Highlight
-
•
7β-hydroxycholesterol and 7-ketocholesterol both correlate with lens cataract.
-
•
Ionising Radiation (IR) exposure increases oxysterols in isolated lens membranes.
-
•
Mouse exposure to low dose IR, increases oxysterols in the living lens.
-
•
Oxysterol increase is dose dependant and transient in living lenses.
-
•
Oxysterol levels higher in older compared to younger lens cells.
Keywords: Eye lens, Age-related cataract, Cholesterol, Lipid rafts, X-rays, Ionising radiation , Occupational exposure threshold, Free radicals, Cholesterol oxidation, Oxysterol formation, Smith-Lemli-Optiz syndrome, Cataractogenic load, Posterior subcapsular cataract
Abstract
Ionising radiation (IR) is a cause of lipid peroxidation, and epidemiological data have revealed a correlation between exposure to IR and the development of eye lens cataracts. Cataracts remain the leading cause of blindness around the world. The plasma membranes of lens fibre cells are one of the most cholesterolrich membranes in the human body, forming lipid rafts and contributing to the biophysical properties of lens fibre plasma membrane. Liquid chromatography followed by mass spectrometry was used to analyse bovine eye lens lipid membrane fractions after exposure to 5 and 50 Gy and eye lenses taken from wholebody 2 Gy-irradiated mice. Although cholesterol levels do not change significantly, IR dose-dependant formation of the oxysterols 7β-hydroxycholesterol, 7-ketocholesterol and 5, 6-epoxycholesterol in bovine lens nucleus membrane extracts was observed. Whole-body X-ray exposure (2 Gy) of 12-week old mice resulted in an increase in 7β-hydroxycholesterol and 7-ketocholesterol in their eye lenses. Their increase regressed over 24 h in the living lens cortex after IR exposure. This study also demonstrated that the IR-induced fold increase in oxysterols was greater in the mouse lens cortex than the nucleus. Further work is required to elucidate the mechanistic link(s) between oxysterols and IR-induced cataract, but these data evidence for the first time that IR exposure of mice results in oxysterol formation in their eye lenses.
Introduction
The eye lens focuses light onto the retina and therefore its transparency and optical function is essential to vision [1]. Opacity and visual impairment are the main clinical characteristics presented in eye lens cataract. As the lens is a system where proteins [2,3] and lipids [4,5] are retained throughout life, post-translational modifications and oxidative damage accumulate in these biomolecules with increasing age (reviewed in [1]). Cataracts are an iconic age-related pathology, but epidemiological data suggest that they can also be caused by exposure to ionising radiation (IR) because there is a clear correlation between IR exposure and cataractogenesis [6], [7], [8]. The mechanisms involved are still under investigation [9], but IR damages macromolecules either directly or indirectly by ionising water into free radicals to then cause lipid, protein and DNA damage. Such damage will collectively contribute to the cataractogenic load upon the lens, which is defined as the biomolecular damage accumulated over a lifetime as a result of lifestyle, genetic and environmental events [10]. Significant advances have been made regarding the effects of IR on DNA and proteins in the eye lens (reviewed by [10]). The IR effect(s) upon cholesterol, the most abundant lipids in the lens cell membranes [11], have not been investigated. Free radicals cause cholesterol oxidation [12], [13], [14] and IR exposure leads to oxysterol formation [15] in a dose-dependant manner [16], which are involved in many other age-related diseases [17] including cataract [18,19].
Doses >0.5 Gy have been shown to cause irreparable double strand breaks (DSBs) in DNA and change the organisation, differentiation and proliferation of lens epithelial cells (LECs) [20], [21], [22]. Low dose IR exposure, including doses <0.5 Gy, changes cell proliferation and consequently cell density as well [23]. IR exposure stimulates protein post-translational modifications and aggregation resulting in loss of transparency and cataract formation in the eye lens [24], [25], [26], [27]. IR would be expected to cause oxysterol formation in the lens, but this remains to be demonstrated. This is important because there is a strong correlation between the presence of oxysterols and cataract formation [18,19] and this is true also for other age related eye diseases such as macular degeneration [17]. Interestingly, Smith-Lemli-Optiz syndrome also has cataract as a phenotype [28] and this is due to a deficiency in 7-dehydrocholesterol reductase that causes a build-up of 7-dehydrocholesterol, a lipid that is particularly sensitive to free radical oxidation [14,28]. This is further evidence that oxysterols are involved in cataract formation and other inborn errors of metabolism affecting cholesterol metabolism have also been linked to cataract [29,30].
The eye lens comprises a single layer of LECs covering the anterior hemisphere of the lens, and lens fibre cells (LFCs) that differentiate from LECs form the major part of the lens. During differentiation, all cell organelles in the LFCs are degraded and the cytoplasm is filled with crystallins [31]. The youngest LFCs are at the lens cortex, and the oldest cells are situated at the centre of the lens, which is known as the lens nucleus. The LFC plasma membrane is one of the most cholesterol rich membranes in the body; it also contains dihydrosphingomyelin as the most abundant phospholipid in humans [11,32]. With age, the levels of dihydrosphingomyelin and cholesterol rise, even crossing the cholesterol saturation limit, leading to membrane lipid raft formation. In contrast, the levels of glycerolipids are found be declined due to the preferential oxidation of glycerophospholipids [33], [34], [35], [36]. Electron paramagnetic resonance analyses showed the molar ratio of cholesterol:phospholipids was up to 4:1 in the human nucleus [37].
In addition to mediating the organisation and function of integral membrane proteins [38], [39], [40], [41], a protective role for the high cholesterol levels against cataract has been suggested due to the decreased oxygen permeability and subsequent reduced oxidative stress in bovine and human eye lenses [34,[42], [43], [44], [45]]. Cholesterol:phospholipid molar ratio measurements demonstrated decreased cholesterol levels in cataractous lenses [37,46]. Moreover, patients with defects in genes coding for essential enzymes in the cholesterol synthesis pathway, such as 7-dehydrocholesterol reductase in Smith–Lemli–Opitz syndrome [47], mevalonate kinase in mevalonic aciduria [48] and lanosterol synthase [49], show a high incidence of cataract, emphasising the importance of cholesterol homoeostasis in lens transparency. Mutations in CYP21A2, a 21 hydroxylase needed to metabolise cholesterol to cortisol and aldosterone has recently been found to cause autosomal dominant cataract [30] as is also the case for CYP27A1 [50]. In animal models, drugs that interfere with cholesterol biosynthesis also cause cataract [51].
Ageing is a major risk factor for cataract formation because of the time-dependent increase in oxidative stress, metabolic ageing (deleteriome; [52]) and loss of reducing potential in the lens [10]. Over time the anti-oxidant defences in the lens become less efficient because of the development of a glutathione barrier between the lens cortex and nucleus [53,54]. Consistent with this concept, a cholesterol oxidation adduct, 7-keto cholesterol, builds up with age in the lens [55]. Cholesterol oxidation products, i.e. 20α-hydroxycholesterol, 25-hydroxycholesterol, 7β-hydroxycholesterol, 7-ketocholesterol and 5, 6-epoxycholesterol, have been found to accumulate in human lenses with cataract as compared with age-matched clear lenses [18]. These oxysterols can be formed via enzymatic or non-enzymatic (autoxidation) processes depending on enzyme availability and the type of reactive oxygen species (ROS) causing the oxidative stress [56], [57], [58]. Given that IR elevates oxidative stress in the eye lens, and oxysterol content correlates with age-related cataract (ARC), investigation of the effects of IR exposure on the lipid membranes of LFCs was performed. ARC-associated oxysterols were identified when isolated bovine lens membrane fractions were exposed to X-rays (5 – 50 Gy). The formation of these oxysterols in vivo in the lenses of mice following whole body exposure to 2 Gy X-rays was also demonstrated and their levels monitored over a 24 h time period. These data revealed that after an initial increase, oxysterol levels decayed in membrane fractions isolated from the lenses IR-exposed mice.
Materials and methods
Bovine LFC membrane fraction preparation
Bovine eyes were obtained from Linden Burradon Food Supply (FSA-approved Scientific Research Material collection No. 2056). To collect the cholesterol enriched lens membranes, a protocol used to purify lens membrane fractions [59] was adapted. Decapsulated eye lenses were aqua-dissected by stirring them in a low salt phosphate buffer (10 mM Na2HPO4 [pH 7.4], 100 mM NaCl and 5 mM EDTA [pH 8.0]) to first collect the cortical fraction of the LFCs followed by the lens nuclear fraction. A series of buffer extractions designed to enrich for integral membrane proteins and the lipid membranes was performed [60]. The LFC membranes were pelleted by centrifugation (31,000 × g @ rmax at 4 °C for 20 min (Beckman JA20 rotor). Membranes were purified using the following buffer extraction sequence; a high salt buffer (10 mM Na2HPO4 [pH 7.4], 1.5 M KCl, 5 mM EDTA [pH 8.0]), an ammonium bicarbonate buffer (100 mM NH4HCO3, 1 mM EDTA [pH 8.0]), a urea buffer (10 mM Na2HPO4 [pH 7.4], 8 M Urea, 5 mM EDTA [pH 8.0]) and lastly a sodium hydroxide buffer (100 mM NaOH), with a low salt phosphate buffer washes in between each step. The final lens cortical (BoC) and nuclear (BoN) membrane fractions were resuspended in the low salt phosphate buffer and stored at 4 °C until required.
Exposure of membrane fractions to X-rays
The BoC and BoN membrane fractions (10 mg wet weight) were resuspended in 1 mL of the low salt phosphate buffer and exposed to 5 and 50 Gy in a single X-ray dose using an X-ray chamber irradiator calibrated to national standard (IRR320, aluminium filtered 320 kV, dose rate 5 Gy/min). EDTA is expected to scavenge free radicals, but a fraction of the water molecules is bound to the phosphocholine headgroups [61] as well as permeating the AQP0 water channels in these lens membrane fractions [62]. Dose delivery was verified at the exact site where the samples were placed with aluminium oxide chips [63].
Mouse irradiation and lens membrane fraction preparation
Female mice C57BL/6JOla/Hsd (C57BL/6J) were obtained from Envigo RMS (UK) Ltd. (Blackthorn, Bicester, Oxfordshire OX25 1TP) and were housed in groups of four. Food (RM3(E), LBS technology) and water were provided ad libitum. All procedures involving mice were performed according to the UK Animals (Scientific Procedures) Act 1986, and ethical approval was obtained from the United Kingdom Home Office and the local Animal Welfare and Ethical Review Body at the UK Health Security Agency. At 3 months of age, female mice were exposed to 100 mGy or 2 Gy in a single X-ray dose (CD160/1, AGO X-ray Ltd., aluminium and copper filtered (∼1 mm) containing a Varian NDI-320 source; 250 kVp; dose rate 0.5 Gy/min). Dosimetry was performed with a calibrated reference ionisation chamber for the exact exposure setup used. The monitoring of the exposures was accomplished with a calibrated UNIDOS E electrometer and ‘in-beam’ monitor ionisation chamber (Physikalisch-Technische Werkstätten (PTW), Freiburg, Germany). To verify whether the dose was delivered to the entire area of the box, spatial dose uniformity was measured with Gafchromic EBT2 films (Vertec Scientific Ltd.). Subsequently, the mice were returned to their cages and received standard care until sacrifice at 2 h, 24 h and 7 days post-irradiation allowing for 4 mice per time point.
Eight lenses were removed and processed together essentially as described above for bovine LFC membrane fractions using the following buffer sequence: low phosphate, urea, low phosphate and sodium hydroxide buffer. Mouse LFC membranes were pelleted by centrifugation (17,000 × g @ rmax at 4 °C for 20 min (Eppendorf refrigerated microfuge). The final lens cortical (MoC) and nuclear (MoN) membrane fractions were resuspended in the low salt phosphate buffer and kept at 4 °C until further analysis.
For the measurement of cholesterol and 7 keto-cholesterol in 6 and 30 month mouse lens samples, LFC membranes were also prepared from wild type C57BL/6J mice.
Sodium dodecyl sulphate polyacrylamide gel electrophoresis (SDS-PAGE)
Samples were resuspended in a SDS buffer (1 mM EDTA pH 7.8, 50 mM Tris pH 6.8 and 1% (w/v) SDS) and then mixed with 4x SDS-PAGE sample buffer (containing 2.854 M β-mercaptoethanol) ([64] as modified in [59]). Equal protein loadings on the gel and samples were separated on 15% (w/v) polyacrylamide gels at 100 V. Proteins were visualised by Coomassie blue staining (0.25% (w/v) Coomassie Brilliant Blue R250, Merck).
Detection of carbonylated proteins
Carbonylated proteins were detected using the OxyBlot Protein Oxidation Detection Kit (S7150, Sigma-Aldrich) following the manufacturer's instructions.
Lipid purification
Lipids were purified using a modified Bligh-Dyer method [65] and oxysterols identified using published procedures [66]. All glassware was rinsed with methanol and hexane and all extraction solvents were sparged with nitrogen for 10 min. The bovine lens membranes (10 mg of wet weight) or mouse lens membrane fractions of eight eye lenses were transferred into clean glass culture tubes and spiked with deuterated internal standards (1 ng of 7β-hydroxycholesterol-D7, 5 ng of 7-ketocholesterol-D7 and 1 ng of 5α,6α-epoxycholestanol-D7) and vortexed. Membrane fractions were mixed with 2 mL of 2:1 methanol:dichloromethane (MeOH:DCM) containing 50 µg/ml Butylated hydroxytoluene (BHT) as recommended [66]. The samples were vortexed for 30 s and left to incubate for 30 min at room temperature (RT). Subsequently, 0.67 mL of DCM and 1.2 mL of 0.9% (w/v) KCl were added consecutively, with a 30 s vortex step after adding each solution. The samples were centrifuged at 1000 g for 5 min at RT and the lower lipid phase layer was transferred into a fresh glass tube with a Pasteur pipette. The lipids were dried under N2 gas.
Liquid chromatography - mass spectrometry (LC-MS)
Our samples were analysed based on the methodology developed and published by McDonald and colleagues [66]. The following standards were used and all were purchased from Sigma-Aldrich unless otherwise stated; cholesterol and cholesterol-D6: 7-ketocholesterol; 7β-hydroxycholesterol (Sigma); 7 dehydrocholesterol-D7 (Cambridge Bioscience) 5, 6 epoxycholesterol, 25-hydroxycholesterol-D6, desmosterol and desmosterol-D6 using retention times and multiple reaction monitoring (MRMs) for identification of sample peaks [67]. Quantitative analysis was performed with a Shimadzu UHPLC system linked to a hybrid triple-quadrupole mass spectrometer (QTRAP 6500, AB Sciex). For oxysterol analysis, the samples were dissolved in 50 µL of 1:1 acetonitrile:isopropanol (ACN:IPA) and separated on a Kinetex C18 HPLC column (150 × 2.1 mm, 2.6 μm particle size; Phenomenex) with mobile phases A (60:40 ACN: H2O, 10 mM NH4HCO3, 0.1% (v/v) HCOOH) and B (50:50 ACN: IPA, 10 mM NH4HCO3, 0.1% (v/v) HCOOH). For cholesterol analysis, the samples were dissolved in 500 µL 1:1 ACN:IPA and run under the same conditions on a Cortecs C18 UPLC column (100 × 2.1 mm, 1.6 μm particle size; Waters). The flow rate was 140 µL/ min, and the column was maintained at 60 °C.
Mouse lens membrane samples were dissolved in 84 µL of 70:30 MeOH:H2O and analysed with a liquid chromatography UltiMate 3000 HPLC system (Dionex, Thermo Scientific Ltd.) coupled on-line with an electrospray tandem triple quadrupole-linear ion trap mass spectrometer (QTrap 5500, AB Sciex) operated in a positive electrospray ionisation (ESI) mode as described previously [68]. Briefly, the samples were separated using a reverse phase C18 column (100 × 3.2 mm, 5.0 μm particle size; Macherey-Nagel) with mobile phases A (70:30 MeOH:H2O 0.1% (v/v) HCOOH) and B (90:10 IPA:MeOH, 0.1% (v/v) HCOOH). The flow rate was 200 µL/ min, and the column was maintained at 45 °C. Ionisation voltage of 5.5 kV, entrance potential of 10 V, and ion source temperature of 300 °C was used to analyse samples. Optimised parameters for collision energy, declustering potential, and exit quadrupole potential for each Q1/Q3 (precursor ion/fragment ion) m/z transition were optimised for each analyte by direct infusion of authentic standard into the mass spectrometer (Supplementary figure 1).
8-isoprostane F2α analysis
8-isoprostane F2α levels were measured in mouse membrane fractions using the commercially available EIA kit (Cayman chemicals, #516,351; Cambridge Bioscience, UK) as a measure of lipid peroxidation in the mouse lens membrane samples.
Quantification and statistical analysis
The areas under the elution curves on the generated chromatograms were integrated to generate quantitative values (analyte peak area) using quantification mode of the Analyst software 1.6.2. The bovine sample experiments were repeated three times, while two biological repeats were produced for the mice experiments. Minitab 18 was used to perform statistical analyses [69] including power analyses, to ensure tests for significance would detect effects in the data collected and uncertainty budget as a quantitative indication of the reliability of the measurements made. General Linear Model Analysis of Variance (ANOVA) and Tukey's post hoc test for pairwise comparison between the distinct factors were applied.
Results
Cholesterol levels do not change significantly following IR exposure
The plasma membrane of LFCs contains up to 4-fold more cholesterol than phospholipids [37] and these high cholesterol levels have been suggested to provide a protection mechanism for the LFCs against oxidative stress [45]. To investigate the effect of IR exposure on these highly cholesterol concentrated membranes, cholesterol levels in IR-exposed bovine eye lens-extracted membrane fractions were measured via LC-MS (Supplementary Figure 1A). The chromatogram of bovine lipid membrane fractions exposed to 5 and 50 Gy X-ray shows that radiation exposure did not lead to a noticeable change in cholesterol levels (Supplementary Figure 1B). The nucleus does contain significantly more cholesterol than the cortex (p = 0.006), but cholesterol levels were not changed as the IR dose increased (Supplementary Figure 1B).
IR exposure induces a gradual increase of various oxysterols
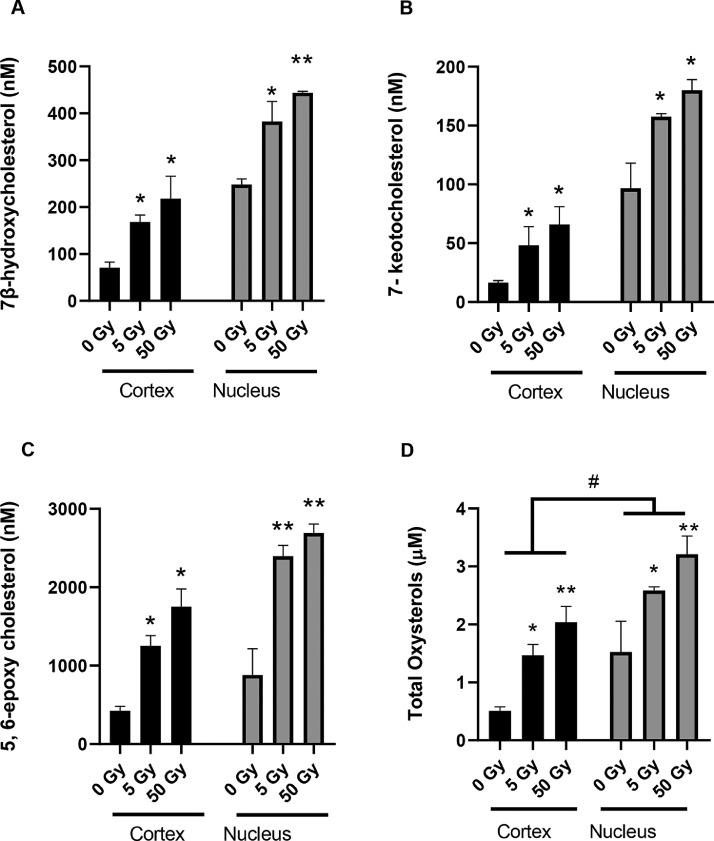
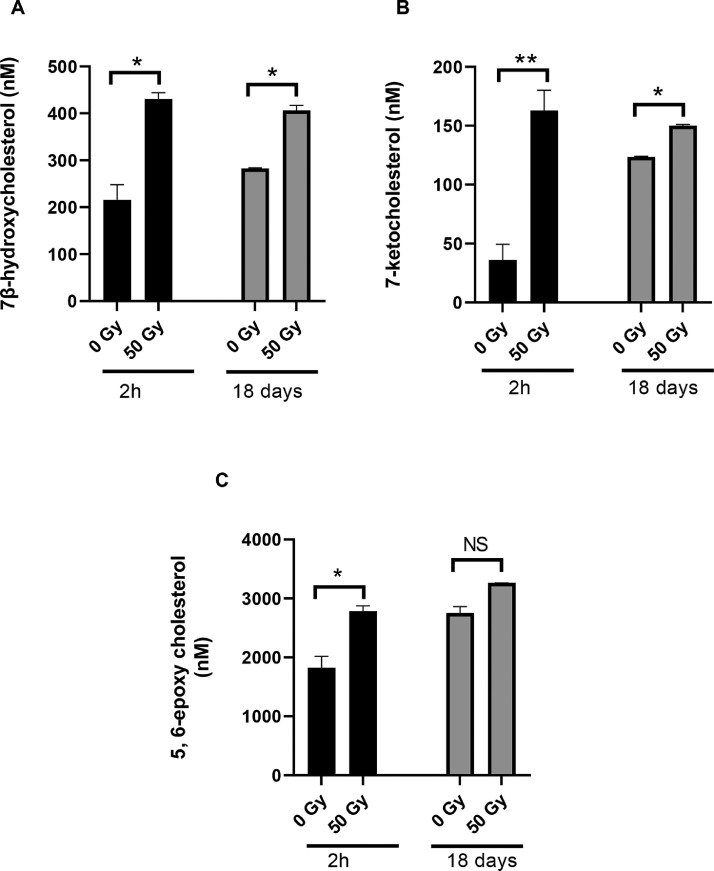
Although the IR exposure of membrane fractions from the cortex and nucleus of bovine lenses didn't change the levels of cholesterol, it did affect three oxysterols (7-ketocholesterol, 7β-hydroxycholesterol and 5, 6-epoxycholesterol; Fig. 1) identified in these lens fractions (Supplementary figure 2). Levels of 7β-hydroxycholesterol (Fig. 2A), 7-ketocholesterol (Fig. 2B) and 5, 6-epoxycholesterol (Fig. 2C) were increased after IR exposure in a dose-dependent manner. Comparison of oxysterol levels in unexposed lens cortex and nucleus membrane samples revealed that the lens nucleus inherently contained more oxysterols than the cortex and this trend was maintained after IR exposure (p< 0.001 Fig. 2D). Interestingly, IR-induced oxysterol increase was more prominent in the cortex (3-fold at 5 Gy and 4-fold at 50 Gy) compared to the nucleus (2-fold at 5 Gy and 3-fold at 50 Gy). To understand clearance of these oxysterols, their levels were analysed after 18 days and compared to the levels seen after 2 h for IR-exposed bovine lens nucleus membrane fractions. The increase in oxysterol levels after IR exposure was found to persist for 7β-hydroxycholesterol (p = 0.869; Fig. 3A) and 7-ketocholesterol (p = 0.180; Fig. 3B), even after 18 days at 37 °C. 5, 6-epoxycholesterol levels increased significantly (p = 0.019; Fig. 3C). The potential contribution of auto-oxidation contributing to these levels of oxysterol during the 18 day incubation in atmospheric oxygen at 37 °C was not investigated.
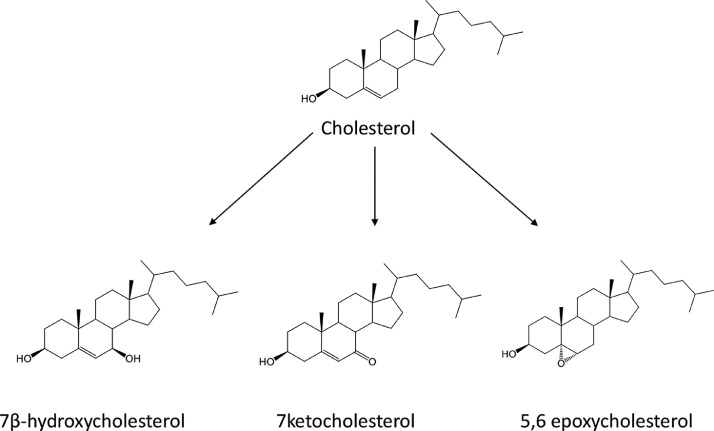
Fig. 1.
Structures of the oxysterols analysed in this study.
Fig. 2.
LC-MS quantification of oxysterols in bovine lens lipid membranes after exposure to IR. A) 7β-hydroxycholesterol, B) 7-ketocholesterol in, C) 5, 6-epoxycholesterol, D) total oxysterols were calculated by combining levels for 7β-hydroxycholesterol, 7-ketocholesterol and 5, 6-epoxycholesterol. Levels of these oxysterols were measured in the membrane fractions from the bovine lens cortex (Cortex) and nucleus (Nucleus) that had been exposed to 0, 5 and 50 Gy IR. General Linear Model Analysis of Variance followed by Tukey pairwise comparison post hoc test using location and dose as independent factors was applied for statistical analysis, *p< 0.05, **p<0.001 compared to untreated control and # p<0.01 cortex compared to nucleus. n = 3,.
Fig. 3.
Comparison of oxysterol levels at 2 h and 18 days post irradiation in the nuclear membrane fraction prepared from bovine lenses after exposure to 50 Gy IR. Levels of 7β-hydroxy cholesterol (A), 7-ketocholesterol (B) and 5,6 epoxycholesterol (C) were measured by LC-MS. General Linear Model Analysis of Variance followed by Tukey pairwise comparison post hoc test using time and dose as independent factors was applied for statistical analysis, *p< 0.05, **p<0.01, NS: not significant. n = 3.
No changes in protein pattern or protein carbonylation are observed following IR exposure
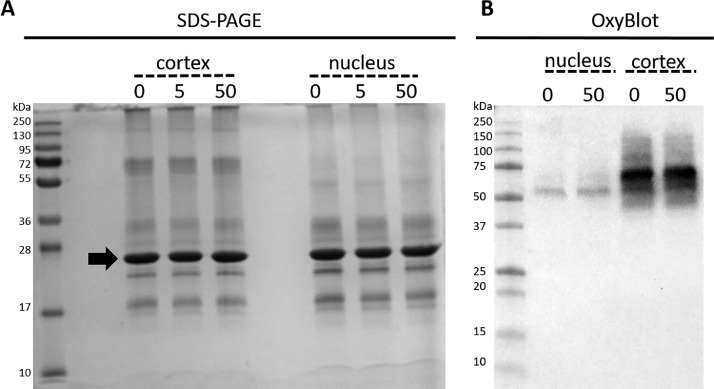
Increasing oxidative stress in the eye lens has been shown to trigger protein oxidation, which results in protein aggregation and increased light scattering through the formation of high molecular weight aggregates [70,71]. To show whether IR-induced oxidation occurred in lens membrane fractions, the protein profile of the IR-exposed lens membranes were analysed by SDS-PAGE. The extraction of lens membranes with sodium hydroxide and urea removes cytoplasmic proteins and the proteins required for protein synthesis but enriches for integral membrane proteins, which in the case of lens membranes is the 26 kDa protein AQP0 [59]. As shown in Fig. 4A, the protein patterns appeared unaltered by both 5 and 50 Gy IR exposure compared with the unexposed membrane protein pattern. The major protein (Fig. 4A, arrow) is the water channel protein AQP0 and its aggregation can be detected by SDS-PAGE [72]. As PTMs may not result in AQP0 aggregation [72], protein carbonylation levels in the 50 Gy IR exposed bovine samples were monitored by OxyBlots. Although lower oxidised protein levels were detected in the nucleus compared with the cortex, no obvious change in protein carbonylation levels were seen after exposure to 50 Gy (Fig. 4B). A comprehensive mass spectrometric analysis is needed to determine the protein specific PTMs that occur after IR exposure [73].
Fig. 4.
Effect of IR upon the proteins present in the lipid membranes isolated from the cortex and the nucleus of bovine eye lenses. A) Coomassie Brilliant Blue stained SDS-PAGE gel of the membrane protein profiles from the lens cortex and nucleus after exposure to 0, 5 and 50 Gy. B) Corresponding OxyBlot of unexposed (0) and 50 Gy exposed nucleus and cortex lens membrane fractions. Note the lack of a positive signal for AQP0 at 26 kDa.
IR induces oxysterol formation in mouse eye lenses
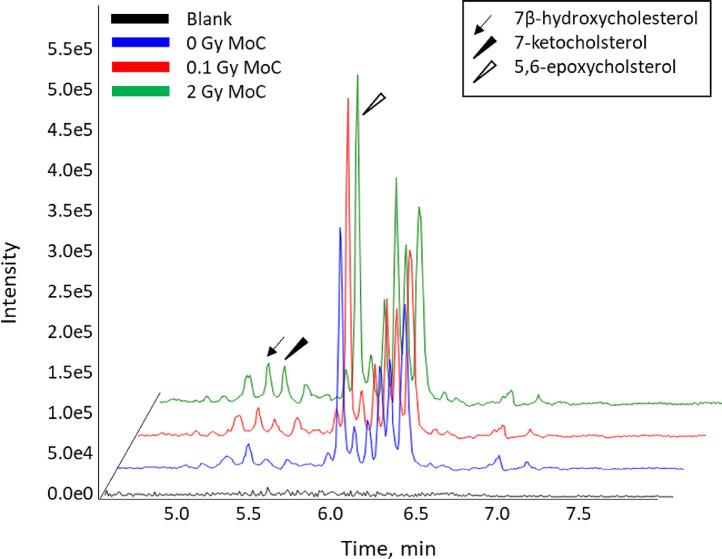
To investigate whether IR-induced oxysterol formation observed in vitro also occurs in vivo, mice were whole-body exposed to IR. Signals for 7β-hydroxycholesterol, 7-ketocholesterol and 5,6-epoxycholesterol were observed in the membrane fractions purified from the lens cortex of IR-exposed mice (Fig. 5 and Supplementary Figure 4) that warrant further investigation to determine their relationship to IR dose and dose rate.
Fig. 5.
LC-MS chromatograms of 0, 0.1 and 2 Gy irradiated mice 2 h post exposure. Eight eye lenses were pooled per samplefor the mouse lens cortex (MoC).
IR-induced oxysterol formation is transient in the lenses of living animals
Our data on isolated bovine lens nucleus membrane fractions suggested that oxysterols would be retained once formed after exposure to IR. Therefore, we investigated the stability of the oxysterols formed in the lenses of living animals after exposure to 2 Gy IR, a dose known to cause cataract in this mouse strain [74]. Four mice were irradiated with 2 Gy and then sacrificed 2 h, 24 h and 7 days later. The eight lenses from the four mice were pooled and the -LFC membranes prepared from the cortical and nuclear lens fractions. Following lipid purification, quantitative LC-MS analysis of cholesterol and oxysterol levels in each sample was made. We also measured lipid peroxidation over the same time course (Supplementary Figure 4). Oxysterol levels between different samples were normalised using the cholesterol analyte peak area as it had already been confirmed that cholesterol levels did not change significantly for IR doses of up to 50 Gy (Supplementary Figure 1A).
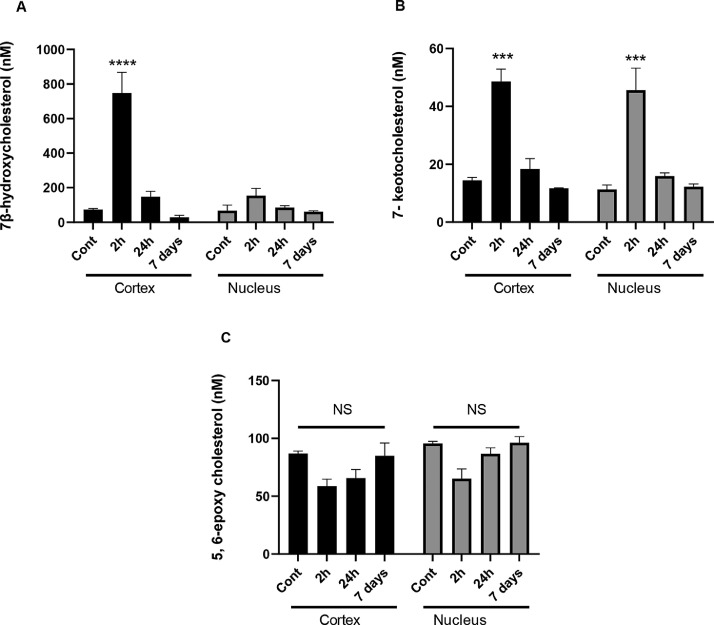
Exposing mice to a single, whole body, acute IR exposure of 2 Gy leads to a rapid increase of 7β-hydroxycholesterol (P< 0.0001; Fig. 6A) in the lens cortex and 7-ketocholesterol in both the lens cortex and nucleus (P<0.005; Fig. 6B). This was also seen for isoprostane levels (Supplementary Figure 5) evidencing a significant increase in lipid peroxidation as a result of IR exposure. After 24 h, the levels of 7β-hydroxycholesterol and 7-ketocholesterol returned to pre-exposure levels (Figs. 6A-C). In contrast to 7β-hydroxycholesterol and 7-ketocholesterol, 5, 6-epoxycholesterol did not show a significant IR-induced change over the 7 days (Fig. 6C). A comparison of the cortical and nuclear lens membrane fractions showed that the changes in oxysterol levels are more pronounced in the cortex than in the nucleus of the mouse lens (Fig. 6A and 6B).
Fig. 6.
LC-MS quantification of in vivo formed oxysterols in eye lenses of mice irradiated with X-rays and sacrificed at 2 h, 24 h and 7 days post-IR exposure. A) 7β-hydroxycholesterol, B) 7-ketocholesterol and C) 5, 6-epoxycholesterol in mouse lens membrane fractions from the cortex (Cortex) and nucleus (Nucleus). General Linear Model Analysis of Variance followed by Tukey pairwise comparison post hoc test using time and location as independent factors was applied for statistical analysis, ***p< 0.005, **** p<0.0001 n = 2 × 8 pooled eye lenses, NS: not significant.
Discussion
LFC membrane cholesterol is a sensor for oxidative damage after IR exposure
The data presented here confirm that cholesterol in eye lens membranes is subject to oxidative damage after exposure to IR (Figs. 2- 6). Importantly, the cholesterol oxidation products (7β-hydroxycholesterol, 7-ketocholesterol and 5, 6-epoxycholesterol) detected after IR exposure are the same as those that have been correlated with ARC [18]. These oxysterols are generated via cholesterol autoxidation [75], [76], [77], [78]. One of the consequences of IR exposure is free radical generation from water radiolysis, which in turn generates hydrogen peroxide. Discriminating between short-lived free radical-mediated events and the IR-induced production of reactive oxygen and nitrogen species is technically challenging, but both are expected to contribute to oxysterol formation. The plasma membranes of LFCs contain high levels of cholesterol and dihydrosphingomyelin [11], but cholesterol is more prone to oxidative damage than dihydrosphingomyelin [19,79]. Cholesterol turnover appears minimal over the lifetime of the lens [4,80] and cholesterol-derived oxysterols are also found in aged, human lenses [18], but their levels are increased in ARC [18]. Cholesterol oxidation is a mark of ARC [19,[81], [82], [83], [84], [85], [86], [87], [88]] and indeed of other age-related pathologies [17,89]. For these reasons, cholesterol can be considered a de facto biosensor of oxygen radical damage in the lens.
Cholesterol in LFC membranes helps protect against IR-induced damage
The high levels of cholesterol present in the plasma membranes of the lens nucleus lead to the formation of lipid rafts and helps protect against oxidative damage by limiting oxygen diffusion into the lens nucleus [37,42,90]. Cholesterol protects membranes against IR-induced damage, by preventing hydroperoxide formation [91,92] and hydroxyl radical mediated damage in artificial membranes [93] and liposomes [94]. Cholesterol could act by intercepting free radicals and interrupting peroxidative chain reactions [95]. The most common oxidation product is 7-ketocholesterol, a product capable of diffusing through membranes and associated with many age-related human diseases [89]. We detected oxysterols in both non-irradiated bovine and mouse eye lenses using LC-MS (Fig. 2, 3, and 6) and others have found them in normal human lenses [18]. We note that exposing un-irradiated bovine nuclear lens membrane extracts to atmospheric oxygen over an 18-day period at 37 °C appeared to increase oxysterol levels (Fig. 3). It is therefore possible that oxidative free radical and enzymatic mechanisms each can contribute to the observed oxysterol levels in these control samples, but their relative contribution or otherwise requires more detailed investigation. For instance, both CYP7A1 and HSD11B1 are expressed in the mouse lens (https://research.bioinformatics.udel.edu/iSyTE/ppi/index.php). It remains to be determined how each might contribute to the age-dependant increase in oxysterols seen in human and animal lenses and how these relate to the observations reported here. The exposure of lipid membranes in vitro to IR caused a fold increase in cholesterol oxidation products that appeared more pronounced in the lens cortex membrane fraction (Fig. 2), the lens region with the lower cholesterol content compared with the lens nucleus [11]. Amongst the oxysterols 7β-hydroxycholesterol, 7-ketocholesterol and 5, 6-epoxycholesterol were identified, all of which have been correlated with human ARC [18]. We have demonstrated here that IR exposure can generate the same oxysterols as those that are observed to be increased in ARC [18].
The IR-dependent increase in oxysterols (Fig. 2) was significant for both 5 and 50 Gy, but the 50 Gy exposure did not produce a 10-fold increase over the 5 Gy sample in the three oxysterols measured (Fig. 2). These data require further investigation. For instance, a more extensive dose range to determine the detail of IR-induced oxysterol formation in terms of its linearity, saturation and threshold. Another area of investigation concerns free radical autoxidation mechanisms from water radiolysis induce oxysterol formation [77]. It also has to be considered whether cholesterol itself is protective [95] because the γ-irradiation of synthetically generated liposomes with a 4:1 phospholipid:cholesterol ratio generated more cholesterol oxidation adducts than liposomes with a 2:1 phospholipid:cholesterol ratio [96], supporting the concept that oxysterol formation is cholesterol ratio-dependent.
There have been multiple studies to confirm that cholesterol-rich synthetic membranes and vesicles are protective against free radicals and oxidation [92], [93], [94]. The lens membrane fractions also contain integral membrane proteins, in particular AQP0, and it has been reported that membrane associated proteins were more susceptible than soluble equivalents to oxidative damage [97]. By SDS-PAGE and OxyBlot, major changes to the protein pattern (Fig. 4A) and protein carbonylation (Fig. 4B) 2 h post IR exposure were not obvious. The lipids surrounding the integral membrane proteins could protect them from the immediate damaging effects of IR-induced oxidation, but this needs to be investigated further. A signal in the region of the Oxyblot where AQP0 would be expected (26 kDa) was conspicuous by its absence given the abundance of this protein in these membrane fractions (Fig. 4A; arrow). The reduced level of protein oxidation in the nucleus compared to the lens cortex as suggested by the OxyBlot data (Fig. 5B) is most likely due to proteolytic processing that trims cytoplasm exposed sequences of the integral membrane proteins in these fractions, which occurs during fibre cell differentiation and ageing in the eye lens [98], [99], [100], [101].
Our data also evidence a time-dependent decay in oxysterol levels after a single IR exposure of the living mouse lens. Fig. 6 shows the increase in both 7β-hydroxycholesterol and 7-ketocholesterol regressed with time in the lens membrane fractions prepared from irradiated mice. The bovine lens nucleus would be expected to contain the oldest lipids [4,5] and it also had the highest oxysterol levels (Fig. 2). These data suggest that oxysterols accumulate with age, which is consistent with aged human lenses [18]. We confirmed that the ratio of oxysterols (7 keto-cholesterol and 5,6 epoxycholesterol) to cholesterol increased significantly with age in the nuclear fraction of C57Bl/6J mouse lenses (Supplementary Figure 6). Here we report the lens cortex membrane fraction from irradiated mice (Fig. 6A) showed a significant reduction in 7β-hydroxycholesterol 24 h after the initial IR exposure. We also noted that isoprostane levels as an indication of lipid peroxidation remained significantly increased 7 days after the exposure (Supplementary Figure 4). Together these data suggest there could be different mechanisms for the age-dependent increase in oxysterols compared to the removal of oxysterols after a single acute IR exposure, resonating with the observation that DSB repair [102,103] is also different for chronic versus acute exposures and oxidative stresses [104], [105], [106].
It is possible that the higher cholesterol content of the lens nucleus helps provide the cell biological environment to effect protection against IR-mediated oxidative damage in line with previous studies using reconstituted membranes and vesicles [91,92]. The data from the bovine lens membrane fractions might seem to contradict this suggestion because in Fig. 2, the levels of 7β-hydroxycholesterol and 7-ketocholesterol were higher in the lens nucleus membrane fraction. In terms of the fold-increase over the baseline, however, the increase in oxysterols was greatest in the lens cortex and not the lens nucleus, so they are consistent with the concept that higher cholesterol levels protect against oxidative damage [37]. The fact that cholesterol is actively synthesized and metabolised in the lens [29,30,107] means that enzymatic activities will also likely contribute to the post IR response of the lens as these oxysterols are produced. The enzymes CYP21A1, DHCR7, EBP (D8D7I) and HDM11B1/2, can metabolise 7-ketocholesterol [108], 7β-hydroxycholesterol [109] and 5,6-epoxycholesteol [110] are expressed in the mouse lens (https://research.bioinformatics.udel.edu/iSyTE/ppi/index.php). Mutations in CYP21A2 cause congenital cataract [30] as further evidence that the lens has the capacity to metabolise the oxysterols detected here and so potentially explain the decline we observed in the mouse lens samples after 7 days. The mechanism by which oxysterols derived from cholesterol contribute to ARC [18] needs further investigation. The range of inherited diseases that alter cholesterol and its derivatives [29,111] or its precursors [112] and that are also linked to cataractogenesis evidence the critical role played by these lipids in eye lens transparency and optical function.
Effects of oxysterols on membrane properties
Oxysterols migrate better than cholesterol through cell membranes and increase water penetration of the bilayer [92]. The initial increase of oxysterol levels with a subsequent decrease could be due to chemical cascade reactions in which hydrophobic cholesterol molecules are oxidised to less hydrophobic oxysterols. These oxysterols will potentially change the permeability of membranes [113,114]. The exchange of molecules between the aqueous humour and the lens [115] provides another potential mechanism by which the lens could clear some IR-induced oxysterols. Dietary anti-oxidants such as α-tocopherol, ascorbic acid and vitamin A as well as the endogenous lenticular glutathione [53,[116], [117], [118]] are all potential chemical antioxidants in the eye lens. Why then should the lens cortex be more sensitive to IR than the nucleus (Fig. 6) is interesting and it resonates with the epidemiological data that reports posterior subcapsular cataracts (PSC) in the lens cortex of IR-exposed individuals [119,120] and also in irradiated mice 18 months after IR exposure [121]. Epidemiological studies show occupational workers exposed to IR can also develop cataract after chronic exposure later in their lifetime [122]. Further studies are needed to evidence how IR dose rate may affect the formation and retention of oxysterols given has been shown to influence lens outcomes [123].
Our study shows that IR can potentially contribute to the cataractogenic load [10] upon the lens by cholesterol oxidation. A better understanding of the mechanism through which IR-induced cataract formation occurs will enable the radiation protection community to develop better dosimetry, refine the occupational exposure threshold to devise future treatment/protection protocols for individuals exposed to high doses such as radiotherapy patients and clean-up workers compared to those with chronic exposures during prolonged periods in their lifetime e.g. healthcare professionals, astronauts, air crew and energy industry workers.
Declaration of Competing Interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
AU, SB, EA and RAQ are part of the LDLensRad project that has received funding from the Euratom research and training programme 2014–2018 in the framework of the CONCERT [grant agreement No. 662287]. This publication reflects only the author's view. Responsibility for the information and views expressed therein lies entirely with the authors. The European Commission is not responsible for any use that may be made of the information it contains. HKID acknowledges the support from Kidney Research UK grant PDF3/2014. GA and MHAM acknowledge the support of the European Union's Horizon 2020 research and innovation programme under the Marie Sklodowska-Curie grant agreement number 675132 (http://cordis.europa.eu/project/rcn/198275_en.html). The financial support of the National Eye Research Foundation (AAK; SAC014) and the EuroCellNet COST Action (CA15214) that provided STSM support to AU, RAQ and GA are also gratefully acknowledged.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.arres.2022.100057.
Contributor Information
Irundika HK Dias, Email: diashki1@aston.ac.uk.
Roy A. Quinlan, Email: r.a.quinlan@durham.ac.uk.
Appendix. Supplementary materials
Data Availability
Data will be made available on request.
References
- 1.Quinlan R.A., Clark J.I. Insights into the biochemical and biophysical mechanisms mediating the longevity of the transparent optics of the eye lens. J. Biol. Chem. 2022 doi: 10.1016/j.jbc.2022.102537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nielsen J., et al. Eye lens radiocarbon reveals centuries of longevity in the Greenland shark (Somniosus microcephalus) Science. 2016;353(6300):702–704. doi: 10.1126/science.aaf1703. [DOI] [PubMed] [Google Scholar]
- 3.Liu, P., et al., Long-lived metabolic enzymes in the crystalline lens identified by pulse-labeling of mice and mass spectrometry. Elife, 2019. 8. [DOI] [PMC free article] [PubMed]
- 4.Hughes J.R., et al. No turnover in lens lipids for the entire human lifespan. Elife. 2015;4 doi: 10.7554/eLife.06003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Borchman D., Stimmelmayr R., George J.C. Whales, lifespan, phospholipids, and cataracts. J. Lipid Res. 2017;58(12):2289–2298. doi: 10.1194/jlr.M079368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hamada N., Azizova T.V., Little M.P. An update on effects of ionizing radiation exposure on the eye. Br. J. Radiol. 2019 doi: 10.1259/bjr.20190829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Azizova T.V., et al. Risk of various types of cataracts in a cohort of Mayak workers following chronic occupational exposure to ionizing radiation. Eur. J. Epidemiol. 2018;33(12):1193–1204. doi: 10.1007/s10654-018-0450-4. [DOI] [PubMed] [Google Scholar]
- 8.Ainsbury E.A., et al. Radiation cataractogenesis: a review of recent studies. Radiat. Res. 2009;172(1):1–9. doi: 10.1667/RR1688.1. [DOI] [PubMed] [Google Scholar]
- 9.Stewart F.A., et al. ICRP publication 118: ICRP statement on tissue reactions and early and late effects of radiation in normal tissues and organs–threshold doses for tissue reactions in a radiation protection context. Ann. ICRP. 2012;41(1–2):1–322. doi: 10.1016/j.icrp.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 10.Uwineza A., et al. Cataractogenic load - a concept to study the contribution of ionizing radiation to accelerated aging in the eye lens. Mutat. Res. 2019;779:68–81. doi: 10.1016/j.mrrev.2019.02.004. [DOI] [PubMed] [Google Scholar]
- 11.Borchman D., Yappert M.C. Lipids and the ocular lens. J. Lipid Res. 2010;51(9):2473–2488. doi: 10.1194/jlr.R004119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pamplona R. Membrane phospholipids, lipoxidative damage and molecular integrity: a causal role in aging and longevity. Biochim. Biophys. Acta. 2008;1777(10):1249–1262. doi: 10.1016/j.bbabio.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 13.Halliwell B., Gutteridge J.M. Oxygen free radicals and iron in relation to biology and medicine: some problems and concepts. Arch. Biochem. Biophys. 1986;246(2):501–514. doi: 10.1016/0003-9861(86)90305-x. [DOI] [PubMed] [Google Scholar]
- 14.Xu L., Porter N.A. Free radical oxidation of cholesterol and its precursors: implications in cholesterol biosynthesis disorders. Free Radic. Res. 2015;49(7):835–849. doi: 10.3109/10715762.2014.985219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dauben W., Payot P. Radiation induced oxidation of cholesterol1. J. Am. Chem. Soc. 1956;78(21):5657–5660. [Google Scholar]
- 16.Lebovics V., et al. Cholesterol oxides in γ-irradiated spray-dried egg powder. J. Sci. Food Agric. 1992;60(2):251–254. [Google Scholar]
- 17.Zarrouk A., et al. Involvement of oxysterols in age-related diseases and ageing processes. Ageing Res. Rev. 2014;18:148–162. doi: 10.1016/j.arr.2014.09.006. [DOI] [PubMed] [Google Scholar]
- 18.Girao H., et al. Cholesterol oxides accumulate in human cataracts. Exp. Eye Res. 1998;66(5):645–652. doi: 10.1006/exer.1998.0465. [DOI] [PubMed] [Google Scholar]
- 19.Vejux A., Samadi M., Lizard G. Contribution of cholesterol and oxysterols in the physiopathology of cataract: implication for the development of pharmacological treatments. J. Ophthalmol. 2011;2011 doi: 10.1155/2011/471947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Worgul B.V., Merriam G.R., Jr., Medvedovsky C. Cortical cataract development–an expression of primary damage to the lens epithelium. Lens Eye Toxic Res. 1989;6(4):559–571. [PubMed] [Google Scholar]
- 21.Hayden J.H., et al. Hypophysectomy exerts a radioprotective effect on frog lens. Experientia. 1980;36(1):116–118. doi: 10.1007/BF02004009. [DOI] [PubMed] [Google Scholar]
- 22.Von Sallmann L. Experimental studies on early lens changes after roentgen irradiation. III. Effect of x-radiation on mitotic activity and nuclear fragmentation of lens epithelium in normal and cysteine-treated rabbits. AMA Arch. Ophthalmol. 1952;47(3):305–320. [PubMed] [Google Scholar]
- 23.Markiewicz, E., et al., Nonlinear ionizing radiation-induced changes in eye lens cell proliferation, cyclin D1 expression and lens shape. 2015. 5(4): p. 150011. [DOI] [PMC free article] [PubMed]
- 24.Fujii N., et al. d-Amino acids in protein: the mirror of life as a molecular index of aging. Biochim. Biophys. Acta. 2018 doi: 10.1016/j.bbapap.2018.03.001. [DOI] [PubMed] [Google Scholar]
- 25.Fujii N., Uchida H., Saito T. The damaging effect of UV-C irradiation on lens alpha-crystallin. Mol. Vis. 2004;10:814–820. [PubMed] [Google Scholar]
- 26.Fujii N., et al. Correlation between the loss of the chaperone-like activity and the oxidation, isomerization and racemization of gamma-irradiated alpha-crystallin. Photochem. Photobiol. 2001;74(3):477–482. doi: 10.1562/0031-8655(2001)074<0477:cbtlot>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 27.Kim I., et al. One-shot LC-MS/MS analysis of post-translational modifications including oxidation and deamidation of rat lens alpha- and beta-crystallins induced by gamma-irradiation. Amino Acids. 2016;48(12):2855–2866. doi: 10.1007/s00726-016-2324-y. [DOI] [PubMed] [Google Scholar]
- 28.Ramachandra Rao S., Fliesler S.J. Cholesterol homeostasis in the vertebrate retina: biology and pathobiology. J. Lipid Res. 2021;62 doi: 10.1194/jlr.TR120000979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barnes S., Quinlan R.A. Small molecules, both dietary and endogenous, influence the onset of lens cataracts. Exp. Eye Res. 2017;156:87–94. doi: 10.1016/j.exer.2016.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Berry V., et al. Pathogenic variants in the CYP21A2 gene cause isolated autosomal dominant congenital posterior polar cataracts. Ophthalmic Genet. 2021:1–6. doi: 10.1080/13816810.2021.1998556. [DOI] [PubMed] [Google Scholar]
- 31.Bassnett S., Mataic D. Chromatin degradation in differentiating fiber cells of the eye lens. J. Cell. Biol. 1997;137(1):37–49. doi: 10.1083/jcb.137.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ferguson S.R., Borchman D., Yappert M.C. Confirmation of the identity of the major phospholipid in human lens membranes. Invest. Ophthalmol. Vis. Sci. 1996;37(8):1703–1706. [PubMed] [Google Scholar]
- 33.Rujoi M., et al. Isolation and lipid characterization of cholesterol-enriched fractions in cortical and nuclear human lens fibers. Invest Ophthalmol Vis Sci. 2003;44(4):1634–1642. doi: 10.1167/iovs.02-0786. [DOI] [PubMed] [Google Scholar]
- 34.Mainali L., et al. Changes in the properties and organization of human lens lipid membranes occurring with age. Curr. Eye Res. 2017;42(5):721–731. doi: 10.1080/02713683.2016.1231325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li L.K., So L., Spector A. Membrane cholesterol and phospholipid in consecutive concentric sections of human lenses. J. Lipid Res. 1985;26(5):600–609. [PubMed] [Google Scholar]
- 36.Huang L., et al. Human lens phospholipid changes with age and cataract. Invest. Ophthalmol. Vis. Sci. 2005;46(5):1682–1689. doi: 10.1167/iovs.04-1155. [DOI] [PubMed] [Google Scholar]
- 37.Mainali L., et al. Properties of membranes derived from the total lipids extracted from clear and cataractous lenses of 61-70-year-old human donors. Eur. Biophys. J. 2015;44(1–2):91–102. doi: 10.1007/s00249-014-1004-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Briones R., Aponte-Santamaria C., de Groot B.L. Localization and ordering of lipids around aquaporin-0: protein and lipid mobility effects. Front. Physiol. 2017;8:124. doi: 10.3389/fphys.2017.00124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gonen T., et al. Lipid-protein interactions in double-layered two-dimensional AQP0 crystals. Nature. 2005;438(7068):633–638. doi: 10.1038/nature04321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tong J., et al. The water permeability of lens aquaporin-0 depends on its lipid bilayer environment. Exp. Eye Res. 2013;113:32–40. doi: 10.1016/j.exer.2013.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang Z., Schey K.L. Proteomic analysis of lipid raft-like detergent-resistant membranes of lens fiber cells. Invest. Ophthalmol. Vis. Sci. 2015;56(13):8349–8360. doi: 10.1167/iovs.15-18273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Widomska J., Raguz M., Subczynski W.K. Oxygen permeability of the lipid bilayer membrane made of calf lens lipids. Biochim. Biophys. Acta. 2007;1768(10):2635–2645. doi: 10.1016/j.bbamem.2007.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Subczynski W.K., et al. Organization of lipids in fiber-cell plasma membranes of the eye lens. Exp. Eye Res. 2017;156:79–86. doi: 10.1016/j.exer.2016.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Subczynski W.K., et al. Functions of cholesterol and the cholesterol bilayer domain specific to the fiber-cell plasma membrane of the eye lens. J. Membr. Biol. 2012;245(1):51–68. doi: 10.1007/s00232-011-9412-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Plesnar E., et al. Is the cholesterol bilayer domain a barrier to oxygen transport into the eye lens? Biochim. Biophys. Acta. 2018;1860(2):434–441. doi: 10.1016/j.bbamem.2017.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jacob R.F., Cenedella R.J., Mason R.P. Evidence for distinct cholesterol domains in fiber cell membranes from cataractous human lenses. J. Biol. Chem. 2001;276(17):13573–13578. doi: 10.1074/jbc.M010077200. [DOI] [PubMed] [Google Scholar]
- 47.Cotlier E., Rice P. Cataracts in the Smith-Lemli-Opitz syndrome. Am. J. Ophthalmol. 1971;72(5):955–959. doi: 10.1016/0002-9394(71)91696-5. [DOI] [PubMed] [Google Scholar]
- 48.Wilker S.C., Dagnelie G., Goldberg M.F. Retinitis pigmentosa and punctate cataracts in mevalonic aciduria. Retin Cases Brief Rep. 2010;4(1):34–36. doi: 10.1097/ICB.0b013e3181a59db6. [DOI] [PubMed] [Google Scholar]
- 49.Chen X., Liu L. Congenital cataract with LSS gene mutations: a new case report. J. Pediatr. Endocrinol. Metab. 2017;30(11):1231–1235. doi: 10.1515/jpem-2017-0101. [DOI] [PubMed] [Google Scholar]
- 50.Tibrewal S., et al. Cerebrotendinous xanthomatosis: early diagnosis on the basis of juvenile cataracts. J. aapos. 2017;21(6):505–507. doi: 10.1016/j.jaapos.2017.07.211. [DOI] [PubMed] [Google Scholar]
- 51.Aleo M.D., et al. Lens cholesterol biosynthesis inhibition: a common mechanism of cataract formation in laboratory animals by pharmaceutical products. J. Appl. Toxicol. 2019;39(9):1348–1361. doi: 10.1002/jat.3822. [DOI] [PubMed] [Google Scholar]
- 52.Golubev A., Hanson A.D., Gladyshev V.N. A tale of two concepts: harmonizing the free radical and antagonistic pleiotropy theories of aging. Antioxid. Redox Signal. 2018;29(10):1003–1017. doi: 10.1089/ars.2017.7105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sweeney M.H., Truscott R.J. An impediment to glutathione diffusion in older normal human lenses: a possible precondition for nuclear cataract. Exp Eye Res. 1998;67(5):587–595. doi: 10.1006/exer.1998.0549. [DOI] [PubMed] [Google Scholar]
- 54.Giblin F.J. Glutathione: a vital lens antioxidant. J. Ocul. Pharmacol. Ther. 2000;16(2):121–135. doi: 10.1089/jop.2000.16.121. [DOI] [PubMed] [Google Scholar]
- 55.Rodriguez I.R., et al. 7-ketocholesterol accumulates in ocular tissues as a consequence of aging and is present in high levels in drusen. Exp. Eye Res. 2014;128:151–155. doi: 10.1016/j.exer.2014.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Iuliano L. Pathways of cholesterol oxidation via non-enzymatic mechanisms. Chem. Phys. Lipids. 2011;164(6):457–468. doi: 10.1016/j.chemphyslip.2011.06.006. [DOI] [PubMed] [Google Scholar]
- 57.Kulig W., et al. Cholesterol oxidation products and their biological importance. Chem. Phys. Lipids. 2016;199:144–160. doi: 10.1016/j.chemphyslip.2016.03.001. [DOI] [PubMed] [Google Scholar]
- 58.Griffiths W.J., et al. Current trends in oxysterol research. Biochem. Soc. Trans. 2016;44(2):652–658. doi: 10.1042/BST20150255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tapodi A., et al. BFSP1 C-terminal domains released by post-translational processing events can alter significantly the calcium regulation of AQP0 water permeability. Exp. Eye Res. 2019;185 doi: 10.1016/j.exer.2019.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gold M.G., et al. AKAP2 anchors PKA with aquaporin-0 to support ocular lens transparency. EMBO Mol. Med. 2012;4(1):15–26. doi: 10.1002/emmm.201100184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chattopadhyay M., et al. Hydration layer of only a few molecules controls lipid mobility in biomimetic membranes. J. Am. Chem. Soc. 2021;143(36):14551–14562. doi: 10.1021/jacs.1c04314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ricci M., Quinlan R.A., Voitchovsky K. Sub-nanometre mapping of the aquaporin-water interface using multifrequency atomic force microscopy. Soft Matter. 2017;13(1):187–195. doi: 10.1039/c6sm00751a. [DOI] [PubMed] [Google Scholar]
- 63.Akselrod M.S., McKeeveer S.W.S. A radiation dosimetry method using pulsed optically stimultaed luminescence. Radiat. Prot. Dosimetry. 1999;81:161–176. [Google Scholar]
- 64.Dos Remedios C., Gilmour D. An historical perspective of the discovery of titin filaments. Biophys Rev. 2017;9(3):179–188. doi: 10.1007/s12551-017-0269-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Bligh E.G., Dyer W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- 66.McDonald J.G., et al. A comprehensive method for extraction and quantitative analysis of sterols and secosteroids from human plasma. J. Lipid Res. 2012;53(7):1399–1409. doi: 10.1194/jlr.D022285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Magomedova L., Cummins C.L. Quantification of oxysterol nuclear receptor ligands by LC/MS/MS. Methods Mol. Biol. 2019;1951:1–14. doi: 10.1007/978-1-4939-9130-3_1. [DOI] [PubMed] [Google Scholar]
- 68.Dias I.H.K., et al. Simvastatin reduces circulating oxysterol levels in men with hypercholesterolaemia. Redox Biol. 2018;16:139–145. doi: 10.1016/j.redox.2018.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.State College, P.M., Inc., Minitab 18 statistical software. [Computer software], 2017.
- 70.Fu S.C., et al. Characterization of lens proteins. IV. Analysis of soluble high molecular weight protein aggregates in human lenses. Exp. Eye Res. 1984;38(5):485–495. doi: 10.1016/0014-4835(84)90126-x. [DOI] [PubMed] [Google Scholar]
- 71.Siew E.L., Opalecky D., Bettelheim F.A. Light scattering of normal human lens. II. Age dependence of the light scattering parameters. Exp. Eye Res. 1981;33(6):603–614. doi: 10.1016/s0014-4835(81)80100-5. [DOI] [PubMed] [Google Scholar]
- 72.Swamy-Mruthinti S., et al. Thermal stress induced aggregation of aquaporin 0 (AQP0) and protection by α-crystallin via its chaperone function. PLoS ONE. 2013;8(11):e80404. doi: 10.1371/journal.pone.0080404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ramkumar S., et al. Comparison of effect of gamma ray irradiation on wild-type and N-terminal mutants of αA-crystallin. Mol. Vis. 2014;20:1002–1016. [PMC free article] [PubMed] [Google Scholar]
- 74.McCarron R.A., et al. Radiation-induced lens opacity and cataractogenesis: a lifetime study using mice of varying genetic backgrounds. Radiat. Res. 2022;197(1):57–66. doi: 10.1667/RADE-20-00266.1. [DOI] [PubMed] [Google Scholar]
- 75.Smith L.L. Cholesterol autoxidation 1981-1986. Chem. Phys. Lipids. 1987;44(2–4):87–125. doi: 10.1016/0009-3084(87)90046-6. [DOI] [PubMed] [Google Scholar]
- 76.Girotti A.W., Korytowski W. Cholesterol as a singlet oxygen detector in biological systems. Methods Enzymol. 2000;319:85–100. doi: 10.1016/s0076-6879(00)19011-1. [DOI] [PubMed] [Google Scholar]
- 77.Zerbinati C., Iuliano L. Cholesterol and related sterols autoxidation. Free Radic. Biol. Med. 2017;111:151–155. doi: 10.1016/j.freeradbiomed.2017.04.013. [DOI] [PubMed] [Google Scholar]
- 78.Jin H., et al. Multifunctional antioxidants for the treatment of age-related diseases. J. Med. Chem. 2010;53(3):1117–1127. doi: 10.1021/jm901381j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ahuja R.P., et al. Effect of oxidation on Ca2+ -ATPase activity and membrane lipids in lens epithelial microsomes. Free Radic. Biol. Med. 1999;27(1–2):177–185. doi: 10.1016/s0891-5849(99)00068-4. [DOI] [PubMed] [Google Scholar]
- 80.Hughes J.R., et al. Instability of the cellular lipidome with age. Age (Dordr) 2012;34(4):935–947. doi: 10.1007/s11357-011-9293-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Borchman D., Yappert M.C. Age-related lipid oxidation in human lenses. Invest. Ophthalmol. Vis. Sci. 1998;39(6):1053–1058. [PubMed] [Google Scholar]
- 82.Bhuyan K.C., Bhuyan D.K. Molecular mechanism of cataractogenesis: III. Toxic metabolites of oxygen as initiators of lipid peroxidation and cataract. Curr. Eye Res. 1984;3(1):67–81. doi: 10.3109/02713688408997188. [DOI] [PubMed] [Google Scholar]
- 83.Bhuyan K.C., Bhuyan D.K., Podos S.M. Lipid peroxidation in cataract of the human. Life Sci. 1986;38(16):1463–1471. doi: 10.1016/0024-3205(86)90559-x. [DOI] [PubMed] [Google Scholar]
- 84.Bhuyan K.C., et al. Molecular mechanisms of cataractogenesis: IV. Evidence of phospholipid . malondialdehyde adduct in human senile cataract. Mech. Ageing Dev. 1986;34(3):289–296. doi: 10.1016/0047-6374(86)90080-1. [DOI] [PubMed] [Google Scholar]
- 85.Micelli-Ferrari T., et al. Role of lipid peroxidation in the pathogenesis of myopic and senile cataract. Br. J. Ophthalmol. 1996;80(9):840–843. doi: 10.1136/bjo.80.9.840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Simonelli F., et al. Lipid peroxidation and human cataractogenesis in diabetes and severe myopia. Exp. Eye Res. 1989;49(2):181–187. doi: 10.1016/0014-4835(89)90088-2. [DOI] [PubMed] [Google Scholar]
- 87.Varma S.D., et al. Oxidative stress on lens and cataract formation: role of light and oxygen. Curr. Eye Res. 1984;3(1):35–57. doi: 10.3109/02713688408997186. [DOI] [PubMed] [Google Scholar]
- 88.Babizhayev M.A., Deyev A.I., Linberg L.F. Lipid peroxidation as a possible cause of cataract. Mech. Ageing Dev. 1988;44(1):69–89. doi: 10.1016/0047-6374(88)90080-2. [DOI] [PubMed] [Google Scholar]
- 89.Anderson A., et al. 7-Ketocholesterol in disease and aging. Redox Biol. 2020;29 doi: 10.1016/j.redox.2019.101380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.McNulty R., et al. Regulation of tissue oxygen levels in the mammalian lens. J. Physiol. 2004;559(Pt 3):883–898. doi: 10.1113/jphysiol.2004.068619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Smith L.L. Another cholesterol hypothesis: cholesterol as antioxidant. Free Radic. Biol. Med. 1991;11(1):47–61. doi: 10.1016/0891-5849(91)90187-8. [DOI] [PubMed] [Google Scholar]
- 92.Parasassi T., et al. Cholesterol protects the phospholipid bilayer from oxidative damage. Free Radic. Biol. Med. 1995;19(4):511–516. doi: 10.1016/0891-5849(95)00038-y. [DOI] [PubMed] [Google Scholar]
- 93.Zhang X., Barraza K.M., Beauchamp J.L. Cholesterol provides nonsacrificial protection of membrane lipids from chemical damage at air-water interface. Proc. Natl. Acad. Sci. U S A. 2018;115(13):3255–3260. doi: 10.1073/pnas.1722323115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Girao H., et al. Cholesterol may modulate membrane susceptibility to oxidation in cataractous lens. Vision Res. 1995;(35):S141. [Google Scholar]
- 95.Girao H., Mota C., Pereira P. Cholesterol may act as an antioxidant in lens membranes. Curr. Eye Res. 1999;18(6):448–454. doi: 10.1076/ceyr.18.6.448.5273. [DOI] [PubMed] [Google Scholar]
- 96.Sevanian A., McLeod L.L. Cholesterol autoxidation in phospholipid membrane bilayers. Lipids. 1987;22(9):627–636. doi: 10.1007/BF02533940. [DOI] [PubMed] [Google Scholar]
- 97.Kim J.H., Jenrow K.A., Brown S.L. Mechanisms of radiation-induced normal tissue toxicity and implications for future clinical trials. Radiat. Oncol. J. 2014;32(3):103–115. doi: 10.3857/roj.2014.32.3.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Han J., Schey K.L. Proteolysis and mass spectrometric analysis of an integral membrane: aquaporin 0. J. Proteome. Res. 2004;3(4):807–812. doi: 10.1021/pr049945w. [DOI] [PubMed] [Google Scholar]
- 99.Korlimbinis A., et al. Protein aging: truncation of aquaporin 0 in human lens regions is a continuous age-dependent process. Exp. Eye Res. 2009;88(5):966–973. doi: 10.1016/j.exer.2008.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Liu K., et al. Altered ubiquitin causes perturbed calcium homeostasis, hyperactivation of calpain, dysregulated differentiation, and cataract. Proc. Natl. Acad. Sci. U S A, 2015;112(4):1071–1076. doi: 10.1073/pnas.1404059112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Grey A.C., Schey K.L. Age-related changes in the spatial distribution of human lens alpha-crystallin products by MALDI imaging mass spectrometry. Invest. Ophthalmol. Vis. Sci. 2009;50(9):4319–4329. doi: 10.1167/iovs.09-3522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Rothkamm K., Lobrich M. Evidence for a lack of DNA double-strand break repair in human cells exposed to very low x-ray doses. Proc. Natl. Acad. Sci. U S A, 2003;100(9):5057–5062. doi: 10.1073/pnas.0830918100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Timm S., et al. Clustered DNA damage concentrated in particle trajectories causes persistent large-scale rearrangements in chromatin architecture. Radiother. Oncol. 2018;129(3):600–610. doi: 10.1016/j.radonc.2018.07.003. [DOI] [PubMed] [Google Scholar]
- 104.Ulyanenko S., et al. Formation of γH2AX and pATM foci in human mesenchymal stem cells exposed to low dose-rate gamma-radiation. Int. J. Mol. Sci. 2019;20(11) doi: 10.3390/ijms20112645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kumareswaran R., et al. Chronic hypoxia compromises repair of DNA double-strand breaks to drive genetic instability. J. Cell Sci. 2012;125(Pt 1):189–199. doi: 10.1242/jcs.092262. [DOI] [PubMed] [Google Scholar]
- 106.Lobachevsky P., et al. Compromized DNA repair as a basis for identification of cancer radiotherapy patients with extreme radiosensitivity. Cancer Lett. 2016;383(2):212–219. doi: 10.1016/j.canlet.2016.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Schaefer E.J., et al. Cerebrotendinous xanthomatosis, sitosterolemia, Smith-Lemli-Opitz syndrome and the seminal contributions of Gerald Salen, MD (1935-2020) J. Clin. Lipidol. 2021;15(4):540–544. doi: 10.1016/j.jacl.2021.05.004. [DOI] [PubMed] [Google Scholar]
- 108.Heo G.Y., et al. Conversion of 7-ketocholesterol to oxysterol metabolites by recombinant CYP27A1 and retinal pigment epithelial cells. J. Lipid Res. 2011;52(6):1117–1127. doi: 10.1194/jlr.M014217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Mitić T., et al. 11β-Hydroxysteroid dehydrogenase type 1 contributes to the balance between 7-keto- and 7-hydroxy-oxysterols in vivo. Biochem. Pharmacol. 2013;86(1):146–153. doi: 10.1016/j.bcp.2013.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Sevanian A., McLeod L.L. Catalytic properties and inhibition of hepatic cholesterol-epoxide hydrolase. J. Biol. Chem. 1986;261(1):54–59. [PubMed] [Google Scholar]
- 111.Raza S.T., et al. Association of angiotensin-converting enzyme, CYP46A1 genes polymorphism with senile cataract. Oman J. Ophthalmol. 2017;10(1):21–25. doi: 10.4103/ojo.OJO_40_2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Cenedella R.J. Cholesterol synthesis inhibitor U18666A and the role of sterol metabolism and trafficking in numerous pathophysiological processes. Lipids. 2009;44(6):477–487. doi: 10.1007/s11745-009-3305-7. [DOI] [PubMed] [Google Scholar]
- 113.Theunissen J.J., et al. Membrane properties of oxysterols. Interfacial orientation, influence on membrane permeability and redistribution between membranes. Biochim. Biophys. Acta. 1986;860(1):66–74. doi: 10.1016/0005-2736(86)90499-2. [DOI] [PubMed] [Google Scholar]
- 114.Vestergaard M.C., et al. The effect of oxycholesterols on thermo-induced membrane dynamics. Biochim. Biophys. Acta. 2011;1808(9):2245–2251. doi: 10.1016/j.bbamem.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 115.Davson H. Nutrition of the lens by way of aqueous humour. J. Physiol. 1954;124(2):42–43. [PubMed] [Google Scholar]
- 116.Varma S.D., Richards R.D. Ascorbic acid and the eye lens. Ophthalmic Res. 1988;20(3):164–173. doi: 10.1159/000266579. [DOI] [PubMed] [Google Scholar]
- 117.Krepler K., Schmid R. Alpha-tocopherol in plasma, red blood cells and lenses with and without cataract. Am. J. Ophthalmol. 2005;139(2):266–270. doi: 10.1016/j.ajo.2004.09.031. [DOI] [PubMed] [Google Scholar]
- 118.Yeum K.J., et al. Measurement of carotenoids, retinoids, and tocopherols in human lenses. Invest. Ophthalmol. Vis. Sci. 1995;36(13):2756–2761. [PubMed] [Google Scholar]
- 119.Miller R.J., Fujino T., Nefzger M.D. Lens findings in Atomic bomb survivors. A review of major ophthalmic surveys at the atomic Bomb Casualty Commission (1949-1962) Arch. Ophthalmol. 1967;78(6):697–704. doi: 10.1001/archopht.1967.00980030699002. [DOI] [PubMed] [Google Scholar]
- 120.Wilde G., Sjostrand J. A clinical study of radiation cataract formation in adult life following gamma irradiation of the lens in early childhood. Br. J. Ophthalmol. 1997;81(4):261–266. doi: 10.1136/bjo.81.4.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kunze S., et al. Posterior subcapsular cataracts are a late effect after acute exposure to 0.5 Gy ionizing radiation in mice. Int. J. Radiat. Biol. 2021;97(4):529–540. doi: 10.1080/09553002.2021.1876951. [DOI] [PubMed] [Google Scholar]
- 122.Little M.P., et al. Occupational radiation exposure and risk of cataract incidence in a cohort of US radiologic technologists. Eur. J. Epidemiol. 2018;33(12):1179–1191. doi: 10.1007/s10654-018-0435-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Barnard S., et al. Lens epithelial cell proliferation in response to ionizing radiation. Radiat. Res. 2022;197(1):92–99. doi: 10.1667/RADE-20-00294.1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.