Abstract
Longitudinal multimodal biomarker studies reveal that the continuum of Alzheimer’s disease (AD) includes a long latent phase, referred to as preclinical AD, which precedes the onset of symptoms by decades. Treatment during the preclinical AD phase offers an optimal opportunity for slowing the progression of disease. However, trial design in this population is complex. In this Review, we discuss the recent advances in accurate plasma measurements, new recruitment approaches, sensitive cognitive instruments and self-reported outcomes that have facilitated the successful launch of multiple phase 3 trials for preclinical AD. The recent success of anti-amyloid immunotherapy trials in symptomatic AD has increased the enthusiasm for testing this strategy at the earliest feasible stage. We provide an outlook for standard screening of amyloid accumulation at the preclinical stage in clinically normal individuals, during which effective therapy to delay or prevent cognitive decline can be initiated.
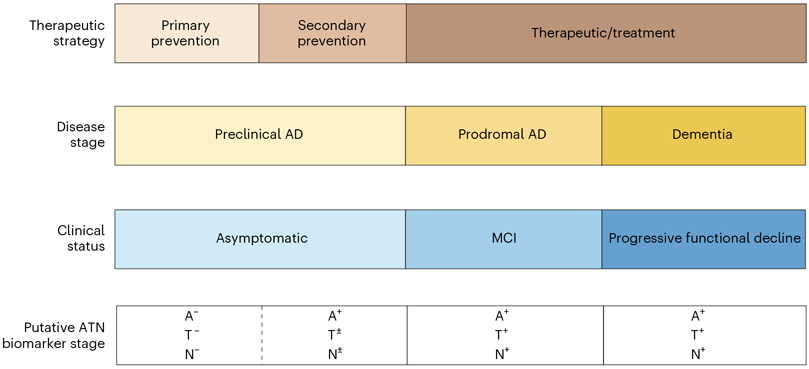
AD is a progressive neurodegenerative disease characterized by the neuropathologic findings of amyloid-β (Aβ) plaques, neurofibrillary tau tangles and neurodegeneration resulting in dementia1. AD is now generally considered to be a seamless continuum that encompasses three broad stages: the ‘preclinical’ or presymptomatic stage, lasting over a decade, during which individuals are asymptomatic yet harbor AD neuropathology; the ‘prodromal’ stage, during which individuals have impairment in at least one cognitive domain but maintain good function; and the more familiar ‘dementia’ stage, in which multiple cognitive domains are affected with an accompanying decline in daily function2 (Fig. 1). These abnormalities are associated with a pathophysiological cascade that results in cytoskeletal changes and neuronal dysfunction with subsequent degeneration and brain atrophy3,4.
Fig. 1 ∣. Continuum of Alzheimer’s disease over 25 years.
Neuropathological changes in Alzheimer’s disease occur decades before the manifestation of clinical symptoms. MCI, mild cognitive impairment.
The neuropathologic features of AD begin 15–20 years before obvious cognitive symptoms in both sporadic and genetic forms of AD5-7. These changes can now be accurately and reliably detected by cerebrospinal fluid (CSF) assays, brain positron emission tomography (PET) imaging and, most recently, by plasma biomarker assays. Preclinical AD may be divided into ‘presymptomatic’ and ‘asymptomatic at risk’ (ref. 8). ‘Presymptomatic preclinical AD’ refers to the state of individuals with genetic forms of AD who will develop AD in the future. Individuals with presymptomatic preclinical AD initially show no overt clinical symptoms but have at least one mutation in the familial AD (fAD) genes (amyloid precursor protein (APP), presenilin 1 (PSEN1), presenilin 2 (PSEN2)) or have trisomy 21 (Down syndrome (DS)) with its resultant extra copy of the APP gene. In all cases of genetically determined AD, there is overproduction and hence accumulation of brain Aβ. ‘Asymptomatic at risk’ may be used to define preclinical AD in individuals in the general population without clinical symptoms but who are positive for AD biomarkers, which are elevated brain Aβ as visualized by PET or, in the CSF, decreased Aβ42 and increased phosphorylated tau (p-tau) species as well as total tau protein8. Data from longitudinal studies indicate that individuals with elevated brain Aβ (that is, preclinical AD) are at a heightened risk for developing cognitive symptoms consistent with dementia on the Clinical Dementia Rating (CDR) scale with long-term follow-up9, suggesting that the preclinical state represents a stage of AD rather than being at risk for developing AD. That is, the presence of Aβ pathology is not merely a risk factor but rather a manifestation of disease10. Therefore, treatment during the preclinical AD stage offers an ideal opportunity for slowing the progression of AD, as the absence of symptoms suggests that extensive irreversible damage is limited. However, trial design in this population is complex for several reasons, including tracking cognitive change in asymptomatic individuals and identification of individuals who are asymptomatic but have elevated brain amyloid11.
Food and Drug Administration (FDA) guidance on developing drugs for ‘early AD’ has been an important catalyst for the development of clinical trial designs for earlier stages of AD. In its 2018 guidance for industry12, the FDA considers that AD biomarker change may form the basis for accelerated approval, while meeting both biomarker and cognitive endpoints will be necessary to receive full approval12. The FDA also recommends that sponsors conduct studies of sufficient duration to evaluate patients as they transition to the next defined AD stage. Aβ reduction is considered ‘reasonably likely to confer clinical benefit’ and is designated by the FDA as a suitable surrogate endpoint for AD clinical trials. Therefore, reduction of Aβ alone is now considered sufficient to obtain accelerated approval for AD interventions. Post-marketing studies supporting clinical benefit are required for full approval.
In this Review, we aim to discuss recent advances in plasma biomarkers, new approaches to recruitment, sensitive cognitive instruments and self-reported outcomes that have facilitated the successful launch of multiple phase 3 trials for preclinical AD. We will also highlight the recent success of anti-Aβ immunotherapy trials in symptomatic AD, which have increased the enthusiasm for testing this strategy at the earliest feasible disease stage. Finally, we provide an outlook for standard screening for Aβ accumulation in the aging population with effective therapy to delay or prevent cognitive decline.
Definition of preclinical AD along the AD continuum
Autopsy evidence
Data from the Alzheimer’s Disease Neuroimaging Initiative (ADNI) and the Australian Imaging, Biomarkers and Lifestyle Flagship Study of Aging (ABLE), among others, have provided converging evidence that biomarker abnormalities consistent with the AD pathophysiological process are detectable before the emergence of overt clinical symptomatology and are, in fact, highly predictive of subsequent cognitive decline. Both Aβ PET and CSF studies suggest that a substantial proportion of clinically normal older individuals demonstrate evidence of elevated brain Aβ13-16, ranging from approximately 20% to 40%, which is consistent with large postmortem series17,18. Interestingly, the percentage of ‘Aβ-positive’ cognitively normal individuals at autopsy detected at a given age appears to closely parallel the percentage of individuals diagnosed with AD dementia a decade later19,20. Similarly, genetic at-risk cohorts demonstrate evidence of elevated brain Aβ many years before detectable cognitive impairment9,21-25.
Controversy: does Aβ accumulation inevitably lead to symptomatic AD?
Multiple longitudinal studies have also consistently found that cognitively normal individuals with elevated brain Aβ display, in general, accelerated cognitive decline compared to individuals with normal brain Aβ levels26-32. Nonetheless, the question as to whether all individuals with elevated brain Aβ will develop AD dementia if they live long enough is difficult to answer. The approximately 10-year delay between accumulation of brain Aβ and the onset of early cognitive impairment, along with the variability in associated cognitive decline, lead to a small percentage of cognitively normal adults with high levels of Aβ who may not experience cognitive decline or dementia33. Across studies, however, those individuals showing abnormalities in both Aβ and additional biomarkers demonstrate more rapid cognitive decline than individuals with no elevation in brain Aβ with respect to their cognitive trajectories over the subsequent decade34-36.
Genetic forms of AD
Autosomal dominant AD
Aside from the sporadic form, AD can also occur because of dominantly inherited mutations. Mutations in the APP gene (located at chromosome region 21q21.2), PSEN1 (located at 14q24.3) or PSEN2 (located at 1q42.13) lead to fAD. fAD accounts for <1% of all AD cases and presents as a classic Mendelian autosomal dominant disease, usually with an early (<65 years) age of onset. Individuals carrying presymptomatic mutations have provided important clues on biomarker trajectories associated with the preclinical state of the disease6. They also represent an important population in which to investigate the efficacy of disease-modifying agents in delaying clinical onset of the disease, as it is possible to estimate when the clinical signs of the disease will appear based on the family history of the carriers, the so called ‘estimated year of onset’ (ref. 6). These familial cases of AD appear to have the same clinical and pathologic phenotypes as sporadic cases37.
DS
People with trisomy 21 (DS) represent the world’s largest population of genetically determined AD7. DS is not a familial form of AD and therefore is not considered part of the 1% represented by fAD. There are approximately 250,000 persons with DS in the USA, representing approximately 4% of the 6.5 million people with AD in the USA38. AD pathology has been described in all adults with full trisomy 21 by the age of 40 years, and its hallmarks are qualitatively similar to those of sporadic AD. Evidence from biomarker studies also suggests that the pathophysiology of the disease in DS is similar to that of the sporadic and autosomal dominant forms of AD7,39. Several studies of individuals with DS have assessed Aβ brain deposition with PET tracers, studied plasma and CSF biomarkers or described the atrophy and cerebral metabolic patterns of AD40-44. These changes begin more than a decade before the onset of dementia, in a strikingly similar order and timing as those described for autosomal dominant AD6,25,45.
Several clinical trials for AD have been conducted in the population with DS46,47. Clinical trials conducted in this population have obvious advantages: the ultra-high risk for developing symptomatic AD and a predictable sequence of events that make this population ideal for prevention trials for AD48.
The amyloid, tau and neurodegeneration (ATN) staging framework
Besides the clinical staging of symptomatic AD, it is now possible to stage patients based on pathology using the ATN framework (Table 1). The cascade of changes in biomarkers that occurs over the AD continuum has been used to define pathological stages of the disease and is referred to as the ATN framework: ‘A’ refers to amyloid pathology, ‘T’ refers to tau pathology, and ‘N’ refers to neurodegeneration49,50. Evaluation of elevated brain Aβ can be performed by use of Aβ PET imaging, CSF measures of Aβ peptides and, most recently, plasma measurements. Additionally, tau pathology in neurofibrillary tangles can be assessed with PET imaging, while total tau as well as its various phosphorylated forms can be measured in both CSF and in plasma. Neurodegeneration can be assessed with fluorodeoxyglucose (FDG) PET imaging and magnetic resonance imaging (MRI), while measurements of neurofilament light (NfL) in the CSF and plasma have been shown to reflect brain atrophy to a certain extent. This framework is being applied across the spectrum of AD including its preclinical stage51,52. The ATN framework allows for targeting the appropriate mechanism at each disease stage and has been shown to aid in providing accurate diagnosis and prognosis53-55 (Fig. 2). With this framework, there is the challenge of dichotomization across various biomarkers and determining cutoffs for each category, and it continues to be refined56.
Table 1 ∣.
Amyloid, tau and neurodegeneration (ATN) classification
| AT(N) profiles |
Biomarker category | Part of Alzheimer’s disease (AD) continuum (yes or no) |
Stable cognition | MCI | Dementia |
|---|---|---|---|---|---|
| A−T−N− | Normal biomarkers | No | Stable cognition + normal AD biomarkers | MCI + normal AD biomarkers | Dementia + normal AD biomarkers |
| A+T−N− | AD pathological change | Yes | Preclinical AD pathological change | MCI + AD pathological change | Dementia + AD pathological change |
| A+T+N− | AD | Yes | Preclinical AD | Prodromal AD | AD + dementia |
| A+T+N+ | AD | Yes | Preclinical AD | Prodromal AD | AD + dementia |
| A+T−N+ | AD and concomitant suspected non-AD pathological change | Yes | Preclinical AD | Prodromal AD | AD + dementia |
| A−T+N− | Non-AD pathological change | No | Preclinical non-AD | MCI not due to AD | Non-AD dementia |
| A−T−N+ | Non-AD pathological change | No | Preclinical non-AD | MCI not due to AD | Non-AD dementia |
| A−T+N+ | Non-AD pathological change | No | Preclinical non-AD | MCI not due to AD | Non-AD dementia |
MCI, mild cognitive impairment.
Fig. 2 ∣. Biomarkers and the amyloid, tau and neurodegeneration (ATN) classification.
Biomarkers can be used for individualized risk profiling and neuropathological staging of patients along the Alzheimer’s disease (AD) continuum. MCI, mild cognitive impairment.
The ATN framework is imperfect in its predictive ability due to the large variability in rate of progression among individuals57. In addition, it leaves room for choice of biomarker and for determining specific thresholds for positivity. Different biomarker modalities and cutoffs can result in different categorizations of individuals. Nevertheless, the ATN framework provides an important and useful starting point as we consider anchoring the clinical phenotype of AD across its spectrum with objective biomarkers of pathology.
Amyloid pathology burden
Several longitudinal follow-up studies have examined the role of AD neuroimaging and biofluid biomarkers to predict subsequent decline in cognition among cognitively normal individuals58-69.
Clinically normal individuals with elevated brain Aβ demonstrate multiregional brain atrophy70,71, cortical thinning72-74, aberrant default network activity and functional MRI connectivity deficits similar to AD75,76, decreased task-induced functional MRI deactivation in the default network regions75, lower performance on a demanding test of associative memory retrieval76 and episodic memory deficits77 as well as longitudinal cognitive decline31. Aβ deposition occurs in the neocortical areas of the brain, one of the first areas affected due to preclinical AD pathology. Across three independent samples of cognitively normal older adults, it has been reported that lower thresholds for Aβ PET in the Centiloid (CL) range of 15.0 to 18.5 are predictive of future Aβ accumulation and cognitive decline. This range appears to correspond to an inflection point in the Aβ PET distribution beyond which Aβ and cognitive trajectories diverge from normal67. For CSF, an Aβ42/Aβ40 ratio of ≤0.062 is considered abnormal68.
Low levels of Aβ42 in CSF reflect higher levels of Aβ plaques, and CSF biomarkers have been found to have good predictive ability for memory decline in clinically normal individuals and are thought to represent one of the earliest changes reflecting AD pathology in the brain65.
Recent work on immunoassays of plasma biomarkers of AD has demonstrated their ability to confirm AD pathology in the brain and to improve prediction of cognitive decline in cognitively unimpaired older populations78-83. Assessment of the plasma Aβ1–42/Aβ1–40 ratio by mass spectrometric methods has now been identified as a sensitive and specific indicator of brain fibrillar Aβ load as determined by Aβ PET. Immunoassays and mass spectrometry measurements of p-tau species, particularly p-tau217 and p-tau231, are also excellent markers of AD pathology84,85.
In preclinical AD, plasma p-tau217 in combination with either plasma Aβ42 or Aβ40 has demonstrated excellent accuracy in predicting elevated brain Aβ86,87. Many of these plasma biomarkers are being implemented in clinical trials for preclinical AD (for example, the AHEAD trial of lecanemab in preclinical AD) to assist with enriching for cognitively normal individuals who are likely to have elevated brain Aβ88.
Tau pathology burden
The hyperphosphorylated paired helical filament that forms neurofibrillary tangles quantified by Braak stages is better correlated with AD severity and neuronal atrophy than Aβ plaques. Both tau and p-tau are increased in AD pathology and are considered to be a measure of neuronal injury. Longitudinal tau PET cohorts in patients with high-risk preclinical AD have provided an understanding of the spatial distribution of neurofibrillary tangles that can allow for staging neurodegeneration according to tau levels in preclinical AD89. Recent work indicates that tau PET may also be an excellent predictor of subsequent cognitive decline in preclinical AD cases10.
Estimates of tau positivity in clinically unimpaired individuals with elevated Aβ vary depending on whether positivity is based on the medial temporal cortex (15%) or the entorhinal cortex alone (40%). Recent work has demonstrated the presence of divergent patterns of tau aggregation in individuals with preclinical AD, which has important implications for participant selection and evaluation of disease modification in prevention trials. Specifically, while the entorhinal cortex has a central role in the early appearance of tau, it may be the inferior temporal cortex that is the critical region for rapid tau accumulation in preclinical AD90. Plasma p-tau181 (ref. 84) and p-tau217 have shown excellent accuracy in predicting elevated brain Aβ in asymptomatic individuals91 as well as subsequent cognitive decline.
Neurodegeneration or neuronal injury
Reductions in regional cerebral glucose metabolism as measured by FDG PET appear to precede the onset of AD symptoms in predisposed individuals, in both genetic early-onset and late-onset AD forms92. Pre-symptomatic persons carrying autosomal dominant genetic mutations associated with early-onset fAD (onset age < 65 years) show the typical AD pattern of hypometabolism compared to age-matched mutation non-carriers93. By monitoring progression to mild cognitive impairment (MCI) and dementia among cognitively normal older people, these studies showed that reductions in regional cerebral glucose metabolism precede the onset of cognitive decline by many years94 and predict cognitive decline from normal cognition to cognitive impairment with over 80% accuracy95. However, it should be noted that the specificity of hypometabolism for AD is confounded by other potential neurodegenerative diseases that may underlie cognitive decline.
PET imaging of SV2A, a presynaptic protein involved in neuro-transmitter release and storage, may serve as a method to quantify functional synapses and, thus, to estimate synaptic density96. Synaptic PET imaging targeting SV2A may provide another biomarker for neurodegeneration in the ATN framework that more closely tracks the progression of the disease and cognitive impairment and is less sensitive to confounding factors such as blood glucose level, stimulation and medication, which affect FDG PET97
NfL and neurogranin (Ng) are promising candidate AD biomarkers, reflecting axonal and synaptic damage, respectively. CSF NfL and Ng concentrations are increased in cognitively normal older adults with biomarker evidence of tau pathology and neurodegeneration98. Elevated NfL chain levels correlate with AD progression99. Combinations of plasma biomarkers are being validated as diagnostic and prognostic tools in population-based cohorts including preclinical AD100.
The most direct method of visualizing and quantifying regional brain neurodegeneration is using MRI volumetrics. Although limited in applicability in preclinical AD101, much work has demonstrated hippocampal atrophy as being helpful in predicting subsequent cognitive decline across the spectrum of AD102-104.
It is worth noting that recent advances in plasma biomarkers, while not currently available in clinical practice, are being rapidly implemented in clinical trials, especially in prevention trials to enrich the sample with individuals who harbor AD brain pathology. Plasma biomarkers are also being validated beyond screening tests but also as potential outcome measures91 and represent a class of biomarkers that is cost effective and much more scalable than PET and CSF-based biomarkers.
Cognitive-assessment measures in preclinical AD
Slight but measurable cognitive decline has been reported during preclinical AD. Both retrospective and prospective studies of cognitively normal individuals who subsequently progressed to AD dementia have found episodic memory decline to be a defining feature of preclinical AD105,106. Sensitive cognitive measures for use in preclinical AD have been developed and validated. These include the Preclinical Alzheimer’s Cognitive Composite107-109, the Alzheimer’s Prevention Initiative Composite Cognitive Test110,111 and the Repeatable Battery for the Assessment of Neuropsychological Status. Each of these assessment tools includes measurement of episodic memory and executive function. These scales have been shown to be useful in detecting AD for clinical trial purposes as they allow for accurate longitudinal measurement of subtle cognitive decline associated with AD.
The Cognitive Function Instrument (CFI) is a 14-item assessment of cognitive status that can be completed by participants or a study partner. Both participant and study partner CFI scores are good predictors of cognitive decline in individuals with normal cognition112,113. While baseline values among the cognitively healthy might be a marker of the risk to progress, change scores including those from study partners appear to be useful outcome measures in predicting decline among individuals with some impairment. Importantly, the CFI administered to the participant appears to be a better initial predictor of decline than study partner CFI in the cognitively normal group113. The CFI score is used as a key secondary outcome in preclinical trials, as a treatment benefit on the CFI would support a clinically meaningful effect.
The ADL Prevention Instrument was designed to detect early impairment of the activities of daily living (ADL) in dementia-prevention trials of clinically normal individuals114. The ADL Prevention Instrument is a subjective self-report and informant-based scale consisting of 15 items. It has been shown to distinguish well between clinically normal individuals and those with preclinical AD and to be associated with future cognitive decline in cognitively normal individuals115.
Participant versus study partner assessments
There exists an important complication in the tracking of early declines in preclinical AD using subjective scales: as an individual becomes more impaired and transitions from preclinical AD to MCI and then to AD dementia, that individual is less likely to provide a reliable self-report of his or her daily functioning, thus necessitating an informant report116. However, before MCI sets in, the individual with preclinical AD may be more attuned to early functional difficulties than an informant. Therefore, at different stages of AD, either self-report or informant-based subjective ADL scales might be more reliable, suggesting that both are required, at least in preclinical AD.
Prevention of preclinical AD
Therapeutic strategies in preclinical AD
Several lifestyle modifications are thought to possibly reduce the risk of developing dementia, including AD dementia117. Specifically, optimal management of hypertension118 and treatment of hearing loss119 as well as participating in regular aerobic exercise120 are thought to contribute to risk reduction for AD dementia. Further work will be needed to evaluate these interventions in midlife and even earlier to assess for sustained long-term benefits. In addition, several therapeutics are targeting the various key biological elements in AD pathophysiological processes, namely, Aβ and tau (Tables 2 and 3).
Table 2 ∣.
Disease-modifying agents targeting amyloid-β (Aβ)
| Target/mechanism | Drug | Study population | Clinical trial identifier |
|---|---|---|---|
| BACE1 inhibitor (β-secretase inhibitor) | Verubecestat | Mild to moderate AD | NCT01739348 |
| Prodromal AD | NCT01953601 | ||
| Lanabecestat | Prodromal AD; mild AD | NCT02245737, NCT02783573 | |
| Atabecestat | Preclinical AD | NCT02569398 | |
| Umibecestat | Preclinical AD | NCT03131453, NCT02565511 | |
| Elenbecestat | Prodromal to mild AD | NCT02956486, NCT03036280 | |
| γ-Secretase inhibitor | Semagacestat | Mild to moderate AD | NCT01035138, NCT00762411, NCT00594568 |
| Avagacestat | Prodromal AD | NCT00890890 | |
| γ-Secretase modulator | Tarenflurbil | Mild AD | NCT00105547 |
| Inhibitors of Aβ aggregation | Scyllo-inositol | Mild to moderate AD | NCT00568776 |
| DS | NCT01791725 | ||
| Tramiprosate | Mild to moderate AD | NCT00088673 | |
| Valiltramiprosate | APOE4/4 early AD | NCT04770220 | |
| Active immunotherapy (anti-Aβ vaccine) | AN-1792 | Mild to moderate AD | NCT00021723 |
| ACI.24 | DS | NCT02738450 | |
| ACI24.060 | Prodromal AD and DS | NCT05462106 | |
| Amilomotide (CAD106) | Preclinical AD | NCT02565511 | |
| Vanutide cridificar (ACC-001) | Mild to moderate AD | NCT00955409, NCT00960531, NCT01238991 | |
| NCT01284387 | |||
| ABvac40 | Prodromal AD | NCT03461276 | |
| Lu AF20513 | Mild AD; prodromal to mild AD | NCT02388152, NCT03819699, NCT03668405 | |
| UB-311 | Mild AD | NCT02551809 | |
| NCT03531710 | |||
| Passive immunotherapy (anti-Aβ antibody) | Bapineuzumab (AAB-001) | Mild to moderate AD | NCT00575055, NCT00574132 |
| Solanezumab (LY2062430) | Mild to moderate AD; mild AD, preclinical AD | NCT00905372, NCT00904683, NCT01900665 | |
| NCT02008357 | |||
| Crenezumab (RG7412) | Prodromal to mild AD | NCT02670083, NCT03114657 | |
| Gantenerumab (RO4909832) | Prodromal to mild AD | NCT03443973, NCT03444870 | |
| Aducanumab (BIIB037) | Prodromal to mild AD | NCT02477800, NCT02484547 | |
| BAN2401 | Prodromal to mild AD | NCT03887455 | |
| Preclinical AD; pre-preclinical AD | NCT04468659 |
AD, Alzheimer’s disease; DS, Down syndrome.
Table 3 ∣.
Anti-tau therapies
| Mechanism | Drug | Population | Clinical trial identifier |
|---|---|---|---|
| Active immunotherapy (anti-tau vaccine) | AADvac1 | Mild AD | NCT02579252 |
| ACI-35 | Mild to moderate AD | NCT04445831 | |
| Passive immunotherapy (anti-tau antibody) | Gosuranemab (BIIB092/BMS-986168) | Prodromal to mild AD | NCT03352557 |
| Tilavonemab (ABBV-8E12/C2N-8E12) | Prodromal to mild AD | NCT02880956 | |
| Passive immunotherapy (anti-tau antibody) | Semorinemab (RO7105705) | Prodromal to mild AD; moderate AD | NCT03289143, NCT03828747 |
| Zagotenemab (LY3303560) | Prodromal to mild AD | NCT03518073 |
AD, Alzheimer’s disease.
Anti-Aβ approaches
For the past 2 decades, AD drug-development efforts have focused on disease modification and have been strongly influenced by the two key neuropathological hallmarks of AD: extracellular deposition of Aβ and the subsequent formation of intraneuronal neurofibrillary tangles. Therapeutic strategies aimed at preventing Aβ formation, lowering its soluble levels in the brain, blocking its aggregation into plaques and disassembling existing Aβ plaques are among the main approaches employed to slow the progression of AD. Key aspects of clinical trials for preclinical AD are the need for a long treatment period (3.5–4.5 years) given the slow rate of change in cognitive decline and the challenge of identifying individuals who are clinically asymptomatic yet harbor elevated brain Aβ.
Anti-Aβ approaches have been criticized as a mechanism of action since their initial development and testing. The number of negative trials over the past 20 years for mild to moderate AD and the limited efficacy observed in early AD trials have raised the question of whether Aβ is the right target or a sufficient target for disease-modifying treatments121. However, anti-Aβ approaches are the furthest along in development and represent the first class of disease-modifying therapeutics to be approved by the FDA. It is expected that earlier intervention in the AD continuum and combination therapies targeting other pathologies including tau, neuroinflammation and cerebrovascular disease will help to increase the likelihood of improved efficacy.
Aducanumab.
Aducanumab is an immunoglobulin (Ig)G1 monoclonal antibody that selectively binds to soluble and insoluble fibrillar Aβ aggregates122. It targets Aβ aggregates, including soluble oligomers and insoluble fibrils123. The phase 1b randomized trial PRIME showed significant reductions in the Aβ PET standard uptake value ratio composite score of aducanumab-treated patients, especially those treated with 10 mg per kg aducanumab at 54 weeks124. The brain Aβ burden decreased in a dose- and time-dependent manner in patients with prodromal or mild AD. Worsening on the CDR sum of boxes (CDR-SB) and Mini Mental State Examination scores was delayed by aducanumab treatment, indicating a positive effect on cognition and clinical progression124. Aducanumab is the first disease-modifying treatment for AD that has been approved by the FDA. It received an accelerated approval due to its dramatic effectiveness in removing Aβ plaque as indicated by Aβ PET, although the approval was mired in controversy. The accelerated approval pathway allows for clinical use of a drug with effects on a surrogate marker considered reasonably likely to predict clinical benefit and requires additional post-approval studies to confirm clinical benefit. The EMERGE and ENGAGE trials were two phase 3, 18-month studies of early AD (prodromal and mild AD). However, both studies were terminated after an interim futility analysis demonstrated that one of the trials (ENGAGE) met futility criteria while the other (EMERGE) was trending positive125. However, while the data from the trials were extracted for futility analysis, participants continued in the studies at the sites for an additional 3 months before the studies were halted. In the larger dataset that included data collected during these 3 additional months, high-dose aducanumab in the EMERGE study showed benefits in the primary outcome, CDR-SB, slowing cognitive decline by 22% and in each of the other secondary outcomes, while low-dose aducanumab did not show benefits compared to placebo125. No benefits were seen for low-dose or high-dose aducanumab in the ENGAGE study in the larger dataset. In both the EMERGE and ENGAGE trials, Aβ PET imaging showed dose-related reductions in brain Aβ, indicating target engagement. Although they had identical designs, EMERGE and ENGAGE differed in the duration of exposure to high-dose aducanumab and the extent of fibrillar Aβ reduction in the brain. The total duration of exposure to high-dose aducanumab was higher in the positive EMERGE study and is thought to have accounted for the divergent outcomes. The FDA gave accelerated approval to aducanumab based on the surrogate endpoint of reduction of Aβ, which is the measure that is thought to predict clinical benefit but is not itself a measure of clinical benefit. The Centers for Medicare and Medicaid Services have declined coverage of aducanumab outside of approved clinical trials, and clinical use of the drug has been severely limited.
Gantenerumab.
Gantenerumab is also an IgG1 monoclonal antibody that binds with high affinity to aggregated Aβ species and removes Aβ plaques via Fcγ receptor-mediated microglial phagocytosis126. Gantenerumab neutralizes the neurotoxic effect of oligomeric Aβ42 in vivo126 and has been shown to reduce Aβ plaques127. Two pivotal phase 3 trials in patients with early AD, GRADUATE 1 (NCT03443973) and GRADUATE 2 (NCT03444870), with subcutaneously administered gantenerumab have been completed. Gantenerumab failed to meet its primary outcome in either study. Gantenerumab cleared only half as much plaque as expected, and fewer participants became Aβ negative as determined by PET scans than in previous trials. Clinical measures trended to improvement with an 8% slowing in cognitive decline. Participants who cleared Aβ below the positivity threshold did the best clinically128. Development of gantenerumab was halted, and a new formulation that uses transferrin transporter to help larger amounts of the antibody cross the blood–brain barrier is under development. Gantenerumab was also used in studies of autosomal dominant AD with negative results129.
Donanemab.
Donanemab is an IgG1 that targets Aβp3–42, an N-terminal pyroglutamate Aβ epitope, present in Aβ plaques. This monoclonal antibody mechanism also reduces plaque through microglial-mediated phagocytosis130. A phase 1b trial showed a significant reduction in Aβ plaque that was observed even after a single dose130, and a phase 2 result was positive for its primary cognitive–functional composite outcome131. The TRAILBLAZER-ALZ-2 (NCT04437511) phase 3 trial is determining the safety and efficacy of donanemab in early AD, and the TRAILBLAZER-ALZ-3 study (NCT05026866) is being conducted in patients with preclinical AD.
Lecanemab.
Lecanemab is an Aβ-targeting monoclonal antibody with relative selectivity for the protofibrillar species of Aβ protein. The phase 2 trial of the antibody showed that it was well tolerated and demonstrated Aβ removal and reduction in cognitive decline, which was proportional to exposure132. The phase 3 trial of lecanemab in early AD has been completed (CLARITY AD). Topline results indicate that the trial was positive, showing a highly significant 27% slowing of clinical progression as measured by the CDR-SB, with consistent results on all key secondary outcomes133. The drug received accelerated approval and is expected to receive full approval in 2023. Additionally, lecanemab is being studied in preclinical AD as well as pre-preclinical AD, in which participants have intermediate elevation in brain Aβ88.
An important adverse event to consider in the treatment of AD across its spectrum is that of amyloid-related imaging abnormalities (ARIA), which can be observed in two distinct presentations via MRI (ARIA-H and ARIA-E), following treatment with monoclonal antibodies that target Aβ plaques. ARIA-H refers to hemosiderin deposits presenting as microhemorrhages (hemorrhages < 10 mm) in the brain parenchyma and/or localized superficial siderosis (located in the subarachnoid space). ARIA-E refers to vasogenic edema in the brain parenchyma or sulcal effusions134,135. ARIA is believed to be driven by immune-mediated dissolution of Aβ aggregates in the cerebral blood vessels and brain parenchyma136. In the case of cerebrovascular Aβ deposits, it is thought to undermine the structural integrity of the blood vessel wall, leading to increased permeability and a localized hemorrhage or vasogenic edema. In most cases, ARIA-H and ARIA-E are asymptomatic, but, in severe cases, they can result in neurological symptoms such as headache, visual changes and death137.
Solanezumab.
Solanezumab is a monoclonal antibody that selectively binds to soluble Aβ monomers to promote Aβ clearance. It slows further accumulation of Aβ but does not remove Aβ deposited in plaques. Solanezumab has also progressed through a large development program including two phase 3 trials that were completed in mild to moderate AD dementia. The phase 3 trials did not meet their primary endpoints138,139. Solanezumab was evaluated in the phase 3 randomized, placebo-controlled Anti-Amyloid in Asymptomatic Alzheimer’s (A4) trial using a higher dose of solanezumab than that in the EXPEDITION trials and for a longer duration. Solanezumab did not slow cognitive decline on the primary outcome, the Preclinical Alzheimer Cognitive Composite, over 4.5 years in preclinical AD140. Overall, 36.1% of participants progressed to CDR Global Score (CDR-GS) > 0, which was similar between groups. Initial results suggest that the baseline Aβ level was the strongest predictor of cognitive decline. On Aβ PET imaging, Aβ continued to accumulate over time in both placebo and solanezumab groups, with less accumulation in the solanezumab group at the endpoint140. Additionally, the LEARN study, which enrolled participants with no elevation in brain Aβ, experienced no AD-associated cognitive decline over 8 years. These data suggest that more aggressive Aβ clearance is required even at the preclinical stage of disease and further strengthen the notion that elevated brain Aβ levels lead to subsequent cognitive decline.
BACE inhibitors.
The discovery of β-site APP-cleaving enzyme 1 (BACE1) as the enzyme that initiates Aβ production and the identification of a mutation at the BACE1 cleavage site of APP that inhibited production of Aβ and was protective of AD141,142 led to the development of several highly potent BACE inhibitors. However, their clinical trials failed to demonstrate efficacy, and most were associated with mild, self-limited, cognitive worsening143-146.
Further evaluation of clinical trial data has raised the possibility that a low dose of BACE inhibition, which results in less-potent but still diminished Aβ production, similar to that observed with the protective mutation, that is, a mutation that reduces risk for AD, may provide a reasonable path forward for asymptomatic patients who do not have substantial plaque burden or associated symptoms147.
Anti-tau approaches
Beyond Aβ as a target, the accumulation of tau aggregates in the brain is a key pathological hallmark of several neurodegenerative diseases, termed tauopathies, including AD. As transcellular spread of pathological tau aggregates has been implicated in disease progression, immunotherapy is being considered as a treatment for tauopathies. Given that tau lesions correlate better with the degree of dementia than do Aβ plaques, their clearance may also be clinically more efficacious once Aβ plaques develop and more proximal to when cognitive deficits become evident in AD. The most active mechanism of action is tau immunotherapy, with two active vaccines (AADvac1 and ACI-35) and six antibodies (LY3303560, RO7105705, BMS-986168, C2N-8E12, JNJ-63733657 and UCB0107) in clinical trials, although most of these therapies are still in the early stages of development and target stages later then preclinical AD148. The use of the ATN disease staging framework offers the potential for testing combination therapy with both anti-Aβ and anti-tau approaches, which may represent tailored interventions for AD across its various stages. Combining anti-Aβ and anti-tau therapies may provide additive or even synergistic benefits.
Current preclinical AD trials
We are now in an unprecedented era in AD therapeutic research. Phase 3 results from the aducanumab, gantenerumab, lecanemab and solanezumab trials and phase 2 results from the donanemab trials provide strong evidence that Aβ has a key role in AD-associated cognitive decline and that reducing brain amyloid is needed for efficacy. By the end of 2023, it is anticipated that there will be three FDA-approved anti-Aβ treatments for early AD. In addition, there are two large ongoing clinical trials, AHEAD and TRAILBLAZER-ALZ-3, in the preclinical AD stage. The AHEAD 3-45 trial (NCT04468659) is testing lecanemab in participants with preclinical AD as well as in individuals at an even earlier stage of AD, defined by an intermediate level of Aβ accumulation88. The SKYLINE trial (NCT05256134) was evaluating subcutaneous gantenerumab in preclinical AD until the negative readout of the GRADUATE 1 and GRADUATE 2 studies128, further supporting the notion that amyloid levels should be significantly reduced in order to achieve clinical efficacy. The TRAILBLAZER-ALZ-3 trial is testing donanemab in asymptomatic individuals between 65 and 80 years old who have evidence of elevated brain Aβ. The study will run for 3 years, with a clinical primary outcome of time to clinical progression as measured by the CDR-GS (NCT05026866). And, finally, prevention trials are ongoing or soon to begin in the populations with autosomal dominant AD or DS.
Recruitment challenges in preclinical AD
Recruitment challenges are especially demanding for trials in populations with preclinical AD, in which the minimal nature or absence of clinical symptoms means that individuals do not seek medical care for memory decline149. While AD dementia trials typically recruit from medical practices and clinics specializing in caring for patients with cognitive disorders, the population with preclinical AD requires a different approach that includes screening many cognitively normal individuals to fully enroll a prevention trial. Thus, trials for preclinical AD require a method to efficiently connect with individuals who are concerned about their risk for AD and prescreening those individuals who are at high risk. Moreover, clinical trials have historically lacked diversity with respect to under-represented racial groups about which there is limited information regarding efficacy of these new therapies and racial differences in biomarkers150.
The Trial-Ready Cohort in Preclinical/prodromal AD (TRC-PAD) project has established a recruitment infrastructure for early-stage AD trials151. The initial outreach effort has enrolled about 52,000 participants who have consented online and enrolled in the Alzheimer Prevention Trials Webstudy (APT Webstudy). The Webstudy is an online tool designed to collect brief information on demographics, family history, medical history and subjective cognitive concerns. Unsupervised cognitive assessment collects data on intellectual and memory function relevant to possible early AD. Participants are asked to return to the website quarterly to provide longitudinal cognitive and subjective data. The APT Webstudy data are analyzed to determine the likelihood of elevation in brain Aβ levels; initial algorithms are based primarily on analysis of the pre-randomization data from the A4 trial152. Based on the risk determination, as well as proximity to active TRC-PAD clinical sites and the entry criteria for available trials, individuals may be invited for a blood draw at a nearby commercial laboratory for plasma AD biomarkers and then an in-person assessment and, based on the updated risk assessment, Aβ testing by Aβ PET or CSF. Those with Aβ results consistent with AD are invited to be cohort participants, are followed in-person longitudinally and are ready for enrollment into trials. Those without Aβ abnormalities continue to be followed remotely in the APT Webstudy to continue to provide data for updated risk assessments.
Future directions: population screening and primary prevention
Two major recent developments in AD therapeutic research, the demonstration that effective targeting of Aβ slows clinical progression and the validation of highly accurate plasma biomarkers of AD neurobiology86, indicate the way forward toward primary prevention of the disease.
While there has been compelling evidence, particularly from genetic considerations, that Aβ accumulation drives AD, it has now been proven that reducing fibrillar Aβ in the brain is clinically beneficial at the early symptomatic stage of the disease. It is reasonable to be optimistic that intervention at the presymptomatic stage, when there is less irreversible neurodegeneration, will yield more than the 27% slowing shown with lecanemab in early AD. The results of the preclinical AD trials will arrive later in the decade.
We can now envision primary prevention of AD. The new plasma assays of Aβ ratios and p-tau species demonstrate abnormalities before measurable fibrillar Aβ accumulation as measured by PET scanning. As this technology continues to improve, we will be able to monitor the general population, perhaps starting around the age of 50 years, for evidence of Aβ dysregulation pointing to eventual brain accumulation. Routine longitudinal monitoring of plasma biomarkers may allow identification of those likely to accumulate Aβ. Candidate primary prevention therapies include low-dose BACE inhibitors, γ-secretase modulators and active anti-Aβ vaccination. Because Aβ is essential to the initiation of the AD neuropathological cascade, we anticipate that accurate monitoring and effective intervention against Aβ dysregulation will have a major impact on the incidence of AD, even greater than the impact of statin therapy on cardiovascular disease. Much work remains to be done to optimize and validate plasma assays for this purpose and demonstrate the safety and efficacy of preventive therapies; implementation of this approach to primary prevention is at least a decade away. The pathway forward is taking shape.
Acknowledgements
Funding support includes U24AG057437 to P.S.A.
Footnotes
Competing interests
M.S.R. reports consulting for AC Immune, Alzheon, Aptah, Biohaven, Ionis and Keystone Bio and grants from the National Institute on Aging P.S.A. received research support from Eisai, Eli Lilly, Janssen, the Alzheimer’s Association, the NIH and the FNIH and has consulted for ImmunoBrain Checkpoint, Merck and Roche.
References
- 1.Knopman DS et al. Alzheimer disease. Nat. Rev. Dis. Primers 7, 33 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aisen PS et al. On the path to 2025: understanding the Alzheimer’s disease continuum. Alzheimers Res. Ther 9, 60 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jack CR Jr et al. Tracking pathophysiological processes in Alzheimer’s disease: an updated hypothetical model of dynamic biomarkers. Lancet Neurol. 12, 207–216 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Raskin J, Cummings J, Hardy J, Schuh K & Dean RA Neurobiology of Alzheimer’s disease: integrated molecular, physiological, anatomical, biomarker, and cognitive dimensions. Curr. Alzheimer Res 12, 712–722 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jack CR Jr et al. Hypothetical model of dynamic biomarkers of the Alzheimer’s pathological cascade. Lancet Neurol. 9, 119–128 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bateman RJ et al. Clinical and biomarker changes in dominantly inherited Alzheimer’s disease. N. Engl. J. Med 367, 795–804 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fortea J. et al. Clinical and biomarker changes of Alzheimer’s disease in adults with Down syndrome: a cross-sectional study. Lancet 395, 1988–1997 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dubois B. et al. Advancing research diagnostic criteria for Alzheimer’s disease: the IWG-2 criteria. Lancet Neurol. 13, 614–629 (2014). [DOI] [PubMed] [Google Scholar]
- 9.Donohue MC et al. Association between elevated brain amyloid and subsequent cognitive decline among cognitively normal persons. JAMA 317, 2305–2316 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ossenkoppele R. et al. Amyloid and tau PET-positive cognitively unimpaired individuals are at high risk for future cognitive decline. Nat. Med 28, 2381–2387 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aisen PS, Jimenez-Maggiora GA, Rafii MS, Walter S & Raman R Early-stage Alzheimer disease: getting trial-ready. Nat. Rev. Neurol 18, 389–399 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.US Department of Health and Human Services, Food and Drug Administration. Guidance for Industry: Early Alzheimer’s Disease: Developing Drugs for Treatment https://www.fda.gov/regulatory-information/search-fda-guidance-documents/alzheimers-disease-developing-drugs-treatment-guidance-industy (2018).
- 13.Rowe CC et al. Amyloid imaging results from the Australian Imaging, Biomarkers and Lifestyle (AIBL) study of aging. Neurobiol. Aging 31, 1275–1283 (2010). [DOI] [PubMed] [Google Scholar]
- 14.Mintun MA et al. [11C]PIB in a nondemented population: potential antecedent marker of Alzheimer disease. Neurology 67, 446–452 (2006). [DOI] [PubMed] [Google Scholar]
- 15.Jack CR Jr et al. 11C PiB and structural MRI provide complementary information in imaging of Alzheimer’s disease and amnestic mild cognitive impairment. Brain 131, 665–680 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.De Meyer G. et al. Diagnosis-independent Alzheimer disease biomarker signature in cognitively normal elderly people. Arch. Neurol 67, 949–956 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arriagada PV, Marzloff K & Hyman BT Distribution of Alzheimer-type pathologic changes in nondemented elderly individuals matches the pattern in Alzheimer’s disease. Neurology 42, 1681–1688 (1992). [DOI] [PubMed] [Google Scholar]
- 18.Morris JC et al. Cerebral amyloid deposition and diffuse plaques in “normal” aging: evidence for presymptomatic and very mild Alzheimer’s disease. Neurology 46, 707–719 (1996). [DOI] [PubMed] [Google Scholar]
- 19.Villemagne VL et al. Aβ deposits in older non-demented individuals with cognitive decline are indicative of preclinical Alzheimer’s disease. Neuropsychologia 46, 1688–1697 (2008). [DOI] [PubMed] [Google Scholar]
- 20.Stonnington CM et al. Fibrillar amyloid correlates of preclinical cognitive decline. Alzheimers Dement. 10, e1–e8 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moonis M. et al. Familial Alzheimer disease: decreases in CSF Aβ42 levels precede cognitive decline. Neurology 65, 323–325 (2005). [DOI] [PubMed] [Google Scholar]
- 22.Klunk WE et al. Amyloid deposition begins in the striatum of presenilin-1 mutation carriers from two unrelated pedigrees. J. Neurosci 27, 6174–6184 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ringman JM et al. Biochemical markers in persons with preclinical familial Alzheimer disease. Neurology 71, 85–92 (2008). [DOI] [PubMed] [Google Scholar]
- 24.Reiman EM et al. Fibrillar amyloid-β burden in cognitively normal people at 3 levels of genetic risk for Alzheimer’s disease. Proc. Natl Acad. Sci. USA 106, 6820–6825 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Boerwinkle AH et al. Comparison of amyloid burden in individuals with Down syndrome versus autosomal dominant Alzheimer’s disease: a cross-sectional study. Lancet Neurol. 22, 55–65 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pike KE et al. β-amyloid imaging and memory in non-demented individuals: evidence for preclinical Alzheimer’s disease. Brain 130, 2837–2844 (2007). [DOI] [PubMed] [Google Scholar]
- 27.Wirth M. et al. The effect of amyloid β on cognitive decline is modulated by neural integrity in cognitively normal elderly. Alzheimers Dement. 9, 687–698 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mormino EC et al. Synergistic effect of β-amyloid and neurodegeneration on cognitive decline in clinically normal individuals. JAMA Neurol. 71, 1379–1385 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lim YY et al. Effect of amyloid on memory and non-memory decline from preclinical to clinical Alzheimer’s disease. Brain 137, 221–231 (2014). [DOI] [PubMed] [Google Scholar]
- 30.Landau SM et al. Amyloid deposition, hypometabolism, and longitudinal cognitive decline. Ann. Neurol 72, 578–586 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Storandt M, Mintun MA, Head D & Morris JC Cognitive decline and brain volume loss as signatures of cerebral amyloid-β peptide deposition identified with Pittsburgh compound B: cognitive decline associated with Aβ deposition. Arch. Neurol 66, 1476–1481 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pietrzak RH et al. Trajectories of memory decline in preclinical Alzheimer’s disease: results from the Australian Imaging, Biomarkers and Lifestyle Flagship Study of Ageing. Neurobiol. Aging 36, 1231–1238 (2015). [DOI] [PubMed] [Google Scholar]
- 33.Jicha GA et al. Preclinical AD Workgroup staging: pathological correlates and potential challenges. Neurobiol. Aging 33, 622.e1–622.e16 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vos SJ et al. Preclinical Alzheimer’s disease and its outcome: a longitudinal cohort study. Lancet Neurol. 12, 957–965 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Knopman DS et al. Short-term clinical outcomes for stages of NIA-AA preclinical Alzheimer disease. Neurology 78, 1576–1582 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Toledo JB et al. CSF Apo-E levels associate with cognitive decline and MRI changes. Acta Neuropathol. 127, 621–632 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ryman DC et al. Symptom onset in autosomal dominant Alzheimer disease: a systematic review and meta-analysis. Neurology 83, 253–260 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.de Graaf G, Buckley F & Skotko BG Estimation of the number of people with Down syndrome in the United States. Genet. Med 19, 439–447 (2017). [DOI] [PubMed] [Google Scholar]
- 39.Rafii MS, Wishnek H & Brewer JB The Down Syndrome Biomarker Initiative (DSBI) pilot: proof of concept for deep phenotyping of Alzheimer’s disease biomarkers in Down syndrome. Front. Behav. Neurosci 9, 239 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Neale N, Padilla C, Fonseca LM, Holland T & Zaman S Neuroimaging and other modalities to assess Alzheimer’s disease in Down syndrome. NeuroImage Clin. 17, 263–271 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Handen BL, Cohen AD & Channamalappa U Imaging brain amyloid in nondemented young adults with Down syndrome using Pittsburgh compound B. Alzheimers Dement. 8, 496–501 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Annus T, Wilson LR & Hong YT The pattern of amyloid accumulation in the brains of adults with Down syndrome. Alzheimers Dement. 12, 538–545 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lao PJ, Betthauser TJ & Hillmer AT The effects of normal aging on amyloid-β deposition in nondemented adults with Down syndrome as imaged by carbon 11-labeled Pittsburgh compound B. Alzheimers Dement. 12, 380–390 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fortea J. et al. Plasma and CSF biomarkers for the diagnosis of Alzheimer’s disease in adults with Down syndrome: a cross-sectional study. Lancet Neurol. 17, 860–869 (2018). [DOI] [PubMed] [Google Scholar]
- 45.Fleisher AS, Chen K & Quiroz YT Associations between biomarkers and age in the presenilin 1 E280A autosomal dominant Alzheimer disease kindred: a cross-sectional study. JAMA Neurol. 72, 316–324 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rafii MS et al. A randomized, double-blind, placebo-controlled, phase II study of oral ELND005 (scyllo-inositol) in young adults with Down syndrome without dementia. J. Alzheimers Dis 58, 401–411 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rafii MS et al. Safety, tolerability, and immunogenicity of the ACI-24 vaccine in adults with Down syndrome: a phase 1b randomized clinical trial. JAMA Neurol. 79, 565–574 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rafii MS Alzheimer’s disease in Down syndrome: progress in the design and conduct of drug prevention trials. CNS Drugs 34, 785–794 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jack CR Jr et al. A/T/N: an unbiased descriptive classification scheme for Alzheimer disease biomarkers. Neurology 87, 539–547 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Knopman DS et al. The National Institute on Aging and the Alzheimer’s Association Research Framework for Alzheimer’s disease: perspectives from the Research Roundtable. Alzheimers Dement. 14, 563–575 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Strikwerda-Brown C. et al. Association of elevated amyloid and tau positron emission tomography signal with near-term development of Alzheimer disease symptoms in older adults without cognitive impairment. JAMA Neurol. 79, 975–985 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.van der Flier WM & Scheltens P The ATN framework—moving preclinical Alzheimer disease to clinical relevance. JAMA Neurol. 79, 968–970 (2022). [DOI] [PubMed] [Google Scholar]
- 53.Soldan A. et al. ATN profiles among cognitively normal individuals and longitudinal cognitive outcomes. Neurology 92, e1567–e1579 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vos SJB & Duara R The prognostic value of ATN Alzheimer biomarker profiles in cognitively normal individuals. Neurology 92, 643–644 (2019). [DOI] [PubMed] [Google Scholar]
- 55.Delmotte K. et al. Prognostic value of amyloid/tau/neurodegeneration (ATN) classification based on diagnostic cerebrospinal fluid samples for Alzheimer’s disease. Alzheimers Res. Ther 13, 84 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Allegri RF et al. Prognostic value of ATN Alzheimer biomarkers: 60-month follow-up results from the Argentine Alzheimer’s Disease Neuroimaging Initiative. Alzheimers Dement. 12, e12026 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Selvackadunco S. et al. Comparison of clinical and neuropathological diagnoses of neurodegenerative diseases in two centres from the Brains for Dementia Research (BDR) cohort. J. Neural Transm. 126, 327–337 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Morris JC et al. Pittsburgh compound B imaging and prediction of progression from cognitive normality to symptomatic Alzheimer disease. Arch. Neurol 66, 1469–1475 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vemuri P. et al. MRI and CSF biomarkers in normal, MCI, and AD subjects: predicting future clinical change. Neurology 73, 294–301 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fagan AM et al. Decreased cerebrospinal fluid Aβ42 correlates with brain atrophy in cognitively normal elderly. Ann. Neurol 65, 176–183 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lowe VJ et al. Association of hypometabolism and amyloid levels in aging, normal subjects. Neurology 82, 1959–1967 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nettiksimmons J. et al. Subtypes based on cerebrospinal fluid and magnetic resonance imaging markers in normal elderly predict cognitive decline. Neurobiol. Aging 31, 1419–1428 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pankratz VS et al. Predicting the risk of mild cognitive impairment in the Mayo Clinic Study of Aging. Neurology 84, 1433–1442 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Stomrud E. et al. Correlation of longitudinal cerebrospinal fluid biomarkers with cognitive decline in healthy older adults. Arch. Neurol 67, 217–223 (2010). [DOI] [PubMed] [Google Scholar]
- 65.Sutphen CL et al. Longitudinal cerebrospinal fluid biomarker changes in preclinical Alzheimer disease during middle age. JAMA Neurol. 72, 1029–1042 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dumurgier J. et al. Alzheimer’s disease biomarkers and future decline in cognitive normal older adults. J. Alzheimers Dis 60, 1451–1459 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Farrell ME et al. Defining the lowest threshold for amyloid-PET to predict future cognitive decline and amyloid accumulation. Neurology 96, e619–e631 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Campbell MR et al. P-tau/Aβ42 and Aβ42/40 ratios in CSF are equally predictive of amyloid PET status. Alzheimers Dement. 13, e12190 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Schindler SE et al. Predicting symptom onset in sporadic Alzheimer disease with amyloid PET. Neurology 97, e1823–e1834 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bourgeat P. et al. β-amyloid burden in the temporal neocortex is related to hippocampal atrophy in elderly subjects without dementia. Neurology 74, 121–127 (2010). [DOI] [PubMed] [Google Scholar]
- 71.Oh H. et al. β-amyloid affects frontal and posterior brain networks in normal aging. NeuroImage 54, 1887–1895 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Dickerson BC et al. The cortical signature of Alzheimer’s disease: regionally specific cortical thinning relates to symptom severity in very mild to mild AD dementia and is detectable in asymptomatic amyloid-positive individuals. Cereb. Cortex 19, 497–510 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sperling RA et al. Amyloid deposition is associated with impaired default network function in older persons without dementia. Neuron 63, 178–188 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hedden T. et al. Disruption of functional connectivity in clinically normal older adults harboring amyloid burden. J. Neurosci 29, 12686–12694 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mormino EC et al. Relationships between β-amyloid and functional connectivity in different components of the default mode network in aging. Cereb. Cortex 21, 2399–2407 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rentz DM et al. Face–name associative memory performance is related to amyloid burden in normal elderly. Neuropsychologia 49, 2776–2783 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chetelat G. et al. Independent contribution of temporal β-amyloid deposition to memory decline in the pre-dementia phase of Alzheimer’s disease. Brain 134, 798–807 (2011). [DOI] [PubMed] [Google Scholar]
- 78.Nakamura A. et al. High performance plasma amyloid-β biomarkers for Alzheimer’s disease. Nature 554, 249–254 (2018). [DOI] [PubMed] [Google Scholar]
- 79.Schindler SE et al. High-precision plasma β-amyloid 42/40 predicts current and future brain amyloidosis. Neurology 93, e1647–e1659 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Li Y et al. Validation of plasma amyloid-β 42/40 for detecting Alzheimer disease amyloid plaques. Neurology 98, e688–e699 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Fandos N. et al. Plasma amyloid β 42/40 ratios as biomarkers for amyloid β cerebral deposition in cognitively normal individuals. Alzheimers Dement. 8, 179–187 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mattsson N, Cullen NC, Andreasson U, Zetterberg H & Blennow K. Association between longitudinal plasma neurofilament light and neurodegeneration in patients with Alzheimer disease. JAMA Neurol. 76, 791–799 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mielke MM et al. Plasma phospho-tau181 increases with Alzheimer’s disease clinical severity and is associated with tau- and amyloid-positron emission tomography. Alzheimers Dement. 14, 989–997 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Janelidze S. et al. Plasma p-tau181 in Alzheimer’s disease: relationship to other biomarkers, differential diagnosis, neuropathology and longitudinal progression to Alzheimer’s dementia. Nat. Med 26, 379–386 (2020). [DOI] [PubMed] [Google Scholar]
- 85.Palmqvist S. et al. Performance of fully automated plasma assays as screening tests for Alzheimer disease-related β-amyloid status. JAMA Neurol. 76, 1060–1069 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Cullen NC et al. Plasma biomarkers of Alzheimer’s disease improve prediction of cognitive decline in cognitively unimpaired elderly populations. Nat. Commun 12, 3555 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ossenkoppele R. et al. Accuracy of tau positron emission tomography as a prognostic marker in preclinical and prodromal Alzheimer disease: a head-to-head comparison against amyloid positron emission tomography and magnetic resonance imaging. JAMA Neurol. 78, 961–971 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Rafii MS et al. The AHEAD 3-45 Study: design of a prevention trial for Alzheimer’s disease. Alzheimers Dement. 10.1002/alz.12748 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Johnson KA et al. Tau positron emission tomographic imaging in aging and early Alzheimer disease. Ann. Neurol 79, 110–119 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Insel PS et al. Tau positron emission tomography in preclinical Alzheimer’s disease. Brain 146, 700–711 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mattsson-Carlgren N. et al. Prediction of longitudinal cognitive decline in preclinical Alzheimer disease using plasma biomarkers. JAMA Neurol. 80, 360–369 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Mosconi L. et al. Hippocampal hypometabolism predicts cognitive decline from normal aging. Neurobiol. Aging 29, 676–692 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kennedy AM et al. Deficits in cerebral glucose metabolism demonstrated by positron emission tomography in individuals at risk of familial Alzheimer’s disease. Neurosci. Lett 186, 17–20 (1995). [DOI] [PubMed] [Google Scholar]
- 94.de Leon MJ et al. Prediction of cognitive decline in normal elderly subjects with 2-[18F]fluoro-2-deoxy-d-glucose/positron-emission tomography (FDG/PET). Proc. Natl Acad. Sci. USA 98, 10966–10971 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Jagust WJ et al. Brain imaging evidence of preclinical Alzheimer’s disease in normal aging. Ann. Neurol 59, 673–681 (2006). [DOI] [PubMed] [Google Scholar]
- 96.Serrano ME, Kim E, Petrinovic MM, Turkheimer F & Cash D Imaging synaptic density: the next holy grail of neuroscience? Front. Neurosci 16, 796129 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Mecca AP et al. In vivo measurement of widespread synaptic loss in Alzheimer’s disease with SV2A PET. Alzheimers Dement. 16, 974–982 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Arvidsson Rådestig M. et al. Cerebrospinal fluid biomarkers of axonal and synaptic degeneration in a population-based sample. Alzheimers Res. Ther 15, 44 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Zetterberg H. et al. Association of cerebrospinal fluid neurofilament light concentration with Alzheimer disease progression. JAMA Neurol. 73, 60–67 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ferreira PCL et al. Plasma biomarkers identify older adults at risk of Alzheimer’s disease and related dementias in a real-world population-based cohort. Alzheimers Dement. 10.1002/alz.12986 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Pettigrew C. et al. Progressive medial temporal lobe atrophy during preclinical Alzheimer’s disease. NeuroImage Clin. 16, 439–446 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Pettigrew C. et al. Cortical thickness in relation to clinical symptom onset in preclinical AD. NeuroImage Clin. 12, 116–122 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Mueller SG et al. Hippocampal atrophy patterns in mild cognitive impairment and Alzheimer’s disease. Hum. Brain Mapp 31, 1339–1347 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.McRae-McKee K. et al. Combining hippocampal volume metrics to better understand Alzheimer’s disease progression in at-risk individuals. Sci. Rep 9, 7499 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Elias MF et al. The preclinical phase of Alzheimer disease: a 22-year prospective study of the Framingham Cohort. Arch. Neurol 57, 808–813 (2000). [DOI] [PubMed] [Google Scholar]
- 106.Saxton J. et al. Preclinical Alzheimer disease: neuropsychological test performance 1.5 to 8 years prior to onset. Neurology 63, 2341–2347 (2004). [DOI] [PubMed] [Google Scholar]
- 107.Donohue MC et al. The preclinical Alzheimer cognitive composite: measuring amyloid-related decline. JAMA Neurol. 71, 961–970 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Bransby L. et al. Sensitivity of a Preclinical Alzheimer’s Cognitive Composite (PACC) to amyloid β load in preclinical Alzheimer’s disease. J. Clin. Exp. Neuropsychol 41, 591–600 (2019). [DOI] [PubMed] [Google Scholar]
- 109.Papp KV et al. Sensitivity of the Preclinical Alzheimer’s Cognitive Composite (PACC), PACC5, and Repeatable Battery for Neuropsychological Status (RBANS) to amyloid status in preclinical Alzheimer’s disease—Atabecestat Phase 2b/3 EARLY Clinical Trial. J. Prev. Alzheimers Dis 9, 255–261 (2022). [DOI] [PubMed] [Google Scholar]
- 110.Ayutyanont N. et al. The Alzheimer’s Prevention Initiative Composite Cognitive Test score: sample size estimates for the evaluation of preclinical Alzheimer’s disease treatments in presenilin 1 E280A mutation carriers. J. Clin. Psychiatry 75, 652–660 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Langbaum JB et al. The Alzheimer’s Prevention Initiative Composite Cognitive Test: a practical measure for tracking cognitive decline in preclinical Alzheimer’s disease. Alzheimers Res. Ther 12, 66 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Amariglio RE et al. Tracking early decline in cognitive function in older individuals at risk for Alzheimer disease dementia: the Alzheimer’s Disease Cooperative Study Cognitive Function Instrument. JAMA Neurol. 72, 446–454 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Li C. et al. The utility of the Cognitive Function Instrument (CFI) to detect cognitive decline in non-demented older adults. J. Alzheimers Dis 60, 427–437 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Galasko D. et al. ADCS Prevention Instrument Project: assessment of instrumental activities of daily living for community-dwelling elderly individuals in dementia prevention clinical trials. Alzheimer Dis. Assoc. Disord 20, S152–S169 (2006). [DOI] [PubMed] [Google Scholar]
- 115.Marshall GA et al. Measuring instrumental activities of daily living in non-demented elderly: a comparison of the new performance-based Harvard Automated Phone Task with other functional assessments. Alzheimers Res. Ther 11, 4 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Weintraub S. et al. Measuring cognition and function in the preclinical stage of Alzheimer’s disease. Alzheimers Dement. 4, 64–75 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Barnes DE & Yaffe K The projected effect of risk factor reduction on Alzheimer’s disease prevalence. Lancet Neurol. 10, 819–828 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ding J. et al. Antihypertensive medications and risk for incident dementia and Alzheimer’s disease: a meta-analysis of individual participant data from prospective cohort studies. Lancet Neurol. 19, 61–70 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Marinelli JP et al. Association between hearing loss and development of dementia using formal behavioural audiometric testing within the Mayo Clinic Study of Aging (MCSA): a prospective population-based study. Lancet Healthy Longev. 3, e817–e824 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Middleton LE, Barnes DE, Lui LY & Yaffe K Physical activity over the life course and its association with cognitive performance and impairment in old age. J. Am. Geriatr. Soc 58, 1322–1326 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Yiannopoulou KG, Anastasiou AI, Zachariou V & Pelidou SH Reasons for failed trials of disease-modifying treatments for Alzheimer disease and their contribution in recent research. Biomedicines 7, 97 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Arndt JW et al. Structural and kinetic basis for the selectivity of aducanumab for aggregated forms of amyloid-β. Sci. Rep 8, 6412 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ferrero J. et al. First-in-human, double-blind, placebo-controlled, single-dose escalation study of aducanumab (BIIB037) in mild-to-moderate Alzheimer’s disease. Alzheimers Dement. 2, 169–176 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Sevigny J. et al. The antibody aducanumab reduces Aβ plaques in Alzheimer’s disease. Nature 537, 50–56 (2016). [DOI] [PubMed] [Google Scholar]
- 125.Budd Haeberlein S. et al. Two randomized phase 3 studies of aducanumab in early Alzheimer’s disease. J. Prev. Alzheimers Dis. 9, 197–210 (2022). [DOI] [PubMed] [Google Scholar]
- 126.Ostrowitzki S. et al. Mechanism of amyloid removal in patients with Alzheimer disease treated with gantenerumab. Arch. Neurol 69, 198–207 (2012). [DOI] [PubMed] [Google Scholar]
- 127.Klein G. et al. Gantenerumab reduces amyloid-β plaques in patients with prodromal to moderate Alzheimer’s disease: a PET substudy interim analysis. Alzheimers Res. Ther 11, 101 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Doody R. Clinical Trial in Alzheimer’s Disease (CTAD) meeting, November 29 (2022). [Google Scholar]
- 129.Salloway S. et al. A trial of gantenerumab or solanezumab in dominantly inherited Alzheimer’s disease. Nat. Med 27, 1187–1196 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Lowe SL et al. Donanemab (LY3002813) phase 1b study in Alzheimer’s disease: rapid and sustained reduction of brain amyloid measured by florbetapir F18 imaging. J. Prev. Alzheimers Dis 8, 414–424 (2021). [DOI] [PubMed] [Google Scholar]
- 131.Mintun MA et al. Donanemab in early Alzheimer’s disease. N. Engl. J. Med 384, 1691–1704 (2021). [DOI] [PubMed] [Google Scholar]
- 132.Swanson CJ et al. A randomized, double-blind, phase 2b proof-of-concept clinical trial in early Alzheimer’s disease with lecanemab, an anti-Aβ protofibril antibody. Alzheimers Res. Ther 13, 80 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.van Dyck CH et al. Lecanemab in early Alzheimer’s disease. N. Engl. J. Med 388, 9–21 (2023). [DOI] [PubMed] [Google Scholar]
- 134.Sperling RA et al. Amyloid-related imaging abnormalities in amyloid-modifying therapeutic trials: recommendations from the Alzheimer’s Association Research Roundtable Workgroup. Alzheimers Dement. 7, 367–385 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Barakos J. et al. Detection and management of amyloid-related imaging abnormalities in patients with Alzheimer’s disease treated with anti-amyloid β therapy. J. Prev. Alzheimers Dis 9, 211–220 (2022). [DOI] [PubMed] [Google Scholar]
- 136.Piazza F. et al. Anti-amyloid β autoantibodies in cerebral amyloid angiopathy-related inflammation: implications for amyloid-modifying therapies. Ann. Neurol 73, 449–458 (2013). [DOI] [PubMed] [Google Scholar]
- 137.Salloway S. et al. Amyloid-related imaging abnormalities in 2 phase 3 studies evaluating aducanumab in patients with early Alzheimer disease. JAMA Neurol. 79, 13–21 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Doody RS et al. Phase 3 trials of solanezumab for mild-to-moderate Alzheimer’s disease. N. Engl. J. Med 370, 311–321 (2014). [DOI] [PubMed] [Google Scholar]
- 139.Honig LS et al. Trial of solanezumab for mild dementia due to Alzheimer’s disease. N. Engl. J. Med 378, 321–330 (2018). [DOI] [PubMed] [Google Scholar]
- 140.Lilly. Lilly provides update on A4 study of solanezumab for preclinical Alzheimer’s disease. investory.lilly.com, https://investor.lilly.com/news-releases/news-release-details/lilly-provides-update-a4-study-solanezumab-preclinical#:~:text=INDIANAPOLIS%2C%20March%208%2C%202023%20%2FPRNewswire%2F%20--%20Eli%20Lilly,known%20as%20the%20preclinical%20stage%20of%20AD%201 (8 March 2023).
- 141.Vassar R. et al. β-secretase cleavage of Alzheimer’s amyloid precursor protein by the transmembrane aspartic protease BACE. Science 286, 735–741 (1999). [DOI] [PubMed] [Google Scholar]
- 142.Jonsson T. et al. A mutation in APP protects against Alzheimer’s disease and age-related cognitive decline. Nature 488, 96–99 (2012). [DOI] [PubMed] [Google Scholar]
- 143.Egan MF et al. Randomized trial of verubecestat for prodromal Alzheimer’s disease. N. Engl. J. Med 380, 1408–1420 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Henley D. et al. Preliminary results of a trial of atabecestat in preclinical Alzheimer’s disease. N. Engl. J. Med 380, 1483–1485 (2019). [DOI] [PubMed] [Google Scholar]
- 145.Wessels AM et al. Efficacy and safety of lanabecestat for treatment of early and mild Alzheimer disease: the AMARANTH and DAYBREAK-ALZ randomized clinical trials. JAMA Neurol. 77, 199–209 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Sperling R. et al. Findings of efficacy, safety, and biomarker outcomes of atabecestat in preclinical Alzheimer disease: a truncated randomized phase 2b/3 clinical trial. JAMA Neurol. 78, 293–301 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.McDade E. et al. The case for low-level BACE1 inhibition for the prevention of Alzheimer disease. Nat. Rev. Neurol 17, 703–714 (2021). [DOI] [PubMed] [Google Scholar]
- 148.Congdon EE & Sigurdsson EM Tau-targeting therapies for Alzheimer disease. Nat. Rev. Neurol 14, 399–415 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Langbaum JB et al. Recommendations to address key recruitment challenges of Alzheimer’s disease clinical trials. Alzheimers Dement. 19, 696–707 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Indorewalla KK, O’Connor MK, Budson AE, Guess DiTerlizzi C & Jackson J Modifiable barriers for recruitment and retention of older adults participants from underrepresented minorities in Alzheimer’s disease research. J. Alzheimers Dis 80, 927–940 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Aisen PS et al. The Trial-Ready Cohort for Preclinical/Prodromal Alzheimer’s Disease (TRC-PAD) project: an overview. J. Prev. Alzheimer’s Dis 7, 208–212 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Aisen PS et al. The Trial-Ready Cohort for Preclinical/Prodromal Alzheimer’s Disease (TRC-PAD) project: an overview. J. Prev. Alzheimers Dis 7, 208–212 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]