ABSTRACT
Exosomes derived from bone marrow mesenchymal stem cells (BMSCs) exhibit considerable therapeutic potential for myocardial regeneration. In our investigation, we delved into their impact on various aspects of myocardial infarction (MI), including cardiac function, tissue damage, inflammation, and macrophage polarization in a murine model. We meticulously isolated the exosomes from TNF-α-treated BMSCs and evaluated their therapeutic efficacy in a mouse MI model induced by coronary artery ligation surgery. Our comprehensive analysis, incorporating ultrasound, serum assessment, Western blot, and qRT-PCR, revealed that exosomes from TNF-α-treated BMSCs demonstrated significant therapeutic potential in reducing MI-induced injury. Treatment with these exosomes resulted in improved cardiac function, reduced infarct area, and increased left ventricular wall thickness in MI mice. On a mechanistic level, exosome treatment fostered M2 macrophage polarization while concurrently suppressing M1 polarization. Hence, exosomes derived from TNF-α-treated BMSCs emerge as a promising therapeutic strategy for alleviating MI injury in a mouse model.
KEYWORDS: BMSCs, exosomes, macrophage polarization, myocardial infarction, TNF-α
Introduction
Myocardial infarction (MI) is a severe condition characterized by blocked blood supply to the heart, resulting in tissue damage and cell death.1 The global prevalence of MI was estimated to be 4.7%-6.4% among adults aged 18 years and older.2,3 The prevalence was higher in men than in women and increased with age.2 Despite advances in reperfusion strategies, the limited regenerative capacity of the adult heart remains a challenge for effective cardiac repair.4
Mesenchymal stem cells (MSCs), especially bone marrow MSCs (BMSCs), have shown promise for myocardial regeneration due to their low immunogenicity and differentiation potential.5–7 BMSCs are one of the most widely studied sources of stem cells for MI treatment, because they have low immunogenicity, higher differentiation ability toward to cardiomyocyte-like cells and endothelial cells, which are essential for cardiac function and vascularization.8–10 However, it is still controversial for the effectiveness of MSCs therapies in the MI treatment due to the limited cell numbers acquired for the treatment, low efficiency, as well as the delicate route and timing of the injection of the cells. Thus, it would be an alternative option to develop MSCs-based therapies for MI treatment.
In recent years, exosomes, small extracellular vehicles secreted by various cell types, have gained significant attention as mediators of intercellular communication and as potential therapeutic agents.11 Exosomes derived from MSCs have been shown to possess regenerative properties and exhibit protective effects in various disease models, including myocardial infarction. ,12–16 Compared to cell-based therapies using MSCs, exosome-based therapy offers several advantages, including high efficiency, low immunogenicity, and ease of long-term storage at low temperatures.17 Furthermore, exosomes derived from MSCs from different sources exhibit a high degree of consistency in their basic morphological and phenotypic characteristics, as well as their ability to repair acute injuries and prevent fibrosis.18,19 These studies suggest it may be more effective using MSCs-derived exosomes instead of MSCs for MI treatment.
Composition and content of exosomes secreted by the same MSCs can significantly vary under different physiological and pathological conditions, and even exhibit contradictory functions.20,21 The paracrine effect of MSCs is significantly enhanced by lipopolysaccharide (LPS) pretreatment, leading to the upregulation of anti-inflammatory cytokines and the promotion of M2 macrophage activation.22,23 Notably, exosomes released by MSCs in response to inflammatory cytokines have been demonstrated to regulate macrophage polarization, which is crucial for myocardial infarction repair.24,25 These findings demonstrate that the anti-inflammatory activities of these exosomes could be enhanced if derived from the cytokine-pretreated MSCs, which may further promote their ability for the MI treatment. In this study, we investigated the impact of TNF-α stimulation on BMSCs in enhancing therapeutic potential of exosomes derived from these cells for myocardial infarction repair. Additionally, we evaluated the effects of these exosomes on macrophage polarization within the microenvironment of myocardial infarction. Our findings contribute to understanding the potential of TNF-α-treated BMSC-derived exosomes as a therapeutic approach for myocardial infarction.
Materials and methods
Animals
Twelve-week-old male C57BL/6 mice were procured from GemPharmatech (Nanjing, China). The mice were housed in a controlled laboratory environment, maintaining a temperature of 22 ± 1°C, humidity ranging between 45% and 55%, and a 12-hour light/12-hour dark cycle. The mice were provided with ad libitum access to food and water throughout the study period. All experimental protocols involving animal subjects were approved by the Ethics Committee of Hebei Medical University of Shijiazhuang People’s Hospital (#2023.09.152).
BMSCs isolation
BMSCs were isolated from the bone marrow of C57BL/6 mice following a standardized protocol.26 In brief, the bone marrow was collected by flushing the femurs and tibias of the mice using a 2% heat-inactivated fetal bovine serum (FBS, Gibco, Grand Island, NY) solution. The collected bone marrow cells were then processed to isolate mononuclear cells (MNCs) using density gradient centrifugation. Subsequently, the MNCs were further purified by isolating mononuclear granulocytes through the utilization of CD11b+ microbeads (StemCell Technologies, Vancouver, Canada). The remaining cells were subjected to culture conditions, by supplementing Minimum Essential Medium α (MEM α, ThermoFisher, Waltham, MA) with 10% FBS and 1% of Penicillin/Streptomycin, at 37°C in an incubator with 5% CO2. Following incubation for 72–96 hours, non-adherent cells were carefully removed, while the adherent BMSCs were maintained and cultured until reaching approximately 75% confluence. These cultured BMSCs were subsequently used for the following experimental procedures.
TNF-α stimulation of BMSCs
Recombinant human TNF-α was procured from Biolegend (San Diego, CA). BMSCs (1.5 × 106 cells/well) at the third passage were subjected to two distinct culture conditions: with or without the addition of 20 ng/ml TNF-α in the 6-well plate. The cells were incubated under these respective conditions for a duration of 48 hours as described previously.15
Exosome isolation
Isolation of BMSCs-derived exosomes followed a previously described method.15 Briefly, the supernatants from BMSC cultures were collected and subjected to a series of centrifugation steps: first, the supernatants were centrifuged at 500 g for 10 minutes to remove cellular debris. The resulting supernatants were then collected and subjected to a second centrifugation at 12,000 g for 20 minutes to pellet larger vesicles. Subsequently, the supernatants were collected again and subjected to ultracentrifugation at 100,000 g for 2 hours to obtain exosome pellets. The isolated exosomes were resuspended in phosphate-buffered saline (PBS) for subsequent analysis. The size distribution of the exosomes was determined using Nanoparticle Tracking Analysis (Malvern Panalytical, Malvern, UK). Exosome identification and profiling reveal that there is no discernible difference in exosome concentration and size between TNF-α-treated BMSCs and their non-treated counterparts.
MI model and treatment
To induce MI, 12-week-old C57BL/6 mice were anesthetized and positioned on an operating table.27 Mice were connected to a respirator to ensure proper ventilation throughout the procedure. Surgical exposure was achieved by beveling the third and fourth ribs, allowing visualization of the hearts. The coronary artery was then ligated using 6–0 nylon thread with three knots. Immediate paleness in the lower left region of the left ventricle confirmed successful ligation. Subsequently, 5 μg of exosomes suspended in 25 μL of PBS were intramyocardially injected at five distinct points surrounding the MI area, following a previously established protocol.23 The incision was carefully sutured, cleaned, and disinfected after the injection. Samples for analysis were collected after a 28-day period following the induction of MI.
Cardiac function analysis
Cardiac function of mice with indicated treatment was detected by using Ruihua ultrasound instrument (Xuzhou, China), including of left ventricular ejection fraction (LVEF), left ventricular fractional shortening (LVFS), left ventricular internal dimension at diastole (LVIDd), and left ventricular internal dimension at systole (LVIDs), as described previously.28
Quantitative reverse transcription PCR (qRT-PCR)
Total RNA was extracted from the heart tissues using TRIzol (Invitrogen, Waltham, MA) and then transcribed to cDNA by using superscript III reverse transcriptase and random primers following the manufacturer's instruction (Invitrogen). Target genes expression levels were determined through SYBR Green PCR Kit using ProFlex Flat PCR System (ThermoFisher). The primers used were as follows (5’-3’):
Cd86
Forward: TCAATGGGACTGCATATCTGCC,
Reverse: GCCAAAATACTACCAGCTCACT;
Cd206
Forward: GGGACTCTGGATTGGACTCA,
Reverse: GCTCTTTCCAGGCTCTGATG;
Gapdh
Forward: AATGGATTTGGACGCATTGGT,
Reverse: TTTGCACTGGTACGTGTTGAT.
Histological and immunofluorescent assays
Hearts were collected at 28 days post-MI, fixed in 4% paraformaldehyde overnight, and subsequently embedded in paraffin. Tissue sections of 5 μm thickness were obtained and subjected to various staining techniques for quantitative analysis. Hematoxylin and eosin staining (H&E) was performed to assess inflammatory cell infiltration. Immunofluorescence staining using anti-CD206 F4/80, and iNOS primary antibodies (1:500 dilution, Abcam, ab178945), and CD206 (1:500 dilution, CST, #24595), F4/80 (1:500 dilution, CST, #70076) were performed to quantify M2 macrophage polarization in the myocardial tissues after MI in mice.29 Image quantification of the sections was carried out using ImageJ.
Western blot
Protein extraction from exosomes performed using radioimmunoprecipitation assay buffer supplemented with protease inhibitors (Sigma-Aldrich, St. Louis, MO). The protein samples were separated by 10% sodium dodecyl sulfate – polyacrylamide gel electrophoresis (Beyotime, Shanghai, China) and subsequently transferred onto PVDF membranes (Life Technology, Waltham, MA). After blocking with 5% skim milk at room temperature for 1 hour, the membranes were incubated overnight at 4°C with the appropriate primary antibodies, CD63 (1:1000 dilution, CST, #52090), CD9 (1:500 dilution, CST, #98327), and Alix (1:1000 dilution, CST, #92880). Following the primary antibody incubation, the membranes were incubated with the corresponding secondary antibody for an additional 1 hour at room temperature. Protein bands were visualized using an ECL chemiluminescence kit (Vazyme, Nanjing, China) according to the manufacturer’s instructions.
Statistical analysis
Statistical analysis was performed on the collected data using appropriate methods. All data, unless specified, were included in the analysis. The results are presented as the mean ± standard deviation (SD). Statistical significance was determined using Brown-Forsythe ANOVA test followed by Dunnett’s T3 multiple comparisons test. A p-value less than 0.05 was considered statistically significant. All statistical analyses were conducted using Prism 8.0.
Results
Exosomes derived from TNF-α-treated BMSCs attenuated cardiac injury after MI in mice
The experimental design commenced with the induction of MI in mice on day 0 through surgical procedures involving ligation of the left anterior descending branch. Subsequently, mice were intramyocardially injected with transfected exosomes (5 μg in 25 μL PBS) at five points surrounding the MI region, within 30 minutes of the surgery (Figure 1a). On day 28 following MI, relevant assessments were conducted. Initially, the levels of cardiac injury biomarkers, including serum lactate dehydrogenase (LDH) (Figure 1b), creatine kinase-MB (CK-MB) (Figure 1c), and Troponin I (Figure 1d), were compared. The results demonstrated that treatment with exosomes effectively reduced the levels of cardiac injury biomarkers in the serum (Figure 2b-d). Furthermore, exosomes derived from TNF-α-treated BMSCs exhibited a more significant therapeutic effect. These data suggested that TNF-α-treated BMSCs-derived exosomes could attenuate MI-induced cardiac injury in mice.
Figure 1.
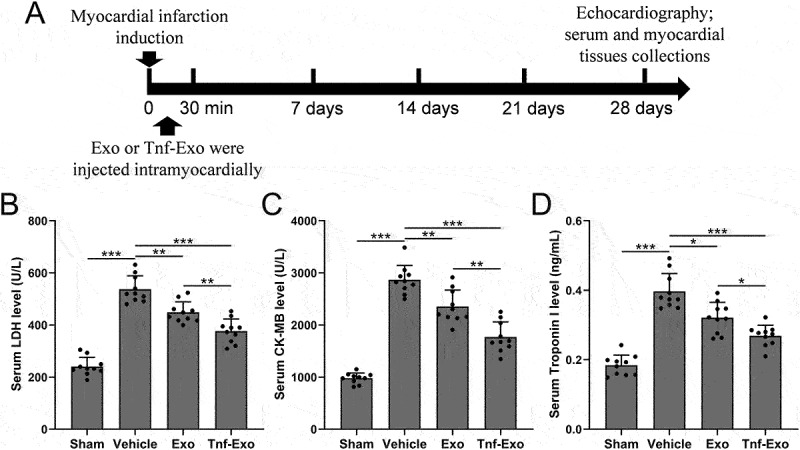
Attenuation of cardiac injury by exosomes from TNF-α-treated BMSCs in the MI mouse model.
(a) Study design of animal experiments to investigate the impact of exosomes derived from TNF-α-treated BMSCs on cardiac injury post-MI. At 28 days post-MI, serum levels of key cardiac injury markers, including LDH (Lactate Dehydrogenase) (b), CK-MB (Creatine Kinase-MB) (c), and Troponin I (d), were quantified. These analyses served as pivotal indicators in assessing the therapeutic efficacy of the administered exosomes. Data were shown with mean ± SD. 10 mice were used for each group. *p < .05, **p < .01, ***p < .001 from Brown-Forsythe ANOVA test followed by Dunnett’s T3 multiple comparisons test.
Figure 2.
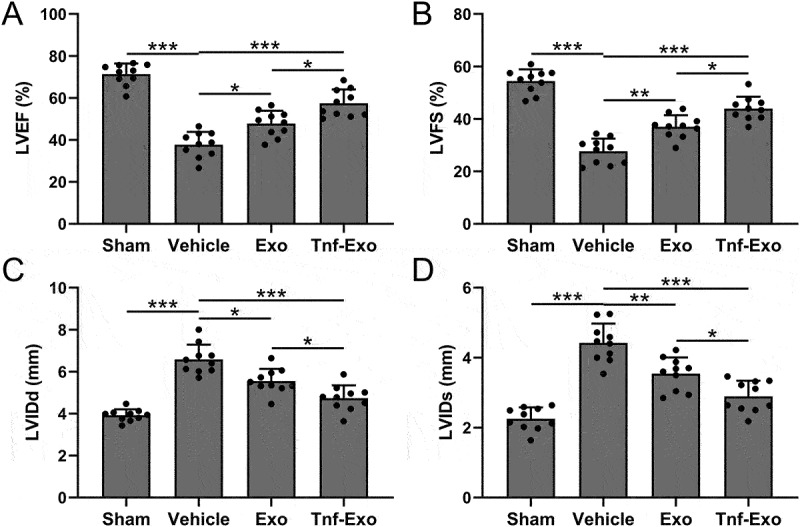
Preservation of cardiac function by exosomes from TNF-α-treated BMSCs in the MI mouse model.
The protective effects of exosomes derived from TNF-α-treated BMSCs on cardiac function post-MI is evidenced through comprehensive assessments of key parameters, including Left Ventricular Ejection Fraction (LVEF) (a), Left Ventricular Fractional Shortening (LVFS) (b), Left Ventricular Internal Diameter at end-diastole (LVIDd) (c), and Left Ventricular Internal Diameter at end-systole (LVIDs) (d) at the 28-day mark post-MI. These comparisons elucidate the therapeutic impact of the administered exosomes on preserving overall cardiac function. Data were shown with mean ± SD. 10 mice were used for each group. *p < .05, **p < .01, ***p < .001 from Brown-Forsythe ANOVA test followed by Dunnett’s T3 multiple comparisons test.
Exosomes derived from TNF-α-treated BMSCs protected cardiac function after MI in mice
Next, we used the small animal echocardiography to analyze the cardiac function of mice in different treatment groups, including LVEF (Figure 2a), LVFS (Figure 2b), LVIDd (Figure 2c), LVIDs (Figure 2d). In comparison with vehicle control group, mice in Tnf-Exo treated had significant improvement of both LVEF and LVFS, accordingly, the LVIDd and LVIDs were remarkedly reduced after Exo and Tnf-Exo treatment (Figure 2a-d). The results revealed that treatment with exosomes effectively improved the deterioration of cardiac function in mice after MI. Importantly, exosomes derived from TNF-α-treated BMSCs exhibited a more pronounced therapeutic effect.
Exosomes derived from TNF-α-treated BMSCs alleviated infarct size after MI in mice
Subsequently, we performed H&E staining to analyze the left ventricular wall thickness and infarct area in mice from different groups after 28 days of MI. The results demonstrated that treatment with exosomes effectively and increased the thickness of the left ventricular wall (Figure 3a) and reduced the extent of the infarct area (Figure 3b). Notably, exosomes derived from TNF-α-treated BMSCs exhibited a more pronounced therapeutic effect (Figure 3a,b). The above data indicated that TNF-α-treated BMSCs-derived exosomes could alleviate infarct size in MI-induced cardiac injury mice.
Figure 3.
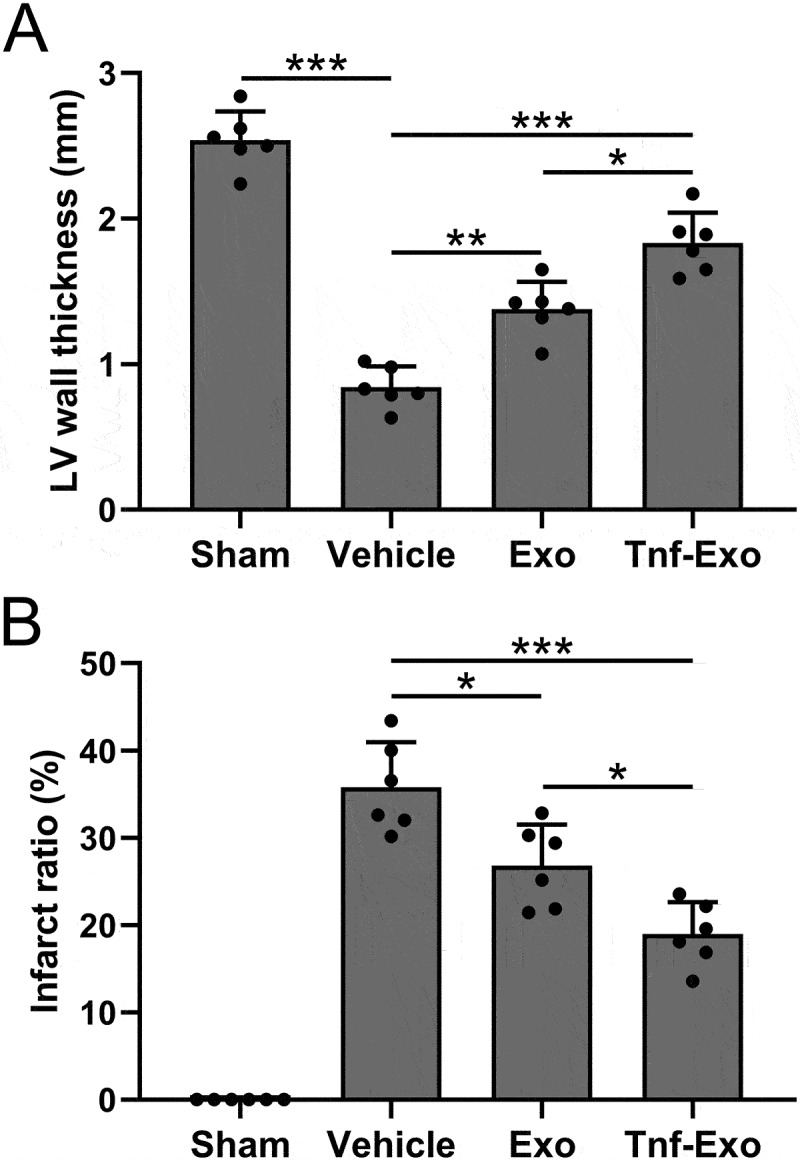
Exosomes derived from TNF-α-treated BMSCs alleviated infarct size after myocardial infarction in mice.
(a) The calculations of left ventricular wall thickness and (b) infarct ratio in the experimental groups. Data were shown with mean ± SD. 6 mice were used for each group. *p < .05, **p < .01, ***p < .001 from Brown-Forsythe ANOVA test followed by Dunnett’s T3 multiple comparisons test.
Exosomes derived from TNF-α-treated BMSCs enhanced M2 macrophage polarization in the myocardial tissues after MI in mice
Next, we analyzed the polarization of macrophages in the infarct area of mice from each group after 28 days of MI. CD206 is a marker of M2 macrophage polarization, while iNOS is a marker of M1 polarization.28,30 The results showed that MI led to the activation of macrophages in the infarcted tissue of mice, with an excessive imbalance between M1 and M2 polarization (Figure 4a,b). However, treatment with exosomes effectively promoted M2 polarization and suppressed M1 polarization (Figure 4a,b). Notably, exosomes derived from TNF-α-treated BMSCs exhibited a more pronounced therapeutic effect (Figure 4a,b).
Figure 4.
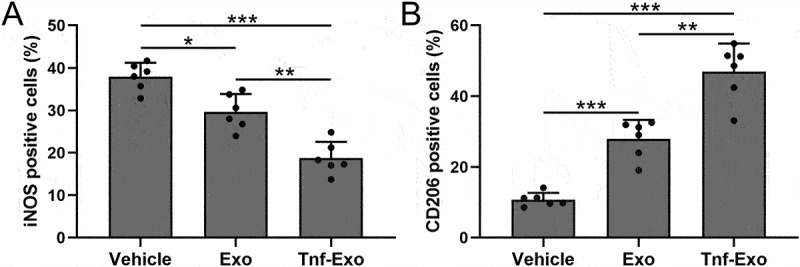
Exosomes derived from TNF-α-treated BMSCs enhanced M2 macrophage polarization in the myocardial tissues after myocardial infarction in mice.
The percentage of M1 (a) and M2 (b) polarized macrophages in the total number of macrophages in the infarct area of myocardial tissues at 28 days post-MI. Data were shown with mean ± SD. 6 mice were used for each group. *p < .05, **p < .01, ***p < .001 from Brown-Forsythe ANOVA test followed by Dunnett’s T3 multiple comparisons test.
Exosomes derived from TNF-α-treated BMSCs enhanced M2 macrophage polarization in the myocardial tissues after MI in mice
We examined the protein levels of the pro-inflammatory cytokine IL-1β, associated with M1 polarization, and the anti-inflammatory cytokine IL-10, associated with M2 polarization, in the infarcted area of mice. The results of our analysis further confirmed that treatment with exosomes effectively promoted M2 polarization while suppressing M1 polarization (Figure 5a,b). Moreover, exosomes derived from TNF-α treated BMSCs exhibited a more significant therapeutic effect (Figure 5a,b). The mRNA expression levels of Cd86 and Cd206, markers of M1 and M2 polarization, respectively, in the infarcted area of mice, provided additional support for the enhanced M2 macrophage polarization by exosomes derived from TNF-α-treated BMSCs in the myocardial tissues following myocardial infarction (Figure 5c,d). These results suggested that TNF-α-treated BMSCs-derived exosomes could enhance M2 macrophage polarization in the myocardial tissues of MI-induced cardiac injury mice.
Figure 5.
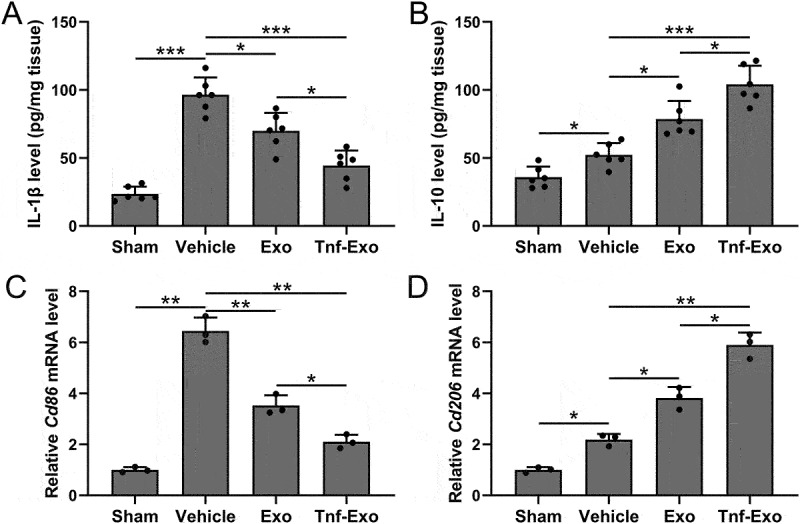
Exosomes derived from TNF-α-treated BMSCs enhanced M2 macrophage polarization in the myocardial tissues after myocardial infarction in mice.
ELISA was used to measure the levels of IL-1β (a) and IL-10 (b) in left ventricular tissues at 28 days post-MI. 6 mice were used for each group. qRT-PCR was used to measure the mRNA expressions of Cd86 (c) and Cd206 (d) in left ventricular tissues at 28 days post-MI. 6 mice were used for each group and the experiments were repeated 3 times using mixed tissue homogenates. Data were shown with mean ± SD. *p < .05, **p < .01, ***p < .001 from Brown-Forsythe ANOVA test followed by Dunnett’s T3 multiple comparisons test.
Discussion
MI poses a formidable challenge due to its detrimental impact on cardiac function, primarily attributed to the loss of viable cardiomyocytes.1 Despite ongoing therapeutic explorations, there remains an imperative for effective strategies to enhance myocardial repair and functional recovery.2,4 In this study, we explored the potential therapeutic role of exosomes derived from TNF-α-treated BMSCs in ameliorating MI injury.
Exosomes, intricate mediators of intercellular communication, have emerged as promising therapeutic agents in the regenerative landscape.11,15 Our results underscored the superior therapeutic efficacy of exosomes from TNF-α-treated BMSCs compared to their untreated counterparts. This observation hints at the modulatory influence of TNF-α treatment on exosomal cargo, heightening their regenerative potential. The multifaceted benefits of exosome treatment were evident on several fronts. First, we observed a significant reduction in myocardial injury markers, including serum levels of LDH, creatine kinase-MB (CK-MB), and troponin I, indicating a protective effect on cardiomyocytes. This finding is consistent with previous studies demonstrating the ability of exosomes to attenuate myocardial damage and preserve cardiac function.15, 23, 24, 31 Furthermore, histological analyses revealed that treatment with exosomes led to a decrease in the infarct area and an increase in left ventricular wall thickness. These findings suggest that exosomes contribute to the inhibition of adverse remodeling processes and promote structural preservation of the myocardium.32,33 The improvement in cardiac function, as substantiated by echocardiography, further bolsters the therapeutic promise of exosomes in MI.
Macrophage polarization plays a crucial role in the inflammatory response following MI.34,35 Excessive M1 polarization and impaired M2 polarization have been associated with adverse cardiac remodeling.36,37 Exosome treatment demonstrated a proclivity toward promoting M2 macrophage polarization while suppressing M1 polarization, as evidenced by altered expression levels of markers such as iNOS and CD206. This shift in polarization potentially contributes to inflammation attenuation and tissue repair promotion. Furthermore, examination of pro-inflammatory cytokine IL-1β and anti-inflammatory cytokine IL-10 in the infarcted area revealed a pronounced increase in IL-10 expression and a reduction in IL-1β levels post-exosome treatment. These findings substantiate the anti-inflammatory and immunomodulatory prowess of exosomes in the MI context.
It is imperative to acknowledge certain limitations. The intricate mechanisms through which exosomes from TNF-α-treated BMSCs exert therapeutic effects necessitate further exploration. Detailed investigation into the specific cargo components, such as proteins and microRNAs, could unravel the molecular intricacies underlying their enhanced regenerative properties. Additionally, delineating the signaling pathways involved in mediating these effects remains an avenue for future studies. Despite these limitations, our findings provide compelling evidence supporting the therapeutic potential of TNF-α-treated BMSCs-derived exosomes in mitigating MI injury and warrant further exploration of their intricate molecular mechanisms.
Conclusion
Our study provides compelling evidence for the therapeutic potential of exosomes derived from TNF-α-treated BMSCs in ameliorating myocardial injury following MI. These exosomes exhibited superior efficacy in preserving cardiac function, reducing myocardial injury, promoting structural preservation, and modulating macrophage polarization. These findings support the notion that exosomes could serve as a promising therapeutic approach for myocardial repair and highlight the importance of TNF-α priming in enhancing the regenerative properties of exosomes derived from BMSCs.
Funding Statement
The study was supported by Hebei Medical Science Research Program (No. 20240119).
Disclosure statement
No potential conflict of interest was reported by the author(s).
Abbreviations
- MSCs
Mesenchymal stem cells
- BMSCs
Bone marrow mesenchymal stem cells
- MI
Myocardial infarction
- MNCs
Mononuclear cells
- MEM α
Minimum Essential Medium α
- PBS
Phosphate-buffered saline
- FBS
Fetal bovine serum
- LVEF
Left ventricular ejection fraction
- LVFS
Left ventricular fractional shortening
- LVIDd
Left ventricular internal dimension at diastole
- LVIDs
Left ventricular internal dimension at systole
- qRT-PCR
Quantitative reverse transcription PCR
- LDH
Lactate dehydrogenase
- CK-MB
Creatine kinase-MB
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
References
- 1.Reed GW, Rossi JE, Cannon CP.. Acute myocardial infarction. Lancet. 2017;389(10065):197–10. doi: 10.1016/S0140-6736(16)30677-8. [DOI] [PubMed] [Google Scholar]
- 2.Salari N, Morddarvanjoghi F, Abdolmaleki A, Rasoulpoor S, Khaleghi AA, Hezarkhani LA, Shohaimi S, Mohammadi M. The global prevalence of myocardial infarction: a systematic review and meta-analysis. BMC Cardiovasc Disord. 2023;23(1):206. doi: 10.1186/s12872-023-03231-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu M, Wang W, Zhang X, Li J. The prevalence of acute stress disorder after acute myocardial infarction and its psychosocial risk factors among young and middle-aged patients. Sci Rep. 2022;12(1):7675. doi: 10.1038/s41598-022-11855-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fioretta ES, Motta SE, Lintas V, Loerakker S, Parker KK, Baaijens FPT, Falk V, Hoerstrup SP, Emmert MY. Next-generation tissue-engineered heart valves with repair, remodelling and regeneration capacity. Nat Rev Cardiol. 2021;18(2):92–116. doi: 10.1038/s41569-020-0422-8. [DOI] [PubMed] [Google Scholar]
- 5.Belien H, Evens L, Hendrikx M, Bito V, Bronckaers A. Combining stem cells in myocardial infarction: the road to superior repair? Med Res Rev. 2022;42(1):343–73. doi: 10.1002/med.21839. [DOI] [PubMed] [Google Scholar]
- 6.Clifford DM, Fisher SA, Brunskill SJ, Doree C, Mathur A, Watt S, Martin-Rendon E. Stem cell treatment for acute myocardial infarction. Cochrane Database Syst Rev. 2012;(2):CD006536. doi: 10.1002/14651858.CD006536.pub3. [DOI] [PubMed] [Google Scholar]
- 7.Fisher SA, Zhang H, Doree C, Mathur A, Martin-Rendon E. Stem cell treatment for acute myocardial infarction. Cochrane Database Syst Rev. 2015;2015(9):CD006536. doi: 10.1002/14651858.CD006536.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cai M, Shen R, Song L, Lu M, Wang J, Zhao S, Tang Y, Meng X, Li Z, He ZX. Bone Marrow Mesenchymal Stem Cells (BM-MSCs) improve heart function in swine myocardial infarction model through paracrine effects. Sci Rep. 2016;6(1):28250. doi: 10.1038/srep31528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miao C, Lei M, Hu W, Han S, Wang Q. A brief review: the therapeutic potential of bone marrow mesenchymal stem cells in myocardial infarction. Stem Cell Res Ther. 2017;8(1):242. doi: 10.1186/s13287-017-0697-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yamada Y, Minatoguchi S, Kanamori H, Mikami A, Okura H, Dezawa M, Minatoguchi S. Stem cell therapy for acute myocardial infarction - focusing on the comparison between Muse cells and mesenchymal stem cells. J Cardiol. 2022;80(1):80–87. doi: 10.1016/j.jjcc.2021.10.030. [DOI] [PubMed] [Google Scholar]
- 11.Krause M, Samoylenko A, Vainio SJ. Exosomes as renal inductive signals in health and disease, and their application as diagnostic markers and therapeutic agents. Front Cell Dev Biol. 2015;3:65. doi: 10.3389/fcell.2015.00065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ala M. The beneficial effects of mesenchymal stem cells and their exosomes on myocardial infarction and critical considerations for enhancing their efficacy. Ageing Res Rev. 2023;89:101980. doi: 10.1016/j.arr.2023.101980. [DOI] [PubMed] [Google Scholar]
- 13.Burke J, Kolhe R, Hunter M, Isales C, Hamrick M, Fulzele S. Stem cell-derived exosomes: a potential alternative therapeutic agent in orthopaedics. Stem Cells Int. 2016;2016:1–6. doi: 10.1155/2016/5802529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Charles CJ, Li RR, Yeung T, Mazlan SMI, Lai RC, de Kleijn DPV, Lim SK, Richards AM. Systemic mesenchymal stem cell-derived exosomes reduce myocardial infarct size: characterization with MRI in a porcine model. Front Cardiovasc Med. 2020;7. doi: 10.3389/fcvm.2020.601990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang S, Dong J, Li L, Wu R, Xu L, Ren Y, Hu X. Exosomes derived from miR-129-5p modified bone marrow mesenchymal stem cells represses ventricular remolding of mice with myocardial infarction. J Tissue Eng Regen Med. 2022;16(2):177–87. doi: 10.1002/term.3268. [DOI] [PubMed] [Google Scholar]
- 16.Das CK, Jena BC, Banerjee I, Das S, Parekh A, Bhutia SK, Mandal M. Exosome as a novel shuttle for delivery of therapeutics across biological barriers. Mol Pharm. 2019;16(1):24–40. doi: 10.1021/acs.molpharmaceut.8b00901. [DOI] [PubMed] [Google Scholar]
- 17.Batrakova EV, Kim MS. Development and regulation of exosome-based therapy products. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2016;8(5):744–57. doi: 10.1002/wnan.1395. [DOI] [PubMed] [Google Scholar]
- 18.Ilaltdinov AW, Gong Y, Leong DJ, Gruson KI, Zheng D, Fung DT, Sun L, Sun HB. Advances in the development of gene therapy, noncoding RNA, and exosome-based treatments for tendinopathy. Ann N Y Acad Sci. 2021;1490(1):3–12. doi: 10.1111/nyas.14382. [DOI] [PubMed] [Google Scholar]
- 19.Gurunathan S, Kang MH, Jeyaraj M, Qasim M, Kim JH. Review of the isolation, characterization, biological function, and multifarious therapeutic approaches of exosomes. Cells. 2019;8(4):307. doi: 10.3390/cells8040307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhao R, Zhao T, He Z, Cai R, Pang W. Composition, isolation, identification and function of adipose tissue-derived exosomes. Adipocyte. 2021;10(1):587–604. doi: 10.1080/21623945.2021.1983242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Heo JS, Choi Y, Kim HO. Adipose-derived mesenchymal stem cells promote M2 macrophage phenotype through exosomes. Stem Cells Int. 2019;2019:1–10. doi: 10.1155/2019/7921760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li YW, Zhang C, Sheng QJ, Bai H, Ding Y, Dou XG. Mesenchymal stem cells rescue acute hepatic failure by polarizing M2 macrophages. World J Gastroenterol. 2017;23(45):7978–88. doi: 10.3748/wjg.v23.i45.7978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hu H, Wu J, Cao C, Ma L. Exosomes derived from regulatory T cells ameliorate acute myocardial infarction by promoting macrophage M2 polarization. Lubmb Life. 2020;72(11):2409–19. doi: 10.1002/iub.2364. [DOI] [PubMed] [Google Scholar]
- 24.Ning H, Chen H, Deng J, Xiao C, Xu M, Shan L, Yang C, Zhang Z. Exosomes secreted by FNDC5-BMMSCs protect myocardial infarction by anti-inflammation and macrophage polarization via NF-kappaB signaling pathway and Nrf2/HO-1 axis. Stem Cell Res Ther. 2021;12(1):519. doi: 10.1186/s13287-021-02591-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xu H, Fu J, Chen L, Zhou S, Fang Y, Zhang Q, Chen X, Yuan L, Li Y, Xu Z. et al. TNF-α enhances the therapeutic effects of MenSC-derived small extracellular vesicles on inflammatory bowel disease through macrophage polarization by miR-24-3p. Stem Cells Int. 2023;2023:1–28. doi: 10.1155/2023/2988907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maridas DE, Rendina-Ruedy E, Le PT, Rosen CJ. Isolation, culture, and differentiation of bone marrow stromal cells and osteoclast progenitors from mice. J Vis Exp. 2018;131:e56750. doi: 10.3791/56750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Borst O, Ochmann C, Schonberger T, Jacoby C, Stellos K, Seizer P, Flogel U, Lang F, Gawaz M. Methods employed for induction and analysis of experimental myocardial infarction in mice. Cell Physiol Biochem. 2011;28(1):1–12. doi: 10.1159/000331708. [DOI] [PubMed] [Google Scholar]
- 28.Choi KM, Kashyap PC, Dutta N, Stoltz GJ, Ordog T, Shea Donohue T, Bauer AJ, Linden DR, Szurszewski JH, Gibbons SJ. et al. CD206-positive M2 macrophages that express heme oxygenase-1 protect against diabetic gastroparesis in mice. Gastroenterology. 2010;138(7):2399–409.e1. doi: 10.1053/j.gastro.2010.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Baudouy D, Michiels JF, Vukolic A, Wagner KD, Wagner N. Echocardiographic and histological examination of cardiac morphology in the mouse. J Vis Exp. 2017;2017(128):e55843. doi: 10.3791/55843-v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang L, Zhang W, Cen R, Yue C, Xiao T, Deng Y, Li L, Sun K, Lei X. ALA-PDT regulates macrophage M1 polarization via ERK/MAPK-NLRP3 pathway to promote the early inflammatory response. Lasers Surg Med. 2022;54(10):1309–20. doi: 10.1002/lsm.23618. [DOI] [PubMed] [Google Scholar]
- 31.Zhu F, Chen Y, Li J, Yang Z, Lin Y, Jiang B, Shao L, Hu S, Shen Z. Human umbilical cord mesenchymal stem cell-derived exosomes attenuate myocardial infarction injury via miR-24-3p-promoted M2 macrophage polarization. Adv Biol (Weinh). 2022;6(11):e2200074. doi: 10.1002/adbi.202200074. [DOI] [PubMed] [Google Scholar]
- 32.Gonzalez A, Ravassa S, Beaumont J, Lopez B, Diez J. New targets to treat the structural remodeling of the myocardium. J Am Coll Cardiol. 2011;58(18):1833–43. doi: 10.1016/j.jacc.2011.06.058. [DOI] [PubMed] [Google Scholar]
- 33.Huang L, Ran L, Zhao P, Tang D, Han R, Ai T, Xia L, Tao Q. MRI native T1 and T2 mapping of myocardial segments in hypertrophic cardiomyopathy: tissue remodeling manifested prior to structure changes. Br J Radiol. 2019;92(1104):20190634. doi: 10.1259/bjr.20190634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim Y, Nurakhayev S, Nurkesh A, Zharkinbekov Z, Saparov A. Macrophage polarization in cardiac tissue repair following myocardial infarction. Int J Mol Sci. 2021;22(5):2715. doi: 10.3390/ijms22052715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mouton AJ, DeLeon-Pennell KY, Rivera Gonzalez OJ, Flynn ER, Freeman TC, Saucerman JJ, Garrett MR, Ma Y, Harmancey R, Lindsey ML. Mapping macrophage polarization over the myocardial infarction time continuum. Basic Res Cardiol. 2018;113(4):26. doi: 10.1007/s00395-018-0686-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jung M, Ma Y, Iyer RP, DeLeon-Pennell KY, Yabluchanskiy A, Garrett MR, Lindsey ML. IL-10 improves cardiac remodeling after myocardial infarction by stimulating M2 macrophage polarization and fibroblast activation. Basic Res Cardiol. 2017;112(3):33. doi: 10.1007/s00395-017-0622-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu W, Zhang X, Zhao M, Zhang X, Chi J, Liu Y, Lin F, Fu Y, Ma D, Yin X. Activation in M1 but not M2 macrophages contributes to cardiac remodeling after myocardial infarction in rats: a critical role of the calcium sensing receptor/NRLP3 inflammasome. Cell Physiol Biochem. 2015;35(6):2483–500. doi: 10.1159/000374048. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.


