Abstract
Sorting of cytoplasmically synthesized proteins to their target compartments usually is highly efficient so that cytoplasmic precursor pools are negligible and a particular gene product occurs at one subcellular location only. Yeast major adenylate kinase (Adk1p/Aky2p) is one prominent exception to this rule. In contrast to most mitochondrial proteins, only a minor fraction (6–8%) is taken up into the mitochondrial intermembrane space, whereas the bulk of the protein remains in the cytosol in sequence-identical form. We demonstrate that Adk1p/Aky2p uses a novel mechanism for subcellular partitioning between cytoplasm and mitochondria, which is based on competition between spontaneous protein folding and mitochondrial targeting and import. Folding is spontaneous and rapid and can dispense with molecular chaperons. After denaturation, enzymatic activity of Adk1p/Aky2p returns within a few minutes and, once folded, the protein is thermally and proteolytically very stable. In an uncoupled cell-free organellar import system, uptake of Adk1p/Aky2p is negligible, but can be improved by previous chaotropic denaturation. Import ensues independently of Hsp70 or membrane potential. Thus, nascent Adk1p/Aky2p has two options: either it is synthesized to completion and folds into an enzymatically active import-incompetent conformation that remains in the cytosol; or, during synthesis and before commencement of significant tertiary structure formation, it reaches a mitochondrial surface receptor and is internalized.
INTRODUCTION
In all organisms, adenylate kinases are abundant enzymes that provide the ADP required for oxidative and substrate chain phosphorylations (Noda, 1973) and play an important role in the maintenance of the “energy charge” equilibrium (Atkinson, 1977). In eucaryotes, three isozymes exist that are similar to one another in primary structure, but differ in subcellular location (reviewed in Schulz, 1987). The so-called short form adenylate kinases (AK1 or myokinase in vertebrates and Ura6p in yeast) (Schricker et al., 1992b) occur exclusively in the cytoplasm, whereas two subtypes of a long isoform, AK2 and AK3, reside in mitochondria. The yeast homolog to AK3, Aky3p, is a mitochondrial matrix protein (Schricker et al., 1992a, 1995). The major adenylate kinase in yeast, Adk1p/Aky2p, displays a dual location. The bulk of the protein resides in the cytoplasm, and a minor fraction (6–8% of the total) is imported into the mitochondrial intermembrane space, where it fulfills an important role in oxidative metabolism (Bandlow et al., 1988; Schricker et al., 1992b). The protein from both locations is encoded by the nuclear ADK1/AKY2 gene (Magdolen et al., 1987), translated without cleavable presequence from a single transcript and modified posttranslationally in identical manner (the N-terminal two amino acids, Met and Ser, are removed; Ser3 is N-acetylated; and the C-terminal Asp amidated) (Tomasselli et al., 1986; Klier et al., 1996). Thus, Aky2p strikingly differs from other cytoplasmically synthesized mitochondrial proteins in that, in general, cytoplasmic precursor pools are too small to measure in the steady state (Ades and Butow 1980; Fujiki and Verner, 1993), whereas the majority of Aky2p remains and is active in the cytosol. This article analyzes the basis for this extraordinary equilibrium of partitioning of Aky2p between the two compartments.
Two principally different routes exist for intermembrane space proteins to reach their destination. One group of proteins, e.g., the nonheme iron protein constituent of the respiratory complex III and cytochromes c1 and b2 have bipartite presequences that are cleaved by the matrix processing peptidase, and their uptake requires a membrane potential over the inner membrane. Mitochondrial import of members of the other group, comprising cytochrome c and c1 heme lyases, dispenses with a cleavable presequence and ΔΨ, and rather ensues in a direct way (Steiner et al., 1995; Diekert et al., 1999). The N-terminal 10 amino acid residues of Aky2p have been shown recently to carry target information because this peptide, fused to two heterologous cytoplasmic passengers, DHFR from mouse and Ura6p from yeast, is sufficient to direct their uptake into mitochondria. The propensity of this peptide to form an α-helix has been found to correlate positively with uptake efficiency, whereas positive or negative charges did not improve or impede import, and the magnitude of the hydrophobic helical moment was of minor importance (Bandlow et al., 1998).
The import of virtually all precursor proteins into mitochondria more or less depends on ATP on the cytoplasmic side of the mitochondrial membrane, indicative of the posttranslational interaction with a molecular chaperon of the Hsp70 class. Chaperon binding keeps precursors partially unfolded for passage through the import channel(s) of outer (and inner) mitochondrial membranes (Kübrich et al., 1995; Lill et al., 1996; Lithgow et al., 1997; Pfanner et al., 1997; Koehler et al., 1999). Aky2p appears to behave differently. To explain the basis of the abnormal import equilibrium of Aky2p, we have examined whether folding of this polypeptide is rapid, spontaneous, and independent of Hsp70. Therefore, we have analyzed mitochondrial import of Aky2p in vivo, determined the influence of previous chaotropic denaturation of Aky2p on import efficiency in vitro, and measured thermal denaturation curves as well as renaturation kinetics after previous denaturation with urea or guanidinium isothiocyanate. In addition, we have measured the importance of molecular chaperons and ATP on renaturation and import in organello. The data show that Aky2p folds rapidly and spontaneously in the absence of molecular chaperons. Uptake of Aky2p into mitochondria and folding in the cytoplasm are, thus, competitive and mutually exclusive processes providing a novel principle for dual subcellular location of a protein in cytoplasm and mitochondria.
MATERIALS AND METHODS
Yeast Strains, Growth Conditions, and Preparation of Mitochondria
The AKY2-disrupted yeast strains DL1-D16 Δaky2 (aky2::LEU2) (Schricker et al., 1992b) or WCG4 Δaky2 served as recipients for all constructs as indicated. For analysis of import efficiency under steady-state conditions in vivo, yeast cells were grown on semisynthetic medium supplemented according to the auxotrophic requirements and 3% lactate as carbon source. Spheroplasts were prepared from mid-logarithmic cultures (1–2 × 107 cells/ml), lysed in 0.6 M mannitol, and nuclei and debris removed (3000 × g, 2 × 5 min). Mitochondria were collected by centrifugation (10,000 × g, 15 min), resuspended in 0.6 M mannitol-containing buffer, further purified by Percoll (28%; Pharmacia, Freiburg, Germany) gradient centrifugation as described previously (Müller and Bandlow, 1989), and analyzed by Western blotting and immunodetection after SDS-PAGE. Outer mitochondrial membranes were disrupted by controlled hypotonic treatment and mitochondria subfractionated into intermembrane space proteins, matrix fraction, and membranes as described previously (Müller and Bandlow, 1989).
Construction of AKY2 Mutants
Construction of AKY2 mutants by site-directed mutagenesis has been described previously (Kunkel et al., 1987; Magdolen et al., 1992). The mature Aky2 wild-type protein has the N-terminal sequence SSESIRMVLIGPPGAGK (Tomasselli et al., 1986). To construct AKY-N10, the 15 amino acids from the N terminus of Aky2p were replaced with the homologous 23 N-terminal residues from Aky3p by using homologous in vitro recombination within the conserved P-loop of the ATP-binding motif. In AKY-N16, Ser2 of Aky2p was exchanged for amino acids 2–67 of cytochrome c1 (in analogy to a similar fusion to the Aky2 isozyme Ura6p; Schricker et al., 1992b). Wild-type gene and mutant constructs were expressed in DL1-D16 Δaky2 or WCG4 Δaky2 transformants (as indicated) from YEp352-based multicopy shuttle plasmids (Hill et al., 1986) under the control of the original AKY2 promoter retaining the authentic context of the ATG translational initiation codon.
In Vitro Transcription-Translation System
The genes of wild-type AKY2, as well as the genes for the control proteins, cytochrome c1 heme lyase (marker of the intermembrane space without cleavable presequence; Steiner et al., 1995), and pSU9-(1-86)DHFR (matrix marker with cleavable presequence; Ungermann et al., 1994) were ligated to pGEM vectors (Stratagene, Heidelberg, Germany) in SP6 promoter orientation. Capped transcripts of the various yeast genes were obtained in vitro by using SP6 polymerase and the Cap Scribe kit (Roche Applied Science, Mannheim, Germany). mRNAs were translated in micrococcus nuclease-pretreated, methionine-depleted rabbit reticulocyte lysates (Promega, Heidelberg, Germany) in the presence of 50 μCi of l-[35S]methionine (1000 Ci/mmol; ICN Biomedicals, Eschwege, Germany) in a final assay volume of 50 μl.
Mitochondrial Import In Vitro
Mitochondria were prepared from spheroplasts of strain D273-10B (ATCC 24657) after osmotic lysis (Daum et al., 1982), suspended in import mix, and used for in organello import studies, either directly or after previous thawing of shock-frozen aliquots. Briefly, 50 μg of mitochondria was suspended in 97 μl of import mix containing 2 mM NADH, 220 mM sucrose, 10 mM 4-morpholinepropanesulfonic acid, 40 mM potassium phosphate, pH 7.4, 80 mM KCl, 2 mM Mg(OAc)2, 1 mM MnCl2, and 3% bovine serum albumin (Ungermann et al., 1994) and, where indicated, 2 mM ATP and/or chaperon (DnaK DnaJ GrpE; Roche Applied Sciences; or S100 supernatant from strain DL1-D16 Δaky2). The reaction was started by the addition of 3 μl of 35S-labeled, urea-denatured (8 M urea) precursor. Other additives were as indicated in the figure legends. Valinomycin was used at a final concentration of 2 μM. After incubation at 25°C for 20 min, the import assays were divided into four aliquots, which were diluted with 10 volumes of 20 mM HEPES, pH 7.4, containing no further additives or proteinase K (generally 50 μg/ml) either in the presence of 250 mM sucrose or without osmotic stabilization or with 1% Triton X-100 (final concentration). Samples were incubated at room temperature (RT) (30 min) and digestions terminated by addition of 2 mM phenylmethylsulfonyl fluoride (final concentration). Mitochondria were collected by centrifugation (4°C, 20,000 × g, 7 min), resuspended in 250 μl of 250 mM sucrose, 20 mM HEPES, pH 7.4, and 150 mM KCl, and recentrifuged. Pellets were dissolved and proteins separated by SDS-PAGE (14% gels) and dried gels exposed to x-ray film (Betamax; Amersham Biosciences, Braunschweig, Germany).
Proteolytic Stability of Proteins
Cells were disrupted by homogenization with glass beads (6 pulses à 30 s with intermittent cooling on ice, in the absence of protease inhibitors). The 4000 × g supernatant was incubated with 1% digitonin in 0.6 M mannitol at 0°C for 1 min and aliquots of the 15,000 × g supernatant shock-frozen. Protein (150 μg) in 100 μl was incubated at RT with 25 μg/ml proteinase K for the times indicated, aliquots withdrawn, and the digestion terminated with 2 mM phenylmethylsulfonyl fluoride (final concentration). Total cellular protein (17 μg) was loaded per slot, separated by SDS-PAGE, and proteins detected by Western blotting with anti-AK or anti-hexokinase antibodies.
Denaturation of Protein
If denaturation of precursor was desired in import experiments, samples were precipitated with 1.2 volumes of neutralized, saturated (NH4)2SO4 solution (0°C, 30 min); precipitates were collected by centrifugation (33,000 × g, 4°C, 10 min), dissolved, and denatured in 1 volume of 8 M urea, 20 mM K/HEPES, pH 7.4, and 100 μM dithiothreitol; and incubated at RT for 30 min. For renaturation experiments, cytoplasmic fractions were prepared from wild-type and mutant strains from the 5000 × g (30 min) supernatants, made 8 M in urea or 2 M in guanidinium isothiocyanate (GuSCN), and incubated at RT for 30 min. Aliquots were withdrawn, diluted 200-fold with TEA buffer (70 mM triethanolamine, 13 mM MgSO4, and 50 mM KCl, pH 8.0) and renatured at RT for the periods indicated in the figures. In some experiments bacterial groEL groES (Roche Applied Sciences) together with 10 mM ATP was included. Adenylate kinase activity was assayed by a coupled enzymatic test (Bandlow et al., 1988).
Miscellaneous Procedures
Anti-Aky2p antiserum was raised in chickens (Schricker et al., 1992a) and purified from eggs (Jensenius et al., 1981). Published procedures were used to predict amphiphilic moments and secondary structure propensities (Chou and Fasman, 1978; Eisenberg et al., 1984), for determining protein concentrations (Bradford, 1976), for mitochondrial subfractionation and compartment-specific markers (Müller and Bandlow, 1989), for Western blotting and immunodecoration, DNA sequencing, PCR of DNA fragments, molecular cloning, and other molecular biological procedures (Sambrook et al., 1989). Densitometric evaluation of Western blots was performed using ImageQuant, version 1.11 (Amersham Biosciences, Freiburg, Germany).
RESULTS
Import Capability of Adenylate Kinase into Mitochondria In Vivo
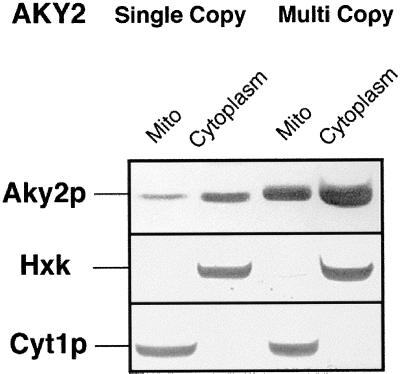
Yeast major adenylate kinase, AKY2, is constitutively expressed, but the polypeptide is poorly imported into mitochondria under any growth condition. To test whether in vivo mitochondrial uptake of Aky2p was attenuated because, under steady-state conditions, the import capacity for Aky2p was already saturated, the cytoplasmic pool size of Aky2p was extended by overexpression. Aky2 wild-type protein was synthesized in the haploid strain WCG4 Δaky2 once from a CEN-based and once from a 2-μ–derived plasmid. Lysed spheroplasts were fractionated into cytoplasm and mitochondria, which were further purified by gradient centrifugation. Mutual contamination of the fractions was controlled using antibodies against specific marker proteins (hexokinase in cytoplasm and cytochrome c1 in mitochondria) (Figure 1). Import of Aky2p into mitochondria in the steady state was measured by Western blotting after SDS-PAGE and quantified densitometrically. Most of the material remained (and was active; Table 1) in the cytoplasm, in agreement with a previous report (Bandlow et al., 1988). Mitochondrial uptake in vivo of Aky2 wild-type protein (Figure 1, Mito) was roughly proportional to the total concentration of precursor in the respective strains. Aky2p levels in the cytoplasmic fraction after expression from a single copy or a multicopy plasmid differed by a factor of 3.1 (68.5 vs. 211.3 × 103 arbitrary units, mean of three fractionations). Mitochondrial import of Aky2p was 3.8-fold increased after overexpression (21.5 vs. 82.2 × 103 units), demonstrating that the mitochondrial import path used by Aky2p in the wild-type was not saturated under steady-state conditions. The same conclusion can be drawn from a comparison of enzymatic activities of Aky2p in cytosol and mitochondria of the wild-type and a multicopy transformant (Table 1, compare DL1 with DL1-D16 [AKY2]). Thus, the inefficiency of Aky2p uptake must have other reasons than saturation of the import machinery with Aky2 precursor.
Figure 1.
Nonsaturation of the mitochondrial import machinery with Aky2p precursors. Aky2p was expressed either from a CEN plasmid (single copy) or a 2-μ plasmid (multicopy), detected by Western blotting after SDS-PAGE and the signals quantified (see text).
Table 1.
Adenylate kinase activity of multicopy [AKY2]-wild-type and mutant transformants and complementation of adenylate kinase deficiency
| Strains | Activity in | Complementation of | ||
|---|---|---|---|---|
| cytoplasm | mitochondriaa | adk1-1 in E. coli | aky2 in yeast | |
| DL1 wild type | 14.7 | 1.2 | ||
| DL1-D16[AKY2] | 69.0 | 3.0 | + | + |
| AKY-N10 | 1.5 | 17.0 | + | + |
| AKY-N16 | 0.2 | 3.6 | − | + |
Activity is given as millimoles of AMP phosphorylated per minute per milligram of protein as described (Bandlow et al., 1988).
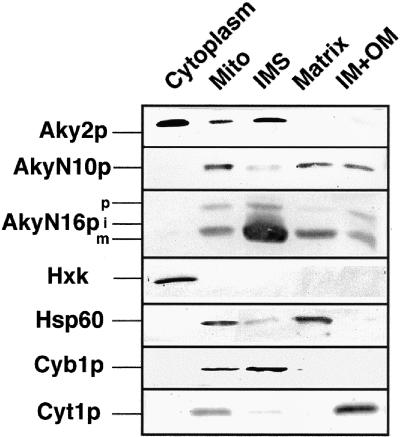
Next, we tested whether import attenuation was due to a structural constraint in Aky2p. To study this issue, the subcellular distribution, and accordingly the compatibility with membrane traversing, of two constructs was examined in vivo. In one construct, the N terminus of Aky2p was replaced with the homologous N-terminal sequence from Aky3p, a matrix-located isozyme of Aky2p without cleavable presequence (construct AKY-N10); and in another, Aky2p was equipped with the cleavable two-partite intermembrane space-targeting sequence of cytochrome c1 (AKY-N16; see MATERIALS AND METHODS). Both chimaeras were transcribed from the AKY2 promoter and translation initiated from the authentic AUG of AKY2 to guarantee identical expression. Cells and mitochondria were subfractionated. Distribution of compartment-specific marker proteins (hexokinase in cytoplasm, cytochrome b2 in intermembrane space, Hsp60 in the matrix, and cytochrome c1 in inner membranes) confirmed that the mutual contamination of the subfractions was low (Figure 2). Both chimeric proteins were efficiently imported into mitochondria, and the precursor was barely detectable in the cytoplasm or superficially associated with the mitochondrial membrane fraction. Most of AKY-N10p was found in the mitochondrial matrix, and AKY-N16p was transported to the intermembrane space very efficiently (cf. Table 1) and correctly processed to the mature form. These results demonstrate that, in principle, the primary sequence of Aky2p is compatible with efficient membrane traversing and does not contain structural barriers that impede or abrogate membrane permeation. The heterologous target sequences evidently effect significantly improved recognition and membrane traversing of the Aky2 passenger compared with the authentic Aky2 protein (see DISCUSSION). Furthermore, the Aky2 passenger protein is sorted to the submitochondrial compartment specified by the presequence and does not contain an active internal signal for being targeted to the intermembrane space. Rather, delivery to this compartment appears to be the consequence of not being sorted to one of the inner mitochondrial compartments, matrix or inner membrane.
Figure 2.
Test of principal competence for membrane passage of Aky2p. Aky2p has been equipped with a heterologous mitochondrial targeting sequence: in AKY-N10 with the noncleaveable N-terminal matrix targeting sequence of Aky3p, and in AKY-N16 with the two-partite intermembrane space-targeting presequence of cytochrome c1. Aky2p, wild-type AKY2 on a multicopy plasmid (control). Steady-state distribution in vivo of Aky2p was analyzed. Spheroplasts were lysed and fractionated into cytoplasm and mitochondria (mito). Mitochondria were further purified and subfractionated into matrix, inner plus outer membranes (IM+OM) and IMS proteins. Compartment-specific topological markers were hexokinase (Hxk in cytosol), Hsp60 (in matrix), cytochrome b2 (Cyb1p in IMS), and cytochrome c1 (Cyt1p in inner membranes).
In Vitro Import into Mitochondria
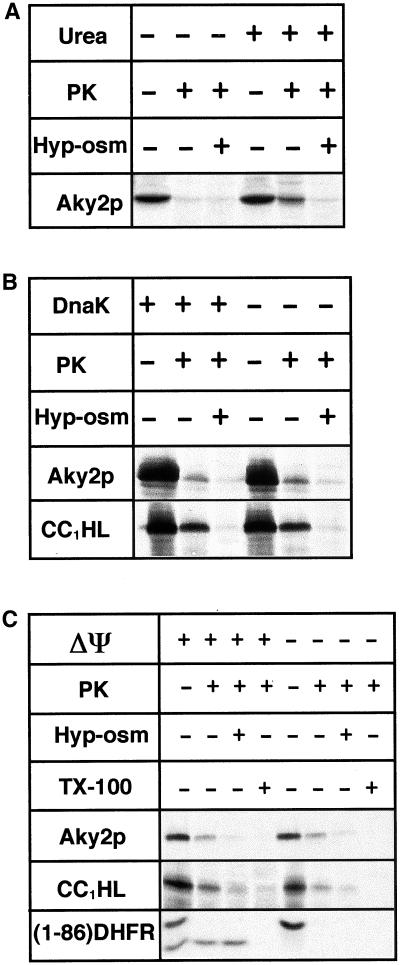
As a third possibility, why Aky2p is inefficiently imported in vivo, we examined whether it folds spontaneously in the cytoplasm and thereby rapidly assumes a rigid tertiary structure that is no longer compatible with import. To test the hypothesis that spontaneous folding counteracts mitochondrial uptake of Aky2p, we examined posttranslational import in vitro. Aky2p precursor was synthesized in a reticulocyte lysate and imported into isolated mitochondria in an uncoupled system. To discriminate whether in vitro-synthesized, radiolabeled precursor was superficially attached to mitochondria or internalized, mitochondria were incubated under isotonic conditions with exogenous protease after the import reaction. Some of the protein is protease resistant. Because Aky2p is not processed upon import, internalization is not readily apparent. However, the controls displayed in Figure 3C reveal that proteinase K degrades all superficially attached precursor (e.g., for a matrix-located control protein [SU9(1-86)DHFR] (Ungermann et al., 1994). In mitoplasts, protease removes all residual cytochrome c1 heme lyase (CC1HL) as an intermembrane space (IMS) control protein as well as Aky2p. It is thus concluded that after previous denaturation, some Aky2p has been transported to the IMS in vitro.
Figure 3.
Test of dependence of Aky2p import into mitochondria on unfolding by urea (A), chaperons (B), or membrane potential (ΔΨ) (C). (A) Radiolabeled precursor had been preincubated with urea or left untreated and then diluted and incubated with isolated mitochondria. (B) Isolated mitochondria were incubated with radiolabeled CC1HL (control) or urea-denatured Aky2p in the absence or presence of chaperon (DnaK DnaJ GrpE plus ATP). (C) Isolated mitochondria were incubated with radiolabeled control proteins or urea-denatured Aky2 wild-type (Aky2p) in the absence (+ΔΨ) or presence (−ΔΨ) of uncoupler. After incubation, mitochondria were sedimented, washed, and treated with proteinase K (PK, 50 μg/ml, 30 min, RT) after swelling (+Hyp-osm) or incubation with Triton X-100 (+TX-100). CC1HL, membrane potential-independent control; (1-86)DHFR, fusion of the N-terminal presequence of ATPase F1 subunit 9 to mouse dihydrofolate reductase (membrane potential-dependent control).
In initial experiments only marginal amounts of radiolabeled Aky2p were imported to a position inaccessible to added proteinase K (Figure 3A). To test whether in the uncoupled system nascent Aky2p had obtained a folded conformation before having had the opportunity to reach an import receptor, we examined whether previous chaotropic denaturation of the radiolabeled precursors and/or addition of chaperons to the import incubation mix improved uptake efficiencies (Figure 3, A and B). Denaturation by urea and 30-fold dilution of the chaotropic agent into the incubation mix yielded significant improvement of uptake (Figure 3A) so that it was used in all further experiments. On the other hand, molecular chaperons (excess native yeast cytoplasmic supernatant as a source of homologous chaperons and presequence-binding proteins or DnaK/DnaJ/GrpE or GroEL/GroES; only DnaK complex is shown) or/and exogenous ATP had no influence on import efficiency (Figure 3B), indicating that, after dilution, the denatured precursor rapidly assumed an import-incompetent state and that the velocity was not affected by chaperons of the Hsp60 or Hsp70 families. Because presequence-binding proteins and molecular chaperons intrinsic to the reticulocyte lysate were denatured by urea, too, it must be concluded that import of Aky2p into mitochondria can dispense with accessory cytoplasmic proteins. In this respect, Aky2p behaves similar to CC1HL (Figure 3B).
It has been reported that rat liver AK2 required a membrane potential over the inner mitochondrial membrane for uptake (Nobumoto et al., 1998). Therefore, it was tested whether import of Aky2p was dependent on ΔΨ (Figure 3C). In energy-coupled mitochondria or mitoplasts, the matrix-imported mature version of SU9(1-86)DHFR control protein is inaccessible to protease and degraded only after complete lysis of mitochondria by detergent (Triton X-100). Dissipation of ΔΨ abrogates import of the matrix control protein SU9(1-86)DHFR completely, but has no detectable influence on the uptake of Aky2 wild-type protein. These data prove that, in contrast to AK2, uptake of wild-type Aky2p ensues independently of membrane potential as has been demonstrated previously for CC1HL (Steiner et al., 1995; see IMS control in Figure 3C).
Aky2p Is Highly Resistant to Proteolysis and Thermal Denaturation
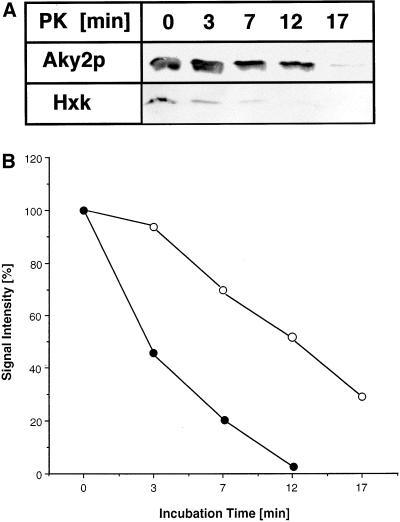
To scrutinize rigid folding of native Aky2p and to challenge independently a possible inverse correlation between protein stability and mitochondrial import efficiency in more detail, we examined the susceptibility of Aky2p in crude extracts (and after radioactive in vitro synthesis in a reticulocyte lysate; our unpublished data) to proteolysis in vitro. At 0°C, radiolabeled in vitro-synthesized Aky2p was almost completely resistant to proteinase K (up to 100 μg/ml, 15 min), whereas the control protein, CC1HL, was completely digested (our unpublished data). Aky2p was, however, degraded at RT by 25 μg/ml proteinase K within 17 min. Aky2p and intrinsic hexokinase, which was used as standard, were measured by Western analysis in total cell extracts (see MATERIALS AND METHODS). Aky2p was significantly more resistant to proteolytic attack than hexokinase (Figure 4, A and B).
Figure 4.
Sensitivity to proteolysis of native Aky2 wild-type and hexokinase. A crude 4000 × g supernatant (20 μg, homogenate) was incubated with 25 μg of proteinase K at room temperature for the periods indicated. (A) After SDS-PAGE and Western blotting, proteins were detected by immunodecoration by using anti-Aky2p antiserum or antihexokinase serum (control) as the primary antibody. Proteolysis was terminated with an excess of phenylmethylsulfonyl fluoride. Experiment has been repeated three times with similar results. (B) Quantitative evaluation of proteolytic stability of Aky2 wild-type protein and hexokinase. (○), Aky2p wild-type; (●), hexokinase.
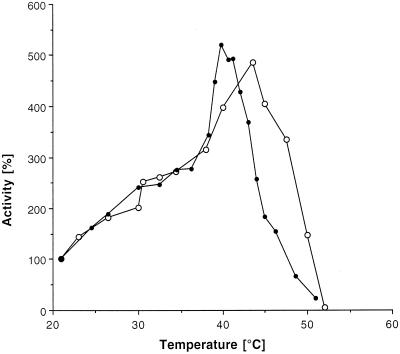
As an additional approach to test folding stability of Aky2p, we measured the temperature optimum of enzymatic activity. Cytoplasmic AK enzymatic activity was followed with increasing temperature (Figure 5). Aky2p was unusually resistant to heat denaturation and displayed a temperature optimum at 44°C and half-inactivation at 49°C. Intrinsic hexokinase, which was measured for comparison, was much more sensitive and had a temperature optimum of activity at 39°C.
Figure 5.
Thermal denaturation of Aky2 wild-type protein and hexokinase. Denaturation was followed by measuring adenylate kinase activity (Bandlow et al., 1988) or hexokinase activity of a 4000 × g crude cytoplasmic supernatant fraction with increasing temperature (new sample measured at each time point). (○), AKY2 wild-type, 100% activity is 57.2 U/mg; (●), hexokinase.
Adenylate Kinase Activity and Renaturation Kinetics
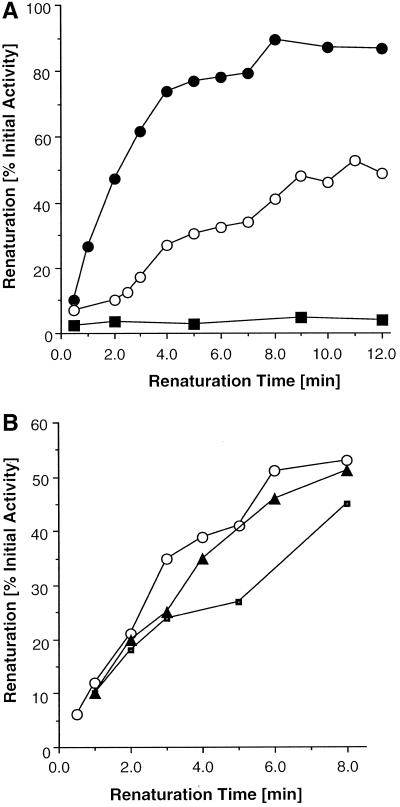
Folded Aky2 wild-type protein was found to be transported into isolated mitochondria only to a marginal extent if at all, and uptake efficiency was only moderate after previous chaotropic denaturation. These results suggest that Aky2p rapidly and spontaneously assumes an import incompetent conformation in vitro and presumably also in vivo. In contrast, both AKY2 mutants, in which the N terminus had been exchanged for either the targeting sequence of Aky3p or cytochrome c1, dramatically improved import efficiency of the Aky2 passenger (Figure 2). This could be due to one or both of two reasons: either the altered target sequence retards folding compared with wild-type Aky2p so that targeting information is exposed to the Tom import receptors for a longer period of time, and/or the import signal is improved so that the affinity of the heterologous presequences to the receptors of the Tom complex is tremendously higher than of the authentic Aky2 N-terminal target peptide and the Aky2 passenger is imported more rapidly. To test the first possibility, three alternative reasons for inefficient in vitro import were considered: 1) denatured Aky2p renatures spontaneously upon dilution into the import assay with very fast kinetics and extremely rapidly assumes an import-incompetent conformation; 2) the denatured protein aggregates upon dilution; or 3) mitochondrial import of Aky2p depends on supernatant proteins also denatured by urea. The last possibility seems unlikely because inclusion of excess yeast cytoplasmic supernatant (from a Δaky2 strain) as a homologous source of such accessory factors as well as the addition of bacterial chaperons to the import assay did not improve import efficiency. To discriminate between the first two possibilities, denaturation-renaturation experiments were performed with wild-type and AKY-N10 mutant proteins (Figure 6). (The precursor AKY-N16 was excluded because it is unstable in yeast and processed inside mitochondria to the mature protein [cf. Figure 2], which is almost identical to Aky2p so that its renaturation could not be followed; see DISCUSSION). In an approximation, renaturation was measured as restoration of catalytic activity. Although this kinetics presumably is much slower than loss of import competence, it gives a maximum period of persistence of the import-competent conformation.
Figure 6.
Renaturation kinetics of Aky2 wild-type and mutant AKY-N10 protein and hexokinase after chaotropic denaturation. AKY-N10 has the N-terminal target sequence of Aky3p. Renaturation was measured as return of enzymatic activity (Bandlow et al., 1988) in percentage of initial activity in a 4000 × g crude cytoplasmic supernatant fraction. (A) Proteins were denatured with 8 M urea for 30 min at RT. Renaturation of Aky2p wild-type (●) 100% activity is 34.5 U/mg before denaturation; (○) AKY-N10p, 100% activity is 8.4 U/mg; hexokinase (▪). It has been verified before the assay that urea at the final concentration in the assay (35 mM) did not affect activity. (B) Renaturation of Aky2p after denaturation with 2 M GuSCN for 3 h (○); renaturation in the presence of 2 mM ATP (▴); or in the presence of 2 mM ATP plus groEL groES (▪).
To follow folding directly in the absence or presence of chaperons, total protein of a crude cytoplasmic extract from a strain overexpressing Aky2 wild-type or mutant proteins was denatured as described in MATERIALS AND METHODS, and the kinetics of spontaneous regain of AK activity was followed after 300-fold dilution of the chaotropic agent (urea in Figure 6A or GuSCN in Figure 6B). The enzymatic activity was close to zero when measured immediately after dilution, indicating efficient denaturation by exposure to the denaturants for 30 min; >50% of the original activity of Aky2p returned within a few minutes under renaturing conditions. The near-to-quantitative regain of activity excluded significant aggregation after dilution of the denatured proteins (Figure 6A). Inclusion of ATP as one of the cosubstrates did not accelerate renaturation kinetics, and also the simultaneous presence of ATP and molecular chaperons (or of excess native yeast supernatant protein from strain DL1-D16; our unpublished data) was without significant effect under any condition of previous denaturation (Figure 6B). In contrast, Aky-N10p renatured only reluctantly, indicating that the heterologous target sequence of Aky3p impeded folding of the chimaera although all the rest of the protein was identical to Aky2p. Hexokinase did not renature to a significant extent under identical conditions.
The results allow to conclude that the import-competent state of Aky2p is maintained in only an extremely narrow time window after synthesis. Thus, it is assumed that rapid spontaneous folding counteracts efficient mitochondrial import. In addition, the affinity of the N-terminal target sequence of Aky2p to the receptors of the Tom complex is low and can be significantly increased and thereby import accelerated by replacement with authentic target sequences from either Aky3p or cytochrome c1.
DISCUSSION
The majority of mitochondrial proteins is encoded by nuclear genes and synthesized on cytoplasmic ribosomes. Because precursor proteins can accumulate in the cytoplasm in vivo after dissipation of membrane potential and be chased into the organelle upon restoration of ΔΨ or imported in vitro in an uncoupled translation/import system, it is generally assumed that most mitochondial proteins are imported in a posttranslational mode. Proteins following this route are mostly synthesized as N-terminally elongated precursors. Among other tasks the presequences serve to allow association with accessory proteins (e.g., the presequence-binding protein Mtf52p, which recruits the mitochondrial import stimulating factor Msf1p, and with heat-shock proteins of the Hsp70 class) to maintain a partially unfolded, import-competent state and to facilitate interaction of the presequence with the mitochondrial import surface receptor complexes (Lill and Neupert, 1996; reviewed in Lithgow et al., 1997). The efficiency of this interaction correlates positively with the propensity of the presequence to form an amphipathic α-helix, which usually is 18 or more amino acids in length (Roise et al., 1988) and with the magnitude of the hydrophobic moment of this helix (Eisenberg et al., 1984; von Heijne, 1986). In addition, several reports exist where presequences have been observed to retard folding of the precursor and thereby help to maintain the unfolded, transport-competent state (Laminet and Plückthun, 1989; Liu et al., 1989; Lithgow et al., 1997). The presequence is removed by the matrix processing peptidase as soon as it has traversed the inner mitochondrial membrane (Glick and Schatz, 1991; reviewed in Kübrich et al., 1995).
Aky2p contrasts with this general concept of import into mitochondria in several important aspects: 1) It dispenses with a cleavable presequence (Bandlow et al., 1988). 2) The N-terminal peptide of only seven amino acids contains (part of) the import information (Bandlow et al., 1998), but secondary structure prediction for this region is higher for β-sheet formation than for α-helix, and β-sheet conformation of the N terminus of the native molecule has been confirmed by x-ray crystallography (Egner et al., 1987). Moreover, the amphiphilic moment of the presumptive N-terminal helix, which may form at the membrane-aqueous interface, is low (Bandlow et al., 1998). 3) As shown herein, folding of Aky2p most likely does not require association with chaperons at the cytoplasmic side. 4) In yeast, the cytoplasmic pool of Aky2 protein comprises >90% of the total, and only a minor fraction is imported into the mitochondrial intermembrane space (Bandlow et al., 1988).
We show that the low import efficiency is due to an extraordinarily high rate of spontaneous folding so that import information can be deciphered by mitochondrial Tom receptors in a narrow time window only. In addition, the quality of the interaction of the N-terminally located target information with the import receptors can be dramatically improved by exchange for the authentic target sequences of Aky3p or cytochrome c1. It must be concluded that the affinity of the wild-type N-terminal sequence of Aky2p to the Tom receptors is low, presumably due to its low propensity to form an α-helix. Both parameters are readily improved and thereby the efficiency of uptake increased by replacement with the authentic target sequences of Aky3p or cytochrome c1. The N-terminal target sequence of Aky3p is longer by 12 residues and, in addition, displays a significantly higher hydrophobic α-helical moment (Aky2p has an average μH of ∼4 over the length of 8 amino acid residues, which is fairly below the value expected for mitochondrial target sequences of μH > 7.5 over a length of 3–5 helical turns; von Heijne, 1986) vs. μH ∼5.5 on the average over a length of 19 residues in AKY-N10p; Bandlow et al., 1998). In addition, we show herein that AKY-N10p folds significantly slower than wild type. In accordance with these observations we conclude that targeting interactions with the mitochondrial import receptors are improved and, hence, efficiency of uptake increased.
Although renaturation velocity of the AKY-N16 precursor cannot be followed directly in yeast because it accumulates only to marginal concentrations (even in the presence of uncoupler) it may be concluded that similar considerations apply for the presequence of AKY-N16 as for AKY-N10: 1) This chimaera has the two-partite mitochondrial intermembrane space-targeting sequence of cytochrome c1, of which the latter is imported highly efficiently so that, in the steady state, no Cyt1 precursor is detectable in the cytoplasm. 2) It effects efficient uptake of a cytoplasmic passenger (Ura6p; Schricker et al., 1992b) into the intermembrane space, indicating strong interaction with the Tom complex. 3) Most significantly, in contrast to Aky2p and AKY-N10, AKY-N16 fails to complement the adk1–1ts deficiency in Escherichia coli (Table 1); although, after processing in yeast, it complements the respiratory deficiency caused by the deletion of AKY2 in yeast (Bandlow et al., 1988) and displays significant adenylate kinase activity. This suggests that, at least in E. coli, the cytochrome c1 presequence interferes with the correct folding and enzymatic activity, whereas in yeast, after import, it is processed to a mature form of Aky2p that is just two amino acids longer at the N terminus than mature wild-type Aky2p and presumably will fold rapidly into the enzymatically active conformation.
The question arises, whether import of Aky2p ensues posttranslationally at all or whether mitochondrial uptake is directly coupled to translation. In fact, ribosomes entangled specifically with the synthesis of mitochondrial proteins have been found attached to the outer mitochondrial membrane (Kellems and Butow, 1974; Kellems et al., 1974a,b; Suissa and Schatz, 1982), and a cotranslational mechanism of uptake has been postulated for at least some proteins (Fujiki and Verner, 1993; Verner, 1993). As the precursor of fumarase (Stein et al., 1994; Knox et al., 1998) and mammalian AK2 (Nobumoto et al., 1998) could not be imported into mitochondria after completion of synthesis either in vivo or in vitro, it has been proposed that these proteins are translated and imported in a coupled manner.
For yeast Aky2p, a strictly vectorial uptake is difficult to reconcile with the observation that the protein from both the cytoplasmic and the mitochondrial location is posttranslationally modified in the identical way: the first two amino acids removed, Ser3 N-acetylated, and the C-terminal Asp amidated (Klier et al., 1996). In addition, traversing of mitochondrial membranes dispenses with a signal recognition/docking mechanism, and translation of messages for mitochondrial proteins lacks a translational arrest to allow the presequence of the nascent polypeptide to contact the import apparatus of the outer membrane, two requirements that are obligatory for cotranslational endoplasmic reticulum membrane traversing.
On the other hand, our data show that, after denaturation, restoration of the enzymatically active conformation is an unusually rapid process that is not significantly influenced by the inclusion of Hsp60 or Hsp70 proteins or excess native cytoplasmic supernatant proteins from AK-deficient yeast as a source of accessory factors. This implies that folding of Aky2p occurs spontaneously in vitro and, presumably, does so also in vivo. Thereby, loss of import competence is presumably even faster than regain of enzymatic activity. Because Aky2p is not imported in an uncoupled translation/import system in vitro, a purely posttranslational mechanism is also not very likely.
The solution to the problem could be that translation and import of Aky2p proceed in a loosely or kinetically coupled manner. According to this model, the nascent Aky2 polypeptide in vivo has two options: If mitochondrial import receptors are too far to reach before commencement of tight folding, the protein is synthesized to completion and released from the ribosome in largely folded form to remain in the cytoplasm (as in the in vitro situation with translation and import uncoupled). Once folded, Aky2p is excluded from mitochondrial import. This is in line with x-ray crystallographic analysis (Egner et al., 1987), which shows that both the N terminus and a presumptive internal import-relevant sequence are concealed in the folded protein structure of the native conformation. Alternatively, during translation or soon after release from the ribosome, nascent Aky2p reaches a mitochondrial import receptor before the attainment of a stably folded tertiary structure and is translocated. The observation that Aky2p is identically modified in both locations (Klier et al., 1996) is explained by the fact that N-terminal processing and N-acetylation are very rapid processes that act on the nascent polypeptide chain (Huang et al., 1987). The data presented herein suggest that import and spontaneous folding of Aky2p are competitive and mutually exclusive processes. In agreement with this conclusion it has been shown that the fraction of Aky2p imported into mitochondria does not equilibrate with the cytoplasmic Aky2p pool to a significant extent, because mutants have been isolated that are unstable and barely detectable in the cytosol, but nevertheless efficiently rescued by uptake into the IMS (Bandlow et al., 1998; our unpublished data).
Other mechanisms have been reported that also allow sorting of a particular protein encoded by a single gene to more than one subcellular location. Among them are transcription of two mRNAs starting from two different promoters of the same gene (Natsoulis et al., 1986) or start of translation from two alternative translational initiation sites (Najarian et al., 1987). Fumarase is synthesized on cytoplasmic ribosomes with a mitochondrial extension. It is sorted to cytoplasm and mitochondrial matrix. However, in contrast to Aky2p, fumarase initially is quantitatively taken up by mitochondria (in a ΔΨ-dependent manner) and arrested in the import channel where it is N-terminally cleaved by the matrix-processing peptidase. Subsequently, part of the molecules is taken up into the matrix, whereas the rest is lost into the cytoplasm by an as-yet-not defined retrograde transport mechanism (Stein et al., 1994; Knox et al., 1998). Thus, a number of completely different mechanisms exist that allow sorting of a particular protein simultaneously to mitochondria (generally the mitochondrial matrix) and cytoplasm. Inefficient sorting to the intermembrane space of Aky2p due to rapid spontaneous folding and attainment of a conformation that is import-incompetent provide a novel mechanism of subcellular sorting.
ACKNOWLEDGMENTS
We thank Drs. R.A. Stuart and J. Herrmann (Adolf-Butenandt-Institute for Physiological Chemistry, The University of Munich, Munich, Germany) for help and discussion, and A.Z. acknowledges the hospitality received during a stay in that laboratory. Rabbit antibodies against hexokinase and citrate synthetase were kindly donated by G. Schatz (The Biocenter, Basel, Switzerland); those directed against Hsp60 and cytochrome b2, as well as plasmid pSU9-(1-86)DHFR were the kind gifts of W. Neupert and J. Herrmann (Adolf-Butenandt-Institute for Physiological Chemistry); anti-DHFR was provided by R. Zimmermann (University of Homburg/Saar, Homburg, Germany). Yeast wild-type strain WCG4 was the gift of D.H. Wolf (Stuttgart, Germany). The work was supported by the Deutsche Forschungsgemeinschaft through grant Ba415/24-1.
Abbreviations used:
- AK
adenylate kinase
- Aky2p
yeast major adenylate kinase
- AKY2
gene encoding Aky2p, YDR226w, GenBank accession number Y00413
- GuSCN
guanidinium isothiocyanate
Footnotes
DOI: 10.1091/mbc.01–08–0396.
REFERENCES
- Ades IZ, Butow RA. The transport of proteins into yeast mitochondria. Kinetics and pools. J Biol Chem. 1980;255:9925–9935. [PubMed] [Google Scholar]
- Atkinson DE. Cellular Energy Metabolism and Its Regulation. New York: Academic Press; 1977. Adenylate control and adenylate energy charge; pp. 85–107. [Google Scholar]
- Bandlow W, Strobel G, Schricker R. Influence of N-terminal sequence variation on the sorting of major adenylate kinase to the mitochondrial intermembrane space in yeast. Biochem J. 1998;329:359–367. doi: 10.1042/bj3290359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandlow W, Strobel G, Zoglowek C, Oechsner U, Magdolen V. Yeast adenylate kinase is active simultaneously in mitochondria and cytoplasm and is required for non-fermentative growth. Eur J Biochem. 1988;178:451–457. doi: 10.1111/j.1432-1033.1988.tb14469.x. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Chou PY, Fasman GD. Prediction of the secondary structure of proteins from their amino acid sequence. Adv Enzymol. 1978;47:45–148. doi: 10.1002/9780470122921.ch2. [DOI] [PubMed] [Google Scholar]
- Daum G, Böhni PC, Schatz G. Import of proteins into mitochondria. Cytochrome b2 and cytochrome c peroxidase are located in the intermembrane space of yeast mitochondria. J Biol Chem. 1982;257:13028–13033. [PubMed] [Google Scholar]
- Diekert K, Kispal G, Guiard B, Lill R. An internal targeting signal directing proteins into the mitochondrial intermembrane space. Proc Natl Acad Sci USA. 1999;12:11752–11757. doi: 10.1073/pnas.96.21.11752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egner U, Tomasselli AG, Schulz GE. Structure of the complex of yeast adenylate kinase with the inhibitor P1,P5-di(adenosine-5′-)pentaphosphate at 2.6 Å resolution. J Mol Biol. 1987;195:649–658. doi: 10.1016/0022-2836(87)90188-4. [DOI] [PubMed] [Google Scholar]
- Eisenberg D, Schwarz M, Komaromy M, Wall R. Analysis of membrane and surface protein sequences with the hydrophobic moment plot. J Mol Biol. 1984;178:125–142. doi: 10.1016/0022-2836(84)90309-7. [DOI] [PubMed] [Google Scholar]
- Fujiki M, Verner K. Coupling of cytosolic protein synthesis and mitochondrial protein import in yeast. Evidence for cotranslational import in vivo. J Biol Chem. 1993;268:1914–1920. [PubMed] [Google Scholar]
- Glick B, Schatz G. Import of proteins into mitochondria. Annu Rev Genet. 1991;25:21–44. doi: 10.1146/annurev.ge.25.120191.000321. [DOI] [PubMed] [Google Scholar]
- Hill JE, Myers AM, Koerner TJ, Tzagoloff A. Yeast/E. coli shuttle vectors with multiple unique restriction sites. Yeast. 1986;2:163–167. doi: 10.1002/yea.320020304. [DOI] [PubMed] [Google Scholar]
- Huang S, et al. Specificity of cotranslational amino-terminal processing of proteins in yeast. Biochemistry. 1987;26:8242–8246. doi: 10.1021/bi00399a033. [DOI] [PubMed] [Google Scholar]
- Jensenius JC, Andersen I, Hau J, Crone M, Koch C. Eggs: conveniently packaged antibodies. Methods for purification of yolk IgG. J Immunol Methods. 1981;46:63–68. doi: 10.1016/0022-1759(81)90333-1. [DOI] [PubMed] [Google Scholar]
- Kellems RE, Allison VF, Butow RA. Cytoplasmic type 80 S ribosomes associated with yeast mitochondria. II. Evidence for the association of cytoplasmic ribosomes with the outer mitochondrial membrane in situ. J Biol Chem. 1974a;249:3297–3303. [PubMed] [Google Scholar]
- Kellems RE, Allison VF, Butow RA. Cytoplasmic type 80 S ribosomes associated with yeast mitochondria. IV. Attachment of ribosomes to outer membranes of isolated mitochondria. J Cell Biol. 1974b;65:1–14. doi: 10.1083/jcb.65.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellems RE, Butow RA. Cytoplasmic type 80 S ribosomes associated with yeast mitochondria. III. Changes in the amount of bound ribosomes in response to changes in metabolic state. J Biol Chem. 1974;249:3304–3310. [PubMed] [Google Scholar]
- Klier H, Magdolen V, Schricker R, Strobel G, Lottspeich F, Bandlow W. Cytoplasmic and mitochondrial forms of yeast adenylate kinase are N-acetylated. Biochim Biophys Acta. 1996;1280:251–256. doi: 10.1016/0005-2736(95)00304-5. [DOI] [PubMed] [Google Scholar]
- Knox C, Sass E, Neupert W, Pines O. Import into mitochondria, folding and retrograde movement of fumarase in yeast. J Biol Chem. 1998;273:25587–25593. doi: 10.1074/jbc.273.40.25587. [DOI] [PubMed] [Google Scholar]
- Koehler CM, Merchant S, Schatz G. How membrane proteins travel across the mitochondrial intermembrane space. Trends Biochem Sci. 1999;24:428–432. doi: 10.1016/s0968-0004(99)01462-0. [DOI] [PubMed] [Google Scholar]
- Kübrich M, Dietmeier K, Pfanner N. Genetic and biochemical dissection of the mitochondrial protein-import machinery. Curr Genet. 1995;27:393–403. doi: 10.1007/BF00311207. [DOI] [PubMed] [Google Scholar]
- Kunkel TA, Roberts JD, Zakour RA. Rapid and efficient site-specific mutagenesis without phenotypic selection. Methods Enzymol. 1987;154:367–382. doi: 10.1016/0076-6879(87)54085-x. [DOI] [PubMed] [Google Scholar]
- Laminet AA, Plückthun A. The precursor of β-lactamase: purification, properties and folding kinetics. EMBO J. 1989;8:1469–1478. doi: 10.1002/j.1460-2075.1989.tb03530.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lill R, Nargang FE, Neupert W. Biogenesis of mitochondrial proteins. Curr Opin Cell Biol. 1996;8:505–512. doi: 10.1016/s0955-0674(96)80028-7. [DOI] [PubMed] [Google Scholar]
- Lill R, Neupert W. Mechanisms of protein import across the mitochondrial outer membrane. Trends Cell Biol. 1996;6:56–61. doi: 10.1016/0962-8924(96)81015-4. [DOI] [PubMed] [Google Scholar]
- Lithgow T, Cuezva JM, Silver PA. Highways for protein delivery to the mitochondrion. Trends Biol Sci. 1997;22:110–113. doi: 10.1016/s0968-0004(97)01007-4. [DOI] [PubMed] [Google Scholar]
- Liu G, Topping TB, Randall LL. Physiological role during export for the retardation of folding by the leader peptide of maltose-binding protein. Proc Natl Acad Sci USA. 1989;86:9213–9217. doi: 10.1073/pnas.86.23.9213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magdolen V, Oechsner U, Bandlow W. The complete sequence of the gene coding for yeast adenylate kinase. Curr Genet. 1987;12:405–411. doi: 10.1007/BF00434817. [DOI] [PubMed] [Google Scholar]
- Magdolen V, Schricker R, Strobel G, Germaier H, Bandlow W. In vivo import of yeast adenylate kinase into mitochondria affected by site directed mutagenesis. FEBS Lett. 1992;299:267–272. doi: 10.1016/0014-5793(92)80129-5. [DOI] [PubMed] [Google Scholar]
- Müller G, Bandlow W. An amphitropic cAMP-binding protein in yeast mitochondria. Synergistic control of the intramitochondrial location by calcium and phospholipid. Biochemistry. 1989;28:9957–9967. doi: 10.1021/bi00452a013. [DOI] [PubMed] [Google Scholar]
- Najarian D, Dihanich ME, Martin NC, Hopper AK. DNA sequence and transcript mapping of MOD5: features of the 5′ region which suggest two translational starts. Mol Cell Biol. 1987;7:185–191. doi: 10.1128/mcb.7.1.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natsoulis G, Hilger F, Fink GR. The HTS1 gene encodes both the cytoplasmic and mitochondrial histidine tRNA synthetases of S. cerevisiae. Cell. 1986;46:235–243. doi: 10.1016/0092-8674(86)90740-3. [DOI] [PubMed] [Google Scholar]
- Nobumoto M, Yamada M, Song S, Inouye S, Nakazawa A. Mechanism of mitochondrial import of adenylate kinase isoenzymes. J Biochem. 1998;123:128–135. doi: 10.1093/oxfordjournals.jbchem.a021899. [DOI] [PubMed] [Google Scholar]
- Noda LH. Adenylate kinase. In: Boyer PD, editor. The Enzymes. Vol. 8. Orlando, FL: Academic Press; 1973. pp. 279–305. [Google Scholar]
- Pfanner N, Craig EA, Hönlinger A. Mitochondrial preprotein translocase. Annu Rev Cell Dev Biol. 1997;13:25–51. doi: 10.1146/annurev.cellbio.13.1.25. [DOI] [PubMed] [Google Scholar]
- Roise D, Theiler F, Horvath SJ, Tomich JM, Richards JB, Allison DS, Schatz G. Amphiphilicity is essential for mitochondrial presequence function. EMBO J. 1988;7:649–653. doi: 10.1002/j.1460-2075.1988.tb02859.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning, A Laboratory Manual. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- Schricker R, Magdolen V, Bandlow W. A new member of the adenylate kinase family in yeast: PAK3 is highly homologous to mammalian AK3 and is targeted to mitochondria. Mol Gen Genet. 1992a;233:363–371. doi: 10.1007/BF00265432. [DOI] [PubMed] [Google Scholar]
- Schricker R, Magdolen V, Kaniak A, Wolf K, Bandlow W. The adenylate kinase family in yeast: identification of URA6 as a multicopy suppressor of deficiency of major AMP kinase. Gene. 1992b;122:111–118. doi: 10.1016/0378-1119(92)90038-q. [DOI] [PubMed] [Google Scholar]
- Schricker R, Magdolen V, Strobel G, Bogengruber E, Breitenbach M, Bandlow W. Strain-dependent occurrence of functional GTP:AMP phosphotransferase (AK3) in Saccharomyces cerevisiae. J Biol Chem. 1995;270:31103–31110. doi: 10.1074/jbc.270.52.31103. [DOI] [PubMed] [Google Scholar]
- Schulz GE. Structural and functional relationships in the adenylate kinase family. Cold Spring Harbor Symp Quant Biol. 1987;52:429–439. doi: 10.1101/sqb.1987.052.01.050. [DOI] [PubMed] [Google Scholar]
- Stein I, Peleg Y, Even-Ram S, Pines O. The single translation product of the FUM1 gene (fumarase) is processed in mitochondria before being distributed between the cytosol and mitochondria in Saccharomyces cerevisiae. Mol Cell Biol. 1994;14:4770–4778. doi: 10.1128/mcb.14.7.4770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner H, Zollner A, Haid A, Neupert W, Lill R. Biogenesis of mitochondrial heme lyases in yeast. Import and folding in the intermembrane space. J Biol Chem. 1995;270:22842–22849. doi: 10.1074/jbc.270.39.22842. [DOI] [PubMed] [Google Scholar]
- Suissa M, Schatz G. Import of proteins into mitochondria. Translatable mRNAs for imported mitochondrial proteins are present in free as well as mitochondria-bound cytoplasmic polysomes. J Biol Chem. 1982;257:13068–13074. [PubMed] [Google Scholar]
- Tomasselli AG, Mast E, Janes W, Schiltz E. The complete amino acid sequence of adenylate kinase from baker's yeast. Eur J Biochem. 1986;155:111–119. doi: 10.1111/j.1432-1033.1986.tb09465.x. [DOI] [PubMed] [Google Scholar]
- Ungermann C, Neupert W, Cyr DM. The role of Hsp70 in conferring unidirectionality on protein translocation into mitochondria. Science. 1994;266:1250–1253. doi: 10.1126/science.7973708. [DOI] [PubMed] [Google Scholar]
- Verner K. Co-translational protein import into mitochondria: an alternative view. Trends Biochem Sci. 1993;18:366–371. doi: 10.1016/0968-0004(93)90090-a. [DOI] [PubMed] [Google Scholar]
- von Heijne G. Mitochondrial targeting sequences may form amphiphilic helices. EMBO J. 1986;5:1335–1342. doi: 10.1002/j.1460-2075.1986.tb04364.x. [DOI] [PMC free article] [PubMed] [Google Scholar]