Abstract
1,25-dihydroxyvitamin D3 (1,25(OH)2D3) has recently been found to have the anti-inflammatory potential to suppress experimental autoimmune encephalomyelitis (EAE), an animal model of multiple sclerosis; however, its direct effect on neural cells is not clear. In the current study we show that 1,25(OH)2D3 treatment effectively suppressed clinical signs of ongoing EAE and reduced inflammation and demyelination scores in the central nervous system (CNS). The treatment significantly decreased production/expression of pro-inflammatory cytokines IFN-γ, GM-CSF and IL-17A, while it increased anti-inflammatory cytokines IL-4 and IL-10. Further, 1,25(OH)2D3 treatment effectively elevated the numbers of neural stem cells, oligodendrocyte precursor cells, as well as oligodendrocytes in disease lesions in the CNS. These results, together with its in vitro effect of inducing oligodendrocyte differentiation as shown in our previous findings, demonstrate that 1,25(OH)2D3 suppressed EAE not only by its immunomodulatory capacity, but also by its effect on oligodendrocyte differentiation and maturation, and thus has potential for remyelination and neural repair.
Keywords: 1, 25-dihydroxyvitamin D3; Oligodendrocytes; Multiple Sclerosis; EAE
Introduction
Multiple sclerosis (MS) is an autoimmune disease of the central nervous system (CNS) (Steinman 2001), whose progression is associated with extensive inflammatory infiltrates and relatively early loss of oligodendrocytes (OLGs), the myelinating cells, in the CNS (Lucchinetti, Bruck et al. 2001, Cudrici, Niculescu et al. 2006). While the etiology of MS is not well understood, the prevalence of this disease is increasing due to a complex interplay between environmental and genetic risk factors in susceptible individuals (Grytten, Glad et al. 2006, Compston and Coles 2008). Experimental autoimmune encephalomyelitis (EAE), an animal model of MS, makes it possible to explore multiple facets of the immune and neural mechanisms of disease, and to test interactions among a variety of mechanisms that lead to key pathological features of MS such as inflammation, demyelination, axonal loss and gliosis (Constantinescu, Farooqi et al. 2011, Lucchinetti, Bruck et al. 2001).
The activated form of vitamin D, 1,25-dihydroxy-vitamin D3 (1,25(OH)2D3) is an environmental factor that can modify multiple functions in the body, and a low level of this vitamin in the diet or low sunlight exposure increases the risk of MS (Munger, Levin et al. 2006, Wergeland, Torkildsen et al. 2011). Clinical effects of 1,25(OH)2D3 are mediated via the vitamin D receptor (VDR), which is widely distributed both in immune cells and CNS resident cells (Mayne, Spanier et al. 2011, Cekic, Sayeed et al. 2009). 1,25(OH)2D3 predominantly mediates immunomodulatory responses in vitro and in vivo (Dehghani, Meamar et al. 2013, Smolders, Thewissen et al. 2009), and effectively suppresses EAE (Cantorna, Humpal-Winter et al. 2000, Smolders, Thewissen et al. 2009, Becklund, Hansen et al. 2009). On the other hand, VDR expression in OLGs may play an important role in maintaining a balance between OLG differentiation and axonal adhesion during brain development, while VDR depletion leads to a slower rate of OLG differentiation with an increased proportion of apoptosis in these cells (Chaudhuri 2005). Our previous study showed that 1,25(OH)2D3 in vitro significantly enhanced proliferation of neural stem cells, and promoted their differentiation into neurons and OLGs, but not astrocytes, likely through inducing production of neurotrophic factors NT-3, BDNF, GDNF and CNTF (Shirazi, Rasouli et al. 2015). However, the effect of this vitamin on OLG differentiation in EAE is not known.
In the present study, we investigated the therapeutic potential of 1,25(OH)2D3 in EAE by studying its effect on cytokine regulation and neuron/OLG differentiation whereby demyelination was reduced in EAE/MS.
Materials and Methods
EAE induction and treatment
Female C57BL/6 mice, 8–12 weeks of age, were purchased from Jackson Laboratory (Bar Harbor, Maine) and used for EAE induction. EAE was induced by subcutaneous injection of 200 μg MOG35-55 (Gen Script, Piscataway, NJ) in complete Freund’s adjuvant (CFA) containing 5 mg/ml Mycobacterium tuberculosis H37Ra (Difco, Detroit, MI). Mice received 200 ng pertussis toxin (Sigma Aldrich, St. Louis, MO) intraperitoneally (i.p.) on days 0 and 2 post immunization (p.i.). Daily clinical observation and grading were recorded by two researchers in a blind manner according to a 0 to 5 scale as follows : 0, clinically normal; 1, limp tail or waddling gait with tail tonicity; 2, waddling gait with a limp tail (ataxia); 2.5, ataxia with partial limb paralysis; 3, full paralysis of one limb; 3.5, full paralysis of one limb with partial paralysis of a second limb; 4, full paralysis of two limbs; 4.5, moribund; and 5, death. All work was performed in accordance with the guidelines for animal use and care at Thomas Jefferson University.
EAE mice received i.p. injection of 0.1 μg of 1,25 (OH)2D3 (Sigma Aldrich) along with 0.1% ethanol in a volume of 0.2 ml of normal saline, every second day from day 15 to 30 p.i., as previously described (Chaudhuri 2005, Dehghani, Meamar et al. 2013). Mice that received the same volume of normal saline with 0.1% ethanol base served as control.
Histological analysis
On day 30 p.i., mice were extensively perfused with PBS, and lumbar spinal cords were harvested and fixed with 4% PFA (Mediatech, Inc., Manassas, VA). The five-micrometer sections of tissues were stained with hematoxylin and eosin (H&E) to determine inflammatory infiltrate cells and with luxol fast blue (LFB) to examine demyelination. Slides were assessed for inflammation and demyelination by two researchers in a blinded manner, as follows. For inflammation: 0, none; 1, a few inflammatory cells; 2, organization of perivascular infiltrates; and 3, increasing severity of perivascular cuffing with extension into the adjacent tissue. For demyelination: 0, none; 1, rare foci; 2, a few areas of demyelination; and 3, large (confluent) areas of demyelination.
Cell culture and flow cytometry
Splenocytes were harvested at day 30 day p.i and cultured with MOG35-55 (25 μg/ml) at a density of 2×106/ml in cell media (IMDM, 5% FBS, Pen-Strep, L-Glutamin) for 3 days. For intracellular cytokine secretion, cells were activated with phorbol 12-myristate 13-acetate (PMA) (50 ng/ml), ionomycin (500 ng/ml) and Golgi Plug (1 μg/ml) (BD Biosciences, San Jose, CA) for 4 hours. Cells were washed and stained with CD4 Pacific Blue (BD Biosciences) and CD8 Percp-Cy5.5 (BD Biosciences), and fixed/permeabilized with Caltag Fix/Perm reagents (Invitrogen). Afterwards, cells were stained for intracellular cytokines with the following antibodies: IL-4-FITC, IL-5-allophycocyanin, IL-10-PE, IL-17A-Alexa Fluor 488, GM-CSF-PE and IFN-γ-allophycocyanin; appropriate isotype antibodies were used as control (all from BD Biosciences). Data were evaluated by FACS Aria II (BD Biosciences, San Jose, CA) and analyzed by FlowJo software (Tree Star).
ELISA and cytokine quantification
Supernatants were harvested 3 days after culture; concentrations of IFN-γ, IL-17A, GM-CSF, IL-4, and IL-10 were quantified by ELISA (R&D system, Minneapolis, MN) according to the manufacturer’s instructions.
Real-time quantitative PCR
Total RNA was extracted from spinal cord tissues (RNeasy Kit, QIAGEN Valencia, CA). RNA concentration and purity were determined (NanoDrop Technologies, Wilmington, DE); cDNA was then synthesized from 1 μg of total RNA according to the manufacturer’s instructions (QuantiTect Reverse Transcription Kit, QIAGEN, Valencia, CA). RT-PCR reactions (QuantiFast SYBR Green PCR Kit, QIAGEN, Valencia, CA) were initiated with denaturation at 95°C for 10s, annealing at 60°C for 30s and polymerization at 72°C for 30s with 40 cycles. The experiment was carried out in triplicate for cytokine gene expression, with glyceraldehyde-3-phosphate dehydrogenase (GAPDH) housekeeping gene primer serving as an endogenous control and internal standards. Primer sequences (Integrated DNA Technologies, Coralville, Iowa) are shown in Table 1. Real-time qRT-PCR was performed with ABI Prism 7000 sequence detection system (Applied Bio-Systems, Foster City, CA).
Table 1:
Sequences of primers used in real time RT-PCR
| Gene Name | Primer sequence |
|---|---|
| IL-4 | Forward : CGA GTT GAC CGT AAC AGA CAT Reverse : CGT CTT TAG CCT TTC CAA GAA G |
| IL-5 | Forward : GGG CTT CCT GCT CCT ATC TA Reverse : CAG TCA TGG CAC AGT CTG AT |
| IL-10 | Forward : CCC TGG GTG AGA AGC TGA AG Reverse : CAC TGC CTT GCT CTT ATT TTC ACA |
| IL-17A | Forward : ACT ACC TCA ACC GTT CCA CG Reverse : AGA ATT CAT GTG GTG GTC CAG |
| GM-CSF | Forward : GCT GCC CAA CCC TGT CAC C Reverse : GGT TGC CCC GTA GAC CCT GC |
| IFN-γ | Forward : TGT TAC TGC CAC GGC ACA GTC ATT Reverse : GTG GAC CAC TCG GAT GAG CTC ATT |
| GAPDH | Forward : ACC ACA GTC CAT GCC ATC AC Reverse : TCC ACC ACC CTG TTG CTG TA |
Immunohistochemistry
Lumbar spinal cords of 1,25(OH)2D3 treated and untreated mice were harvested (n=5 each group) at day 30 p.i. and fixed with 4% paraformaldehyde (Mediatech, Inc., Manassas, VA). Paraffin embedded tissues were cut into 5-μm thick sections. Immunohistochemistry staining was performed on three serial sections of spinal cords per mouse. Sections were deparaffinized/dehydrated with xylene and ethanol and washed with PBS. Samples were blocked with 8% horse serum and 3% BSA in PBS for 30 minutes at room temperature and incubated with the following primary antibodies at 4°C overnight: anti-nestin (1:500), anti-GalC (1:50), anti-NG2 (1:300), anti-MBP or myelin basic protein (1:100), anti-CD4 (1:500), anti-CD11b (1:400) and anti-VDR (1:50). Sections were washed and incubated with secondary antibody (1:500, Alexa Fluor 488 X IgG H&L; all of the above from Abcam, Cambridge, MA, USA) for 1 hour at room temperature. Serial sections of each sample were used as negative controls by using only secondary antibodies. Sections were washed and mounted with ProLong Diamond Antifade mounting media (Life Technologies, Grand Island, NY) and visualized by fluorescence microscope (Nikon Eclipse E600, Optical Apparatus Co. Dewitt, Iowa), (Helming, Bose et al. 2005, Yang, Yan et al. 2010). Mean pixel intensity of biomarker expressions and the number of CD4 and CD11b cells in lesion areas were quantified according to “counting of single color image” method by Image J software (Adzemovic, Zeitelhofer et al. 2013; Yang, Yan et al. 2014).
Statistical analysis
Clinical scores were analyzed by calculating the area under the curve for each mouse over the clinical period of the experiment. Differences between multiple groups were evaluated by the Kruskal-Wallis one-way analysis of variance. Experiments with two groups for statistical significance were tested by using unpaired, two-tailed, Student’s t-tests and Two-way ANOVA. P< 0.05 was considered to be statistically significant. Experimental data are presented as the mean ± SEM of three independent experiments. Graphs of the original data were produced using GraphPad Prism software, version 6.00 for Windows GraphPad, USA.
Results
1, 25 (OH)2D3 suppresses inflammation and demyelination in EAE.
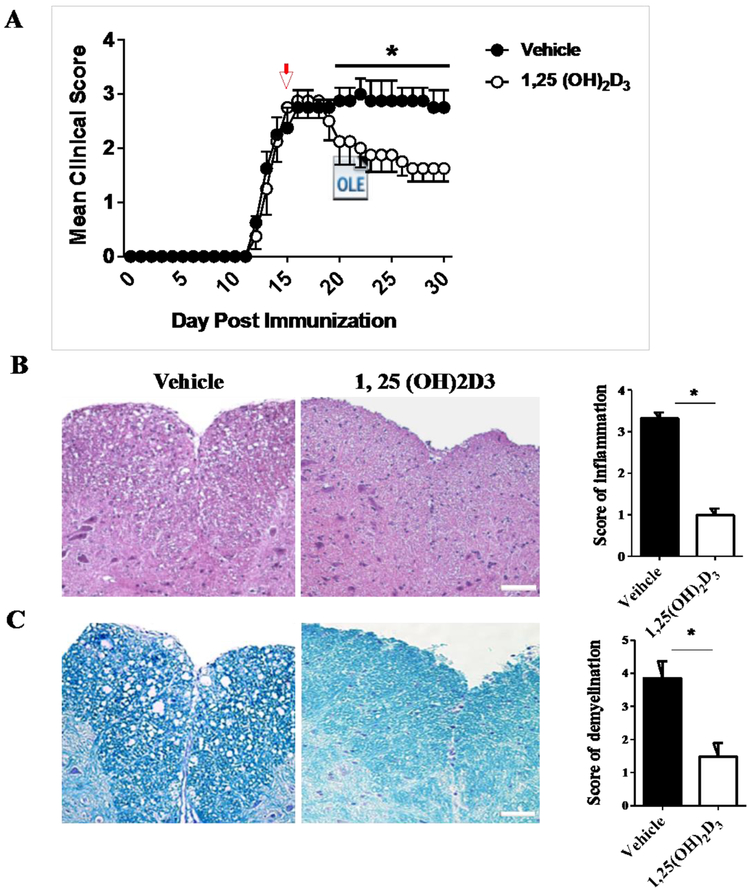
To evaluate the effect of 1,25(OH)2D3 on remyelination in vivo, EAE was induced and mice received 1,25(OH)2D3 at day 15 p.i and for additional 15 days. Vehicle-treated mice developed chronic EAE and 1,25(OH)2D3 significantly reduced clinical scores and halted disease progression (Fig. 1A). Next, we sacrificed the mice at day 30 and the lumbar part of spinal cords was analyzed for EAE-associated CNS pathology. Our data demonstrate that 1,25(OH)2D3 significantly reduces inflammation (Fig. 1B) and demyelination (Fig. 1C) when compared with controls.
Figure 1. 1,25 (OH)2D3 ameliorates clinical signs of EAE and suppresses inflammation and demyelination in the CNS.
(A) Clinical EAE scores were evaluated daily according to a 0-5 severity scale in 1,25(OH)2D3 treated and vehicle mice. Spinal cords were harvested at day 30 p.i. and stained with (B) H&E for inflammation and (C) Luxol fast blue (LFB) (for degree of demyelination. Scale bars= 20 μm. Data represent mean± SEM (n=5/group). *P<0.05. One representative of three independent experiments is shown.
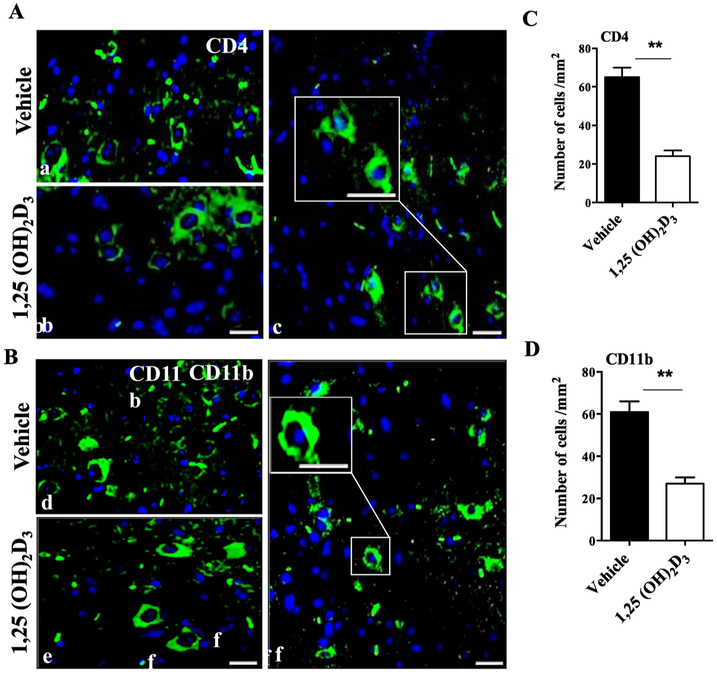
Our histopathology results demonstrate that 1,25(OH)2D3-treated mice had less inflammation in the white matter of the spinal cord. To analyze infiltrating cells, we stained serial sections of the lumbar part of spinal cord for CD4 and CD11b. Our immunohistochemistry results show that 1,25(OH)2D3 significantly decreased the numbers of both infiltrating CD4+ (Fig. 2A) and CD11b+ cells (Fig. 2B) when compared to the vehicle group.
Figure 2. 1,25(OH)2D3 treatment reduces numbers of CD4+ and CD11b+ cells in the CNS.
Lumbar spinal cords of 1,25(OH)2D3 treated and vehicle EAE mice were harvested at day 30 p.i. for immunostaining. Representative images indicate (A) CD4+ T cells and (B) CD11b+ microglia/macrophages. Nuclei were stained with DAPI (blue). Quantitative analysis of numbers of (C) CD4+ and (D) CD11b+ cells per mm2 in the spinal cord. Scale bars at c and f are 20 μm in small square and 40 μm in large square, respectively. Data represent mean ± SEM (n=5/group). **P< 0.01. One representative of three experiments is shown.
1,25(OH)2D3 enhances expression of immunoregulatory cytokines and decreases pro-inflammatory cytokines in EAE.
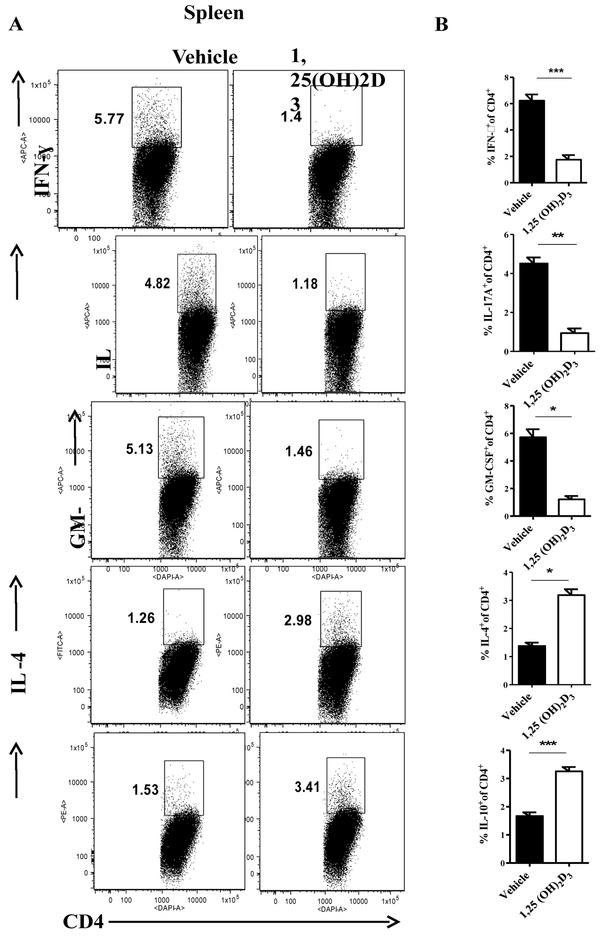
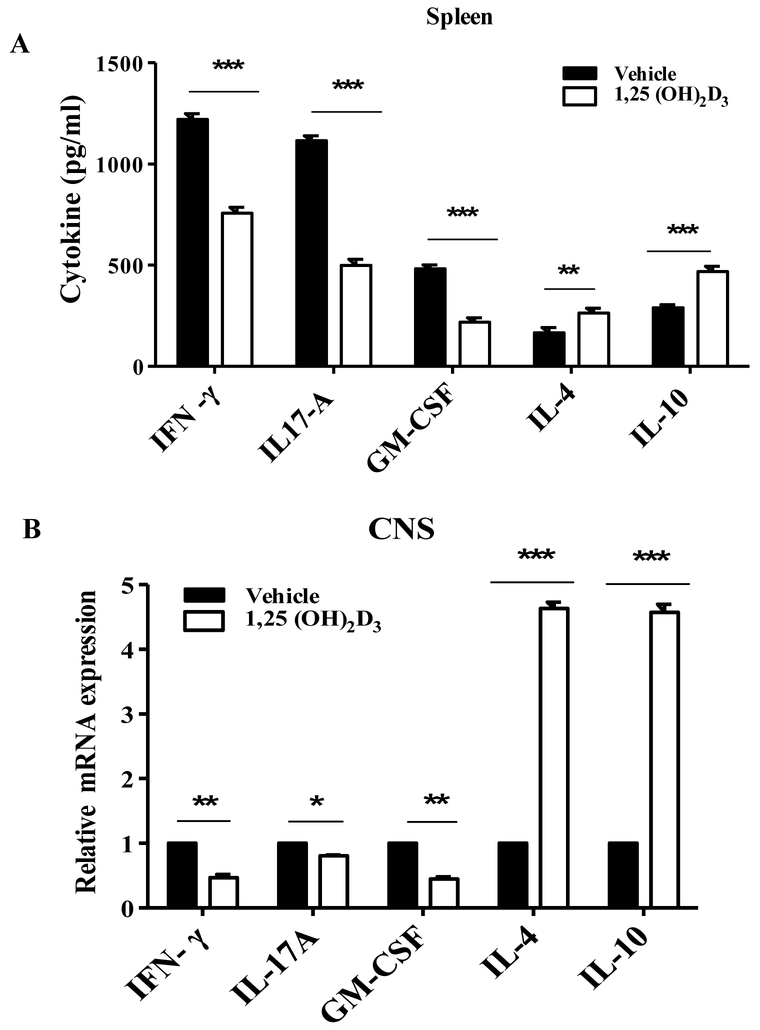
Next, we evaluated the effect of 1,25(OH)2D3 on cytokine profile in the secondary lymphoid organs and the CNS of EAE mice. 1,25(OH)2D3 significantly increased the percentages of myelin specific IL-4- and IL-10-producung CD4+ T cells in the spleen of EAE mice. 1,25(OH)2D3 treted mice had a lower proportion of IFN-γ+, IL-17A+, and GM-CSF+ CD4+ T cells compared with the vehicle group (Fig. 3A, B). We also measured cytokine production by myelin-specific splenocytes. Similar to flow cytometry results, 1,25(OH)2D3 dramatically reduced production of pro-inflammatory cytokines such as IFN-γ, GM-CSF and IL-17A, while it induced production of IL-4 and IL-10 (Fig. 4A).
Figure 3. 1,25(OH)2D3 induces immunoregulatory cytokine and decreases pro-inflammatory cytokines expression in EAE.
Splenocytes of 25(OH)2D3 treated and vehicle EAE mice were stimulated with PMA, ionomycin and Golgi Plug for 4 hours, stained, and analyzed by flow cytometry. Percentages of IFN-γ+, IL-17A+, GM-CSF+, IL-4+ and IL-10+ cells in gated CD4+ T cells were determined (A), and statistically analyzed (B). Data represent mean±SEM (n=5/group), *P< 0.05, **P< 0.01, and ***P< 0.001. One representative of three experiments is shown.
Figure 4. 1,25(OH)2D3 increases immunoregulatory cytokine production/expression.
(A) Splenocytes of 25(OH)2D3 treated and vehicle EAE mice were culture with MOG35-55 for three days and supernatants were collected and analyzed for cytokine production by ELISA. (B) Spinal cord was collected and mRNA expression of IFN-γ, IL-17A, GM-CSF, IL-4 and IL-10 was quantified by RT-PCR. Data represent mean± SEM (n=5/group), *P<0.05, **P< 0.01, and ***p<0.001. One representative of three experiments is shown.
To determine the effect of 1,25(OH)2D3 on cytokine levels in the CNS of EAE mice, total RNA was extracted from spinal cords and mRNA expression was quantified by real-time PCR. Consistent with cytokine production in splenocytes, 1,25(OH)2D3 significantly enhanced the expression of IL-4 and IL-10, while it inhibited the expression of IFN-γ, GM-CSF and IL-17A (Fig. 4B). Together, these results indicate that 1,25(OH)2D3 has an immunomodulatory effect in EAE.
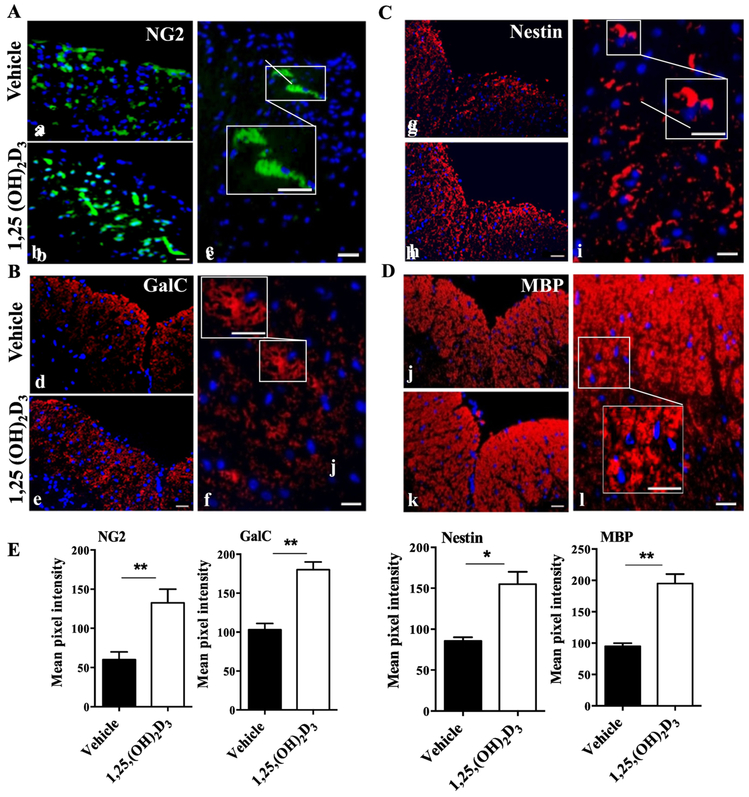
1,25(OH)2D3 treatment enhances differentiation/survival of oligodendrocyte progenitor cell/oligodendrocyte (OPC/OLG) lineage
Our previous study showed that 1,25(OH)2D3 promoted neural stem cell (NSC) proliferation and OPC/OLG lineage differentiation in vitro (Shirazi, Rasouli et al. 2015). To study the effect of 1,25(OH)2D3 on NSC proliferation/survival and OPC/OLG differentiation in vivo, we stained the lumbar part of the spinal cord of EAE mice for NG2 (OPCs marker; Fig. 5A), GalC (OLGs marker; Fig. 5B), nestin (NSCs marker; Fig. 5C), and MBP (myelin sheath marker; Fig. 5D) and analyzed by immunofluorescence microscopy. 1,25(OH)2D3-treated mice had higher expression levels of NG2, GalC, Nestin, and MBP in their spinal cords when compared with vehicle-treated mice (Fig. 5E). These results indicate that 1,25(OH)2D3, similar to its effects in vitro, effectively induces NSC proliferation/survival and their differentiation into OPC/OLG lineage in vivo.
Figure 5. 1,25(OH)2D3 enhances remyelination.
Lumbar spinal cords of 25(OH)2D3 treated and vehicle EAE mice were harvested at day 30 p.i., and 3 sections from each mouse were stained and analyzed by confocal microscopy. Representative images indicate (A) OPCs (NG2+; green), (B) OLGs (GalC+; red), (C) neural stem cells (nestin+; red), and (D) Myelin sheath (MBP+, red). Nuclei were counterstained with DAPI (blue). (E) Image J was used to perform quantitative analysis for the pixel intensity of NG2, GalC, nestin and MBP at random areas of spinal cord lesions. Scale bars =10 μm, 20 μm, and 40 μm, respectively. Data represent mean± SEM (n=5 each group). *P<0.05 and **P< 0.01. One representative of three experiments is shown.
Discussion
The present study provides evidence that 1,25(OH)2D3 treatment significantly suppressed ongoing EAE, through both its immunomodulatory capacity and direct promoting effect for the differentiation of NSCs into OPC/OLG lineage.
In addition to its fundamental role in calcium homeostasis and bone metabolism, 1,25(OH)2D3 plays a particular immunoregulatory role in the treatment of autoimmune diseases. 1,25(OH)2D3 skews pro-inflammatory Th1/Th17 cell-mediated cytokines to an anti-inflammatory Th2 cell-mediated state (Matheu, Back et al. 2003, Tang, Zhou et al. 2009, Helming, Bose et al. 2005). The administration of 1,25(OH)2D3 can initiate a biological process that reduces the number of pathogenic CD4 cells and weakens their activity (Cantorna, Snyder et al. 2015), while a high vitamin D3 content is associated with attenuated white matter microglia activation/macrophage infiltration during OLG death and demyelination (Wergeland, Torkildsen et al. 2011, Huang, Ho et al. 2015). Moreover, the active metabolite of 1,25(OH)2D3 exerts immunomodulatory effects by inhibiting differentiation of dendritic cells and desensitizing them to maturation stimuli (Griffin, Lutz et al. 2000). This process effectively controlled T cell responses and induced rapid apoptosis of inflammatory cells (Pombo, Barettino et al. 1999, Disanto, Sandve et al. 2012). These data, together with our observations in ongoing EAE, indicate that 1,25(OH)2D3 is a potential therapy to inhibit Th1/Th17 cells and antigen presenting cells, thus suppressing the progression of EAE/MS.
We and others have shown that 1,25(OH)2D3 reduces demyelination in EAE, an effect that can be explained by two possible mechanisms: First, demyelination in the CNS lesions of MS/EAE takes place in a pro-inflammatory environment that is intrinsically hostile to the OPC/OLG lineage (Franklin and Ffrench-Constant 2008). 1,25(OH)2D3 may reduce autoimmune response by converting a hostile environment into an anti-inflammatory milieu that induces remyelination. Indeed, Th1 and Th17 cells play a central role in the development of EAE (Pikor, Prat et al. 2015) and are toxic to NSCs and OPCs (Li, Li et al. 2013, Helming, Bose et al. 2005). A pronounced suppression of IFN-γ and IL-17A by 1,25(OH)2D3 treatment would therefore protect these cells from the harmful environment created by pro-inflammatory cytokines in the disease foci. On the other hand, although IL-4 does not directly prevent demyelination (Dumitrascu, Mott et al. 2014), increased IL-4 production by 1,25(OH)2D3 treatment would indirectly contribute to remyelination through inhibiting Th1 and Th17 cells (Annunziato, Romagnani et al. 2015). Furthermore, the IL-10-IL-10R pathway is essential for suppression of EAE by 1,25(OH)2D3 (Spach, Nashold, et al. 2006). 1,25(OH)2D3 induces IDO+ tolerogenic DCs and enhances Treg, reducing the severity of EAE (Farias, Spagnol, et al., 2013). In addition, 1,25(OH)2D3 induces IL-10 production by splenocytes in vitro (Waddell, Zhao, et al., 2015). Our results indicate that 1,25(OH)2D3 treatment increases IL-10 expression in the CNS and its production by splenic CD4 T cells. In addition to its anti-inflammatory effect, IL-10 also protects OLGs from inflammation-induced damage (Molina-Holgado, Grencis et al. 2001), and the increased IL-10 level induced by 1,25(OH)2D3 may directly interact with resident (endogenous) OLGs and neurons locally to promote survival of these cells (Molina-Holgado, Grencis et al. 2001). Thus, the anti-inflammatory effects of 1,25(OH)2D3 treatment can result in a significantly increased number of OLGs, which provides a foundation for enhanced remyelination (Lin, Bailey et al. 2007).
The second possible mechanism of action of 1,25(OH)2D3 on demyelination can be through its direct effect on OPC/OLG lineage. As a therapeutic factor, 1,25(OH)2D3 can infiltrate into the CNS through the blood-brain barrier (BBB), thereby exerting a direct protective effect on neurons and OLGs (Zahir, Klassen et al. 2005, DeLuca, Kimball et al. 2013). 1,25(OH)2D3 could also induce remyelinating through rapid proliferation of OPCs in response to injuries within the CNS (Gacias and Casaccia 2013, Li and Leung 2015). It has also been shown that 1,25(OH)2D3 treatment increased OPC proliferation and OLG differentiation in demyelinated areas leading to remyelination (Gacias and Casaccia 2013). Direct proof that 1,25(OH)2D3 promotes remyelination comes from a cuprizone-induced demyelination model, in which 1,25(OH)2D3 actually promoted the repair process, possibly through a stimulating effect on OLG maturation and astrocyte activation (Nystad, Wergeland et al. 2014). Our own in vitro study demonstrated that 1,25(OH)2D3 has a direct effect on NSC proliferation, survival, and neuron/OLG differentiation, through upregulating neurotrophic factors such as NT-3, BDNF, GDNF and CNTF (Shirazi, Rasouli et al. 2015), providing a mechanism for the observations in the present study in vivo. These results are also consistent with an observation showing that 1,25(OH)2D3 can modulate the transcription of target genes to increase certain locally derived factors, such as basic fibroblast growth factor (Gagelin, Pierre et al. 1995) and, consequently, ciliary neurotrophic factor (Zahir, Klassen et al. 2005). Together, these observations provide a basis for a direct protective and remyelinating effect of 1,25(OH)2D3 treatment in EAE.
It has been suggested that immunomodulation should be considered as a combinatorial therapeutic approach along with a strategy to protect OLGs and promote remyelination (Helming, Bose et al. 2005). Certain repurposed drugs are showing promise for protecting OLGs and for remyelination, and pharmacological targets have shifted from cellular replacement to endogenous repair enhancement (Helming, Bose et al. 2005). 1,25(OH)2D3, in addition to its immunomodulatory capacity, could therefore be a potential neuroregenerative agent through promoting NSC differentiation into OPC/OLG linage and/or the survival of these cells.
Acknowledgments
This study was supported by a grant from the National Institutes of Health (NIH). We thank Katherine Regan for editorial assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adzemovic MZ, et al. (2013). γEfficacy of vitamin D in treating multiple sclerosis-like neuroinflammation depends on developmental stage." Exp Neurol 249: 39–48. [DOI] [PubMed] [Google Scholar]
- Annunziato F, et al. (2015). "The 3 major types of innate and adaptive cell-mediated effector immunity." J Allergy Clin Immunol 135(3): 626–635. [DOI] [PubMed] [Google Scholar]
- Becklund BR, et al. (2009). "Enhancement of 1,25-dihydroxyvitamin D3-mediated suppression of experimental autoimmune encephalomyelitis by calcitonin." Proceedings of the National Academy of Sciences of the United States of America 106(13): 5276–5281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantorna MT, et al. (2000). "In Vivo Upregulation of Interleukin-4 Is One Mechanism Underlying the Immunoregulatory Effects of 1,25-Dihydroxyvitamin D3." Archives of Biochemistry and Biophysics 377(1): 135–138. [DOI] [PubMed] [Google Scholar]
- Cantorna MT, et al. (2015). "Vitamin D and 1,25(OH)2D regulation of T cells." Nutrients 7(4): 3011–3021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cekic M, et al. (2009). "Combination treatment with progesterone and vitamin D hormone may be more effective than monotherapy for nervous system injury and disease." Frontiers in neuroendocrinology 30(2): 158–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhuri A (2005). "Why we should offer routine vitamin D supplementation in pregnancy and childhood to prevent multiple sclerosis." Medical hypotheses 64(3): 608–618. [DOI] [PubMed] [Google Scholar]
- Compston A and Coles A (2008). "Multiple sclerosis." Lancet 372(9648): 1502–1517. [DOI] [PubMed] [Google Scholar]
- Constantinescu CS, et al. (2011). "Experimental autoimmune encephalomyelitis (EAE) as a model for multiple sclerosis (MS)." Br J Pharmacol 164(4): 1079–1106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cudrici C, et al. (2006). "Oligodendrocyte cell death in pathogenesis of multiple sclerosis: Protection of oligodendrocytes from apoptosis by complement." J Rehabil Res Dev 43(1): 123–132. [DOI] [PubMed] [Google Scholar]
- Dehghani L, et al. (2013). "Can vitamin d suppress endothelial cells apoptosis in multiple sclerosis patients?" International journal of preventive medicine 4(Suppl 2): S211–215. [PMC free article] [PubMed] [Google Scholar]
- DeLuca GC, et al. (2013). "Review: the role of vitamin D in nervous system health and disease." Neuropathol Appl Neurobiol 39(5): 458–484. [DOI] [PubMed] [Google Scholar]
- Disanto G, et al. (2012). "Vitamin D receptor binding, chromatin states and association with multiple sclerosis." Hum Mol Genet 21(16): 3575–3586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumitrascu OM, et al. (2014). "A comparative study of experimental mouse models of central nervous system demyelination." Gene Ther 21(6): 599–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farias AS, et al. (2013). "Vitamin D3 induces IDO+ tolerogenic DCs and enhances Treg, reducing the severity of EAE." CNS Neurosci Ther. 19(4):269–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin RJ and Ffrench-Constant C (2008). "Remyelination in the CNS: from biology to therapy." Nat Rev Neurosci 9(11): 839–855. [DOI] [PubMed] [Google Scholar]
- Gacias M and Casaccia P (2013). "Promoting return of function in multiple sclerosis: An integrated approach." Mult Scler Relat Disord 2(4). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagelin C, et al. (1995). "Rapid TGF beta 1 effects on actin cytoskeleton of astrocytes: comparison with other factors and implications for cell motility.” Glia 13(4): 283–293. [DOI] [PubMed] [Google Scholar]
- Griffin MD, et al. (2000). "Potent inhibition of dendritic cell differentiation and maturation by vitamin D analogs." Biochem Biophys Res Commun 270(3): 701–708. [DOI] [PubMed] [Google Scholar]
- Grytten N, et al. (2006). "A 50-year follow-up of the incidence of multiple sclerosis in Hordaland County, Norway." Neurology 66(2): 182–186. [DOI] [PubMed] [Google Scholar]
- Helming L, et al. (2005). "1alpha,25-Dihydroxyvitamin D3 is a potent suppressor of interferon gamma-mediated macrophage activation." Blood 106(13): 4351–4358. [DOI] [PubMed] [Google Scholar]
- Huang YN, et al. (2015). "1,25-Dihydroxyvitamin D3 attenuates endotoxin-induced production of inflammatory mediators by inhibiting MAPK activation in primary cortical neuron-glia cultures." J Neuroinflammation 12: 147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N and Leung GK (2015). "Oligodendrocyte Precursor Cells in Spinal Cord Injury: A Review and Update." Biomed Res Int 2015: 235195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, et al. (2013). "Inhibitory effect of IL-17 on neural stem cell proliferation and neural cell differentiation." BMC Immunol 14: 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin W, et al. (2007). "The integrated stress response prevents demyelination by protecting oligodendrocytes against immune-mediated damage." J Clin Invest 117(2): 448–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucchinetti C, et al. (2001). "Multiple sclerosis: recent developments in neuropathology, pathogenesis, magnetic resonance imaging studies and treatment." Curr Opin Neurol 14(3): 259–269. [DOI] [PubMed] [Google Scholar]
- Matheu V, et al. (2003). "Dual effects of vitamin D-induced alteration of TH1/TH2 cytokine expression: enhancing IgE production and decreasing airway eosinophilia in murine allergic airway disease." J Allergy Clin Immunol 112(3): 585–592. [DOI] [PubMed] [Google Scholar]
- Mayne CG, et al. (2011). "1,25-Dihydroxyvitamin D3 acts directly on the T lymphocyte vitamin D receptor to inhibit experimental autoimmune encephalomyelitis." Eur J Immunol 41(3): 822–832. [DOI] [PubMed] [Google Scholar]
- Molina-Holgado F, et al. (2001). "Actions of exogenous and endogenous IL-10 on glial responses to bacterial LPS/cytokines." Glia 33(2): 97–106. [PubMed] [Google Scholar]
- Munger KL, et al. (2006). "Serum 25-hydroxyvitamin D levels and risk of multiple sclerosis." JAMA 296(23): 2832–2838. [DOI] [PubMed] [Google Scholar]
- Nystad AE, et al. (2014). "Effect of high-dose 1.25 dihydroxyvitamin D3 on remyelination in the cuprizone model." APMIS 122(12): 1178–1186. [DOI] [PubMed] [Google Scholar]
- Pikor NB, et al. (2015). "Meningeal Tertiary Lymphoid Tissues and Multiple Sclerosis: A Gathering Place for Diverse Types of Immune Cells during CNS Autoimmunity." Front Immunol 6: 657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pombo PM, et al. (1999). "Stimulation of the myelin basic protein gene expression by 9-cis-retinoic acid and thyroid hormone: activation in the context of its native promoter." Brain Res Mol Brain Res 64(1): 92–100. [DOI] [PubMed] [Google Scholar]
- Shirazi HA, et al. (2015). "1,25-Dihydroxyvitamin D3 enhances neural stem cell proliferation and oligodendrocyte differentiation." Exp Mol Pathol 98(2): 240–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smolders J, et al. (2009). "Vitamin D status is positively correlated with regulatory T cell function in patients with multiple sclerosis." PLoS ONE 4(8): e6635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spach KM, et al. (2006). "IL-10 signaling is essential for 1,25-dihydroxyvitamin D3-mediated inhibition of experimental autoimmune encephalomyelitis." J Immunol 177(9):6030–6037. [DOI] [PubMed] [Google Scholar]
- Steinman L (2001). "Multiple sclerosis: a two-stage disease." Nat Immunol 2(9): 762–764. [DOI] [PubMed] [Google Scholar]
- Takahashi K, et al. (2007). "TREM2-transduced myeloid precursors mediate nervous tissue debris clearance and facilitate recovery in an animal model of multiple sclerosis." PLoS Med 4(4): e124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang J, et al. (2009). "Calcitriol suppresses antiretinal autoimmunity through inhibitory effects on the Th17 effector response." Journal of immunology (Baltimore, Md : 1950) 182(8): 4624–4632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waddell A, et al. (2015). "NKT cells can help mediate the protective effects of 1,25-dihydroxyvitamin D3 in experimental autoimmune encephalomyelitis in mice." Int Immunol. 27(5):237–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wergeland S, et al. (2011). "Dietary vitamin D3 supplements reduce demyelination in the cuprizone model." PLoS ONE 6(10): e26262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, et al. (2010). "Evaluation of bone marrow- and brain-derived neural stem cells in therapy of central nervous system autoimmunity." Am J Pathol 177(4): 1989–2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, et al. (2014). "Neurotrophin 3 transduction augments remyelinating and immunomodulatory capacity of neural stem cells." Mol Ther 22(2): 440–450. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Zahir T, et al. (2005). "Effects of ciliary neurotrophic factor on differentiation of late retinal progenitor cells." Stem Cells 23(3): 424–432. [DOI] [PubMed] [Google Scholar]