Abstract
Astroviruses have been found in cattle and other species with encephalitis. Our objective was to determine the frequency of neurotropic bovine astrovirus (BoAstV) in cases of encephalitis in cattle ≥ 4-mo-old. Of 56 cases of idiopathic lymphocytic encephalitis examined retrospectively (1988–2019), fixed brain from 11 cases (19%) tested positive by semi-quantitative RT-PCR for BoAstV CH13/NeuroS1. None of the control cases tested positive, including 32 with other forms of encephalitis and 40 with no neurologic disease. Most astrovirus-positive cases were 1–2-y-old, with a range of 7 mo to 7 y, and affected both beef and dairy breeds with wide geographic distribution. BoAstV-positive cases had acute onset of neurologic signs of 12 h to 7 d before death or euthanasia. Affected cattle had lymphocytic inflammation throughout the brain including cerebrum, thalamus, midbrain, cerebellum, medulla oblongata, and spinal cord, and affecting gray and white matter. Further PCR testing identified a possible cause in 9 of the 45 (20%) remaining idiopathic cases of lymphocytic encephalitis, including eastern equine encephalitis virus, Listeria monocytogenes, bovine viral diarrhea virus, bovine alphaherpesvirus 1, and ovine gammaherpesvirus 2 (malignant catarrhal fever); we found no cases of infection by West Nile virus, rabies virus, or Chlamydia spp. No cause was identified in 36 of 56 (64%) cases of lymphocytic encephalitis. We frequently identified neurotropic BoAstV in cases of lymphocytic encephalitis that had no previously identified cause. Neurotropic BoAstV infections had gone undetected for decades, but the frequency of BoAstV infections has not increased among contemporary cases.
Keywords: brain, bovine astrovirus, Canada, encephalitis, emerging infectious disease, neurologic
Lymphocytic or lymphoplasmacytic encephalitis is identified by finding lymphocytes and other mononuclear inflammatory cells around blood vessels and in the neuroparenchyma, with or without neuronal necrosis, focal or diffuse microglial proliferation, and increased astrocyte size and number.7,8 Infectious causes of lymphocytic or lymphoplasmacytic encephalitis in cattle (excluding fetuses and neonates) include rabies virus (RABV; Rhabdoviridae, Lyssavirus rabies), which must be ruled out as it causes a fatal zoonosis 22 ; bovine alphaherpesvirus 1 (BoAHV1; Orthoherpesviridae, Varicellovirus bovinealpha1), typically in calves < 6-mo-old19,30; bovine alphaherpesvirus 5 (BoAHV5; Orthoherpesviridae, Varicellovirus bovinealpha5), which causes sporadic bovine necrotizing meningoencephalitis in calves and yearlings11,35; ovine gammaherpesvirus 2 (OvGHV2; Orthoherpesviridae, Macavirus ovinegamma2) causing malignant catarrhal fever; Listeria monocytogenes, particularly in chronic lesions in which neutrophils can be infrequent or absent 33 ; eastern equine encephalitis virus North American (EEEV-NA; Togaviridae, Eastern equine encephalitis virus), for which neutrophils may not be a prominent feature in adult cattle compared to other species8,31; Chlamydia pecorum, which has caused sporadic bovine encephalomyelitis in the United States, typically in calves < 6-mo-old, but recently reported to cause abortion, lymphoplasmacytic encephalitis, and vasculitis in adult cattle 42 ; bovine viral diarrhea virus (BVDV; Flaviviridae, Pestivirus), which causes congenital cerebellar hypoplasia but has been linked to encephalitis in an adult animal 3 ; and neurotropic strains of bovine astrovirus (BoAstV; Astroviridae). West Nile virus (WNV; Flaviviridae, Orthoflavivirus nilense) is also anecdotally reported to cause lymphocytic encephalitis in cattle. 8 Viruses exotic to Canada can also cause lymphocytic encephalitis including suid alphaherpesvirus 1 (formerly pseudorabies virus; Orthoherpesviridae, Varicellovirus suidalpha1), Akabane virus (Peribunyaviridae, Orthobunyavirus akabaneense), and Borna disease virus (Bornaviridae, Orthobornavirus bornaense).9,12,27
European sporadic bovine encephalitis (ESBE) is a type of lymphohistiocytic encephalitis first described in 1961 that is proposed to be caused by neurotropic strains of BoAstV.38,43 The initial discovery of a novel astrovirus in cases of encephalitis in cattle was made in parallel in the United States (BoAstV NeuroS1) and Switzerland (BoAstV CH13).6,28 These viruses are often referred to as BoAstV CH13/NeuroS1 in later literature. In situ hybridization (ISH) on intestinal sections of affected animals was negative, 28 suggesting a novel route of infection rather than atypical spread of an intestinal astrovirus infection. Among historical cases (1958–1976) of ESBE in Switzerland, 12 of 14 tested cases were positive using PCR and ISH for BoAstV CH13/NeuroS1. 38 These new bovine astroviruses were most closely related to the ovine astrovirus prototype6,28, and to human astrovirus VA1 and mink astrovirus SMS that are both associated with neurologic disease.4,34 Subsequent investigation of cases from Switzerland identified a second astrovirus named BoAstV CH15, which had < 65% sequence homology to CH13 but was phylogenetically related to the same human–mink–ovine clade. 40 BoAstV BH89/14, later discovered in Germany in a 15-mo-old cow with neurologic signs and encephalitis, had only 69% sequence homology with BoAstV CH13/NeuroS1. 37
Subsequent reports identified additional cases. In 2 cases of lymphocytic encephalitis in adult cattle in eastern Canada, BoAstV was identified by reverse-transcription PCR (RT-PCR), with 94% homology to BoAstV CH13 and 91% homology to BoAstV NeuroS1. 41 ISH for BoAstV CH13/NeuroS1 labeled neurons in 4 of 9 cases of nonsuppurative encephalitis in feedlot cattle in western Canada. 39 Astrovirus has also been found in cases of encephalitis in cattle in Japan, Uruguay, Italy, and South Korea.16,20,27,45 In the Japanese study, other tissues tested, including lung, liver, and gastrointestinal tissue, were negative for BoAstV, lending further evidence of its neurotropism and shedding no light on possible routes of transmission. 20
Our objectives were 1) to compare the frequency of BoAstV CH13/NeuroS1 infection in cases of lymphocytic encephalitis, other forms of encephalitis, or non-neurologic disease, 2) to summarize the clinical and histologic findings in BoAstV-positive cases, 3) to characterize the frequency of lymphocytic encephalitis associated with BoAstV over time in Ontario, and 4) to identify alternative or concurrent causes of this histologic diagnosis.
Materials and methods
Selection of cases and controls
Postmortem case material and records were examined retrospectively, including cases of lymphocytic encephalitis, other forms of encephalitis, and non-neurologic disease. For all study groups, the inclusion criteria were as follows: cattle that were ≥ 4-mo-old at the time of death, and for which the whole body or tissues had been submitted to the Ontario Veterinary College or the Animal Health Laboratory from a farm in Ontario. Only one animal per premises was included. There were 3 study groups: 1) cattle with a history of neurologic disease and a histologic diagnosis of lymphocytic encephalitis or lymphocytic meningoencephalitis for which a cause was not identified (cases were excluded if inflammation was limited to the meninges); 2) cattle with a history of neurologic disease, and a histologic diagnosis of encephalitis or meningoencephalitis that was not lymphocytic (neutrophilic, histiocytic, or mixed encephalitis), for which a cause was not identified (animals were excluded if inflammation was limited to the meninges); and 3) cattle with no clinical history of neurologic disease and no histologic lesions in the CNS (hemorrhage from captive-bolt euthanasia was not considered a lesion).
Using the above criteria, the Animal Health Laboratory and Ontario Veterinary College pathology archives (January 1998–December 2019) were searched to identify cases and controls. Search terms to identify cattle, histologic lesions in the CNS, and any form of inflammation identified 258 such submissions (1998–2019), of which 50 fit the above criteria of a case of lymphocytic encephalitis, and 54 as other forms of encephalitis. Most of the 154 excluded were because a specific cause of encephalitis had been identified; others had only meningitis without encephalitis, or were too autolyzed. From submissions with no neurologic disease in the archive of 15,838 bovine pathology submissions (1998–2019), we selected 205 based on criteria for age, location, history, and availability of archived slides and paraffin blocks. A random number generator was used to select a similar number of negative control animals (n = 40 in total) matched by year to the lymphocytic encephalitis group.
H&E-stained histologic sections from all groups were evaluated (D Comeau), and questionable or suspect cases were reviewed (D Comeau and JL Caswell or RA Foster) to ensure that sufficient brain tissue was available and that the histologic lesions fit the criteria. As a retrospective study of diagnostic case material, the number and anatomic location of brain sections varied from case to case. For the included cases and controls, the paraffin block for the slide with the most histologically severe lesions was used for PCR testing. For cases with missing histology slides, new 4-μm sections were cut from the paraffin blocks and routinely stained with H&E. Samples were missing for 15 cases and 22 controls and these were excluded. In total, 35 cases of lymphocytic encephalitis, 32 controls with other forms of encephalitis, and 40 controls with no neurologic disease were included in the study from the 1998–2019 archive.
An additional 21 cases of lymphocytic encephalitis were selected from an older archive (1988–1998) using similar search terms and the same inclusion criteria as above. Control animals were not retrieved from this older archive because the objective was to compare the frequency of BoAstV infection in contemporary versus older cases.
Nucleic acid extraction and PCR testing
Samples were tested at the University of Guelph Animal Health Laboratory (AHL; Guelph, Ontario, Canada). Total nucleic acids were extracted from 128 formalin-fixed, paraffin-embedded (FFPE) tissue blocks. Tissue scrolls were prepared, transferred to 1.5-mL tubes, and ATL buffer (939011; Qiagen) followed by proteinase K (19131; Qiagen) and deparaffinization solution (19093; Qiagen) were added and incubated in a shaking heat block. Once samples cooled, 50 μL of the bottom phase was collected, and total nucleic acids were extracted (AM1836, MagMAX viral RNA isolation kit; MagMAX Express-96 magnetic particle processor; Thermo Fisher). Armored RNA enterovirus-like particles (42050; Asuragen) were added to the extraction kit lysis solution as an internal control.
PCR testing for BoAstV CH13/NeuroS1 and CH15 was performed on nucleic acids from 128 blocks, including 35 from the lymphocytic encephalitis group, 32 from the other encephalitis group, and 40 that had no brain disease, and an additional 21 cases of lymphocytic encephalitis from the older archive (for a total of 56 in the lymphocytic encephalitis group). Of the BoAstV-positive cases, only one (case 14) had additional non-neurologic tissues available, and the block containing multiple pieces of small intestine was also tested for BoAstV. The primers and probe for detecting BoAstV in FFPE tissue were designed by the AHL and run as a single-target assay (Suppl. Tables 1, 2). The design was based on sequences obtained from 4 cases of lymphocytic encephalitis and confirmed to have BoAstV via conventional PCR and sequencing. 41 Using these sequences and additional sequences available in GenBank, PCR primers were designed to target the ORF2 of BoAstV CH13/NeuroS1, and the ORF2 of CH15. BLAST analysis (https://blast.ncbi.nlm.nih.gov/Blast.cgi) of these primers and probe confirmed their target. This test was compared with a published assay for bovine neurotropic astroviruses.29,40 The test was run as a triplex assay that included the AHL-designed primers, the primers from the published assay, and the internal control (armored enterovirus). The positive control was case material known to be positive for BoAstV by conventional PCR and sequencing. Serial dilutions made from this control were used to generate a curve. If the cycle threshold (Ct) value for the positive control fell outside of 30 ± 5, the run was discarded and repeated. The negative control was PBS buffer without nucleic acids.
Histology and immunohistochemistry
All BoAstV-positive cases were re-examined histologically to allow a descriptive summary of the distribution and character of the lesions found in cases of astroviral encephalitis. Quantitative analysis of the lesions was not an objective of our study. The 4 recent positive cases (cases 11–14) were further characterized by immunohistochemistry (IHC) to identify the types of leukocytes within the inflammatory foci. Only recent cases were included because of concerns of poor antigen preservation and retrieval in older cases with unknown duration of formalin fixation and prolonged FFPE tissue storage. For each case, the block with the most significant lesions was selected for IHC. The antibodies used included ionized calcium-binding adapter molecule 1 (Iba1; rabbit polyclonal, Waco), CD3 (A0452, rabbit polyclonal; Dako/Agilent), and PAX5 (clone 24 [formerly known as BC/24], mouse monoclonal; Biocare Medical). The negative controls were the sections of brain processed without the primary antibody, and the positive controls were canine lymph node, spleen, and tonsil, as these antibodies were previously validated and used in routine test panels at the AHL.
Cases positive for EEEV and BVDV on PCR testing were further examined using IHC for these agents. BVDV (15C5, mouse monoclonal antibody; Idexx) and EEEV (1A4B-6, mouse monoclonal antibody; Public Health Agency of Canada) were run following the procedures for routine testing as previously validated through the AHL. The negative controls were sections of the brain processed without the primary antibody, and the positive control tissues were equine brain (EEEV) and bovine ear notch tissue (BVDV).
Testing for additional pathogens
Following the PCR results for BoAstV, all cases of lymphocytic encephalitis were tested by PCR for other viruses and bacteria known to cause lymphocytic encephalitis in juvenile or adult cattle in Ontario, including RABV, OvGHV2 BoAHV1, WNV, EEEV, BVDV, L. monocytogenes, and Chlamydia pecorum. Bovine adenovirus (BAdV; Adenoviridae), bovine respiratory syncytial virus (BRSV; Pneumoviridae, Orthopneumovirus bovis), and bovine parainfluenza virus 3 (BPIV3; Paramyxoviridae, Respirovirus bovis) were also tested in these multiplex PCR panels. The samples were tested by previously described real-time PCR methods used routinely at the AHL for BVDV, EEEV, WNV, and RABV.2,24–26,32 Chlamydia (all species) were tested using unpublished primers that were previously validated at the AHL for diagnostic cases (Andre Hamel, pers. comm., 2020). Primers and probes for OvGHV2, BoAHV1, BRSV, and BPIV3 PCR assays were designed in-house (Suppl. Table 1) and had been validated by the AHL. OvGHV2, L. monocytogenes, and the pan-Chlamydia PCR tests were single-target assays; BoAHV1, BRSV, and BPIV3 PCR tests were triplex assays. PCR for all viruses was carried out in 25-µL reactions (Ag Path-ID one-step RT-PCR kit, Thermo Fisher; Light Cycler 480, Roche; Suppl. Table 2). Samples with Ct < 37 were considered positive. For L. monocytogenes testing, the PCR was performed (208454, QuantiNova multiplex PCR kit; Qiagen) as described previously 18 (Suppl. Tables 3, 4).
Statistical analysis
A chi-square test was used to compare the frequency of positive versus negative BoAstV results between the cases and each control group. To compare the frequency of BoAstV over time, the cases were divided into 2 groups encompassing the same number of years (1990 to mid-2005 and mid-2005 to 2019) and tested by chi-square. Analyses were performed in Prism (v.6; GraphPad).
Results
Frequency of astroviral infection
Of the 35 cases of previously diagnosed idiopathic lymphocytic encephalitis (1998–2019), 4 cases (11%) were positive by RT-qPCR for BoAstV CH13/NeuroS1. The Ct for these cases was 30.3–35.1. One case occurred in 2017 (case 14), 2 in 2016 (cases 12, 13), and 1 in 2014 (case 11; Table 1, Suppl. Table 5). All cases had severe lymphocytic inflammation throughout all sections of the brain examined, including cerebrum, thalamus, midbrain, cerebellum, and medulla oblongata (described in more detail below). The intestinal tissue available from case 14 tested negative. None of the samples tested positive for BoAstV CH15.
Table 1.
Animal, clinical, and laboratory details for bovine lymphocytic encephalitis cases that tested positive for bovine astrovirus CH13/NeuroS1.
| Case | Year | Age | Sex | Breed | Area of Ontario | Clinical signs | Brain region tested | BoAstV PCR Ct | Coinfection(s) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 1990 | 12 mo | M | Limousin cross | Unknown | 12-h history of opisthotonos and loss of sensation in hindlimbs | Thalamus | 36.8 | BVDV |
| 2 | 1990 | 15 mo | F | Holstein | Southwest | Ongoing herd production losses, neurologic signs not specified | Cerebrum | 36.5 | EEEV |
| 3 | 1994 | 12 mo | F | Hereford | Northeast | 3-d history of ataxia, 12-h history of blindness | Cerebrum | 27.6 | NA |
| 4 | 1995 | 5 y | F | Holstein | South | 24-h history of mania, followed by an inability to rise and trembling | Thalamus | 24.7 | BCoV |
| 5 | 1995 | 16 mo | F | Holstein | South | Sudden death | Cerebrum | 36.5 | BCoV |
| 6 | 1996 | 12 mo | M | Limousin | Southeast | Repetitive yawning and tongue movements, crouching | Thalamus | 35.5 | NA |
| 7 | 1996 | 15 mo | F | Simmental | Southeast | 4-d history of depression, ataxia, and weakness | Thalamus | 28.4 | BCoV |
| 11 | 2014 | 7 mo | M | Beef breed | Southwest | Fell into feedbunk 1 wk prior to euthanasia, 1 wk history of ataxia | Cerebellum | 35.1 | NA |
| 12 | 2016 | 2 y | M | Limousin | South | Ongoing herd production losses, neurologic signs not specified | Medulla oblongata, cerebrum | 34.4 | NA |
| 13 | 2016 | 12 mo | M | Hereford | Southwest | Unspecified period of tonic-clonic seizures, repetitive licking and chewing movements | Midbrain | 32.8 | BCoV |
| 14 | 2017 | 7 y | F | Holstein | Southeast | 2-d history of paddling, staggering, tremors, hyper-excitability, and circling | Midbrain | 30.3 | OvGHV2, BCoV |
BoAstV = bovine astrovirus CH13/NeuroS1; BCoV = bovine coronavirus; BVDV = bovine viral diarrhea virus; Ct = cycle threshold; EEEV = eastern equine encephalitis virus; F = female; M = male; NA = not applicable; OvGHV2 = ovine gammaherpesvirus 2 (malignant catarrhal fever). Case numbers are as listed in Suppl. Table 5.
None of the animals with other forms of encephalitis (n = 32) or without encephalitis (n = 40) tested positive for BoAstV. The frequency of BoAstV-positive PCR tests in animals with lymphocytic encephalitis (4 of 35) was significantly different from those with other forms of encephalitis (p = 0.048, chi-square test) and those without encephalitis (p = 0.028).
Of the additional 21 older cases of lymphocytic encephalitis (1988–1998), 7 (33%) tested positive. An additional 3 cases (14%) were inconclusive with Ct of 37.1, 38.5, and 39.2; all 3 cases tested negative on repeat testing of new scrolls, from different blocks of brain if available, and were thus considered negative. Thus, in total (1988–2019), 56 cases of idiopathic lymphocytic encephalitis were tested via RT-qPCR for BoAstV, and 11 (19%; 95% CI: 10–32%) were positive (Table 1; Suppl. Table 5).
The frequency of BoAstV-positive cases was compared between older (1990 to mid-2005) and more recent cases (mid-2005 to 2020). There was no significant difference in the proportion of astrovirus infections in cases from 1990–2005 (7 of 34; 21%) versus 2005–2020 (4 of 22, 18%; p = 0.82, chi-square test).
Characterization of BoAstV-positive cases
Of the 11 BoAstV-positive animals, 1 was < 1-y-old (7 mo), 8 were 1–2-y-old, and 2 were > 2-y-old. Both sexes, dairy and beef breeds, and different regions of the province were represented (Table 1). One case was found dead; the others with available information had neurologic signs of at least 12 h duration, with a disease course lasting up to 1 wk before death or euthanasia (Table 1).
The hallmark histologic lesions of viral encephalitis, which formed the inclusion criteria for lymphocytic encephalitis cases in our study, include perivascular cuffing by lymphocytes with or without plasma cells, diffuse and/or focal gliosis, and neuronal necrosis.7,8 Cases varied in the number of sections and regions of brain available. The BoAstV-positive cases had lesions in all levels of the brain, including 9 of 11 sections of cerebrum, 5 of 6 of thalamus, 9 of 10 of midbrain, 5 of 7 of cerebellum, and 4 of 5 of medulla oblongata (Table 2). Perivascular cuffs were present in both the gray and white matter, often at the border between these 2 regions. Overall, perivascular cuffing was more frequent in the white matter (Figs. 1, 2), but the predominance of gray versus white matter lesions varied among cases and among brain regions within individual cases. The perivascular cuffs ranged from subtle aggregates of small lymphocytes to florid cuffs up to 15 cells thick, composed predominantly of small lymphocytes with admixed large lymphocytes and rare plasma cells (Figs. 1, 2). Occasionally, large perivascular cuffs contained nuclear debris, suggesting cell death, and rare individual neutrophils. Lymphocytes occasionally extended out of the perivascular space into the adjacent neuroparenchyma. Lymphocytes frequently formed cuffs around leptomeningeal vessels and aggregates within the leptomeninges. In both the meninges and brain parenchyma, affected blood vessels were often lined by plump, reactive endothelium, but vasculitis was not identified. The ependyma of the ventricles was unaffected when present (artifactual loss was common in the examined sections), and lesions in the periventricular neuroparenchyma were limited.
Table 2.
Anatomic localization of inflammation and neuronal damage for bovine astrovirus–positive cases, and summary of brain sections available.
| Case | Cerebrum | Thalamus | Midbrain | Cerebellum | Medulla | No. of sections |
|---|---|---|---|---|---|---|
| 1 | Yes* | Yes | Yes* | Yes | Yes | 5 |
| 2 | No | No | Yes | No | No | 5 |
| 3 | Yes* | NA | NA | NA | NA | 1 |
| 4 | Yes* | NA | Yes* | Yes* | NA | 3 |
| 5 | Yes | NA | No | No | NA | 3 |
| 6 | No | NA | Yes | NA | NA | 2 |
| 7 | Yes | NA | Yes* | NA | NA | 2 |
| 11 | Yes* | Yes* | Yes* | Yes | Yes | 5 |
| 12 | Yes | Yes | Yes* | Yes | Yes* | 5 |
| 13 | Yes* | Yes* | Yes | NA | Yes | 4 |
| 14 | Yes | Yes | Yes | Yes* | NA | 4 |
| Total | 11 | 6 | 10 | 7 | 5 | 39 |
Medulla = medulla oblongata; NA = no tissue available; No = tissue present, no inflammation present; Yes = tissue present, inflammation present. Case numbers are as listed in Suppl. Table 5.
Neuronal necrosis present.
Figures 1–6.
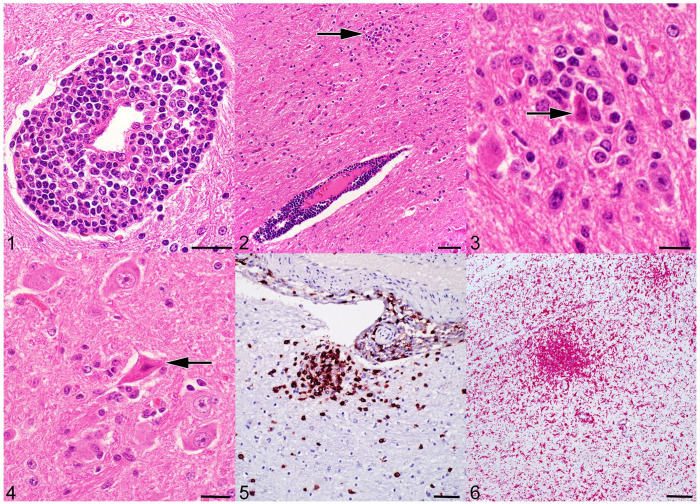
Encephalitis in bovine astrovirus (BoAstV)-positive cattle. Figure 1. Perivascular cuff of leukocytes. H&E. Bar = 20 μm. Figure 2. Perivascular cuff of leukocytes near a glial nodule (arrow), in the region of the thalamus and basal nuclei. H&E. Bar = 50 μm. Figure 3. Higher magnification of Fig. 2; a glial nodule with neuronophagia (arrow). H&E. Bar = 10 μm. Figure 4. Necrotic neuron with pyknotic nucleus and shrunken, angular, hypereosinophilic cytoplasm (arrow), and gliosis of the neuroparenchyma. H&E. Bar = 20 μm. Figure 5. CD3-immunoreactive T lymphocytes in a perivascular cuff and in a glial nodule, with scattered CD3-positive T cells in the adjacent neuroparenchyma. Midbrain. Immunohistochemistry (IHC) for CD3. Bar = 50 μm. Figure 6. A glial nodule contains Iba1-immunoreactive macrophages. Dispersed Iba1-positive cells in the surrounding neuroparenchyma are consistent with microglia. Cerebrum. IHC for Iba1. Bar = 100 μm.
Glial nodules were occasionally present in the gray matter adjacent to perivascular cuffs, or more rarely as isolated lesions within the gray matter (Fig. 2). Neuronophagia was not prominent but was noted within these nodules in several cases (Figs. 2, 3). Individual dead neurons were present in 8 of 11 cases and located throughout the neuroparenchyma in all regions of the brain. Affected neurons ranged from swollen, pale cells with loss of Nissl substance, to shrunken, angular, and hypereosinophilic, with loss of nuclear detail consistent with cell death (Fig. 4). In some older cases (cases 2, 5), histologic lesions consisted only of rare, subtle cuffs or of minimal foci of gliosis and individual necrotic neurons.
In the 4 recent positive cases (cases 11–14), the cell populations were further characterized by IHC for CD3 (T lymphocytes), PAX5 (B lymphocytes), and Iba1 (microglia and macrophages). Most cells within the perivascular cuffs and the glial nodules had strong membranous immunoreactivity for CD3. The estimated percentage of CD3-positive cells within individual cuffs was 30–90%; 10–80% of the glial nodules were of CD3-positive cells (Fig. 5). Scattered individual CD3-positive cells were present throughout the neuroparenchyma. There were frequent cells with strong membranous immunoreactivity for Iba1 in the perivascular cuffs, comprising 10–30% of the cells. The glial nodules frequently contained many Iba1-immunopositive cells, which comprised up to 75% of the cells (Fig. 6). Additionally, there were numerous strongly Iba1-immunoreactive cells throughout the neuroparenchyma, consistent with microglia. These neuroparenchymal Iba1-positive cells often formed denser clusters in areas of marked perivascular cuffing and glial nodule formation, typical of focal gliosis. The perivascular cuffs and glial nodules also had a few individual cells with moderate nuclear immunoreactivity for PAX5; ~50% of the perivascular cuffs had cells with weak-to-moderate immunoreactivity for PAX5, comprising 5–15% of the cuff.
In the one available section of spinal cord (case 14), there were frequent perivascular cuffs of lymphocytes and fewer plasma cells located predominantly in the gray matter of the dorsal horns and rarely of the ventral horns.
Testing for additional pathogens
Of the 45 cases of idiopathic lymphocytic encephalitis that tested negative for BoAstV, 1 or more agents were identified by additional PCR testing in 9 (20%) cases (Table 3; Suppl. Table 5). One was positive for OvGHV2 (case 38), 1 for BoAHV1 (case 28), 3 for BVDV (cases 21, 27, 49), 3 for EEEV (cases 27, 46, 55), and 2 for L. monocytogenes (case 47 [which was also positive for EEEV], case 50). No samples tested positive for WNV, RABV, or Chlamydia spp.
Table 3.
Cases of lymphocytic encephalitis in which additional pathogens were identified. Ct values are shown for cases with positive tests. Bovine astrovirus–positive cases that were negative for other pathogens are not included in this table. Coronavirus and adenovirus were not included as these were not considered causes of encephalitis.
| Case | Year | BoAHV1 | BoAstV | BVDV | EEEV | OvGHV2 | Listeria |
|---|---|---|---|---|---|---|---|
| 1 | 1990 | – | 36.8 | 28.5 | – | – | – |
| 2 | 1990 | – | 36.5 | – | 32.6 | – | – |
| 14 | 2017 | – | 30.3 | – | – | 36.3 | – |
| 21 | 2002 | – | – | 30.9 | – | – | – |
| 27 | 2001 | – | – | 31.8 | – | – | – |
| 28 | 2001 | 34.6 | – | – | – | – | – |
| 38 | 2014 | – | – | – | – | 30.0 | – |
| 46 | 1991 | – | – | – | 32.9 | – | – |
| 47 | 1992 | – | – | – | 32.4 | – | 31.3 |
| 49 | 1993 | – | – | 26.1 | – | – | – |
| 50 | 1993 | – | – | – | – | – | 34.8 |
| 55 | 1998 | – | – | – | 32.8 | – | – |
BoAHV1 = bovine alphaherpesvirus 1; BoAstV = bovine astrovirus CH13/NeuroS1; BVDV = bovine viral diarrhea virus; EEEV = eastern equine encephalitis virus; Listeria = Listeria monocytogenes; OvGHV2 = ovine gammaherpesvirus 2 (malignant catarrhal fever); – = negative.
Case numbers are as listed in Suppl. Table 5.
Of the 11 cases that tested positive for BoAstV, 1 also tested positive for BVDV (case 1), 1 for EEEV (case 2), and 1 for OvGHV2 (malignant catarrhal fever; case 14; Table 3). IHC for EEEV and BVDV on brain sections from 1 of the EEEV-positive cases and 3 of the BVDV-positive cases was done to further investigate these agents. The EEEV case was negative with a scant amount of nonspecific background labeling but no specific immunolabeling. For BVDV, one case had strong immunolabeling throughout all neurons and some of the endothelium, one case had scattered moderate labeling of some neurons, and one case had no immunolabeling.
No samples tested positive for either BRSV or BPIV3, which were included as part of the respiratory panel used to test for BoAHV1. Bovine coronavirus (BCoV; Coronaviridae, Betacoronavirus 1) and BAdV were included as part of the gastrointestinal panel used to test for BVDV. Five of 11 BoAstV-positive cases (cases 4, 5, 7, 13, 14) and 2 of 11 BoAstV-negative cases (case 47 [also positive for EEEV and L. monocytogenes], case 53) tested positive for BCoV. One of 45 BoAstV-negative cases tested positive for BAdV (case 46, also positive for EEEV).
Discussion
Our main objective was to identify the frequency of BoAstV infection in cases of idiopathic lymphocytic encephalitis in cattle, compared to that in cattle with other forms of encephalitis or no encephalitis. Eleven of 56 cases of idiopathic lymphocytic encephalitis tested positive for BoAstV (19%; 95% CI: 10–32%). Cases 12–14 were reported previously. 41 Most cases had neurologic signs of up to one-week duration, which frequently included ataxia. There was perivascular cuffing throughout all levels of the brain examined. In contrast, none of the cattle with other forms of encephalitis (e.g., suppurative encephalitis) tested positive, nor did any of the cattle that had no brain disease. Failure to find BoAstV in any of the cattle that died with no neurologic disease or with other types of encephalitis suggests that BoAstV is not widely present in brain tissue of cattle. Combined with previous studies that co-localized viral antigen or nucleic acid within the histologic lesions, 5 our results further support BoAstV as a cause of lymphocytic encephalitis in cattle. Furthermore, if the archived laboratory population is representative of the general population, then this suggests that BoAstV CH13/NeuroS1 is an important cause of encephalitis in cattle in Ontario. BoAstV CH15, associated with some cases of disease in Europe, was not detected in our sample of this population. We found that BoAstV has been present in Ontario cattle since at least 1990, but there was no significant difference in the number of astrovirus-positive animals in 1990–2005 versus 2005–2020. Therefore, we did not identify any evidence that the disease is becoming more or less frequent.
In our study, the positive cases appeared to cluster, with an 18-y gap (1996–2014) with no positive cases. The reason for the gap in BoAstV cases is unclear. As positive cases were found as early as 1990, degradation of RNA in stored samples is unlikely to be the cause for multiple false-negative samples in 1996–2014. A greater frequency of coinfections is a possible explanation because detecting another causative agent would have excluded the case from our study.
Examination of the BoAstV-positive cases indicated variation in lesion severity. Cases with mild lesions were limited to the older archived material. The cause for this difference compared to more recent cases is unknown. Occasional cases had minimal lesions consisting of 1–2 small perivascular cuffs and rare neuronal necrosis. These milder cases might represent an earlier stage of disease before development of fulminant lesions, or alternatively represent resolution of older infections. It is also possible that more florid lesions were present in other sections because not all of the slides and blocks could be located. This limitation might also explain why the distribution of lesions in our study was slightly more rostral than described in previous studies28,39 (perhaps because the caudal brain regions were under-represented in our study).
The lymphocyte population within the brain was overwhelmingly composed of T lymphocytes. Although T-lymphocyte subsets were not investigated, these could include cytotoxic T lymphocytes, which are key players in the immune response to viral infection. 21 Their presence in the glial nodules suggests that they could be acting directly to kill virus-infected cells in the brain. The T lymphocytes could also include helper T lymphocytes, which produce cytokines that stimulate inflammatory responses and direct the activation of other T-cell subsets, B cells, and macrophages. 44
To further investigate the remaining cases of idiopathic lymphocytic encephalitis, samples were tested by PCR assays for other known and plausible causes. PCR testing detected a potential cause in 9 (20%) of the 45 BoAstV-negative cases tested. This left 36 of our initial 56 (64%) cases in the lymphocytic encephalitis group as idiopathic despite extensive testing. This is consistent with the historical difficulty in identifying the causes of lymphocytic encephalitis.17,36 For example, the human California encephalitis project (1998–2000) found a cause in only 12% of 334 human cases. 17 Excluding those cases considered to be autoimmune, 62% of encephalitis cases had no known cause. Another study tested 37 cattle with “idiopathic non-suppurative” encephalitis and found a cause in only one. 36 Thus, there are still many cases of idiopathic bovine encephalitis that could represent unknown pathogens, non-infectious causes, clearance of the pathogen before death, or degradation of microbial nucleic acid in archived samples.10,14
The limited positive results for L. monocytogenes and OvGHV2 in our study might reflect that cases with an etiologic diagnosis when the case was first examined would have been excluded from the study. It is notable that positive results for L. monocytogenes were only found in the material from the older archive (1990–1998), which may reflect improved availability or sensitivity of IHC and PCR testing over time. Thus, our data do not represent the overall frequency of these additional pathogens among cattle with lymphocytic encephalitis.
The number of positive results for EEEV and BVDV highlights the importance of testing for these uncommon agents when more common causes have been eliminated. There are rare case reports of eastern equine encephalitis in cattle, which describe lesions of lymphocytic or lymphohistiocytic perivascular cuffs with gliosis. 31 The EEEV-positive cases were from different areas of Ontario, perhaps related to the widespread distribution of the insect vectors that spread this agent. In 2 of the 4 EEEV-positive cases, an additional cause was found (BoAstV, L. monocytogenes). BVDV is best known for causing congenital cerebellar hypoplasia and hydrocephalus. BVDV antigen has been detected in the brains of persistently infected adult cattle that had no neurologic disease or histologic lesions. 15 A case report attributed neurologic disease to BVDV infection in an adult cow with lymphohistiocytic perivascular cuffs in the forebrain, with relative sparing of the cerebellum and medulla oblongata. 3 In our BVDV-positive cases, all had lesions in the forebrain, with fewer lesions in the cerebellum and medulla oblongata. Not all cases had hindbrain available for examination. The perivascular cuffs were predominantly located in the white matter. The extensive labeling in one case (case 1) is suspected to represent a persistently infected animal due to the diffuse nature of the neuronal labeling in areas with and without inflammatory lesions, consistent with reports of the distribution of viral antigen in persistently infected animals. 15 Therefore, the encephalitis in case 1 is presumed to be caused by BoAstV and not BVDV. BVDV is a possible cause of encephalitis in the other positive case (case 27), as it had patchy scattered immunolabeling of neurons for BVDV and no other agents were detected. The PCR-positive, IHC-negative case (case 21) may reflect absence of viral antigen at the time of death, or that the distribution of antigen was patchy and was not detected in the single block examined.
No cattle in our study tested positive for WNV, which has been anecdotally reported as a possible cause of encephalitis in cattle 8 and was thus tested in our study. Antibodies to WNV have been found rarely in cattle in Ontario in serologic studies 1 ; however, we did not identify any primary published studies describing encephalitis in cattle due to WNV infection.
The BAdV- and BCoV-positive cases were detected because these were tested as part of a PCR panel. These viruses were not considered causal because they are not known causes of encephalitis in cattle, and all but one also tested positive for another known cause of encephalitis (BoAstV, EEEV, L. monocytogenes, or OvHGV2). We included only cattle > 4-mo-old in our study, and we did not test for BoAHV5 or Neospora caninum because these are not known to cause encephalitis in juvenile or adult cattle.
Three of our BoAstV-positive cases tested positive for another known cause of encephalitis (OvGHV2, EEEV, BVDV). One EEEV-positive, BoAstV-negative case also tested positive for L. monocytogenes. We found no published evidence of synergism among viral infections causing encephalitis in cattle. Reports are rare even in human cases with severe immunocompromise.13,23 The cattle in our study had no evidence of immunosuppressive conditions. In the case with BoAstV and OvGHV2, the absence of vasculitis or clinical signs compatible with malignant catarrhal fever suggests that OvGHV2 infection may have been incidental. In the case with BoAstV and EEEV infection, it is uncertain which was the cause of encephalitis because the expected clinical and histologic presentations are similar.
The use of archived laboratory samples introduced some limitations to our study. Laboratory archives are subject to submission biases that could alter the apparent frequency of the disease, and the excluded cases introduced potential bias to the study population. However, the population covered by the archived material represents a reasonable cross-section of cattle across the province with respect to geographic location, types of operations (dairy and beef), and breeds of cattle. Selection criteria ensured that specific herds were not overrepresented. The availability of brain tissue for testing is a key benefit to archived samples, which is especially useful because encephalitis is an uncommon diagnosis. Because the overall prevalence of BoAstV encephalitis is low, this approach provided more cases than a prospective study. Furthermore, the archive allowed investigation of how long neurotropic BoAstV has been present in the Ontario cattle population.
Knowledge of BoAstV as a common cause of bovine neurologic disease can allow clinicians and diagnostic laboratories to better pursue appropriate testing in suspect cases, and lead to more frequent definitive diagnoses in cases of encephalitis. Given that rabies is an important differential diagnosis for lymphocytic encephalitis, an alternative diagnosis of BoAstV encephalitis could inform preventive interventions for in-contact humans and animals. For some causes of lymphocytic encephalitis, the circumstances and route of infection are well known, such as exposure to sheep for OvGHV2, mosquito bites for WNV and EEEV, and animal bites for RABV. For neurotropic BoAstV, the method of transmission and route of infection are not known. A fecal–oral route is the method of transmission for enteric astroviral strains, but it is not known if neurotropic astroviruses are transmitted by the same route. Previous studies have not detected neurotropic BoAstV in the bovine digestive tract,20,28 nor was it detected in the single case with available intestinal tissue in our study (case 14). Our findings, along with prior studies, establish neurotropic BoAstV as a frequent cause of lymphocytic encephalitis in cattle, and emphasize that many cases with this lesion remain idiopathic despite improvements in diagnostic test methods.
Supplemental Material
Supplemental material, sj-pdf-1-vdi-10.1177_10406387241237192 for Bovine astrovirus and its role in lymphocytic encephalitis in cattle in Ontario, Canada, 1988–2019 by Dominique Comeau, Maria T. Spinato, Davor Ojkic, Robert A. Foster and Jeff L. Caswell in Journal of Veterinary Diagnostic Investigation
Acknowledgments
We thank Anna Marom for technical assistance with PCR testing, Jan Sargeant for assistance with study design, and Brandon Plattner for contributions to initial development of the project.
Footnotes
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: Funding for this project was provided by the Ontario Agrifood Innovation Alliance through the Ontario Ministry of Agriculture, Food, and Rural Affairs (2018-3086).
ORCID iDs: Davor Ojkic  https://orcid.org/0000-0002-4429-4843
https://orcid.org/0000-0002-4429-4843
Jeff L. Caswell  https://orcid.org/0000-0002-1991-3219
https://orcid.org/0000-0002-1991-3219
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Dominique Comeau, Department of Pathobiology, University of Guelph, Guelph, Ontario, Canada; Animal Health Laboratory, University of Guelph, Guelph, Ontario, Canada.
Maria T. Spinato, Animal Health Laboratory, University of Guelph, Guelph, Ontario, Canada
Davor Ojkic, Animal Health Laboratory, University of Guelph, Guelph, Ontario, Canada.
Robert A. Foster, Department of Pathobiology, University of Guelph, Guelph, Ontario, Canada
Jeff L. Caswell, Department of Pathobiology, University of Guelph, Guelph, Ontario, Canada.
References
- 1. Allen SE, et al. Serologic evidence of arthropod-borne virus infections in wild and captive ruminants in Ontario, Canada. Am J Trop Med Hyg 2020;103:2100–2107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Baxi M, et al. A one-step multiplex real-time RT-PCR for detection and typing of bovine viral diarrhea viruses. Vet Microbiol 2006;116:37–44. [DOI] [PubMed] [Google Scholar]
- 3. Blas-Machado U, et al. Bovine viral diarrhea virus type 2-induced meningoencephalitis in a heifer. Vet Pathol 2004;41:190–194. [DOI] [PubMed] [Google Scholar]
- 4. Blomström A-L, et al. Detection of a novel astrovirus in brain tissue of mink suffering from shaking mink syndrome by use of viral metagenomics. J Clin Microbiol 2010;48:4392–4396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Boujon CL, et al. Development and validation of an immunohistochemistry procedure for the detection of a neurotropic bovine astrovirus. J Virol Methods 2017;239:26–33. [DOI] [PubMed] [Google Scholar]
- 6. Bouzalas IG, et al. Neurotropic astrovirus in cattle with nonsuppurative encephalitis in Europe. J Clin Microbiol 2014;52:3318–3324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Callan RJ, Van Metre DC. Viral diseases of the ruminant nervous system. Vet Clin North Am Food Anim Pract 2004;20:327–362. [DOI] [PubMed] [Google Scholar]
- 8. Cantile C, Youssef S. Nervous system. In: Maxie MG, ed. Jubb, Kennedy & Palmer’s Pathology of Domestic Animals. 6th ed. Vol. 1. Elsevier, 2016:350–396. [Google Scholar]
- 9. Caplazi P, et al. Borna disease in naturally infected cattle. J Comp Pathol 1994;111:65–72. [DOI] [PubMed] [Google Scholar]
- 10. Debiasi RL, Tyler KL. Molecular methods for diagnosis of viral encephalitis. Clin Microbiol Rev 2004;17:903–925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. d’Offay JM, et al. Diagnosis of encephalitic bovine herpesvirus type 5 (BHV-5) infection in cattle: virus isolation and immunohistochemical detection of antigen in formalin-fixed bovine brain tissues. J Vet Diagn Invest 1995;7:247–251. [DOI] [PubMed] [Google Scholar]
- 12. Dow C, McFerran JB. The pathology of Aujeszky’s disease in cattle. J Comp Pathol Ther 1962;72:337–347. [Google Scholar]
- 13. Dupuis M, et al. Molecular detection of viral causes of encephalitis and meningitis in New York State. J Med Virol 2011;83:2172–2181. [DOI] [PubMed] [Google Scholar]
- 14. Evers DL, et al. Paraffin embedding contributes to RNA aggregation, reduced RNA yield, and low RNA quality. J Mol Diagn 2011;13:687–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fernandez A, et al. Viral antigen distribution in the central nervous system of cattle persistently infected with bovine viral diarrhea virus. Vet Pathol 1989;26:26–32. [DOI] [PubMed] [Google Scholar]
- 16. Giannitti F, et al. The first case of bovine astrovirus-associated encephalitis in the southern hemisphere (Uruguay), uncovers evidence of viral introduction to the Americas from Europe. Front Microbiol 2019;10:1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Glaser CA, et al. In search of encephalitis etiologies: diagnostic challenges in the California encephalitis project, 1998–2000. Clin Infect Dis 2003;36:731–742. [DOI] [PubMed] [Google Scholar]
- 18. Hage E, et al. Identification of six Listeria species by real-time PCR assay. Lett Appl Microbiol 2014;58:535–540. [DOI] [PubMed] [Google Scholar]
- 19. Hall WT, et al. The pathogenesis of encephalitis caused by the infectious bovine rhinotracheitis virus. Aust Vet J 1966;42:229–237. [DOI] [PubMed] [Google Scholar]
- 20. Hirashima Y, et al. Whole genome analysis of a novel neurotropic bovine astrovirus detected in a Japanese black steer with non-suppurative encephalomyelitis in Japan. Arch Virol 2018;163:2805–2810. [DOI] [PubMed] [Google Scholar]
- 21. Juno JA, et al. Cytotoxic CD4 T cells—friend or foe during viral infection? Front Immunol 2017;8:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. King AA, Turner GS. Rabies: a review. J Comp Pathol 1993;108:1–39. [DOI] [PubMed] [Google Scholar]
- 23. Kumar M, et al. Acute encephalitis syndrome child patient with multi-viral co-infection: a rare case report. J Med Allied Sci 2019;9:100–102. [Google Scholar]
- 24. La Rocca SA, Sandvik T. A short target real-time RT-PCR assay for detection of pestiviruses infecting cattle. J Virol Methods 2009;161:122–127. [DOI] [PubMed] [Google Scholar]
- 25. Lambert AJ, et al. Detection of North American eastern and western equine encephalitis viruses by nucleic acid amplification assays. J Clin Microbiol 2003;41:379–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lanciotti RS, et al. Rapid detection of West Nile virus from human clinical specimens, field-collected mosquitoes, and avian samples by a TaqMan reverse transcriptase-PCR assay. J Clin Microbiol 2000;38:4066–4071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lee JK, et al. Encephalomyelitis associated with Akabane virus infection in adult cows. Vet Pathol 2002;39:269–273. [DOI] [PubMed] [Google Scholar]
- 28. Li L, et al. Divergent astrovirus associated with neurologic disease in cattle. Emerg Infect Dis 2013;19:1385–1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lüthi R, et al. Accurate and precise real-time RT-PCR assays for the identification of astrovirus associated encephalitis in cattle. Sci Rep 2018;8:9215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Marin MS, et al. Distribution of bovine herpesvirus type 1 in the nervous system of experimentally infected calves. Vet J 2016;209:82–86. [DOI] [PubMed] [Google Scholar]
- 31. McGee ED, et al. Eastern equine encephalomyelitis in an adult cow. Vet Pathol 1992;29:361–363. [DOI] [PubMed] [Google Scholar]
- 32. Nadin-Davis SA, et al. Development of real-time reverse transcriptase polymerase chain reaction methods for human rabies diagnosis. J Med Virol 2009;81:1484–1497. [DOI] [PubMed] [Google Scholar]
- 33. Oevermann A, et al. Neuropathogenesis of naturally occurring encephalitis caused by Listeria monocytogenes in ruminants. Brain Pathol 2010;20:378–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Quan PL, et al. Astrovirus encephalitis in boy with X-linked agammaglobulinemia. Emerg Infect Dis 2010;16:918–925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Rissi DR, et al. Neurological disease in cattle in southern Brazil associated with Bovine herpesvirus infection. J Vet Diagn Invest 2008;20:346–349. [DOI] [PubMed] [Google Scholar]
- 36. Sánchez S, et al. A retrospective study of non-suppurative encephalitis in beef cattle from western Canada. Can Vet J 2013;54:1127–1132. [PMC free article] [PubMed] [Google Scholar]
- 37. Schlottau K, et al. Detection of a novel bovine astrovirus in a cow with encephalitis. Transbound Emerg Dis 2016;63:253–259. [DOI] [PubMed] [Google Scholar]
- 38. Selimovic-Hamza S, et al. Detection of astrovirus in historical cases of European sporadic bovine encephalitis, Switzerland 1958–1976. Front Vet Sci 2016;3:91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Selimovic-Hamza S, et al. Bovine astrovirus infection in feedlot cattle with neurological disease in western Canada. Can Vet J 2017;58:601–603. [PMC free article] [PubMed] [Google Scholar]
- 40. Seuberlich T, et al. Identification of a second encephalitis-associated astrovirus in cattle. Emerg Microbes Infect 2016;5:e71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Spinato MT, et al. Identification of bovine astrovirus in cases of bovine non-suppurative encephalitis in eastern Canada. Can Vet J 2017;58:607–609. [PMC free article] [PubMed] [Google Scholar]
- 42. Struthers JD, et al. Meningoencephalitis, vasculitis, and abortions caused by Chlamydia pecorum in a herd of cattle. Vet Pathol 2021:58:549–557. [DOI] [PubMed] [Google Scholar]
- 43. Theil D, et al. Neuropathological and aetiological studies of sporadic non-suppurative meningoencephalomyelitis of cattle. Vet Rec 1998;143:244–249. [DOI] [PubMed] [Google Scholar]
- 44. Wan Z, et al. Regulatory T cells and T helper 17 cells in viral infection. Scand J Immunol 2020;91:e12873. [DOI] [PubMed] [Google Scholar]
- 45. Zaccaria G, et al. Detection of astrovirus in a cow with neurological signs by nanopore technology, Italy. Viruses 2020;12:530. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-vdi-10.1177_10406387241237192 for Bovine astrovirus and its role in lymphocytic encephalitis in cattle in Ontario, Canada, 1988–2019 by Dominique Comeau, Maria T. Spinato, Davor Ojkic, Robert A. Foster and Jeff L. Caswell in Journal of Veterinary Diagnostic Investigation