Abstract
Objective The aim of Placental Assessment in Response to Environmental Pollution Study (PARENTs) was to determine whether imaging of the placenta by novel multiparametric magnetic resonance imaging (MRI) techniques in early pregnancy could help predict adverse pregnancy outcomes (APOs) due to ischemic placental disease (IPD). Additionally, we sought to determine maternal characteristics and environmental risk factors that contribute to IPD and secondary APOs.
Study Design Potential patients in their first trimester of pregnancy, who agreed to MRI of the placenta and measures of assessment of environmental pollution, were recruited into PARENTs, a prospective population-based cohort study. Participants were seen at three study visits during pregnancy and again at their delivery from 2015 to 2019. We collected data from interviews, chart abstractions, and imaging. Maternal biospecimens (serum, plasma, and urine) at antepartum study visits and delivery specimens (placenta, cord, and maternal blood) were collected, processed, and stored. The primary outcome was a composite of IPD, which included any of the following: placental abruption, hypertensive disease of pregnancy, fetal growth restriction, or a newborn of small for gestational age.
Results In this pilot cohort, of the 190 patients who completed pregnancy to viable delivery, 50 (26%) developed IPD. Among demographic characteristics, having a history of prior IPD in multiparous women was associated with the development of IPD. In the multiple novel perfusion measurements taken of the in vivo placenta using MRI, decreased high placental blood flow (mL/100 g/min) in early pregnancy (between 14 and 16 weeks) was found to be significantly associated with the later development of IPD.
Conclusion Successful recruitment of the PARENTs prospective cohort demonstrated the feasibility and acceptability of the use of MRI in human pregnancy to study the placenta in vivo and at the same time collect environmental exposure data. Analysis is ongoing and we hope these methods will assist researchers in the design of prospective imaging studies of pregnancy.
Key Points
MRI was acceptable and feasible for the study of the human placenta in vivo .
Functional imaging of the placenta by MRI showed a significant decrease in high placental blood flow.
Measures of environmental exposures are further being analyzed to predict IPD.
Keywords: placental imaging, ischemic placental disease, adverse pregnancy outcomes, MRI, environmental exposures
Healthy development and function of the placenta, from conception and implantation to birth, are paramount to preserving the life of the mother and in ensuring the long-term health of children, from the fetal stages, even into adulthood. Unfortunately, 30% of all pregnancies are complicated by the development of adverse pregnancy outcomes (APOs), which together account for more than one half of maternal deaths globally. 1 As more than 80% of women give birth at least once in their lives, 1 identification of APOs in early pregnancy may have an important impact on the development of prevention strategies for improved health of mothers and future generations.
To address current knowledge gaps in understanding the human placenta, in 2015, the Eunice Kennedy Shriver National Institute of Child Health and Human Development launched the “Human Placenta Project,” 2 3 4 to develop new placental imaging and genomic biomarker assessment technologies to assess placental structure and function throughout gestation. As part of that effort, we studied a cohort of pregnant participants who agreed to undergo two magnetic resonance imaging (MRI) scans to help develop the real-time assessment of normal and abnormal placental development and function. In addition to imaging, participants agreed to ultrasound Doppler evaluation of the uterine artery blood flow, assessment of environmental exposures, and longitudinal collection of biosamples including blood, urine, and placenta for later transcriptomic analysis ( Table 1 ).
Table 1. Biospecimen collection.
| Study visit (GA) | Delivery | |||
|---|---|---|---|---|
| Study visit 1 (<16 wk) | Study visit 2 (20–29 wk) | Study visit 3 (36+ wk) | ||
| Clinical visits | ||||
| Ultrasound evaluation of uterine artery Dopplers | X | X | ||
| mp-MRI (GA: 14–16 and 19–22 wk) | X | X | ||
| Environmental exposures | ||||
| Environmental surveys | X | X | X | |
| Air quality monitors | X | X | X | |
| Biosamples | ||||
| Blood processed (10 mL) to plasma (3 mL) | X | X | X | X |
| Blood processed (20 mL) to serum (6 mL) | X | X | X | X |
| Urine (50 mL) | X | X | X | X |
| Placenta (frozen and formalin fixed) | X | |||
| Fetal membranes | X | |||
| Umbilical cord segment | X | |||
| Placenta processed with RNA later | X | |||
| Umbilical cord (2 cm) | X | |||
| Cord blood (EDTA for plasma) | X | |||
| Cord blood (blue top for plasma) | X | |||
| Cord blood red top for serum | X | |||
Abbreviations: GA, gestational age; mp-MRI, multiparametric magnetic resonance imaging.
Hypertensive disorders of pregnancy (e.g., preeclampsia), placental abruption, fetal growth restriction (FGR), and most small-for-gestational-age (SGA) newborns, together are classified as ischemic placental diseases (IPDs) because they share similar pathophysiology, arising from the failure of physiologic transformation of the maternal spiral arteries in early pregnancy causing limited blood flow to the placenta and uteroplacental ischemia. 5 6 More than half of indicated preterm births (PTBs) are due to IPD. 7 In addition to IPD, another APO, gestational diabetes mellitus (GDM), is thought to have beginnings early in pregnancy; chronic low-grade inflammation in early pregnancy is thought to play a role in the pathophysiology GDM. 8 This study focused on IPD and secondarily on GDM, as these complications are thought to have modifiable risk factors that impact early pregnancy, yet these are currently undiagnosable until mid- to late pregnancy, when treatment cannot reverse a morbid disease course. An adequate understanding of placental development and function in both normal and abnormal pregnancies is critical to identify patients, to determine biomarkers, and to develop targeted therapeutics for pregnancies at risk. Currently, however, accurate assessment of the placenta is largely limited to postdelivery as imaging in vivo is limited to poor resolution, and functional assessment during gestation is indirect, or imposes a potential unacceptable risk to both mother and fetus.
We designed this prospective cohort study to help build new predictive models for IPD during early gestation, based on the synergy of MRI-based and transcriptomic features, and data collected regarding environmental exposures. We have previously reported that decreased high placental blood flow (hPBF) in early pregnancy (between 14 and 16 weeks) is potentially associated with the later development of the primary study outcome of IPD. 9 The goal of these ongoing early, noninvasive assessments of the placental function is to help develop future strategies of preventive and interventional therapies, to target reversal before encountering detrimental consequences to mother and infant.
Materials and Methods
Participants and Recruitment
Potential patients who sought prenatal care and planned to deliver their infant at the University of California, Los Angeles (UCLA) hospitals were screened for eligibility in the first trimester of pregnancy and followed prospectively once consent was obtained. Our Institutional Review Board approved the Placental Assessment in Response to Environmental Pollution Study (PARENTs) protocol and the study was listed by ClinicalTrials.gov ( https://clinicaltrials.gov/ct2/show/NCT02786420 ). Participants provided informed consent only after trained study personnel explained the study in the participant's native language. Eligibility for enrollment included a viable singleton with known dating by obstetrical criteria confirmed by study staff, and ability to provide informed consent to perform placental imaging by MRI and study protocol. Exclusion criteria were maternal age <18 years, fetal malformation evident before enrollment, known fetal chromosomal abnormality, twin pregnancy, plan to terminate the pregnancy, or inability or unwilling to provide consent.
Study Visits and Procedures
Study visits and timing for questionnaires and biosample collection are shown in Fig. 1 . To decrease the number of visits to the clinics, study visits generally correlated with times when participants were scheduled to see their providers for clinical care visits in the first trimester, early to mid-second trimester, third trimester, delivery, and postpartum. Participants were compensated for their time and effort. Detailed interviews were performed using standardized questionnaires to collect demographic characteristics, medical history and perceived stress in pregnancy, diet, medication and supplement use, alcohol, tobacco use, household environment, and occupational information. We used the National Institutes of Health Diet History Questionnaire (DHQ) II ( https://epi.grants.cancer.gov/diet/shortreg ) and the Healthy Eating Index (HEI)-2015 ( https://www.fns.usda.gov/healthy-eating-index-hei ) to measure dietary exposures and compliance with the U.S. Dietary Guidelines for Americans. The HEI-2015 contains 13 components that sum to a total maximum score of 100 points. Each of the components is scored on a density basis out of 1,000 calories, except for fatty acids, which comprised a ratio of unsaturated to saturated fatty acids. We calculated the HEI-2015 for all of the 148 women who completed DHQ II during pregnancy.
Fig. 1.
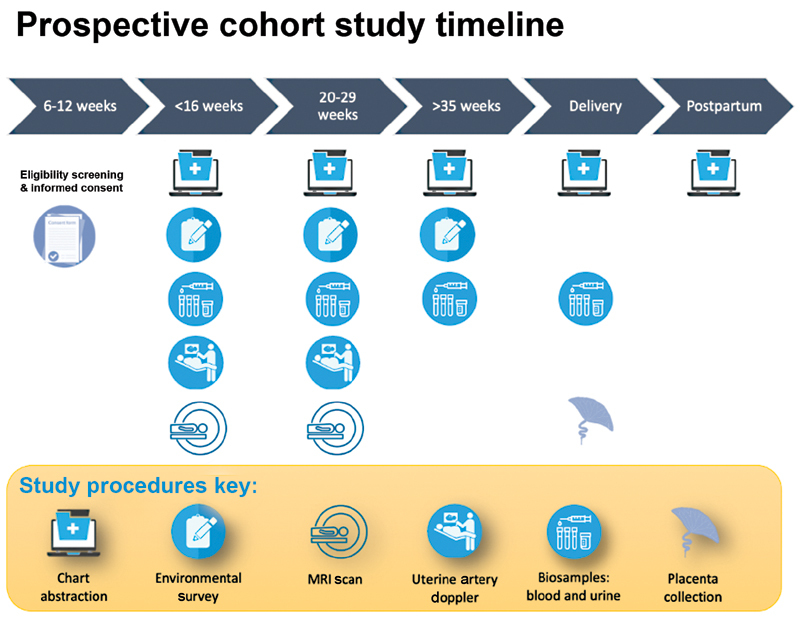
Timeline for data and biospecimen collection.
At the time of delivery, medical history, medication, indications for labor admission, and mode of delivery were updated. Maternal blood samples and urine were collected before birth. At delivery, an umbilical cord blood sample was obtained, and the placenta, membranes, and umbilical cord were processed for histologic study. Labor course, Apgar scores, and any APOs were recorded. Newborn outcomes included anomalies/malformations, FGR/SGA, neonatal intensive care unit course, respiratory distress syndrome, ventilator support, continuous positive airway pressure, transient tachypnea of the newborn, oxygen therapy, hypoglycemia requiring intervention, confirmed neonatal sepsis (early onset, late onset), suspected neonatal sepsis (early onset, late onset), necrotizing enterocolitis (stage 1, 2, or 3), intraventricular hemorrhage (grade I, II, III, or IV), chronic lung disease/bronchopulmonary dysplasia, newborn seizures, retinopathy of prematurity, and infant discharge status.
Three-month follow-up : infants were followed up until 3 months of age to ensure the health status until a month following their 2-month immunizations. In addition to growth parameters (body weight, length, and head circumference), any acquisition of infections or perturbations to health was recorded using medical chart reviews.
Environmental Assessment
A large international literature suggests that environmental stressors, particularly air pollution, contribute to APOs. 10 Relatively little is known, however, about the biological mechanisms of how air pollution affects fetal development and birth outcomes. In this study, we hypothesized that chronic exposures to high levels of environmental pollution increase the risk of adverse placental development, which can be assessed by placental MRI during early pregnancy. We planned to confirm placental ischemia through further study of pregnancy/neonatal outcome and postparturient placental pathology. Our study location in Los Angeles, where the levels of environmental pollution are relatively high compared with other locations in the United States, provides us with a setting to apply our air pollution measure to adverse pregnancy events with the addition of measurements from the newly developed placental MRI technology. In addition, the sprawling landscape of Los Angeles results in a high dependency on automobiles for transportation. This combined with the presence of a major and several smaller airports and two major seaports that unload thousands of containers onto trucks each day creates highly variable levels of traffic and transportation-related pollution. 11
We assessed air pollution using low-cost PurpleAir particle monitors ( www.purpleair.com ) that we placed in- and outdoors at participants homes. Recent evaluations by the South Coast Air Quality management district have shown these monitors to have very strong correlations with high-end reference monitors in both field studies and a laboratory chamber experiment. R 2 between the reference instruments and the PurpleAir monitors ranged from 0.93 to 0.99 in field and laboratory settings ( http://www.aqmd.gov/docs/default-source/aq-spec/summary/purpleair-pa-ii—summary-report.pdf?sfvrsn=16 ). Each deployment lasted an average of 4 months. We deployed the monitors indoors in the bedroom and outdoors in a shaded area in their yard or on patios. These data allow us to assess the indoor particulate air pollution, which usually represents a mixture of pollutants from indoor sources such as gas burning appliances, cooking, wood burning, and outdoor air particles that infiltrate into the homes. People spend the majority of their time indoors in their homes. 12 PurpleAir monitors assess the light scattering properties of the particles and give estimates for particle concentrations of the following sizes: PM 0.3 , PM 0.5 , PM 1 , and PM 2.5 . We are utilizing these data to develop estimates of indoor air pollution and to assess how correlated the measures of indoor air particles are with the particles that may have penetrated from the outdoors.
Imaging
Uterine artery Doppler measurement was first used as a reference standard. Uterine artery pulsatility index (UTPI; arbitrary unit) 13 was obtained at approximately 12 to 14 and 20 weeks of gestational age using the following technique: a sagittal image of the cervical canal was obtained abdominally and the internal cervical os was identified. The transducer was tilted from side to side and color flow mapping was applied to visualize the uterine arteries aliasing alongside the cervix and the uterus. The flow velocity waveforms were obtained from the ascending branch of the uterine artery as close to the internal os as possible. The PI was measured and the mean PI of the left and the right arteries was determined as the final UTPI. 14
MRI was used to scan the placenta of the study participants twice, with the first MRI scan between 14 and 18 weeks, and the second scan between 19 and 24 weeks. All MRI scans were performed on one of the two 3 Tesla MRI scanners (Prisma or Skyra, Siemens Healthcare, Erlangen, Germany) using a body array coil. Patients were positioned in a feet-first, supine position, and were breathing normally without physiological monitoring during MRI scans. No contrast agents were administered. A T 2 -weighted half-Fourier single-shot turbo spin-echo (T2-HASTE) MRI sequence was performed to identify the placenta, uterus, and other relevant anatomical structures, and a free-breathing pseudo-continuous arterial spin labeling (pCASL) sequence 9 15 16 was used to measure placenta perfusion. A clinical fellow (B.L.), supervised by an experienced maternal-fetal medicine specialist (C.J., with 20 years of experience), annotated the placental regions of interest on both T2-HASTE and pCASL to measure a placental volume (cm 3 ) and hPBF (mL/100 g/min), blinded to the pregnancy outcome. We have published details and preliminary results of the newly developed placenta MRI protocol in this population and its feasibility to predict the subsequent development of IPD; these studies are ongoing. 9 15 16 17
Placental Pathology
Pathologic examination was performed based on the Amsterdam Placental Workshop Group Consensus Statement. 18 Placental weight (trimmed of membranes and cord), maximal linear dimension (length), greatest dimension perpendicular to this length, disc thickness, shortest distance of membrane rupture, and cord insertion to disc edge, cord length, diameter, and number of cord vessels were determined on the fresh, unfixed specimens. Any description of color, cord strictures, twists, membrane insertion, any fetal/maternal surface or cut section lesional findings was performed on the placenta after fixation in 10% buffered formalin. Standard sampling of cord, membrane, and three unremarkable full-thickness sections taken within the central two-thirds of the disc (including one adjacent to cord insertion) were taken for every placenta. Focal/mass lesion findings were recorded by location, percent of disc volume involved by lesion, color, and consistency of lesion(s). All focal/mass lesions were sampled for microscopic examination.
Histologic review was performed blinded to clinical history by two separate reviewers using Amsterdam criteria. Findings recorded include the presence or absence of any of the following: features of amniotic fluid infection (including acute subchorionitis, chorionitis, chorioamnionitis, chorionic or umbilical vasculitis), recent or remote infarction, increased perivillous fibrin deposition (including percentage and location of fibrin deposition), vascular thrombosis or obliteration, villous stromal-vascular karyorhexis, villitis (including type of inflammatory cell, location of inflammation, grade, and distribution), avascular villi (including amount and associated lesion), chorangiosis, decidual arteriopathy (including location and specific microscopic finding such as fibrinoid necrosis), and other miscellaneous findings.
Scoring : Pathologic score was assigned to each case as follows: inflammation score (0–2) : one point each—any villitis, any membrane, or cord inflammation. Hypoxic score (0–6) : one point each—abnormal maturation (accelerated or delayed), infarction, villous stromal karyorhexis, thrombus, chorangiosis, decidual arteriopathy.
Outcomes and Measures
The primary outcome of the study was defined as pregnancy complicated by IPD (preeclampsia, gestational hypertension [GHTN], FGR/SGA, and placental abruption). Secondary analysis is planned to study APOs more broadly to include GDM.
APOs were defined as pregnancy complicated by IPD or GDM.
IPD is present with one or more of the following complications: placental abruption, hypertensive disease of pregnancy (preeclampsia and GHTN), FGR, or a newborn of SGA. 5
Preeclampsia is defined as blood pressure (BP) 140/90 or higher on two occasions at least 4 hours apart after 20 weeks of gestation with previously normal BP, and proteinuria of 300 mg/24 hours or more. 19 In the absence of proteinuria, preeclampsia is defined as new-onset hypertension with new onset of thrombocytopenia, renal insufficiency (serum creatinine greater than 1.1 mg/dL), impaired liver function (elevated liver transaminases to twice the normal concentration), pulmonary edema, cerebral or visual symptoms. GHTN is hypertension developing after 20 weeks of gestation not associated with systemic features of preeclampsia (e.g., proteinuria, liver involvement, etc.). Chronic hypertension (CHTN), on the other hand, is defined as BP 140/90 or higher mm Hg that either predates pregnancy or develops before 20 weeks of gestation. Patients diagnosed with these conditions were managed according to American College of Obstetricians and Gynecologists (ACOG) practice guidelines. 20
PTB : PTB was defined as a live-born or stillborn infant for any cause between 20 and 37 weeks' gestation. Spontaneous PTB is defined as delivery that occurs subsequent to spontaneous onset of preterm labor or premature rupture of the membranes or fetal membrane prolapse.
Indicated PTB was defined as delivery after induction or cesarean delivery between 20 and 37 weeks' gestation. The indication for induction was noted.
GDM was defined as any degree of glucose intolerance with an initial recognition during pregnancy. 21 The majority of participants were diagnosed by the two-step Carpenter–Coustan criteria between 24 and 28 weeks of gestation. This is based on an initial screen of a 50-g glucose challenge test. Patients with glucose values >135 mg/dL underwent a fasting diagnostic 3-hour 100-g glucose tolerance test. Patients diagnosed with GDM were managed according to the ACOG practice guidelines. 21
FGR was defined as fetuses with an estimated fetal weight or abdominal circumference that was less than the 10th percentile for gestational age, with subclassification of <3rd percentile and presence of abnormal umbilical artery Doppler velocimetry. 22 SGA was defined as a newborn birth weight less than the 10th percentile per Fenton's growth charts with subclassification of <3rd percentile. Further proportional versus disproportional SGA was classified by assessing the percentiles of length and head circumference.
Power to Predict Ischemic Placental Disease Using MRI Perfusion Studies
Prior to recruiting this prospective cohort, we based our power analysis on published ultrasound differences found in IPD. The uterine artery PI obtained from ultrasound previously had the mean value 1.7 (standard deviation [SD] = 0.5) and 2.1 (SD = 0.7) in normal and pregnancies complicated by placental insufficiency from 11 to 14 weeks, respectively, 23 corresponding to a Cohen's d of 0.66. If 36 participants (12%) developed the composite primary outcome of IPD, we estimated that a sample size of 300 would have ≥ 96% statistical power to detect effect sizes of 0.66 or higher between normal and IPD, using a two-sided two-sample t -test at the significance level of 0.05.
Statistical Analysis
Participant data were summarized for the full sample, as well as stratified by IPD status. Comparisons between IPD and non-IPD women were performed using two-sample t -tests for quantitative variables, and chi-squared or Fisher's exact test as appropriate for categorical variables. A two-sided significance level of 0.05 was used, and analyses were performed using R v. 4.1.0 ( http://www.r-project.org/ ).
Results
From October 1, 2015, until November 6, 2019, 841 patients were screened with 199 participants ultimately enrolled in PARENTs ( Fig. 2 ). Of the 199 participants enrolled, there were 5 cases of pregnancy termination/early fetal demise prior to imaging. Four more patients were lost to follow-up or withdrew from the study before completion. Of the final 190 patients who completed pregnancy to viable delivery, 50 (26%) developed the IPD. Table 2 displays the number of participants according to each subtype of IPD. The majority of IPD cases (38/50) met criteria by the development of hypertensive disease of pregnancy (76%). Of the preeclampsia cases, two patients developed preterm preeclampsia (delivery <37 weeks of gestation), and the remainder delivered after 37 weeks.
Fig. 2.
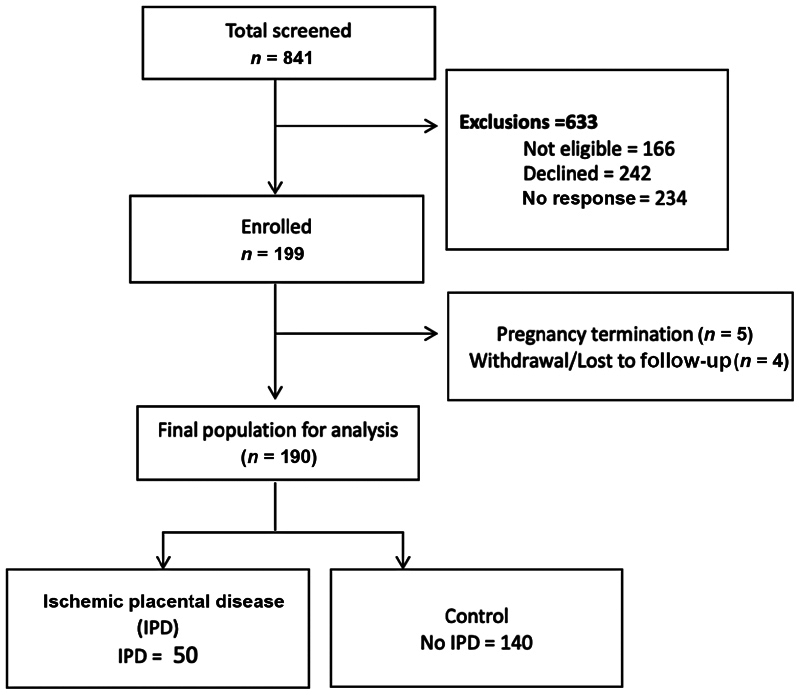
Placental Assessment in Response to Environmental Pollution study flow chart: screening, enrollment, and study population.
Table 2. IPD composite, N = 50 .
| Composite of IPD |
Total (
n
= 50)
n (%) |
|---|---|
| GHTN | 20 (40) |
| Preeclampsia a | 18 (36) |
| Diagnosed FGR | 8 (16) |
| SGA only | 4 (8) |
| Abruption | 0 |
Abbreviations: FGR, fetal growth restriction; GHTN, gestational hypertension; IPD, ischemic placental disease; SGA, small for gestational age.
Three patients with preeclampsia also with FGR + SGA.
Study Population
Demographic and clinical information for the cohort stratified by the primary outcome of IPD is displayed in Table 3 . The mean age of the participants was 34 years (SD of 4.1 years). The race/ethnicity of the final cohort included 47% non-Hispanic whites, 19% Hispanic, 27% Asian, 6% black, and <1% American Indian or Alaska Native. This breakdown reflected the racial/ethnic make-up of the patients who seek care at our clinics at UCLA.
Table 3. Clinical and demographic characteristics of cohort stratified by IPD.
| Predictor |
Total (
n
= 190)
n (%) |
IPD (
n
= 50)
n (%) |
No IPD (
n
= 140)
n (%) |
p -Value |
|---|---|---|---|---|
| Maternal age (wk) | ||||
| 18–34 | 115 (60.5) | 26 (52) | 89 (64) | 0.2 |
| > 34 | 75 (39.5) | 24 (48.0) | 51 (36.4) | |
| Maternal race/ethnicity | ||||
| American Indian or Alaska Native | 1 (1) | 0 | 1 (1) | 0.9 |
| Asian | 51 (27) | 14 (28) | 37 (26) | |
| Black | 12 (6) | 3 (6) | 9 (6) | |
| Hispanic or Latinx | 37 (19) | 8 (16) | 29 (21) | |
| White, non-Hispanic | 89 (47) | 25 (50) | 64 (46) | |
| Insurance type ( N = 187) | ||||
| Private | 180 (96) | 48 (98) | 132 (96) | 0.7 |
| Public | 7 (4) | 1 (2) | 6 (4) | |
| Prepregnancy BMI | ||||
| Nonobese (BMI <30 kg/m 2 ) | 167 (88) | 43 (86) | 124 (89) | 0.8 |
| Obesity (BMI ≥ 30 kg/m 2 ) | 23(12) | 7 (14) | 16 (11) | |
| Parity | ||||
| 0 (primiparous) | 91 (48) | 27 (54) | 64 (46) | 0.4 |
| ≥1 (multiparous) | 99 (52) | 23 (46) | 76 (54) | |
| Multiparous and history of IPD | ||||
| Yes | 9 (5) | 6 (12) | 3 (2) | 0.01 |
| No | 181 (85) | 44 (88) | 137 (98) | |
| Newborn birth weight, g (median, IQR) | 3,340 (3,060–3,670) | 3,129 (2,685–3,348) | 3,403 (3,128–3,750) | 0.01 |
Abbreviations: BMI, body mass index; IPD, ischemic placental disease; IQR, interquartile range.
Note: a p -value of <0.05 is marked as bold.
Maternal clinical risk factors alone were not significantly associated with the development of IPD in this small cohort (e.g., nonwhite, maternal age >35, nulliparity, obesity, and preexisting CHTN). 13 There were 99 multiparous patients who completed the study. Of these, nine had a history of IPD. As expected, the development of IPD was associated with having a history of IPD in multiparous patients (relative risk = 2.5 [0.97–6.2]).
Imaging
Of our final cohort of 190 participants, 96% successfully completed the first MRI study ( n = 182) conducted between 14 and 16 weeks' gestational age, and 91% completed both the first and second MRI scans ( n = 173). Measurements of uterine artery resistance by ultrasound Doppler in early second trimester (14–16 weeks) and late second trimester (19–22 weeks) were not associated with the development of IPD ( Fig. 3 ). However, in terms of our primary study outcome of IPD, of the multiple novel placenta perfusion measurements taken of the in vivo placenta using MRI, hPBF (mL/100 g/min) in early pregnancy between 14 and 16 weeks was significantly decreased and found to be statistically associated ( p < 0.05) with the later development of IPD ( Table 4 ). 9
Fig. 3.
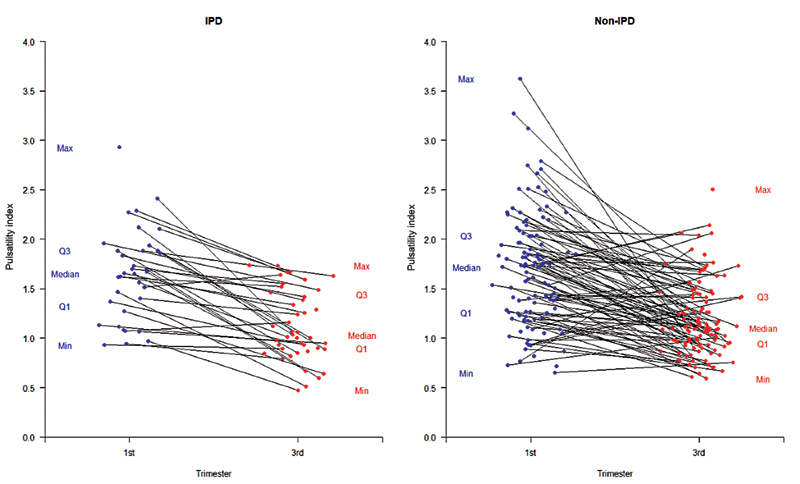
Ultrasound Doppler evaluation of uterine artery pulsatility index (UTPI) in first and third trimesters. UTPI showing no difference between non-IPD cases (control) versus IPD. IPD, ischemic placental disease.
Table 4. Imaging UTPI1.
| Imaging | Total | IPD | No IPD | p -Value |
|---|---|---|---|---|
| hPBF1 (mL/100 g/min) | 221.0 ± 102.6 ( n = 163) | 189.4 ± 76.9 ( n = 45) | 233 ± 108.8 ( n = 118) | 0.03 a |
| hPBF2 (mL/100 g/min) | 246.7 ± 131.9 ( n = 153) | 226.4 ± 87.7 ( n = 42) | 254.4 ± 145 ( n = 111) | 0.24 |
| Volume 1 (cm 3 ) | 137.9 ± 47.9 ( n = 164) | 131.7 ± 46.6 ( n = 47) | 138.8 ± 48.5 ( n = 117) | 0.53 |
| Volume 2 (cm 3 ) | 257.9 ± 72.8 ( n = 156) | 251.7 ± 81.9 ( n = 44) | 258.6 ± 69.8 ( n = 112) | 0.65 |
| UTPI1 | 1.7 (1.3–2.0; n = 141) | 1.65 (1.2–1.9; n = 37) | 1.73 (1.3–2.0; n = 104) | 0.43 |
| UTPI2 | 1.1 (0.9–1.4; n = 155) | 1.0 (0.9–1.5; n = 46) | 1.1 (0.9–1.4; n = 109) | 0.6 |
Abbreviations: hPBF1, MRI human placenta blood flow at 16 weeks; hPBF2, MRI human placenta blood flow at 20 weeks; UTPI1, ultrasound uterine artery PI at 14 weeks; UTP2, ultrasound uterine artery PI at 20 weeks.
Pathology
Thus far, 90 placentas have been examined histologically (28 IPD and 62 non-IPD cases were selected at random). Table 5 shows that through blind standardized scoring using Amsterdam criteria, more than half (58%) of normal outcome (non-IPD) pregnancies have microscopic findings that may be associated with placental mal-perfusion (hypoxic score more than 0). Of the IPD patients ( n = 28 examined to date), a large proportion (almost half) had unremarkable microscopic findings (hypoxic score 0). There was a nonsignificant trend of lower weight in IPD placental weight compared with controls (nonsignificant, p = 0.28). Microscopic examination of remaining cohort is ongoing.
Table 5. Placenta pathology, stratified by IPD.
| Pathology |
Total (
n
= 190)
n . (%) |
IPD (
n
= 50)
n (%) |
No IPD (
n
= 140)
n (%) |
p -Value |
|---|---|---|---|---|
| Placental weight (g; median, IQR) | 438 (382–510) | 428 (374–497) | 440 (385–517) | 0.28 |
| Histologic examination | n = 90 | n = 28 | n = 62 | |
| Hypoxic score = 0 | 43 (48) | 13 (46) | 30 (48) | 0.33 |
| Hypoxic score > 0 | 47 (52) | 15 (54) | 32 (52) | 0.53 |
Abbreviations: IPD, ischemic placental disease; IQR, interquartile range.
Environmental Assessment
Of the initial 199 participants, 110 patients agreed to host PurpleAir monitors indoors and outdoors at the site of their homes. After patient drop out and excluding those monitors with error/breakage, a total of 84 participants had a complete set of data available ( Fig. 4 ). Of the 143 patients who completed diet analysis, for most components and total HEI-2015 scores, no statistically significant differences (cut-off p -value of 0.05) were observed between IPD cases and noncases except for dairy intake with HEI-2015 score of 5.3 out of 10 for IPD cases and 6.3 for noncases ( Table 6 ).
Fig. 4.
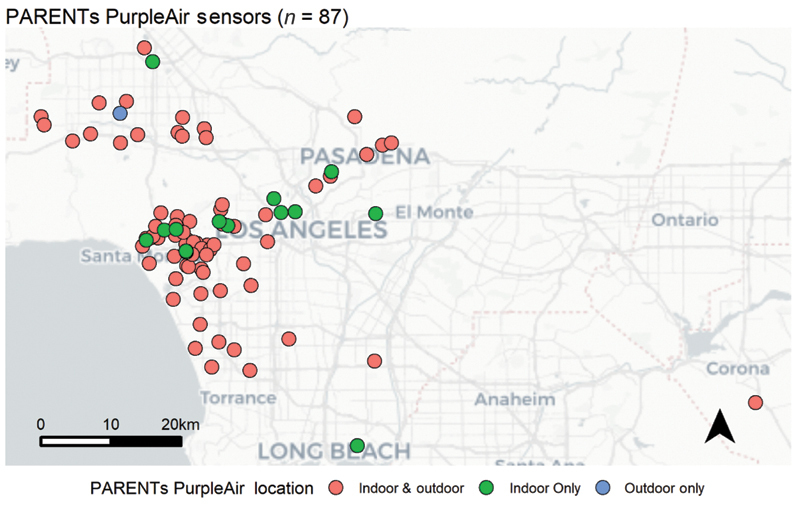
Location of PurpleAir monitors placed in the homes of Placental Assessment in Response to Environmental Pollution Study (PARENTs) patients ( n = 84 of 87 with complete data). Patients were given monitors either indoors (green), outdoors (blue), or both indoors and outdoors (red). Sensor locations were shifted by a random distance between 0 and 1 km to protect patient privacy.
Table 6. HEI-2015 a scores stratified by IPD .
| Diet scores | Maximum points | Mean of HEI-2015 score | p -Value | ||
|---|---|---|---|---|---|
| Total ( n = 143) | IPD ( n = 40) | No IPD ( n = 103) | |||
| Adequacy | |||||
| Total fruits b | 5 | 4.19 | 4.16 | 4.21 | 0.83 |
| Whole fruits c | 5 | 4.59 | 4.49 | 4.63 | 0.42 |
| Total vegetables d | 5 | 4.23 | 4.22 | 4.23 | 0.92 |
| Greens and beans d | 5 | 4.35 | 4.11 | 4.44 | 0.16 |
| Whole grains | 10 | 2.58 | 2.48 | 2.62 | 0.65 |
| Dairy e | 10 | 6.02 | 5.27 | 6.31 | 0.03 a |
| Total frotein foods f | 5 | 4.59 | 4.55 | 4.61 | 0.68 |
| Seafood and plant proteins f, g | 5 | 4.34 | 4.26 | 4.37 | 0.62 |
| Fatty acids h | 10 | 6.00 | 6.07 | 5.97 | 0.85 |
| Moderation | |||||
| Refined grains | 10 | 7.72 | 7.54 | 7.79 | 0.57 |
| Sodium | 10 | 5.07 | 5.08 | 5.06 | 0.97 |
| Added sugars | 10 | 8.52 | 8.81 | 8.40 | 0.38 |
| Saturated fats | 10 | 5.07 | 4.96 | 5.12 | 0.78 |
| Total HEI-2015 score | 100 | 67.27 | 66.01 | 67.76 | 0.25 |
Abbreviations: HEI, Healthy Eating Index; IPD, ischemic placental disease.
Intakes between the minimum and maximum standards are scored proportionately.
Includes 100% fruit juice.
Includes all forms except juice.
Includes legumes (beans and peas).
Includes all milk products, such as fluid milk, yogurt, and cheese, and fortified soy beverages.
Includes legumes (beans and peas).
Includes seafood, nuts, seeds, soy products (other than beverages), and legumes (beans and peas).
Ratio of poly- and monounsaturated fatty acids (PUFAs and MUFAs) to saturated fatty acids (SFAs).
Blood and Urine Sample Analysis
Multiple studies were performed which included genome-wide assessments of cell-free placenta specific DNA methylation and transcriptomics, 24 and targeted and untargeted metabolomics (to be reported). Urine samples were subjected to testing for oxidants and transcriptomics with analysis ongoing.
Discussion
Principal Findings
In this study, we provide a description of the study design, methods, and preliminary demographic characteristics of the cohort. These results demonstrated that implementation of MRI and biosample collection in a prospective population-based study of pregnant patients was feasible and well tolerated. We believe the majority our patients accepted the two MRI procedures because each of them was free breathing and our protocol took less than 30 minutes at a time.
The objective of PARENTs was to develop and evaluate a suit of cutting-edge placental MRI technologies and translate these novel placental imaging modalities to predict a composite of APOs including IPD (primary outcome) and GDM (planned secondary analysis). We also hypothesized that chronic exposure to high rates of environmental pollution, independent of socioeconomic status, increases the risk of IPD due to early gestational development of adverse placental structure/function as detected by placental MRI technological advances.
Strengths and Limitations
Our study has several notable strengths including the prospective design with close longitudinal follow-up of mothers and newborns 3 months postpartum. Because of the high resolution of MRI measurement, the size of our pilot cohort was powered to study the primary study outcome of IPD. In addition, a composite of multiple measurements of environmental pollution were also predictive of IPD (results submitted separately for publication). In addition, our survey/questionnaires captured the breadth of multiple adverse social determinants of health.
This study demonstrates that in a relatively small cohort, MRI of the placenta shows a significant decrease in hPBF in early pregnancy. This lends credence to the hypothesis that IPD is associated to measurable physiological changes related to placental development. In a small cohort, clinical risk factors, other than the history of IPD, were not predictive of the development of IPD, but further biomarkers (imaging) and those found in blood, urine, and saliva will supplement current screening methods.
Histologically, scoring placentas ex vivo by Amsterdam criteria identified a high percentage of placental lesions in clinically normal patients. Our analysis thus far did not show statistically significant clinicopathologic correlations between IPD and normal pregnancies. This demonstrates the need for further refinement of reporting thresholds for placental lesions as further biomarkers may be needed to stratify risks to newborns and future pregnancies.
Limitations of the study include the low prevalence of African American and Hispanic populations, which may limit the generalizability of findings to those who are known to suffer a disproportionate amount of IPD and their sequalae, and the highest incidence of maternal mortality. In this cohort, black and Hispanic populations were more likely to be ineligible because of late entry to prenatal care and missing enrollment prior to 14 weeks' gestational age. In ongoing studies, we plan to increase early outreach to enroll another prospective cohort enriched for increased risk factors for APOs, including women with adverse social determinants of health that pose a barrier to care in pregnancy.
Our findings thus far indicate several areas for future research. We must continue recruitment of a larger prospective cohort and recruit participants at highest risk so that we can further learn how to mitigate those risks. In addition, a larger cohort will help us stratify data by risk factors for IPD. Data from our cohort will be used to study the relationship between IPD and various environmental exposures, including NO 2 and PM 2.5 species.
Conclusion
In summary, this was a multidisciplinary study involving human patients that has shown the feasibility of developing a noninvasive measurement for prediction of IPD. Studies on collected blood and urine samples await final analysis and publication.
Acknowledgment
We are indebted to the patients who participated in this study.
Funding Statement
Funding This work was supported by the NIH-U01 HD087221 (multi-PI to S.U.D., K.S., and C.J.) and NICHD HD089714 and HD100015 (to S.U.D.).
Conflict of Interest None declared.
These authors are the equal contributors to the overarching study concept, design, methods, and execution of the study.
References
- 1.Rich-Edwards J W, Fraser A, Lawlor D A, Catov J M. Pregnancy characteristics and women's future cardiovascular health: an underused opportunity to improve women's health? Epidemiol Rev. 2014;36:57–70. doi: 10.1093/epirev/mxt006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guttmacher A E, Spong C Y.The Human Placenta Project: it's time for real time Am J Obstet Gynecol 2015213(4, suppl):S3–S5. [DOI] [PubMed] [Google Scholar]
- 3.Guttmacher A E, Maddox Y T, Spong C Y. The Human Placenta Project: placental structure, development, and function in real time. Placenta. 2014;35(05):303–304. doi: 10.1016/j.placenta.2014.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sadovsky Y, Clifton V L, Burton G J. Invigorating placental research through the “Human Placenta Project”. Placenta. 2014;35(08):527. doi: 10.1016/j.placenta.2014.06.367. [DOI] [PubMed] [Google Scholar]
- 5.Ananth C V. Ischemic placental disease: a unifying concept for preeclampsia, intrauterine growth restriction, and placental abruption. Semin Perinatol. 2014;38(03):131–132. doi: 10.1053/j.semperi.2014.03.001. [DOI] [PubMed] [Google Scholar]
- 6.Ananth C V, Vintzileos A M. Maternal-fetal conditions necessitating a medical intervention resulting in preterm birth. Am J Obstet Gynecol. 2006;195(06):1557–1563. doi: 10.1016/j.ajog.2006.05.021. [DOI] [PubMed] [Google Scholar]
- 7.Vintzileos A M, Ananth C V. First trimester prediction of ischemic placental disease. Semin Perinatol. 2014;38(03):159–166. doi: 10.1053/j.semperi.2014.03.006. [DOI] [PubMed] [Google Scholar]
- 8.Brink H S, van der Lely A J, van der Linden J. The potential role of biomarkers in predicting gestational diabetes. Endocr Connect. 2016;5(05):R26–R34. doi: 10.1530/EC-16-0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu D, Shao X, Danyalov A et al. Human placenta blood flow during early gestation with pseudocontinuous arterial spin labeling MRI. J Magn Reson Imaging. 2020;51(04):1247–1257. doi: 10.1002/jmri.26944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bekkar B, Pacheco S, Basu R, DeNicola N. Association of air pollution and heat exposure with preterm birth, low birth weight, and stillbirth in the US: a systematic review. JAMA Netw Open. 2020;3(06):e208243. doi: 10.1001/jamanetworkopen.2020.8243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Su J G, Meng Y Y, Chen X, Molitor J, Yue D, Jerrett M. Predicting differential improvements in annual pollutant concentrations and exposures for regulatory policy assessment. Environ Int. 2020;143:105942. doi: 10.1016/j.envint.2020.105942. [DOI] [PubMed] [Google Scholar]
- 12.Zota A, Adamkiewicz G, Levy J I, Spengler J D. Ventilation in public housing: implications for indoor nitrogen dioxide concentrations. Indoor Air. 2005;15(06):393–401. doi: 10.1111/j.1600-0668.2005.00375.x. [DOI] [PubMed] [Google Scholar]
- 13.Poon L C, Shennan A, Hyett J A et al. Erratum to “The International Federation of Gynecology and Obstetrics (FIGO) initiative on pre-eclampsia: a pragmatic guide for first-trimester screening and prevention” [Int J Gynecol Obstet 145 Suppl. 1 (2019) 1–33] Int J Gynaecol Obstet. 2019;146(03):390–391. doi: 10.1002/ijgo.12802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martin A M, Bindra R, Curcio P, Cicero S, Nicolaides K H. Screening for pre-eclampsia and fetal growth restriction by uterine artery Doppler at 11-14 weeks of gestation. Ultrasound Obstet Gynecol. 2001;18(06):583–586. doi: 10.1046/j.0960-7692.2001.00594.x. [DOI] [PubMed] [Google Scholar]
- 15.Shao X, Liu D, Martin T et al. Measuring human placental blood flow with multidelay 3D GRASE pseudocontinuous arterial spin labeling at 3T. J Magn Reson Imaging. 2018;47(06):1667–1676. doi: 10.1002/jmri.25893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Martin T, Janzen C, Li X et al. Characterization of uterine motion in early gestation using MRI-based motion tracking. Diagnostics (Basel) 2020;10(10):E840. doi: 10.3390/diagnostics10100840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Armstrong T, Liu D, Martin T et al. 3D [formula: see text] mapping of the placenta during early gestation using free-breathing multiecho stack-of-radial MRI at 3T. J Magn Reson Imaging. 2019;49(01):291–303. doi: 10.1002/jmri.26203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Khong T Y, Mooney E E, Ariel I et al. Sampling and definitions of placental lesions: Amsterdam Placental Workshop Group Consensus Statement. Arch Pathol Lab Med. 2016;140(07):698–713. doi: 10.5858/arpa.2015-0225-CC. [DOI] [PubMed] [Google Scholar]
- 19.Hypertension in pregnancy. report of the American College of Obstetricians and Gynecologists' Task Force on hypertension in pregnancy. Obstet Gynecol. 2013;122(05):1122–1131. doi: 10.1097/01.AOG.0000437382.03963.88. [DOI] [PubMed] [Google Scholar]
- 20.American College of Obstetricians and Gynecologists' Committee on Practice Bulletins—Gynecology . Female sexual dysfunction: ACOG Practice Bulletin Clinical Management Guidelines for Obstetrician-Gynecologists, Number 213. Obstet Gynecol. 2019;134(01):e1–e18. doi: 10.1097/AOG.0000000000003324. [DOI] [PubMed] [Google Scholar]
- 21.Committee on Practice B-O. ACOG Practice Bulletin No . ACOG Practice Bulletin No. 190: gestational diabetes mellitus. Obstet Gynecol. 2018;131(02):e49–e64. doi: 10.1097/AOG.0000000000002501. [DOI] [PubMed] [Google Scholar]
- 22.ACOG Practice Bulletin No . ACOG Practice Bulletin No. 227: fetal growth restriction: correction. Obstet Gynecol. 2021;137(04):754. doi: 10.1097/AOG.0000000000004350. [DOI] [PubMed] [Google Scholar]
- 23.Gómez O, Figueras F, Martínez J M et al. Sequential changes in uterine artery blood flow pattern between the first and second trimesters of gestation in relation to pregnancy outcome. Ultrasound Obstet Gynecol. 2006;28(06):802–808. doi: 10.1002/uog.2814. [DOI] [PubMed] [Google Scholar]
- 24.Del Vecchio G, Li Q, Li W et al. Cell-free DNA methylation and transcriptomic signature prediction of pregnancies with adverse outcomes. Epigenetics. 2021;16(06):642–661. doi: 10.1080/15592294.2020.1816774. [DOI] [PMC free article] [PubMed] [Google Scholar]


