Abstract
Background:
Computed tomography (CT) imaging has a large portion in the dose of patients from radiological procedures; therefore, accurate calculation of radiation risk estimation in this modality is inevitable. In this study, a method for determining the patient-specific effective dose using the dose–length product (DLP) index in lung CT scan using Monte Carlo (MC) simulation is introduced.
Methods:
EGSnrc/BEAMnrc MC code was used to simulate a CT scanner. The DOSxyznrc simulation code was used to simulate a specific voxelized phantom from the patient’s lungs and irradiate it according to X-ray parameter of routing lung CT scan, and dose delivered to thorax organs was calculated. Three types of phantoms were simulated according to three different body habits (slim, standard, and fat patients) in two groups of men and women. A factor was used to convert the relative dose per particle in MC code to the absolute dose. The dose was calculated in all lung organs, and the effective dose was calculated for all three groups of patient body habits. DLP index and volume CT dose index (CTDIvol) were extracted from the patient’s dose report in the CT scanner. The DLP to effective dose conversion factor (k-factor) for patients with different body habitus was calculated.
Results:
Lung radiation dose in slim, standard, and fat patients in men was 0.164, 0.103, and 0.078 mGy/mAs and in women was 0.164, 0.105, and 0.079 mGy/mAs, respectively. The k-factor in the group of slim patients, especially in women, was higher than in other groups.
Conclusions:
CT scan dose indexes for slim patients are reported to be underestimated in studies. The dose report in CT scan systems should be modified in proportion to the patient’s body habitus, to accurately estimate the radiation risk.
Keywords: Computed tomography scan, dose–length product, effective dose, organ dose, simulation
Introduction
With the advent of high-speed, high-resolution multislice computed tomography (CT) technology, the use of this system in the diagnosis of diseases has increased significantly.[1] About 20% of radiological diagnostic methods in the world belong to CT scans, whereas about 70% of the cumulative dose received by the community is from this imaging modality.[2] In CT scan imaging procedures, the radiation dose has been reported up to 52 times compared to conventional radiography and up to 122 times higher in some special techniques.[3] Therefore, the issue of dose optimization, dose reduction techniques, and accurate dose measurement are very necessary to determine the risk of CT scan imaging.[4] The biologic effects of ionizing radiation (BEIR) VII report on the risk of ionizing radiation in the diagnostic energy range in 2005 suggests that accurate radiation risk estimation requires measurement of body organ dose.[5] The International Commission on Radiological Protection (ICRP) 103 report also states that accurate radiation risk is possible by determining the dose of organs.[6] By calculating the dose of body organs, in the irradiated area, the effective dose can be calculated. The effective dose is a quantity that can be used to estimate the relative risk and biological effects on the body in a nonuniform ionizing radiation field.[7] The most important physical indicators in dose evaluation in volume CT dose index (CTDIvol) and the dose–length product (DLP). These quantities are merely an indicator of the output and intensity of the X-ray beam in the CT scan and are not a patient-specific index.[8] There are several methods for estimating the effective dose in CT scan imaging. In the study of Christner et al.,[9] the dose of the several anatomical regions was calculated using ImPACT software (St George’s Healthcare, London, UK), and the effective dose was calculated using the ICRP-specific methodology in reports 26, 60, and 103 based on tissue-weighting coefficients. The tissue-weighting coefficient is a quantity that represents the relative radiation sensitivity of body tissues.[7] Another method for calculating the effective dose in CT scan, which is very common, was used in the study by Shrimpton et al.[10] In this study, the DLP was first measured, and then the effective dose was calculated using the DLP to effective dose conversion factors (k-factor). In Abuhaimed and Martin’s study,[11] the dose in CT scan was calculated according to the physical characteristics of the patient’s body and CTDIvol value as size-specific dose estimates. In Borbinha’s study,[12] a digital patient model was used to increase the accuracy in organ dose calculation. In the mentioned methods, the calculated organ dose is done on a mathematical phantom or voxelized phantom with regular geometric shapes or on the tissue-equivalent phantom, which is different from the morphology of the organs. In our study, the thorax organ dose was calculated directly in a patient-specific phantom, by the Monte Carlo (MC) simulation method for three groups of patients with different body habitus, and the specific effective dose was obtained. By knowing the DLP CT dose index, the patient-specific conversion factor was calculated. Obviously, by calculating the specific effective dose of patients, it is possible to estimate the radiation risk more accurately.
Methods
In this study, the following steps were performed.
Monte Carlo simulation
The EGSnrc codes system was used for the MC simulation. All head components of the CT scan system (64 slices general electric [GE] light speed volumetric computed tomography [VCT]) such as X-ray tubes, filters, Bowtie filters, and collimators were simulated by the component module of BEAMnrc user code of EGSnrc. Two scoring planes, one after the Bowtie filter and the other in the center of the gantry, were considered to record phase space files. These files involve data about transporting photons and particles. One of these files was used as a beam source in subsequent analyzes (to irradiate the patient’s phantom) and the other one, which was placed in the center of the gantry, was used to extract the X-ray spectrum in the tube. Five hundred million electrons were accelerated from the cathode to the anode of the X-ray tube to produce photons. All parameters related to low-energy particle interactions in the diagnostic radiology range were activated in the BEAMnrc code. Beam data processor, one of the EGSnrc subroutine, was used to analyze the data, draw the particle energy flux, and check the slice thickness (field size) and spectrum profile. Validation of CT scanner modeling methods in MC simulation for evaluation of the X-ray beam data (X-ray beam slice thickness, X-ray spectrum, and beam profile) and X-ray tube components (beam-shaping filter) has been investigated in our previous study.[13] In this study, using computed radiography (CR) cassette and thermoluminescent dosimeter (TLD) chips, the dose profile was measured in the direction of the gantry diameter axis (from center to the peripheral of gantry isocenter). After simulating the CT scan system, an air phantom was simulated, and the dose profile calculated in the MC code was compared with the measured value. The X-ray spectrum produced in the tube was compared with Ipem78 software.[14]
Patient-specific phantom design and organ dose measurements
In this study, the axial mode of the scan was used to irradiate the thorax region of phantom and calculate the radiation dose of organs. Based on the technical parameters of the lung CT scan, the dose value of organs in the thorax was calculated. In this study, patients (man and woman) were divided into three groups based on physical body habitus, including fat patients with a body mass index (BMI) between 25 and 29.5 kg/m2, standard patients with a BMI between 18.5 and 24.5 kg/m2, and slim patients with BMI below 18 kg/m2. The CT scan image of the patient’s lung was used as a specific phantom to calculate the radiation dose in the simulation code. The CT scan images of the patients were imported to the MC code as a voxelized phantom. An input file was created in DOSxyznrc, in which the voxelized phantom specifications were entered manually. The dimension of each voxel was 6.25 cm × 6.25 cm × 6.25 cm and the size of the three-dimensional (3D) matrix was 64 × 64 × 20. The pegs4 file, which contains information about the cross-section of collision and attenuation coefficients of all materials in the phantom, was created using EGSnrcMP user code. The organs of the thorax included lungs, esophagus, adipose (the layer of fat under the skin), spinal cord, heart, breast, muscle, ribs, bone marrow, and cortex of the spine. The phantom was designed in the egsphant file format, and the X-ray source in the phase space file format was introduced to the DOSxyznrc code. The energy of the beam (in kV) and beam intensity (in mAs) were selected according to the technical parameters of CT scan imaging of the lung. After irradiating the phantom, the output of the DOSxyznrc code as a 3D dose file, which includes the dose calculated per particle in each voxel, was analyzed using the DOSCTP software.[15] In MC simulation, the dose of each voxel is reported per incident particles (Gy/particles). To convert the relative dose to the absolute dose, a conversion factor must be used. In this study, to calculate the conversion factor, a direct measurement of the dose in air was implemented at the isocenter of the CT scan gantry using the ion chamber dosimeter, and the calculation was performed with the same geometrical setup used in the MC simulation. By dividing the measured dose by the dose calculated in the simulation, the conversion factor was calculated in terms of particles/mAs. To ensure the validity of the calculated conversion factor, a direct measurement was performed in the CTDI phantom with a diameter of 32 cm an using ion chamber detector (CT Chamber PTW 30009), then the same phantom was designed in the simulation code. By comparing the dose value obtained from the MC code after applying the conversion factor and the measured value, the accuracy of the calculation of the conversion factor was confirmed.
Calculation of effective dose from dose–length product and volume computed tomography dose index
The patient dose index can be displayed as CTDIvol in the imaging system. CTDIvol and DLP values were extracted from patients’ dose report files. The specific effective dose of the patients, in the defined groups, was calculated from the product of the dose of each organ in the thorax region and the weighting factor of the tissues present in the ICRP report 103.[6] The following equation was used to calculate the effective dose.
E = ∑WT HT = ∑WT ∑ WR DT. R
WT = Tissue-weighting factor
WR = Radiation-weighting coefficient (1 for photons)
DT. R = Average absorbed dose to tissue T from beam R
By dividing the effective dose by the DLP value, patient-specific conversion factors were calculated.
Results
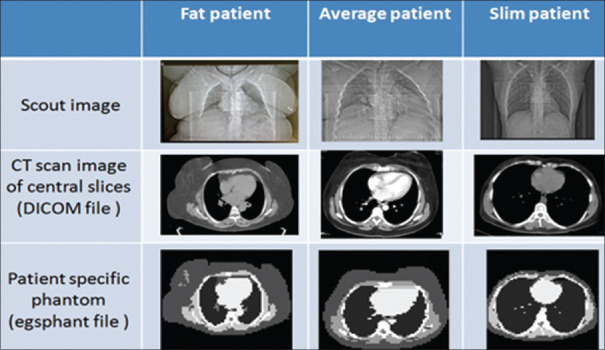
In this study, the specific dose for patients with different body habitus, obese, medium, and thin, in male and female patient groups, was studied in the CT scan of the lung. Figure 1 shows the images of the CT scan and the tomographic phantom that was used to calculate the organ dose using MC simulation.
Figure 1.
Specific phantom of the lung of fat, standard, and slim patients in the group of female to calculate the dose in the DOSxyznrc code. Computed tomography images are from the central slice of the thorax region
To estimate the dose of the organs, the conversion factors shown in Table 1 were used to convert the calculated dose value to the absolute dose of the organs.
Table 1.
Conversion factor of relative dose to absolute dose
| Photon energy (kV) | mAs | X-ray beam width (mm) | Dose value in gantry isocenter (mGy/mAs) | Calculated dose using MC in air (mGy/incident particle) | Conversion factor (particle/mAs) |
|---|---|---|---|---|---|
| 120 | 100 | 40 | 0.184 | 6.06E-18 | 3.04E+16 |
MC: Monte Carlo
Table 2 shows the technical parameters of the CT scan of the lung in the 64-slice CT scan system. The effective dose to DLP conversion factor was calculated based on the values presented in Table 2.
Table 2.
Technical parameters in computed tomography scan imaging of lungs in axial mode, in three groups of patients, fat, average, and slim
| Patient | kv | Effective mAs | Collimation (mm) | CTDIvol (mGy) | DLP (mGy.cm) |
|---|---|---|---|---|---|
| Slim | 120 | 87 | 40 | 4 | 103.4±11.5 |
| Average | 120 | 147 | 40 | 10.6 | 274.1±30.6 |
| Fat | 120 | 232 | 40 | 37.4 | 966.9±107.8 |
DLP: Dose–length product, CTDIvol: Volume CT dose index
With the technical parameters of Table 2, the organ dose was calculated for three groups of patients. Radiation organ doses for patients with three different body habits, separated by men and women, are shown in Table 3.
Table 3.
Mean dose of organs in the thorax per mAs in three patient body habits, separately for male and female groups in computed tomography scan imaging of the lung
| Thorax organs | Mean organ dose (mGy/mAs) Patients groups | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Slim | Standard | Fat | ||||
|
|
|
|
||||
| Man | Woman | Man | Woman | Man | Woman | |
| Esophagus | 0.117 | 0.117 | 0.066 | 0.069 | 0.055 | 0.057 |
| Spinal cord | 0.093 | 0.094 | 0.063 | 0.067 | 0.057 | 0.061 |
| Heart | 0.166 | 0.164 | 0.087 | 0.086 | 0.068 | 0.070 |
| Lung | 0.164 | 0.164 | 0.103 | 0.105 | 0.078 | 0.079 |
| Muscle | 0.195 | 0.193 | 0.099 | 0.097 | 0.159 | 0.161 |
| Breast | - | 0.198 | - | 0.237 | - | 0.172 |
| Adipose | 0.189 | 0.189 | 0.196 | 0.205 | 0.148 | 0.149 |
| Spine (bone surface) | 0.346 | 0.346 | 0.246 | 0.246 | 0.208 | 0.208 |
| Spin (bone marrow) | 0.256 | 0.274 | 0.170 | 0.170 | 0.158 | 0.158 |
| Rib | 0.614 | 0.599 | 0.484 | 0.482 | 0.372 | 0.372 |
-: Data not available
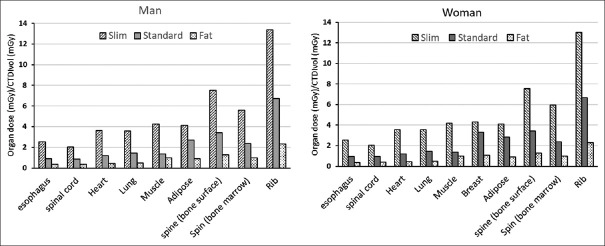
According to the technical parameters of Table 2, the CT dose index was extracted from the CT scan system in the patient dose report data. CTDIvol is one of the most important parameters in evaluating patient dose. In this study, the dose of each organ was calculated per CTDIvol. This quantity is displayed in Figure 2.
Figure 2.
The mean dose of organs in the thorax per volume computed tomography dose index in three body habits separately for men and women in computed tomography scan imaging of the lung
Table 4 shows the effective dose and DLP to effective dose conversion factors for three groups of patients.
Table 4.
Mean effective dose of thoracic organs and effective dose conversion factor per dose–length product in three groups of patients, separately for male and female groups in lung computed tomography scan imaging
| Organ | Weighting factor (6) | Organ effective dose (mSv) | |||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Slim patient | Standard patient | Fat patient | |||||
|
|
|
|
|||||
| Man | Woman | Man | Woman | Man | Woman | ||
| Esophagus | 0.04 | 0.41 | 0.41 | 0.39 | 0.41 | 0.51 | 0.53 |
| Spinal cord | 0.0092 | 0.07 | 0.08 | 0.09 | 0.09 | 0.12 | 0.13 |
| Heart | 0.0092 | 0.13 | 0.13 | 0.12 | 0.12 | 0.15 | 0.15 |
| Lung | 0.12 | 1.71 | 1.71 | 1.82 | 1.85 | 2.17 | 2.20 |
| Muscle | 0.0092 | 0.16 | 0.15 | 0.13 | 0.13 | 0.34 | 0.34 |
| Breast | 0.12 | - | 2.07 | - | 4.18 | - | 4.79 |
| Adipose | 0.0092 | 0.15 | 0.15 | 0.27 | 0.28 | 0.32 | 0.32 |
| Spine (bone surface) | 0.01 | 0.30 | 0.30 | 0.36 | 0.36 | 0.48 | 0.48 |
| Spin (bone marrow) | 0.12 | 2.67 | 2.86 | 3.00 | 3.00 | 4.40 | 4.40 |
| Rib | 0.12 | 6.41 | 6.25 | 8.54 | 8.50 | 10.36 | 10.36 |
| Whole body effective dose (mSv) | 12.02 | 14.11 | 14.70 | 18.92 | 18.84 | 23.70 | |
| Effective dose/DLP (mSv/mGy.cm) | 0.12 | 0.14 | 0.05 | 0.07 | 0.02 | 0.02 | |
DLP: Dose length product
Discussion
In this study, a patient-specific organ dose calculation method has been presented to calculate the organ dose of the thorax region in the CT scan imaging. The aim of our study is to design a method that can be used to calculate the effective dose of patients specifically from the dose indicators in CT scan as DLP, using the conversion factor. One of the most important advantages of effective dose calculation is the comparison of patient dose and radiation risk in CT scan compared to other radiological diagnostic procedures.[16] In our study, three conversion factors were introduced according to the three patient body habits. In the present study, three groups of voxel phantoms were made from CT scan images of patients. These phantoms became the basis for calculating the patient-specific dose [Figure 1]. Since men and women have different anatomical characteristics, it is expected that they have different DLP to effective dose conversion factor (k-factor). In most studies, only one reference phantom is used to calculate patient-specific dose in CT scan.[17,18] Martin[7] reports that the uncertainty in estimating the effective dose (using organ doses), based on the reference patient and without considering the diversity of the patient’s body habits, was about ± 40%. Several mathematical methods have been used to calculate the dose distribution in each voxel of the organs, using the values of the attenuation coefficient of the tissues and the geometry of the body organs from the CT image information in the digital imaging and communications in medicine (DICOM) file format.[19,20] Our findings show that the dose of organs in the group of slim patients is significantly higher than in other groups. In slim patients, there is a thin layer of fat tissue and muscle tissue around the thorax, so the organs in this area are exposed to a higher dose. In our study, the k-factor obtained in the group of slim patients was more than in other groups. Therefore, in CT scan imaging of patients with slim body habits, the selection of technical imaging parameters should be done with careful consideration compared to other body habitus groups. The conversion factor of DLP to effective dose (k) is calculated based on information extracted from scanners with different models and is a specific value for each CT scan.[9] Table 5 shows the conversion factors, obtained in our study compared to other studies.
Table 5.
Dose–length product to effective dose conversion coefficients obtained in our study compared to other studies, all values are for lung computed tomography scan imaging and 120 kVp
Different software is used to measure the k-factor in similar studies. The calculation of the k-factor, which was done using Impact Dose, ImPACT, and CT-Expo dosimetry software, shows a difference of about 5%.[23] In all these software packages, an anthropomorphic mathematical phantom is used, which is very different from the real conditions of the patient’s body. Our study shows Table 5 that it is necessary to use a specific k-factor to calculate the effective dose in the group of women. The reason for the higher the k-factor of women compared to men in CT scan imaging of the lungs is that the effective dose of women is higher in this procedure because the radiation-sensitive organs in women receive a higher dose. In Saltybaeva et al.’s study,[24] there was a 20% difference between male and female k-factor in CT angiography, whereas this difference in our study was 21% for all physical habits [Table 5].
Studies show that changing the kV in CT scan examination has very little effect on the conversion factor.[23,24] In the report of Lee et al.[22] in the CT scan of the lung at 120 kVp, the lung tissue dose for the female group was 10.6 mGy/100 mAs which is almost the same as the dose obtained for the same organ in our study. In Lee et al.’s study,[22] the effective dose of the lungs was calculated as 4.6 mSv/100 mAs, whereas in our study, the effective dose was calculated as 12 mSv/100 mAs, which shows a higher value, and the reason for this difference can be the type of phantom (pediatric hybrid phantoms representing newborn, 1-, 5-, 10-, and 15-year-old males and females in Lee’s study), and the way of calculation and imaging parameter (CT scanner model, beam collimation, and CTDIvol). According to the findings of our study, there is a significant difference in the k-factor value of patients with different body habits, and calculating the radiation risk based on a reference patient and generalizing it to all patients does not seem reasonable. One of the limitations of the present study is the impossibility of real-time organ dose reporting in patients specifically because the MC calculation method is time-consuming. However, the report of dose indicators can be done comparatively and can include a wide range of patients in one of the mentioned groups and check the dose of organs.
Conclusions
In this study, a special method of calculating the effective dose from the DLP value, using patient-specific tomographic phantom, is proposed. The k-factor of the slim patients’ group was more than other groups; therefore, in CT scan imaging of slim patients, optimization of radiation technical parameters is more important. The conversion factor of DLP into effective dose and organ doses are different among patient groups, and the patient’s body habitus should be taken into account to calculate the radiation risk. It is suggested to mention the effective dose for three groups of physical habits in the patients’ dose report file in CT scan imaging.
Financial support and sponsorship
This research was financially supported by the University of Mazandaran.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Fearon T, Xie H, Cheng JY, Ning H, Zhuge Y, Miller RW. Patient-specific CT dosimetry calculation: A feasibility study. J Appl Clin Med Phys. 2011;12:3589. doi: 10.1120/jacmp.v12i4.3589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Radiation. U. N. S. C. O. T. E. O. A., Effects of Ionizing Radiation, United Nations Scientific Committee on the Effects of Atomic Radiation (UNSCEAR) 2006 Report, Volume I: Report to the General Assembly, Scientific Annexes A and B 2008: United Nations [Google Scholar]
- 3.Anam C, Haryanto F, Widita R, Arif I, Dougherty G, McLean D. Volume computed tomography dose index (CTDIvol) and size-specific dose estimate (SSDE) for tube current modulation (TCM) in CT scanning. Int J Radiat Res. 2018;16:289–97. [Google Scholar]
- 4.Kalender WA. X-ray computed tomography. Phys Med Biol. 2006;51:R29–43. doi: 10.1088/0031-9155/51/13/R03. [DOI] [PubMed] [Google Scholar]
- 5.National Research Council. Washington: National Research Council; 2006. Health Risks from Exposure to Low Levels of Ionizing Radiation: BEIR VII Phase 2. [Google Scholar]
- 6.ICRP, 2007. The 2007 Recommendations of the International Commission on Radiological Protection. ICRP Publication 103. Ann. ICRP 37 (2-4) doi: 10.1016/j.icrp.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 7.Martin CJ. Effective dose: How should it be applied to medical exposures? Br J Radiol. 2007;80:639–47. doi: 10.1259/bjr/25922439. [DOI] [PubMed] [Google Scholar]
- 8.Huda W, Mettler FA. Volume CT dose index and dose-length product displayed during CT: What good are they? Radiology. 2011;258:236–42. doi: 10.1148/radiol.10100297. [DOI] [PubMed] [Google Scholar]
- 9.Christner JA, Kofler JM, McCollough CH. Estimating effective dose for CT using dose-length product compared with using organ doses: Consequences of adopting International Commission on Radiological Protection publication 103 or dual-energy scanning. AJR Am J Roentgenol. 2010;194:881–9. doi: 10.2214/AJR.09.3462. [DOI] [PubMed] [Google Scholar]
- 10.Shrimpton PC, Hillier MC, Lewis MA, Dunn M. Doses from computed tomography (CT) examinations in the UK-2003 review 67. Chilton: NRPB; 2005. [Google Scholar]
- 11.Abuhaimed A, Martin CJ. Estimation of size-specific dose estimates (SSDE) for paediatric and adults patients based on a single slice. Phys Med. 2020;74:30–9. doi: 10.1016/j.ejmp.2020.05.001. [DOI] [PubMed] [Google Scholar]
- 12.Borbinha J, Di Maria S, Madeira P, Belchior A, Baptista M, Vaz P. Increasing organ dose accuracy through voxel phantom organ matching with individual patient anatomy. Radiat Phys Chem. 2019;159:35–46. [Google Scholar]
- 13.Fallah Mohammadi GR, Riyahi Alam N, Geraily G, Paydar R. Thorax organ dose estimation in computed tomography based on patient CT data using Monte Carlo simulation. Int J Radiat Res. 2016;14:313–21. [Google Scholar]
- 14.Cranley K. Catalogue of Diagnostic X-ray Spectra and other Data. The Institute of Physics and Engineering in Medicine Report; 1997. [Google Scholar]
- 15.Chow JC, Leung MK. A graphical user interface for calculation of 3D dose distribution using Monte Carlo simulations. J Phys Conf Series. 2008;102:012003. [Google Scholar]
- 16.Radiation, U. N. S. C. O. T. E. O. A., Sources and Effects of Ionizing Radiation, United Nations Scientific Committee on the Effects of Atomic Radiation (UNSCEAR) 2000 Report, Volume I: Report to the General Assembly, with Scientific Annexes-Sources. United Nations; 2000. [Google Scholar]
- 17.Ono K, Hiraoka T, Ono A, Komatsu E, Shigenaga T, Takaki H, et al. Low-dose CT scan screening for lung cancer: comparison of images and radiation doses between low-dose CT and follow-up standard diagnostic CT. Springerplus. 2013;2:393. doi: 10.1186/2193-1801-2-393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Takahashi F, Sato K, Endo A, Ono K, Yoshitake T, Hasegawa T, et al. WAZA-ARI: computational dosimetry system for X-ray CT examinations. I. Radiation transport calculation for organ and tissue doses evaluation using JM phantom. Radiat Prot Dosimetry. 2011;146:241–3. doi: 10.1093/rpd/ncr160. [DOI] [PubMed] [Google Scholar]
- 19.Deak P, van Straten M, Shrimpton PC, Zankl M, Kalender WA. Validation of a Monte Carlo tool for patient-specific dose simulations in multi-slice computed tomography. Eur Radiol. 2008;18:759–72. doi: 10.1007/s00330-007-0815-7. [DOI] [PubMed] [Google Scholar]
- 20.DeMarco JJ, Cagnon CH, Cody DD, Stevens DM, McCollough CH, O’Daniel J, et al. AMonte Carlo based method to estimate radiation dose from multidetector CT (MDCT): Cylindrical and anthropomorphic phantoms. Phys Med Biol. 2005;50:3989–4004. doi: 10.1088/0031-9155/50/17/005. [DOI] [PubMed] [Google Scholar]
- 21.Jessen KA, Panzer W, Shrimpton PC, Bongartzm G, Geleijns J, Golding S, et al. EUR 16262: European guidelines on quality criteria for computed tomography. Luxembourg: Office for Official Publications of the European Communities; 2000. [Google Scholar]
- 22.Lee C, Kim KP, Long DJ, Bolch WE. Organ doses for reference pediatric and adolescent patients undergoing computed tomography estimated by Monte Carlo simulation. Med Phys. 2012;39:2129–46. doi: 10.1118/1.3693052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huda W, Ogden KM, Khorasani MR. Converting dose-length product to effective dose at CT. Radiology. 2008;248:995–1003. doi: 10.1148/radiol.2483071964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Saltybaeva N, Jafari ME, Hupfer M, Kalender WA. Estimates of effective dose for CT scans of the lower extremities. Radiology. 2014;273:153–9. doi: 10.1148/radiol.14132903. [DOI] [PubMed] [Google Scholar]