas P, Pal SK, et al. Utilization of systemic therapy for treatment of advanced urothelial carcinoma: lessons from real world experience. Cancer Treat Res Commun 2021; 27: 100325–100325. [DOI] [PubMed] [Google Scholar]
- 5. Powles T, Park SH, Voog E, et al. Avelumab maintenance therapy for advanced or metastatic urothelial carcinoma. N Engl J Med 2020; 383: 1218–1230. [DOI] [PubMed] [Google Scholar]
- 6. Tagawa ST, Balar AV, Petrylak DP, et al. TROPHY-U-01: a phase II open-label study of sacituzumab govitecan in patients with metastatic urothelial carcinoma progressing after platinum-based chemotherapy and checkpoint inhibitors. J Clin Oncol 2021; 39: 2474–2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fu Z, Li S, Han S, Shi C, et al. Antibody drug conjugate: the “biological missile” for targeted cancer therapy. Signal Transduct Target Ther 2022; 7: 93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Shastry M, Gupta A, Chandarlapaty S, et al. Rise of antibody-drug conjugates: the present and future. Am Soc Clin Oncol Educ Book 2023; 43: e390094. [DOI] [PubMed] [Google Scholar]
- 9. Giugliano F, Corti C, Tarantino P, et al. Bystander effect of antibody–drug conjugates: fact or fiction? Curr Oncol Rep 2022; 24: 809–817. [DOI] [PubMed] [Google Scholar]
- 10. Pettinato MC. Introduction to antibody-drug conjugates. Antibodies 2021; 10: 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Najminejad Z, Dehghani F, Mirzaei Y, et al. Clinical perspective: antibody-drug conjugates for the treatment of HER2-positive breast cancer. Mol Ther 2023; 31: 1874–1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Brown MP, Staudacher AH. Could bystander killing contribute significantly to the antitumor activity of brentuximab vedotin given with standard first-line chemotherapy for Hodgkin lymphoma? Immunotherapy 2014; 6: 371–375. [DOI] [PubMed] [Google Scholar]
- 13. Li F, Emmerton KK, Jonas M, et al. Intracellular released payload influences potency and bystander-killing effects of antibody-drug conjugates in preclinical models. Cancer Res 2016; 76: 2710–2719. [DOI] [PubMed] [Google Scholar]
- 14. Staudacher AH, Brown MP. Antibody drug conjugates and bystander killing: is antigen-dependent internalisation required? Br J Cancer 2017; 117: 1736–1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Burke JM, Morschhauser F, Andorsky D, et al. Antibody–drug conjugates for previously treated aggressive lymphomas: focus on polatuzumab vedotin. Expert Rev Clin Pharmacol 2020; 13: 1073–1083. [DOI] [PubMed] [Google Scholar]
- 16. Liu BA, Olson D, Snead K, et al. Abstract 5581: Enfortumab vedotin, an anti-Nectin-4 ADC demonstrates bystander cell killing and immunogenic cell death anti-tumor activity mechanisms of action in urothelial cancers. Cancer Res 2020; 80(Suppl. 16): 5581–5581. [Google Scholar]
- 17. López De Sá A, Díaz-Tejeiro C, et al. Considerations for the design of antibody drug conjugates (ADCs) for clinical development: lessons learned. J Hematol OncolJ Hematol Oncol 2023; 16: 118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Calabretta E, Hamadani M, Carlo-Stella C. The antibody-drug conjugate loncastuximab tesirine for the treatment of diffuse large b-cell lymphoma. Blood 2022; 140: 303–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zammarchi F, Corbett S, Adams L, et al. ADCT-402, a PBD dimer–containing antibody drug conjugate targeting CD19-expressing malignancies. Blood 2018; 131: 1094–1105. [DOI] [PubMed] [Google Scholar]
- 20. Alley SC, Harris JR, Cao A, et al. Abstract 221: Tisotumab vedotin induces anti-tumor activity through MMAE-mediated, Fc-mediated, and Fab-mediated effector functions in vitro. Cancer Res 2019; 79(Suppl. 13): 221–221. [Google Scholar]
- 21. Markham A. Tisotumab vedotin: first approval. Drugs 2021; 81: 2141–2147. [DOI] [PubMed] [Google Scholar]
- 22. Moore KN, Martin LP, O’Malley DM, et al. Safety and activity of mirvetuximab soravtansine (IMGN853), a folate receptor alpha–targeting antibody–drug conjugate, in platinum-resistant ovarian, fallopian tube, or primary peritoneal cancer: a phase I expansion study. J Clin Oncol 2017; 35: 1112–1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bennett G, Brown A, Mudd G, et al. MMAE delivery using the bicycle toxin conjugate BT5528. Mol Cancer Ther 2020; 19: 1385–1394. [DOI] [PubMed] [Google Scholar]
- 24. Rigby M, Bennett G, Chen L, et al. BT8009; a nectin-4 targeting bicycle toxin conjugate for treatment of solid tumors. Mol Cancer Ther 2022; 21: 1747–1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mudd GE, Scott H, Chen L, et al. Discovery of BT8009: a nectin-4 targeting bicycle toxin conjugate for the treatment of cancer. J Med Chem 2022; 65: 14337–14347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021: 372: n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Challita-Eid PM, Satpayev D, Yang P, et al. Enfortumab vedotin antibody–drug conjugate targeting nectin-4 is a highly potent therapeutic agent in multiple preclinical cancer models. Cancer Res 2016; 76: 3003–3013. [DOI] [PubMed] [Google Scholar]
- 28. Klümper N, Ralser DJ, Ellinger J, et al. Membranous NECTIN-4 expression frequently decreases during metastatic spread of urothelial carcinoma and is associated with enfortumab vedotin resistance. Clin Cancer Res 2023; 29: 1496–14505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Heath EI, Rosenberg JE. The biology and rationale of targeting nectin-4 in urothelial carcinoma. Nat Rev Urol 2021; 18: 93–103. [DOI] [PubMed] [Google Scholar]
- 30. Kumar A, White J, James Christie R, et al. Antibody-drug conjugates. Annual Reports in Medicinal Chemistry: Platform Technologies in Drug Discovery and Validation; 2017; 50: pp. 441–80. [Google Scholar]
- 31. O’Donnell P, Galsky MD, Rosenberg JE, et al. 746P EV-201: long-term results of enfortumab vedotin monotherapy for locally advanced or metastatic urothelial cancer previously treated with platinum and PD-1/PD-L1 inhibitors. Ann Oncol 2020; 31: S579–S580. [Google Scholar]
- 32. Rosenberg JE, O’Donnell PH, Balar AV, et al. Pivotal trial of enfortumab vedotin in urothelial carcinoma after platinum and anti-programmed death 1/programmed death ligand 1 therapy. J Clin Oncol 2019. Oct; 37: 2592–2600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. McGregor BA, Balar AV, Rosenberg JE, et al. Enfortumab vedotin in cisplatin-ineligible patients with locally advanced or metastatic urothelial cancer who received prior PD-1/PD-L1 inhibitors: an updated analysis of EV-201 cohort 2. J Clin Oncol 2021; 39(Suppl. 15): 4524. [Google Scholar]
- 34. Petrylak DP, Flaig TW, Mar N, et al. Study EV-103 cohort H: ANTitumor activity of neoadjuvant treatment with enfortumab vedotin monotherapy in patients (pts) with muscle invasive bladder cancer (MIBC) who are cisplatin-ineligible. J Clin Oncol 2022; 40(Suppl. 6): 435. [Google Scholar]
- 35. O’Donnell PH, Rosenberg JE, Hoimes CJ, et al. Enfortumab vedotin (EV) alone or in combination with pembrolizumab (P) in previously untreated cisplatin-ineligible patients with locally advanced or metastatic urothelial cancer (la/mUC): subgroup analyses of confirmed objective response rate (cORR) from EV-103 cohort K. J Clin Oncol 2023; 41(Suppl 6.): 499–499. [Google Scholar]
- 36. Rosenberg JE, Powles T, Sonpavde GP, et al. Long-term outcomes in EV-301: 24-month findings from the phase 3 trial of enfortumab vedotin versus chemotherapy in patients with previously treated advanced urothelial carcinoma. J Clin Oncol 2022; 40(Suppl. 16): 4516. [DOI] [PubMed] [Google Scholar]
- 37. Matsubara N, Yonese J, Kojima T, et al. Japanese subgroup analysis of EV-301: an open-label, randomized phase 3 study to evaluate enfortumab vedotin versus chemotherapy in subjects with previously treated locally advanced or metastatic urothelial carcinoma. Cancer Med 2023; 12: 2761–2771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Powles T, Valderrama BP, Gupta S, et al. Enfortumab Vedotin and Pembrolizumab in Untreated Advanced Urothelial Cancer. N Engl J Med 2024; 390: 875–88. [DOI] [PubMed] [Google Scholar]
- 39. Powles T, Rosenberg JE, Sonpavde GP, et al. Enfortumab vedotin in previously treated advanced urothelial carcinoma. N Engl J Med 2021; 384: 1125–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Koshkin VS, Henderson N, James M, et al. Efficacy of enfortumab vedotin in advanced urothelial cancer: analysis from the urothelial cancer network to investigate therapeutic experiences (UNITE) study. Cancer 2022; 128: 1194–1205. [DOI] [PubMed] [Google Scholar]
- 41. Koshkin VS, Henderson N, Kilari D, et al. Enfortumab vedotin (EV) outcomes with and without immediate prior immune checkpoint inhibitor (ICI) in patients (pts) with advanced urothelial carcinoma (aUC). J Clin Oncol 2023; 41(Suppl. 6): 514. [Google Scholar]
- 42. O’Donnell PH, Milowsky MI, Petrylak DP, et al. Enfortumab vedotin with or without pembrolizumab in cisplatin-ineligible patients with previously untreated locally advanced or metastatic urothelial cancer. J Clin Oncol 2023; 41: 4107–4117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. van der Heijden MS, Gupta S, Galsky MD, et al. 798TiP Study EV-302: a 3-arm, open-label, randomized phase III study of enfortumab vedotin plus pembrolizumab and/or chemotherapy, versus chemotherapy alone, in untreated locally advanced or metastatic urothelial cancer. Ann Oncol 2020; 31: S605–S606. [Google Scholar]
- 44. Milowsky MI, O’Donnell PH, Hoimes CJ, et al. Patient-reported outcomes (PROs) in cisplatin-ineligible patients (pts) with locally advanced or metastatic urothelial cancer (la/mUC) treated with enfortumab vedotin (EV) alone or in combination with pembrolizumab (P) in the phase 1b/2 EV-103 cohort K study. J Clin Oncol 2023; 41(Suppl. 6): 439.36469836 [Google Scholar]
- 45. McGregor B, O’Donnell PH, Balar A, et al. Health-related quality of life of patients with locally advanced or metastatic urothelial cancer treated with enfortumab vedotin after platinum and PD-1/PD-L1 inhibitor therapy: results from cohort 1 of the phase 2 EV-201 clinical trial. Eur Urol 2022; 81: 515–522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Mamtani R, Rosenberg JE, Powles T, et al. Quality of life, functioning, and symptoms in patients with previously treated locally advanced or metastatic urothelial carcinoma from EV-301: a randomized phase 3 trial of enfortumab vedotin versus chemotherapy. J Clin Oncol 2021; 39(Suppl. 15): 4539. [Google Scholar]
- 47. Memorial Sloan Kettering Cancer Center. Enfortumab vedotin in combination with pembrolizumab for locally advanced and/or node positive urothelial carcinoma prior to surgery (EV-ECLIPSE) [Internet]. Report No: NCT05239624. https://clinicaltrials.gov/study/NCT05239624 (2023, accessed 11 September 2023).
- 48. Nazha B. Phase II single-arm study of enfortumab vedotin (EV) plus pembrolizumab in the treatment of locally advanced or metastatic bladder cancer of variant histology [Internet]. Report No: NCT05756569. https://clinicaltrials.gov/study/NCT05756569 (2023, accessed 12 September 2023).
- 49. Jia X, Yan B, Tian X, et al. CD47/SIRPα pathway mediates cancer immune escape and immunotherapy. Int J Biol Sci 2021; 17: 3281–3287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Jain RK, Kim Y, Rembisz J, et al. Phase Ib trial of erdafitinib (E) combined with enfortumab vedotin (EV) following platinum and PD-1/L1 inhibitors for metastatic urothelial carcinoma (mUC) with FGFR2/3 genetic alterations (GAs). J Clin Oncol 2022; 40(Suppl. 6): TPS595. [Google Scholar]
- 51. Bilen M. A Phase I/Ib open label, single-arm study of cabozantinib in combination with enfortumab vedotin (EV) in the treatment of locally advanced or metastatic urothelial cancer [Internet]. Report No: NCT04878029. https://clinicaltrials.gov/study/NCT04878029 (2023, accessed 11 September 2023).
- 52. McGregor BA, Kwak L, Mantia C, et al. Sacituzumab govitecan (SG) plus enfortumab vedotin (EV) for metastatic urothelial carcinoma (UC) progressing on platinum-based chemotherapy and PD1/L1 inhibitors (ICB): double antibody drug conjugate (DAD) phase I trial. J Clin Oncol 2022; 40(Suppl. 6): TPS588. [Google Scholar]
- 53. Drakaki A, Rezazadeh Kalebasty A, Lee JL, et al. Phase Ib/II umbrella trial to evaluate the safety and efficacy of multiple 2L cancer immunotherapy (CIT) combinations in advanced/metastatic urothelial carcinoma (mUC): MORPHEUS-mUC. J Clin Oncol 2020; 38(Suppl. 6): TPS591. [Google Scholar]
- 54. Kamat AM, Steinberg GD, Inman BA, et al. Study EV-104: phase 1 study of intravesical enfortumab vedotin for treatment of patients with non-muscle invasive bladder cancer (NMIBC)—trial in progress. J Clin Oncol 2023; 41(Suppl. 6): TPS582. [Google Scholar]
- 55. Hoimes CJ, Rosenberg JE, Petrylak DP, et al. Study EV-103: new cohorts testing enfortumab vedotin alone or in combination with pembrolizumab in muscle invasive urothelial cancer. J Clin Oncol 2020; 38(Suppl. 6): TPS595. [Google Scholar]
- 56. Hoimes CJ, Flaig TW, Srinivas S, et al. Study EV-103 cohort L: evaluating perioperative enfortumab vedotin monotherapy in cis-ineligible muscle invasive bladder cancer (MIBC) (trial in progress). J Clin Oncol 2022; 40(Suppl. 6): TPS587. [Google Scholar]
- 57. Hoimes CJ, Loriot Y, Bedke J, et al. Perioperative enfortumab vedotin (EV) plus pembrolizumab (pembro) versus chemotherapy in cisplatin-eligible patients (pts) with muscle-invasive bladder cancer (MIBC): phase 3 KEYNOTE-B15/EV-304. J Clin Oncol 2023; 41(Suppl. 6): TPS588. [Google Scholar]
- 58. Powles T, Drakaki A, Teoh JYC, et al. A phase 3, randomized, open-label, multicenter, global study of the efficacy and safety of durvalumab (D) + tremelimumab (T) + enfortumab vedotin (EV) or D + EV for neoadjuvant treatment in cisplatin-ineligible muscle-invasive bladder cancer (MIBC) (VOLGA). J Clin Oncol 2022; 40(Suppl. 6): TPS579. [Google Scholar]
- 59. Kobayashi T. A phase 2, open-label, multi-institutional study to evaluate the efficacy of induction therapy with MK-3475 and ASG-22CE followed by radiation therapy with MK-3475 in patients with MIBC who are unfit for or refuse radical cystectomy [Internet]. Report No: NCT05879653. https://clinicaltrials.gov/study/NCT05879653 (2023, accessed 11 September 2023).
- 60. Necchi A, Bedke J, Galsky MD, et al. Phase 3 KEYNOTE-905/EV-303: perioperative pembrolizumab (pembro) or pembro + enfortumab vedotin (EV) for muscle-invasive bladder cancer (MIBC). J Clin Oncol 2023; 41(Suppl. 6): TPS585. [Google Scholar]
- 61. ALX Oncology Inc. A phase 1, open-label, multicenter, safety, pharmacokinetic, pharmacodynamic study of ALX148 in combination with enfortumab vedotin and/or other anticancer therapies in subjects with urothelial carcinoma (ASPEN-07) [Internet]. Report No.: NCT05524545, https://clinicaltrials.gov/study/NCT05524545 (2023, accessed 31 December 2022)
- 62. Baldini C, Goldschmidt V, Brana I, et al. BT8009-100: a phase I/II study of novel bicyclic peptide and MMAE conjugate BT8009 in patients (pts) with advanced malignancies associated with nectin-4 expression, including urothelial cancer (UC). J Clin Oncol 2023; 41(Suppl. 6): 498. [Google Scholar]
- 63. Galsky MD, Hoimes CJ, Necchi A, et al. Perioperative pembrolizumab therapy in muscle-invasive bladder cancer: phase III KEYNOTE-866 and KEYNOTE-905/EV-303. Future Oncol 2021; 17: 3137–3150. [DOI] [PubMed] [Google Scholar]
- 64. Fan Y, Li Q, Shen Q, et al. Head-to-head comparison of the expression differences of NECTIN-4, TROP-2, and HER2 in urothelial carcinoma and its histologic variants. Front Oncol 2022; 12: Article 858865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Tomiyama E, Fujita K, Nakano K, et al. Trop-2 in upper tract urothelial carcinoma. Curr Oncol 2022; 29: 3911–3921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Chou J, Trepka K, Sjöström M, et al. TROP2 Expression across molecular subtypes of urothelial carcinoma and enfortumab vedotin-resistant cells. Eur Urol Oncol 2022; 5: 714–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Bardia A, Messersmith WA, Kio EA, et al. Sacituzumab govitecan, a Trop-2-directed antibody-drug conjugate, for patients with epithelial cancer: final safety and efficacy results from the phase I/II IMMU-132-01 basket trial. Ann Oncol 2021; 32: 746–756. [DOI] [PubMed] [Google Scholar]
- 68. Tagawa ST, Faltas BM, Lam ET, et al. Sacituzumab govitecan (IMMU-132) in patients with previously treated metastatic urothelial cancer (mUC): results from a phase I/II study. J Clin Oncol 2019; 37(Suppl. 7): 354. [Google Scholar]
- 69. Petrylak DP, Tagawa ST, Jain RK, et al. Primary analysis of TROPHY-U-01 cohort 2, a phase 2 study of sacituzumab govitecan (SG) in platinum (PT)-ineligible patients (pts) with metastatic urothelial cancer (mUC) that progressed after prior checkpoint inhibitor (CPI) therapy. J Clin Oncol 2023; 41(Suppl. 6): 520. [Google Scholar]
- 70. Grivas P, Pouessel D, Park CH, et al. Primary analysis of TROPHY-U-01 cohort 3, a phase 2 study of sacituzumab govitecan (SG) in combination with pembrolizumab (Pembro) in patients (pts) with metastatic urothelial cancer (mUC) that progressed after platinum (PT)-based therapy. J Clin Oncol 2023; 41(Suppl. 6): 518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Tagawa ST, Balar AV, Petrylak DP, et al. Updated outcomes in TROPHY-U-01 cohort 1, a phase 2 study of sacituzumab govitecan (SG) in patients (pts) with metastatic urothelial cancer (mUC) that progressed after platinum (PT)-based chemotherapy and a checkpoint inhibitor (CPI). J Clin Oncol 2023; 41(Suppl. 6): 526. [Google Scholar]
- 72. Vulsteke C, Grivas P, Tagawa ST, et al. TROPiCS-04: study of sacituzumab govitecan (SG) in patients (pts) with locally advanced (LA) unresectable or metastatic urothelial cancer (mUC) that has progressed after prior platinum (PLT) and checkpoint inhibitor (CPI) therapy. J Clin Oncol 2022; 40(Suppl. 6): TPS582. [Google Scholar]
- 73. Tagawa ST, Grivas P, Petrylak DP, et al. TROPHY-U-01 cohort 4: sacituzumab govitecan (SG) in combination with cisplatin (Cis) in platinum (PLT)-naïve patients (pts) with metastatic urothelial cancer (mUC). J Clin Oncol 2022; 40(Suppl. 6): TPS581. [Google Scholar]
- 74. Powles T, Necchi A, Duran I, et al. TROPHU-U-01 cohort 5: evaluation of maintenance sacituzumab govitecan (SG) plus zimberelimab (ZIM), ZIM, or avelumab in cisplatin-eligible patients (pts) with unresectable or metastatic urothelial cancer (mUC). J Clin Oncol 2023; 41(Suppl. 6): TPS598. [Google Scholar]
- 75. Duran I, Necchi A, Powles T, et al. TROPHY-U-01 cohort 6: sacituzumab govitecan (SG), SG plus zimberelimab (ZIM), SG plus ZIM plus domvanalimab (DOM), or carboplatin (CARBO) + gemcitabine (GEM) in cisplatin-ineligible patients (pts) with treatment-naive metastatic urothelial cancer (mUC). J Clin Oncol 2023; 41(Suppl. 6): TPS592. [Google Scholar]
- 76. Hoffman-Censits J, Grivas P, Powles T, et al. 665 JAVELIN Bladder Medley: a phase 2 trial of avelumab in combination with other antitumor drugs as first-line maintenance therapy for advanced urothelial carcinoma. BMJ 2022; 10: A694. [Google Scholar]
- 77. Jain RK, Yang Y, Chadha J, et al. Phase I/II study of ipilimumab plus nivolumab combined with sacituzumab govitecan in patients with metastatic cisplatin-ineligible urothelial carcinoma. J Clin Oncol 2023; 41(Suppl. 6): 521–521. [Google Scholar]
- 78. Combination of Ipi/Nivo plus sacituzumab govitecan in metastatic cisplatin ineligible urothelial carcinoma patients - full text view - ClinicalTrials.gov [Internet]. https://clinicaltrials.gov/ct2/show/NCT04863885 (2021, accessed 11 September 2023).
- 79. Necchi A, Raggi D, Bandini M, et al. SURE: an open label, sequential-arm, phase II study of neoadjuvant sacituzumab govitecan (SG), and SG plus pembrolizumab (pembro) before radical cystectomy, for patients with muscle-invasive bladder cancer (MIBC) who cannot receive or refuse cisplatin-based chemotherapy. J Clin Oncol 2021; 39(Suppl. 6): TPS506. [Google Scholar]
- 80. Mian O. Adaptive radiation therapy with concurrent sacituzumab govitecan (SG) for bladder preservation in patients with MIBC (RAD-SG). [Internet]. Report No: NCT05833867. https://clinicaltrials.gov/study/NCT05833867 (2023, accessed 11 September 2023).
- 81. University of Washington. A pilot single arm trial with sacituzumab govitecan as neoadjuvant therapy in pts with non-urothelial muscle invasive bladder cancer [Internet]. clinicaltrials.gov. Report No: NCT05581589. https://clinicaltrials.gov/study/NCT05581589 (2023, 11 September 2023)
- 82. Daiichi Sankyo. First-in-human study of DS-1062a for advanced solid tumors (TROPION-PanTumor01) - full text view - ClinicalTrials.gov [Internet]. https://clinicaltrials.gov/ct2/show/NCT03401385 (2018, accessed 11 September 2023).
- 83. Sanguedolce F, Zanelli M, Palicelli A, et al. HER2 Expression in bladder cancer: a focused view on its diagnostic, prognostic, and predictive role. Int J Mol Sci 2023; 24: 3720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Sheng X, Yan X, Wang L, et al. Open-label, multicenter, phase ii study of RC48-ADC, a HER2-targeting antibody–drug conjugate, in patients with locally advanced or metastatic urothelial carcinoma. Clin Cancer Res 2021; 27: 43–51. [DOI] [PubMed] [Google Scholar]
- 85. Modi S, Jacot W, Yamashita T, et al. Trastuzumab deruxtecan in previously treated HER2-low advanced breast cancer. N Engl J Med 2022; 387: 9–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Powles T, Yu EY, Iyer G, et al. Phase 2 clinical study evaluating the efficacy and safety of disitamab vedotin with or without pembrolizumab in patients with HER2-expressing urothelial carcinoma (RC48G001). J Clin Oncol 2023; 41(Suppl. 6): TPS594. [Google Scholar]
- 87. de Vries EGE, Rüschoff J, Lolkema M, et al. Phase II study (KAMELEON) of single-agent T-DM1 in patients with HER2-positive advanced urothelial bladder cancer or pancreatic cancer/cholangiocarcinoma. Cancer Med 2023; 12: 12071–12083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Sheng X, He Z, Han W, et al. An open-label, single-arm, multicenter, phase II study of RC48-ADC to evaluate the efficacy and safety of subjects with HER2 overexpressing locally advanced or metastatic urothelial cancer (RC48-C009). J Clin Oncol 2021; 39(Suppl. 15): 4584. [Google Scholar]
- 89. Sheng X, He Z, Shi Y, et al. RC48-ADC for metastatic urothelial carcinoma with HER2-positive: combined analysis of RC48-C005 and RC48-C009 trials. J Clin Oncol 2022; 40(Suppl. 16):4520. [Google Scholar]
- 90. Meric-Bernstam F, Makker V, Oaknin A, et al. Efficacy and safety of trastuzumab deruxtecan (T-DXd) in patients (pts) with HER2-expressing solid tumors: DESTINY-PanTumor02 (DP-02) interim results. J Clin Oncol 2023; 41(Suppl. 17): LBA3000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Petrylak D, Heath E, Sonpavde G, et al. Interim analysis of a phase I dose escalation trial of the antibody drug conjugate (ADC) AGS15E (ASG-15ME) in patients (Pts) with metastatic urothelial cancer (mUC). Ann Oncol 2016; 27: vi269. [Google Scholar]
- 92. Bendell JC, Wang JSZ, Bashir B, et al. BT5528-100 phase I/II study of the safety, pharmacokinetics, and preliminary clinical activity of BT5528 in patients with advanced malignancies associated with EphA2 expression. J Clin Oncol 2020; 38(Suppl. 15): TPS3655–TPS3655. [Google Scholar]
- 93. Narayan P, Dilawari A, Osgood C, et al. US food and drug administration approval summary: fam-trastuzumab deruxtecan-nxki for human epidermal growth factor receptor 2-low unresectable or metastatic breast cancer. J Clin Oncol 2023; 41: 2108–2116. [DOI] [PubMed] [Google Scholar]
- 94. Meric-Bernstam F, Anoka C, Dobrowolska A, et al. 1869TiP A phase II, multicenter, open-label study evaluating trastuzumab deruxtecan (T-DXd) for the treatment of select HER2-expressing solid tumors (DESTINY-PanTumor02). Ann Oncol 2021; 32: S1253–S1254. [Google Scholar]
- 95. Liu X, Liu Y, Liu Z, et al. Identification of SLITRK6 as a Novel Biomarker in hepatocellular carcinoma by comprehensive bioinformatic analysis. Biochem Biophys Rep 2021; 28: 101157–101157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Chang E, Weinstock C, Zhang L, et al. FDA approval summary: enfortumab vedotin for locally advanced or metastatic urothelial carcinoma. Clin Cancer Res 2021; 27: 922–927. [DOI] [PubMed] [Google Scholar]
- 97. Jindal T, Kilari D, Alhalabi O, et al. Biomarkers of response to enfortumab vedotin (EV) in patients (pts) with advanced urothelial carcinoma (aUC): analysis of the UNITE study. J Clin Oncol 2023; 41(Suppl. 6):450–450. [Google Scholar]
- 98. Darr C, Zschäbitz S, Niegisch G, et al. 1759P Enfortumab vedotin in metastatic urothelial carcinoma: retrospective multicentre patient cohort. Ann Oncol 2022; 33: S1342–S1342. [Google Scholar]
- 99. Zschäbitz S, Biernath N, Hilser T, et al. Enfortumab vedotin in metastatic urothelial carcinoma: survival and safety in a european multicenter real-world patient cohort. Eur Urol Open Sci 2023; 53: 31–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Koshkin VS, Sun Y, Osterman CK, et al. 771P Efficacy of enfortumab vedotin in populations of interest among patients with advanced urothelial cancer. Ann Oncol 2020; 31: S594–S594. [Google Scholar]
- 101. Khoury R, Saleh K, Khalife N, et al. Mechanisms of resistance to antibody-drug conjugates. Int J Mol Sci 2023; 24: 9674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Benjamin DJ, Lythgoe M, Raymakers AJN, et al. Cost effectiveness of newly approved second/third-line agents in metastatic urothelial carcinoma (mUC). J Clin Oncol 2022; 40(Suppl. 6): 574–574. [Google Scholar]