Abstract
Background
At the end of 2022, globally, only 46% of children (aged 0–14 years) on ART had suppressed viral loads. Viral load suppression is crucial to reduce HIV-related deaths. To suppress the viral load at the expected level, children must be retained in ART treatment. Nevertheless, lost to follow-up from ART treatment continues to be a global challenge, particularly, in developing countries. Previously, primary studies were conducted in Ethiopia to assess the incidence of lost to follow-up among HIV-positive children on ART treatment. However, variations have been seen among the studies. Therefore, this systematic review and meta-analysis aimed to estimate the pooled incidence of lost to follow-up among HIV-positive children on ART and identify its associated factors in Ethiopia.
Methods
We searched PubMed, HINARI, Science Direct, Google Scholar, and African Journals Online to obtain articles published up to November 20, 2023. Critical appraisal was done using the Joanna Briggs Institute checklist. Heterogeneity was identified using I-square statistics. Funnel plot and Egger’s tests were used to identify publication bias. Data was presented using forest plots and tables. Random and fixed-effect models were used to compute the pooled estimate.
Results
Twenty-four studies were included in the final analysis. The pooled incidence of lost to follow-up among HIV-positive children on ART was 2.79 (95% CI: 1.99, 3.91) per 100-child-year observations. Advanced HIV disease (HR: 2.20, 95% CI: 1.71, 2.73), having opportunistic infection (HR: 2.59, 95% CI: 1.39; 4.78), fair or poor ART treatment adherence (HR: 2.92, 95% CI: 1.31; 6.54) and children aged between 1–5 years (HR: 2.1,95% CI: 1.44; 2.95) were factors associated with lost to follow up among HIV positive children on ART.
Conclusions
The overall pooled incidence of lost to follow-up among HIV-positive children on ART is low in Ethiopia. Therefore, counseling on ART drug adherence should be strengthened. Moreover, emphasis has to be given to children with advanced HIV stage and opportunistic infection to reduce the rate of lost to follow up among HIV-positive children on ART.
Trial registration
Registered in PROSPERO with ID: CRD42024501071.
Introduction
Human Immunodeficiency Virus (HIV) continues to be one of the global public health concerns, particularly in sub-Saharan Africa. At the end of 2022, an estimated 1.5 million children aged 0–14 years were living with HIV infection and about 130,000 of them were newly infected [1]. In the same year, globally, about 84,000 children died from HIV-related diseases [2]. Despite multiple efforts made, about 43% of children living with HIV were not receiving treatment [3].
To avert the burden of HIV, different efforts have been made globally. In 2015, the United Nations, under its sustainable development goal (SDGS), set a global target to end HIV epidemic by 2030 through the provision of antiretroviral therapy (ART) [4]. ART has profound importance in prolonging the life of people living with HIV infection, mainly, by suppressing the viral load, improving the immune system, and reducing the risk of opportunistic infections [5]. To increase the accessibility and coverage of ART, currently, the United Nations has commenced a 95-95-95 ambitious treatment target for the year 2025, which implies that 95% of people living with HIV know their status, 95% of people living with HIV who know their status are receiving treatment and 95% of people on treatment have suppressed viral loads by the year 2025 [6].
To achieve the aforementioned ambitious treatment targets, HIV-infected children must be retained in a cohort of ART treatment and have regular follow-ups [7]. During follow-up, children are checked for clinical progression, ART side effects, drug adherence, and viral load suppression and should be counseled for optimal drug utilization [8].
Lost to follow-up (LTFU) from ART is one of the global public health concerns, particularly in developing countries. A systematic review conducted in resource-limited settings found that about 5–29% of children living with HIV were lost from ART within the first 12 months of ART initiation [9]. In Sub-Saharan African countries, the proportion of LTFU among HIV-positive children after two years of ART initiation varied from the lowest 9.0% in Southern Africa to the highest 21.8% in West Africa [10]. Children who drop out of the treatment are at an increased risk of drug-resistant virus and treatment failure. These can jeopardize the effectiveness of HIV treatments and increase HIV-related deaths [11, 12].
In Ethiopia, there is a paucity of evidence regarding the national incidence of LTFU among HIV-positive children on ART. Moreover, previous primary studies also confirmed that the incidence of LTFU among HIV-positive children on ART varied across regions in Ethiopia [13–21], ranging from 3.3 per 100 child years in the Oromia region [21] to 6.3 per 100 child years in the Amhara region [15]. Conducting an aggregated study using those primary studies is important to know about the national burden of LTFU among HIV-positive children on ART. Therefore, the main objective of this systematic review and meta-analysis is to estimate the pooled incidence of LTFU among HIV-positive children on ART and identify its associated factors in Ethiopia.
Methods
Search strategy
The result was reported using the Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) guideline [22]. We searched PubMed, HINARI, Science Direct, Google Scholar, and African Journals Online to obtain relevant studies. Online database searching was done on November 20, 2023. The following terms and phrases search as “incidence rate”, “lost to follow-up”, “LTFU”, “treatment outcome”, “attrition”, “pediatrics”, “children” “child”, “newborn”, “Human Immunodeficiency virus”, “HIV”, “ART”, “antiretroviral therapy” “antiretroviral drugs”, “AIDS drugs”, “Anti-HIV drugs” and “Ethiopia” were the main key search terms used in this systematic review and meta-analysis. We used Boolean operators such as “AND” and “OR” during database searching (S1 File).
Eligibility criteria
The inclusion criteria were: 1) studies conducted in Ethiopia, 2) studies that report the incidence of LTFU or the number of LTFU for children aged 0–14 years living with HIV, 3) studies that report the child person year, 4) studies published in English languages, 5) studies that report at least one predictors with hazard function and 6) studies available at the electronic source up to November 20, 2023 were included in the study. On the other hand, studies that didn’t report the child person’s years or studies that report predictors other than hazard function were excluded from the study. Furthermore, citations without abstract and/or full-text, anonymous reports, editorials, and qualitative studies were excluded from the analysis.
Data extraction
All studies identified via the online database were exported to EndnoteX7 to identify and remove duplication. A standardized data extraction tool was used and independently extracted by four authors (GF, ZA, GM, and MS). Any disagreements among the data extractors were discussed and it was handled by the principal authors (DG). From each study, the author’s name, publication year, the event or number of LTFU, study region, study design, the total person year, incidence rate, and the predictor of LTFU with hazard ratios were extracted.
Quality assessment/Critical appraisal
Quality appraisal was done using the Joanna Briggs Institute (JBI) Critical Appraisal Checklist for cohort study design [23]. The qualities of the primary studies were independently assessed by two authors (LG and DA). Any discrepancy between the two authors was handled by taking the mean score of the two authors. The tool has Yes, No, Unclear, and Not Applicable options: “1” is given for “Yes” and “0” is given for other options. The scores were summed and changed to percentages. Studies with >50% quality scores were included in this meta-analysis. Finally, twenty-four studies that received a quality score of >50% were included in the final analysis (S2 File).
Outcome measurement
The first outcome was the incidence of LTFU among HIV-positive children on ART. The incidence of LTFU among HIV-positive children on ART was calculated by dividing the number of children who lost from ART treatment for one to three months by the total child follow-up years and multiplying it by 100. Identifying the associated factors of LTFU among HIV-positive children on ART was the second outcome of this study. Accordingly, the hazard ratio of predictors with its 95% confidence intervals (CI) was extracted from the original studies to compute the pooled hazard ratio of predictors.
Lost to follow-up
When HIV-positive children miss an appointment or drug pick-up for one month to three months and are not yet classified as dead or transferred out [24].
Follow-up period (time)
Is measured from the beginning of the study until the event (LTFU) occurs, transferred-out, death, and the study ends.
Advanced HIV disease
Children older than five years whose WHO clinical stages are III and IV. Whereas, children younger than five years living with HIV are considered as having advanced HIV disease, regardless of the clinical stages. Mild WHO clinical stages: HIV-positive children whose WHO clinical stages are stages I and II [24].
ART adherence. Good (> 95%)—if missed doses is ≤ 2 doses of 30 doses or ≤ 3 doses of 60 doses; Fair: (85–94%) if missing doses is between 3–4 of 30 doses or 4–9 of 60 doses; poor: (< 85%) if missed doses are >5 doses of 30 doses or 10 and above doses of 60 doses of ART drug [24].
Statistical analysis
Data entry was done using Microsoft Excel 2013 and then imported into R software version 4.1.3 for further analysis. Meta-package was used to analyze the data. I-square was used to check heterogeneity between studies [25]. Heterogeneity was declared as low, medium, and high if the I2 value was 25%, 50%, and 75%, respectively [26]. Subgroup analysis was done using the study region with evidence of heterogeneity. Univariate meta-regression analysis was done using publication years and sample size to identify the possible source of heterogeneity. Sensitivity analyses were done by omitting individual studies to detect the contribution of each study in the final pooled incidence of LTFU among HIV-positive children on ART. Funnel plot visual inspection was done to identify publication bias. Finally, the Egger test was done to assess any significant publication bias. Further, Trim and fill analyses were conducted to correct publication bias. Tables and forest plots were used to present data. The random and fixed effect models were used to compute the pooled estimates. Results are presented using the random effect model (if there is heterogeneity among studies) and fixed-effect (if there is no heterogeneity among studies).
Results
Characteristics of included studies
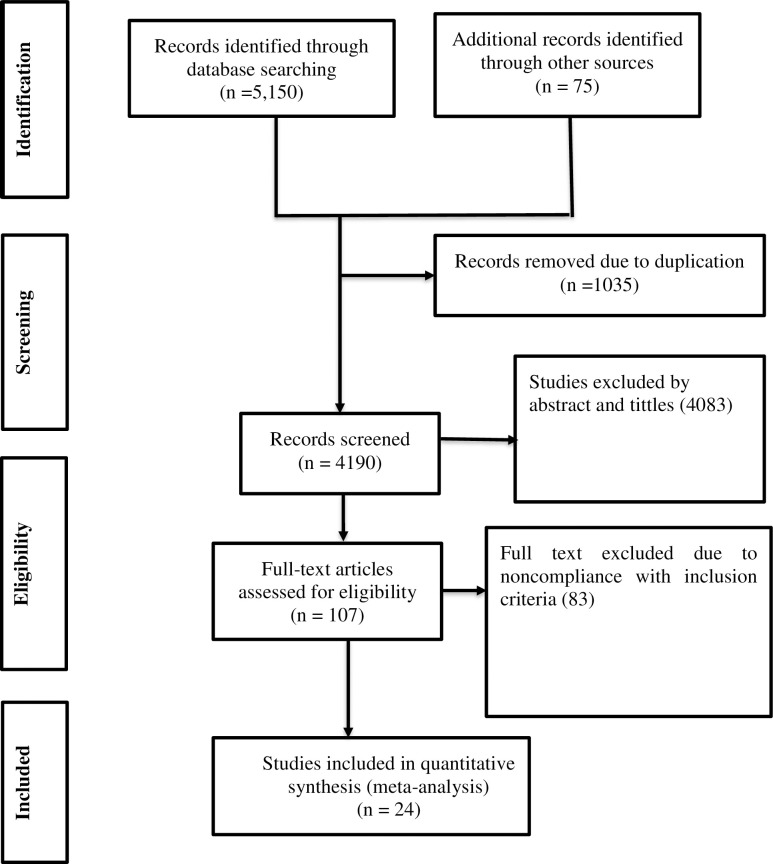
A total of 5,225 articles were identified from PubMed, HINARI, Science Direct, Google Scholar, and African Journals Online. Of these, 2,514 studies were from PubMed, 1,127 studies were from HINARI, 1,509 studies were from Science Direct, and the rest 75 studies were identified from Google Scholar and African Journals online. From these studies, 1035 studies were excluded due to duplication. From the remaining 4190 articles, 4083 studies were excluded as not being relevant to the study after reviewing the titles and abstracts. The rest 107 articles were assessed by reviewing the full text. Finally, twenty-four studies were eligible and incorporated in the final analysis (Fig 1) [13–21, 27–41]. All studies were conducted using the cohort study design. These studies were done from different parts of Ethiopia (Addis Ababa, Amhara, Oromia, SNNPR (South Nation, Nationalities and People Region), and, Tigray) (Table 1).
Fig 1. PRISMA flow chart describing screening protocols of studies for meta-analysis.
Table 1. Characteristics of studies included in the meta-analysis for the pooled incidence of LTFU among HIV-positive children on ART, Ethiopia, 2023.
| Author | Region | Sample sizes | Number of LTFU | Follow-up time in month (IQR) | PMO | PYO | IR per 100 child years |
|---|---|---|---|---|---|---|---|
| Mulgeta et al (2017) [27] | Addis Ababa | 757 | 92 | 62.0–83.0 | 49344 | 4112 | |
| Edessa et al (2015) [28] | Oromia | 305 | 22 | 18–30 | 7, 312 | 609 | --- |
| Adem et al (2014) [29] | Oromia | 560 | 46 | 29–62 | 24936 | 2078 | --- |
| Bimer et al (2021) [13] | SNNPR | 254 | 70 | 1–84 | 8145.33 | 678.78 | --- |
| Chanie et al (2022) [14] | Amhara | 344 | 76 | (4–167 | 19,081 | 1590.1 | 4.8 |
| Menshw et al (2021) [15] | Amhara | 488 | 101 | ------- | ------- | --- | 6.3 |
| Fetene et al(2018) [16] | Amhara | 533 | 46 | 42–11 | 15288 | 1274 | 3.6 |
| Sidamo et al (2017) [30] | SNNPR | 421 | 43 | 24–80 | 21,175 | 1764.58 | --- |
| Haile(2021) [31] | SNNPR | 429 | 38 | 32–122 | 30595.2 | 2549.6 | --- |
| Fisiha et al (2020) [17] | Amhara | 361 | 79 | 14–70 | 15369.6 | 1280.8 | 6.2 |
| Sifr et al (2021) [18] | SNNPR | 143 | 18 | ------- | ------- | 356.06 | 5 |
| Bankere et al (2022) [21] | Oromia | 269 | 43 | 24–92 | 15588 | 1299 | 3.3 |
| Alebel et al (2020) [32] | Amhara | 538 | 38 | 14,600 | 1216 | --- | |
| Hibstie et al (2020) [19] | Amhara | 408 | 70 | 2–136 | 18,755 | 1562.9 | 4.5 |
| Melaku et al (2017) [33] | Amhara | 6815 | 2090 | ------ | ------ | --- | --- |
| Koye et al (2012) [34] | Amhara | 549 | 32 | 1–62 | 12300 | 1025 | --- |
| Gebremedihn et al (2013) [35] | Tigray | 416 | 23 | 17–50 | 14,235 | 1186.25 | --- |
| Gemech et al (2022) [36] | SNNPR | 284 | 32 | 1–120 | 15086.04 | 1257.17 | --- |
| Tagesse et al (2020) [37] | Addis Ababa | 410 | 20 | 18–44 | 13236 | 1103 | --- |
| Biru et al (2018) [20] | Adiss Ababa | 304 | 18 | 10–13 | 3452.4 | 287.7 | 9.12 |
| Atallel et al (2018) [38] | Amhara | 271 | 25 | 1–144 | 14012.04 | 1167.67 | --- |
| Biyazin et al (2022) [39] | Amhara | 251 | 16 | 60 | 7512 | 626 | --- |
| seid (2023) [40] | SNNPR | 261 | 11 | 43–107 | 18,955 | 9477.5 | --- |
| Alemu et al(2022) [41] | Amhara | 415 | 18 | 3–48 | 8700.5 | 725.04 | --- |
LTFU: Lost to follow up, IQR: Inter Quartile Range, PMO: Person Month Observation, PYO: Person Year Observation, IR: incidence rate
The pooled incidence of LTFU among HIV‑positive children on ART
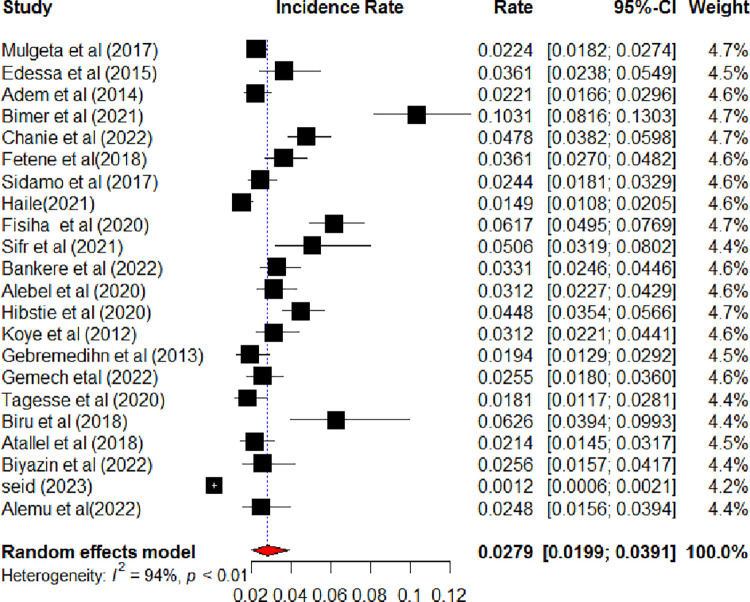
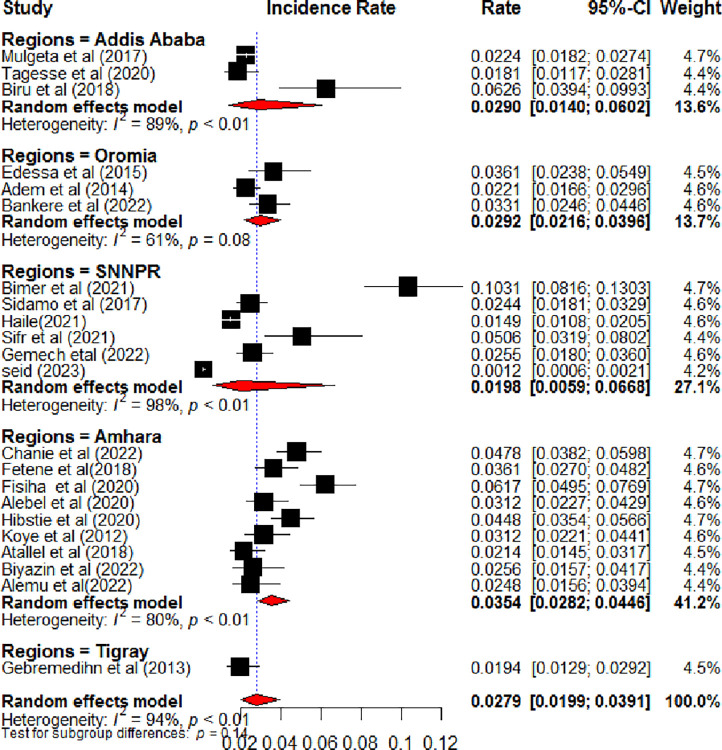
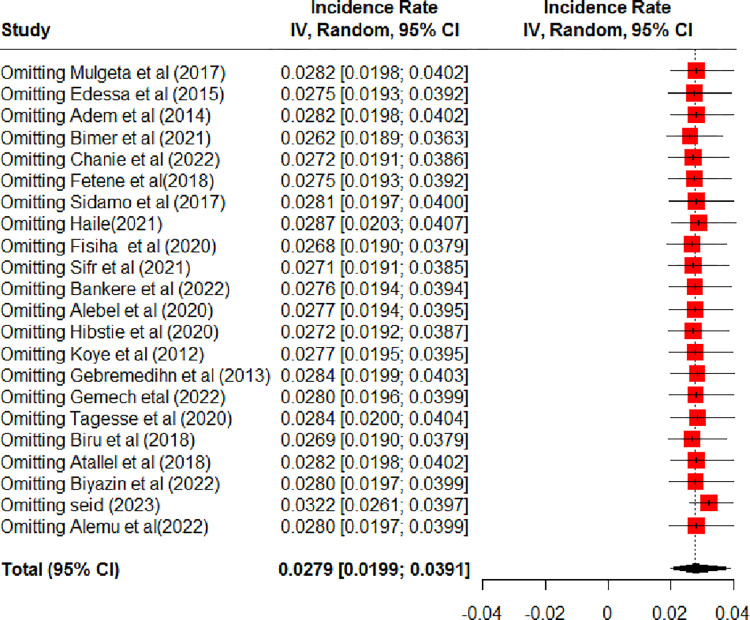
Twenty-two studies were included to estimate the pooled incidence of LTFU among HIV-positive children on ART in Ethiopia [13, 14, 16–21, 27–32, 34–41]. Accordingly, the pooled incidence of LTFU among HIV-positive children on ART in Ethiopia was 2.79 (95% CI: 1.99, 3.91) per 100-child-year observations using a random effect model. Heterogeneity (I2 = 94%, P-value <0.01) was identified (Fig 2). Hence, subgroup analysis was done based on the study regions. Accordingly, the incidence of LTFU among HIV-positive children on ART ranged from 1.98 per 100 child years in SNNPR to 3.54 per 100 child years in the Amhara region (Fig 3). Univariate meta-regression analysis was done to identify the possible source heterogeneity using the publication years and sample size. Of these factors, none of them were statistically significant (Table 2). Finally, sensitivity analysis was done. In sensitivity analysis, except for one study [40], nearly all studies have equal contributions to the pooled incidence of LTFU among HIV-positive children on ART in Ethiopia (Fig 4).
Fig 2. The forest plots show the incidence of lost follow-up among HIV-positive children on ART, Ethiopia, 2023.
Fig 3. Forest plot shows the subgroup analysis of the incidence of lost to follow-up among HIV-positive children on ART by study regions, Ethiopia, 2023.
Table 2. Meta-regression analysis using publication year and sample size for the possible source of heterogeneity of LTFU among HIV-positive children on ART, Ethiopia, 2023.
| Variables | Coefficients | P-value |
|---|---|---|
| Publication years | -0.0405 (-0.1613, 0.0519) | 0.3 |
| Sample size | 0.0010 (- 0.0006,0.0026) | 0.23 |
Fig 4. Sensitivity analysis for the incidence of lost to follow-up among HIV-positive children on ART, Ethiopia, 2023.
Publication bias
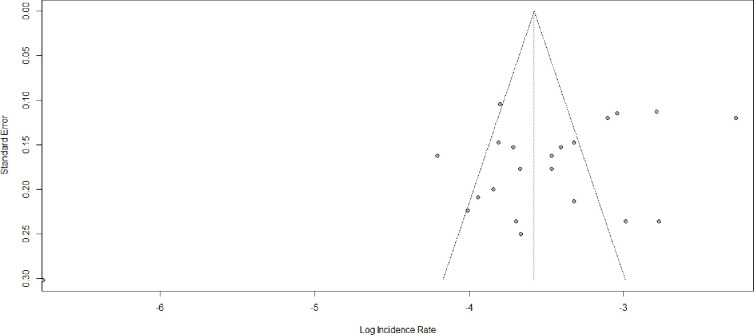
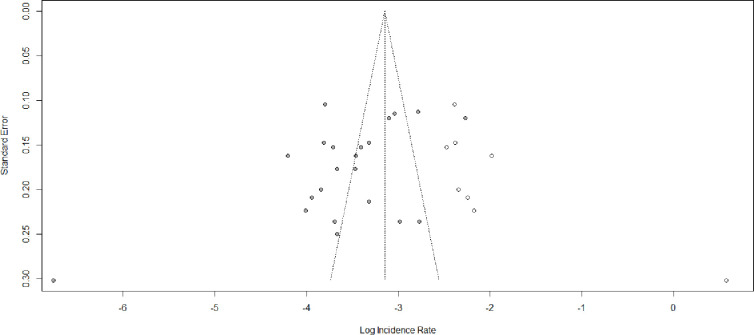
Asymmetric distribution was displayed in the funnel plot visual inspection (Fig 5). The Egger test also shows a statistically significant publication bias with B0 = -2.36, p-value = 0.02. Due to the presence of statically significant publication bias, meta-trim and fill analysis were done. Hence, eight studies were filled and the pooled incidence of LTFU among HIV-positive children on ART became 4.29 (95% CI: 2.87; 6.41) per 100 child years (Fig 6).
Fig 5. Funnel plot showing publication bias among studies used to compute the pooled incidence of lost to follow-up among HIV-positive children on ART, Ethiopia, 2023.
Fig 6. Shows the trim fill analysis for the incidence of lost to follow-up among HIV-positive children on ART, Ethiopia, 2023.
Factors associated with LTFU among HIV-positive children on ART
In this systematic review and meta-analysis, seven studies were incorporated to identify the factors associated with LTFU among children on ART [15–17, 19–21, 33]. Advanced HIV disease, poor or fair ART treatment adherence, history of opportunistic infection, and age between 1–5 years were factors associated with a higher hazard of LTFU among HIV-positive children on ART. Accordingly, the likelihood of LTFU was 2.20 times (HR: 2.20, 95% CI: 1.71, 2.73) higher among children with advanced HIV disease as compared to children with mild WHO clinica stages [15, 17, 33]. The hazard of LTFU was 2.92 times (HR: 2.92, 95% CI: 1.31; 6.54) higher among children with poor or fair ART treatment adherence as compared to children with good ART treatment adherence [15, 17, 19]. The hazard of LTFU was 2.1 times (HR: 2.1,95% CI:1.44; 2.95) higher among children aged between 1–5 years as compared to children aged ≥ 5 years [15, 16, 20]. The likelihood of LTFU was 2.59 times (HR: 2.59, 95% CI:1.39; 4.78) higher among children who had opportunistic infection as compared to their counterparts [16, 21] (Table 3).
Table 3. Predictors of lost to follow up among HIV positive children on ART, Ethiopia, 2023.
| Predictors | Included studies | HR (95% CI) | Pooled HR (95% CI) | Heterogeneity | |
|---|---|---|---|---|---|
| Advanced HIV disease | Menshw et al (2021) | 2.2 0 (1.40; 3.34) | 2.20 (1.71,2.73) | I2 = 0%, p-value = 0.94 | |
| Fisiha et al (2020) | 2.00 (1.10; 3.10) | ||||
| Melaku et al (2017) | 2.20 (1.6; 3.10) | ||||
| Poor or fair ART treatment adherence | Menshw et al (2021) | 6.6 0 (4.11; 10.56) | 2.92 (1.31; 6.54) | I2 = 90.8%, p-value < 0.001 | |
| Fisiha et al (2020) | 1.70 (1.10; 2.11) | ||||
| Hibstie et al (2020) | 2.30 (1.40; 3.70) | ||||
| Having Opportunistic infection | Fetene et al(2018) | 2.26(1.08; 4.71) | 2.59 (1.39; 4.78) | I2 = 0%, p-value = 0.51 | |
| Bankere et al (2022) | 3.54 (1.152; 10.87) | ||||
| Age between 1–5 years | Menshw et al (2021) | 1.60 (1.05; 2.46) | 2.10 (1.44; 2.95) | I2 = 57.5%, p-value = 0.095 | |
| Fetene et al(2018) | 3.86 (1.73; 8.61) | ||||
| Biru et al (2018) | 3.76 (1.16; 12.27) | ||||
| Rural residence | Hibstie et al (2020) | 3.20 (2.00; 5.50) | 1.15 (0.15; 8.84) | I2 = 95%. p-value < 0.001 | |
| Melaku et al (2017) | 0.40 (0.20; 0.90) | ||||
| Biru et al (2018) | 3.57 (11.64; 11.64) | ||||
Discussion
This systematic review and meta-analysis unveiled the pooled incidence of LTFU among HIV-positive children on ART in Ethiopia. Accordingly, the pooled incidence of LTFU among HIV-positive children on ART is found to be 2.79 (95% CI: 1.99, 3.91) per 100-child-year observations. The incidence is lower than the studies conducted in Asia (4.2 per 100 child years) [42], in Uganda (12.6 per 100 child years) [43], in Kenya (14.65 per 100 child years [44], in South Africa (10.8 per 100 person-years) [45], in Malawi (12.6/ per 100 person-years) [46], in Tanzania (18.2 per 100 person-years) [47], in South Africa (5.0 per 100 person-years) [48], in Nigeria (40 per 100 child-years) [49], in western Kenya (18.4 per 100 person-years) [50], in Côte d’Ivoire’s(9.3 per 100 person-years) [51] and in Mozambique (6.9/per 100 person-years) [52]. This can be justified that LTFU was considered when HIV-positive children interrupt ART treatment and with unknown tracing outcomes. Thus, the low incidence of LTFU among HIV-positive children on ART might be due to the improvement in tracing, recording, and reporting systems of health institutions for people living with HIV.
In this systematic review and meta-analysis, the hazard of LTFU is higher among children with advanced HIV disease as compared to children with mild WHO clinical stages. The finding is supported by studies conducted elsewhere [49, 53–55]. This can be justified as children with advanced HIV stage are at higher risk of developing opportunistic infections that cause unregistered HIV-related morbidity and mortality.
In this systematic review and meta-analysis, children with poor or fair ART treatment adherence have a higher hazard of LTFU than children with good ART treatment adherence. The finding is consistent with studies conducted elsewhere [56–58]. This is the fact that ART can suppress viral replication, boost immune function, and prevent opportunistic infection [59, 60]. Such that, fair or poor ART treatment adherence can open a window for viral replication, cause HIV viral resistance, decrease drug effectiveness, and cause treatment failure. This increases the risk of opportunistic infection and unregistered deaths.
The hazard of LTFU is higher among children who develop opportunistic infections as compared to their counterparts. The fact that opportunistic infections occur in advanced HIV disease, which further exacerbates the clinical outcome of people living with HIV [61]. Thus, the poor improvement in children with opportunistic infection may make parents feel hopeless or careless and they may fail to bring their child for treatment follow-up. This implies that strict and frequent follow-ups are needed for children with opportunistic infection than children without opportunistic infection.
In this systematic review and meta-analysis, children aged between 1–5 years are at an increased risk of LTFU from ART treatment as compared to children aged ≥ 5 years. The finding is supported by studies conducted elsewhere [55, 62, 63]. This might be due to the immature immune system in infants and young children increases the risk of rapid progression of HIV disease than older children. Thus, infants and young children are highly susceptible to opportunistic infections, which indirectly increases the incidence of LTFU from ART treatment.
The clinical and public health implications of this systematic review and meta-analysis are to take prompt intervention against the identified factors and response to reduce the burden of LTFU among HIV-positive children on ART and increase ART retention, later, to reduce HIV-related deaths. Therefore, researchers, program implementers, and policymakers should consider the aforementioned factors in their strategic plans.
Limitations
This systematic review and meta-analysis have the following limitations: in this analysis, articles published only in English were included. Only five regions were included in the analysis, such that some regions may not be represented. Moreover, some variables associated with LTFU among HIV-positive children on ART were excluded from the analysis because it reported in only one primary article and/or classified in a different way from the included articles. Furthermore, only seven studies reported the predictors of LTFU among HIV-positive children on ART, such that limited factors were identified. Finally, because of the previous primary studies didn’t report the event (number of LTFU) at 6 months, 12 months, 24 months, and more, the segregated incidence of LTFU among HIV-positive children on ART was not pooled for the respective months.
Conclusion
The overall pooled incidence of LTFU among HIV-positive children on ART is low in Ethiopia. Advanced HIV disease, having an opportunistic infection, fair or poor ART treatment adherence, and children aged between 1–5 years were factors associated with LTFU among HIV-positive children on ART. Therefore, counseling on ART drug adherence should be strengthened. Moreover, emphasis has to be given to children with advanced HIV stage and opportunistic infection to reduce the rate of LTFU among HIV-positive children on ART.
Supporting information
(DOCX)
(DOCX)
(DOCX)
Abbreviations
- ART
Antiretroviral therapy
- HIV
Human immunodeficiency virus
- AIDS
Acquired Immune Deficiency Syndrome
- PYO
Person-year observation
- PMO
Person-month observation
- WHO
World Health Organization
- SNNPR
South Nation, Nationalities and People Region
- JBI
The Joanna Briggs Institute Critical Appraisal Checklist
- PRISMA
Preferred Reporting Items for Systematic Review and Meta-Analysis Statement
- LTFU
lost to follow u
- HR
hazard ratio and
- CI
confidence interval
Data Availability
The data used for this study was publicly available at the Harvard Dataverse Network repository: URL: https://doi.org/10.7910/DVN/BQ6MU0.
Funding Statement
The author(s) received no specific funding for this work.
References
- 1.UNAIDS. Global HIV & AIDS statistics—Fact sheet. 2023 [cited 2023 December,26]; Available from: https://www.unaids.org/en/resources/fact-sheet.
- 2.World Health Organazation. HIV data and statistics. 2023 [cited 2023 December,26]; Available from: https://www.who.int/teams/global-hiv-hepatitis-and-stis-programmes/hiv/strategic-information/hiv-data-and-statistics.
- 3.UNAIDS, The path that ends AIDS: UNAIDS Global AIDS Update 2023. Geneva: Joint United Nations Programme on HIV/AIDS. Licence: CC BY-NC-SA 3.0 IGO. 2023.
- 4.United Nations, Transforming our world: the 2030 Agenda for Sustainable Development 2015.
- 5.World Health Organazation. Global HIV Programme—treatment and care. [cited 2023 October,28]; Available from: https://www.who.int/teams/global-hiv-hepatitis-and-stis-programmes/hiv/treatment.
- 6.UNAIDS. Global aids strategy 2021–2026 End inequalities. End aids. 2021 [cited 2023 October,25]; Available from: https://www.unaids.org/sites/default/files/media_asset/global-AIDS-strategy-2021-2026-summary_en.pdf.
- 7.Hser Y.-I., et al., Relationship between drug treatment services, retention, and outcomes. Psychiatric Services, 2004. 55(7): p. 767–774. doi: 10.1176/appi.ps.55.7.767 [DOI] [PubMed] [Google Scholar]
- 8.World Health Organization, Pocket book of hospital care for children: guidelines for the management of common childhood illnesses. 2013: World Health Organization. [PubMed] [Google Scholar]
- 9.Abuogi L.L., Smith C., and McFarland E.J., Retention of HIV-infected children in the first 12 months of anti-retroviral therapy and predictors of attrition in resource limited settings: a systematic review. PloS one, 2016. 11(6): p. e0156506. doi: 10.1371/journal.pone.0156506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Leroy V., et al., Outcomes of antiretroviral therapy in children in Asia and Africa: a comparative analysis of the IeDEA pediatric multiregional collaboration. Journal of acquired immune deficiency syndromes (1999), 2013. 62(2): p. 208. doi: 10.1097/QAI.0b013e31827b70bf [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jewell B.L., Smith J.A., and Hallett T.B., Understanding the impact of interruptions to HIV services during the COVID-19 pandemic: a modelling study. EClinicalMedicine, 2020. 26. doi: 10.1016/j.eclinm.2020.100483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thomadakis C., et al., The effect of HIV treatment interruption on subsequent immunological response. American Journal of Epidemiology, 2023: p. kwad076. doi: 10.1093/aje/kwad076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bimer K.B., et al., Incidence and predictors of attrition among children attending antiretroviral follow-up in public hospitals, southern Ethiopia, 2020: a retrospective study. BMJ Paediatrics Open, 2021. 5(1). doi: 10.1136/bmjpo-2021-001135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chanie E.S., Tesgera Beshah D., and Ayele A.D., Incidence and predictors of attrition among children on antiretroviral therapy at University of Gondar Comprehensive Specialized Hospital, Northwest Ethiopia, 2019: Retrospective follow-up study. SAGE Open Medicine, 2022. 10: p. 20503121221077843. doi: 10.1177/20503121221077843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Menshw T., et al., Incidence and predictors of loss to follow-up among children attending ART clinics in northeast Ethiopia: A retrospective cohort study. HIV/AIDS-Research and Palliative Care, 2021: p. 801–812. doi: 10.2147/HIV.S320601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tamene F., incidence and predictors of lost to follow up among children on antiretiroviral therapy at east and west gojjam zone referral hospitals, amhara regional state, 2018: a retrospective cohort study. 2018, Addis Ababa University. [Google Scholar]
- 17.Fisiha Kassa S., et al., Incidence of loss to follow-up and its predictors among children with HIV on antiretroviral therapy at the University of Gondar Comprehensive Specialized Referral Hospital: a retrospective data Analysis. HIV/AIDS-Research and Palliative Care, 2020: p. 525–533. doi: 10.2147/HIV.S269580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sifr Z., et al., Level of attrition from antiretroviral therapy among human immune deficiency virus-infected children: The cases of Sidama zone, southern Ethiopia. HIV/AIDS-Research and Palliative Care, 2021: p. 813–822. doi: 10.2147/HIV.S317117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hibstie Y.T., et al., Nearly one in every six HIV-infected children lost from ART follow-up at Debre Markos Referral Hospital, Northwest Ethiopia: A 14-year retrospective follow-up study. PLoS One, 2020. 15(9): p. e0239013. doi: 10.1371/journal.pone.0239013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Biru M., et al., Rates and predictors of attrition among children on antiretroviral therapy in Ethiopia: A prospective cohort study. PloS one, 2018. 13(2): p. e0189777–e0189777. doi: 10.1371/journal.pone.0189777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bankere A.W., et al., Lost to Follow-Up and Its Predictors among Human Immune Deficiency Virus Infected Children on Antiretroviral Therapy, Southern Oromia, and Ethiopia: A Five Year Retrospective Cohort Study. 2022. [Google Scholar]
- 22.Liberati A., et al., The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Annals of internal medicine, 2009. 151(4): p. W-65–W-94. [DOI] [PubMed] [Google Scholar]
- 23.Lockwood C., et al., Chapter 7: systematic reviews of etiology and risk. JBI Manual for Evidence Synthesis [Internet]. Joanna Briggs Institute, 2017. [Google Scholar]
- 24.Ethiopia F., National consolidated guidelines for comprehensive HIV prevention, care and treatment. Addis Ababa: Fmoh, 2018: p. 1–238. [Google Scholar]
- 25.Rücker G., et al., Undue reliance on I 2 in assessing heterogeneity may mislead. BMC medical research methodology, 2008. 8: p. 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huedo-Medina T.B., et al., Assessing heterogeneity in meta-analysis: Q statistic or I2 index? Psychological methods, 2006. 11(2): p. 193. [DOI] [PubMed] [Google Scholar]
- 27.Mulugeta A., et al., Determinants of survival among HIV positive research article open access children on antiretroviral therapy in public hospitals, addis ababa, ethiopia. Qual Prim Care, 2017. 25: p. 235–41. [Google Scholar]
- 28.Edessa D., Asefa F., and Sheikahmed J., Early mortality among HIV-positive children initiated anti-retroviral therapy in eastern Ethiopia: a retrospective cohort study. Science, Technology and Arts Research Journal, 2015. 4(2): p. 157–163. [Google Scholar]
- 29.Adem A.K., Alem D., and Girmatsion F., Factors affecting survival of HIV positive children taking antiretroviral therapy at Adama Referral Hospital and Medical College, Ethiopia. Journal of AIDS and Clinical Research, 2014. 5(3). [Google Scholar]
- 30.Sidamo N., et al., Incidence and predictors of mortality among children on anti-retroviral therapy in public health facilities of Arba Minch town, Gamo Gofa zone, southern Ethiopia; retrospective cohort study. Clin Mother Child Health, 2017. 14(3). [Google Scholar]
- 31.Haile T., Incidence and Predictors of Mortality among HIV-Positive Children Enrolled to Art Clinic in Public Hospitals of Hawassa Town, Sidama, Ethiopia. 2021, HU. [Google Scholar]
- 32.Alebel A., et al., Mortality rate among HIV-positive children on ART in Northwest Ethiopia: a historical cohort study. BMC Public Health, 2020. 20(1): p. 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Melaku Z., et al., Outcomes among HIV‐infected children initiating HIV care and antiretroviral treatment in Ethiopia. Tropical Medicine & International Health, 2017. 22(4): p. 474–484. doi: 10.1111/tmi.12834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Koye D.N., Ayele T.A., and Zeleke B.M., Predictors of mortality among children on antiretroviral therapy at a referral hospital, Northwest Ethiopia: a retrospective follow up study. BMC pediatrics, 2012. 12(1): p. 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gebremedhin A., et al., Predictors of mortality among HIV infected children on anti-retroviral therapy in Mekelle Hospital, Northern Ethiopia: a retrospective cohort study. BMC public health, 2013. 13: p. 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gemechu J., et al., Predictors of mortality among TB-HIV co-infected children attending anti-retroviral therapy clinics of selected public hospitals in southern, Ethiopia: retrospective cohort study. Archives of Public Health, 2022. 80(1): p. 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tagesse N. and Abebe W., predictors of mortality in children and adolescents living with hiv on antiretroviral therapy, ethiopia: a retrospective cohort study. Ethiopian Journal of Pediatrics and Child Health, 2020. 15(2). [Google Scholar]
- 38.Atalell K.A., Birhan Tebeje N., and Ekubagewargies D.T., Survival and predictors of mortality among children co-infected with tuberculosis and human immunodeficiency virus at University of Gondar Comprehensive Specialized Hospital, Northwest Ethiopia. A retrospective follow-up study. PloS one, 2018. 13(5): p. e0197145. doi: 10.1371/journal.pone.0197145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Biyazin Y., et al., Survival and predictors of mortality among HIV-positive children on antiretroviral therapy in public hospitals. Journal of Pharmaceutical Policy and Practice, 2022. 15(1): p. 48. doi: 10.1186/s40545-022-00448-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Seid R., Survival Time and Its Predictors among Hiv Infected Children on Anti Retroviral Therapy in Hawassa City Public Hospitals, Sidama Region, Southern Ethiopia: Retrospective Cohort Study. 2023, HU. [Google Scholar]
- 41.Alemu G.G., et al., Survival time and predictors of death among HIV infected under five children after initiation of anti-retroviral therapy in West Amhara Referral Hospitals, Northwest Ethiopia. BMC pediatrics, 2022. 22(1): p. 670. doi: 10.1186/s12887-022-03693-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hansudewechakul R., et al., Antiretroviral therapy outcomes of HIV-infected children in the TREAT Asia pediatric HIV observational database. JAIDS Journal of Acquired Immune Deficiency Syndromes, 2010. 55(4): p. 503–509. doi: 10.1097/QAI.0b013e3181f5379a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Massavon W., et al., Attrition and loss to follow-up among children and adolescents in a community home-based care HIV programme in Uganda. Pediat Therapeut, 2013. 3(183): p. 2161–2665. [Google Scholar]
- 44.McLigeyo A. and Wekesa P., Factors Associated with Treatment Outcomes Among Children and Adolescents Living with HIV Receiving Antiretroviral Therapy in Central Kenya. 2022. 38(6): p. 480–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chandiwana N., et al., High loss to follow-up of children on antiretroviral treatment in a primary care HIV clinic in Johannesburg, South Africa. Medicine, 2018. 97(29). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ardura-Garcia C., et al., Implementation and operational research: early tracing of children lost to follow-up from antiretroviral treatment: true outcomes and future risks. Journal of Acquired Immune Deficiency Syndromes (1999), 2015. 70(5): p. e160. doi: 10.1097/QAI.0000000000000772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McCormick N.M., et al., Implementation and operational research: risk factors of loss to follow-up among HIV-positive pediatric patients in Dar es Salaam, Tanzania. JAIDS Journal of Acquired Immune Deficiency Syndromes, 2015. 70(3): p. e73–e83. doi: 10.1097/QAI.0000000000000782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sengayi M., et al., Predictors of loss to follow-up among children in the first and second years of antiretroviral treatment in Johannesburg, South Africa. Global health action, 2013. 6(1): p. 19248. doi: 10.3402/gha.v6i0.19248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Okunuga O.C., Predictors of Lost to Follow-Up (LTFU) among Paediatrics on Antiretroviral Therapy (ART) in Nigeria. 2022. [Google Scholar]
- 50.Braitstein P., et al., Retention of HIV‐infected and HIV‐exposed children in a comprehensive HIV clinical care programme in Western Kenya. Tropical Medicine & International Health, 2010. 15(7): p. 833–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Auld A.F., et al., Temporal trends in mortality and loss to follow-up among children enrolled in Cote d’Ivoire’s national antiretroviral therapy program. The Pediatric infectious disease journal, 2014. 33(11): p. 1134–1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Auld A.F., et al., Temporal trends in patient characteristics and outcomes among children enrolled in Mozambique’s national antiretroviral therapy program. The Pediatric infectious disease journal, 2015. 34(8): p. e191. doi: 10.1097/INF.0000000000000741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ekouevi D.K., et al., 12-month mortality and loss-to-program in antiretroviral-treated children: The IeDEA pediatric West African Database to evaluate AIDS (pWADA), 2000–2008. BMC public health, 2011. 11: p. 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fetzer B.C., et al., Predictors for mortality and loss to follow‐up among children receiving anti‐retroviral therapy in Lilongwe, Malawi. Tropical medicine & international health, 2009. 14(8): p. 862–869. doi: 10.1111/j.1365-3156.2009.02315.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Okomo U., et al., Mortality and loss to programme before antiretroviral therapy among HIV-infected children eligible for treatment in The Gambia, West Africa. AIDS research and therapy, 2012. 9(1): p. 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tih P.M., et al., High Incidence and Predictors of Loss to follow-up among children and adolescents on Life Long Antiretroviral therapy in the conflict-affected Northwest and Southwest Regions of Cameroon: A Retrospective cohort study. 2022. [Google Scholar]
- 57.Telayneh A.T., et al., Time to lost to follow-up and its predictors among adult patients receiving antiretroviral therapy retrospective follow-up study Amhara Northwest Ethiopia. Scientific Reports, 2022. 12(1): p. 2916. doi: 10.1038/s41598-022-07049-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gebremichael M.A., et al., Predictors of loss to follow-up among HIV-infected adults after initiation of the first-line antiretroviral therapy at Arba Minch General Hospital, Southern Ethiopia: a 5-year retrospective cohort study. BioMed Research International, 2021. 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Alemu A., et al., The Effect of Long-Term HAART on the Incidence of Tuberculosis Among People Living with HIV in Addis Ababa, Ethiopia: A Matched Nested Case–Control Study. Infection and Drug Resistance, 2021: p. 5189–5198. doi: 10.2147/IDR.S345080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zinyakatira N., The impact of antiretroviral therapy on tuberculosis incidence. 2019, Faculty of Health Sciences. [Google Scholar]
- 61.Vaillant A.A.J. and Naik R., HIV-1 associated opportunistic infections, in StatPearls [Internet]. 2023, StatPearls Publishing. [Google Scholar]
- 62.Ditekemena J., et al., Antiretroviral treatment program retention among HIV-infected children in the Democratic Republic of Congo. PLoS One, 2014. 9(12): p. e113877. doi: 10.1371/journal.pone.0113877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Nimkar S., et al., Loss to follow‐up and mortality among HIV‐infected adolescents receiving antiretroviral therapy in Pune, India. HIV medicine, 2018. 19(6): p. 395–402. doi: 10.1111/hiv.12605 [DOI] [PMC free article] [PubMed] [Google Scholar]