Abstract
Some metazoans have evolved the capacity to survive severe oxygen deprivation. The nematode, Caenorhabditis elegans, exposed to anoxia (0 kPa, 0% O2) enters into a recoverable state of suspended animation during all stages of the life cycle. That is, all microscopically observable movement ceases including cell division, developmental progression, feeding, and motility. To understand suspended animation, we compared oxygen-deprived embryos to nontreated embryos in both wild-type and hif-1 mutants. We found that hif-1 mutants survive anoxia, suggesting that the mechanisms for anoxia survival are different from those required for hypoxia. Examination of wild-type embryos exposed to anoxia show that blastomeres arrest in interphase, prophase, metaphase, and telophase but not anaphase. Analysis of the energetic state of anoxic embryos indicated a reversible depression in the ATP to ADP ratio. Given that a decrease in ATP concentrations likely affects a variety of cellular processes, including signal transduction, we compared the phosphorylation state of several proteins in anoxic embryos and normoxic embryos. We found that the phosphorylation state of histone H3 and cell cycle–regulated proteins recognized by the MPM-2 antibody were not detectable in anoxic embryos. Thus, dephosphorylation of specific proteins correlate with the establishment and/or maintenance of a state of anoxia-induced suspended animation.
INTRODUCTION
Metazoans that survive severe oxygen deprivation arrest energy requiring processes and improve energetic efficiency of metabolic processes until normal oxygen concentrations are restored (Sick et al., 1993; Hochachka et al., 1996; Storey, 1996). Invertebrate embryos such as Artemia franciscana and Drosophila melanogaster maintain a prolonged period of developmental arrest in response to anoxia (0 kPa, 0% O2) and then resume development when reexposed to oxygen (Foe and Alberts, 1985; Hand, 1993; Clegg, 1997). Similarly, zebrafish embryos (Danio rerio) exposed to anoxia enter into a reversible state of suspended animation; all observable movement cease including cell division, developmental progression, and heartbeat (Padilla and Roth, 2001). On return to a normoxic environment, cellular and developmental processes continue normally and the embryos grow into healthy adult fish. The molecular mechanisms that allow metazoans to coordinately arrest a wide range of energy requiring processes such as movement, cell division, and developmental progression in response to anoxia are not completely understood.
Extreme hypoxia is central to the pathology of several diseases such as cardiac and pulmonary dysfunction (Lipton, 1999). Additionally, it is known that within solid tumors, cancerous cells that are severely deprived of oxygen are often more resistant to radiation and chemotherapy (Brown, 1996). Given this, there is much interest in identifying the cellular and molecular response organisms have to various levels of oxygen deprivation (Brown, 1996; Guillemin and Krasnow, 1997; Semenza et al., 2000). In both mammals and Caenorhabditis elegans the hypoxia inducing factor (HIF-1) responds to a decrease in oxygen concentrations and contributes to the ability to survive hypoxia. The regulation of HIF-1a, through a VHL-prolyl hydroxylase pathway, is conserved between mammals and nematodes (Epstein et al., 2001; Ivan et al., 2001; Jaakkola et al., 2001; Jiang et al., 2001; Semenza, 2001). These studies support the idea that the response of gene expression changes due to chronic hypoxic exposure evolved before the divergence of invertebrates and vertebrates.
Through mechanisms unknown, zebrafish and fly embryos exposed to anoxia arrest at specific stages of the cell cycle. In the case of the zebrafish embryo, the rapidly dividing blastomeres exposed to anoxia do not arrest in mitosis. Further evaluation of zebrafish embryos exposed to anoxia, by flow cytometry analysis, indicates that blastomeres arrest during the S and G2 phases of the cell cycle (Padilla and Roth, 2001). Blastomeres of D. melanogaster embryos exposed to anoxia arrest during interphase and all stages of mitosis except anaphase (Foe and Alberts, 1985). Fly embryos exposed to hypoxia contain blastomeres that only arrest in interphase or metaphase (DiGregorio et al., 2001; Douglas et al., 2001). Nitric oxide is thought to play a role in the response fly embryos have to hypoxia (Wingrove and O'Farrell, 1999; DiGregorio et al., 2001; Douglas et al., 2001). Zebrafish embryos, unlike fly embryos, do not arrest during mitosis, suggesting that there are vertebrate specific responses to anoxia. It is not completely understood how a reduction in oxygen concentration signals blastomeres within a developing organism to arrest at specific stages of the cell cycle.
C. elegans is a useful model system in biology because it has the ability to do forward genetics, its genome is completely sequenced, and because RNA-mediated interference (RNAi) has been applied to inactivate individual genes systematically on a genomic scale ( C. elegans Sequencing Consortium, 1998; Fraser et al., 2000). It has been shown that C. elegans can survive a wide range of oxygen concentrations, including anoxia (Paul et al., 2000; Van Voorhies and Ward, 2000). In this report we demonstrate that, like zebrafish embryos, nematodes survive anoxia by entering into a reversible state of suspended animation in which cell division, developmental progression, and movement ceased. We found that the mechanism leading to suspended animation in anoxia may not use signaling pathways that overlap with responses to hypoxia because the hypoxia-induced transcription factor HIF-1 is not required for nematodes to enter into or exit from anoxia-induced suspended animation. We suggest that the coordinated arrest and recovery of embryos is achieved by metabolic responses to anoxia because we find that ATP levels fall in anoxic embryos and that the state of phosphorylation of proteins that are normally phosphorylated in mitosis is not detected when embryos are arrested.
MATERIALS AND METHODS
Oxygen Deprivation Environments
For all studies we used the anaerobic bio-bag type A environmental chamber according to manufactures instructions (Becton Dickinson, Cockeysville, MD; Padilla and Roth, 2001). This method contains a resazurin indicator that allows one to determine when the anoxic environment is established. We used a second method to verify suspended animation results. This method involved use of a chamber perfused with 100% N2 gas (Airgas or Byrne Gas, Seattle, WA) and monitored for oxygen using a Fyrite O2 gas analyzer (Bacharach, Pittsburgh, PA) and a resazurin indicator (Becton Dickinson). To produce a hypoxic environment a chamber was perfused with 0.5% O2 balanced with N2 gas (Byrne Gas, Seattle). These methods required ∼60–90 min to establish the oxygen deprivation environment. During most of this transition period animals behaved and developed at a rate comparable to untreated nematodes.
Viability of Nematodes in Anoxia
Bristol strain N2 was cultured as described (Sulston and Hodgkin, 1988). Synchronized populations of animals used in viability studies were obtained as embryos from hypochlorite-treated adults and allowed development to the life cycle stage of interest. Adult studies were done using young hermaphrodites that were ∼24 h after the L4 larvae stage. Dauer larvae were collected using 1% SDS, placed on a nematode growth medium (NGM) plate with food, and immediately placed into anoxia, thus allowing dauer larvae to be exposed to food upon recovery from the anoxic environment (Sulston and Hodgkin, 1988). Embryos were obtained by allowing young adults to lay eggs on an NGM plate with food for 2 hours before removal of the adults and exposure to anoxia. Two methods were used to collect L1 larvae for viability assays. The first method involved hypochlorite treatment of adults, hatching embryos to the L1 stage in sterile M9 Ringer's media overnight. These L1 larvae were placed on a plate with food and immediately put into anoxia. In this method the L1 larvae were not exposed to food except immediately before anoxic exposure. The second method involved placing young adults on plates with food, allowing embryos to be laid overnight, and collecting the L1 larvae that had hatched onto the food plate the following morning. The L1 larvae were placed onto an NGM plate with food and immediately put into anoxia. All viability assays were done at 20°C.
Viability of the hif-1 Mutant in Anoxia
The hif-1 (ia04) mutation is a 1231-base pair deletion of the second, third, and fourth exons (Jiang et al., 2001). For viability assays, twenty hif-1 (ia04) young adults were placed onto an NGM plate with food and allowed to lay eggs. The adults were removed from the plate within 1.5 h, and embryos were either placed into a hypoxic, anoxic, or normoxic environment. The same 20 adults were used to produce embryos for viability assessment in all conditions (normoxia, anoxia, and hypoxia). On return to a normoxic environment, oxygen deprived embryos were counted and scored for capacity to hatch and develop to adulthood. At least three independent experiments were performed for each condition.
Antibodies
The following antibodies were used: mAb414, which recognizes the FG repeats present in a subset of nuclear pore complexes (Babco, Berkley, CA; Lee et al., 2000), mAb MPM-2, which recognizes many phosphoproteins in mitotic cells (DAKO, Carpinteria, CA; Davis et al., 1983; Moore et al., 1999), phos H3, which recognizes histone H3 when it is phosphorylated on Ser 10 (Upstate Biotechnology, Lake Placid, NY; Hsu et al., 2000), acetylated H3, which recognizes histone H3 when it is acetylated (Dan Gottschling), anti–HCP-1, which recognizes a kinetochore protein (Moore et al., 1999), and Phos SR, which recognizes the phosphorylated form of SR proteins (Neugebauer and Roth, 1997).
Cell Cycle and Cell Biological Analysis
The stage of mitotic arrest was determined by use of the DNA-binding dye DAPI and an mAb (mAb414) that recognizes nuclear envelope pore complexes in a variety of organisms including C. elegans (Browning and Strome, 1996; Pitt et al., 2000). In >30-cell embryos, mAb414 stains the nuclear envelop during interphase, prophase, and telophase, but diminishes during metaphase and is completely absent during anaphase; thus, this antibody can be used to distinguish between blastomeres in anaphase or telophase (Lee et al., 2000; Moore and Roth, 2001). Embryo collection and immunofluroescence was done as described below. Three independent experiments, with a total of ∼200–300 mitotic blastomeres for each environmental condition were examined.
Immunostaining, PAGE, and Western Blot Analysis
Embryos were collected, by chopping adults with razor blades and filtering embryos away from the adult body fragments by using 43- and 15-μm nitex filters (TEKTO, Elmsford, NY). Embryos, mixed with enough M9 Ringer's to prevent dehydration, were equally divided onto three NGM plates (Sulston and Hodgkin, 1988), two of which were placed in an anoxic environment. Control embryos (normoxia) were collected when the experimental embryos placed within the anoxic environment transitioned from a normoxic to an anoxic environment. For immunostaining experiments, embryos remained in the anoxic environment for 24 h and were either immediately (<1 min) collected onto glass slides and frozen on dry ice (anoxia) or allowed to recover in air for 1 h before collection onto glass slides and frozen on dry ice (postanoxia). Immunostaining of embryos was performed essentially as described as previously described (Moore et al., 1999; Moore and Roth, 2001). Microscopy was done on a Zeiss axioscope (Thornwood, NY). Images were collected and analyzed by using QED Imaging (Pittsburgh, PA) and Adobe Photoshop 5.5 (San Jose, CA). For PAGE and protein blot experiments, control embryos (normoxia) were collected when the experimental embryos placed within the anoxic environment transitioned from a normoxic to an anoxic environment. Experimental embryos (anoxia) remained in the anoxic environment for 24 h and were immediately collected and washed with 100% acetone, followed by an 80% acetone wash, and then suspended in protein sample buffer. Protein extracts were subjected to SDS-PAGE, transferred to an Optitran nitrocellulose membrane (Keene, NH) and immunoblotted with specific antibodies in a similar manner as previously described (Neugebauer and Roth, 1997). The protein blot experiment with anoxia-exposed embryos was repeated three times.
Nucleotide Ratio Concentrations
Embryos collected in the same way as was done for cell biology studies were distributed into three eppindorf tubes and placed into either a normoxic or an anoxic environment before processing. Untreated embryos (normoxia) were processed 90 min after collection. Experimental embryos were exposed to anoxia for 24 h and then processed either immediately (anoxia) or after 1 h of recovery in air (post-anoxia). Nucleotide levels were determined by methods described previously (Zager et al., 1999). Briefly, the embryos were processed by placing into 66% TCA, followed by eight intervals of 30-s sonication followed by 30 s on ice. After brief centrifugation the supernatant was extracted using freon triocytl amine, and the upper phase was filtered and quantified by HPLC.
RESULTS
Nematodes Survive Anoxia by Entering a Reversible State of Suspended Animation
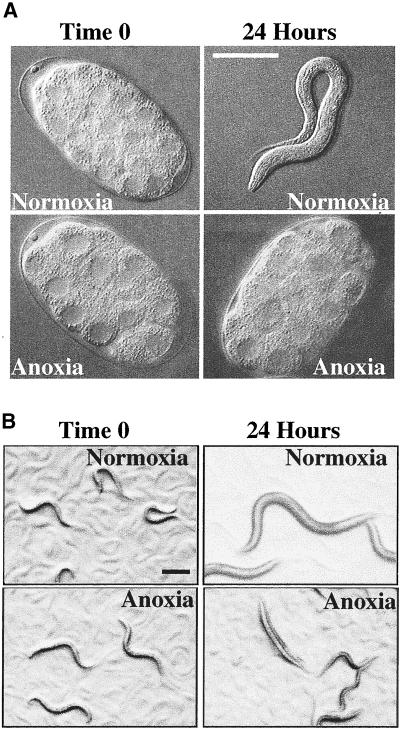
Zebrafish embryos can survive 24 h of anoxia by entering into a state of suspended animation, where all observable movement cease including cell division, developmental progression, and motility (Padilla and Roth, 2001). To determine if C. elegans have a similar response to anoxia as zebrafish embryos do, we exposed embryonic and postembryonic nematodes to anoxia. We found that nematodes, in normal culture and temperature conditions, enter a reversible state of suspended animation in response to anoxia. In the anoxic environment, nematode development stopped and postembryonic nematodes became immobile, stopped feeding, and in the case of adults, did not lay eggs (Figure 1, A and B). After reexposure to a normoxic (21 kPa, 21% O2) environment, nematode development continued and postembryonic nematodes moved and fed in a manner indistinguishable from untreated nematode controls. To determine whether exposure to anoxia caused any long-term effects, we raised >200 embryos, which were exposed to 24 h of anoxia, to sexually mature adults. These nematodes were capable of producing offspring and displayed no detectable defects.
Figure 1.
C. elegans arrest when exposed to anoxia. (A) Two-cell embryos were collected and exposed to either a normoxic (21 kPa, 21% O2) environment or an anoxic (0 kPa, 0% O2) environment. Nematodes were visualized using Nomarski optics. Images were collected and analyzed using NIH image and Adobe Photoshop 5.5. Embryos are ∼50 μm in length. Bar, 50 μm for image showing L1 larva only. (B) L3 larvae were collected and exposed to either a normoxic or an anoxic environment for 24 h. Nematodes were visualized using a dissecting microscope. Images were collected and analyzed using Metamorph and Adobe Photoshop 5.5. Bar, 200 μm. For A and B, methods used to establish the anoxic environment required ∼60–90 min. During most of this transition period animals behaved and developed at a rate comparable to untreated nematodes. Time 0 is the time nematodes began to arrest development. The second time point is 24 h after time 0.
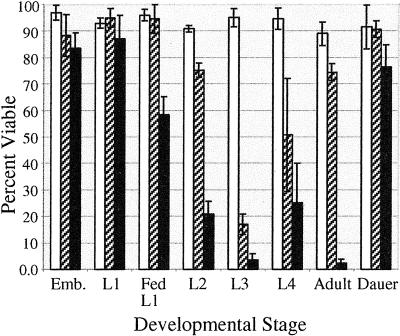
Organisms such as D. melanogaster, A. franciscana, and zebrafish embryos survive anoxia during specific times in development (Foe and Alberts, 1985; Clegg, 1997; Padilla and Roth, 2001). In C. elegans, others have shown that embryos, L2 larvae, L4 larvae, dauer larvae, and adult nematodes survive anoxia (Anderson, 1978; Van Voorhies and Ward, 2000). We confirm that embryos, L2 larvae, L4 larvae, dauer larvae, and adult nematodes survive 24 h of anoxia at a rate of ≥90% (Figure 2). To completely describe the anoxia survival rate of nematodes at all stages of development and to determine if specific stages of development are less sensitive to anoxia, we subjected embryonic and postembryonic nematodes to anoxia for 24, 48, and 72 h. We determined that the L1 larvae and L3 larvae stages survive 24 h of anoxia at a rate of ≥90% (Figure 2). With the exception of embryos, starved L1 larvae, and dauer larvae, the nematode capacity to survive 72 h of anoxia decreased. Nematodes at the L3 larvae stage appear to be more sensitive, in comparison to other developmental stages, to 48 h of anoxia. Embryos laid from adults have high survival rates in anoxia. Embryos removed from an adult can survive 24 h of anoxia, however, the ability to survive prolonged anoxia decreased, suggesting that the treatment of embryos or developmental stage may influence anoxia survival (our unpublished results). L1 larvae that were starved have a higher survival rate to anoxia than L1 larvae that had been fed, which could be due to stage of larvae development or a link between metabolism and anoxia survival. Our studies conclude that all developmental stages can survive 24 h of anoxia; however, the ability to survive prolonged exposure to anoxia is influenced by the stage of development or treatment of the nematode.
Figure 2.
Viability of C. elegans exposed to anoxia. Survival of nematodes in anoxia for 24 h (white bars), 48 h (slashed bars), or 72 h (black bars) was determined for all stages of development. L1 larvae were either starved or fed before placed into anoxia (see MATERIALS AND METHODS). Adult hermaphrodites were collected ∼24 h after the L4 larvae stage. The data shown are representative of three independent experiments, with a total of more than 400 nematodes for each of the postembryonic stages and more than 200 embryos. Error bar represents SD. All experiments were done at 20°C.
HIF-1 Function Is Not Required for Anoxia-induced Suspended Animation
Responses to oxygen deprivation involve the hypoxia-inducing factor HIF-1 in both mammals and C. elegans (Epstein et al., 2001). It has been shown that in C. elegans a loss of function mutation in HIF-1 specifically renders nematodes sensitive to a hypoxic environment with no phenotype observed in a normoxic environment (Jiang et al., 2001). To determine whether HIF-1 function is required for anoxia-induced suspended animation, we exposed the hif-1 mutant embryos to anoxia. We determined that the hif-1 mutant is capable of surviving prolonged periods of anoxia (Table 1). The hif-1 mutant embryos exposed to anoxia arrest development and are capable of maintaining this arrested state for at least 3 d at a level comparable to wild type. On return to normoxia the hif-1 mutant animals developed into sexually mature adults. When hif-1 mutant embryos were cultured in hypoxic conditions (0.51 kPa, 0.5% O2) ∼67% of the embryos did not survive embryogenesis, and only ∼7% are capable of progressing in development to adulthood (Table 1), which agrees with what others have shown (Jiang et al., 2001). The majority of wild-type N2 embryos exposed to an oxygen tension of 0.51 kPa hatch, and ∼92% survive to adulthood. Our results demonstrate that although HIF-1 function is required for hypoxia survival, it is not required for anoxia-induced suspended animation. This suggests that the molecular mechanisms for anoxia survival are different from those that regulate hypoxia survival.
Table 1.
Viability of hif-1(ia04) in anoxia
| Strain (condition, time)a | Hatched embryos (%)b | Survive to Adulthood (%)b | nc |
|---|---|---|---|
| hif-1 (anoxia, 1 d) | 99.1 ± 0.9 | 97.2 ± 2.6 | 362 |
| hif-1 (anoxia, 3 d) | 95.5 ± 2.6 | 87.0 ± 10.0 | 475 |
| hif-1 (hypoxia, 1 d) | 66.9 ± 9.8 | 7.7 ± 5.3 | 354 |
| N2 (anoxia, 1 d) | 98.8 ± 1.3 | 97.0 ± 2.8 | 287 |
| N2 (anoxia, 3 d) | 95.7 ± 2.0 | 83.5 ± 5.9 | 405 |
| N2 (hypoxia, 1 d) | 99.3 ± 0.6 | 91.9 ± 7.2 | 285 |
Anoxia is 0% oxygen (0 kPa); hypoxia is .5% oxygen (0.51 kPa).
Values are mean ± SD.
n is total number of embryos evaluated from 3 independent experiments.
Blastomeres of Nematode Embryos in a State of Suspended Animation Do Not Arrest during Anaphase
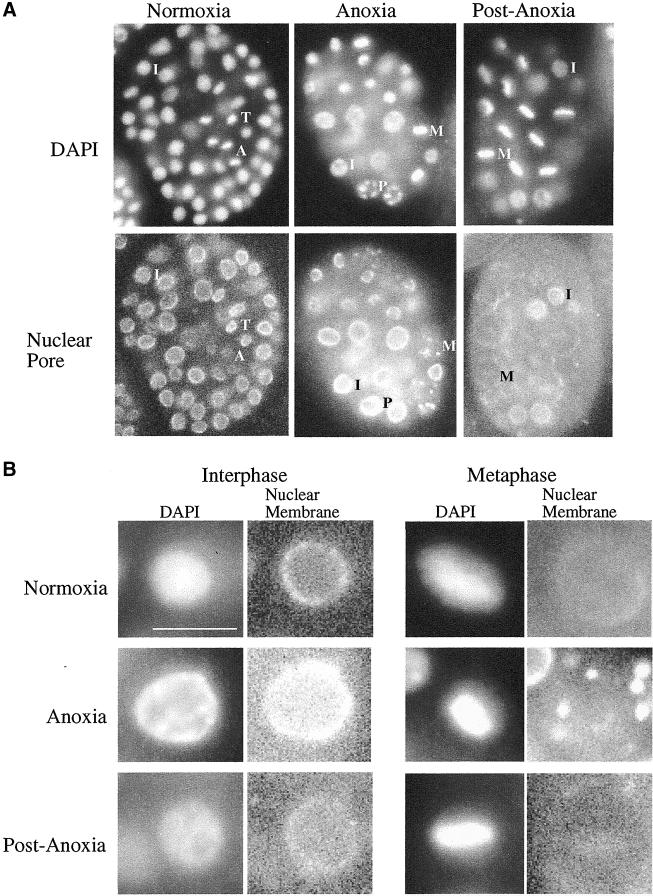
Given that a well-known responder to oxygen deprivation, HIF-1, does not appear to be required for anoxia-induced suspended animation in nematodes we decided to better characterize nematodes exposed to anoxia as an initial way to further understand anoxia induced suspended animation. Blastomeres from zebrafish and fly embryos exposed to oxygen deprivation arrest at a specific point in the cell cycle (Foe and Alberts, 1985; DiGregorio et al., 2001; Douglas et al., 2001; Padilla and Roth, 2001). To determine if nematode embryos in a state of suspended animation arrest at specific points in the cell cycle, we compared embryos exposed to anoxia with untreated embryos. The stage of mitotic arrest was determined by use of the DNA-binding dye DAPI, and the mAb mAb414, which recognizes nuclear envelope pore complexes in a variety of organisms including C. elegans. In >30-cell embryos, mAb414 stains the nuclear envelope during interphase, prophase, and telophase, but diminishes during metaphase and is completely absent during anaphase, thus this antibody along with DAPI can be useful for distinguishing the mitotic stage of blastomeres (Lee et al., 2000; Moore and Roth, 2001). Blastomeres of anoxic embryos, at a variety of stages of embryogenesis, arrested in interphase and at all stages of mitosis except anaphase (Figure 3A and Table 2). The number of blastomeres in telophase was substantially reduced, in anoxic embryos versus untreated embryos (Table 2). When arrested embryos were allowed to recover in air development progressed with a frequency of blastomeres in anaphase and telophase comparable to untreated embryos (Table 2). That blastomeres from anoxia-exposed C. elegans embryos can arrest in mitosis contrasts with studies in zebrafish embryos that arrest only in the S and G2 phases of the cell cycle (Padilla and Roth, 2001). However, the C. elegans results are similar to studies in Drosophila, showing that embryos deprived of oxygen do not arrest in anaphase (Foe and Alberts, 1985; DiGregorio et al., 2001; Douglas et al., 2001). These data suggest that invertebrate and vertebrate embryos may not have an identical response to anoxia and that the mechanism(s) regulating anoxia-induced arrest is dependent on cell cycle position.
Figure 3.
Cell biology analysis of anoxia-exposed embryos versus untreated embryos. (A) Image of blastomeres, at various stages of the cell cycle, of embryos exposed to either a normoxic or anoxic environment. Representatives of blastomeres in prophase (P), metaphase (M), anaphase (A), telophase (T), and interphase (I) are left of the letter denoting such. Embryos are ∼50 μm in length. (B) Enlarged images of blastomeres, in metaphase or interphase stages of the cell cycle, of embryos exposed to either a normoxic or anoxic environment. Bar, ∼5 μm for enlarged images of interphase and metaphase nuclei. For A and B control embryos (normoxia) were collected when the experimental embryos arrested development, ∼90 min. Experimental embryos remained in the anoxic environment for 24 h and were either immediately collected (anoxia) or allowed a 1-h recovery period in air (postanoxia). Embryos were stained with the DNA binding dye DAPI and nuclear pore complex antibody mAb414.
Table 2.
Mitotic stage of blastomeres in anoxic embryos
| Prophase (%) | Metaphase (%) | Anaphase (%) | Telophase (%) | na | |
|---|---|---|---|---|---|
| Normoxia | 37.0 | 24.0 | 19.4 | 19.7 | 262 |
| Anoxia | 47.6 | 48.6 | 0.0 | 5.0 | 318 |
| Postanoxia | 44.1 | 29.6 | 12.5 | 13.7 | 190 |
n is total number of blastomeres evaluated from three independent experiments.
Blastomeres in Nematode Embryos in a State of Suspended Animation Display an Alteration in Cellular Structures
Through mechanisms unknown, interphase blastomeres from zebrafish and fly embryos exposed to anoxia contain chromosomal DNA that is not uniformly distributed throughout the nucleus (Foe and Alberts, 1985; Padilla and Roth, 2001). To determine if such an alteration occurs in nematodes, we analyzed nematode blastomeres from embryos exposed to anoxia. We found that in comparison to untreated embryos, the chromosomal DNA of interphase arrested blastomeres is not uniformly distributed throughout the nucleus (Figure 3B). When arrested embryos were allowed to recover in air the chromosomal DNA from interphase nuclei became more uniform through out the nucleus (Figure 3B). Our results further support the idea that the alteration of chromosomal DNA distribution, in interphase blastomeres of embryos exposed to anoxia, is conserved between vertebrates and invertebrates (Foe and Alberts, 1985; Padilla and Roth, 2001).
Blastomeres from nematode embryos exposed to anoxia were further examined to determine if other alterations occur within the nucleus. Untreated embryos display a reduction of mAb414 staining during metaphase, indicating nuclear pore complex breakdown during this stage of the cell cycle (Lee et al., 2000). However, in metaphase blastomeres of anoxia-treated embryos the mAb414 antibody recognized an aggregate of structures surrounding the chromosomes, suggesting that the nuclear-pore-complex breakdown is altered in comparison to untreated embryos at a similar stage of the cell cycle (Figure 3B). This altered mAb414 staining was observed in anoxia-arrested embryos regardless of the stage of embryogenesis. When arrested embryos were allowed to recover in air, mAb414 no longer recognized an aggregate of structures surrounding the chromosomes in metaphase blastomeres (Figure 3B). Our findings indicate that alteration in nuclear structures such as nuclear pore complexes and chromosomal DNA occurs in embryos exposed to anoxia, which are easily recognized markers for the state of suspended animation.
The ATP Pools Decrease in Embryos in a State of Suspended Animation
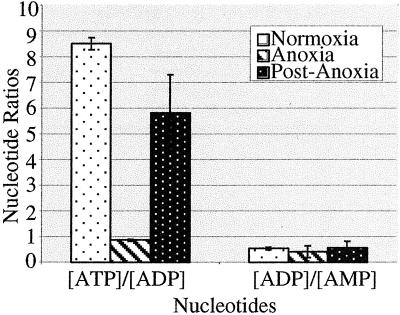
Some organisms survive severe oxygen deprivation by downregulating energy turnover and upregulating the efficiency of ATP producing pathways (Hochachka, 1986; Hochachka and Lutz, 2001). Given that oxygen is required for biosynthesis of ATP through oxidative phosphorylation, known physiological responses to anoxia include a decrease in ATP concentrations and matching of ATP hydrolysis with anaerobic ATP synthesis (Wegener and Krause, 1993). To determine if energy availability decreased in nematode embryos exposed to anoxia, we assayed the ratio of adenine nucleotides. We found that embryos exposed to anoxia exhibit a large decrease in the ATP to ADP ratio in comparison to untreated embryos (Figure 4). However, a significant deviation in the ratio of ADP to AMP did not occur in embryos exposed to anoxia. In embryos that were exposed to anoxia and then allowed to recover in air the ratio of ATP to ADP increased (Figure 4). Our data suggest that the energy availability of ATP, decreases in nematodes exposed to anoxia but will increase after reexposure to oxygen.
Figure 4.
Nucleotide ratios in embryos exposed to anoxia. Embryos were collected and exposed to either a normoxic or an anoxic environment. Control embryos (normoxia) were collected and processed when experimental embryos arrested development. Experimental embryos (anoxia) remained in the anoxic environment for 24 h and were either immediately collected or allowed to recover in air for 1 h (postanoxia). Nucleotide ratios of embryos were determined using HPLC. Error bar represents SD.
Dephosphorylation of Specific Proteins Occurs in Embryos in a State of Suspended Animation
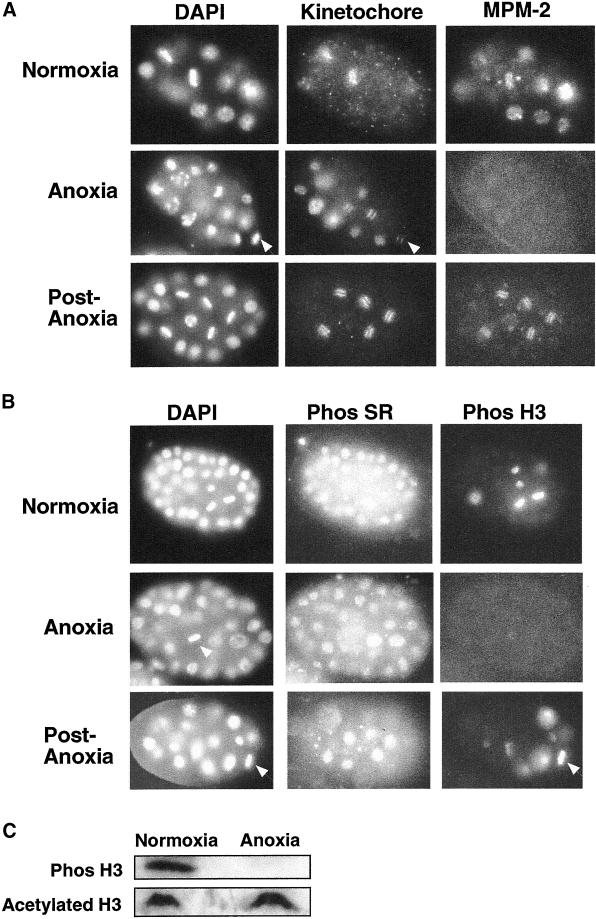
A reduction of intracellular ATP levels in embryos exposed to anoxia likely affects a variety of cellular processes, including posttranslational modifications. To determine if phosphoepitopes are altered in embryos exposed to anoxia, we used antibodies that recognize phosphoepitopes on the SR-protein splicing factors (Neugebauer and Roth, 1997) and two antibodies that recognize phosphoproteins specific for cells in mitosis (Phos H3 and MPM-2; Davis et al., 1983; Hsu et al., 2000). In several organisms including C. elegans embryos, cells in mitosis contain an increase in phosphorylation of histone H3 at serine 10 (Hsu et al., 2000). The MPM-2 antibody recognizes a variety of proteins phosphorylated during mitosis in organisms such as nematodes, yeast, and mammalian cells (Davis et al., 1983; Moore et al., 1999). In nematodes the MPM-2 antibody also recognizes proteins associated with P-granules in the germline blastomere (our unpublished results). Untreated embryos contain mitotic blastomeres with positive immunofluroescence with MPM-2 and antiphosphohistone H3, whereas arrested embryos exposed to anoxia contain mitotic blastomeres with no detectable antibody staining with MPM-2 or antiphosphohistone H3 (Figure 5, A and B). P-granules within the germ cell of embryos exposed to anoxia were also not detected with the MPM-2 antibody (our unpublished results). In evaluation of at least 100 embryos exposed to anoxia, at least 92% of the embryos contained no blastomeres with detectable antibody staining with antiphosphohistone H3 or MPM-2, whereas embryos in normoxic conditions <8% of the embryos contain blastomeres with no detectable antibody staining. An antibody specific for a kinetochore protein (HCP-1) that exhibits dynamic changes in distribution during mitosis was used as a control (Moore et al., 1999). In contrast to the diminished antibody staining with MPM-2 and antiphosphohistone H3, we were able to detect staining in arrested embryos using antiphospho SR antibody (Figure 5B). Embryos that were allowed to recover in air (postanoxia) contained detectable staining with MPM-2 and antiphosphohistone H3 (Figure 5, A and B). Closer examination of postanoxia embryos indicates that there is a slight difference in MPM-2 staining of postanoxia embryos in comparison to normoxic embryos, in that the staining of blastomeres in metaphase of embryos recovering from anoxia is more pronounced and sharp in comparison to normoxic embryos. This could be due to the fact that postanoxic embryos are in a recovering state. Finally, embryos exposed to hypoxia (0.51 kPa, 0.5% O2) for 2 h contain mitotic blastomeres with positive immunofluroescence with MPM-2 and antiphosphohistone H3 (our unpublished results). Our results indicate the inability to detect staining for a subset of phosphoepitopes is associated with anoxia-induced suspended animation.
Figure 5.
Phosphoepitopes on some proteins are reduced in arrested embryos. Embryos were collected exposed to either a normoxic or an anoxic environment. Control embryos (normoxia) were collected when experimental embryos arrested development. Experimental embryos remained in the anoxic environment for 24 h and were either immediately collected (anoxia) or allowed a 1-h recovery period in air (postanoxia). For A and B after embryo collection, embryos were fixed and stained with (A) DAPI, a kinetochore protein antibody (anti–HCP-1) and mAb MPM-2, or (B) DAPI, phos SR, and phos H3 antibodies. White arrows point to examples of blastomeres in metaphase. (C) Western blot analysis of total proteins from control embryos (normoxia) or experimental embryos exposed to anoxia for 24 h (anoxia). Protein blot was probed with phos H3 antibody and acetylated H3 antibody.
Several possibilities could account for the loss of staining, including loss of phosphate residues on the proteins, loss of the proteins, or sequestration of phosphoepitopes. To distinguish among these possibilities we used PAGE and Western blot analysis to examine both the abundance and phosphorylation state of serine 10 on histone H3. Histone H3 abundance was similar in untreated embryos versus embryos exposed to anoxia, as seen by Coomassie staining and by Western blot analysis using an antibody that binds the acetylated form of histone H3 (Figure 5C). Additionally, we were able to detect the acetylated form of histone H3 in embryos exposed to anoxia using indirect immunofluroescence (our unpublished results). These results confirm that there is no loss of histone H3 in embryos arrested in anoxia. The phosphorylated form of histone H3 was detected in untreated embryos. However the phosphorylated form of histone H3 was not detected in embryos exposed to anoxia (Figure 5C). These results indicate that anoxia-induced suspended animation is correlated with removal of phosphates on histone H3.
DISCUSSION
In this article we characterize the capacity of C. elegans to respond to anoxia by entering into a reversible state of suspended animation. In anoxia, nematode cell division and developmental progression ceases until reexposed to normal oxygen concentrations. We determined that the phosphorylated form of cell cycle–regulated proteins such as histone H3, and proteins recognized by the MPM-2 antibody were not detected in embryos exposed to anoxia. Moreover, we demonstrate that the capacity of nematodes to survive anoxia-induced suspended animation is not dependent on the well-known hypoxia-inducing factor, HIF-1. This suggest that nematodes do not require induction of HIF-1 targets to enter into a state of suspended animation induced by anoxia and that the molecular mechanisms to survive hypoxia may be different that those required to survive anoxia.
Nematode Survival in Anoxia
We determined that nematodes at all stages of the life cycle survive anoxia, which is similar to results from another group (Van Voorhies and Ward, 2000). The results differ, in that we demonstrate a high survival rate of embryos exposed to anoxia, which may be explained by methods of embryo collection or developmental stage of embryos. Also, we show a greater sensitivity of adults to prolonged anoxia, which may be due to the difference in age of adult scored. We show embryos, starved L1, and dauer larvae display a high survival rate to prolonged anoxia. These three developmental stages had not been feeding for extended periods of time before anoxic exposure, suggesting a link between metabolism and sensitivity to anoxia. It is possible that a metabolic product is altered in response to lack of food or depletion of nutrient stores that aids in an increase in anoxia survival. Alternatively, something inherent about these stages of development may allow better survival to stress.
C. elegans is a good model system for studying hypoxia because hypoxic responses involving HIF-1 is conserved between mammals and nematodes (Epstein et al., 2001; Jiang et al., 2001). We found that HIF-1 is not required for nematode embryos to survive anoxia. This suggests that the molecular mechanisms required for survival of anoxia are different than those required for hypoxia survival. HIF-1 protein accumulation is likely not the signal for anoxia-induced suspended animation in developing organisms. The use of C. elegans as a model system to study oxygen deprivation will aid in our ability to distinguish between the molecular mechanisms required for various levels of oxygen deprivation in a metazoan.
Cell Biological Characterization of Embryos Exposed to Anoxia
We determined that nematode embryos exposed to anoxia contain blastomeres that arrest during interphase, prophase, metaphase, and to a lesser extent telophase but not anaphase. The fact that embryos exposed to anoxia contain blastomeres that arrest at specific points in the cell cycle suggests that the mechanism regulating developmental arrest may be dependent on cell cycle position. Anaphase is the stage of mitosis when chromosomes are being segregated and proper segregation is essential for decreasing chromosomal abnormalities. It is possible that cell-cycle checkpoint mechanisms could be activated in the metaphase to anaphase transition in response to low oxygen concentrations. In yeast a spindle checkpoint delays the metaphase to anaphase transition; however, such a checkpoint mechanism, in the developing nematode embryo, has not been demonstrated (Hardwick, 1998). An alternate explanation as to why blastomeres from nematode embryos exposed to anoxia arrest at specific stages of the cell cycle is that arrest occurs at positions where energy requirements for further progression is greatest. It remains to be determined what mechanisms control arrest of the cell cycle in response to anoxia.
Similar to D. melanogaster embryos exposed to anoxia, nematodes exposed anoxia did not arrest in anaphase (Foe and Alberts, 1985). In contrast, blastomeres of zebrafish embryos exposed to anoxia arrest in the S and G2 phases of the cell cycle (Padilla and Roth, 2001). Zebrafish embryos have G1 and G2 phases of the cell cycle after midblastula (Zamir et al., 1997). However, it is unknown if C. elegans have G1 and G2 phases of the cell cycle during embryogenesis. Thus, the differences in cell cycle stage of arrest in zebrafish versus nematodes or flies may be due to the pattern of the cell cycle for the particular embryo exposed to anoxia.
We identified cell biological markers for C. elegans in a state of anoxia-induced suspended animation. Similar to work shown in flies and zebrafish embryos, anoxic nematode embryos contain interphase blastomeres with a redistribution of the DNA within the nucleus (Foe and Alberts, 1985; Padilla and Roth, 2001). This may indicate that anoxia exposure reduces transcription and DNA synthesis, which is known to cause a redistribution of the DNA in the nucleus. We also demonstrate that the nuclear pore complex accumulation is altered in blastomeres in metaphase of arrested embryos. Perhaps this nuclear pore complex alteration occurs because cells arrest during the early stages of metaphase, before all of the nuclear pore complexes are disassembled or that there is an insufficient level of energy or other metabolites within the cell to allow disassembly of these complexes. The alteration of nuclear pore complexes and DNA from interphase nuclei is reversed when the anoxia-exposed embryo is allowed to recover in air. The role of these cellular alterations in suspended animation is not understood.
Suspended Animation Correlates with Dephosphorylation of Cell Cycle regulated Proteins
We found that nematodes in anoxia arrest energy requiring processes such as developmental progression, cell division, and movement, which is paralleled with physiological and cellular changes such as a decrease in ATP and dephosphorylation of cell cycle–regulated proteins such as histone H3 and proteins recognized by the MPM-2 antibody. The cellular signal(s) to allow such diverse responses to anoxia within a developing organism are not understood, but the ability of an organism to enter into and exit from suspended animation must involve a coordination of many cellular processes. A model that may help to explain how coordinated stopping occurs has been suggested from studies of the metabolic responses of crayfish, mollusks, and turtles to anoxia (Cowan and Storey, 2001; Storey, 1993, 1996). In studies of the anoxia-tolerant crayfish Orconectes virilis it has been shown that prolonged anoxia stimulates a decrease in the activity of cAMP-dependent protein kinase (PKA) and an increase in protein phosphatases 1, 2A, and 2C activity (Cowan and Storey, 2001). Such alterations in kinase and phosphatase activity may stimulate energy conservation responses by altering the phosphorylation state of glycolytic enzymes (Storey, 1996). We suggest that a similar model may help explain our results. That is, in nematodes subjected to prolonged anoxia oxidative phosphorylation stops, which leads to diminished ATP levels. This in turn leads to a diminished activity of some kinases and increased activity of phosphatases, which may alter the phosphorylated state of key regulatory proteins involved in processes such as cell cycle progression.
ACKNOWLEDGMENTS
The authors thank Jim Priess, Brian Buckwitz, Landon Moore, Jesse Goldmark, Rafal Ciosk, and Jeff Stear for discussions and comments regarding work in this article; Jo Anne Powell-Coffman for providing us with the hif-1 (ia04) mutant; and Dan Gottschling for providing us with the antibody that recognizes the acetylated form of histone H3. This work is supported in part by a grant from the National Institutes of Health (GM48435) to M.B.R, a NSF minority postdoctoral research fellowship to P.A.P, and a NIGMS training grant fellowship to T.G.N.
Footnotes
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.01–12-0594. Article and publication date are at www.molbiolcell.org/cgi/doi/10.1091/mbc.01–12–0594.
REFERENCES
- The C. elegans Sequencing Consortium. Genome sequence of the nematode C. elegans: a platform for investigating biology. Science. 1998;282:2012–2018. doi: 10.1126/science.282.5396.2012. [published errata appear in Science 1999 Jan 1;283(5398):35 and 1999 Mar 26;283(5410):2103 and 1999 Sep 3;285(5433):1493]. [DOI] [PubMed] [Google Scholar]
- Anderson GL. Responses of dauer larvae of Caenorhabditis elegans (Nematoda: Rhabditidae) to thermal stress and oxygen deprivation. Can J Zool. 1978;56:1786–1791. [Google Scholar]
- Brown JM. Tumor hypoxia: problems and opportunities. In: Bertino J R, editor. Encyclopedia of Cancer. New York: Academic Press; 1996. pp. 1883–1898. [Google Scholar]
- Browning H, Strome S. A sperm-supplied factor required for embryogenesis in C. elegans. Development. 1996;122:391–404. doi: 10.1242/dev.122.1.391. [DOI] [PubMed] [Google Scholar]
- Clegg J. Embryos of Artemia franciscana survive four years of continuous anoxia: the case for complete metabolic rate depression. J Exp Biol. 1997;200:467–475. doi: 10.1242/jeb.200.3.467. [DOI] [PubMed] [Google Scholar]
- Cowan KJ, Storey KB. Protein kinase and phosphatase responses to anoxia in crayfish, Orconectes virilis: purification and characterization of cAMP-dependent protein kinase. Comp Biochem Physiol B Biochem Mol Biol. 2001;130:565–577. doi: 10.1016/s1096-4959(01)00467-5. [DOI] [PubMed] [Google Scholar]
- Davis FM, Tsao TY, Fowler SK, Rao PN. Monoclonal antibodies to mitotic cells. Proc Natl Acad Sci USA. 1983;80:2926–2930. doi: 10.1073/pnas.80.10.2926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiGregorio PJ, Ubersax JA, O'Farrell PH. Hypoxia and nitric oxide induce a rapid, reversible cell cycle arrest of the Drosophila syncytial divisions. J Biol Chem. 2001;276:1930–1937. doi: 10.1074/jbc.M003911200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas RM, Xu T, Haddad GG. Cell cycle progression and cell division are sensitive to hypoxia in Drosophila melanogaster embryos. Am J Physiol Regul Integr Comp Physiol. 2001;280:R1555–R1563. doi: 10.1152/ajpregu.2001.280.5.R1555. [DOI] [PubMed] [Google Scholar]
- Epstein AC, Gleadle JM, McNeill LA, Hewitson KS, O'Rourke J, Mole DR, Mukherji M, Metzen E, Wilson MI, Dhanda A, et al. C. elegans EGL-9 and mammalian homologs define a family of dioxygenases that regulate HIF by prolyl hydroxylation. Cell. 2001;107:43–54. doi: 10.1016/s0092-8674(01)00507-4. [DOI] [PubMed] [Google Scholar]
- Foe VE, Alberts BM. Reversible chromosome condensation induced in Drosophila embryos by anoxia: visualization of interphase nuclear organization. J Cell Biol. 1985;100:1623–1636. doi: 10.1083/jcb.100.5.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser AG, Kamath RS, Zipperlen P, Martinez-Campos M, Sohrmann M, Ahringer J. Functional genomic analysis of C. elegans chromosome I by systematic RNA interference. Nature. 2000;408:325–330. doi: 10.1038/35042517. [DOI] [PubMed] [Google Scholar]
- Guillemin K, Krasnow MA. The hypoxic response: huffing and HIFing. Cell. 1997;89:9–12. doi: 10.1016/s0092-8674(00)80176-2. [DOI] [PubMed] [Google Scholar]
- Hand SC. pHi and anabolic arrest during anoxia in Artemia franciscana embryos. In: Hochachka PW, Sick T, Rosenthal M, van den Thillart G, editors. Surviving Hypoxia: Mechanisms of Control and Adaptation, L.P.L. Boca Raton, FL: CRC Press; 1993. pp. 171–185. [Google Scholar]
- Hardwick KG. The spindle checkpoint. Trends Genet. 1998;14:1–4. doi: 10.1016/S0168-9525(97)01340-1. [DOI] [PubMed] [Google Scholar]
- Hochachka PW. Defense strategies against hypoxia and hypothermia. Science. 1986;231:234–241. doi: 10.1126/science.2417316. [DOI] [PubMed] [Google Scholar]
- Hochachka PW, Buck LT, Doll CJ, Land SC. Unifying theory of hypoxia tolerance: molecular/metabolic defense and rescue mechanisms for surviving oxygen lack. Proc Natl Acad Sci USA. 1996;93:9493–9498. doi: 10.1073/pnas.93.18.9493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochachka PW, Lutz PL. Mechanism, origin, and evolution of anoxia tolerance in animals (*) Comp Biochem Physiol B Biochem Mol Biol. 2001;130:435–459. doi: 10.1016/s1096-4959(01)00408-0. [DOI] [PubMed] [Google Scholar]
- Hsu JY, Sun ZW, Li X, Reuben M, Tatchell K, Bishop DK, Grushcow JM, Brame CJ, Caldwell JA, Hunt D F, et al. Mitotic phosphorylation of histone H3 is governed by Ipl1/aurora kinase and Glc7/PP1 phosphatase in budding yeast and nematodes. Cell. 2000;102:279–291. doi: 10.1016/s0092-8674(00)00034-9. [DOI] [PubMed] [Google Scholar]
- Ivan M, Kondo K, Yang H, Kim W, Valiando J, Ohh M, Salic A, Asara JM, Lane WS, Kaelin WG., Jr HIFalpha targeted for VHL-mediated destruction by proline hydroxylation: implications for O2 sensing. Science. 2001;292:464–468. doi: 10.1126/science.1059817. [DOI] [PubMed] [Google Scholar]
- Jaakkola P, Mole DR, Tian YM, Wilson MI, Gielbert J, Gaskell SJ, Kriegsheim A, Hebestreit HF, Mukherji M, Schofield C J, et al. Targeting of HIF-alpha to the von Hippel-Lindau ubiquitylation complex by O2-regulated prolyl hydroxylation. Science. 2001;292:468–472. doi: 10.1126/science.1059796. [DOI] [PubMed] [Google Scholar]
- Jiang H, Guo R, Powell-Coffman JA. The Caenorhabditis elegans hif-1 gene encodes a bHLH-PAS protein that is required for adaptation to hypoxia. Proc Natl Acad Sci USA. 2001;98:7916–7921. doi: 10.1073/pnas.141234698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KK, Gruenbaum Y, Spann P, Liu J, Wilson KL. C. elegans nuclear envelope proteins emerin, MAN1, lamin, and nucleoporins reveal unique timing of nuclear envelope breakdown during mitosis. Mol Biol Cell. 2000;11:3089–3099. doi: 10.1091/mbc.11.9.3089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipton P. Ischemic cell death in brain neurons. Physiol Rev. 1999;79:1431–1568. doi: 10.1152/physrev.1999.79.4.1431. [DOI] [PubMed] [Google Scholar]
- Moore LL, Morrison M, Roth MB. HCP-1, a protein involved in chromosome segregation, is localized to the centromere of mitotic chromosomes in Caenorhabditis elegans. J Cell Biol. 1999;147:471–480. doi: 10.1083/jcb.147.3.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore LL, Roth MB. HCP-4, a CENP-C-like protein in Caenorhabditis elegans, is required for resolution of sister centromeres. J Cell Biol. 2001;153:1199–1208. doi: 10.1083/jcb.153.6.1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neugebauer KM, Roth MB. Distribution of pre-mRNA splicing factors at sites of RNA polymerase II transcription. Genes Dev. 1997;11:1148–1159. doi: 10.1101/gad.11.9.1148. [DOI] [PubMed] [Google Scholar]
- Padilla PA, Roth MB. Oxygen deprivation causes suspended animation in the zebrafish embryo. Proc Natl Acad Sci USA. 2001;12:12. doi: 10.1073/pnas.131213198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul RJ, Gohla J, Foll R, Schneckenburger H. Metabolic adaptations to environmental changes in Caenorhabditis elegans. Comp Biochem Physiol B Biochem Mol Biol. 2000;127:469–479. doi: 10.1016/s0305-0491(00)00284-4. [DOI] [PubMed] [Google Scholar]
- Pitt JN, Schisa JA, Priess JR. P granules in the germ cells of Caenorhabditis elegans adults are associated with clusters of nuclear pores and contain RNA. Dev Biol. 2000;219:315–333. doi: 10.1006/dbio.2000.9607. [DOI] [PubMed] [Google Scholar]
- Semenza GL. Hif-1, o(2), and the 3 phds. How animal cells signal hypoxia to the nucleus. Cell. 2001;107:1–3. doi: 10.1016/s0092-8674(01)00518-9. [DOI] [PubMed] [Google Scholar]
- Semenza GL, Agani F, Feldser D, Iyer N, Kotch L, Laughner E, Yu A. Hypoxia, HIF-1, and the pathophysiology of common human diseases [In Process Citation] Adv Exp Med Biol. 2000;475:123–130. doi: 10.1007/0-306-46825-5_12. [DOI] [PubMed] [Google Scholar]
- Sick TJ, Perez-Pinzon M, Lutz PL, Rosenthal M. Maintaining Coupled Metabolism and Membrane Function in Anoxic Brain: A Comparison between the Turtle and Rat. Boca Raton, FL: CRC Press; 1993. [Google Scholar]
- Storey KB. Molecular mechanisms of metabolic arrest in mollusks. In: Hochachka PW, Lutz PL, Sick T, Rosenthal M, van den Thillart G, editors. Surviving Hypoxia: Mechanisms of Control and Adaptation. Boca Raton, FL: CRC Press; 1993. pp. 253–269. [Google Scholar]
- Storey KB. Metabolic adaptations supporting anoxia tolerance in reptiles: recent advances. Comp Biochem Physiol B Biochem Mol Biol. 1996;113:23–35. doi: 10.1016/0305-0491(95)02043-8. [DOI] [PubMed] [Google Scholar]
- Sulston J, Hodgkin J. Methods. In: Wood W, editor. The Nematode Caenorhabditis elegans. Plainview, NY: Cold Spring Harbor Laboratory Press; 1988. pp. 587–606. [Google Scholar]
- Van Voorhies WA, Ward S. Broad oxygen tolerance in the nematode Caenorhabditis elegans. J Exp Biol. 2000;203(Pt 16):2467–2478. doi: 10.1242/jeb.203.16.2467. [DOI] [PubMed] [Google Scholar]
- Wegener G, Krause U. Environmental and exercise anaerobiosis in frogs. In: Hochachka PW, Lutz PL, Sick T, Rosenthal M, van den Thillart G, editors. Surviving Hypoxia: Mechanisms of Control and Adaptation. Boca Raton, FL: CRC Press; 1993. pp. 217–252. [Google Scholar]
- Wingrove JA, O'Farrell PH. Nitric oxide contributes to behavioral, cellular, and developmental responses to low oxygen in Drosophila. Cell. 1999;98:105–114. doi: 10.1016/S0092-8674(00)80610-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zager RA, Burkhart KM, Johnson AC, Sacks BM. Increased proximal tubular cholesterol content: implications for cell injury and “acquired cytoresistance.”. Kidney Int. 1999;56:1788–1797. doi: 10.1046/j.1523-1755.1999.00745.x. [DOI] [PubMed] [Google Scholar]
- Zamir E, Kam Z, Yarden A. Transcription-dependent induction of G1 phase during the zebra fish midblastula transition. Mol Cell Biol. 1997;17:529–536. doi: 10.1128/mcb.17.2.529. [DOI] [PMC free article] [PubMed] [Google Scholar]