Abstract
Photodynamic therapy is an approved treatment for primary, superficial, and small nodular basal cell carcinomas with a thickness of < 2 mm located on low-risk sites. Histologically verified basal cell carcinomas clinically assessed as suited for photodynamic therapy were included. The study aimed to investigate the agreement between clinical and histological assessments of basal cell carcinoma subtypes and thickness of tumours selected for photodynamic therapy with histopathological evaluation as a reference. A total of 343 tumours were included. The agreement between clinical and histological diagnosis of basal cell carcinoma subtype was 72% (p < 0.001). Clinical assessment of subtype had a sensitivity of 93% and specificity of 55% for superficial tumours and a sensitivity of 55% and specificity of 85% for nodular tumours. The mean ± SD thickness values by clinical and histological assessments were 0.95 ± 0.53 and 0.86 ± 0.75. The difference of 0.09 mm was statistically significant (p = 0.017), but not considered to be clinically relevant, although the differences between specific subgroups could be relevant. Among basal cell carcinomas clinically diagnosed as superficial, 91% were histologically consistent with the current photodynamic therapy criteria. The main results suggest that histopathological evaluation should precede photodynamic therapy to ensure selection of suitable basal cell carcinomas. In selected cases, the clinical diagnosis alone may be adequate before proceeding with photodynamic therapy.
SIGNIFICANCE
Basal cell carcinoma is a common skin cancer and photodynamic therapy is an approved treatment for thin basal cell carcinomas of specific subtypes. Although a histological evaluation of the tumour is recommended before photodynamic therapy, many basal cell carcinomas are treated without prior biopsy. There are few reports on the agreement between clinical and histological assessments of basal cell carcinoma subtypes and thickness. We aimed to investigate whether clinical assessment is reliable for selecting basal cell carcinomas for photodynamic therapy. Reliable selection of basal cell carcinomas can improve photodynamic therapy efficacy, and reduce costs and patient burden associated with recurrence. The results show the importance of histological assessment of basal cell carcinomas before treatment.
Key words: basal cell carcinoma, photodynamic therapy, subtype, thickness, clinical diagnosis
Basal cell carcinoma (BCC) is the most common type of skin cancer in the white population and has a rising incidence (1). Metastatic tumours are extremely rare (2), but thick and/or aggressive tumour types with local invasive properties can cause significant tissue destruction, patient morbidity, and a major burden on healthcare systems (3, 4). Although surgical excision is the most effective treatment, the use of topical treatment modalities with a good therapeutic and cosmetic outcome may be favoured as BCCs often appear on sun-exposed areas such as the head, face, and neck (5, 6).
Photodynamic therapy (PDT) is such a treatment option. This method is based on red light activation of a topically applied photosensitiser that, in the presence of oxygen through the release of reactive oxygen species, causes selective destruction of tumour cells (7). However, tumour thickness limits treatment response to PDT because both the red light and particularly the photosensitiser have limited skin penetration abilities (8–11). Thus, the current guidelines restrict the recommendations of topical PDT to superficial and small, nodular BCC with a thickness of ≤ 2 mm located on low-risk sites (12–14). Therefore, a reliable pre-PDT assessment of BCC subtype and thickness is needed.
Punch biopsy for histological tumour evaluation is a simple, clinically supportive method often used to obtain important information on BCC before treatment selection (15). Nevertheless, a number of tumours are diagnosed clinically without biopsy for histological investigation before treatment (16), and the practice seems to vary (17). It has been suggested that the omission of punch biopsy before treatment may be justified in selected cases, for example if the physician has a high degree of confidence in their diagnosis of the BCC subtype (18). However, a few earlier studies on the agreement between clinical and histological assessments of BCC subtype and thickness reported that histological examination is more sensitive and specific in diagnosing BCC subtypes, particularly the aggressive subtypes, and that the agreement between clinical and histological assessments of tumour thickness may be poor (18–20).
The aim of this study was to evaluate the agreement between clinical and histological assessments of BCC subtype and thickness in respect of clinical diagnosis in BCC selected for PDT.
MATERIALS AND METHODS
This comparative study uses data from a randomised controlled study investigating the treatment efficacy of simplified versus standard PDT for BCC (21) and was conducted in 7 dermatology centres. The BCC diagnosis was histologically verified before the tumours were included. Inclusion criteria included patients of both sexes over the age of 18 with tumours histologically diagnosed as BCC and clinically assessed as superficial or nodular < 2 mm thick, thereby meeting the international PDT criteria (12, 13). Exclusion criteria included tumours with prior treatment, mid-face-H-area location, or longest diameter > 15 mm on the face and scalp, > 30 mm on the trunk, or > 20 mm on the limbs or tumours clinically evaluated as pigmented or morphea types. Furthermore, patients with Gorlin syndrome, porphyria, xeroderma pigmentosum, or a history of arsenic exposure, being on immunosuppressive medication, or with child-bearing potential were also excluded. The study was approved by the Regional Committees for Medical and Health Research Ethics Central (2011/2048). It was performed in accordance with the Declaration of Helsinki. Written informed consent was obtained from all patients before study entry.
One investigator at each of the 7 dermatology centres performed the clinical examinations. All clinical investigators were certified dermatologists and members of the Norwegian PDT group, each with 15–20 years of experience in PDT. They were aware of the histological diagnosis of BCC but were blinded to any further information in the histological report before the clinical examination. Tumour sizes were clinically defined as the mean of the maximum length and width measurements. The clinical assessment of the BCC subtype was based on recognised clinical features (22), and the estimation of BCC thickness was based on inspection and palpation of the tumours.
A biopsy from each tumour was obtained before inclusion using a disposable 3- or 4-mm biopsy punch. The biopsies were taken from the part of the tumour clinically considered to be the thickest. Two sequential histological assessments from each biopsy were performed. The first histological assessment was prospective and routinely performed by pathologists affiliated with each of the dermatological centres aiming to verify the BCC diagnosis, but did not exclude other histological information concerning the tumours, such as subtype and thickness. Later, after treatment of all included BCCs, another histological assessment was performed retrospectively, aiming to assess the subtype and thickness. For this second examination, the original biopsy wax blocks were retrieved from each of the pathological laboratories and sent to St. Olav’s University Hospital for tumour subtype and thickness assessment. Preparation was done at the Cellular and Molecular Imaging Core Facility, the Norwegian University of Science and Technology, Trondheim, by orienting the biopsy sample so that the epidermis was aligned with the longest axis of the wax block and cut with a microtome into 3 parallel, interspersed sections. The sections were stained with haematoxylin, eosin, and saffron, and examined under a microscope by a pathologist at St. Olav’s University Hospital with extensive experience in histological BCC assessment. The initial biopsy sections evaluated by the local pathologists from the first histological examination were not re-evaluated.
In the second histological examination, the tumours were subclassified into 3 categories, superficial, nodular, or aggressive types, according to the histological criteria proposed by J. J. Rippey (23). The aggressive category included morpheaform, infiltrative, and micronodular types. In tumours presenting mixed-growth patterns, the tumour was classified according to the most aggressive component. Any information on the BCC subtype from the first histological assessments was not considered as the local pathologists were not compelled to follow the same criteria for subclassification of tumours used in this study.
BCC thickness was measured on the stained slides from the base of the stratum corneum to the bottom of the tumour nest using 2 different methods: an oculometer (1 mm squares) and an ocular micrometre to a precision of 0.1 mm (Vernier method) (24). For each tumour, the greatest measurement from either the first initial histopathological investigation or the second examination was considered to best reflect the “true” tumour thickness and was defined as the maximum tumour thickness.
Statistical analysis
Analyses were performed using SPSS® Statistics (Version 28, IBM Corp, Armonk, NY, USA). Visual inspection of histograms showed the variable “clinical thickness” and “histological thickness” not to be normally distributed, while “difference between clinical and histological thickness” and “age” were normally distributed.
The overall agreement between clinical and histological assessments of subtype and the difference between clinical and histological assessments of thickness were statistically tested using generalized linear mixed models with a data structure where tumours were nested within patients. The null hypotheses were (a) no significant difference between the subtype assessment from 0.5 (as 50% agreement is to be expected by chance) and (b) no significant difference between thickness assessment. A p-value < 0.05 was regarded as statistically significant; p-values are presented with a 95% confidence interval (CI). The results from each study centre were presented as error bar plots with 95% CI. A 1-sample binomial test, the Clopper–Pearson exact test, was used to calculate CIs with respect to subtype assessments while the statistical software calculated the 95% CIs automatically with regard to the thickness assessment.
A comparison between superficial BCC with nodular/aggressive BCC and nodular BCC with superficial/aggressive BCC was performed to calculate clinical sensitivity and specificity for subtyping, using histological subtypes as reference. Sensitivity and specificity with 95% CI were calculated using the MedCalc Statistical Software (https://www.medcalc.org/) diagnostic test evaluation calculator (25). The relationship between histological and clinical thicknesses was illustrated using scatterplots.
The number of tumours suitable for PDT was calculated by summing up the number of tumours meeting the PDT criteria, superficial and nodular BCC with a thickness of < 2 mm, with histological results as reference and calculating ratios with corresponding percentages.
RESULTS
A total of 202 patients with a mean (range) age of 66 (26–92) participated. Of these, 108 were males with a mean age of 67 (26–91) years, and 94 were females with a mean age of 65 (40–92) years. The number of BCCs per patient was 1, 2, 3, 4, 5, 6, and 7 in 202, 76, 35, 18, 8, 3 and 1 patients, respectively. All tumours had a histologically verified diagnosis before being clinically selected for PDT. A total of 343 BCCs with information on both clinical and histological subtype and thickness were included in this study. The mean ± SD (range) clinical lesion size was 11.3 mm ± 4.5 (5.0–30.0 mm). Tumours were localised on the trunk in 240 cases, head/neck in 36 cases and extremities in 67 cases. Investigators from centres 1 to 7 included and conducted clinical examinations of 90, 8, 55, 45, 30, 68, and 47 BCC cases, respectively.
Subtype analysis
Clinically, 258 (75%) tumours were superficial and 85 (25%) were nodular. Histologically, 217 (63%) tumours were superficial, 83 (24%) were nodular, and 43 (13%) were aggressive (35 infiltrative, 6 morphea, and 2 micronodular). The aggressive tumours were located on the head/neck, trunk, and extremities in 7, 28, and 10 cases, respectively. The overall agreement between clinical and histological diagnosis of BCC subtype was 72% (95% CI 67–77, p < 0.001). The sensitivity and specificity for the clinical diagnosis of superficial BCC were 93% (201/217) (95% CI 88–96) and 55% (69/126) (95% CI 46–64), respectively. The sensitivity and specificity for the clinical diagnosis of nodular BCC were 54% (45/83) (95% CI 43–65) and 84% (220/260) (95% CI 80–89). The proportions between the 2 different clinical and the 3 histological subtypes are presented in Fig. 1. In more detail, the agreement for the different centres is presented in Fig. 2.
Fig. 1.
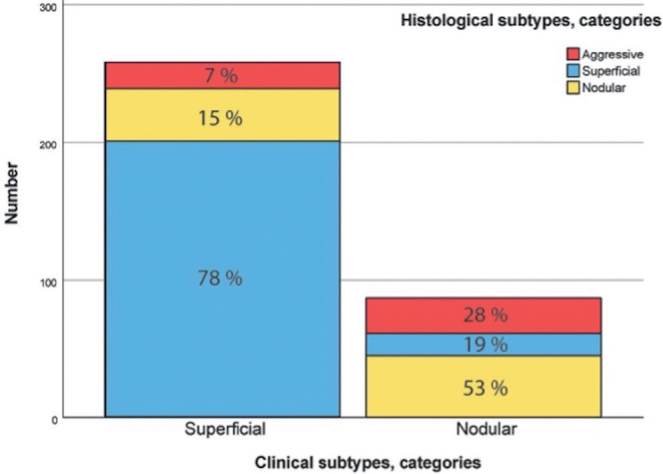
Proportion of histologically diagnosed basal cell carcinoma subtypes among tumours clinically diagnosed as superficial or nodular type.
Fig. 2.
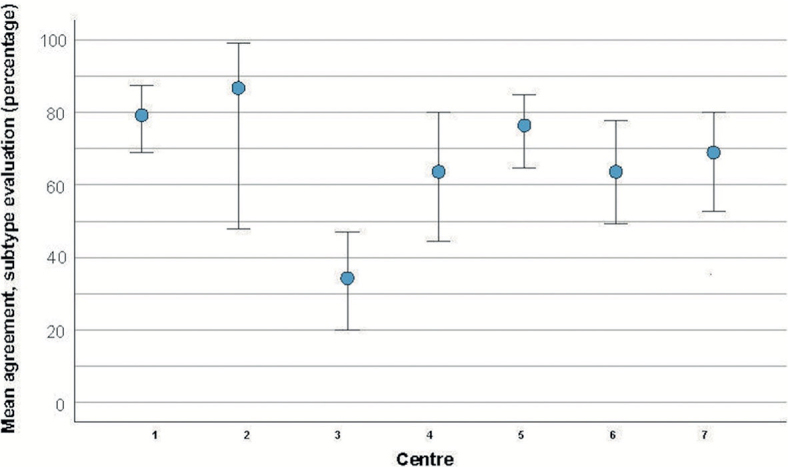
Mean agreement between clinical and histological basal cell carcinoma subtype evaluation for 7 dermatology centres. The blue markers represent the mean values, and the whiskers represent the 95% confidence intervals of the mean.
Thickness analysis
The first histological report included assessments of tumour thickness in 110 cases, of which 23 were included as they gave the largest measurements compared with the second examination. The overall mean ± SD thickness values by clinical and histological assessments were 0.95 ± 0.53 and 0.86 ± 0.75, respectively. The difference was 0.09 mm (95% CI 0.02–0.17, p = 0.017).
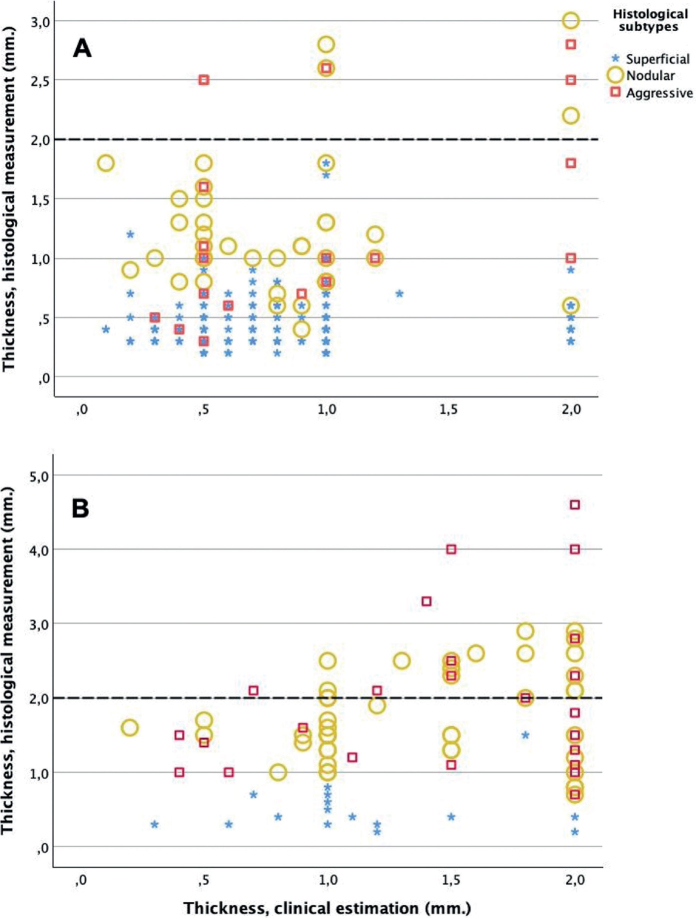
Scatterplots with corresponding clinical and histological tumour thickness measurements are presented in Fig. 3. The 2 clinical subtype diagnoses are presented separately, each with the histological diagnosis marked. The plots show a widening scatter as the clinical assessment of tumour thickness increases, particularly for histologically nodular and aggressive subtypes, reflecting a difference in clinical and histological results. The differences between clinical estimations and histological measurements of tumour thickness for the different centres are presented in Fig. 4. Clinical assessment overestimated the histological thickness of superficial BCCs and underestimated the thickness of nodular and aggressive subtypes. The mean differences in BCC thickness for each clinical subtype against each histological subtype are presented in Table I.
Fig. 3.
Scatterplots showing the corresponding clinical and histological measurements of basal cell carcinoma (BCC) thickness. The 3 histological tumour subtypes are distinguished with different markers. BCCs depicted in panel A were clinically evaluated as superficial, while those in panel B were clinically evaluated as nodular subtype. A dotted line at 2 mm is included to signify the thickness threshold pertinent to current recommendations for photodynamic therapy.
Fig. 4.
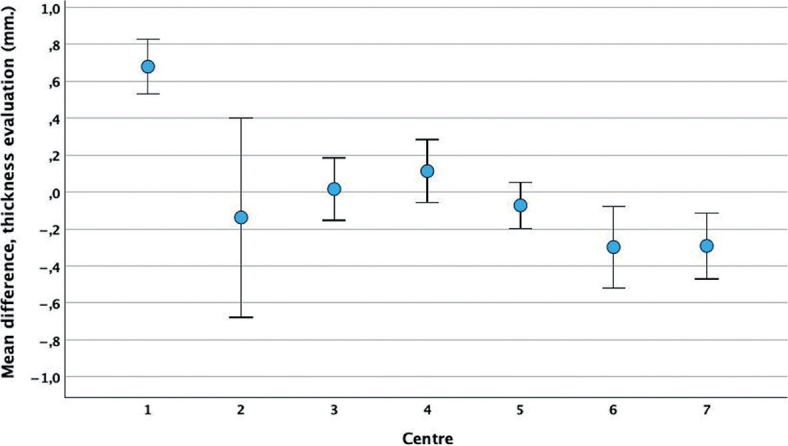
Mean difference between clinical estimations and histological measurements of basal cell carcinoma thickness for 7 dermatology centres. The blue markers represent the mean values, and the whiskers represent the 95% confidence intervals of the mean.
Table I.
Mean difference in basal cell carcinoma (BCC) thickness for each clinical subtype against each histological subtype
| Clinical assessment | Histological assessment | ||
|---|---|---|---|
| Superficial BCC | Nodular BCC | Aggressive BCC | |
| Superficial BCCa, mean, mm (95% CI) | 0.37 (0.31–0.44) | –0.43 (–0.64 to –0.22) | –0.39 (–0.78 to –0.01) |
| Nodular BCCa, mean, mm (95% CI) | 0.57 (0.31–0.84) | –0.33 (–0.78 to –0.01) | –0.55 (–1.00 to –0.10) |
Difference between clinical and histological thickness. CI: confidence interval.
Combined analysis: photodynamic therapy suitability
All BCCs included in the study were clinically assessed as suitable for PDT in respect of the subtype and thickness. Among 258 tumours clinically assessed to be superficial, 235 (91%) were histologically in line with the current PDT criteria. Among 85 tumours clinically assessed as nodular, 46 (54%) were histologically in line with the current PDT criteria. Further information is given in Table II.
Table II.
Clinical and histological basal cell carcinoma (BCC) assessments with number of tumours in different subtype and thickness categories
| Histological assessment | ||||||||
|---|---|---|---|---|---|---|---|---|
| Superficial BCC (n) | Nodular BCC (n) | Aggressive BCC (n) | Within PDT criteria, ratio (%) | |||||
| Clinical assessment | Thickness (mm) | < 2 | > 2 | < 2 | > 2 | < 2 | > 2 | |
| Superficial BCC (n) | ≤ 1.0 | 183 | 0 | 31 | 2 | 11 | 3 | 214/230 (93%) |
| ≤ 1.5 | 184 | 0 | 33 | 2 | 12 | 3 | 217/234 (93%) | |
| ≤ 2.0 | 201 | 0 | 34 | 4 | 14 | 5 | 235/258 (91%) | |
| Nodular BCC (n) | ≤ 1.0 | 9 | 0 | 18 | 2 | 5 | 1 | 27/35 (77%) |
| ≤ 1.5 | 13 | 0 | 23 | 6 | 7 | 6 | 36/55 (65%) | |
| ≤ 2.0 | 16 | 0 | 30 | 15 | 14 | 10 | 46/85 (54%) | |
All BCC were clinically assessed to meet PDT criteria. The table presents the ratio with corresponding percentages of clinically assessed tumours that met the histological PDT criteria.
n: number of tumours.
DISCUSSION
To the best of our knowledge, this is the first study to investigate the agreement between clinical and histological assessments of both BCC subtype and thickness and evaluate whether the clinical diagnosis is reliable for selecting BCCs suitable for PDT. The study results imply that a punch biopsy for histopathological investigation is generally a better diagnostic tool than clinical diagnosis for obtaining pre-PDT information on BCC. We regard the overall agreement of 72% between clinical and histological diagnosis of BCC subtype as poor, as 50% agreement would be expected by chance. The clinical assessment of superficial BCCs showed a relatively high sensitivity but a low specificity. For nodular BCCs the results were even poorer. In addition, clinical assessment missed the diagnosis of the 13% histologically aggressive subtypes perceived clinically to be either superficial or nodular. This suggests that a significant number of aggressive tumours can be overlooked and incorrectly selected for topical therapy if clinical assessment only is used as a diagnostic method.
Overall, we found a small statistically significant difference between clinical and histopathological thickness assessments. We do not consider the overall difference to be clinically relevant; however, differences between specific subgroups may be relevant. Clinical assessment overestimated the thickness of histologically superficial BCCs and underestimated the thickness of nodular and aggressive tumour types.
A number of nodular BCCs with a clinical thickness of > 1 mm were, by visual inspection of the plot, histologically found to be > 2 mm. For histologically aggressive types of BCC, clinically misclassified as non-aggressive, a number of tumours with a clinical thickness of > 0.5 mm were histologically found to be > 2 mm thick.
An underestimation of tumour thickness can result in thick tumours incorrectly being selected for topical therapy, which, in turn, may increase the chances of post-treatment failure. Conversely, overestimated thickness can lead to unnecessary invasive treatment such as excision. There were considerable variations between the centres (investigators) in the assessment of both tumour subtype and thickness. Altogether, the study findings support the practice that a biopsy punch for histopathological assessment of BCC subtype and thickness should generally precede the use of PDT.
Only a few previous studies have investigated the agreement between clinical and histological diagnoses of BCC subtype. In 2 studies, punch and excision biopsies were taken with the histological diagnosis from excision biopsies used as the reference and tumours were histologically classified according to acknowledged criteria (22, 23). The first study included 191 BCCs and demonstrated that the sensitivity and specificity for the clinical diagnosis of superficial BCC were 89% and 64% (18). Furthermore, the results showed that the proportion of aggressive BCCs that was misdiagnosed was much higher for clinical diagnosis than histological punch biopsy diagnosis (44% vs 15%). The second study, which included 43 BCCs, demonstrated that the sensitivity and specificity for clinical diagnosis of superficial and nodular tumours were 73% and 94%, and 95% and 62%, respectively (20). Additionally, 7 of the 10 aggressive tumours were misdiagnosed as non-aggressive based on clinical assessment only. Amici et al. (26) studied the agreement between clinical and histological diagnoses on 2,274 BCCs designated for surgery using excision biopsy as the reference standard. Clinical and histological subtyping agreed in 57% of cases. Of BCCs histologically diagnosed as aggressive, 6% were clinically diagnosed as superficial tumours and 72% as nodular tumours. Clinical accordance for superficial, nodular, and aggressive BCC was 50%, 86%, and 22%, respectively. The results of these 3 studies are not completely comparable due to the lack of uniformity in study design, such as the use of different tumour classification systems and different methods of collecting and using tissue samples. However, all these studies showed that a diagnostic biopsy is overall a better diagnostic tool than clinical assessment for diagnosing BCC subtypes, particularly for detecting aggressive tumours.
To the best of our knowledge, only 1 small-scale study including 55 BCCs exists on the agreement between clinical and histological BCC thicknesses (19). The results showed a clinical overestimation of histologically superficial tumours and an underestimation of histologically nodular and aggressive tumours with an increasing discrepancy between thickness estimations between the 2 methods as the tumour thickness increased. This finding is consistent with the present study, adding strength to the recommended use of biopsy before PDT. However, the results of the present study also indicate the possibility of permitting selected BCCs, clinically assessed as superficial tumours with a thickness of < 2 mm, to be treated with PDT without a prior biopsy as 91% of such cases were histologically found to meet the current PDT criteria. With such use, follow-up of the treatment area is necessary, particularly concerning the risk of clinical underdiagnosis of aggressive subtypes.
Strength and limitations
Strengths of this study are the sample size, the clinical evaluation by experienced dermatologists from multiple centres, and clinical and histological subtyping of BCC based on established criteria (22, 23). The present study has some limitations. The histological assessment was based on punch biopsies, which provide a limited view of the tumour tissue, is highly reliant on biopsy site (20), and has reduced accuracy for diagnosis of mixed subtypes (27, 28). A systematic review has shown that punch biopsy predicts BCC subtypes correctly in 61–85% of cases, with excision as the reference standard (28). Furthermore, the disparity between thickness measurements from punch and excision biopsies increases with the increase in tumour thickness (29). However, it must be stated that, in the context of PDT, excision histology is not relevant as the reference standard. Other limitations were the selection of BCCs that were limited to tumours which clinically met the PDT criteria and the investigators’ knowledge of histologically confirmed BCC diagnosis before the clinical assessment of subtype and thickness.
Conclusion
To conclude, the results imply that a biopsy for histopathological assessment is advisable before PDT. For selected BCCs clinically assessed by an experienced dermatologist to be superficial with a thickness of ≤ 2 mm, a clinical diagnosis may be sufficient before performing PDT.
ACKNOWLEDGEMENTS
The study has been approved by the REK Central in Norway, number 2011/2048. It was performed in accordance with the Declaration of Helsinki. Written informed consent was obtained from all patients before study entry.
Funding Statement
Funding sources The study has been funded by the Liaison Committee for Education, Research and Innovation in Central Norway (Samarbeidsorganet).
Footnotes
The authors have no conflicts of interest to declare.
REFERENCES
- 1.Lomas A, Leonardi-Bee J, Bath-Hextall F. A systematic review of worldwide incidence of nonmelanoma skin cancer. Br J Dermatol 2012; 166: 1069–1080. [DOI] [PubMed] [Google Scholar]
- 2.Ganti AK, Kessinger A. Systemic therapy for disseminated basal cell carcinoma: an uncommon manifestation of a common cancer. Cancer Treat Rev 2011; 37: 440–443. [DOI] [PubMed] [Google Scholar]
- 3.Cameron MC, Lee E, Hibler BP, Barker CA, Mori S, Cordova M, et al. Basal cell carcinoma: epidemiology; pathophysiology; clinical and histological subtypes; and disease associations. J Am Acad Dermatol 2019; 80: 303–317. [DOI] [PubMed] [Google Scholar]
- 4.Housman TS, Feldman SR, Williford PM, Fleischer AB Jr, Goldman ND, Acostamadiedo JM, et al. Skin cancer is among the most costly of all cancers to treat for the Medicare population. J Am Acad Dermatol 2003; 48: 425–429. [DOI] [PubMed] [Google Scholar]
- 5.Berking C, Hauschild A, Kolbl O, Mast G, Gutzmer R. Basal cell carcinoma-treatments for the commonest skin cancer. Dtsch Arztebl Int 2014; 111: 389–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cameron MC, Lee E, Hibler BP, Giordano CN, Barker CA, Mori S, et al. Basal cell carcinoma: contemporary approaches to diagnosis, treatment, and prevention. J Am Acad Dermatol 2019; 80: 321–339. [DOI] [PubMed] [Google Scholar]
- 7.Morton CA, Szeimies RM, Basset-Seguin N, Calzavara-Pinton P, Gilaberte Y, Haedersdal M, et al. European Dermatology Forum guidelines on topical photodynamic therapy 2019 Part 1: treatment delivery and established indications – actinic keratoses, Bowen’s disease and basal cell carcinomas. J Eur Acad Dermatol Venereol 2019; 33: 2225–2238. [DOI] [PubMed] [Google Scholar]
- 8.Sandberg C, Halldin CB, Ericson MB, Larko O, Krogstad AL, Wennberg AM. Bioavailability of aminolaevulinic acid and methylaminolaevulinate in basal cell carcinomas: a perfusion study using microdialysis in vivo. Br J Dermatol 2008; 159: 1170–1176. [DOI] [PubMed] [Google Scholar]
- 9.Peng Q, Soler AM, Warloe T, Nesland JM, Giercksky KE. Selective distribution of porphyrins in skin thick basal cell carcinoma after topical application of methyl 5-aminolevulinate. J Photochem Photobiol B 2001; 62: 140–145. [DOI] [PubMed] [Google Scholar]
- 10.Ahmadi S, McCarron PA, Donnelly RF, Woolfson AD, McKenna K. Evaluation of the penetration of 5-aminolevulinic acid through basal cell carcinoma: a pilot study. Exp Dermatol 2004; 13: 445–451. [DOI] [PubMed] [Google Scholar]
- 11.Peng Q, Warloe T, Berg K, Moan J, Kongshaug M, Giercksky KE, et al. 5-aminolevulinic acid-based photodynamic therapy: clinical research and future challenges. Cancer 1997; 79: 2282–2308. [DOI] [PubMed] [Google Scholar]
- 12.Wong TH, Morton CA, Collier N, Haylett A, Ibbotson S, McKenna KE, et al. British Association of Dermatologists and British Photodermatology Group guidelines for topical photodynamic therapy 2018. Br J Dermatol 2019; 180: 730–739. [DOI] [PubMed] [Google Scholar]
- 13.Peris K, Fargnoli MC, Garbe C, Kaufmann R, Bastholt L, Seguin NB, et al. Diagnosis and treatment of basal cell carcinoma: European consensus-based interdisciplinary guidelines. Eur J Cancer 2019; 118: 10–34. [DOI] [PubMed] [Google Scholar]
- 14.Christensen E, Warloe T, Kroon S, Funk J, Helsing P, Soler AM, et al. Guidelines for practical use of MAL-PDT in non-melanoma skin cancer. J Eur Acad Dermatol Venereol 2010; 24: 505–512. [DOI] [PubMed] [Google Scholar]
- 15.Elston DM, Stratman EJ, Miller SJ. Skin biopsy: biopsy issues in specific diseases. J Am Acad Dermatol 2016; 74: 1–16; quiz 17–18. [DOI] [PubMed] [Google Scholar]
- 16.Backman E, Oxelblom M, Gillstedt M, Dahlen Gyllencreutz J, Paoli J. Basal cell carcinoma: epidemiological impact of clinical versus histopathological diagnosis. J Eur Acad Dermatol Venereol 2023; 37: 521–527. [DOI] [PubMed] [Google Scholar]
- 17.Flohil SC, Proby CM, Forrest AD, van Tiel S, Saksela O, Pitkanen S, et al. Basal cell carcinomas without histological confirmation and their treatment: an audit in four European regions. Br J Dermatol 2012; 167: 22–28. [DOI] [PubMed] [Google Scholar]
- 18.Roozeboom MH, Kreukels H, Nelemans PJ, Mosterd K, Winnepenninckx VJ, Abdul Hamid MA, et al. Subtyping basal cell carcinoma by clinical diagnosis versus punch biopsy. Acta Derm Venereol 2015; 95: 996–998. [DOI] [PubMed] [Google Scholar]
- 19.Christensen E, Mjones P, Grimstad O, Rordam OM, Foss OA. Comparison of clinical and histopathological evaluations of basal cell carcinoma thickness. Br J Dermatol 2015; 173: 578–580. [DOI] [PubMed] [Google Scholar]
- 20.Christensen E, Mjones P, Grimstad O, Rordam OM, Foss OA. Diagnostic accuracy in subtyping basal cell carcinoma by clinical diagnosis compared with punch biopsy. Acta Derm Venereol 2016; 96: 862–863. [DOI] [PubMed] [Google Scholar]
- 21.Christensen E, Mork E, Foss OA, Mork C, Kroon S, Dotterud LK, et al. New, simplified versus standard photodynamic therapy (PDT) regimen for superficial and nodular basal cell carcinoma (BCC): a single-blind, non-inferiority, randomised controlled multicentre study. PLoS One 2024; 19: e0299718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Crowson AN. Basal cell carcinoma: biology, morphology and clinical implications. Mod Pathol 2006; 19 Suppl 2: S127–147. [DOI] [PubMed] [Google Scholar]
- 23.Rippey JJ. Why classify basal cell carcinomas? Histopathology 1998; 32: 393–398. [DOI] [PubMed] [Google Scholar]
- 24.Kirkham N, Cotton DW. Measuring melanomas: the Vernier method. J Clin Pathol 1984; 37: 229–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.MedCalc Software Ltd . Diagnostic test evaluation calculator. Version 20.011 ed. www.MedCalc.org: MedCalc; 2021. [Google Scholar]
- 26.Amici JM, Dousset L, Battistella M, Vergier B, Bailly JY, Cogrel O, et al. Clinical factors predictive for histological aggressiveness of basal cell carcinoma: a prospective study of 2274 cases. Ann Dermatol Venereol 2021; 148: 23–27. [DOI] [PubMed] [Google Scholar]
- 27.Wolberink EA, Pasch MC, Zeiler M, van Erp PE, Gerritsen MJ. High discordance between punch biopsy and excision in establishing basal cell carcinoma subtype: analysis of 500 cases. J Eur Acad Dermatol Venereol 2013; 27: 985–989. [DOI] [PubMed] [Google Scholar]
- 28.Kadouch DJ, van Haersma de With A, Limpens J, van der Wal AC, Wolkerstorfer A, Bekkenk MW, et al. Is a punch biopsy reliable in subtyping basal cell carcinoma? A systematic review. Br J Dermatol 2016; 175: 401–403. [DOI] [PubMed] [Google Scholar]
- 29.Christensen E, Mjones P, Foss OA, Rordam OM, Skogvoll E. Pre-treatment evaluation of basal cell carcinoma for photodynamic therapy: comparative measurement of tumour thickness in punch biopsy and excision specimens. Acta Derm Venereol 2011; 91: 651–654. [DOI] [PubMed] [Google Scholar]