Abstract
Practical relevance:
Ultrasonography is an important tool for the detection of kidney disorders, which are among the most common health problems suffered by cats. It is more accurate than radiography for this purpose and is considered to be the reference modality for imaging the feline kidney, providing excellent visualisation of renal size, shape and internal architecture. Compared with more advanced imaging modalities, such as computed tomography or magnetic resonance imaging, ultrasonography is more accessible, less expensive, does not require general anaesthesia and allows real-time procedures to be performed.
Clinical challenges:
On ultrasound examination, focal or multifocal disorders may be readily identified, but diffuse changes are more challenging. B-mode ultrasonography is of limited use for differentiating between benign and malignant focal lesions. However, based on the presence and pattern of vascularity as an indicator of malignancy, contrast-enhanced ultrasonography allows distinction between benign and malignant focal renal lesions.
Audience:
This review provides a framework for the ultrasonographic approach to feline renal and perirenal disorders for the general practitioner.
Evidence base:
Drawing on current literature relating to ultrasonographic examination of feline kidneys, the aim is to summarise ultrasonographic technique, anatomy and changes associated with renal and perirenal diseases.
Technique for ultrasonography of the feline kidney
B-mode ultrasonography
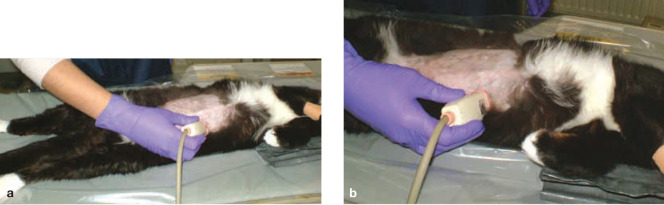
B-mode ultrasonography is often the modality of choice for imaging the feline kidney.1–3 It provides excellent visualisation of renal size, shape and internal architecture.2,4,5 Due to their superficial position, the kidneys can be imaged from both a lateral and ventral approach with the animal in lateral or dorsal recumbency, respectively. The cat can be sedated or manually restrained. The abdomen is prepared by clipping the hair and applying acoustic coupling gel to the skin. High frequency (7.5–10 MHz) linear transducers are mandatory for renal imaging in cats and only light transducer pressure is applied to prevent the loosely attached kidneys being displaced from their normal anatomical location.4,6–9 The transducer is held parallel to the long axis of the animal for longitudinal images and perpendicular to the spine for transverse images (Figure 1), though because feline kidneys are relatively mobile, this is not an absolute rule. 7
Figure 1.
Positioning for ultrasound examination of the kidneys. The cat is lying in dorsal recumbency with the transducer held parallel to the long axis for the longitudinal image (a) and perpendicular to the spine for the transverse image (b)
Each kidney should be imaged in two orthogonal planes as a minimum.4,7 Imaging of the entire parenchyma is important to detect any focal renal lesions. 6 Additionally, ultrasonography allows guided fine-needle aspiration or core biopsy to be performed in real time.
Contrast-enhanced ultrasound examination
Contrast-enhanced ultrasound (CEUS) is a more specialised technique involving the use of contrast agents consisting of gas-filled microbubbles encapsulated by a shell. These highly reflective microbubbles substantially increase contrast between parenchymal tissues and their blood-containing vessels or sinusoids. Hence, parenchymal microcirculation can also be detected, 10 though this requires the use of dedicated software and ultrasound probes.11,12
Ultrasonographic anatomy of the normal kidney
Size
Kidney measurements in cats have been reported to range between 3.0 and 4.3 cm, but may be up to 5.3 cm. Both kidneys should be similar in length.4,5,9,20 Renal size varies depending on sex and sexual status: females have smaller kidneys than males, and neutered cats have larger kidneys than intact cats.3,7,21
Only two studies describe cortical and medullary thickness in cats. Walter et al reported a cortical thickness of 0.82 ± 0.14 cm and a medullary thickness of 0.59 ± 0.06 cm. 20 Park et al recorded a smaller cortical thickness of 0.47 ± 0.08 cm and a similar medullary thickness of 0.55 ± 0.07 for the left kidney and 0.50 ± 0.07 for the right kidney. 13
Anatomical location
The left kidney is located immediately caudal to the fundus of the stomach, caudomedial to the head of the spleen and lateral to the aorta. The cranial pole of the right kidney can be found in the renal fossa of the caudate lobe of the liver, ventral and often medial to the duodenum and lateral to the caudal vena cava.7,8 Both feline kidneys are situated entirely caudal to the ribs and are therefore highly accessible to ultrasound examination. 4
Shape
A normal kidney is oval or bean-shaped, smooth in outline and well defined. 7 The renal capsule is a thin linear hyperechoic structure, but is usually not visible at the poles where the tissue interfaces are parallel to the ultrasound beam. Sometimes edge shadowing may be present.4,7
Internal architecture
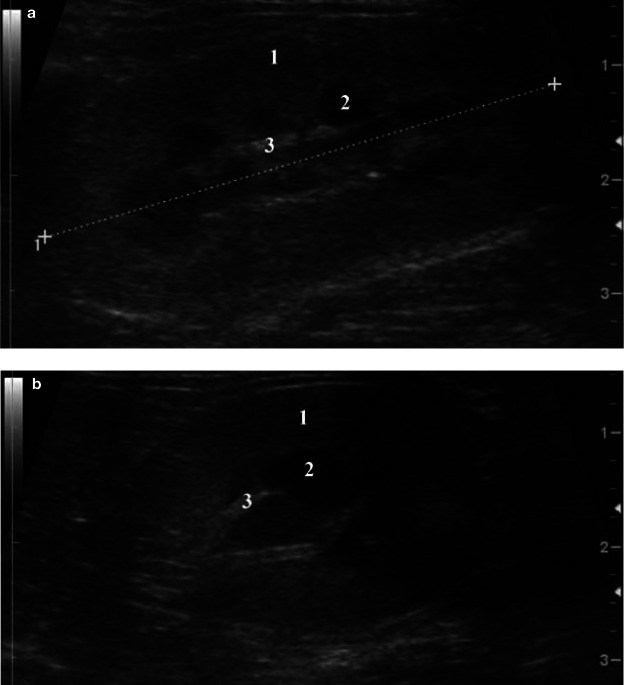
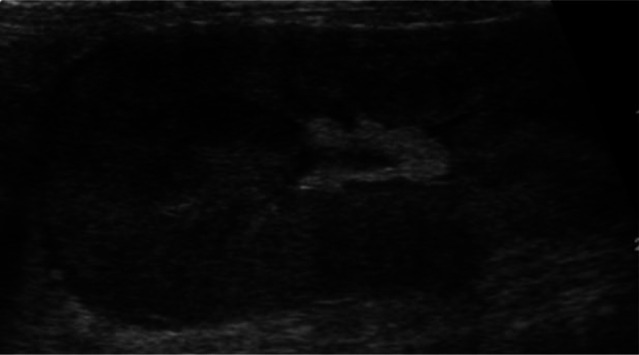
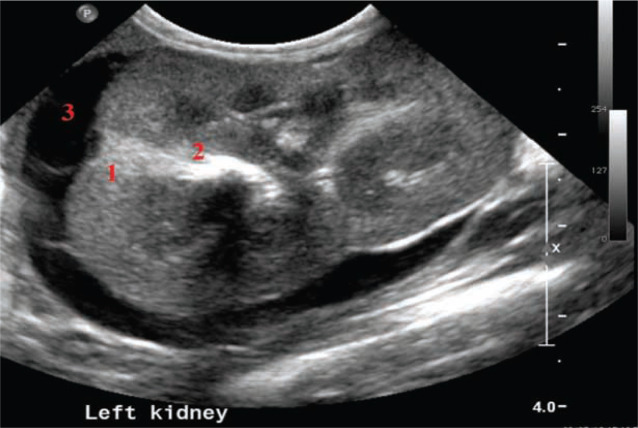
Three distinct regions can be identified: renal cortex, medulla and sinus (Figure 2).5,7 The renal pelvis is usually not visualised.
Figure 2.
Sagittal (a) and transverse (b) scan of a normal kidney, showing cortex (1), medulla (2) and sinus (3)
Cortex The normal renal cortex is uniformly echoic, usually slightly hypo- or isoechoic when compared with the liver parenchyma (right kidney) and hypoechoic when compared with the splenic parenchyma (left kidney). It shows a finely granular echotexture. 7 However, in normal cats, fat accumulation in the cytoplasm of the proximal tubular epithelium can cause increased cortical echogenicity.2,7,22 In these cases, the renal cortex may become hyperechoic compared with the liver.4,6 These kidneys also tend to be larger. 23 Drost et al found that the amount of fat in the renal cortex is related to sex hormones and age, but not to body weight. 22 An increased cortical echogenicity may therefore be identified in elderly, castrated male cats and pregnant females without renal dysfunction. 2 Normally, a clear demarcation between cortex and medulla is present, with a hyperechoic cortex compared with the medulla.4,6
-
Medulla The renal medulla, which surrounds the renal pelvis, is very hypoechoic, to the point of being almost anechoic. It is transected by linear echogenicities (recesses or diverticula) and accompanying interlobar vessels. These vessels pass through the medulla from the sinus to the cortex and may generate acoustic shadowing. This should not be mistaken for calculi. 7
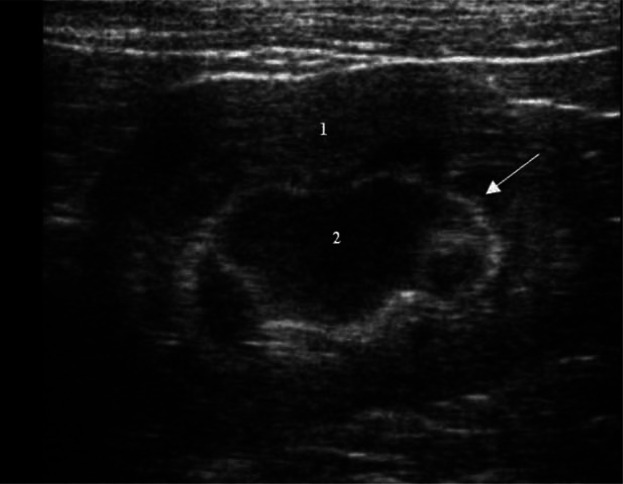
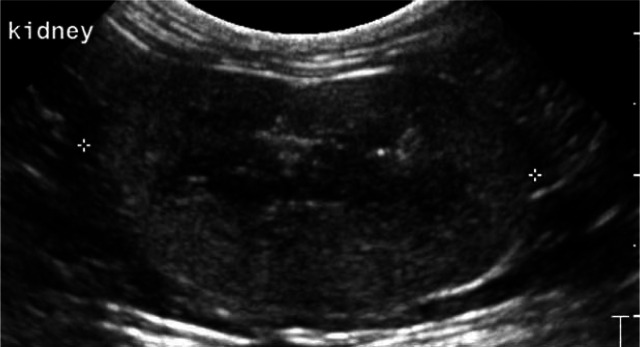
Some normal cats show a thin linear, complete or incomplete hyperechoic band that parallels the corticomedullary junction in the outer zone of the medulla (the so-called medullary rim sign) (Figure 3).5,6,8 Histologically this is due to calcium deposits in the lumen of the proximal renal tubules.4,5,8 The medullary rim sign can also be a feature of multiple pathological conditions such as acute tubular necrosis (ethylene glycol toxicity), pyogranulomatous vasculitis due to feline infectious peritonitis, chronic interstitial nephritis and renal calcification secondary to hypercalcaemia.24,25
Sinus The renal sinus, centrally located at the renal hilus and surrounding the renal pelvis, is hyperechoic due to the presence of peripelvic fat and dense fibrous connective tissue.4,7 It is particularly prominent in obese cats and can show faint distal acoustic shadowing.5,9 Care should be taken not to confuse the normal fat and fibrous tissue with areas of mineralisation. 8
-
Pelvis Although usually not visualised, the renal pelvis is sometimes visible as a narrow anechoic fissure, 1–2 mm wide, in the centre of the sinus.4,5 The lumen of the renal pelvis is Y- or V-shaped and is best seen on transverse or sagittal plane images. 5 A mild degree of pyelectasia (up to 2 mm) may be visible during intravenous fluid administration and should not be interpreted as a pathological finding.5,9
Interlobar arteries and veins lie in grooves within the pelvic diverticula and are seen as linear hypo/anechoic structures running from the renal pelvis towards the cortex. The arcuate arteries appear as small hyperechoic foci with anechoic centres adjacent to the corticomedullary junction. Shadowing or refraction often results in acoustic shadowing and should not be mistaken for areas of mineralisation. 5
Figure 3.
Dorsal ultrasound scan of a normal kidney, showing cortex (1), medulla (2) and a medullary rim sign (arrow)
Perirenal structures
The ureters are normally not identified.4,7 The renal artery and vein entering and leaving the hilus should be identifiable. 6
A mild degree of pyelectasia may be visible during intravenous fluid administration.
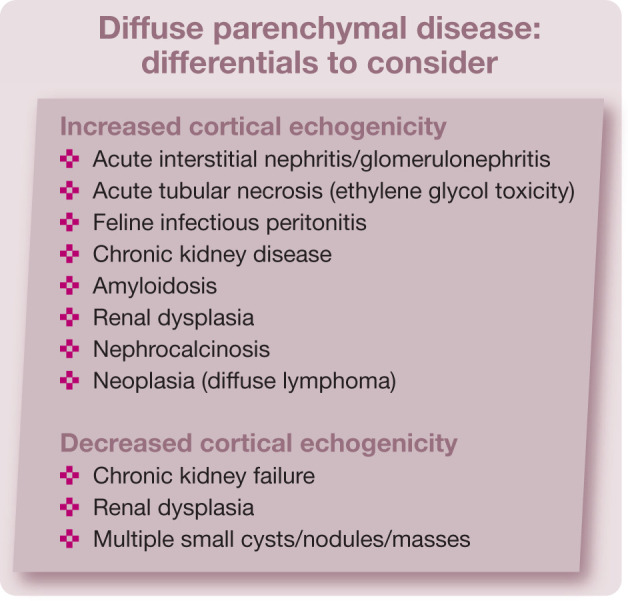
Diffuse parenchymal abnormalities
Diffuse parenchymal abnormalities can be a diagnostic challenge. 2 The ultrasound appearance of diffuse parenchymal renal disease may be normal or there may be increased or decreased cortical echogenicity, with enhanced or reduced corticomedullary differentiation.2,4 Ultrasonographic patterns and echogenicity are often non-specific for diffuse renal diseases. 8
Increased cortical echogenicity
Increased cortical echogenicity is one of the most common findings in cats (and dogs) with renal insufficiency. 9 It is a non-specific finding and can occur with different conditions. In many cases the cortex becomes hyperechoic with enhanced corticomedullary differentiation. With more progressive diseases the medulla may also increase in opacity, resulting in overall poor definition of the renal architecture. 4
Acute kidney disease
Acute kidney problems (acute glomerulonephritis, interstitial nephritis, acute tubular necrosis or nephrosis) often result in increased cortical echogenicity, while corticomedullary distinction is maintained. Often the kidneys are enlarged and retain a smooth contour.2,7,9,25
Acute tubular necrosis or nephrosis secondary to ethylene glycol toxicity
Ethylene glycol toxicity will cause oxalate crystal deposition in the renal cortex and medulla, 8 and typically results in severely hyperechoic cortices. 4 Within 5–8 h of uptake the renal cortices become iso- to hyperechoic to the spleen. In most cases the medulla will also be affected and may become isoechoic to the cortex, leading to a progressive loss of corticomedullary distinction. Thereafter, a thin hypoechoic zone (halo sign) may appear at the corticomedullary junction. This is a poor prognostic indicator and is associated with anuria.5,26
Feline infectious peritonitis
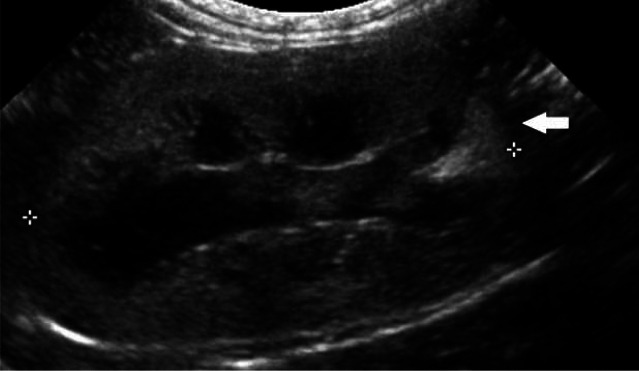
Feline infectious peritonitis is regarded to be caused by a mutated form of the feline enteric coronavirus. Two forms have been described: effusive and non-effusive. The effusive form is an immune-mediated vasculitis with resultant loss of protein-rich fluid into the pleural space, peritoneal cavity, pericardial space and subcapsular space of the kidney. The non-effusive form is characterised by pyogranulomatous inflammation that affects multiple organs (eyes, brain, kidney, omentum and liver). 27 The latter will result in severe generalised renomegaly and irregularly outlined kidneys.4,5,27 On ultrasonography, focal hyperechoic nodules or a diffuse increase in cortical echogenicity will be seen. 5 Occasionally, there may be a thin hypoechoic subcapsular rim surrounding one or both kidneys (Figure 4). This rim is hypothesised to be due to cellular infiltration of the subcapsular space. 27
Figure 4.
Sagittal ultrasound scan of the left kidney of a cat with feline infectious peritonitis showing renomegaly (5.1 cm), which was bilateral, and an irregular contour. The kidney is markedly increased in echogenicity and there is poor corticomedullary distinction. A thin, crescent-shaped, hypoechoic subcapsular rim is present
Chronic kidney disease
Chronic kidney disease (CKD) is the most common disorder involving the kidneys of cats (and dogs). Common causes are glomerulonephritis, polycystic kidney disease (PKD), autoimmune diseases, nephrotoxins, tubular diseases, pyelonephritis, interstitial nephritis and systemic or glomerular hypertension. CKD results in small, irregularly outlined (end-stage) kidneys which show normal or increased echogenicity of the cortex and medulla, decreased corticomedullary distinction and poorly discernible internal architecture (Figure 5).2,4,8,25,28 In human patients, cortical thinning is often present and can precede changes in kidney size. Cortical thinning, in humans, is used as a means of differentiating between acute and chronic kidney failure.29,30
Figure 5.
Irregular kidney contour and poor corticomedullary distinction, consistent with chronic kidney disease
In advanced stages, small areas of mineralisation can be seen on ultrasound images as hyperechoic foci with or without distal acoustic shadowing. 5 These are calcium and phosphorus deposits, triggered by longstanding elevated parathyroid hormone concentrations associated with decreased kidney function.
Amyloidosis
Amyloidosis is a diverse group of diseases characterised by deposition of inert, insoluble extracellular protein fibrils (amyloid) in different organs. These accumulations cause interference with tissue structure and function and sometimes this becomes clinically significant. 31 Familial amyloidosis in Abyssinian cats, which affects females more often than males, causes accumulation of amyloid protein AA, primarily in the interstitium of the renal medulla, although glomerular involvement can also occur. Eventually, amyloid deposition results in medullary fibrosis, papillary necrosis and finally CKD.31,32 Affected kidneys are commonly hyperechoic and can vary in size according to the chronicity of the disease. 9 In cats, reduced renal size and increased medullary echogenicity are often present. 5
Systemic amyloidosis is described in Siamese and Oriental cats. In contrast to familial amyloidosis in Abyssinians, accumulation of amyloid occurs primarily in the liver, leading to hepatic rupture and haemorrhage. However, disseminated amyloid deposits, including renal amyloidosis, may also be encountered in these breeds. 31
Renal dysplasia
Renal dysplasia describes a disorganised development of the renal parenchyma due to anomalous differentiation. It has been reported in various breeds of dogs and in Ragdoll and Persian cats.8,32,33 It may be familial, secondary to fetal/neonatal infection (panleukopenia virus) or a teratogenetic effect. The diagnosis is based on the young age of the animal and renal biopsy. 9 Ultrasonographic findings are similar to any chronic infiltrative renal disease, although kidneys may be unremarkable in the early stages.8,9 Generally, the kidneys are small, misshapen and hyperechoic. The internal architecture is abnormal and there is poor corticomedullary differentiation. Cysts and dilated ureters may be present. 8
The extent of abnormalities varies from a grossly disorganised multicystic dysplasia involving the whole kidney to a less severe segmental change, in which part of the kidney is unaffected. 33
Nephrocalcinosis
Nephrocalcinosis is characterised by dystrophic mineralisation within the renal parenchyma and is sometimes difficult to differentiate from nephrolithiasis. Both disorders cause mineral opacities with bright interfaces and distal acoustic shadowing. It is very common to find faint linear mineralisations in the renal parenchyma adjacent to the diverticula and renal crest in older patients. 4 On ultrasonography (Figure 6) a diffuse increase in cortical and/or medullary echogenicity and a hyperechoic band at the corticomedullary junction can be seen.5,28
Figure 6.
Ultrasound image of the left kidney of a cat presented with hypercalcaemia of unknown origin. Note the irregular contour, hyperechoic cortices and reduced corticomedullary distinction, compatible with nephrocalcinosis
Causes of nephrocalcinosis include CKD, ethylene glycol toxicity, primary or secondary hyperparathyroidism, hypercalcaemia and hypervitaminosis D.5,28
Neoplasia
Diffuse neoplastic infiltrates (lymphoma) and metastatic squamous cell carcinoma can result in an increased cortical echogenicity with retained corticomedullary distinction.2,7,9,25 Lymphoma is the most common renal neoplasm in cats. 34 It often results in a diffuse increase in cortical echogenicity, usually in combination with diffuse renal enlargement, with or without distortion of the kidney contour.4,5 Focal or multifocal lymphomatous lesions can also occur (see later).
Decreased cortical echogenicity
A decrease in cortical echogenicity is not a specific finding and has been described with necrosis, lymphoma infiltration, CKD and renal dysplasia. It can also be associated with multifocal changes due to the presence of multiple small hypoechoic cysts, nodules or masses.4,6
Focal parenchymal abnormalities
In general, focal abnormalities are more easily identified than diffuse renal diseases, although small parenchymal lesions which do not distort the renal contour or the internal architecture are more difficult to detect. 7 The ultrasonographic appearance and internal characteristics of a focal renal mass usually do not permit a definitive diagnosis. 2 B-mode ultrasonography is also not able to differentiate between benign and malignant lesions.4,28 However, by using the presence of vascularity as an indicator of malignancy, CEUS is able to distinguish benign from malignant focal renal lesions. 11
Renal cysts
Cysts are usually round or ovoid structures containing anechoic fluid, and have a smooth, thin and well-defined wall. They may show edge shadowing, distal acoustic enhancement and slice thickness artefacts, mimicking the presence of sediment.7,9 Cysts can be solitary (Figure 7) or multiple (Figure 8) and commonly involve both kidneys. They may be located within medulla or within cortex.8,25 Solitary cysts may be seen in normal, particularly older, animals as an incidental finding. Large and multiple cysts often distort the contour of the kidney and may cause displacement and distortion of the collecting system.6–8
Figure 7.
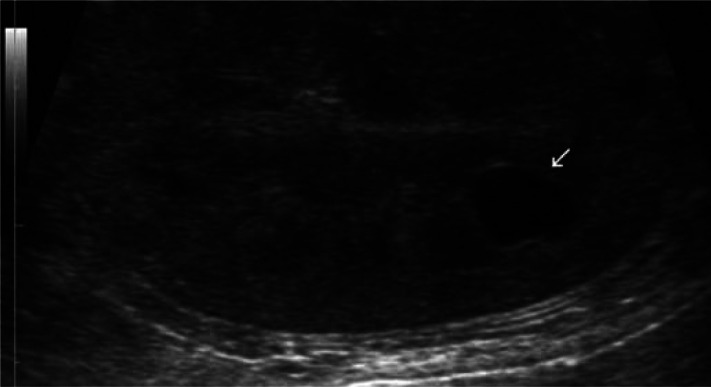
Sagittal ultrasound scan showing an anechoic cyst (arrow) and decreased corticomedullary distinction
Figure 8.
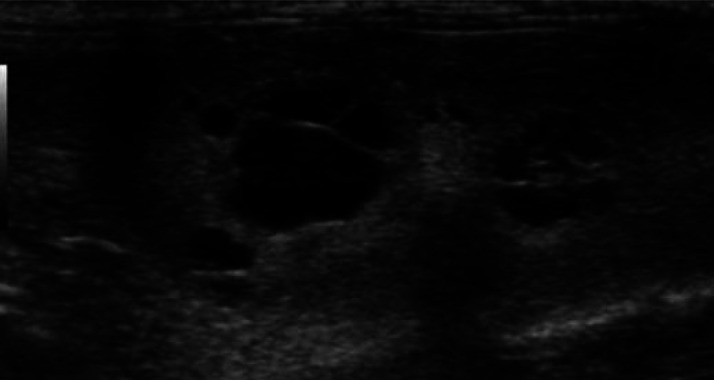
Polycystic kidney disease: multiple, variably sized, well-defined, rounded anechoic structures are present
When the cyst wall is thick or irregular, when internal septations are present or when the content is not completely anechoic, other differentials need to be considered such as an abscess, haematoma, neoplasm or atypical cyst.2,8
Polycystic kidney disease
PKD is one of the most common genetic disorders in cats and is typically seen in Persians or Persian crossbreeds, but can also affect other breeds (eg, Ragdoll, domestic shorthair).35–38 Cats with PKD eventually develop chronic kidney failure with marked renomegaly. Clinical signs become apparent in young to middle-aged adults. 2 Anechoic, variably sized, thin-walled cysts, mostly with acoustic enhancement, are noted in the cortex, corticomedullary junction and/or medulla of one or both kidneys (Figure 8). There is progressive displacement of normal renal tissue by multiple, gradually enlarging cysts. 4 The presence of small cysts lower than the resolution of the ultrasound probe may be missed.
Biller et al found that the sensitivity of ultrasonography for identification of PKD was reduced from 91% in cats over 36 weeks of age to 75% in cats less than 16 weeks old. 39 Wills et al reported that ultrasound scanning at 10 months of age with a high resolution probe and experienced ultrasonographer was a very reliable method of PKD diagnosis. 40
Occasionally, cysts may also be present in the liver and pancreas. 36 In some cases, multiple very small cysts are seen in cats with PKD, producing a coarse echotexture with diffusely increased echogenicity. 7
Renal neoplasia
Most solid kidney masses are neoplastic in origin (lymphoma, cystadenocarcinoma, sarcoma, metastatic tumour). 2 They appear as homo- or heterogeneous, hypo-, iso- or hyperechoic, regular or irregular lesions with variable well-defined margins.2,9 Their ultrasound pattern is non-specific although uniformly hypoechoic masses are often lymphomatous. Usually neoplasia causes renomegaly with distortion of the normal contour.2,7,8 Fine-needle aspiration or biopsy is required in most cases to achieve a precise diagnosis.
Lymphoma can result in focal or multifocal enlargement with hypoechoic cortices or hypoechoic nodules/masses.4,41 A subcapsular hypoechoic rim or crescent surrounding the renal cortex has been reported (Figure 9), although this can also be seen with renal carcinoma and feline infectious peritonitis.4,41 On CEUS, small vessels are observed enhancing at the periphery of the lesion during the early arterial phase, which differs from the arterial vascularity noted with renal cell carcinoma. Lymphoma lesions become gradually hypoechoic during the late corticomedullary phase of enhancement, with earlier washout of contrast agent compared with renal carcinoma. 11
Figure 9.
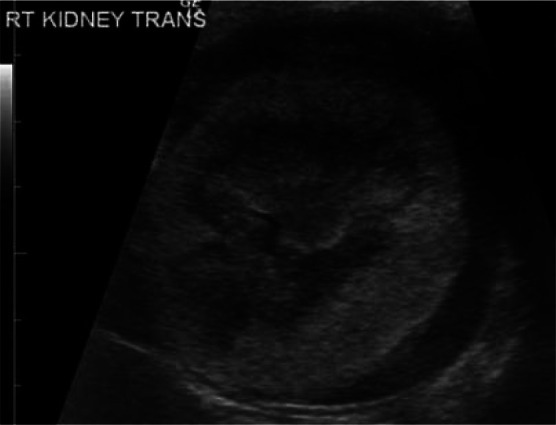
Transverse ultrasound scan showing a severely enlarged (6.5 cm) kidney. The cortex is hyperechoic and surrounded by a thick subcapsular hypoechoic rim, compatible with lymphoma
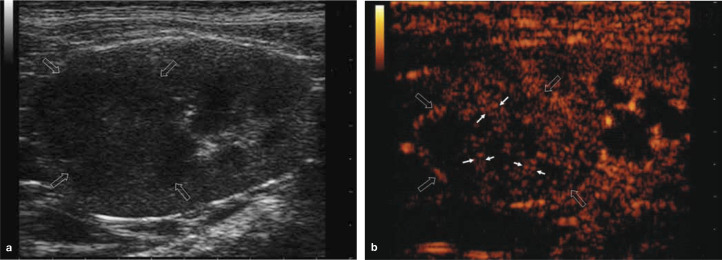
Renal carcinoma usually begins at one pole of the kidney and generally produces focal hyperechoic lesions. 10 On CEUS they are typically characterised by larger arterial vessels with a random distribution, enhancing slightly earlier than normal vessels in some patients, reflecting malignant neoangiogenesis. During the late corticomedullary phase, carcinomas have intense homo- or heterogeneous, iso- or slightly hypoechoic enhancement, which decreases progressively (Figure 10). 11
Figure 10.
Renal cell carcinoma in a cat. (a) B-mode ultrasound scan showing a hypoechoic lesion with internal echoes (open arrows), which could represent malignant tissue or alternatively debris/haemorrhage in a benign lesion. (b) On CEUS, there is uptake of contrast in the arterial phase, with the presence of feeding vessels (small arrows) indicating malignancy
Isoechoic masses may not be recognised unless a distinct outline, change in echotexture, distortion of internal architecture or change in renal contour is identified. 2
Hyperechoic masses are rare and seem to be associated with well vascularised tumours (haemangiosarcomas, haemangioma, metastatic thyroid carcinoma, chondrosarcoma). 7
Renal infarcts
Renal infarcts may be an incidental finding in older animals and are more commonly seen in dogs than cats. 5 Acute infarcts usually present as mass-like lesions with decreased or mixed echogenicity and may be seen within 24 h of occlusion of a blood vessel. 4 These lesions gradually become hyperechoic with increasing fibrosis (chronic infarction) and eventually depressions appear in the cortex. Chronic infarcts can be seen as hyperechoic wedge-shaped or triangular lesions in the cortex, with the tip of the infarct located at the corticomedullary junction, directed towards the renal hilus (Figure 11).4,9
Figure 11.
Renal infarct visible as a small hyperechoic triangular area (arrow) in the cortex at the caudal pole of the kidney
Renal abscesses and haematomas
Renal abscesses and haematomas are a rare cause of focal renal disease. Abscesses may develop secondarily to adjacent infection or haematogenous spread of bacteria, which results in irregular renal enlargement. Their ultrasound appearance is variable; most present as cavitated masses of mixed echogenicity with a thick, irregular wall and anechoic or hyperechoic contents. If gas is located within the abscess, hyperechoic shadowing is visible.4,7
Haematomas may arise secondarily to trauma, coagulopathy or renal biopsy and can be located within the parenchyma or in the subcapsular area. 4 They are visible as hypoechoic or complex masses (ie, contain a mixture of anechoic, hypoechoic and hyperechoic components) and can be associated with perinephric haemorrhage. 5
Renal pelvis abnormalities
Hydronephrosis
Hydronephrosis describes dilation of the renal pelvis and the presence of diverticula (dilation of more than 3 mm is abnormal). Often dilation of the proximal ureter (hydroureter) is present concurrently. It is caused by chronic infection (pyelonephritis, ureteritis), obstruction of the urinary tract distal to the kidney (bladder trigone neoplasia, ureteral calculi/ neoplasia, ureteral stricture secondary to trauma or chronic ureteritis, renal calculi, pelvic neoplasia) or ectopic ureters.5,8 In cats, clinical experience suggests that obstruction of the ureter by a ureteral calculus is more common than other causes. 2 Kidneys are enlarged, uni- or bilaterally, and retain a smooth contour. The degree of distension ranges from minimal to severe.8,25
On ultrasonography, accumulation of anechoic fluid in the renal pelvis is seen, displaying acoustic enhancement (Figure 12). As the severity of the dilation increases, progressive compression of the surrounding renal parenchyma results in cortical atrophy. 6 At this stage, there is widening of the diverticula and cortical atrophy, ultimately producing a septated, cystic appearance. 5 In severe cases (eg, with complete ureteral obstruction) only a small rim of cortical tissue surrounding the markedly dilated pelvis is observed. 4
Figure 12.
Transverse image of a kidney with secondary severe hydroureter and hydronephrosis (pelvis = 9 mm) due to bladder trigone neoplasia
Pyelonephritis
Inflammation of the renal pelvis and renal parenchyma (pyelonephritis) is associated with bacterial infection. Ultrasonographically, it can manifest as a mildly dilated pelvis and proximal ureter with anechoic or hyperechoic debris and blunted, poorly defined diverticula. 4 In cases of acute pyelonephritis no detectable pelvic/ureteral dilation may be present. 2 Renal size can be normal, slightly increased (acute cases) or slightly decreased (chronic cases). In both acute and chronic conditions the cortex can become hyperechoic (Figure 13). 4 Renal pelvic and proximal ureteral dilation, and a hyperechoic line along the renal crest, have been described as the principal findings. 8 However, the ultrasound appearance of pyelonephritis is very variable and a range of changes can be seen; for example, a hyperechoic line within the pelvis parallel to the renal sinus fat, (multi)focal hyper- or hypoechoic areas in the cortex, (multi)focal hyperechoic areas in the medulla and a less defined corticomedullary junction. 4
Figure 13.
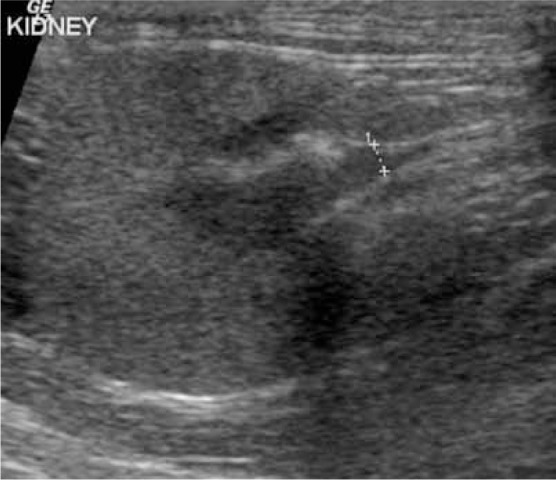
Pyelonephritis in a cat. Transverse scan showing an irregularly outlined kidney of normal size (3.6 cm). The cortex is thickened and hyperechoic. The pelvis is less than 2 mm in thickness but contains some hyperechoic material (crosses)
Nephrolithiasis
Renal calculi possess a strongly reflective surface and display distal acoustic shadowing (Figure 14), depending on the size of the calculus and the frequency of the transducer. 6 The calculi are located in diverticula or the pelvis or extend into the proximal ureter. Ultrasonography can, in contrast to radiography, aid in the detection of both radiolucent and radiopaque calculi. If they cause an obstruction, dilation with fluid will be present proximal to the affected area. 4
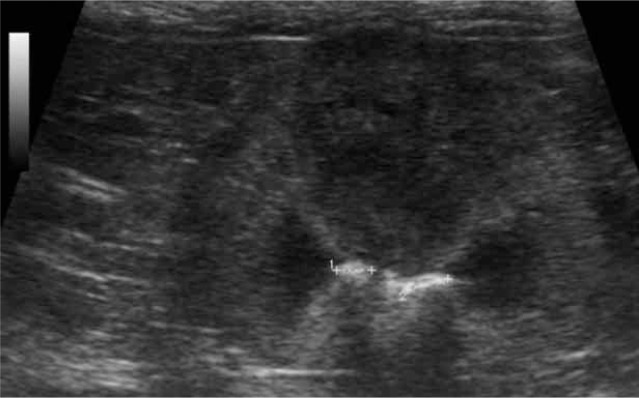
Figure 14.
Dorsal scan of a left kidney showing two calculi (crosses), measuring 0.34 cm and 0.29 cm, with acoustic shadowing in the pelvis, compatible with nephrolithiasis
It may be difficult to differentiate calculi from parenchymal mineralisations. However, calculi are often associated with pelvic dilation and can be confidently diagnosed if they are surrounded by fluid or are clearly visible within the renal pelvis.5,28
Perirenal abnormalities
This group of conditions includes the presence of perinephric pseudocysts, abscesses and haematomas surrounding the kidneys.
Perinephric pseudocysts
Perinephric (perirenal) pseudocysts are a rare cause of renomegaly and abdominal distension in cats, and are mostly seen in elderly male cats. The cause often remains unclear but these lesions may be associated with trauma, surgery, neoplasia or venous congestion.4,42 The characteristic ultrasonographic finding is the presence of a variable amount of anechoic fluid between the renal capsule and the renal parenchyma in fibrous sacs surrounding one or both kidneys (Figure 15).42,43 The walls of the pseudocysts are composed of multi-layered bands of connective tissue without an epithelial layer. 42 The fluid is mostly a low protein transudate, less frequently urine. Exudate or pure blood is never detected.
Figure 15.
Image of the left kidney of a cat presented with an abdominal mass effect. The kidney is enlarged (4.5 cm), irregular in outline, and shows a hyperechoic cortex and a retained corticomedullary junction. One hyperechoic wedge-shaped area (1) is present in the cortex (infarct). Two large mineralisations (2) are visible in the medulla. A large anechoic accumulation of perirenal fluid (3) is present around the kidney contour
Often perinephric pseudocysts occur in association with chronic kidney failure. 44 Renal hyperechogenicity may be artefactual, resulting from the surrounding fluid or secondary to diffuse renal disease.
Perirenal abscesses and haematomas
Perirenal abscesses are a rare finding in cats, and are typically unilateral. They are often associated with chronic pyelonephritis. Perirenal haematomas develop after blunt abdominal trauma or as a complication of renal biopsy.
Both conditions are associated with variable ultrasound patterns, depending on the age of the lesions. They are usually described as complex perirenal masses with hyper-, hypo- or anechoic content. 45
Footnotes
Funding: The authors received no grant from any funding agency in the public, commercial or not-for-profit sectors for the preparation of this review.
The authors do not have any potential conflicts of interest to declare.
Key Points
B-mode ultrasonography is the reference modality for imaging the feline kidney. It provides excellent visualisation of renal size, shape and internal architecture.
Focal or multifocal disorders may be readily diagnosed, while diffuse changes are more challenging.
Diffuse acute renal disorders induce an increase in renal size and mostly also in echogenicity. Diffuse chronic renal disorders also induce an increase in echogenicity but in these cases the kidneys are often reduced in size and irregular in outline.
The ultrasonographic appearance and internal characteristics of diffuse renal diseases or focal renal masses usually do not permit a definitive diagnosis. Fine-needle aspiration or core biopsy is often needed.
Contrast-enhanced ultrasound allows differentiation between benign and malignant focal renal lesions by using the presence of vascularity as a distinguishing factor.
References
- 1. Walter PA, Johnston GR, Feeney DA, O’Brien T. Applications of ultrasonography in the diagnosis of parenchymal kidney disease in cats: 24 cases (1981–1986). J Am Vet Med Assoc 1988; 192: 92–98. [PubMed] [Google Scholar]
- 2. Nyland TG, Mattoon JS, Herrgesell EJ, Wisner ER. Urinary tract. In: Nyland TG, Mattoon JS. (eds). Veterinary diagnostic ultrasound. 1st ed. Philadelphia: WB Saunders, 1995, pp 158–195. [Google Scholar]
- 3. Seyrek-Intas D, Kramer M. Renal imaging in cats. Vet Focus 2008; 18: 23–30. [Google Scholar]
- 4. Larson MM. The kidneys and ureters. In: O’Brien R, Barr F. (eds). BSAVA manual of canine and feline abdominal imaging. 1st ed. Gloucester: British Small Animal Veterinary Association, 2009, pp 185–204. [Google Scholar]
- 5. Dennis R, McConnell F. Diagnostic imaging of the urinary tract. In: Elliott J, Grauer GF. (eds). BSAVA manual of canine and feline nephrology and urology. 2nd ed. Gloucester: British Small Animal Veterinary Association, 2007, pp 126–141. [Google Scholar]
- 6. Dennis R, Kirberger RM, Barr F, Wrigley RH. Handbook of small animal radiology and ultrasound: techniques and differential diagnoses. 2nd ed. Edinburgh: Elsevier, 2010, pp 305–308. [Google Scholar]
- 7. Mannion P. Diagnostic ultrasound in small animal practice. 1st ed. Oxford: Blackwell Science, 2006, pp 109–127. [Google Scholar]
- 8. Anderson KL. Ultrasonography of the upper urinary tract. http://www.academic-server.cvm.umn.edu/radiology/CVM6105/2011/Anderson/pdf/USofUUT.pdf.
- 9. d’Anjou M. Kidneys and ureters. In: Penninck D, d’Anjou M. (eds). Atlas of small animal ultrasonography. 1st ed. Ames: Blackwell Publishing, 2008, pp 339–364. [Google Scholar]
- 10. Calliada F, Campani R, Bottinelli O, Bozzini A, Sommaruga MG. Ultrasound contrast agents: basic principles. Euro J Radiol 1998; 27: 157–160. [DOI] [PubMed] [Google Scholar]
- 11. Haers H, Vignoli M, Paes G, Rossi F, Taeymans O, Daminet S, et al. Contrast harmonic ultrasonographic appearance of focal space-occupying renal lesions. Vet Radiol Ultrasound 2010; 51: 516–522. [DOI] [PubMed] [Google Scholar]
- 12. Kinns J, Aronson L, Hauptman J, Seiler G. Contrast-enhanced ultrasound of the feline kidney. Vet Radiol Ultrasound 2010; 51: 168–172. [DOI] [PubMed] [Google Scholar]
- 13. Park I, Lee H, Kim J, Nam S, Choi R, Oh K, et al. Ultrasonographic evaluation of renal dimension and resistive index in clinically healthy Korean domestic short-hair cats. J Vet Sci 2009; 9: 415–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tublin ME, Bude RO, Platt JF. The resistive index in renal doppler sonography: where do we stand? Am J Roentgenol 2003; 180: 885–892. [DOI] [PubMed] [Google Scholar]
- 15. Novellas R, Espada Y, Ruiz de Gopegui R. Doppler ultrasonographic estimation of renal and ocular resistive and pulsatility indices in normal dogs and cats. Vet Radiol Ultrasound 2007; 48: 69–73. [DOI] [PubMed] [Google Scholar]
- 16. Webster N. Ultrasonography of the urogenital tract in dogs and cats. In Pract 2009; 31: 210–217. [Google Scholar]
- 17. Rivers BJ, Walter PA, O’Brien TD, Polzin DJ. Duplex Doppler estimation of Pourcelot resistive index in arcuate arteries of sedated normal cats. J Vet Intern Med 1996; 10: 28–33. [DOI] [PubMed] [Google Scholar]
- 18. Rivers BJ, Walter PA, Polzin DJ, King VL. Duplex doppler estimation of intrarenal pourcelot resistive index in dogs and cats with renal disease. J Vet Intern Med 1997; 11: 250–260. [DOI] [PubMed] [Google Scholar]
- 19. Novellas R, Ruiz de Gopegui R, Espada Y. Assessment of renal vascular resistance and blood pressure in dogs and cats with renal disease. Vet Rec 2010; 166: 618–623. [DOI] [PubMed] [Google Scholar]
- 20. Walter PA, Feeney DA, Johnston GR, Fletcher TF. Feline renal ultrasonography: quantitative analyses of imaged anatomy. Am J Vet Res 1987; 48: 596–599. [PubMed] [Google Scholar]
- 21. Shiroma JT, Gabriel JK, Carter RL, Scruggs SL, Stubbs PW. Effect of reproductive status on feline renal size. Vet Radiol Ultrasound 1999; 40: 242–245. [DOI] [PubMed] [Google Scholar]
- 22. Drost WT, Henry GA, Meinkoth JH, Woods JP, Lehenbauer TW. Quantification of hepatic and renal cortical echogenicity in clinically normal cats. Am J Vet Res 2000; 61: 1016–1020. [DOI] [PubMed] [Google Scholar]
- 23. Yeager AE, Anderson WI. Study of association between histologic features and echogenicity of architecturally normal cat kidneys. Am J Vet Res 1989; 50: 860–863. [PubMed] [Google Scholar]
- 24. Biller DS, Bradley GA, Partington BP. Renal medullary rim sign: ultrasonographic evidence of renal disease. Vet Radiol Ultrasound 1992; 33: 286–290. [Google Scholar]
- 25. Mantis P. Ultrasonography of the urinary and genital system of the dog and cat. Iranian J Vet Surg 2008; Suppl for the 2nd ISVS and 7th ISVAR: 63–71. [Google Scholar]
- 26. Adams WH, Toal RL, Breider MA. Ultrasonographic findings in dogs and cats with oxalate nephrosis attributed to ethylene glycol intoxication: 15 cases (1984–1988). J Am Vet Med Assoc 1991; 199: 492–496. [PubMed] [Google Scholar]
- 27. Lewis KM, O’Brien RT. Abdominal ultrasonographic findings associated with feline infectious peritonitis: a retrospective review of 16 cases. J Am Anim Hosp Assoc 2010; 46: 152–160. [DOI] [PubMed] [Google Scholar]
- 28. Burk RI, Feeney DA. Small animal radiology and ultrasonography: a diagnostic atlas and text. 3rd ed. St Louis: WB Saunders, 2003, pp 262–265, 360–388. [Google Scholar]
- 29. Ozmen CA, Akin D, Bilek SU, Bayrak AH, Senturk S, Nazaroglu H. Ultrasound as a diagnostic tool to differentiate acute from chronic renal failure. Clin Nephrol 2010; 74: 46–52. [PubMed] [Google Scholar]
- 30. Adibi A, Emami Naini A, Salehi H, Matinpour M. Renal cortical thickness in adults with normal renal function measured by ultrasonography. Iran J Radiol 2008; 5: 163–166. [Google Scholar]
- 31. Sparkes A. Amyloidosis. In: Norsworthy GD, Fooshee GS, Crystal MA, Tilley LP. (eds). The feline patient. 4th ed. Iowa: Wiley-Blackwell, 2011, pp 14–15. [Google Scholar]
- 32. Lees GE. Congenital renal diseases. Vet Clin North Am Small Anim Pract 1996; 26: 1379–1399. [DOI] [PubMed] [Google Scholar]
- 33. Aresu L, Zanatta R, Pregel P, Caliari D, Tursi M, Valenza F, et al. Bilateral juvenile renal dysplasia in a Norwegian Forest Cat. J Feline Med Surg 2009; 11: 326–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Mooney SC, Hayes AA, Matus RE, MacEwen EG. Renal lymphoma in cats: 28 cases (1977–1984). J Am Vet Med Assoc 1987; 191: 1473–1477. [PubMed] [Google Scholar]
- 35. Gubbels J, Prins P. Polycystic kidney disease (PKD) in cats [German]. Tijdschr Diergeneeskd 2005; 130: 184–185. [PubMed] [Google Scholar]
- 36. Bonazzi M, Volta A, Gnudi G, Bottarelli E, Gazzola M, Bertoni G. Prevalence of the polycystic kidney disease and renal and urinary bladder ultrasonographic abnormalities in Persian and exotic shorthair cats in Italy. J Feline Med Surg 2007; 9: 387–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Domanjko-Petri A, Cernec D, Cotman M. Polycystic kidney disease: a review and occurrence in Slovenia with comparison between ultrasound and genetic testing. J Feline Med Surg 2008; 10: 115–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Paepe D, Saunders JH, Bavegems V, Paes G, Peelman LJ, Makay C, et al. Screening of ragdoll cats for kidney disease – a retrospective evaluation. J Small Anim Pract. Epub ahead of print 2 August 2012. DOI: 10.1111/j.1748-5827.2012.01254.x. [DOI] [PubMed] [Google Scholar]
- 39. Biller DS, DiBartola SP, Eaton KA, Pflueger S, Wellman ML, Radin MJ. Inheritance of polycystic kidney disease in Persian cats. J Heredity 1996; 87: 1–5. [DOI] [PubMed] [Google Scholar]
- 40. Wills SJ, Barrett EL, Barr FJ, Bradley KJ, Helps CR, Cannon MJ, et al. Evaluation of the repeatability of ultrasound scanning for detection of feline polycystic kidney disease. J Feline Med Surg 2009; 11: 993–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Valdés-Martinez A, Cianciolo R, Mai W. Association between renal hypoechoic subcapsular thickening and lymphosarcoma in cats. Vet Radiol Ultrasound 2007; 48: 357–360. [DOI] [PubMed] [Google Scholar]
- 42. Essman SC, Drost WT, Hoover JP, Lemire TD, Chalman JA. Imaging of a cat with perirenal pseudocysts. Vet Radiol Ultrasound 2000; 41: 329–334. [DOI] [PubMed] [Google Scholar]
- 43. Beck JA, Bellenger CR, Lamb WA, Churcher RK, Hunt GB, Nicoll RG, et al. Perirenal pseudocysts in 26 cats. Aust Vet J 2000; 78: 166–171. [DOI] [PubMed] [Google Scholar]
- 44. Ochoa VB, DiBartola SP, Chew DJ, Westropp J, Carothers M, Biller D. Perinephric pseudocysts in the cat: a retrospective study and review of the literature. J Vet Intern Med 1999; 13: 47–55. [PubMed] [Google Scholar]
- 45. Zatelli A, D’Ippolito P. Bilateral perirenal abcesses in a domestic neutered shorthair cat. J Vet Intern Med 2004; 18: 902–903. [DOI] [PubMed] [Google Scholar]