Abstract
Background
MEDI7247 is a first-in-class antibody-drug conjugate (ADC) consisting of an anti-sodium-dependent alanine-serine-cysteine transporter 2 antibody-conjugated to a pyrrolobenzodiazepine dimer.
Objective
This first-in-human phase 1 trial evaluated MEDI7247 in patients with hematological malignancies.
Patients and methods
Adults with acute myeloid leukemia (AML), multiple myeloma (MM), or diffuse large B-cell lymphoma (DLBCL) relapsed or refractory (R/R) to standard therapies, or for whom no standard therapy exists, were eligible. Primary endpoints were safety and determination of the maximum tolerated dose (MTD). Secondary endpoints included assessments of antitumor activity, pharmacokinetics (PK), and immunogenicity.
Results
As of 26 March 2020, 67 patients were treated (AML: n = 27; MM: n = 18; DLBCL: n = 22). The most common MEDI7247-related adverse events (AEs) were thrombocytopenia (41.8%), neutropenia (35.8%), and anemia (28.4%). The most common treatment-related grade 3/4 AEs were thrombocytopenia (38.8%), neutropenia (34.3%), and anemia (22.4%). Anticancer activity (number of responders/total patients evaluated) was observed in 11/67 (16.4%) patients. No correlation was observed between ASCT2 expression and clinical response. Between-patient variability of systemic exposure of MEDI7247 ADC and total antibody were high (AUCinf geometric CV%: 62.3–134.2, and 74.8–126.1, respectively). SG3199 (PBD dimer) plasma concentrations were below the limit of quantification for all patients after Study Day 8. Anti-drug antibody (ADA) prevalence was 7.7%, ADA incidence was 1.9%, and persistent-positive ADA was 5.8%.
Conclusions
Thrombocytopenia and neutropenia limited repeat dosing. Although limited clinical activity was detected, the dose-escalation phase was stopped early without establishing an MTD.
The study was registered with ClinicalTrials.gov (NCT03106428).
Supplementary Information
The online version contains supplementary material available at 10.1007/s11523-024-01054-z.
Key Points
| An unmet medical need exists for effective and tolerable therapies for the treatment of patients with relapsed and refractory hematologic malignancies, for whom survival outcomes are poor. |
| This phase 1, first-in-human trial studied the safety, maximum tolerated dose, antitumor activity, pharmacokinetics, and immunogenicity of a first-in-class, selective antibody-drug conjugate that is in development for patients with relapsed and refractory hematologic malignancies. |
| Targeting the sodium-dependent alanine-serine-cysteine transporter 2, which is often overexpressed in hematologic malignancies, may provide clinical benefit to patients who have relapsed or are refractory to standard treatments, or for whom there are no other treatments available. |
Introduction
The sodium-dependent alanine-serine-cysteine transporter 2 (ASCT2, also known as SLC1A5) is a member of the solute carrier 1A (SLC1A) family, and it preferentially transports the amino acid glutamine across the plasma membrane [1]. Glutamine is considered “conditionally essential” in cells with a high proliferative rate (e.g., immune cells, stem cells, and tumor cells) [1, 2]. ASCT2-overexpression has been reported in a range of solid malignancies, including squamous cell carcinoma of the head and neck, squamous cell carcinoma of the lung, non-small cell lung cancer, prostate cancer, pancreatic cancer, and breast cancer, and is associated with poor prognoses [3–8]. ASCT2 is also overexpressed in many hematologic malignancies, including acute myeloid leukemia (AML), multiple myeloma (MM), and diffuse large B-cell lymphoma (DLBCL) [9, 10]. Rates of ASCT2-positive tumors range from 95 to 100% in AML, MM, and DLBCL [9, 10]; and 45–95% in other cancer types [3–5, 8]. Therefore, ASCT2 represents a potentially attractive, novel pharmacological target for anticancer therapy [1], particularly in patients with hematologic malignancies for whom chemotherapy remains the standard of care but provides limited disease control [11, 12].
Although several ASCT2 inhibitors have been synthesized and characterized, they have low potency (affinities in the low micromolar range) and do not effectively inhibit ASCT2 glutamine transport in vivo [13]. Furthermore, the atomic-resolution structure of the transporter in the outward-facing conformation has not been experimentally determined, which presents a challenge for the development of effective ASCT2 small molecule inhibitors [13].
MEDI7247 is a first-in-class antibody-drug conjugate (ADC) comprising an anti-ASCT2 antibody site-specifically conjugated to the pyrrolobenzodiazepine (PBD) dimer SG3199, via a protease-cleavable linker, with a drug-to-antibody ratio (DAR) of close to two [10]. PBDs are a class of highly potent DNA cross-linking agents that bind to the minor groove of DNA and cross-link specific DNA sites, blocking cell division [14]. Hematologic cell lines are more sensitive to the PBD dimer SG3199, compared with solid tumor cell lines [15]. The short half-life of the free PBD warhead may restrict the bystander effect, limiting the potential for off-target toxicity caused by systemic accumulation of free drug [15]. However, due to their potency, target expression must be minimal in normal tissue exposed to PBD-bound ADC complexes.
Previously approved ADCs mainly target lineage-specific cell surface markers (e.g., CD70, CD33, CD30, transmembrane receptor tyrosine kinases, human epidermal growth factor receptor 2, and epidermal growth factor receptor) [16]. MEDI7247 demonstrates specificity for ASCT2 expressed on the cell surface and does not exhibit affinity for other members of the SLC1 transporter family, including ASCT1 [10]. Once bound, MEDI7247 is internalized and trafficked to the lysosomes, where the PBD warhead releases, and triggers cell death [10]. ASCT2 function is not compromised by MEDI7247; rather, the ADC enables targeted delivery of the PBD warhead to cancer cells via the glutamine transporter. Preclinical in vitro cytotoxicity studies demonstrated that the free ASCT2 antibody did not inhibit cell proliferation or significantly reduce glutamine transport (manuscript in progress).
Preclinical studies showed that MEDI7247 exerts potent antitumor activity [9]. Moreover, MEDI7247 demonstrated antitumor efficacy across all tumor types tested, with varying levels of ASCT2 expression (AML, MM, acute lymphoblastic leukemia, and Burkitt lymphoma) [9]. In vivo efficacy studies using mouse models of hematologic malignancies demonstrated a significant, dose-dependent survival benefit in MEDI7247-treated animals relative to untreated controls [9]. This first-in-human, multicenter, open-label, phase 1 study evaluated the safety and efficacy of single-agent MEDI7247 in adult patients with relapsed/refractory (R/R) hematologic malignancies AML, MM, and DLBCL.
Methods
Patient Eligibility
This open-label, multicenter, phase 1 study of MEDI7247 was conducted at 14 centers globally, from 29 March 2017 to 3 January 2020. Eligible patients were aged ≥ 18 years with a clinical history of AML, MM, or DLBCL and who had disease that relapsed after, or was refractory to, standard therapy, with no salvage regimen; had an Eastern Cooperative Oncology Group (ECOG) performance status of 0–1; hepatic alanine aminotransferase (ALT) and aspartate aminotransferase (AST) ≤ 3 × upper limit of normal (ULN), and serum total bilirubin levels ≤ 1.5 × ULN, unless consistent with Gilbert’s syndrome (wherein the ratio between the total and direct bilirubin was > 5) and for which total bilirubin levels ≤ 2.5 × ULN was allowed; international normalized ratio (INR) < 1.5 × ULN; and creatinine clearance ≥ 40 mL/min (per 24-h urine or calculated by the Cockroft and Gault equation). Additional information regarding blood counts by disease as well as histological- and hematologic-specific inclusion criteria are included in the Online Supplementary Material (OSM). Key exclusion criteria are also presented in the OSM.
All patients provided written informed consent, and the study was conducted in accordance with the principles of the Declaration of Helsinki and the International Conference on Harmonisation/Good Clinical Practice. The study protocol was approved by an institutional review board or independent ethics committee at each study site and is included in the OSM. This study was registered with clinicaltrials.gov, NCT03106428.
Study Design and Assessments
The study consisted of a dose-escalation phase and a planned dose-expansion phase; the latter was not initiated due to the hematologic toxicity observed during dose escalation (OSM Fig. 1). In the dose-escalation phase, patients with R/R AML, MM, or DLBCL received MEDI7247 intravenously (IV) once every 3 weeks (Q3W). A protocol amendment was introduced following review of the Q3W data, and patients were enrolled consecutively to receive a fractionated dosing schedule in parallel (three doses per cycle, with either 21- or 28-day cycles). Patients could receive MEDI7247 for a maximum of 2 years. The starting dose for MEDI7247 was 0.016 mg/kg Q3W; and the fractionated dosing schedule used a starting dose of 0.03 mg/kg/day for AML and DLBCL and a starting dose of 0.01 mg/kg/day for MM, with doses given on days 1, 2, and 3 of a 21-day cycle (Q3W), or days 1, 8, and 15 of a 28-day cycle (Q4W). Dose escalation or de-escalation was determined using a modified toxicity probability interval algorithm, with a target dose-limiting toxicity (DLT) rate of 30% and equivalence interval of (25%, 35%) [17]. A dose level was considered unsafe—with no additional patients enrolled at that dose level—if it had an estimated 95% or more probability of exceeding the target DLT rate of 30% with at least three patients treated at that dose level. DLTs were evaluated at different timepoints, depending on the disease cohort and dosing schedule.
In patients with AML, the DLT evaluation period was up to 42 days. In patients with MM or DLBCL, the DLT evaluation period was up to 21 or 28 days, depending on cycle length. Additional information regarding DLT definitions is summarized in the OSM.
Safety was assessed by the occurrence of adverse events (AEs), serious AEs (SAEs), and DLTs. AEs were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI CTCAE, v4.03) and are described by system organ class and preferred term using the Medical Dictionary for Regulatory Activities (MedDRA, v22.1). Best overall response (BOR) was based on all post-baseline disease assessments that occurred prior to the initiation of subsequent anticancer treatment. Classification of disease response differed by disease cohort. For AML, classification and treatment was based on the revised European LeukemiaNet (ELN) recommendations for diagnosis and management of AML in adults [18]; for MM, classification was based on the International Myeloma Working Group consensus criteria [19]; for DLBCL, classification was based upon the Lugano Response Criteria for Non-Hodgkin’s lymphoma [20]. For MM and DLBCL, the objective response rate (ORR) was defined as the proportion of patients with a BOR of complete response (CR) or partial response (PR). For AML, ORR was defined as the proportion of patients with a BOR of CR, CR with incomplete hematological recovery (CRi), morphologic leukemia-free state (MLFS), and PR.
ASCT2 expression levels were analyzed retrospectively via an ASCT2 immunohistochemistry (IHC) assay in archival bone marrow aspirates, depending upon availability. The IHC staining protocol was developed using an ASCT2 monoclonal antibody (mAb, Cell Signaling Technology, Inc., Danvers, MA, USA). Baseline ASCT2 expression was not an inclusion criterion for this trial. H-scores were determined by pathologist evaluation of stained slides, each containing a minimum of 100 cells. The percentage of tumor cells with membrane staining at various intensity levels was estimated. Membrane staining intensity (0, 1+, 2+, or 3+) was determined for each cell in a fixed field. H-scores (ranging from 0 to 300) were obtained by using the formula: [1 × (% cells 1+) + 2 × (% cells 2+) + 3 × (% cells 3+)].
To explore genetic correlates of response to MEDI7247 in patients with AML, genomic DNA profiles were generated from bone marrow aspirates collected at screening. Genomic DNA was extracted (sample preparation summarized in OSM) and amplicon-based targeted next-generation sequencing (NGS) was performed using an AML-focused panel of 54 target genes (Focus:Myeloid™ NGS Panel; Cancer Genetics, Inc., Rutherford, NJ) (OSM Table 1). Data meeting quality standards and minimum coverage requirements were analyzed (> 90% of amplicons with minimum coverage of 500×). In addition, an independent fragment analysis assay was performed to detect FMS-like tyrosine kinase 3-internal tandem duplication (FLT3-ITD) (Cancer Genetics, Inc., Rutherford, NJ, USA).
Outcomes
The primary endpoint was safety and determination of the maximum tolerated dose (MTD). Secondary endpoints included antitumor activity, including BOR and ORR, pharmacokinetics, and immunogenicity. Exploratory endpoints included the relationship between baseline ASCT2 protein levels and clinical outcome.
Pharmacokinetics
Non-compartmentalized analysis of plasma PK data was collected during cycle 1 of MEDI7247 administration at a dose of 0.016–0.18 mg/kg/day ×3 Q4W, and 0.03 mg/kg/day ×3 Q3W. On Day 1, plasma samples were collected predose (within 30 min prior to the start of infusion), and immediately post end of infusion (± 10 min), 2 h (± 10 min), and 6 h (± 15 min) post end of infusion; on Day 2, predose (within 30 min prior to the start of infusion) and at the end of infusion (± 10 min); on Day 3, predose (within 30 min prior to the start of infusion), at end of infusion (± 10 min), 6 h (± 10 min), and 24 h (± 10 min) post infusion; and two additional plasma samples were collected on Days 8 and 15 (± 1 day), respectively. Samples were analyzed using a validated immunoassay to determine MEDI7247 ADC and total antibody concentrations. Free warhead (SG3199) concentrations were measured using a validated liquid-chromatography-couple mass spectrometry method. Individual PK parameters for MEDI7247 ADC and total antibody after the first dose of MEDI7247 included area under the curve from time zero to infinity (AUCinf), area under the concentration-time curve from the start of dosing to the time of the last quantifiable concentration (AUClast), maximum concentration (Cmax), time to maximum concentration (Tmax), terminal half-life (T1/2), systemic clearance (CL), and volume of distribution (Vss).
Immunogenicity
Blood samples were collected predose within 30 min prior to the start of the first infusion on Days 1, 22, 43 (± 3 days), and then Q3W through Day 127, followed by Q12W, starting on Day 211 for anti-MEDI7247 antibody determination, and were analyzed using a validated immunoassay.
Statistical Analysis
The safety and efficacy analyses were based on the as-treated population for each cohort, defined as all patients who received any dose of MEDI7247. Upon completion of the dose-escalation phase, the study planned to determine the MTD via isotonic regression analysis [17] applied to the DLT rates observed during the dose-escalation phase. The MTD was based on the DLT-evaluable population, defined as all patients who were enrolled in the dose-escalation phase, received MEDI7247, and completed the safety follow-up through the DLT evaluation period, or who experienced any DLT during the DLT evaluation period. Categorical data are summarized by the number and percentage of patients in each category and continuous variables by descriptive statistics. ORR and the 95% confidence interval (CI) were estimated using the exact probability method. Individual MEDI7247, total antibody, and SG3199, concentrations were tabulated by dose cohort along with descriptive statistics. Non-compartmental PK data analysis was performed from each dose cohort with scheduled PK sample collection. Relevant descriptive statistics of non-compartmentalized PK parameters were provided, including AUC, Cmax, Tmax, and T1/2. For each disease type, the immunogenic potential of MEDI7247 was assessed by summarizing the number and percentage of patients who developed detectable ADAs. SAS version 9.4 (SAS Institute Inc., Cary, NC, USA) was used for statistical analyses.
Role of the Funding Source
The study sponsor had a role in the study design, data collection, analysis, and interpretation. All authors had full access to the data, reviewed the manuscript, and agreed to submit for publication.
Results
Patient Demographics and Clinical Characteristics
As of 26 March 2020, a total of 67 patients were treated (AML, n = 27; MM, n = 18; DLBCL, n = 22) (Fig. 1). Of these patients, 54 were included in the DLT-evaluable population. Within the AML cohort, 25 patients received MEDI7247 Q3W and two patients received fractionated dosing. Within the MM cohort, 13 patients received MEDI7247 Q3W, and five patients received fractionated dosing. Within the DLBCL group, 18 patients received MEDI7247 Q3W, and four patients received fractionated dosing. Patient demographics and baseline disease characteristics are presented in Table 1. Forty-four (65.7%) patients received at least three prior treatment regimens. Disease-specific baseline characteristics are presented in OSM Table 2.
Fig. 1.
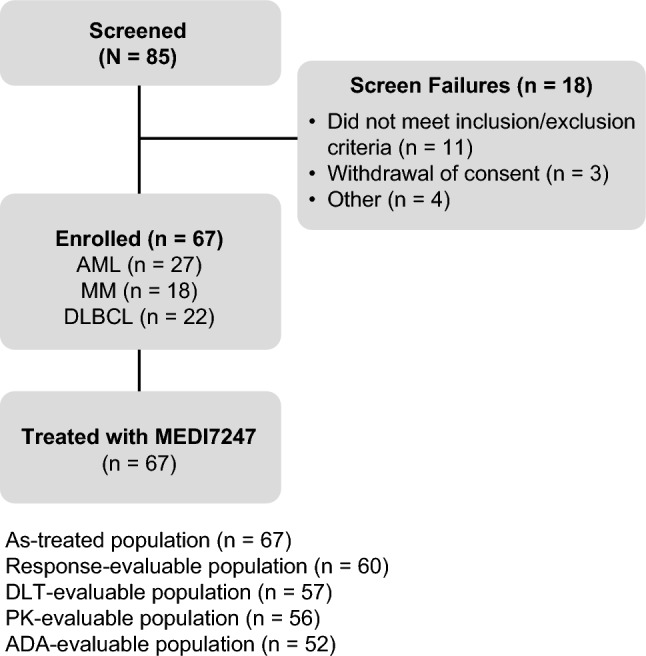
Patient flow diagram. ADA anti-drug antibody, AML acute myeloid leukemia, DLBCL diffuse large B-cell lymphoma, DLT dose-limiting toxicity, MM multiple myeloma, PK pharmacokinetic. See Online Supplementary Material Table 3 for patient disposition
Table 1.
Patient demographics and baseline characteristics, as-treated population
| Parameter | AML (n = 27) | MM (n = 18) | DLBCL (n = 22) | Total (N = 67) |
|---|---|---|---|---|
| Age, years, median (min, max) | 68.0 (37, 79) | 62.5 (51, 75) | 69.0 (43, 88) | 68.0 (37, 88) |
| Sex | ||||
| Female | 8 (29.6) | 4 (22.2) | 5 (22.7) | 17 (25.4) |
| Male | 19 (70.4) | 14 (77.8) | 17 (77.3) | 50 (74.6) |
| ECOG PS | ||||
| 0 | 6 (22.2) | 6 (33.3) | 7 (31.8) | 19 (28.4) |
| 1 | 21 (77.8) | 11 (61.1) | 14 (63.6) | 46 (68.7) |
| 2 | 0 | 1 (5.6) | 1(4.5) | 2 (3.0) |
| Racea | ||||
| n | 25 | 15 | 19 | 59 |
| Asian | 1 (4.0) | 0 | 8 (42.1) | 9 (15.3) |
| African American | 1 (4.0) | 1 (6.7) | 0 | 2 (3.4) |
| White | 21 (84.0) | 13 (86.7) | 10 (52.6) | 44 (74.6) |
| Other | 2 (8.0) | 1 (6.7) | 1 (5.3) | 4 (6.8) |
| Ethnicity | ||||
| n | 24 | 14 | 19 | 57 |
| Not Hispanic or Latino | 24 (100) | 14 (100) | 19 (100) | 57 (100) |
| Line of therapy for recurrent disease | ||||
| First line | 8 (29.6) | 0 | 2 (9.1) | 10 (14.9) |
| Second line | 9 (33.3) | 2 (11.1) | 2 (9.1) | 13 (19.4) |
| Third line or greater | 10 (37.0) | 16 (88.9) | 18 (81. 8) | 44 (65.7) |
| Prior stem cell/bone marrow transplant type | ||||
| Autologous | 0/3 | 12/13 (92.3) | 3/3 (100) | 15/19 (78.9) |
| Allogeneic | 3/3 (100) | 2/13 (15.4) | 0/3 | 5/19 (26.3) |
Data are presented as n (%) unless otherwise specified
AML acute myeloid leukemia, DLBCL diffuse large B-cell lymphoma, ECOG PS Eastern Cooperative Oncology Group performance status, max maximum, min minimum, MM multiple myeloma
aEach race category counts subjects who selected only that category
Dose-Limiting Toxicities and Maximum Tolerated Dose
Seven of 54 (13.0%) DLT-evaluable patients experienced a total of nine MEDI7247-related DLTs. DLTs were neutropenia (n = 2; 0.01 mg/kg/day × 3 and 0.12 mg/kg), thrombocytopenia (n = 6; 0.09 mg/kg: n = 1, 0.12 mg/kg: n = 5), and prostatitis (n = 1; 0.09 mg/kg). Four DLTs occurred in the MM cohort, and five DLTs occurred in the DLBCL cohort. Across disease cohorts, the maximum dose of MEDI7247 administered was 0.18 mg/kg (AML). An MTD was not determined because the dose escalation was stopped early.
Safety
The median duration of exposure to MEDI7247 was 2.0 cycles (range 1.0–4.0) for AML; 2.0 cycles (range 1.0–18.0) for MM; and 2.0 cycles (range 1.0–6.0) for DLBCL. Treatment discontinuations are summarized in OSM Table 3 and a summary of AEs is in Table 2. Overall, a total of 29 of 67 patients had dose omissions (median: 1.0; range: 1.0-3.0), 23 (34.3%) of whom had dose omissions due to an AE (Table 2). The most common treatment-emergent AEs (TEAEs) were thrombocytopenia (52.2%), anemia (47.8%), neutropenia (41.8%), fatigue (31.3%), and nausea (22.4%) (OSM Table 4). The most common MEDI7247-related AEs—occurring in ≥ 10% of patients—were thrombocytopenia (41.8%), neutropenia (35.8%), anemia (28.4%), fatigue (14.9%), nausea (13.4%), and febrile neutropenia (10.4%). In patients who experienced blood and lymphatic system AEs, there was one patient with DLBCL that later developed myelodysplastic syndrome.
Table 2.
Summary of adverse events (AEs), as-treated population
| Patients with n (%) | AML (n = 27) | MM (n = 18) | DLBCL (n = 22) | Total (N = 67) |
|---|---|---|---|---|
| At least one AE | 26 (96.3) | 18 (100.0) | 22 (100.0) | 66 (98.5) |
| At least one treatment-related AE | 17 (63.0) | 17 (94.4) | 18 (81.8) | 52 (77.6) |
| At least one grade 3-4 AE | 23 (85.2) | 15 (83.3) | 19 (86.4) | 57 (85.1) |
| At least one grade 3-4 treatment-related AE | 13 (48.1) | 14 (77.8) | 16 (72.7) | 43 (64.2) |
| At least one serious AE | 18 (66.7) | 6 (33.3) | 10 (45.5) | 34 (50.7) |
| Treatment-related death | 1 (3.7) | 0 | 0 | 1 (1.5) |
| At least one AE leading to discontinuation | 6 (22.2) | 9 (50.0) | 8 (36.4) | 23 (34.3) |
| At least one treatment-related AE leading to discontinuation | 3 (11.1) | 7 (38.9) | 6 (27.3) | 16 (23.9) |
| At least one AE leading to dose interruption | 0 | 1 (5.6) | 0 | 1 (1.5) |
| At least one treatment-related AE leading to dose interruption | 0 | 1 (5.6) | 0 | 1 (1.5) |
| At least one AE leading to dose delay | 8 (29.6) | 8 (44.4) | 10 (45.5) | 26 (38.8) |
| At least one treatment-related AE leading to dose delay | 5 (18.5) | 8 (44.4) | 9 (40.9) | 22 (32.8) |
| At least one AE leading to dose omission | 4 (14.8) | 9 (50.0) | 10 (45.5) | 23 (34.3) |
| At least one treatment-related AE leading to dose omission | 3 (11.1) | 9 (50.0) | 10 (45.5) | 22 (32.8) |
AML acute myeloid leukemia, DLBCL diffuse large B-cell lymphoma, MM multiple myeloma
Of the 67 patients in the as-treated population, 16 patients (23.9%) discontinued treatment due to treatment-related AEs (thrombocytopenia, n = 10; neutropenia; febrile neutropenia; pancytopenia; liver function test elevation; platelet count decreased; blister; rash; rash papular; n = 1, each respectively). MEDI7247-related AEs of grade 3/4 severity occurred in 43 of 67 (64.2%) patients (AML, n = 13; MM, n = 14; DLBCL, n = 16). The most commonly reported MEDI7247-related grade 3/4 TEAEs were thrombocytopenia (38.8%), neutropenia (34.3%), anemia (22.4%), and febrile neutropenia (10.4%) (Table 3). SAEs were reported in 34 of 67 (50.7%) patients and included febrile neutropenia (n = 8) and thrombocytopenia (n = 2) (OSM Table 5). During the treatment phase, no patients with febrile neutropenia or thrombocytopenia received hematopoietic growth factors or thrombopoietin receptor agonists and erythropoiesis-stimulating agents, respectively. Pleural effusion occurred in two patients in the AML cohort and malignant pleural effusion occurred in one patient in the MM cohort. There was one MEDI7247-related death that occurred outside of the DLT period in a patient with R/R AML who developed hepatobiliary disease (suspected veno-occlusive disease (VOD)) following two doses of 0.18 mg/kg MEDI7247. The patient had been previously treated with two induction cycles of liposomal daunorubicin and cytarabine. None of the remaining deaths of patients with AML were noted as related to study drug.
Table 3.
MEDI7247-related adverse events of severity grade 3/4 by system organ class and preferred term in ≥ 5% of patients in any group, as-treated population
| System organ class and preferred termb | AML (n = 27) | MM (n = 18) | DLBCL (n = 22) | Total (N = 67) | ||||
|---|---|---|---|---|---|---|---|---|
| All grades | Grades 3/4a | All grades | Grades 3/4a | All grades | Grades 3/4a | All grades | Grades 3/4a | |
| Blood and lymphatic system disorders | ||||||||
| Anemia | 3 (11.1) | 3 (11.1) | 6 (33.3) | 5 (27.8) | 10 (45.5) | 7 (31.8) | 19 (28.4) | 15 (22.4) |
| Febrile neutropenia | 3 (11.1) | 3 (11.1) | 2 (11.1) | 2 (11.1) | 2 (9.1) | 2 (9.1) | 7 (10.4) | 7 (10.4) |
| Leukopenia | 1 (3.7) | 1 (3.7) | 1 (5.6) | 1 (5.6) | 0 | 0 | 2 (3.0) | 2 (3.0) |
| Neutropenia | 2 (7.4) | 2 (7.4) | 11 (61.1) | 11 (61.1) | 11 (50.0) | 10 (45.5) | 24 (35.8) | 23 (34.3) |
| Thrombocytopenia | 5 (18.5) | 5 (18.5) | 11 (61.1) | 9 (50.0) | 12 (54.5) | 12 (54.5) | 28 (41.8) | 26 (38.8) |
| Investigations | ||||||||
| Electrocardiogram QT prolonged | 0 | 0 | 0 | 0 | 2 (9.1) | 1 (4.5) | 2 (3.0) | 1 (1.5) |
| GGT increased | 0 | 0 | 1 (5.6) | 0 | 1 (4.5) | 1 (4.5) | 2 (3.0) | 1 (1.5) |
| Lipase increased | 0 | 0 | 0 | 0 | 1 (4.5) | 1 (4.5) | 1 (1.5) | 1 (1.5) |
| Neutrophil count decreased | 0 | 0 | 1 (5.6) | 1 (5.6) | 3 (13.6) | 3 (13.6) | 4 (6.0) | 4 (6.0) |
| Platelet count decreased | 0 | 0 | 0 | 0 | 2 (9.1) | 1 (4.5) | 2 (3.0) | 1 (1.5) |
| White blood cell count decreased | 0 | 0 | 0 | 0 | 2 (9.1) | 2 (9.1) | 2 (3.0) | 2 (3.0) |
| Reproductive system and breast disorders | ||||||||
| Prostatitis | 0 | 0 | 1 (5.6) | 1 (5.6) | 0 | 0 | 1 (1.5) | 1 (1.5) |
| Respiratory, thoracic, and mediastinal disorders | ||||||||
| Dyspnea | 1 (3.7) | 0 | 2 (11.1) | 1 (5.6) | 0 | 0 | 3 (4.5) | 1 (1.5) |
| Skin and subcutaneous tissue disorders | ||||||||
| Blister | 0 | 0 | 1 (5.6) | 1 (5.6) | 1 (4.5) | 0 | 2 (3.0) | 1 (1.5) |
| Rash | 0 | 0 | 3 (16.7) | 1 (5.6) | 1 (4.5) | 0 | 4 (6.0) | 1 (1.5) |
| Rash maculo-papular | 1 (3.7) | 0 | 2 (11.1) | 0 | 1 (4.5) | 1 (4.5) | 4 (6.0) | 1 (1.5) |
| Rash popular | 0 | 0 | 1 (5.6) | 1 (5.6) | 0 | 0 | 1 (1.5) | 1 (1.5) |
Data are presented as n (%)
AML acute myeloid leukemia, DLBCL diffuse large B-cell lymphoma, GGT gamma-glutamyltransferase, MedDRA Medical Dictionary for Regulatory Activities, MM multiple myeloma
aGrade 3–4 events include grade 3 (Severe) and grade 4 (Life Threatening)
bPatients are counted once for each System Organ Class and Preferred Term (MedDRA v22.1) regardless of the number of events
Efficacy
Median duration of follow-up was 22.8 months for patients with AML, 15.0 months for patients with MM, and 17.0 months for patients with DLBCL. The ORR was 22.2% in the AML cohort; 11.1% in the MM cohort; and 13.6% for the DLBCL cohorts (OSM Tables 6, 7, and 8).
Overall, two patients with MM achieved a PR (DOR of 419 days and time to response (TTR) of 185 days with 0.09 mg/kg; DOR of 1 day and TTR of 24 days with 0.12 mg/kg), in addition to one patient with DLBCL (DOR of 64 days and TTR of 58 days with 0.09 mg/kg). Two patients achieved CRs (both with germinal center B-cell-like DLBCL). One patient with DLBCL achieved a CR following CAR T therapy and one patient with DLBCL achieved CR following stem cell transplantation (DOR of 78 days and TTR of 58 days with 0.03 mg/kg/day × 3; DOR of 26 days and TTR of 68 days with 0.12 mg/kg). In the AML cohort, one patient had a CRi (DOR of nine days and TTR of 15 days with 0.18 mg/kg) and five patients achieved a MLFS, with DOR ranging from 8 to 88 days. The anticancer activity of MEDI7247 across all cohorts (defined as the number of responders/total patients evaluated) was 16.4% (11/67). Treatment response by cohort is summarized in Fig. 2.
Fig. 2.
MEDI7247 treatment response for patients with AML (A), MM (B), and DLBCL (C). For AML, PD includes PD and TF. AML acute myeloid leukemia, CR complete remission, CRi complete response with incomplete hematologic recovery, DLBCL diffuse large B-cell lymphoma, MM multiple myeloma, MLFS morphologic leukemia-free state, MR minimal response, PD progressive disease, SD stable disease, TF treatment failure
Translational Endpoints
ASCT2 expression in bone marrow was relatively high in patients with AML or MM, with IHC H-scores ≥ 100 in 12/12 patients (AML, n = 10; MM, n = 2). In patients with DLBCL (n = 12), IHC H-scores ranged from 2 to 300, with 50% (6/12) patients ≥ 100 (OSM Fig. 2). There was no correlation observed between ASCT2 expression and clinical response (OSM Fig. 2). Mutation profiles were retrospectively obtained for a subset of AML patients (n = 15) where sufficient pretreatment bone marrow aspirates were available after prospective clinical and IHC assessments.
A total of 44 unique somatic variants (20 genes) were identified in 13/15 patients with evaluable NGS data (OSM Table 9). An average of 3.5 mutations were detected in each patient (OSM Fig. 3). Of the 15 patients, 10 (66.6%) carried at least one mutation in one of three genes (TP53, ASXL1, or RUNX1), associated with poor clinical outcomes for patients with AML (OSM Fig. 3)[21]. TP53 and RUNX1 mutations were not detected in either of the two responders, but they were detected in 8/12 (66.7%) of non-responders (OSM Fig. 3). Mutations that typically occur in early leukemogenesis and persist through remission and relapse (ASXL1, RUNX1, SRSF2, TET2, IDH1, and IDH2) were detected in 10/15 (66.7%) patients [22].
Pharmacokinetics
Summary PK parameters of MEDI7247 ADC and total antibody are presented in OSM Table 10. Following a single dose of MEDI7247 in cycle 1, MEDI7247 ADC and total antibody concentrations rapidly declined with a geometric mean T1/2 ranging from 0.56 to 0.70 days and 0.56 to 0.68 days, respectively. Individual T1/2 values ranged from 0.33 to 1.97 days across doses of 0.03–0.18 mg/kg for MEDI7247 ADC, and from 0.31 to 2.11 days across all doses for total antibody. For patients whose apparent terminal elimination phase was well characterized, CL and Vss for MEDI7247 ADC and total antibody were similar across doses of MEDI7247 from 0.03 to 0.18 mg/kg. Between-patient variability of systemic exposures to MEDI7247 ADC and total antibody were high, with a Cmax geometric CV% of 40.9% to 156.7%, and 52.4% to 135.9%, respectively; an AUClast geometric CV% ranging from 66.0% to 175.9%, and 71.7% to 352.5%, respectively; and an AUCinf geometric CV% from 62.3% to 134.2%, and 74.8% to 126.1%, respectively (OSM Table 10).
Plasma concentrations of SG3199 in cycle 1 were below the 20 pg/mL limit of quantification for all patients after Study Day 8. For doses with quantifiable concentrations of SG3199 for > 50% of patients in the dose cohort (ie, 0.09 to 0.18 mg/kg Q3W), the maximum SG3199 concentration was observed at 2 h postdose on Day 1, and the geometric mean was 15.98, 20.28, and 39.10 pg/mL, for the 0.09, 0.12, and 0.18 mg/kg doses, respectively.
Immunogenicity
A summary of ADA responses to MEDI7247 are presented in OSM Table 11. The ADA prevalence to MEDI7247 (ie, the proportion of patients who were evaluable for ADA and were positive for MEDI7247 ADA at any point) was 7.7% (4/52 patients). ADA incidence (ie, the proportion of patients who were evaluable for ADA and were treatment-emergent ADA positive) was 1.9% (1/52 patients). Persistent positive ADA was 5.8% (3/52 patients), one of whom had dose omissions due to treatment-related AEs.
Discussion
This phase 1 dose-escalation study evaluated the safety and efficacy of MEDI7247, a novel ADC targeting ASCT2 with the potential to treat a wide variety of hematological malignancies. In the present study, MEDI7247 treatment led to treatment-related AEs in 52 of 67 (77.6%) patients; cytopenias and anemias were among the most commonly reported DLTs reported in 7 of 54 (13.0%) patients, and there was one treatment-related death in a patient with AML that was clinically consistent with VOD. Notably, VOD has been reported in patients with acute leukemias (e.g., acute lymphocytic leukemia and AML) who were treated with ADCs of varying targets and warheads [23–25]. The development of cytopenias (i.e., thrombocytopenia and neutropenia) limited repeat dosing in patients. Consequently, the dose-escalation phase was stopped early without establishing an MTD for MEDI7247.
The potency of the PBD warhead may have contributed to the bone marrow toxicity observed in this study. It is unclear whether a less toxic warhead could lead to less marrow suppression, while still providing antitumor efficacy. It is unlikely that aggressive myeloid or megakaryocyte growth factor support could have mitigated the cumulative marrow toxicity of MEDI7247 in this study.
Most of the AEs observed in this study were similar to those reported in other published studies of PBD-containing agents, either alone (e.g., SJG-136) or as warheads in ADCs (e.g., rovalpituzumab tesirine) [26–28]. Hepatobiliary toxicity has been associated with ADCs for leukemia, regardless of the target or warhead [29, 30]. Thrombocytopenia is also commonly associated with ADC therapies [26, 31]; however, the underlying mechanism remains unknown. One hypothesis is that a bystander effect may occur via cell death-mediated cytokine release or through the uptake of apoptotic vesicles by healthy, untreated cells [32, 33]. The PBD warhead released after lysosomal degradation binds DNA and triggers apoptosis. Apoptotic bodies may then be phagocytosed by adjacent cells, transferring the toxic PBD to those cells and potentially contributing to observed cytopenias. In AML, MM, and some lymphomas, the bone marrow is heavily infiltrated by tumor cells expressing high levels of the ASCT2 transporter [10], facilitating the transfer of apoptotic bodies to the remaining normal marrow cells within their vicinity.
Clinical activity for MEDI7247 was observed with ORRs ranging from 11.1 to 22.2% across the three cohorts and in heavily pretreated patients at multiple dose levels. It is notable that responses were observed across dose levels, even with lower doses. Two patients with germinal center B-cell-like DLBCL had a CR, one patient with AML had a CRi, and five patients achieved an MLFS. One patient with DLBCL achieved a CR after relapse from prior CAR T therapy, and one patient with DLBCL achieved CR after relapse from prior stem cell transplantation, suggesting some clinical activity in these patient groups. Two patients with MM achieved a PR, in addition to one patient with DLBCL. Overall, the safety and efficacy results of this study should be interpreted with caution as they are based on a small sample size, multiple diseases, and multiple dose levels.
In a subset of patients for whom pre-treatment bone marrow aspirates were available, there was no observed correlation between ASCT2 expression and clinical response. ASCT2 expression levels in bone marrow were consistent with preclinical findings [10, 34]. Mutational profiles acquired via NGS were generally similar to those previously reported for patients with R/R AML [21, 22], with mutations characteristic of poor prognosis and early leukemogenesis detected. In two patients with AML, an independent fragment analysis identified FLT3-ITD, a common driver mutation and a poor prognostic factor in patients with AML [35]. Given the small sample size, results from the mutational analyses should be interpreted with caution.
The PK of MEDI7247 for both the ADC and total antibody was well characterized across doses ranging from 0.06 to 0.18 mg/kg, with linear single-dose kinetics observed. Mean half-life values across these doses were low, with MEDI7247 ADC and total antibody concentrations declining rapidly following administration of a single dose. However, at lower doses of MEDI7247 (i.e, 0.016 and 0.03 mg/kg) there were insufficient samples to facilitate a full PK profile characterization. Due to the low numbers of evaluable patients for ADA, no general conclusions can be made regarding the effect of ADA on safety, efficacy, and PK of MEDI7247.
In conclusion, MEDI7247-related AEs precluded repeat dosing and durability of response. As such, the study was terminated early due to the limited clinical activity and the overall benefit-risk profile observed. Continued development of MEDI7247 for the treatment of R/R hematological malignancies is not supported. However, evaluation of alternative warheads (e.g., less potency, non-cleavable linker) and other strategies to improve the therapeutic index are warranted.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors thank the patients, their families, and study-site personnel for their participation; and they thank Yi Wang (AstraZeneca) for statistical analysis support. This study was funded by AstraZeneca. Medical writing and editorial support, conducted in accordance with Good Publication Practice (GPP3) and the International Committee of Medical Journal Editors (ICMJE) guidelines, were provided by April Suriano, PhD, of Oxford PharmaGenesis Inc., Newtown, PA, and funded by AstraZeneca, Gaithersburg, MD.
Declarations
Funding
AstraZeneca.
Conflict of interest
Michael Maris: None. Gilles Salles: Financial compensation for participating in advisory boards or consulting from: Abbvie, BMS/Celgene, Debiopharm, Epizyme, Genentech/Roche, Incyte, Janssen, Kite/Gilead, Epizyme, Miltenyi, Morphosys, Novartis, VelosBio; and for participation in educational events from: Kite Pharma/Gilead Sciences. Won Seog Kim: Nothing to disclose. Tae Min Kim: Advisory or consultant roles for AstraZeneca, Novartis, Takeda, Sanofi, Roche/Genentech, and Boryung. Research funding from AstraZeneca and Korea Health Industry Development Institute outside this work. Roger Lyons: Consultancy and Advisory boards for ABBVie, Argenx, AstraZeneca, BriGene, Celgene, INCYTE, IQVIA, Karyopharm, Taiho, and Takeda. Martha Arellano: Advisory board member for Kite Pharma, Inc, Gilead Sciences, Inc, and Syndax Pharmaceuticals, Inc. Reem Karmali: Consulting Fees: Celgene Corporation, Gilead Sciences, Inc, Juno Therapeutics, Kite Pharma, OncLive, Verastem, Janssen, Karyopharm, Morphosys; Grants/Research; Support: Celgene Corporation, Juno Therapeutics, BMS, Takeda, BeiGene, AstraZeneca; Speakers Bureau: Celgene Corporation, Gilead Sciences, Inc, Juno Therapeutics, Kite Pharma, AstraZeneca, BeiGene, Morphosys. Gary Schiller: Stock and Other Ownership Interests: Bristol Myers Squibb, Amgen, Johnson & Johnson; Consulting or Advisory Role: Ono Pharmaceutical, Agios, Celgene, Incyte, Jazz Pharmaceuticals, Novartis, AbbVie, Astellas Pharma; Speakers' Bureau: Astellas Pharma, Kite, a Gilead Company, Jazz Pharmaceuticals, Stemline Therapeutics, Bristol Myers Squibb, Sanofi, Karyopharm Therapeutics, Incyte, AbbVie; Research Funding: AbbVie, Actinium Pharmaceuticals, Actuate Therapeutics, Arog, Astellas Pharma, Bristol Myers Squibb/Celgene, Celator, Constellation Pharmaceuticals, Daiichi Sankyo, Deciphera, Delta-Fly Pharma, FORMA Therapeutics, Fujifilm, Gamida Cell, Genentech/Roche, Geron, Incyte, Karyopharm Therapeutics, Kite, a Gilead Company, Mateon Therapeutics, Onconova Therapeutics, Pfizer, Precog, REGiMMUNE, Samus Therapeutics, Sangamo Bioscience, SELLAS Life Sciences, Stemline Therapeutics, Takeda, Tolero Pharmaceuticals, Trovagene, Agios, Amgen, Jazz Pharmaceuticals, ElevateBio, Ono Pharmaceutical, Novartis, Sanofi, AVM Biotechnology, Syros Pharmaceuticals. Elizabeth Cull: Speaker for Bristol Myers Squibb. Camille N. Abboud: Clinical research support from Novartis, Gilead, and Ryvu. Connie Batlevi: Research Funding: Janssen, Novartis, Epizyme, Xynomics, Bayer, Autolus, Roche; Consulting/Advisory Boards: Life Sci, GLG, Juno/Celgene, Seattle Genetics, Kite Pharma, Karyopharm, TG Therapeutics, ADC Therapeutics; Honorarium: Dava Oncology, TouchIME, Medscape; Stock ownership: BMS, Pfizer, Viatris, Regeneron, Moderna, Novavax. Ioannis Kagiampakis: Employee of AstraZeneca and may own stock or stock options. Marlon C. Rebelatto: Employee of AstraZeneca and may own stock or stock options. Young Lee: Employee of AstraZeneca and may own stock or stock options. Lyndon C. Kirby: Employee of AstraZeneca and may own stock or stock options. Fujun Wang: Employee of AstraZeneca and may own stock or stock options. Current equity holder in a publicly traded company. John Bothos: Employee of AstraZeneca and may own stock or stock options. Danielle M Townsley: Employee of AstraZeneca and may own stock or stock options. Amir T. Fathi: Consulting/Advisory Fees: Celgene/BMS, Foghorn, Kite Pharma, Morphosys, Abbvie, Agios, Genentech, Takeda, Pfizer, Trillium, Kura Oncology, Blueprint, Astellas; Grants/Research Support: Celgene/BMS, Abbvie, Agios. Vincent Ribrag: Advisory boards for Gilead, Infinity, MSD, BMS, Nanostring, Incyte, Roche, AstraZeneca, Servier; Research support from Argen-X and Astex.
Availability of data and material
Data underlying the findings described in this article may be obtained in accordance with AstraZeneca’s data-sharing policy, described at: https://astrazenecagrouptrials.pharmacm.com/ST/Submission/Disclosure.
Ethics approval
The study protocol was approved by an institutional review board or independent ethics committee at each study site. The study was conducted in accordance with the principles of the Declaration of Helsinki and the International Conference on Harmonisation/Good Clinical Practice.
Informed Consent
All patients provided written informed consent.
Author contributions
Investigators on the trial: MM, GS, WSK, TMK, RML, MA, RK, GS, EC, CAN, CB, ATF, VR. Members of the Steering Committee: MM. Provided clinical and scientific input to the study design and study protocol: MM, YL (design and protocol for TM exploratory biomarker analyses), MR (design and protocol for PD-L1 IHC assessment). Trial statistician: FW. Interpreted the data: YL, IK, MR, MM. TM contributors: IK, MR, YL. All authors had full access to the data and analyses and vouch for the accuracy and integrity, and for the fidelity of the study to the protocol. All authors were involved in review of each draft of the manuscript and the decision to submit.
Footnotes
Amir T. Fathi and Vincent Ribrag contributed equally to this work.
References
- 1.Scalise M, Pochini L, Console L, Losso MA, Indiveri C. The Human SLC1A5 (ASCT2) amino acid transporter: from function to structure and role in cell biology. Front Cell Dev Biol. 2018;6:96. doi: 10.3389/fcell.2018.00096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Liu Y, Zhao T, Li Z, Wang L, Yuan S, Sun L. The role of ASCT2 in cancer: a review. Eur J Pharmacol. 2018;837:81–87. doi: 10.1016/j.ejphar.2018.07.007. [DOI] [PubMed] [Google Scholar]
- 3.Hassanein M, Hoeksema MD, Shiota M, Qian J, Harris BK, Chen H, et al. SLC1A5 mediates glutamine transport required for lung cancer cell growth and survival. Clin Cancer Res. 2013;19(3):560–570. doi: 10.1158/1078-0432.CCR-12-2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nikkuni O, Kaira K, Toyoda M, Shino M, Sakakura K, Takahashi K, et al. Expression of amino acid transporters (LAT1 and ASCT2) in patients with stage III/IV laryngeal squamous cell carcinoma. Pathol Oncol Res. 2015;21(4):1175–1181. doi: 10.1007/s12253-015-9954-3. [DOI] [PubMed] [Google Scholar]
- 5.Kaira K, Sunose Y, Arakawa K, Sunaga N, Shimizu K, Tominaga H, et al. Clinicopathological significance of ASC amino acid transporter-2 expression in pancreatic ductal carcinoma. Histopathology. 2015;66(2):234–243. doi: 10.1111/his.12464. [DOI] [PubMed] [Google Scholar]
- 6.Wang Q, Hardie RA, Hoy AJ, van Geldermalsen M, Gao D, Fazli L, et al. Targeting ASCT2-mediated glutamine uptake blocks prostate cancer growth and tumour development. J Pathol. 2015;236(3):278–289. doi: 10.1002/path.4518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van Geldermalsen M, Wang Q, Nagarajah R, Marshall AD, Thoeng A, Gao D, et al. ASCT2/SLC1A5 controls glutamine uptake and tumour growth in triple-negative basal-like breast cancer. Oncogene. 2016;35(24):3201–3208. doi: 10.1038/onc.2015.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shimizu K, Kaira K, Tomizawa Y, Sunaga N, Kawashima O, Oriuchi N, et al. ASC amino-acid transporter 2 (ASCT2) as a novel prognostic marker in non-small cell lung cancer. Br J Cancer. 2014;110(8):2030–2039. doi: 10.1038/bjc.2014.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Monks NR, Schifferli KP, Tammali R, Borrok MJ, Coats SR, Herbst R, et al. MEDI7247, a novel pyrrolobenzodiazepine ADC targeting ASCT2 with potent in vivo activity across a spectrum of hematological malignancies. Cancer Res. 2018;78(13_Supplement):LB-295. doi: 10.1158/1538-7445.AM2018-LB-295. [DOI] [Google Scholar]
- 10.Pore N, Schifferli KP, Monks NR, Tammali R, Borrok M, Hurt E, et al. Discovery and development of MEDI7247, a novel pyrrolobenzodiazepine (PBD)-based antibody drug conjugate targeting ASCT2, for treating hematological cancers. Blood. 2018;132(Supplement 1):4071. doi: 10.1182/blood-2018-99-119836. [DOI] [Google Scholar]
- 11.Crump M, Neelapu SS, Farooq U, Van Den Neste E, Kuruvilla J, Westin J, et al. Outcomes in refractory diffuse large B-cell lymphoma: results from the international SCHOLAR-1 study. Blood. 2017;130(16):1800–1808. doi: 10.1182/blood-2017-03-769620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Luppi M, Fabbiano F, Visani G, Martinelli G, Venditti A. Novel agents for acute myeloid leukemia. Cancers (Basel) 2018 doi: 10.3390/cancers10110429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ndaru E, Garibsingh RA, Shi Y, Wallace E, Zakrepine P, Wang J, et al. Novel alanine serine cysteine transporter 2 (ASCT2) inhibitors based on sulfonamide and sulfonic acid ester scaffolds. J Gen Physiol. 2019;151(3):357–368. doi: 10.1085/jgp.201812276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mecklenburg L. A brief introduction to antibody-drug conjugates for toxicologic pathologists. Toxicol Pathol. 2018;46(7):746–752. doi: 10.1177/0192623318803059. [DOI] [PubMed] [Google Scholar]
- 15.Hartley JA, Flynn MJ, Bingham JP, Corbett S, Reinert H, Tiberghien A, et al. Pre-clinical pharmacology and mechanism of action of SG3199, the pyrrolobenzodiazepine (PBD) dimer warhead component of antibody-drug conjugate (ADC) payload tesirine. Sci Rep. 2018;8(1):10479. doi: 10.1038/s41598-018-28533-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nejadmoghaddam MR, Minai-Tehrani A, Ghahremanzadeh R, Mahmoudi M, Dinarvand R, Zarnani AH. Antibody-drug conjugates: possibilities and challenges. Avicenna J Med Biotechnol. 2019;11(1):3–23. [PMC free article] [PubMed] [Google Scholar]
- 17.Ji Y, Liu P, Li Y, Bekele BN. A modified toxicity probability interval method for dose-finding trials. Clin Trials. 2010;7(6):653–663. doi: 10.1177/1740774510382799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dohner H, Estey E, Grimwade D, Amadori S, Appelbaum FR, Buchner T, et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood. 2017;129(4):424–447. doi: 10.1182/blood-2016-08-733196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kumar S, Paiva B, Anderson KC, Durie B, Landgren O, Moreau P, et al. International Myeloma Working Group consensus criteria for response and minimal residual disease assessment in multiple myeloma. Lancet Oncol. 2016;17(8):e328–e346. doi: 10.1016/S1470-2045(16)30206-6. [DOI] [PubMed] [Google Scholar]
- 20.Cheson BD, Fisher RI, Barrington SF, Cavalli F, Schwartz LH, Zucca E, et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J Clin Oncol. 2014;32(27):3059–3068. doi: 10.1200/JCO.2013.54.8800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Papaemmanuil E, Dohner H, Campbell PJ. Genomic classification in acute myeloid leukemia. N Engl J Med. 2016;375(9):900–901. doi: 10.1056/NEJMc1608739. [DOI] [PubMed] [Google Scholar]
- 22.Rothenberg-Thurley M, Amler S, Goerlich D, Kohnke T, Konstandin NP, Schneider S, et al. Persistence of pre-leukemic clones during first remission and risk of relapse in acute myeloid leukemia. Leukemia. 2018;32(7):1598–1608. doi: 10.1038/s41375-018-0034-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yilmaz M, Richard S, Jabbour E. The clinical potential of inotuzumab ozogamicin in relapsed and refractory acute lymphocytic leukemia. Ther Adv Hematol. 2015;6(5):253–261. doi: 10.1177/2040620715596715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wynne J, Wright D, Stock W. Inotuzumab: from preclinical development to success in B-cell acute lymphoblastic leukemia. Blood Adv. 2019;3(1):96–104. doi: 10.1182/bloodadvances.2018026211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yu B, Liu D. Gemtuzumab ozogamicin and novel antibody-drug conjugates in clinical trials for acute myeloid leukemia. Biomark Res. 2019;7:24. doi: 10.1186/s40364-019-0175-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Phillips T, Barr PM, Park SI, Kolibaba K, Caimi PF, Chhabra S, et al. A phase 1 trial of SGN-CD70A in patients with CD70-positive diffuse large B cell lymphoma and mantle cell lymphoma. Invest New Drugs. 2019;37(2):297–306. doi: 10.1007/s10637-018-0655-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Janjigian YY, Lee W, Kris MG, Miller VA, Krug LM, Azzoli CG, et al. A phase I trial of SJG-136 (NSC#694501) in advanced solid tumors. Cancer Chemother Pharmacol. 2010;65(5):833–838. doi: 10.1007/s00280-009-1088-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morgensztern D, Besse B, Greillier L, Santana-Davila R, Ready N, Hann CL, et al. Efficacy and safety of rovalpituzumab tesirine in third-line and beyond patients with DLL3-expressing, relapsed/refractory small-cell lung cancer: results from the phase II TRINITY study. Clin Cancer Res. 2019;25(23):6958–6966. doi: 10.1158/1078-0432.CCR-19-1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McDonald GB, Freston JW, Boyer JL, DeLeve LD. Liver complications following treatment of hematologic malignancy with anti-CD22-calicheamicin (inotuzumab ozogamicin) Hepatology. 2019;69(2):831–844. doi: 10.1002/hep.30222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chau CH, Steeg PS, Figg WD. Antibody-drug conjugates for cancer. Lancet. 2019;394(10200):793–804. doi: 10.1016/S0140-6736(19)31774-X. [DOI] [PubMed] [Google Scholar]
- 31.Zhao H, Gulesserian S, Ganesan SK, Ou J, Morrison K, Zeng Z, et al. Inhibition of megakaryocyte differentiation by antibody-drug conjugates (ADCs) is mediated by macropinocytosis: implications for ADC-induced thrombocytopenia. Mol Cancer Ther. 2017;16(9):1877–1886. doi: 10.1158/1535-7163.MCT-16-0710. [DOI] [PubMed] [Google Scholar]
- 32.Ramesh R, Marrogi AJ, Munshi A, Abboud CN, Freeman SM. In vivo analysis of the 'bystander effect': a cytokine cascade. Exp Hematol. 1996;24(7):829–838. [PubMed] [Google Scholar]
- 33.Freeman SM, Abboud CN, Whartenby KA, Packman CH, Koeplin DS, Moolten FL, et al. The "bystander effect": tumor regression when a fraction of the tumor mass is genetically modified. Cancer Res. 1993;53(21):5274–5283. [PubMed] [Google Scholar]
- 34.Cancer Cell Line Encyclopedia. 2021. https://portals.broadinstitute.org/ccle.
- 35.Lagunas-Rangel FA, Chavez-Valencia V. FLT3-ITD and its current role in acute myeloid leukaemia. Med Oncol. 2017;34(6):114. doi: 10.1007/s12032-017-0970-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



