Abstract
Objectives
The purpose of this study was to establish a method for feline splenic measurement on abdominal radiographs and evaluate for correlation between the radiographic measurements and ultrasonographic measurements.
Methods
One hundred cats with normal abdominal radiographs and ultrasound (US) studies of the spleen were evaluated. The hypothesis was that the measurement of the spleen on the radiographs would correlate with the measurement of the spleen on US. The radiographic and ultrasonographic measurements were tabulated and compared using linear regression and t-tests using unequal variances.
Results
The measurement of the spleen on the ventrodorsal projection was characterized as one of three shapes (A, B or C), and thereby based on the thickest part of the spleen (when corrected for radiographic magnification: A = 9.9 ± 2.2 mm; B = 8.1 ± 1.8 mm; C = 8.0 ± 2.3 mm). There were 48 cats where the head of the spleenwas seen on the right lateral (n = 10), the left lateral (n = 24) or both (n = 14) projections. On one left lateral, both the head and tail of the spleen were seen. There was weak correlation between the radiographic and US measurements(R ⩼0.6). Splenic thickness of shape A on the ventrodorsal projection was significantly greater than categories B and C.
Conclusions and relevance
Radiographic measurement of the spleen is not a reliable indicator of its ultrasonographic measurement. The ultrasonographic measurements seen in this study (mean of 8.0 ± 1.6 mm) were similar to measurements of the spleen reported in previous studies. It is rare to see the tail of the spleen on lateral feline abdominal radiographs.
Introduction
The feline spleen is identified and evaluated on radiographs as a triangular soft tissue opacity located caudal and lateral to the gastric fundus and craniolateral to the left kidney on the ventrodorsal projection.1–3 The amount of the spleen visualized on ventrodorsal radiographs, and its shape, is variable owing to its mobility and other factors, including body condition, positioning, gastric contents and abdominal pathology. The feline splenic head can also be evaluated radiographically on lateral projections, caudal and dorsal to the gastric fundus.1,3 Radiographic assessment of splenic size is subjective and the ability to identify the tail of the spleen on lateral projections is often used as a method for declaring splenomegaly in the cat. Ultrasound (US) is considered the current gold standard for assessing the spleen as it allows evaluation of size, shape and parenchymal echotexture.
Two studies evaluating diagnostic imaging measurements of the normal feline spleen have been performed and both are focused on its normal ultrasonographic measurements and echogenicity.4,5 The first study established a baseline of 8.2 ± 1.4 mm for splenic height in cats prior to anesthesia. 4 A second study found the mean height of the splenic head was 7.1 ± 1.2 mm, mean height of the body was 9.3 ± 1.5 mm and mean height of the splenic tail was 8.7 ± 1.5 mm. 5 Objective radiographic methods for measuring the feline spleen and standard values have not been described in the veterinary literature. Information available on evaluating feline splenic size is largely found in published textbooks or manuals, which reflect the clinical experience of experts.
The purpose of this study was to establish a method for radiographic splenic measurements, and to correlate these radiographic measurements with ultrasonographic measurements of the spleen. The null hypothesis is that the splenic measurements will not correlate between abdominal radiographs and abdominal US.
Materials and methods
A database search was performed through the Radiology Information System (Fuji Synapse; Fuji Medical Systems) at the University of Florida College of Veterinary Medicine. Inclusion criteria consisted of domestic cats, over 1 year of age, that had undergone both abdominal radiographs and US without a diagnosis of splenomegaly in either imaging study. The abdominal radiographic studies consisted of three projections: right lateral (RL), left lateral (LL) and ventrodorsal (VD). The inclusion criteria also required that the spleen be identifiable on at least the VD with good serosal margin detail and lack of radiographically apparent splenic or adjacent pathology. The abdominal radiographs and US images were reviewed and measured within the Picture Archive and Communication System (PACS; Merge Healthcare). The radiographs and US of each cat were performed within 5 days of each other, and performed between 14 October 2013 and 27 September 2015. All studies contained reports reviewed by a Diplomate of the American College of Veterinary Radiology (ACVR) and only studies with no mention of splenomegaly in both reports were included. No exclusion was based on previous/concurrent illness, sex, breed or sedation status.
Radiographic measurements
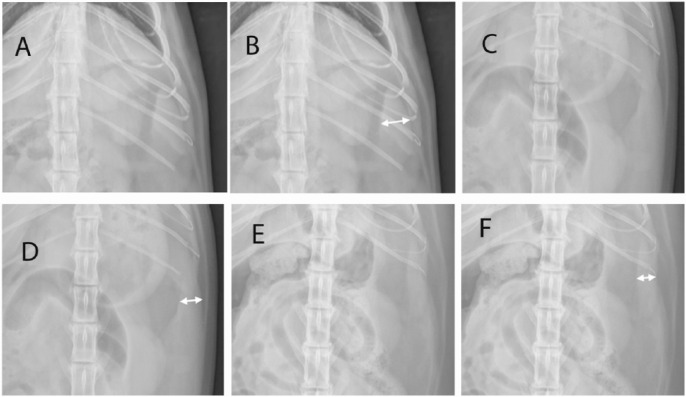
All radiographs were taken using a digital radiography system (Canon Imaging System, CXDI-50G digital radiography detectors). A protocol was developed to measure consistently the splenic thickness on radiographs while addressing the variability in spleen position. All cases were required to have an identifiable portion of the spleen for measurement on the VD projection, and if the head or tail was visible on either lateral projection it was also measured. All measures were made by consensus between all three authors. Measurements were made using digital calipers. Each measurement of the spleen was made in a consistent method based on a given shape. The spleen shapes as seen on the VD projection were placed into one of three possible categories, labeled A, B or C (Figure 1).
Figure 1.
Comparative images of the three categories of spleen shape noted on ventrodorsal abdominal radiographs. Shape A: a triangular soft tissue opacity, caudolateral to the gastric fundus and cranial to the left kidney; shown without (A) and with (B) an arrow indicating how the measurement was made. Shape B: two contiguous soft tissue opaque structures with a triangular, soft tissue opacity centered between; shown without (C) and with (D) an arrow indicating how the measurement was made. Shape C: a soft tissue opaque, fusiform shape along the body wall with tapering ends; shown without (E) and with (F) an arrow indicating how the measurement was made
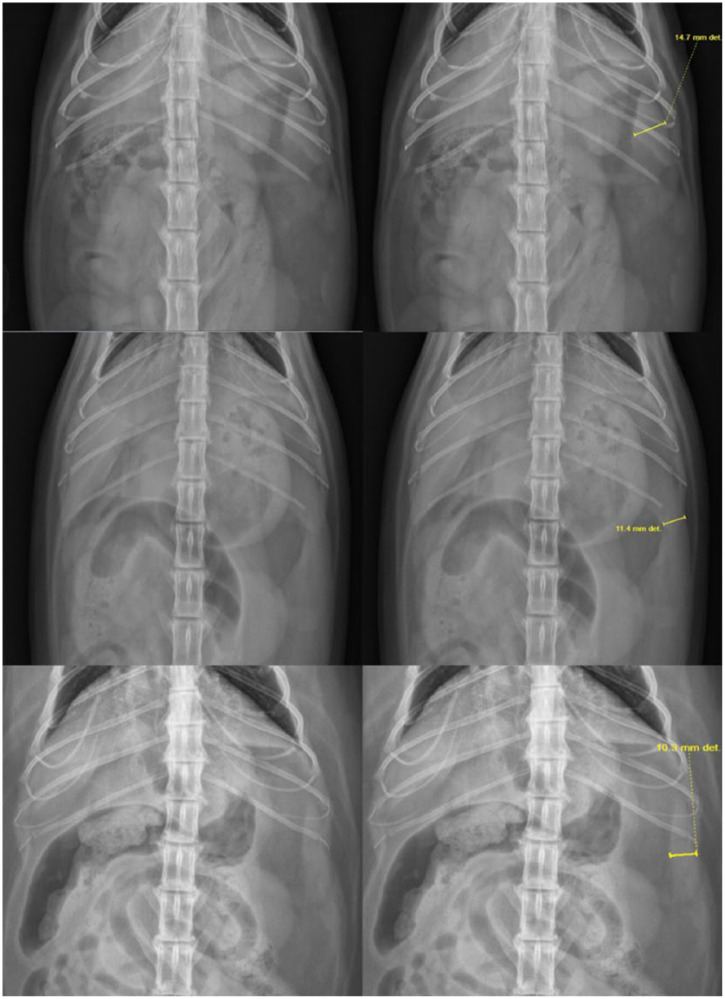
Shape A was assigned when the spleen was identified solely as a triangular soft tissue opacity, caudolateral to the gastric fundus and cranial to the left kidney. The triangle was measured at its widest point, in mm, perpendicular to its long axis of the spleen. Shape B was assigned when the spleen was seen as two contiguous soft tissue structures with a triangular, soft tissue opacity centered between, representative of the splenic head and body as they curved and overlapped along the body wall. The measurement was made in mm at the widest point of the triangle, perpendicular to its long axis. Shape C was assigned when the spleen was seen as a soft tissue, fusiform shape along the body wall with tapering ends. The measurement was made in mm, at its widest point, perpendicular to the long axis (Figure 2).
Figure 2.
Collimated ventrodorsal abdominal radiographs exhibiting the three different spleen shape categories before and after measurements of the spleen were performed using digital calipers
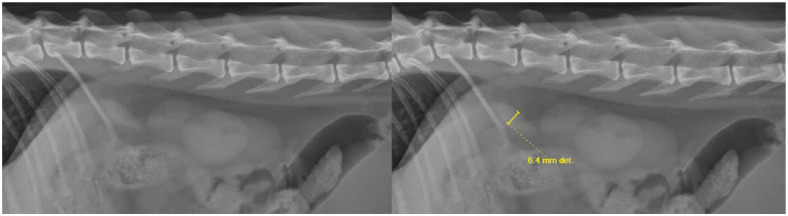
On right and left lateral projections, the presence or absence of the splenic head and tail was recorded and measured when visible. The splenic head was seen as an elongated, oval shape, caudal to the gastric fundus and cranial to the kidneys. Measurement of the splenic head was performed by measuring perpendicular to the long axis, at its widest point (Figure 3).
Figure 3.
Collimated left lateral abdominal radiographs before and after measurement of the splenic head was performed using digital calipers
In order to estimate magnification retrospectively, the average thickness of a feline abdomen was estimated to be 8 cm, and the feline spleen was estimated to be positioned in the mid abdomen, 4 cm from the table. A radiograph of a quarter was then made, with the quarter sitting on a radiographic positioning sponge, 4 cm away from the table in order to mimic the location of the spleen. The radiographic image of the quarter was measured three times using digital calipers, and the average of the three measurements was determined to be the image size (26.6 mm). The actual diameter of a quarter is 24.26 mm. The magnification factor was calculated by dividing the image diameter by the object diameter, and was determined to be 1.08.
Ultrasonographic measurements
All abdominal US (iU22; Philips Medical) were performed by either a Diplomate of ACVR or radiology resident in training, using either a curvilinear (C8-5 MHz) or linear (L12-5 MHz) transducer. The thickness of the spleen (referred to as ‘height’ in other studies) on US was measured on the image captured, at its widest point, with the spleen in short axis. Captured images were based on the mid-body of the spleen. US data for the study were derived from either the captured image with measurement created by the ultrasonographer or, in the event the measurement was not saved, by taking the measurement on the captured image while viewing the image in PACS. If the authors did not agree with the presaved measurement, the measurements were repeated (Figure 4).
Figure 4.
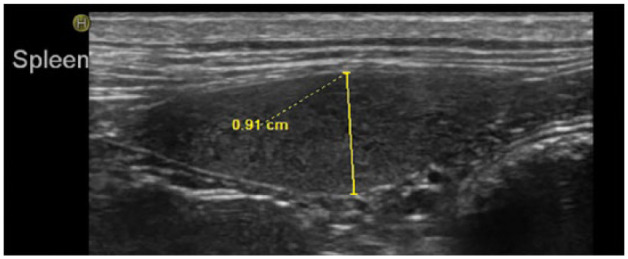
Ultrasound spleen measurement performed retrospectively when original measurement image was not saved. Cranial is to the left side of the image and caudal is to the right. The ultrasound image of the spleen was captured using a linear transducer. The splenic shape and echotexture are within normal limits. Measurements were made by recording the thickness of the spleen, at its widest point, perpendicular to the long axis
Data collection and analysis
Data were tabulated and analyzed using Microsoft Excel. A total of 100 cases were used. The average and SD of each type of radiographic measurement (shape A, shape B, shape C, RL head, LL head) and US measurements were calculated and recorded. Linear regression was used to observe the relationship between each radiographic splenic shape (A, B or C) and the US measurement, between the thickest splenic measurement of each radiographic projection and the US measurement and between all VD spleen measurements (regardless of category) and the US measurements both before and after radiographic magnification was estimated. A correlation coefficient (R) ⩾0.8 was considered a strong correlation. An independent t-test with unequal variances (Welch test) was performed between the spleen shapes: A and B, B and C, C and A. Frequency of splenic head identification on the lateral projections was evaluated and linear regression analysis was performed to compare the splenic head measurements (right and left) with the US measurements. An independent t-test with unequal variances was also performed between the right splenic head and left splenic head measurements. A P value <0.05 was considered significant.
Results
The cats ranged in age from 1–20.5 years old with an average age (± SD) of 9.2 ± 4.8 years, and a median of 9.3 years. Of the 100 VD splenic measurements, the majority were categorized as shape B (52 cases), followed by shape A (27 cases) and shape C (21 cases). After radiographic magnification was accounted for, the mean (± SD) thickness of all VD measurements was 8.6 ± 2.2 mm. The average (± SD) VD spleen thickness by shape was A = 9.9 ± 2.2 mm, B = 8.1 ± 1.8 mm and C = 8.0 ± 2.3 mm. The mean (± SD) thickness of the splenic head was 8.0 ± 2.0 mm on the RL and 7.1 ± 2.0 mm on the LL. The mean (± SD) thickness measured on US was 8.0 ± 1.6 mm (Table 1).
Table 1.
Radiographic and ultrasound (US) mean measurements with SD
| Type of measurement | Mean ± SD (mm) | MeanM ± SD (mm)* |
|---|---|---|
| Shape A | 10.7 ± 2.4 | 9.9 ± 2.2 |
| Shape B | 8.7 ± 1.9 | 8.1 ± 1.8 |
| Shape C | 8.6 ± 2.4 | 8.0 ± 2.3 |
| All VD | 9.2 ± 2.3 | 8.6 ± 2.2 |
| RL head | 8.6 ± 2.1 | 8.0 ± 2.0 |
| LL head | 7.7 ± 2.2 | 7.1 ± 2.0 |
| US | 8.0 ± 1.6 | – |
Shapes A, B and C represent mean ± SD of their respective shapes on the ventrodorsal (VD) view
MeanM ± SD indicates the mean ± SD acquired following adjustment for magnification
All VD = mean ± SD of all VD radiographic measurements; RL = mean ± SD of right lateral splenic head measurements; LL = mean ± SD of left lateral splenic head measurements; US = mean ± SD of spleen on all US images
The spleen was identified only on the VD projection in 52 cats, and on more than one projection in 48 cats. Of those 48 cats, the splenic head was identified on only the left lateral and VD in 24 cats, only the right lateral and VD in 10 cats and both laterals and the VD in 14 cats. Therefore, of the cases in which the splenic head could be visualized on the lateral, it could be seen in the left lateral in 38 cats (79%). On only one image, a left lateral, were both the splenic head and tail visualized together. A measurement of the tail thickness was performed and recorded by measuring perpendicular to the long axis at its widest point.
All R value or P values were the same before and after radiographic magnification was accounted for. No statistically significant correlation was found between any of the radiographic and US measurements (R ⩽0.6). The relationship of shape A and US measurement had the highest correlation (R = 0.6) (Table 2).
Table 2.
Correlation coefficients (R) comparing the relationships between radiographic and ultrasound (US) measurements
| Relationship | R value | R valueM* |
|---|---|---|
| All VD vs US | 0.4 | 0.4 |
| Thickest vs US | 0.4 | 0.4 |
| Shape A vs US | 0.6 | 0.6 |
| Shape B vs US | 0.4 | 0.4 |
| Shape C vs US | 0.2 | 0.2 |
| RL vs US | 0.2 | 0.2 |
| LL vs US | 0.1 | 0.1 |
Shapes A, B and C are based on the shape of the spleen on the ventrodorsal (VD) image
All VD = all ventrodorsal radiographic measurements; RL = right lateral; LL = left lateral
R valueM represents the R value acquired using measurements adjusted for magnification
Independent samples t-tests with unequal variances were performed among the different splenic shapes A (n = 27), B (n = 52) and C (n = 21) to test the null hypothesis that they were not statistically different in size (Table 3). The independent samples t-test results showed a statistical difference between the category A and category B measurements (P = 0.0006), as well as between category A and C measurements (P = 0.004). However, the relationship between B and C categories was similar and not significantly different (P = 0.8). An independent samples t-test with unequal variances was also performed to compare RL splenic head and LL splenic head radiographic measurements. There was not a significant difference between these measurements (P = 0.12).
Table 3.
Unequal variance t-test between different radiographic measurements
| Comparison | P value* | P valueM† |
|---|---|---|
| A to B | 0.0006 | 0.0006 |
| B to C | 0.8 | 0.8 |
| A to C | 0.004 | 0.004 |
| RL head to LL head | 0.1 | 0.1 |
A, B and C based on the ventrodorsal spleen shape
<0.05 significance level
P valueM represents the P value acquired using measurements adjusted for magnification
RL = right lateral; LL = left lateral
Discussion
Results of our analysis accepted the null hypothesis and showed no strong correlation between any of the radiographic and ultrasonographic spleen measurements. Our study also showed that measuring the spleen on VD projections as shape A is not equivalent to measuring the spleen as shape B or C. Shapes B and C, however, were similar in thickness. The shape categories were created based on a repeated pattern noted by the authors. The cat spleen’s long narrow body with widening of the distal tail (referred to as ‘spoon shape’) and mobility creates different areas at which the spleen can fold on itself, creating the unique soft tissue shapes that were seen. The discrepancies between shape A and both shapes B and C is, in large part, due to positioning and folding of the spleen, and the measurable soft tissue opacity is that formed. Shape A is seen when the spleen is vertically oriented relative to the long axis of the cat’s body, with the splenic head positioned dorsally and summating with a portion of the splenic body. Both shapes B and C are due to tissue summation of splenic body along the long axis as it travels caudally along the body wall. Therefore, the increased thickness of measurements in category A relative to B and C is suspected to be due to a degree of summation and folding rather than the true thickness of the spleen.
The average US measurement of 8.0 ± 1.6 mm (range 5.0–11.2 mm) in this study was similar to that established in normal cats by previous studies: 8.2 ± 1.4 mm (range 5.3–11.1 mm) 4 and 7.1 ± 1.19 mm (range 5.1–9.1 mm). 5 When compared with splenic US measurements of the first study, only two cats, with measurements of 11.1 and 11.2 mm, exceed the range of two SDs (5.4–11.0mm); and only three cats with measurements of 4.3, 5.0 and 5.0 mm, respectively, fell below the range. 4
Multiple studies have been performed evaluating feline splenic disease and the associated diagnostic imaging characteristics; however, these studies do not define the criteria used when describing splenomegaly in the cat.6–10 Diagnosing the feline spleen as enlarged on US is often aided by using the upper limit of 10 mm and the observer’s subjective opinion of its shape, margination and echogenicity. This cut-off of 10 mm is usually cited as the author’s opinion or referencing the first study on feline US spleen measurement.1,11 In the second study on feline US measurement, the authors suggest utilizing 9.1 mm as the upper limit of normal for the height, or thickness, of the splenic head on US.
Of the cases in which the splenic head could be identified on a lateral projection, it was 1.5 times more likely that the head would be visualized on the left lateral than the right lateral. This is not surprising because the splenic head is fixed in the left abdominal cavity via the gastrosplenic ligament.1,2,12
Typically, identification of the splenic tail on a lateral projection is abnormal and considered suggestive of splenomegaly. 13 Our findings support the concept that it is rare to see the splenic tail on a lateral projection (1%), but based on our study design, we cannot conclude that seeing the splenic tail on a lateral projection correlates to splenomegaly. There was only one projection in which the splenic head and tail were simultaneously identified, and it occurred on the left lateral. The cat had urethral obstruction and a markedly distended urinary bladder, which was causing rightward displacement of the descending colon. It is possible that the tail of the spleen was more easily identified due to displacement of the splenic tail from the distended urinary bladder.
A possible contributing factor for our inability to identify a correlation between radiographic and ultrasonographic measurements of splenic size is the lack of a standard method for measuring the spleen on radiographs based on the different splenic positions seen on the VD image. This presented a challenge when performing retrospective, radiographic splenic measurements. Categorizing splenic shapes on the VD projection was useful in maintaining consistency of where to measure the thickest portion of the spleen on the VD image. Admittedly, there is subjectivity in the process of measuring the feline spleen on radiographs, and it is likely that intra- and inter-observer variability exists in performing measurements. These were not tested in this study.
As an additional limitation, the retrospective nature of this study confounded our ability to account for radiographic magnification as magnification markers are not routinely included in collimation on abdominal radiographs at our institution. The technique used in this study provides only a rough estimate of the true radiographic magnification. In reality, there is variability in magnification based on patient sizes and positioning of the patient (LL, RL or VD). Because magnification should affect all radiographic measurements similarly within each patient, we would not expect comparative values between the radiographic measurements to be affected. However, the effect of magnification should be considered when assessing absolute radiographic measurements of the spleen and when making comparisons between radiographic and ultrasonographic measurements. That said, the correlation between radiographic and ultrasonographic measurements was not improved when radiographic magnification was accounted for.
Although the sample size of 100 cases helps account for abnormal outliers, the sample is only representative of a population of cats seen by the hospital with spleens that were visible on VD radiographic projections, and not necessarily healthy cats. In excluded cases, the majority of the reasons that the spleen could not be identified on the VD projection were border effacement with peritoneal effusion, a decrease in serosal margin detail due to thin body condition and recent surgery leading to mixed peritoneal gas and effusion, abdominal mass and distension of the gastric lumen. These reasons further draw attention to the fact that the abdominal radiographs included in this study were not all normal studies, absent of pathology. Furthermore, splenic cytology or histopathology was not inclusion criteria for this study. Imaging studies were not included if splenomegaly had been diagnosed on the imaging report; however, no further efforts were made to ensure that the spleens evaluated in this study were absent of disease. Therefore, it should be noted that findings in this study are not representative of a normal, healthy feline population and the data are not intended to provide a normal reference interval for splenic size. These data do provide a representative example of the variable radiographic and ultrasonographic appearances of feline spleens at our teaching hospital during a given time period.
Sedation status was not considered as part of the inclusion criteria in this study. In the first study measuring feline spleens on US, sevoflurane as the sole method of general anesthesia led to a statistically significant increase in height, width and cross-sectional area of the feline spleen. However, the changes were considered minimal and it was questioned whether they were clinically relevant. 4 Studies have been carried out to demonstrate canine splenomegaly secondary to anesthetic agents with variable results.14–16 It would not be fair to make similar assumptions about the feline spleen as histologic and physiologic differences in the feline spleen may alter its response to sedation. Theoretically, if the spleen size was altered owing to sedation, that proportion of change would be the same on both the radiographic and ultrasonographic measurements on those cats in which the imaging studies were performed consecutively under one dose of sedation, and would therefore not affect the degree of correlation between the two. Owing to the variable methods of sedation used in the cats, and the retrospective nature of this study, we could not reliably classify the cases based on sedation status.
Conclusions
This study did not detect a significant correlation between radiographic and ultrasonographic measurements of the spleen. In this study, we have established three basic shape categories for identifying the feline spleen radiographically on the VD image. The radiographic splenic appearance and method of measurement in cats determined by this study may serve as a comparison for future studies. More studies of the radiographic measurement of the feline spleen would be beneficial in establishing a standard method. The average splenic US measurements found in this study is similar to that found in previous studies of a normal feline population.
Footnotes
Accepted: 2 August 2016
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Larson MM. The liver and spleen. In: Thrall DE. (ed). Textbook of veterinary diagnostic radiology. 6th ed. Philadelphia, PA: Elsevier, 2013, pp 694–701. [Google Scholar]
- 2. Kealy JK, McAllister H, Graham JP. The abdomen. In: Kealy JK, McAllister H, Graham JP. (eds). Diagnostic radiology and ultrasonography of the dog and cat. 5th ed. Philadelphia, PA: Sauders Elsevier, 2011, pp 23–198. [Google Scholar]
- 3. Coulson A, Lewis N. (eds). An atlas of interpretative radiographic anatomy of the dog and cat. Oxford: Blackwell Science, 2002, pp 514–517. [Google Scholar]
- 4. Reese SL, Zekas LJ, Iazbk MC, et al. Effect of sevoflurane anesthesia and blood donation on the sonographic appearance of the spleen in 60 healthy cats. Vet Radiol Ultrasound 2013; 54: 168–175. [DOI] [PubMed] [Google Scholar]
- 5. Sayre RS, Spaulding KA. Formulation of a standardized protocol and determination of the size and appearance of the spleen in healthy cats. J Feline Med Surg 2014; 16: 326–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Spangler WL, Culbertson MR. Prevalence and type of splenic diseases in cats: 455 cases (1985–1991). J Am Vet Med Assoc 1992; 201: 773–776. [PubMed] [Google Scholar]
- 7. Lamb CR, Hartzband LE, Tidwell AS, et al. Ultrasonographic findings in hepatic and splenic lymphosarcoma in dogs and cats. Vet Radiol Ultrasound 1991; 32: 117–120. [Google Scholar]
- 8. O’Keefe DA, Couto CG. Fine-needle aspiration of the spleen as an aid in diagnosis of splenomegaly. J Vet Intern Med 1987; 1: 102–109. [DOI] [PubMed] [Google Scholar]
- 9. Patel RT, Caceres A, French AF, et al. Multiple myeloma in 16 cats: a retrospective study. Vet Clin Pathol 2005; 34: 341–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hanson JA, Papageorges M, Girard E, et al. Ultrasonographic appearance of splenic disease in 101 cats. Vet Radiol Ultrasound 2001; 42: 441–445. [DOI] [PubMed] [Google Scholar]
- 11. Hecht S, Mai W. Spleen. In: Penninck D, D’Anjou MA. (eds). Atlas of small animal ultrasonography. 2nd ed. Ames, IA: John Wiley, 2015, pp 239–258. [Google Scholar]
- 12. Armbrust L. The spleen. In: O’Brien R, Barr F. (eds). BSAVA manual of canine and feline abdominal imaging. Quedgeley: BSAVA, 2009, pp 167–176. [Google Scholar]
- 13. Farrow CS, Green R, Shively M. (eds). Radiology of the cat. St Louis, MO: Mosby-Year Book, 1994, p 159. [Google Scholar]
- 14. Baldo CF, Garcia-Pereira FL, Nelson NC, et al. Effects of anesthetic drugs on canine splenic volume determined via computed tomography. Am J Vet Res 2012; 73: 1715–1719. [DOI] [PubMed] [Google Scholar]
- 15. Petroianu A. Drug-induced splenic enlargement. Acta Med Port 2011; 24: 977–982. [PubMed] [Google Scholar]
- 16. Wilson DV, Evans AT, Carpenter RA, et al. The effect of four anesthetic protocols on splenic size in dogs. Vet Anaesth Analg 2004; 31:102–108. [DOI] [PubMed] [Google Scholar]