Abstract
Introduction
Matching-adjusted indirect comparisons (MAIC) were used to assess the relative efficacy of bimekizumab 160 mg every 4 weeks (Q4W) compared to guselkumab 100 mg Q4W or every 8 weeks (Q8W) at 48/52 weeks in patients with psoriatic arthritis (PsA) who were biologic disease-modifying antirheumatic drug-naïve (bDMARD-naïve) or with previous inadequate response or intolerance to tumor necrosis factor inhibitors (TNFi-IR).
Methods
Relevant trials were identified as part of a systematic literature review. For patients who were bDMARD-naïve, individual patient data (IPD) from BE OPTIMAL (N = 431) was matched to summary data from DISCOVER-2 (Q4W, n = 245; Q8W, n = 248). For patients who were TNFi-IR, IPD from BE COMPLETE (n = 267) and summary data from COSMOS (Q8W, N = 189). Trial populations were re-weighted using propensity scores. Unanchored comparisons of recalculated bimekizumab and guselkumab 48- or 52-week non-responder imputation outcomes for 20/50/70% improvement in American College of Rheumatology score (ACR20/50/70) and minimal disease activity (MDA) index were analyzed.
Results
In patients who were bDMARD-naïve, bimekizumab was associated with a greater likelihood of ACR50 (odds ratio [95% confidence interval] 1.62 [1.07, 2.44]; p = 0.021), ACR70 (2.20 [1.43, 3.38]; p < 0.001), and MDA (1.82 [1.20, 2.76]; p = 0.005) compared to guselkumab Q4W at week 52. Bimekizumab also had a greater likelihood of ACR70 response (2.08 [1.34, 3.22]; p = 0.001) and MDA (2.07 [1.35, 3.17]; p < 0.001) compared to guselkumab Q8W at week 52. In patients who were TNFi-IR, bimekizumab had a greater likelihood in achieving all evaluated outcomes compared to guselkumab Q8W at week 48/52 (ACR20, 1.77 [1.15, 2.72]; p = 0.010; ACR50, 1.56 [1.03, 2.36]; p = 0.037; ACR70, 1.66 [1.05, 2.61]; p = 0.028; and MDA, 1.95 [1.27, 3.02]; p = 0.003).
Conclusions
According to MAICs, bimekizumab demonstrated greater or comparable efficacy on ACR50/70 and MDA outcomes than guselkumab in patients with PsA who were bDMARD-naïve and TNFi-IR at week 48/52. Bimekizumab had a more favorable likelihood than guselkumab in achieving more stringent treatment outcomes.
Trial Registrations
NCT03895203, NCT03896581, NCT04009499, NCT03158285, NCT03796858.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40744-024-00659-0.
Keywords: ACR, Bimekizumab, Biologics, Guselkumab, IL-17, IL-23, MAIC, MDA, Psoriatic arthritis
Key Summary Points
| Why carry out this study? |
| There is currently no direct head-to-head evidence of the long-term efficacy of bimekizumab compared to interleukin (IL)-23 inhibitors in psoriatic arthritis (PsA). |
| This study uses matching-adjusted indirect comparisons (MAICs) to compare the efficacy of bimekizumab 160 mg every 4 weeks (Q4W) and guselkumab 100 mg every 4 or 8 weeks (Q4W or Q8W) at 52 weeks for the treatment of PsA in patients who were naïve to biologic disease-modifying antirheumatic drugs (bDMARD-naïve) or patients with previous inadequate response or intolerance to tumor necrosis factor inhibitors (TNFi-IR). |
| What was learned from this study? |
| In patients who were bDMARD-naïve, bimekizumab had a greater likelihood of achieving at least a 70% improvement according to American College of Rheumatology response criteria (ACR70) and minimal disease activity (MDA) outcomes than guselkumab Q4W, and a greater likelihood of achieving ACR50, ACR70, and MDA outcomes than guselkumab Q8W at week 52. |
| In patients who were TNFi-IR, bimekizumab had a greater likelihood of achieving all ACR and MDA outcomes compared to guselkumab Q8W at 52 weeks. |
| Bimekizumab had a more favorable likelihood than guselkumab in achieving more stringent and long-term treatment outcomes in PsA in patients who were both bDMARD-naïve and TNFi-IR. |
Introduction
Approximately 30% of patients with psoriasis develop psoriatic arthritis (PsA), a chronic and systemic disease characterized by inflammation across a range of tissue domains, particularly cutaneous and musculoskeletal [1]. According to the European League Against Rheumatism and the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA), biologic or targeted synthetic disease-modifying antirheumatic drugs (b/tsDMARDs), such as interleukin (IL)-17 or IL-23 inhibitors, are recommended for the treatment of PsA [2, 3]. With an increasing number of targeted therapies becoming available in PsA, clinicians face treatment decisions concerning the variety of modes of action available to them.
Recently, the efficacy and safety of bimekizumab, a humanized monoclonal IgG1 antibody that selectively inhibits IL-17F in addition to IL-17A, were established in two phase 3 randomized controlled trials (RCTs): BE OPTIMAL (NCT03895203) [4] in patients who were naïve to biologic disease-modifying antirheumatic drugs (bDMARD-naïve) and BE COMPLETE (NCT03896581) [5] in patients with previous inadequate response or intolerance to tumor necrosis factor inhibitors (TNFi-IR). An open-label extension (OLE) of both trials, BE VITAL (NCT04009499) [6], is currently underway. Guselkumab is a selective IL-23 inhibitor that has demonstrated efficacy and safety in the treatment of patients with active PsA in the DISCOVER-2 (NCT03158285) [7] and COSMOS (NCT03796858) [8] RCTs. Given their potentially shared broader cytokine inhibitory profile over IL-17A monospecific inhibitors, there is particular interest in evaluating the relative efficacy of IL-17A/F compared with IL-23 inhibition [9].
Head-to-head studies of available treatments for PsA are sparse, and no head-to-head trials have been conducted between bimekizumab and guselkumab. Without head-to-head data, it has become standard to evaluate the relative effectiveness of several treatments by conducting a network meta-analysis (NMA) of placebo-controlled trials. Although the comparative efficacy of bimekizumab with other b/tsDMARDs over a shorter treatment period of up to 24 weeks has been established in a recently published NMA [10], there are no analyses for long-term comparative efficacy because of the lack of a placebo common comparator after 24 weeks [11, 12].
In this study, MAICs were conducted to assess the relative efficacy of bimekizumab vs guselkumab at 52 weeks in patients with PsA who were bDMARD-naïve and TNFi-IR. This MAIC analysis aims to provide additional long-term comparative data of bimekizumab and guselkumab following the findings of a recently completed NMA up to week 24 [10].
Methods
Systematic Literature Review and Source Data
A systematic literature review (SLR) was conducted according to the Preferred Reporting Items for Systematic Reviews (PRISMA) guidelines [13] to identify relevant clinical evidence for existing bDMARD therapies in PsA published from January 1991 to December 2022 and was used as the basis for this MAIC analysis. Details on SLR eligibility criteria and reasons for inclusion/exclusion were previously published [10]. From this SLR, all available IL-23 inhibitors in PsA were selected as potential comparators. Guselkumab was chosen as the comparator for this analysis owing to its longer period of availability in the PsA treatment market in Europe [14]. The DISCOVER-2 (for patients who were bDMARD-naïve) and COSMOS (for patients who were TNFi-IR) RCTs were identified as most relevant for this MAIC analysis owing to their alignment with the target patient populations in the BE OPTIMAL and BE COMPLETE trials, as well as alignment with the European Medicines Agency guidance on dosing for guselkumab [14]. In this analysis, the efficacy of bimekizumab dosed at 160 mg every 4 weeks (Q4W) was compared to guselkumab dosed at 100 mg every 4 or 8 weeks (Q4 or 8W) in patients who were bDMARD-naïve and 100 mg Q8W in patients who were TNFi-IR. Patients at higher risk of joint damage, according to clinical judgment, received the Q4W dose [14]. BE OPTIMAL and BE COMPLETE were conducted in accordance with the Declaration of Helsinki and the International Conference on Harmonisation Guidance for Good Clinical Practice. Ethical approval was obtained from the relevant institutional review boards at participating sites, and all patients provided written informed consent in accordance with local requirements.
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Selection of Baseline Characteristics for Matching
Adjustment variables were selected on the basis of a review of previous MAICs in PsA [15, 16], consensus agreement with clinical experts (n = 5), and adherence to established MAIC guidelines [17]. Exploratory univariate sensitivity analyses evaluated the impact of all adjustment variables. To adjust for cross-trial differences, patients using bimekizumab in the BE OPTIMAL and BE COMPLETE trials were re-weighted to match the baseline characteristics of the patients using guselkumab in the DISCOVER-2 and COSMOS trials. Weights were determined on the basis of age, sex, methotrexate (MTX) use, Health Assessment Questionnaire Disability Index (HAQ-DI) score, percentage with psoriasis affecting at least 3% body surface area (BSA ≥ 3%), swollen joint count–68 joints (SJC 68), tender joint count–66 joints (TJC 66), and disease duration. Adjustments for race, weight, and DMARD use at baseline were excluded as they were well balanced across trials and their adjustment impact was minimal. Adjustments for dactylitis and enthesitis at baseline were excluded as the impact of the effective sample size (ESS) was assessed to be too large, leading to an unbalanced distribution of weights.
Adjustment of IPD to Aggregate Data and Pairwise Comparisons
The MAIC methodology described by Signorovitch et al. [18] was followed and analyses were conducted in accordance with the National Institute for Health and Care Excellence Decision Support Unit Technical Support Document 18 (NICE DSU TSD 18) to create a robust population-adjusted indirect treatment comparison (ITC) [17]. Inverse propensity score weighting was used to form weighted mean estimators of the expected mean outcomes for bimekizumab in guselkumab trial populations, where the propensity scores are found using a method of moments [18]. All analyses were conducted with R version 3.6.2. The R program from the NICE DSU TSD 18 was used to implement this MAIC.
For patients who were bDMARD-naïve, IPD from the bimekizumab arm of BE OPTIMAL were matched to summary data from DISCOVER-2. For patients who were TNFi-IR, IPD from the bimekizumab arm of BE COMPLETE and BE VITAL were matched to summary data from COSMOS (Fig. 1).
Fig. 1.
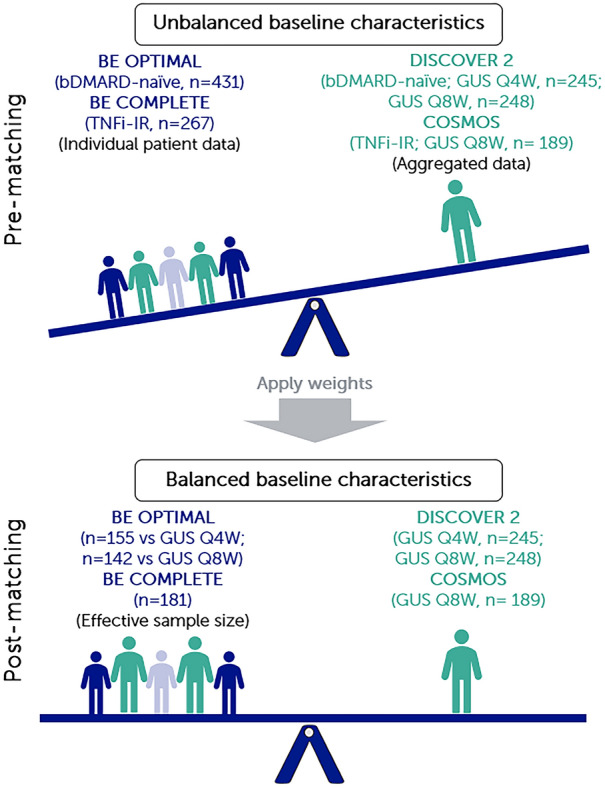
Summary of MAIC matching. Note: MAICs use IPD from trials of one treatment to match baseline aggregate statistics reported from trials of another treatment. Use of propensity score weighting techniques to balance trial population characteristics allows indirect comparisons to be made. Trial populations adjusted for age, sex, disease duration, MTX use, HAQ-DI, BSA ≥ 3%, SJC, and TJC. bDMARD biologic disease-modifying antirheumatic drug, BSA body surface area, GUS guselkumab, HAQ-DI Health Assessment Questionnaire Disability Index, IPD individual patient data, MAIC matching-adjusted indirect comparison, MTX methotrexate, Q4W every 4 weeks, Q8W every 8 weeks, SJC swollen joint count, TJC tender joint count, TNFi-IR tumor necrosis factor inhibitor-inadequate response or intolerant
Outcomes
The outcomes reported were the proportion of patients with 20/50/70% improvement in the American College of Rheumatology criteria (ACR20/50/70) and minimal disease activity (MDA, minimum 5 out of 7 domains achieved) scores. These were selected in line with the Outcomes Measures in Rheumatology (OMERACT) [19] and the Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA) [3] guidelines. For this MAIC analysis, the closest available time points at the time of the analysis in the guselkumab trials (week 52 for DISCOVER-2 and week 48 for COSMOS) were used for the comparison with week 52 data from the bimekizumab trials.
Analyses of Psoriasis Area and Severity Index (PASI) scores, enthesitis resolution, dactylitis resolution, and inhibition of radiographic progression outcomes were not feasible as the baseline characteristics of the respective patient subsets were not sufficiently reported in their respective RCTs.
Reporting of Missing Data
Published outcomes were taken from the intent-to-treat population in all relevant trials (DISCOVER-2, COSMOS, BE OPTIMAL, BE COMPLETE, and BE VITAL). Missing binary outcome data (ACR20/50/70 and MDA) were handled using non-responder imputation (NRI) methods.
Non-Placebo-Adjusted Outcome Comparisons
All patients randomized to placebo in the bimekizumab and guselkumab RCTs were allowed to receive active treatment from week 16 onwards, resulting in the absence of placebo as a common comparator in all RCTs after week 16. Non-placebo-adjusted (unanchored) outcomes at week 52 from DISCOVER-2 and week 48 from COSMOS (no data at 52 weeks for COSMOS) were directly compared with recalculated outcomes from the bimekizumab-randomized arms in BE OPTIMAL and BE COMPLETE/BE VITAL.
Reporting of Results
After matching, the ESS indicates the number of independent, non-weighted individuals that would be required to give an estimate with the same precision as the weighted sample estimate and is expressed as a proportion of the original sample size (OSS) from the source trials. Recalculated outcomes were reported as adjusted response rates and the relative effects of bimekizumab vs guselkumab in different patient groups were reported as odds ratios (ORs) with 95% confidence intervals (95% CI, based on ESS). A standard value of p ≤ 0.05 was considered as the threshold for concluding statistical significance (i.e., greater/lesser likelihood or comparable at achieving an outcome response compared to guselkumab).
Results
Patient baseline values for adjusted characteristics prior to matching are provided in Table 1 for both bDMARD-naïve and TNFi-IR patient subgroups in the bimekizumab and guselkumab RCTs. Post-matching adjustment baseline values for bimekizumab-treated patients are provided in Table S1. Prior to matching, patients in the BE OPTIMAL and BE COMPLETE/BE VITAL trials had longer disease duration, lower proportion of patients with psoriasis covering BSA ≥ 3%, lower proportion of patients who were receiving MTX therapy, lower HAQ-DI scores, and lower SJC/TJC scores compared to patients in the corresponding subgroups in DISCOVER-2 and COSMOS.
Table 1.
Baseline characteristics of patients from bimekizumab (BE OPTIMAL/BE COMPLETE/BE VITAL) and guselkumab (DISCOVER-2/COSMOS) trials before matching
| bDMARD-naïve | TNFi-IR | ||||
|---|---|---|---|---|---|
| Mean (SD) unless stated | BE OPTIMAL | DISCOVER-2 (Q4W) | DISCOVER-2 (Q8W) | BE COMPLETE/BE VITAL | COSMOS |
| N = 431 | N = 245 | N = 248 | N = 267 | N = 189 | |
| Age, years | 49 (13) | 46 (12) | 45 (12) | 50 (12) | 49 (12) |
| Male, % | 47 | 58 | 52 | 49 | 46 |
| Time since diagnosis, years | 6.0 (7.3) | 5.5 (5.9) | 5.1 (5.5) | 9.6 (9.9) | 8.3 (7.8) |
| MTX use, % | 59 | 69 | 69 | 45 | 56 |
| SJC (of 66 joints) | 9.0 (6.2) | 12.9 (7.8) | 11.7 (6.8) | 9.7 (7.5) | 10.0 (7.0) |
| TJC (of 68 joints) | 16.8 (11.8) | 22.4 (13.5) | 19.8 (11.9) | 18.4 (13.5) | 21.0 (13.0) |
| HAQ-DI score | 0.82 (0.59) | 1.2 (0.6) | 1.3 (0.6) | 0.97 (0.59) | 1.3 (0.6) |
| BSA ≥ 3%, % | 50 | 75 | 71 | 66 | 70 |
bDMARD biologic disease-modifying antirheumatic drug, BSA body surface area, HAQ-DI Health Assessment Questionnaire Disability Index, MTX methotrexate, Q4W every 4 weeks, Q8W every 8 weeks, SD standard deviation, SJC swollen joint count, TJC tender joint count, TNFi-IR tumor necrosis factor inhibitor-inadequate response or intolerant
bDMARD-Naïve Patient Subgroup
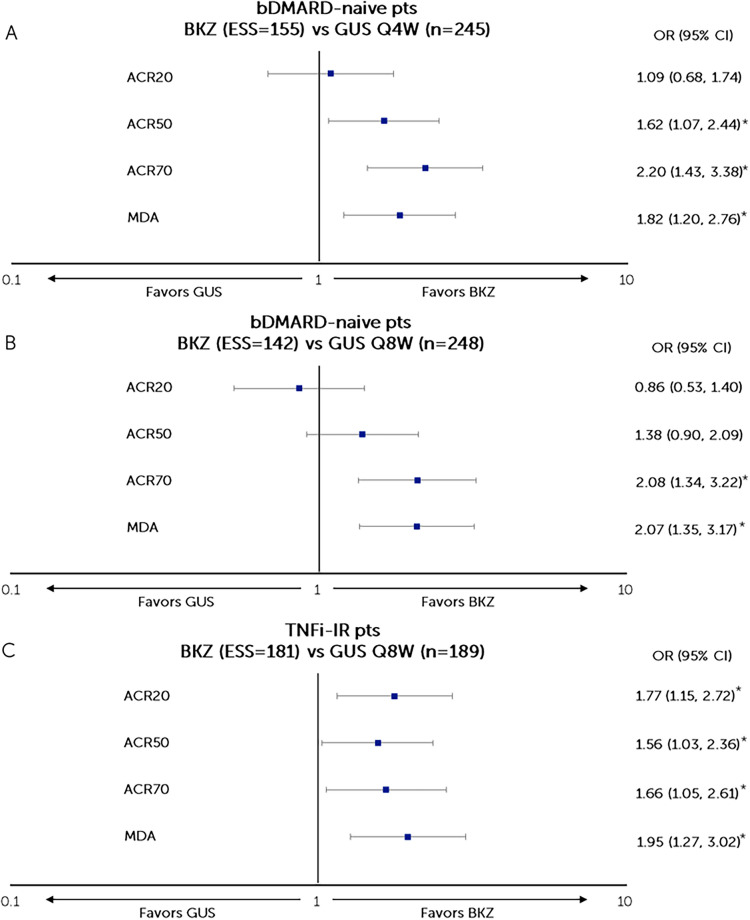
Patients who were bDMARD-naïve from BE OPTIMAL (bimekizumab, n = 431) were matched to patients from DISCOVER-2 (guselkumab Q4W, n = 245; guselkumab Q8W, n = 248). The post-matching ESSs for bimekizumab were 155.08 (36.0% of OSS) and 142.04 (33.0% of OSS) and for comparisons to guselkumab Q4W and Q8W, respectively (Fig. 2a, b and Table S2).
Fig. 2.
Matching-adjusted odds ratio comparison of bimekizumab vs guselkumab (Q4W and Q8W) at week 52 (NRI). a BKZ 160 mg Q4W vs GUS 100 mg Q4W in bDMARD-naïve patients with PsA, b BKZ 160 mg Q4W vs GUS 100 mg Q8W in bDMARD-naïve patients with PsA, c BKZ 160 mg Q4W vs GUS 100 mg Q8W in TNFi-IR patients with PsA. *Indicates statistical significance. Figure shows a logarithmic scale. ACR American College of Rheumatology, ACR20/50/70 at least a 20/50/70% improvement according to the ACR response criteria, bDMARD biologic disease-modifying antirheumatic drugs, BKZ bimekizumab, CI confidence interval, ESS effective sample size, GUS guselkumab, NRI non-responder imputation, OR odds ratio, pts, patients, Q4W every 4 weeks, Q8W every 8 weeks, TNFi-IR tumor necrosis factor inhibitor-inadequate response or intolerant
Compared to guselkumab Q4W, bimekizumab had a greater likelihood of achieving ACR50 (OR [95% CI] 1.62 [1.07, 2.44]; p = 0.021]), ACR70 (2.20 [1.43, 3.38]; p < 0.001), and MDA (1.82 [1.20, 2.76]; p = 0.005) responses and was comparable in achieving ACR20 (1.09 [0.68, 1.74]; p = 0.734) response at week 52 (Fig. 2a). Compared to guselkumab Q8W, bimekizumab had a greater likelihood of achieving ACR70 (2.08 [1.34, 3.22]; p = 0.001) and MDA (2.07 [1.35, 3.17]; p < 0.001) responses and was comparable in achieving ACR20 (0.86 [0.53, 1.40]; p = 0.548) and ACR50 (1.38 [0.90, 2.09]; p = 0.135) responses at week 52 (Fig. 2b).
TNFi-IR Patient Subgroup
Patients who were TNFi-IR from BE COMPLETE/BE VITAL (bimekizumab, n = 267) were matched to patients from COSMOS (guselkumab Q8W, n = 189). The post-matching ESS for bimekizumab was 180.84 (67.7% of OSS) (Fig. 2c and Table S3).
Compared to guselkumab Q8W, bimekizumab had a greater likelihood of achieving ACR20 (1.77 [1.15, 2.72]; p = 0.010), ACR50 (1.56 [1.03, 2.36]; p = 0.037), ACR70 (1.66 [1.05, 2.61]; p = 0.028), and MDA (1.95 [1.27, 3.02]; p = 0.003) responses at week 52 (Fig. 2c).
Unadjusted response rates and ORs for both treatments are available in Tables S2 and S3 in the supplementary information. The adjusted ORs were similar to unadjusted ORs for all outcomes, providing further support for the validity of these findings.
Discussion
This study used a MAIC analysis to assess the comparative efficacy of bimekizumab 160 mg Q4W against guselkumab 100 mg Q4W or Q8W at 52 weeks. Patients treated with bimekizumab who were bDMARD-naïve had a greater likelihood of achieving ACR70 and MDA responses compared to those treated with guselkumab Q8W/Q4W, and patients who were TNFi-IR had a greater likelihood of achieving all ACR and MDA responses than those receiving guselkumab Q8W. For more stringent composite outcomes such as MDA, bimekizumab maintained better efficacy compared to guselkumab.
These findings are consistent with a recently published NMA in which bimekizumab ranked higher in efficacy than guselkumab on most joint outcomes at 16 to 24 weeks [10]. Although both IL-17 and IL-23 pathways are distinctly implicated in the pathogenesis of PsA, IL-23 is involved in the upstream regulation of IL-17A production in the psoriatic inflammatory cascade. IL-17-specific treatments (such as bimekizumab) that directly target the IL-17 pathway further down this cascade may provide more disease specificity and a rapid onset of action than those that target the IL-23 pathway [20]. The results of this study suggest that inhibition of IL-17A in addition to IL-17F may provide better long-term efficacy outcomes for skin and joint manifestations compared to IL-23 mono-inhibition for the treatment of PsA; however, treatment decisions will still be driven by clinicians on a case-by-case basis.
Study Limitations
This MAIC analysis has limitations, both intrinsic to the methodology and specific to this analysis. This MAIC analysis required the use of TNFi-IR patient-level data from the BE VITAL OLE trial. The efficacy analyses used for BE VITAL were conducted by NRI using the total patient population that started BE COMPLETE, thereby reducing uncertainties introduced from using OLE data. All patients completing week 16 in BE COMPLETE were eligible to enroll in BE VITAL and patients receiving placebo were switched to bimekizumab. Although observed patient variables at baseline could be matched, it was not possible to control unobserved or unreported variables. The low recalculated ESS (33–36%) for the analyses in patients who were bDMARD-naïve is an indicator of a limited overlap in patient characteristics between the bimekizumab and guselkumab trials; however, this was not expected to have a significant impact on the interpretation of the results. For this analysis, 48-week data from the COSMOS trial [8] were used in the absence of 52-week data; however, this was not expected to substantially impact the results as response rates were observed to be stable from week 28 onwards for bimekizumab (ACR50 NRI response rate 50.6% at week 40 vs 51.7% at week 52). There were also differences in the duration of the placebo-controlled segment between RCTs (16 weeks for BE OPTIMAL/BE COMPLETE, and to 24 weeks for DISCOVER-2/COSMOS). There was variation in the study designs (active treatment blind [BE OPTIMAL/DISCOVER-2/COSMOS] vs open-label [BE COMPLETE/BE VITAL]) at week 52, although none of the studies were placebo-controlled at week 48/52, hence all patients included in this MAIC were aware that they were receiving active treatment. Analyses of PASI scores, enthesitis resolution, dactylitis resolution, and inhibition of radiographic progression were not feasible as outcomes assessed in the RCTs were only based on a subset of the trial population for which the baseline characteristics were insufficiently reported. Safety outcomes could not be analyzed as the original guselkumab trials (DISCOVER-2 and COSMOS) did not provide safety data stratified by subgroups of interest (bDMARD-naïve or TNFi-IR).
Conclusion
According to established MAIC methods, bimekizumab demonstrated a higher likelihood of achieving stringent clinical efficacy outcomes than guselkumab Q4W and Q8W in patients with PsA who were bDMARD-naïve (for ACR50, ACR70, and MDA) and guselkumab Q8W (for all ACR and MDA) in patients who were TNFi-IR. The results of this analysis should be viewed in the context of the limitations for an indirect comparison, yet the use of IPD and established MAIC methodology provides comparative evidence in the absence of a confirmatory head-to-head RCT.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors thank the clinical investigators who provided advice on the design and implementation of this study.
Medical Writing, Editorial, and Other Assistance.
The authors would like to acknowledge Heather Edens, PhD, UCB Pharma, Smyrna, GA, USA, and Costello Medical, UK for publication coordination and editorial assistance and Darryl Low, PhD, Cytel Inc, UK for medical writing and editorial assistance based on the authors’ input and direction. Support for third-party writing assistance for the article was funded by UCB Pharma.
Author Contributions
Substantial contributions to study conception and design: Damon Willems, Vanessa Taieb, Jason Eells; substantial contributions to analysis and interpretation of the data: Damon Willems, Nikos Lyris, Vanessa Taieb, Jason Eells; drafting the article or revising it critically for important intellectual content: Richard B. Warren, Iain B. McInnes, Peter Nash, Jean-Marie Grouin, Damon Willems, Nikos Lyris, Vanessa Taieb, Jason Eells, Philip J. Mease; final approval of the version of the article to be published: Richard B. Warren, Iain B. McInnes, Peter Nash, Jean-Marie Grouin, Damon Willems, Nikos Lyris, Vanessa Taieb, Jason Eells, Philip J. Mease.
Funding
This study and its publication, including the Journal’s Rapid Service Fee, was sponsored by UCB Pharma. This article was based on the original studies BE OPTIMAL (NCT03895203), BE COMPLETE (NCT03896581), and BE VITAL (NCT04009499) sponsored by UCB Pharma. Support for third-party writing assistance for this article was funded by UCB Pharma and provided by Darryl Low, PhD, Cytel Inc, UK, in accordance with ISMPP Good Publication Practice (GPP 2022) guidelines [21].
Data Availability
Data from the bimekizumab clinical trials used in this analysis may be requested by qualified researchers 6 months after product approval in the USA and/or Europe, or global development is discontinued, and 18 months after trial completion. Investigators may request access to anonymized individual patient data and redacted study documents which may include: raw datasets, analysis-ready datasets, study protocol, blank case report form, annotated case report form, statistical analysis plan, dataset specifications, and clinical study report. Prior to the use of the data, proposals need to be approved by an independent review panel at www.Vivli.org and a signed data-sharing agreement will need to be executed. All documents are available in English only, for a pre-specified time, typically 12 months, on a password-protected portal.
Declarations
Conflict of Interest
Richard B. Warren: Supported by the Manchester NIHR Biomedical Research Centre; consulting fees from AbbVie, Almirall, Amgen, Arena, Astellas, Avillion, Biogen, BMS, Boehringer Ingelheim, Celgene, Eli Lilly, GSK, Janssen, LEO Pharma, Novartis, Pfizer, Sanofi, and UCB Pharma; research grants to his institution from AbbVie, Almirall, Janssen, LEO Pharma, Novartis, and UCB Pharma; and honoraria from Astellas, DiCE, GSK, and Union. Iain B. McInnes: Consulting fees and honoraria from AbbVie, AstraZeneca, BMS, Boehringer Ingelheim, Cabaletta, Causeway Therapeutics, Celgene, Evelo, Janssen, Novartis, Lilly, Moonlake, and UCB Pharma; and research support from BMS, Boehringer Ingelheim, Celgene, Janssen, Novartis, and UCB Pharma. Peter Nash: Research grants, clinical trials and honoraria for advice and lectures on behalf of AbbVie, Boehringer Ingelheim, BMS, Eli Lilly, Galapagos/Gilead, GSK, Janssen, Novartis, Pfizer, Samsung, Sanofi, and UCB Pharma. Jean-Marie Grouin: Consulting fees and honoraria from Acticor, Chugai, BeiGene, Inflectis, Inotrem, Ipsen, Janssen, Otsuka, SpikImm, UCB Pharma. Nikos Lyris, Damon Willems, Jason Eells, Vanessa Taieb: Employee and stockholder of UCB Pharma. Philip J. Mease: Research grants from AbbVie, Acelyrin, Amgen, BMS, Eli Lilly, Gilead, Janssen, Novartis, Pfizer, Sun Pharma and UCB Pharma; consultancy fees from AbbVie, Acelyrin, Aclaris, Alumis, Amgen, BMS, Boehringer Ingelheim, Eli Lilly, Galapagos, Gilead, GSK, Janssen, Moonlake Pharma, Novartis, Pfizer, Sun Pharma, Takeda, and UCB, and Ventyx Pharma; speakers’ bureau from AbbVie, Amgen, Eli Lilly, Janssen, Novartis, Pfizer, and UCB Pharma.
Ethical Approval
BE OPTIMAL and BE COMPLETE were conducted in accordance with the Declaration of Helsinki and the International Conference on Harmonisation Guidance for Good Clinical Practice. Ethical approval was obtained from the relevant institutional review boards at participating sites, and all patients provided written informed consent in accordance with local requirements. This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors. All the results presented in this article are in aggregate form, and no personally identifiable information was used for this study.
Footnotes
Prior Presentation: The data in this manuscript was previously shared at the 32nd European Academy of Dermatology and Venereology Congress 2023, Berlin, Germany, 11–14 October 2023 (Poster No. P0757).
References
- 1.Mease PJ, Gladman DD, Papp KA, et al. Prevalence of rheumatologist-diagnosed psoriatic arthritis in patients with psoriasis in European/North American dermatology clinics. J Am Acad Dermatol. 2013;69(5):729–735. doi: 10.1016/j.jaad.2013.07.023. [DOI] [PubMed] [Google Scholar]
- 2.Gossec L, Baraliakos X, Kerschbaumer A, et al. EULAR recommendations for the management of psoriatic arthritis with pharmacological therapies: 2019 update. Ann Rheum Dis. 2020;79(6):700–712. doi: 10.1136/annrheumdis-2020-217159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Coates LC, Soriano ER, Corp N, et al. Group for Research and Assessment of Psoriasis and Psoriatic Arthritis (GRAPPA): updated treatment recommendations for psoriatic arthritis 2021. Nat Rev Rheumatol. 2022;18(8):465–479. doi: 10.1038/s41584-022-00798-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ritchlin CT, Coates LC, McInnes IB, et al. Bimekizumab treatment in biologic DMARD-naive patients with active psoriatic arthritis: 52-week efficacy and safety results from the phase III, randomised, placebo-controlled, active reference BE OPTIMAL study. Ann Rheum Dis. 2023 doi: 10.1136/ard-2023-224431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Merola JF, Landewe R, McInnes IB, et al. Bimekizumab in patients with active psoriatic arthritis and previous inadequate response or intolerance to tumour necrosis factor-alpha inhibitors: a randomised, double-blind, placebo-controlled, phase 3 trial (BE COMPLETE) Lancet. 2023;401(10370):38–48. doi: 10.1016/S0140-6736(22)02303-0. [DOI] [PubMed] [Google Scholar]
- 6.Coates LC, Landewe R, McInnes I, Mease P, Ritchlin C, Tanaka Y. Sustained efficacy and safety of bimekizumab in patients with active psoriatic arthritis and prior inadequate response to tumour necrosis factor inhibitors: results from the phase 3 BE COMPLETE study and its open-label extension up to 1 year EULAR. Lancet. 2023 doi: 10.1016/S0140-6736(22)02303-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McInnes IB, Rahman P, Gottlieb AB, et al. Efficacy and safety of guselkumab, an interleukin-23p19-specific monoclonal antibody, through one year in biologic-naive patients with psoriatic arthritis. Arthritis Rheumatol. 2021;73(4):604–616. doi: 10.1002/art.41553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coates LC, Gossec L, Theander E, et al. Efficacy and safety of guselkumab in patients with active psoriatic arthritis who are inadequate responders to tumour necrosis factor inhibitors: results through one year of a phase IIIb, randomised, controlled study (COSMOS) Ann Rheum Dis. 2022;81(3):359–369. doi: 10.1136/annrheumdis-2021-220991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vecellio M, Hake VX, Davidson C, Carena MC, Wordsworth BP, Selmi C. The IL-17/IL-23 axis and its genetic contribution to psoriatic arthritis. Front Immunol. 2020;11:596086. doi: 10.3389/fimmu.2020.596086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mease PJ, Gladman DD, Merola JF, et al. Comparative effectiveness of bimekizumab in patients with psoriatic arthritis: results from a systematic literature review and network meta-analysis. Rheumatology. 2024 doi: 10.1093/rheumatology/kead705. [DOI] [PubMed] [Google Scholar]
- 11.Deodhar A. Mirror, mirror, on the wall, which is the most effective biologic of all? J Rheumatol. 2018;45(4):449–450. doi: 10.3899/jrheum.171279. [DOI] [PubMed] [Google Scholar]
- 12.Dias S, Sutton AJ, Ades AE, Welton NJ. Evidence synthesis for decision making 2: a generalized linear modeling framework for pairwise and network meta-analysis of randomized controlled trials. Med Decis Mak. 2013;33(5):607–617. doi: 10.1177/0272989X12458724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.EMA. Guselkumab - Summary of Product Characteristics: EMA; 2022. https://www.ema.europa.eu/en/documents/product-information/tremfya-epar-product-information_en.pdf. Accessed 15 Oct 2023.
- 15.Nash P, McInnes IB, Mease PJ, et al. Secukinumab versus adalimumab for psoriatic arthritis: comparative effectiveness up to 48 weeks using a matching-adjusted indirect comparison. Rheumatol Ther. 2018;5(1):99–122. doi: 10.1007/s40744-018-0106-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Strand V, McInnes I, Mease P, et al. Matching-adjusted indirect comparison: secukinumab versus infliximab in biologic-naive patients with psoriatic arthritis. J Comp Eff Res. 2019;8(7):497–510. doi: 10.2217/cer-2018-0141. [DOI] [PubMed] [Google Scholar]
- 17.Phillippo DM, Ades AE, Dias S, Palmer S, Abrams KR, Welton NJ. Methods for population-adjusted indirect comparisons in health technology appraisal. Med Decis Mak. 2018;38(2):200–211. doi: 10.1177/0272989X17725740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Signorovitch JE, Sikirica V, Erder MH, et al. Matching-adjusted indirect comparisons: a new tool for timely comparative effectiveness research. Value Health. 2012;15(6):940–947. doi: 10.1016/j.jval.2012.05.004. [DOI] [PubMed] [Google Scholar]
- 19.Tillett W, Eder L, Goel N, et al. Enhanced patient involvement and the need to revise the core set - report from the psoriatic arthritis working group at OMERACT 2014. J Rheumatol. 2015;42(11):2198–2203. doi: 10.3899/jrheum.141156. [DOI] [PubMed] [Google Scholar]
- 20.Menter A, Krueger GG, Paek SY, Kivelevitch D, Adamopoulos IE, Langley RG. Interleukin-17 and interleukin-23: a narrative review of mechanisms of action in psoriasis and associated comorbidities. Dermatol Ther (Heidelb) 2021;11(2):385–400. doi: 10.1007/s13555-021-00483-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.DeTora LM, Toroser D, Sykes A, et al. Good publication practice (GPP) guidelines for company-sponsored biomedical research: 2022 update. Ann Intern Med. 2022;175(9):1298–1304. doi: 10.7326/M22-1460. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data from the bimekizumab clinical trials used in this analysis may be requested by qualified researchers 6 months after product approval in the USA and/or Europe, or global development is discontinued, and 18 months after trial completion. Investigators may request access to anonymized individual patient data and redacted study documents which may include: raw datasets, analysis-ready datasets, study protocol, blank case report form, annotated case report form, statistical analysis plan, dataset specifications, and clinical study report. Prior to the use of the data, proposals need to be approved by an independent review panel at www.Vivli.org and a signed data-sharing agreement will need to be executed. All documents are available in English only, for a pre-specified time, typically 12 months, on a password-protected portal.