Transitions of care, such as those that occur at hospital admission and discharge, are vulnerable times for patients with respect to medication safety.1–3 Medication errors are common during these times of transition, resulting from a number of factors, including discontinuity of providers and information,4–6 multiple changes in medication regimens,5,7–9 rushed discharge processes,5 and inadequate or ineffective patient/caregiver counseling.4,10–12
Of particular concern are medication errors due to unexplained differences in documented medication regimens across different sites of care, occurring in up to 70% of patients at admission or discharge.13–17 These differences in documentation can then easily lead to unexplained discrepancies in actual medication orders. Of these discrepancies, nearly one third have potential to cause patient harm (that is, potential adverse drug events).17 Adverse drug events (ADEs) resulting from medication discrepancies include unintended medication side effects and potentially unnecessary utilization of resources, such as prolonged hospital stays and increased emergency room visits or hospital readmissions.18,19
Medication reconciliation, the “process of identifying the most accurate list of all medications a patient is taking … and using this list to provide correct medications for patients anywhere within the health care system,” is a strategy to reduce the occurrence of medication discrepancies.20 In 2005, in recognition of the potential impact of reconciling medications during care transitions, The Joint Commission added medication reconciliation to its list of National Patient Safety Goals—“Accurately and completely reconcile medications across the continuum of care.”21 Although straightforward in concept, the process of medication reconciliation is complex, and therefore, this mandate proved difficult for health care institutions to implement, and in 2011 The Joint Commission modified its requirements.22 Although some literature reports successful medication reconcilation implementation efforts,23 the practices have yet to be widely accepted and disseminated.
In March 2009 the Society of Hospital Medicine (SHM) convened an Agency for Healthcare Research and Quality (AHRQ)–sponsored conference focused on identifying and addressing (1) barriers to implementation of a successful medication reconciliation program, (2) opportunities to identify best practices surrounding medication reconciliation, (3) the role of partnerships among traditional health care sites and nonclinical and other community-based organizations, and (4) metrics for measuring processes involved in medication reconciliation and their impact on preventing harm to patients. The results of the conference were summarized in a white paper outlining a set of key action items and recommendations for making progress in this area.24
Using these recommendations, a team of researchers and advisory staff were recruited by SHM to operationalize medication reconciliation efforts on the basis of these key action items and evaluate their effects on patient safety. In September 2010 funding was obtained from AHRQ to carry out a study, entitled the Multi-Center Medication Reconciliation Quality Improvement Study (MARQUIS), which aims to (1) develop a toolkit of the best-practice recommendations for medication reconciliation, conduct a multihospital mentored quality improvement (QI) project in which each site adapts the tools for its own environment and implements them, (3) assess the effects of that intervention on unintentional medication discrepancies with potential for patient harm, and (4) conduct rigorous program evaluation to determine the most important components of a medication reconciliation program and the best methods of implementation. The study’s steering committee includes representation from various health care professions (medicine, nursing, pharmacy) and, importantly, includes many of the original SHM/AHRQ conference organizers and attendees, allowing for continuity in converting the suggested recommendations into actual interventions. In this article, we describe how the intervention toolkit was created, outline the components of the toolkit, and report experience with implementation at the first two sites and lessons learned to date. We describe the study’s methodology elsewhere.25
The MARQUIS team consists of clinician investigators and interdisciplinary steering committee members who collectively have content expertise in medication reconciliation and experience with conducting rigorous evaluations of medication safety interventions. Central project management support is provided by SHM, an organization that has substantial experience in conducting multihospital mentored QI projects.26
This project uses a mentored implementation strategy, as has been modeled in other SHM–led projects, including Project BOOST (Better Outcomes by Optimizing Safe Transitions),27 the Venous Thromboembolism Prevention Collaborative (VTE PC),28 and the Glycemic Control Mentored Implementation (GCMI) project.29 This approach was recognized by The Joint Commission and the National Quality Forum with the John M. Eisenberg Patient Safety and Quality Award.26 The overarching strategy of the mentored implementation model is to employ a small group of hospitalists with QI expertise to serve as mentors to help groups of hospitals achieve specific goals through implementation of a set of best-practice interventions using QI methods. As part of the mentoring process for MARQUIS specifically, the hospitalist QI experts/mentors engage in monthly phone calls with QI team leaders at each site. In addition, the mentor visits each site twice (3 to 4 months and 12 months after the start of the intervention) to directly observe the interventions and meet with QI team members and other clinicians as well as hospital leadership. Similar to other SHM–led mentored implementation QI programs funded by a foundation or governmental agency, the toolkit created by the team is freely available via the Internet for download and use to facilitate widespread dissemination of medication reconciliation QI efforts across a broad range of health care organizations and systems.
Tool Development
From October 2010 through January 2011, the MARQUIS research team members started by performing a collaborative review of the previously determined medication reconciliation action items and recommendations from the white paper24 and transforming each item into draft components of the intervention toolkit. Concurrently, a systematic review of the literature on most effective practices of inpatient medication reconciliation was performed.23 Following these initial steps, the research team and steering committee members further developed the content for each draft intervention component, synthesizing evidence from the literature, expert opinion, and any available examples of best practices, including results from the Medications at Transitions and Clinical Handoffs (MATCH) study.30 A two-day steering committee conference was held in January 2011, during which the content for each intervention component was presented and discussed by the group to foster consensus.
Following the conference, investigators participated in weekly phone meetings with quarterly input from steering committee members to further refine the components and create specific tools and measurements that comprise the MARQUIS toolkit.
Tool Description
The toolkit (Table 1, pages 373–374) is composed of the following three major sections:
Table 1.
Multi-Center Medication Reconciliation Quality Improvement Study (MARQUIS) Toolkit Components*
| A. First Steps for Success: Preparation and Site Assessment | Examples |
|---|---|
| Review of Basic Quality Improvement Principles Additional materials available in Appendix (1, 2) |
▪ Start preimplementation planning ▪ Clarify key stakeholders ▪ Obtain institutional support |
| Define Steps of Medication Reconciliation process | ▪ Take BPMH on admission ▪ Reconcile BPMH with admission orders ▪ At time of transfer or discharge, reconcile BPMH and current medication orders ▪ At discharge, provide discharge medication counseling to the patient and an accurate medication list to patient/caregiver ▪ At discharge, communicate medication list with outpatient provider |
| Brief Literature Review | ▪ Referenced† |
| Assemble Team and Develop a Strategy | ▪ Identify team members, including team leader, QI team facilitator, etc. ▪ Map current medication reconciliation process, perform gap analysis ▪ Create specific, measurable goals |
| B. Intervention Components | Examples |
| I. Assigning Roles and Responsibilities | ▪ Identify resources available for each person to complete task ▪ Outline required knowledge, skills, and behaviors for person performing each med reconciliation step ▪ Encourage ideal use of personnel to increase efficiency ▪ Assign “ownership” for the overall medication reconciliation process |
| II. Improve Access to Preadmission Medication Sources | ▪ Empower patients/caregivers to own medication list ▪ Improve skills of inpatient medication history-takers ▪ Improve IT access to sources of medication information |
| III. Patient-Owned Medication Lists Additional materials available in Appendix 4 |
▪ Provide patient with templated medication list on discharge ▪ Provide patients with medication lists in the ambulatory setting to support admission list creation |
| IV. Guidelines for Taking a BPMH Instructional video available Additional materials available in Appendix (3, 4) |
▪ Use at least two different sources of information in compiling BPMH (for example, patient, medication list, pharmacy, etc.) ▪ Resolve any discrepancies between sources ▪ Use probing questions |
| V. Discharge Counseling Instructional video available Additional materials available in Appendix (4) |
▪ Correctly identify the “active learner” ▪ Review the entire medication list, including new meds, changes and discontinued meds, instructions, and potential side effects ▪ Use the “teach-back” technique |
| VI. Risk Stratification | ▪ Stratify patients into high vs. low-intermediate risk based on patient characteristics (for example, number of preadmission medications, etc.) such that high-risk patients receive additional resources (see VII.) |
| VII. Use of Medication Reconciliation Bundle Additional materials available in Appendix (6, 7) |
▪ Ensure that high-risk patients have medication histories obtained from and medication counseling performed by the most highly skilled personnel with time and resources to perform an “intensive” medication reconciliation bundle |
| VIII. Application of IT, Ideal Features Additional materials available in Appendix (7) |
▪ Increase access to sources of preadmission medication information ▪ Facilitate comparison and reconciliation of medication lists |
| IX. Phased Implementation | ▪ Phase in interventions by location or service, patient risk, or component |
| X. Social Marketing/Engagement of Community Additional materials available in Appendix (8) |
▪ Determine target audience (for example, inpatient or outpatient providers, patients) |
| C. Appendix (Supplemental Material) | Examples |
| 1. Making Business Case for Medication Reconciliation | ▪ Demonstrate potential for return on investment |
| 2. MARQUIS Institutional Site Assessment | ▪ Assess institutional support ▪ Assess existence of on-site study pharmacist ▪ Assess policies and procedures for medication reconciliation |
| 3. Best Possible Medication History (BPMH) Toolkit | ▪ Case for role-play |
| 4. Patient-Friendly Discharge Material | ▪ Examples provided |
| 5. Patient-Owned Medication Lists | ▪ Adopt a medication template, provide patient/caregiver with template on discharge |
| 6. Paper Medication Reconciliation Forms | ▪ Examples provided |
| 7. Vendors of Electronic Medication Reconciliation Products | ▪ Examples provided |
| 8. Social Marketing Materials | ▪ Examples provided |
Appendices refer to those available with the implementation manual at Society of Hospital Medicine. Overview: Multi-center Medication Reconciliation Quality Improvement Study (MARQUIS). Accessed Jun 27, 2013. http://www.hospitalmedicine.org/MARQUIS. BPMH, best possible medication history; QI, quality improvement; IT, information technology.
Mueller SK, et al. Hospital-based medication reconciliation practices: A systematic review. Arch Intern Med. 2012 Jul 23;172(14):1057–1069.
Section A. First steps a hospital should undertake before beginning any interventions, including preparation and site assessment, to allow for maximum likelihood of successful implementation
Section B. MARQUIS intervention components
Section C. Appendix material, which supplements the narrative components of the implementation guide with ready-to-use tools.
Unlike many intervention projects, several components of this toolkit are intended to be customized as needed at each site on the basis of existing personnel and work-flow structures and previous medication reconciliation QI efforts, thus enhancing applicability, generalizability, and “shelf life.”
Section A. First Steps For Success: Preparation and Site Assessment
This section of the guide reviews key QI principles necessary for successful implementation, including the importance of preimplementation planning, identifying key stakeholders, obtaining institutional support, and assembling an effective multidisciplinary QI team to carry out the project. Because implementation of this type of intervention requires hospital-level commitment, resources, and time, we emphasize the talking points necessary to obtain institutional support, including ongoing benefits to patient safety and the return on investment (ROI) in terms of decreased inpatient ADEs and hospital readmissions. In the appendix (Section C), we also include links to a spreadsheet so that sites can customize their own ROI calculations. The implementation guide also highlights the importance of understanding the institution’s current practices of medication reconciliation and ongoing QI efforts in this area. Recommendations to achieve this understanding include performing process mapping and a gap analysis between current and ideal processes.
The ideal medication reconciliation process, as proposed by the MARQUIS team (Figure 1, page 375, and available in color in online article), is provided in this section of the toolkit to assist with these efforts. For example, on the basis of the literature, the guide recommends robust involvement of pharmacists in medication reconciliation processes, communicating with postdischarge providers, and focusing efforts on patients at highest risk for ADEs. Descriptions of each step of medication reconciliation and the skills required can help sites in matching individual tasks to the personnel and roles best able to complete those tasks at their site. The toolkit also includes a site assessment, adapted from another AHRQ–funded toolkit,31 to be used before implementation to help the QI teams assess their current environment and readiness.
Figure 1.
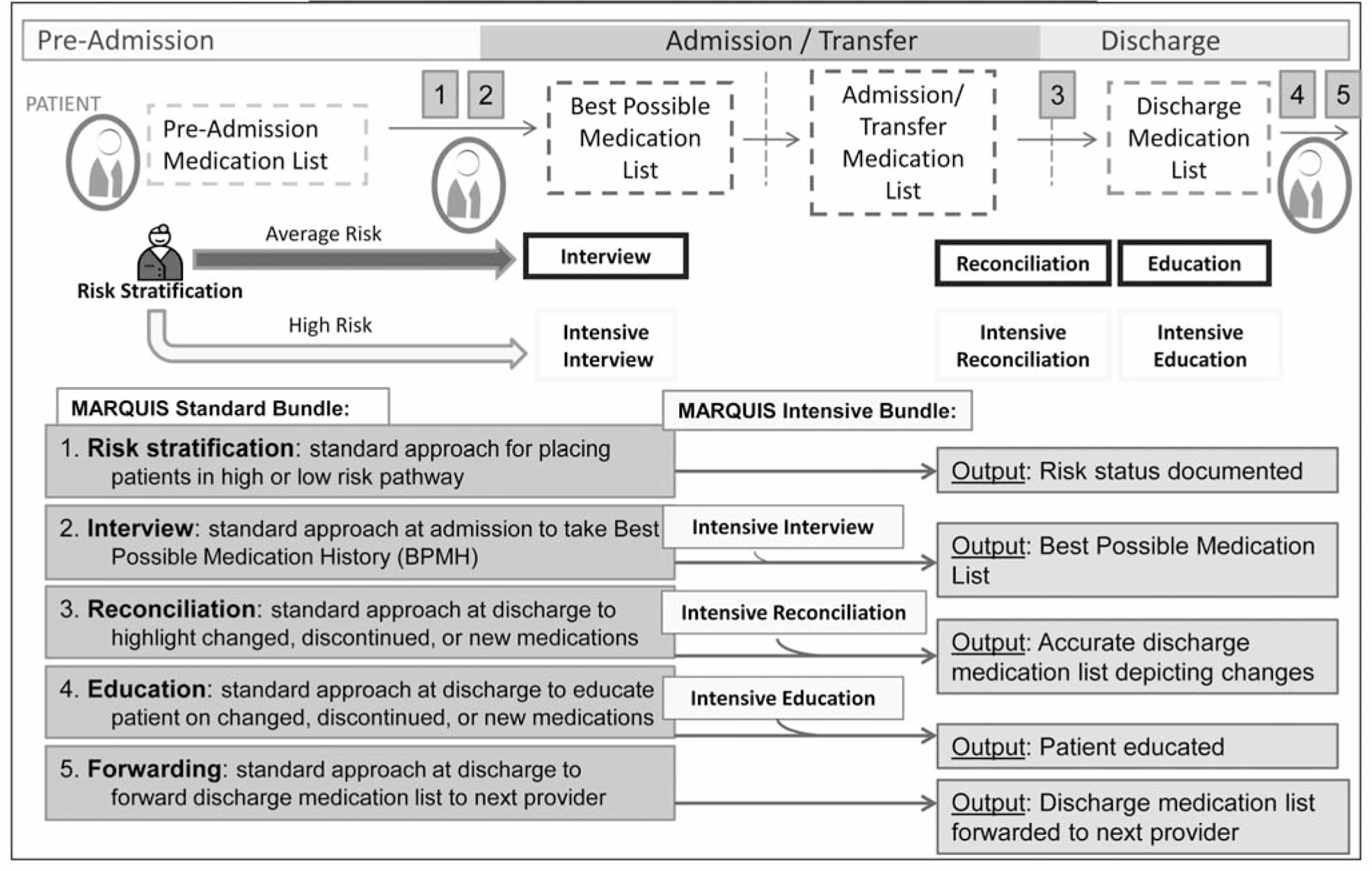
The ideal medication reconciliation process begins at time of patient admission, where patients are stratified into average- versus high-risk pathways, based on particular baseline patient characteristics. Both average- and high-risk patients then proceed through the same subsequent four steps in the medication reconciliation process that differ only in the additional dedicated time and expertise that is required for the intensive medication reconciliation bundle. These subsequent four steps are (2) Admission interview to obtain the best possible medication history (BPMH); (3) Standard approach toward discharge reconciliation of medications, where medication changes, discontinuations, and new medications are highlighted; (4) Standard approach to education of patient at time of discharge on any changed, discontinued, or new medications; and (5) Standard approach of forwarding discharge medication list to the follow-up provider(s). Notably, in the ideal medication reconciliation process, reconciliation occurs at time of admission, any transfer of service during patient hospitalization, and discharge. (Available in color in online article).
Section B. Intervention Components
Individual intervention components in the toolkit address all aspects of the proposed ideal medication reconciliation process, including methods, tools, and guidance for implementation, as well as specific metrics for measuring its effectiveness. Certain intervention components (that is, those that are the most evidence-based) can be grouped together to comprise a core set of interventions, while other components may be chosen on the basis of the institution’s self-assessment, process mapping, and gap analysis. The individual components include methods of obtaining an accurate medication history from the patient or other sources (including how to perform a “best possible medication history” [BPMH]),32,33 methods of empowering patients or their caregivers to take ownership of the medication list, discharge counseling techniques, and patient risk stratification for intensification of resources for high-risk patients. The intervention components also reemphasize basic QI principles, including the importance of assigning roles and responsibilities to clinical care team members and stressing the importance of phased implementation.
Additional intervention components highlight various high-risk/high-reward features of the medication reconciliation process, including incorporation of effective information technology (IT) components and social marketing techniques. These components may require substantial resources, planning, and institutional commitment. Therefore, sites are encouraged to decide early whether or not they wish to pursue these efforts, and include these decisions when obtaining institutional support for this intervention.
Intervention components believed to be high yield by the MARQUIS team and most likely to achieve rapid and substantial improvements in medication safety are highlighted throughout the implementation guide, including (1) training clinical personnel in taking a BPMH and in performing health literacy-sensitive discharge medication education, (2) risk-stratifying patients, and (3) providing high-risk patients with an intensive medication reconciliation bundle (as depicted in Figure 1). It is emphasized in the guide that having adequate time and personnel dedicated to performing these tasks is essential. Finally, as part of the mentored implementation process, mentors emphasize with sites the need for “functional standardization,” that is, agreement on a specific set of goals to be achieved with each intervention component, even as sites are given some flexibility to determine exactly how each goal will be achieved.34 For example, although training clinical personnel in taking a BPMH is a standardized component of this intervention, the personnel used to perform this task is flexible, depending on the particular site-specific resources available.
Section C. Appendix, Supplemental Material
To supplement the narrative components of the intervention toolkit, we also include an appendix with ready-to-use tools that assist in various intervention components, including instructional material on how to obtain senior leadership buy-in (with ROI calculations), perform a site assessment, and train personnel on taking a BPMH. Examples are also provided for patient-friendly discharge instructions, patient-owned medication lists, paper and electronic medication reconciliation forms, vendors of products that can enhance the medication reconciliation and discharge process, and social marketing materials.
Finally, this toolkit section includes links to several additional useful references, including the following:
An instructional video on how to perform high-quality discharge education, which emphasizes approaches for effective patient communication, including use of the “teach-back” technique.35 This video also provides examples performed by actors of both inadequate medication education and effective medication education, allowing for reflection on both examples.
Materials on how to take a BPMH, including an instructional video that models the process, didactic slide deck, a case study for role-playing, and pocket cards for clinicians (see link below for all MARQUIS materials).
Tool Application Settings
The toolkit is currently designed to be used in non–intensive care medical/surgical units caring for adult hospitalized patients. The toolkit would need further customization to be used for pediatric or ICU settings. In the case of pediatrics, more emphasis would need to be placed on the involvement of parents as the primary caregivers, clinical examples (for example, for videos and role-plays) would need to be modified, and attention would need to paid to issues specific to pediatrics (such as weight-based dosing and emancipated minors). In the ICU setting, the toolkit would need to emphasize situations in which patients are often unable to communicate, not taking oral medications, ordered intravenous medications with potential drug-drug interactions for a short period of time, and then transferred to medical/surgical wards or step-down units.
How-To
The implementation guide toolkit and the supplemental educational materials are now available for public use.36
To date, the intervention has been implemented for the longest period of time (more than 22 months) at two of the six participating MARQUIS sites. Case summaries of their experiences at the 13-month point of implementation are now provided.
SITE 1
This 110-bed community hospital affiliate of an academic tertiary care center has an engaged medication reconciliation QI committee, led by its chief quality officer. Their QI committee also includes hospitalists, nursing unit directors, the pharmacy director, a discharge education nurse specialist, and nurses from the emergency department (ED) and inpatient units. This team initially chose to work on four components of the intervention: (1) moving primary responsibility for medication histories from the ED to nurses on inpatient units, (2) training nurses on how to take a BPMH, (3) training providers on effective discharge medication counseling for average-risk patients and their families and caregivers, and (4) risk-stratifying patients and performing intensive medication history-taking and discharge medication counseling in these patients.
Site 1 has made progress in several areas:
Provider training in taking a BPMH. A formal training program has been put in place, including a case presentation highlighting a sentinel case at their hospital that was due to medication reconciliation errors, a slide presentation on obtaining a BPMH followed by a discussion, and distribution of laminated BPMH pocket cards (from the MARQUIS toolkit) to nurses and ordering providers. A “train the trainer” model was established to widely implement this intervention.
Risk stratification. The MARQUIS risk assessment tool was customized for use at their site. Nursing staff on target units accepted responsibility for completing the tool and faxing the form to the pharmacy office to prompt pharmacy involvement with high-risk patients. To date, this intervention has been limited in scope, and currently, only patients with congestive heart failure receive intensive discharge counseling, in this case, from a dedicated education nurse specialist on the heart failure service. In the future, it is hoped that pharmacy technicians can play a more active role in taking medication histories and that team-based pharmacists can perform intensive discharge medication counseling in high-risk patients without congestive heart failure.
Provider training in discharge education and feedback. The educational video on discharge counseling was widely shown to nursing staff. The nurse specialist who is providing discharge counseling on the heart failure service has been training nurses and other providers in best practices in medication counseling through direct observation, feedback, and coaching.
Patient-owned medication lists and community engagement. The QI committee explored branding a medication list wallet-sized card to be used by patients. Current plans include working with the community to promote use of the card and explain to the public why it is important to keep an updated medication list with them at all times.
Improved bidirectional flow of medication information. This site has begun conversations with their highest-volume post–acute care facilities and local pharmacies to improve flow of medication information to and from the hospital. It is also planning to develop a memorandum of understanding between the hospital and these facilities to facilitate timely sharing of medication lists at admission and discharge.
This site’s mentor noted several contributors to success with its early efforts, including effective identification and dissemination of information about serious ADEs that had recently occurred at the hospital because of poor medication reconciliation processes. This transparency raised awareness and enhanced motivation among staff and leadership. The site has also benefited from the enthusiasm and support of nursing leadership, who have been willing to champion this effort among their staff. A third facilitating factor was a trusting and productive relationship between the leader of the QI committee and the MARQUIS mentor. Barriers and challenges to success identified by the QI team have included leadership turnover; a shortage of clinicians to perform medication reconciliation, including pharmacists and nurses on the intervention units and limited availability of pharmacy technicians; and competing priorities, including implementation of a new electronic medical record and other QI projects. The new electronic medical record also made it difficult to integrate a risk stratification form into work flow.
SITE 2
The QI team at this 535-bed community nonteaching hospital, which is associated with a large regional health care system, is led by the vice president for clinical improvement, with other members of the team consisting of hospitalists, clinical pharmacists, nurses, and the on-site study pharmacist. The team’s initial intervention targets included training in taking a BPMH and good discharge medication education, as well as giving feedback to providers on the quality of their medication histories.
The site had made progress in the following four areas:
BPMH training. BPMH training video and pocket cards are used by nursing and pharmacy staff on target units to perform medication histories for patients who are directly admitted, transferred from other hospitals, or admitted on weekend nights. The BPMH training complements a preexisting initiative that uses specially trained pharmacy technicians, called Medication Reconciliation Assistants (MRAs), supervised by a pharmacist, to take medication histories for all ED patients admitted during the week and weekend days.
Risk stratification. Development of an automated screening tool to identify high-risk patients and having clinical pharmacists attend daily interdisciplinary rounds to determine which patients require high-intensity discharge education.
High-intensity bundle in high-risk patients. Plans are in place to formally train a cohort of pharmacists and discharging providers to provide high-intensity discharge education for high-risk patients, thus providing additional capacity to offer this intervention to a greater number of patients.
Discharge education. The discharge education training video was placed on the organization’s intranet to complement existing education modules on “teach-back” and “Ask Me 3,” a patient education program designed to promote communication between patients and clinicians.37 Clinical pharmacists can now flag high-risk patients for intensive discharge counseling in their work flow database and see which high-risk patients have received in-hospital intensive counseling and which have not. This complements an existing program that provides postdischarge counseling by telephone for a subset of patients (for example, older patients with a primary care physician employed by the hospital system, discharged home, referred to the program, or on multiple or high-risk medications) run by the regional health care system.
Key factors promoting success include the dedication of the QI team leader and on-site study pharmacist, full support from the leadership of the regional health care system, and integration of the intervention components with previously adopted site initiatives, such as the use of MRAs and the postdischarge education program for high-risk patients. Barriers have included an insufficiently large QI team to lead intervention efforts, difficulty in engaging frontline staff, limited availability of MRAs on weekend nights and for patients not admitted through the ED (and overreliance on MRAs even for patients in whom they are not available), lack of access to administrative data to help guide next steps, and lack of educators trained in the use of teach back.
Results and Lessons Learned
Throughout these efforts to date, several lessons have been learned. First, tools such as those provided by MARQUIS were helpful only if frontline staff were motivated to use them. Hearing stories of patient harm from medication reconciliation errors resonated with staff and facilitated buy-in. Early data collection can be used to help identify these cases in the absence of a case already obvious to frontline staff. Second, early data collection and analyses can also be used to help sites understand current gaps in care. For example, Site 2 needed to know medication discrepancy rates among patients not affected by MRAs (for example, weekend night admissions, direct admissions) in comparison with those of other patients to be able to appreciate the need to devote more staff to those patients. Third, nursing leadership support was critical for nursing staff to take on additional roles and responsibilities regarding medication reconciliation, given their existing workload. Fourth, the need for further educational tools also became apparent. In response, we added a video on how to take a BPMH, as well as a process map/“swim lane” diagram (Figure 2, pages 378–379) that can be customized for each site to allow QI and clinical personnel to document a shared understanding of the envisioned ideal medication reconciliation process, including everyone’s role throughout the hospitalization. A certification program to evaluate and document competency in these new skills (that is, whether staff have integrated what they have learned into actual practice) is in development. Fifth, these experiences have also highlighted the fact that measuring medication processes of care can be difficult. Wherever possible, measurement should be built into the activity (for example, using a form for risk stratification or the high-intensity medication reconciliation bundle that creates a “paper trail”). For some processes, direct observation and brief interviews with providers may be necessary. More difficult to measure are those processes whose success is in the eye of the beholder. For example, did a patient receive the desired education and learn the appropriate material? In these cases, targeted patient interviews may be needed. Finally, the site visits were invaluable for objectively seeing how a process really works. For example, the visit to Site 1 allowed the mentor to see the need for certification of competency in medication history-taking and patient counseling, enabling the mentor to give advice on how to make the process more efficient, and creating clarity on what next steps were necessary. It was also invaluable for facilitating buy-in from staff and leadership. In the future, site visits might be scheduled earlier in the project time line (for example, soon after a QI team has been assembled).
Figure 2.
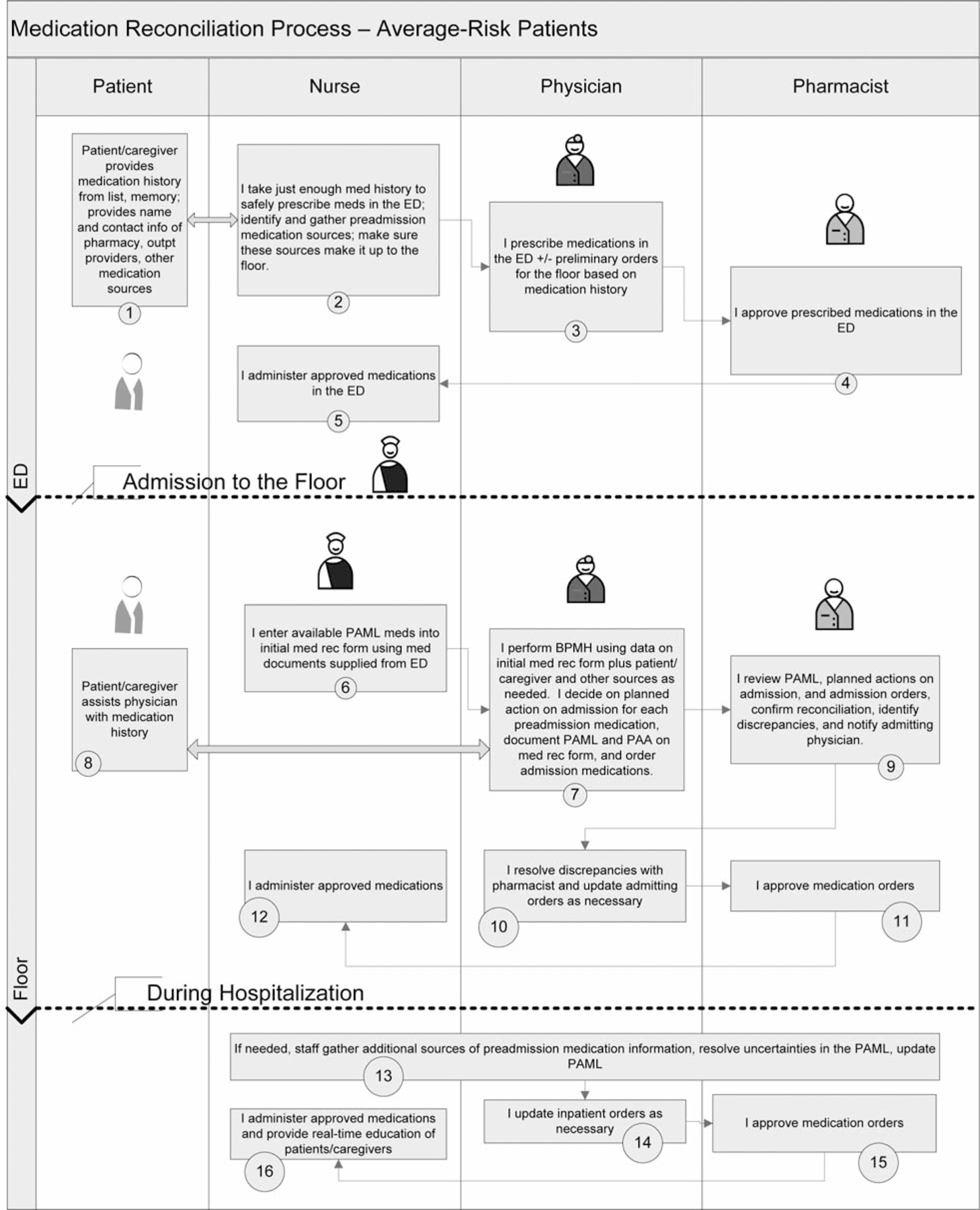
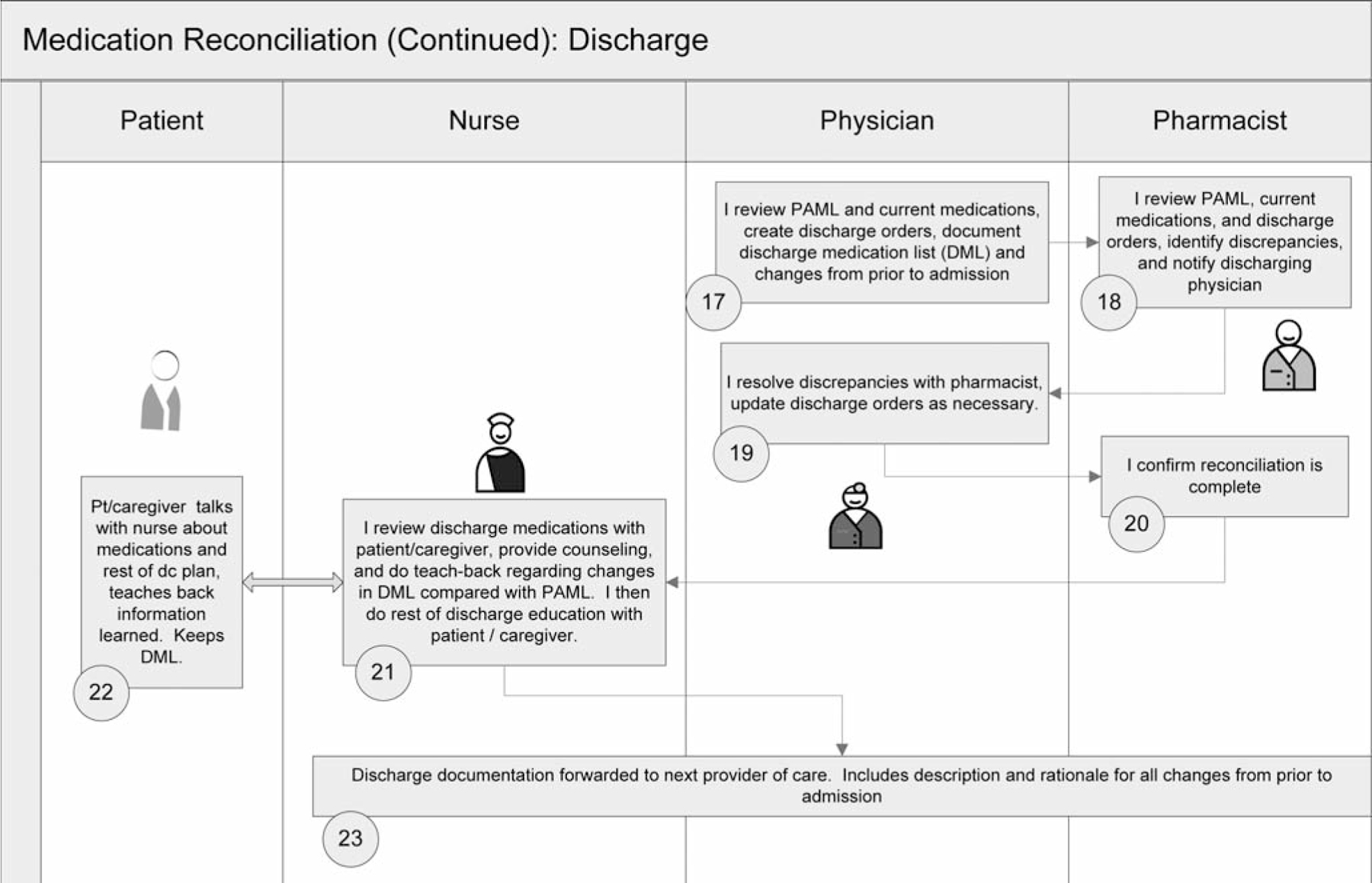
This process map/“swim lane” diagram can be customized for each site that allows QI and clinical personnel to visualize and document a shared understanding of the envisioned ideal medication reconciliation process, including everyone’s role throughout the hospitalization and discharge. ED, emergency department, PAML, preadmission medication list; BPMH, best possible medication history; PAA, planned action on admission; DML, discharge medication list. (Available in color in online article.)
SATISFACTION/FEEDBACK ON THE INTERVENTION
Nine months into the intervention, site leaders at both sites were surveyed to assess satisfaction and feedback on the program. Feedback was most positive regarding the mentorship process and the two training videos (taking a BPMH and discharge counseling). Tools from the MARQUIS Implementation Guide were slightly less positively rated, and the Web-based Data Center (for entering data and viewing reports) received mixed reviews. Perception of the study’s impact on patient outcomes to date varied somewhat by site. Site 1 was more positive about the perceived impact of MARQUIS on the quality and efficiency of medication reconciliation. Both sites commented on the amount of work required to improve medication reconciliation and cited limited resources as a challenge to implementation.
Summary and Next Steps
The goal of the MARQUIS study is to consolidate what is known about best clinical practice; provide a set of guidelines, tools, and approaches that can be readily adapted by sites to improve their local efforts; and evaluate the effects of this intervention on medication safety. In this article we describe our efforts to date in addressing the first two aims of this study; namely, developing a toolkit of the best-practice recommendations for medication reconciliation, and conducting a multihospital mentored QI project in which each site adapts the tools for its own environment and implements them. Next steps will involve addressing the second two aims of this study, including formal evaluation of the effectiveness of the toolkit in improving patient safety (Aim 3) and evaluating which intervention components and approaches to implementation are most important to the success of this effort (Aim 4).
To assess the effectiveness of this intervention on improving patient safety, we are examining how this intervention affects unintentional medication discrepancies with potential for patient harm. This is determined by a trained on-site pharmacist taking a “gold standard” medication history on a random sample of patients (20–25 per month), which is then compared with the primary team’s medication history and to admission and discharge orders. Pharmacists review all discrepancies, including discussion with the medical team if necessary, to distinguish intentional versus unintentional discrepancies. Physician adjudicators, blinded to the status of intervention implementation, then record and categorize unintentional medication discrepancies with respect to (1) timing (admission versus discharge), (2) type (for example, omission, additional medication, change in dose), (3) reason (history versus reconciliation error), (4) potential for harm, and (5) potential severity.
To determine Aim 4 (conduct rigorous program evaluation to determine the most important components of a medication reconciliation program and the best methods of implementation), we will evaluate the influence of contextual factors (such as patient safety culture and staffing levels), intervention fidelity (that is, the faithfulness with which each component of the intervention is implemented in actual practice), and intervening variables (such as perceived effectiveness of training) on implementation and outcomes using a mixed methods approach. Data will be collected by a combination of staff and site surveys, direct observation during site visits, focus groups, and interviews. To assess the most important components of the intervention, monthly “scoring system” surveys of site leaders are conducted to determine the state of each component of medication reconciliation at their site (informed by surveys of providers when necessary), and then using statistical models to determine their temporal impact on changes in discrepancy rates (for example, a sudden improvement in discrepancy rates after a certain intervention component is turned on).
While these evaluations are ongoing, we invite other hospitals to use the provided intervention components, adapt and adopt the tools for their own use, evaluate their use, and give us feedback on how to further improve these tools. We hope to extend mentored implementation to additional hospitals in the future.
Limitations
There are several limitations to this study. First, we are assessing potential and not actual ADEs. An evaluation of ADEs would have greatly increased the time and expense of the study and would have increased the data collection burden of sites and their patients. The relationship between potential and actual ADEs has been established in other studies.38 Second, we do not formally evaluate the effects of patient education (with the exception of using teach-back as part of the intervention to confirm patient understanding). Several other studies conducted by these investigators have shown the effects of counseling interventions on patients’ understanding, adherence, and management of medications.39–44 Lastly, we did not engage primary care physicians to learn their opinions of the interventions on the subsequent care of these patients. This would be a fruitful avenue for further study.
Conclusion
Medication safety during transitions in care remains a major issue in patient safety, resulting in calls to action to improve the process of medication reconciliation. Pending further refinement and evaluation, this toolkit and associated mentored implementation has the potential to substantially improve the medication safety of patients during and following hospitalization.
Supplementary Material
Acknowledgments
This study was provided grant support from the US Agency for Healthcare Research and Quality (R18 HS019598-01).
Contributor Information
Stephanie K. Mueller, Division of General Medicine, Brigham and Women’s Hospital and Harvard Medical School, Boston.
Sunil Kripalani, Section of Hospital Medicine, Department of Medicine, Vanderbilt University, Nashville, Tennessee.
Jason Stein, Clinical Operations Program and Associate Director for Quality, Division of Hospital Medicine, Emory Hospital; Emory University School of Medicine, Atlanta.
Peter Kaboli, Veterans Rural Health Resource Center (VRHRC), VA Office of Rural Health; Division of General Internal Medicine, University of Iowa Carver College of Medicine, Iowa City.
Tosha B. Wetterneck, University of Wisconsin School of Medicine and Public Health; Center for Quality and Productivity Improvement, University of Wisconsin, Madison.
Amanda H. Salanitro, Geriatric Research, Education, and Clinical Center, VA Tennessee Valley Healthcare System and Section of Hospital Medicine, Vanderbilt University, Nashville.
Jeffrey L. Greenwald, Inpatient Clinician Educator Service, Department of Medicine, Massachusetts General Hospital; Harvard Medical School, Boston.
Mark V. Williams, Division of Hospital Medicine, Feinberg School of Medicine, Northwestern University; Project BOOST, Chicago.
Edward Etchells, University of Toronto Centre for Patient Safety; Information Services and Staff Physician, Division of General Internal Medicine; University of Toronto.
Daniel J. Cobaugh, American Society of Health System Pharmacists Research and Education Foundation, Bethesda, Maryland.
Lakshmi Halasyamani, Quality and Systems Improvement, Saint Joseph Mercy Health System, Livonia, Michigan.
Stephanie Labonville, Brigham and Women’s Hospital, Boston.
David Hanson, Cardiovascular Services, Methodist Hospital, Clarian Health Partners; American Association of Critical-Care Nurses, Indianapolis.
Hasan Shabbir, Emory Johns Creek Hospital; Emory Division of Hospital Medicine, Emory University School of Medicine, Atlanta.
John Gardella, Novant Health - Greater Charlotte Market, Charlotte.
Rebecca Largen, Novant Health Presbyterian Medical Center, Charlotte.
Jeffrey Schnipper, Clinical Research of the Hospitalist Service at Brigham and Women’s Hospital; and an Associate Professor of Medicine at Harvard Medical School, Boston.
References
- 1.Coleman EA, et al. Posthospital medication discrepancies: Prevalence and contributing factors. Arch Intern Med 2005. Sep 12;165(16):1842–1847. [DOI] [PubMed] [Google Scholar]
- 2.Smith JD, Coleman EA, Min SJ. A new tool for identifying discrepancies in postacute medications for community-dwelling older adults. Am J Geriatr Pharmacother 2004;2(2):141–147. [DOI] [PubMed] [Google Scholar]
- 3.Cua YM, Kripalani S. Medication use in the transition from hospital to home. Ann Acad Med Singapore. 2008;37(2):136–145. [PMC free article] [PubMed] [Google Scholar]
- 4.Forster AJ, et al. The incidence and severity of adverse events affecting patients after discharge from the hospital. Ann Intern Med 2003. Feb 4;138(3):161–167. [DOI] [PubMed] [Google Scholar]
- 5.Kripalani S, et al. Promoting effective transitions of care at hospital discharge: A review of key issues for hospitalists. J Hosp Med 2007;2(5):314–323. [DOI] [PubMed] [Google Scholar]
- 6.Kripalani S, et al. Deficits in communication and information transfer between hospital-based and primary care physicians: implications for patient safety and continuity of care. JAMA 2007. Feb 28;297(8):831–841. [DOI] [PubMed] [Google Scholar]
- 7.Beers MH, et al. Influence of hospitalization on drug therapy in the elderly. J Am Geriatr Soc 1989;37(8):679–683. [DOI] [PubMed] [Google Scholar]
- 8.Omori DM, Potyk RP, Kroenke K. The adverse effects of hospitalization on drug regimens. Arch Intern Med 1991;151(8):1562–1564. [PubMed] [Google Scholar]
- 9.Schachtner JM, et al. Prevalence and cost savings of therapeutic interchange among U.S. hospitals. Am J Health Syst Pharm 2002;59(6):529–533. [DOI] [PubMed] [Google Scholar]
- 10.Bond CA, Raehl CL. Clinical pharmacy services, pharmacy staffing, and adverse drug reactions in United States hospitals. Pharmacotherapy. 2006; 26(6):735–747. [DOI] [PubMed] [Google Scholar]
- 11.Makaryus AN, Friedman EA. Patients’ understanding of their treatment plans and diagnosis at discharge. Mayo Clin Proc 2005;80(8):991–994. [DOI] [PubMed] [Google Scholar]
- 12.Calkins DR, et al. Patient-physician communication at hospital discharge and patients’ understanding of the postdischarge treatment plan. Arch Intern Med 1997. May 12;157(9):1026–1030. [PubMed] [Google Scholar]
- 13.Cornish PL, et al. Unintended medication discrepancies at the time of hospital admission. Arch Intern Med 2005. Feb 28;165(4):424–429. [DOI] [PubMed] [Google Scholar]
- 14.Gleason KM, et al. Reconciliation of discrepancies in medication histories and admission orders of newly hospitalized patients. Am J Health Syst Pharm 2004. Aug 15;61(16):1689–1695. [DOI] [PubMed] [Google Scholar]
- 15.Pippins JR, et al. Classifying and predicting errors of inpatient medication reconciliation. J Gen Intern Med 2008;23(9):1414–1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tam VC, et al. Frequency, type and clinical importance of medication history errors at admission to hospital: a systematic review. CMAJ 2005. Aug 30;173(5):510–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wong JD, et al. Medication reconciliation at hospital discharge: Evaluating discrepancies. Ann Pharmacother. 2008;42(10):1373–1379. [DOI] [PubMed] [Google Scholar]
- 18.Forster AJ, et al. Adverse drug events occurring following hospital discharge. J Gen Intern Med 2005;20(4):317–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Johnson JA, Bootman JL. Drug-related morbidity and mortality. A cost-of-illness model. Arch Intern Med 1995. Oct 19;155(18):1949–1956. [PubMed] [Google Scholar]
- 20.Resar R, Midlefort L. Medication Reconciliation Review. Cambridge, MA: Institute for Healthcare Improvement, 2004. Accessed Jun 27, 2013. http://www.ihi.org/knowledge/Pages/Tools/MedicationReconciliationReview.aspx. [Google Scholar]
- 21.Joint Commission on Accreditation of Healthcare Organizations. Comprehensive Accreditation Manual for Hospitals 2005: The Official Handbook. Oak-brook Terrace, IL: Joint Commission Resources, 2004. [Google Scholar]
- 22.The Joint Commission. Approved: Modifications to National Patient Safety Goal on reconciling medication information. Jt Comm Perspect. 2011;31(1)1–7. [PubMed] [Google Scholar]
- 23.Mueller SK, et al. Hospital-based medication reconciliation practices: A systematic review. Arch Intern Med 2012. Jul 23;172(14):1057–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Greenwald JL, et al. Making inpatient medication reconciliation patient centered, clinically relevant and implementable: A consensus statement on key principles and necessary first steps. J Hosp Med 2010;5(8):477–485. [DOI] [PubMed] [Google Scholar]
- 25.Salanitro AH, et al. Rationale and design of the Multicenter Medication Reconciliation Quality Improvement Study (MARQUIS). BMC Health Serv Res 2013. Jun 25;13(1):230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maynard GA, et al. 2011 John M. Eisenberg Patient Safety and Quality Awards. Mentored implementation: Building leaders and achieving results through a collaborative improvement model. Innovation in patient safety and quality at the national level. Jt Comm J Qual Patient Saf 2012;38(7):301–310. [DOI] [PubMed] [Google Scholar]
- 27.Society of Hospital Medicine. BOOSTing Care Transitions Resource Room: Overview. 2008. Accessed Jun 27, 2013. http://www.hospitalmedicine.org/ResourceRoomRedesign/RR_CareTransitions/CT_Home.cfm. [Google Scholar]
- 28.Society of Hospital Medicine. SHM VTE Prevention Collaborative. 2012. Accessed Jun 27, 2013. http://www.hospitalmedicine.org/AM/Template.cfm?Section=QI_Current_Initiatives_and_Training_Opportunities&Template=/CM/HTMLDisplay.cfm&ContentID=14406. [Google Scholar]
- 29.Society of Hospital Medicine. Glycemic Control Resource Room: SHM’s Glycemic Control Program. 2008. Accessed Jun 27, 2013. http://www.hospitalmedicine.org/ResourceRoomRedesign/html/GC_RR_Mentor_Program.cfm. [Google Scholar]
- 30.Gleason KM, et al. Results of the Medications at Transitions and Clinical Handoffs (MATCH) study: An analysis of medication reconciliation errors and risk factors at hospital admission. J Gen Intern Med 2010;25(5):441–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Agency for Healthcare Research and Quality. Is Our Pharmacy Meeting Patients’ Needs? Pharmacy Health Literacy Assessment Tool User’s Guide. Jacobson K, et al. Oct. 2007. Accessed Jun 27, 2013. http://www.ahrq.gov/professionals/quality-patient-safety/pharmhealthlit/pharmlit/index.html. [Google Scholar]
- 32.Canadian Patient Safety Institute (CPSI). Medication Reconciliation in Acute Care: Getting Started Kit. Sep 2011. Ottawa: CPSI. Accessed Jun 27, 2013. http://www.saferhealthcarenow.ca/EN/Interventions/medrec/Pages/resources.aspx. [Google Scholar]
- 33.Kripalani S, et al. ; Multi-center Medication Reconciliation Quality Improvement Study (MARQUIS). Taking a Good Medication History. Video. Accessed Jun 27, 2013. http://www.hospitalmedicine.org/MARQUIS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hawe P, Shiell A, Riley T. Complex interventions: How “out of control” can a randomised controlled trial be? BMJ 2004;328(7455):1561–1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kripalani S, et al. ; Multi-center Medication Reconciliation Quality Improvement Study (MARQUIS). Good Discharge Counseling. Video. Accessed Jun 27, 2013. http://www.hospitalmedicine.org/MARQUIS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Society of Hospital Medicine. Overview: Multi-center Medication Reconciliation Quality Improvement Study (MARQUIS). Accessed Jun 27, 2013. http://www.hospitalmedicine.org/MARQUIS. [Google Scholar]
- 37.National Patient Safety Foundation. Ask Me 3. Accessed Jun 27, 2013. http://www.npsf.org/for-healthcare-professionals/programs/ask-me-3/. [Google Scholar]
- 38.Bates DW, et al. Relationship between medication errors and adverse drug events. J Gen Intern Med 1995;10(4):199–205. [DOI] [PubMed] [Google Scholar]
- 39.Kripalani S, et al. Medication use among inner-city patients after hospital discharge: Patient-reported barriers and solutions. Mayo Clin Proc 2008; 83(5):529–535. [DOI] [PubMed] [Google Scholar]
- 40.Kripalani S, et al. Development and evaluation of the Adherence to Refills and Medications Scale (ARMS) among low-literacy patients with chronic disease. Value Health. 2009;12(1):118–123. [DOI] [PubMed] [Google Scholar]
- 41.Kripalani S, et al. Development of an illustrated medication schedule as a low-literacy patient education tool. Patient Educ Couns 2007;66(3):368–377. [DOI] [PubMed] [Google Scholar]
- 42.Kripalani S, et al. Effect of a pharmacist intervention on clinically important medication errors after hospital discharge: A randomized trial. Ann Intern Med 2012. Jul 3;157(1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.. Kripalani S, et al. Factors associated with patients’ understanding of their preadmission medication regimen. Presented at the Society of General Internal Medicine Annual Meeting, Minneapolis, 2010. [Google Scholar]
- 44.Schnipper JL, et al. Role of pharmacist counseling in preventing adverse drug events after hospitalization. Arch Intern Med 2006. Mar 13;166(5):565–571. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


