Abstract
Practical relevance:
Acute kidney injury (AKI) is a frequently recognized disease process in cats that requires immediate and aggressive intervention. A thorough understanding of the pathophysiologic processes underlying AKI and familiarity with the most common etiologies are essential for providing the most effective and timely therapy. Possessing this knowledge will also allow a more accurate prognosis to be given, and afford the best chance of a favorable outcome.
Clinical challenges:
Feline patients often present with vague signs of AKI, which may delay treatment and adversely affect the prognosis. Their response to injury and treatment is often different to that of other species.
Audience:
This two-part review article is directed at small animal practitioners as well as specialists. Part 1 reviews mechanisms underlying AKI in the cat, as well as etiologies and treatments related to some specific causes of AKI.
Evidence base:
The veterinary literature is limited with regards to the pathophysiology of AKI unique to the cat. However, there are numerous feline studies evaluating causes of AKI.

New terminology – and a new emphasis
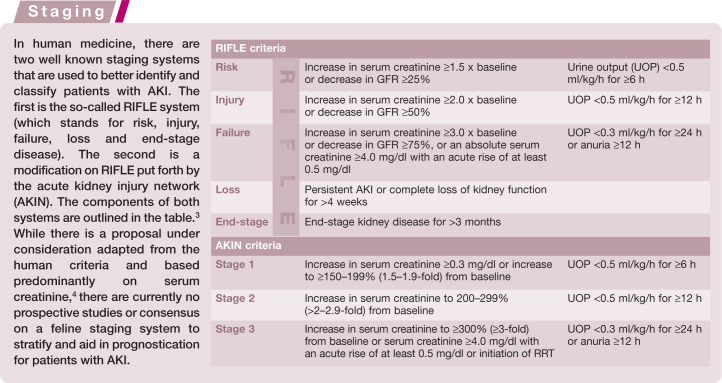
Acute kidney injury (AKI) is a relatively new term in the nephrology literature that largely replaces the use of ‘acute renal failure’ (ARF). Originally introduced in the human literature, AKI allows for greater stratification of cases with regard to severity and prognosis. By suggesting that a patient has injury, rather than failure, one can recognize the potential for earlier treatment and recovery. In human medicine, the older term ‘ARF’ refers only to patients requiring renal replacement therapy (RRT). Additionally, the more common term ‘kidney’, rather than ‘renal’, facilitates communication with and understanding by clients of AKI.
The incidence of AKI in cats is not known, but it is not uncommon and can be caused by a variety of different insults. A uniform definition for AKI does not exist in the veterinary literature and varies among publications. Generally accepted criteria include an abrupt reduction in kidney function resulting in alterations in glomerular filtration, urine production and tubular function. These alterations result in an inability to maintain fluid, electrolyte and acid–base balance, and may lead to azotemia.
Pathophysiology
The pathophysiology of AKI is complex, but can be described by four stages: initiation, extension, maintenance and recovery.
Initiation
The initiation phase begins with the original insult to the kidney, which can be due to several causes including ischemia, toxins, infection, neoplasia, obstruction or the effects of sepsis. This phase can last from hours to a few days, during which time the glomerular filtration rate (GFR) falls but clinical signs are often absent.
Although the underlying cause of the initial insult may vary, all causes lead to a combination of direct damage to the renal tubular cells and ischemia (Figure 1). Hypoxia ensues, leading to a depletion of ATP. This has the most significant effect on the renal tubular epithelial cells in the S3 segment of the proximal convoluted tubule and the thick ascending loop of Henle, due to their location in the outer medulla, which has a relatively low oxygen tension compared with the rest of the kidney. Low ATP leads to an increased intra-cellular calcium concentration, which activates proteases and phospholipases. Reactive oxygen species and free radicals are generated, resulting in cellular damage. In response to this, the kidney mounts an inflammatory reaction involving the production of inflammatory cytokines and chemokines. These are intended to be protective, but can also have deleterious effects.
Figure 1.
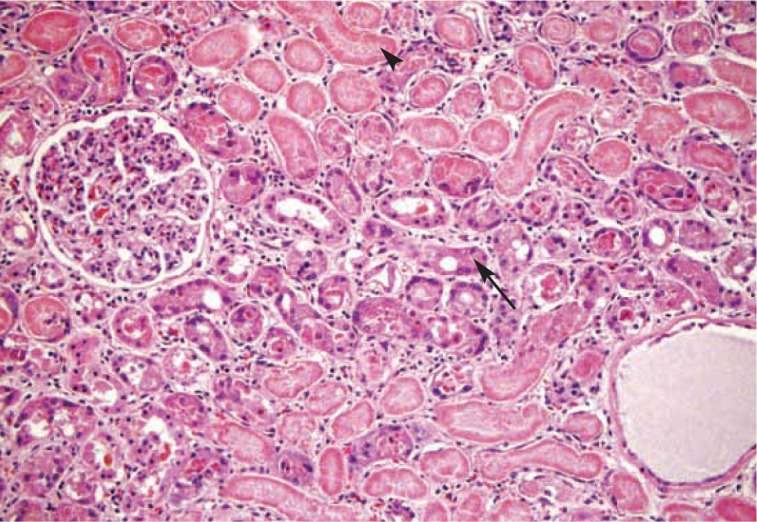
Hypoxic renal tubular necrosis in a cat with AKI of unknown etiology that was suspected to be secondary to an ischemic event under anesthesia. Tubules in the center of the field (arrow) exhibit degenerative changes such as dilatation and casts, as well as isolated epithelial cell necrosis. Tubules in other areas (arrowhead) exhibit regional pan-tubular necrosis, as characterized by diffuse eosinophilia and lack of cellular detail.
Courtesy of John Keating
One consequence of the processes just described is structural disruption of the cytoskeleton of renal tubular epithelial cells.1,2 There is loss of apical microvilli and the absorptive brush border. Cell polarity is lost as Na+/K+-ATPase pumps relocate from their normal position on the basolateral cell membrane to the cytoplasm and apical cell membrane due to loss of the anchoring cytoskeleton. This leads to abnormal solute and electrolyte handling as well as increased sodium delivery to the macula densa in the distal tubule. Tubulo-glomerular feedback is activated, causing afferent arteriolar vasoconstriction and worsened renal ischemia. Integrin dysfunction also plays a role. These glycoproteins mediate adhesion of tubular epithelial cells to the tubular basement membrane, and in AKI they move from the basolateral to the apical cell membrane. Cells lose their connection to the basement membrane and desquamate into the tubular lumen. The tubules become obstructed with casts resulting in backleak of filtrate into the peritubular interstitium and increased glomerular back pressure, both of which decrease GFR. 1 Loss of tight junction integrity between tubular epithelial cells due to cytoskeletal dysfunction also contributes to backleak of filtrate into the interstitium.
Extension
The second stage of AKI is extension, which is characterized by amplification of the initial renal insult by ongoing hypoxia from ischemia and the resulting inflammatory response. 5 Its duration is thought to be 1–2 days, during which renal tubular cells undergo apoptosis and necrosis, and the GFR continues to fall. Cellular injury mechanisms, as described earlier, continue, but endothelial cell damage and dysfunction are thought to play the primary role in this phase.
Damage to peritubular capillary endothelial cells causes enhanced vascular reactivity to vasoconstrictive agents and decreased vascular reactivity to vasodilating agents, with the overall result being exacerbation of ischemia and hypoxia. Damaged endothelial cells also upregulate expression of P- and E-selectin on their surface, as well as ICAM-1 (intercellular adhesion molecule-1). 6 These are mediators of endothelial cell–leukocyte interaction, and increased expression leads to enhanced adhesion and activation of leukocytes. This process accelerates the inflammatory cascade, resulting in vascular congestion and worsened ischemia.
Overall, the consequence of endothelial cell dysfunction is continued damage to the tubular epithelial cells. The extension phase is proposed to be an opportune time for therapeutic intervention to arrest ongoing cellular damage, but the window is short and difficult to identify in a timely manner.
Prompt identification of the underlying etiology in AKI is important to deliver the most effective therapy and give the most accurate prognosis.
Maintenance
The third stage, of maintenance, is characterized by stabilization of the GFR at its nadir and lasts 1–2 weeks. During this time apoptosis continues but renal blood flow returns to normal, leading to cellular repair. Proliferation and migration of renal tubular cells occurs, beginning the process of re-establishing cell polarity and tubular integrity. Uremic complications may be most noticeable during this phase.
Recovery
Maintenance is followed by the recovery phase, during which time the GFR rises as cellular repair continues. Depending on the severity of the renal insult, GFR may fully recover or there may be residual chronic kidney disease. The onset of polyuria is sometimes a marker for the onset of the recovery phase, which can last for weeks to months. It is very important to avoid any additional renal injury during this period.
Etiologies
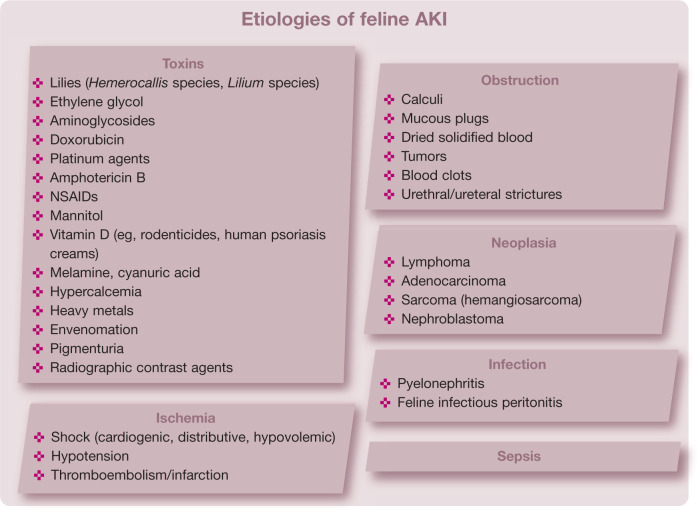
While the exact cause of AKI remains undetermined in about one-third of feline patients, 7 it is imperative to make every effort to identify the etiology due to its potential influence on treatment and prognosis. Of the various types of insults that are known to result in AKI, the most common are toxins, which are the inciting cause in over 50% of feline patients with AKI. 7 Cats may be exposed to a variety of nephrotoxic substances including (but not limited to) lilies, non-steroidal anti-inflammatory drugs (NSAIDs), ethylene glycol (EG), aminoglycosides, doxorubicin, vitamin D, and food contaminants such as melamine and cyanuric acid. Other possible etiologies for AKI include ischemic injuries, infection of the upper urinary tract (pyelonephritis), neoplasia, prolonged ureteral or urethral obstruction, and sepsis.
Toxic nephropathy
Lily plants
Lilies have been recognized as nephrotoxicants in cats for about 20 years, 8 but many owners are not aware of the toxic risk of these decorative house plants (Figure 2). All members of the genera Lilium and Hemerocallis should be considered nephrotoxic to cats.8,9 This includes the Easter lily (Lilium longiflorum), tiger lily (Lilium species) and daylily (Hemerocallis species), among many others. Conversely, calla lilies (Zantedeschia aethiopica) and peace lilies (Spathiphyllum species) are not true lilies and have not been associated with nephrotoxicity. While the toxic dose and exact compound are still not known, any part of the plant should be considered toxic, including the leaves, flowers, seeds, pollen and stem. One study of experimental lily-induced nephrotoxicity identified that the flowers are much more toxic than the leaves and that the compound responsible for toxicity is water-soluble. 10
Figure 2.

Asiatic lily (Lilium asiatic). All members of the genera Lilium and Hemerocallis, and all parts of the lily plant, should be considered nephrotoxic to cats
Lily intoxication results in diffuse renal tubular necrosis, with the proximal tubules being more severely affected than the more distal parts. Due to cell sloughing, the tubules often fill with cellular debris from exfoliated cells, granular casts and hyaline casts. 10 The outcome is severe azotemia and some report a marked elevation in creatinine in relation to blood urea nitrogen. 11 Urinalysis will generally reveal changes consistent with proximal tubular injury before development of azotemia, including isosthenuria, glucosuria, tubular proteinuria and cylindruria. Unlike EG toxi cosis, these patients do not usually develop crystalluria and they are normocalcemic.
After ingestion, cats often develop a transient lily-induced gastritis within about 3 h, causing vomiting and lethargy. 10 If left untreated, signs may temporarily improve and evidence of AKI including polyuria, polydipsia, depression, anorexia, urine chemistry and sediment changes, and azotemia will develop within about 24–72 h. 10 This often progresses to oliguria or anuria and death. Hadley et al reported that 77% of affected cats present with vomiting and hypersalivation, 36% present with neurological dysfunction (seizures, tremors, ataxia) and 32% with AKI. 9
Decontamination and diuresis prevents kidney injury secondary to lily plant ingestion if instituted within 6 h.
If treatment with decontamination and fluid diuresis is instituted within 6 h, full recovery can be expected in cats without evidence of kidney insult. 8 Unfortunately, there is no known antidote for lily toxicosis. If treatment is delayed for longer than 18 h, AKI will develop. Death is expected within 3–7 days if no renal support intervention is made. Mortality rate for cats with AKI secondary to lily ingestion is estimated to be 50–100%. 9 As with many types of AKI, chronic kidney disease (CKD) is a possible sequela in cats surviving AKI. 11
NSAIDs
NSAIDs exert their therapeutic effect through inhibition of cyclo-oxygenase enzymes, COX-1 and/or COX-2, to interrupt production of prostaglandins. COX-1 is expressed constitutively and is involved in the production of prostaglandins needed for normal physiologic functions in several organs including the kidneys. COX-2 expression is inducible by bacterial endotoxins, cytokines and growth factors but this enzyme is also expressed constitutively in the kidneys in the absence of inflammatory stimulation. COX-2 expression is greatly increased in dogs that are volume or sodium depleted; therefore, administration of NSAIDs that preferentially inhibit COX-2 may cause kidney damage, particularly in dehydrated patients. 12
In a hemodynamically normal patient without pre-existing renal compromise, appropriate dosages of NSAIDs should not result in AKI and do not affect GFR, as demonstrated by iohexol clearance studies in cats given a 5-day course of meloxicam. 13 However, in states of low renal blood flow, prostaglandins play a crucial role in maintaining kidney function and GFR. Prostaglandins E2 and I2 exert vasodilatory effects at the level of the kidney that are important in its blood flow regulation, especially during periods of hypovolemia or decreased renal perfusion.
Kidney insult associated with NSAID use is due to an interruption in this prostaglandin pathway during times of decreased renal blood flow, resulting in decreased renal perfusion and subsequent ischemic nephrosis. All NSAIDs represent this risk to renal function regardless of reported COX-selectivity.
NSAID-induced kidney injury is recognized with increasing frequency due to the popularity of these medications and the formulation of flavored tablets and liquids designed to improve patient compliance. There is currently a limited number of NSAIDs that are labeled for usage in cats and, in the United States, these products are not labeled for extended use. In fact, in 2010, the US Food and Drug Administration announced the addition of a boxed warning on labels for Metacam (meloxicam, Boehringer Ingelheim) usage in cats, stating: ‘Repeated use of meloxicam in cats has been associated with acute renal failure and death. Do not administer additional injectable or oral meloxicam to cats.’ 14 Conversely, a recent study showed no evidence of renal decompensation when meloxicam was given to older cats with or without kidney disease. 15 Certainly, this product is not the only one of its class with the potential to induce a nephrotoxic insult in at-risk patients and caution should be exercised with any extra-label NSAID usage in cats. The International Society of Feline Medicine (ISFM) and American Association of Feline Practitioners (AAFP) recently provided consensus guidelines on the long-term use of NSAIDs in cats that serve as an additional reference on this subject. 16 These state that, based on data from cats and other species, the risk of AKI developing during appropriate therapeutic NSAID use in cats is low.
With excessive NSAID doses, gastrointestinal signs occur within a few hours of exposure and AKI may not become apparent until up to 3–5 days after exposure. 17 While the nephrotoxic dosage is not known for all NSAIDs, ibuprofen toxicity is estimated to occur at doses of above 87 mg/kg in cats, or about half of the toxic dose in dogs, because of the limited glucuronyl conjugating capacity in cats. 17
If recent NSAID exposure sufficient to result in toxicity is suspected, emesis or gastric lavage followed by treatment with activated charcoal, proton pump inhibitors, misoprostol and intravenous fluid therapy for at least 48 h is recommended. It is important to note that NSAIDs undergo enterohepatic recirculation, which may prolong their half-life.
Renal injury is potentially reversible if medications are discontinued and treatment is instituted promptly. Dialytic therapies may be indicated if azotemia is non-responsive to diuresis or in oliguric/anuric patients. However, due to the high degree of protein binding seen with NSAIDs, removal of the toxin with dialysis can only be reliably accomplished with hemoperfusion utilizing charcoal filters, which are generally too large for use in cats and have limited availability.
NSAIDs should not be used if hypovolemia, dehydration or hypotension are present.
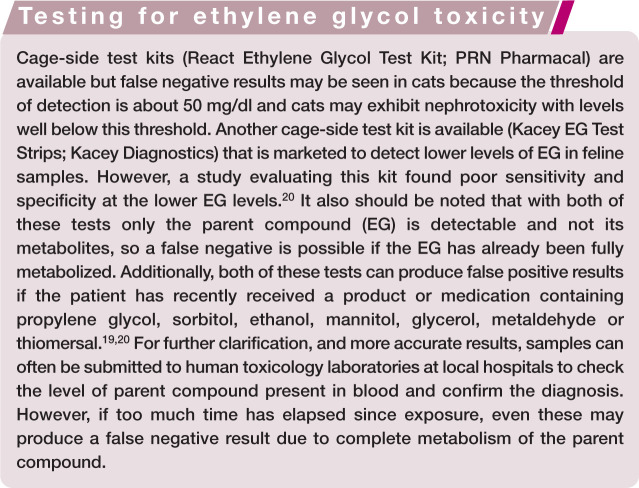
Ethylene glycol
EG is a highly nephrotoxic substance found in antifreeze and other industrial solvents. Cats are considered more susceptible to the toxic effects of EG than dogs and those that consume as little as 1.4 ml/kg may experience severe and life-threatening renal injury. 18 EG is metabolized in the liver by alcohol dehydrogenase and broken down into several toxic metabolites including glycoaldehyde, glycolate, glyoxylate and oxalate. Toxicity develops as a result of the direct actions of these metabolites on the tubular epithelium as well as deposition of calcium oxalate crystals in the renal tubular lumen and interstitium. The parent compound (EG) is rapidly absorbed in the stomach and, in cats, the blood concentration peaks within 1 h of ingestion. 18 It is almost completely metabolized and excreted within about 24 h. 18
Laboratory findings consistent with recent ingestion include increased serum osmolality and an elevated anion gap within 1 h due to the presence of EG and its metabolites; metabolic acidosis; calcium oxalate monohydrate crystalluria within 3 h; isosthenuria within 3 h due to osmotic diuresis; hypocalcemia due to chelation of calcium by oxalate and the subsequent development of calcium oxalate crystalluria; and hyperglycemia, which may be due to inhibition of glucose metabolism by aldehyde metabolites of EG, as well as stress. 19 If left untreated, azotemia, hyperphosphatemia, hyperkalemia and oliguria/anuria will develop.
There are three potential treatments for EG intoxication in addition to standard supportive therapies (see box on page 780). If the patient is already azotemic at presentation and/or if oliguria or anuria is present, the prognosis is poor for recovery.
Aminoglycosides
Many medications have the potential to induce nephrotoxicity but this complication is most well known and frequently encountered with the use of aminoglycoside antimicrobials. These drugs can result in AKI through direct injury to the proximal tubular cells and ensuing tubular necrosis. They are primarily eliminated through glomerular filtration and enter the renal tubular lumen. Here they bind to the brush border of the proximal renal tubular cells and are endocytosed by a receptor known as megalin. 22 As drug accumulates within the proximal tubular cells of the S1 and S2 tubular segments, the intracellular lysosomes concentrate the drug, causing eventual lysosomal rupture into the cytoplasm and cellular damage and necrosis.
Risk factors for nephrotoxicity include the type of aminoglycoside, elevated serum levels, the cumulative dose, the duration and frequency of administration, concurrent use of furosemide or other nephrotoxic drugs, hypoalbuminemia, hypovolemia and pre-existing kidney disease. 23
All species are susceptible to this type of nephrotoxicity, but there is limited data specifically addressing toxicity in felids. In a case series in 1999, AKI was recognized in four cats receiving oral paromomycin for treatment of infectious enteritis/colitis. This aminoglycoside is not well absorbed through the gastrointestinal tract but may have resulted in nephrotoxicity due to increased absorption in the face of intestinal disease. All four of the cats eventually recovered and their azotemia resolved over several months. 24 Another case report identified AKI in a cat after lavage of a wound with gentamicin, which resulted in serum peak concentrations six times the recommended level following systemic absorption. 25 Reportedly, vestibular disease or hearing loss may develop prior to nephrotoxicity in cats and may be an indicator of impending renal toxicity. 26
To reduce toxicity risk, these drugs should be avoided in patients with pre-existing renal disease, and adequate hydration and volume resuscitation should be ensured prior to administration. They should be given concurrently with fluids, only once daily, and for no more than 5–7 days, if possible. Because the proximal tubular uptake of aminoglycosides is a saturable process, once daily administration limits the amount of drug that reaches the tubular lysosomes to result in nephrotoxicity. Monitoring of fresh urine sediments should be performed prior to and throughout administration so that the medication may be discontinued if casts are noted. Additionally, some clinicians recommend assessing urinary γ-glutamyl transpeptidase activity as a sensitive method for detecting nephrotoxicity in patients requiring therapy with aminoglycoside antibiotics. 27
Ischemic nephropathy
Ischemia is another common cause of renal injury in feline patients with AKI. 7 This type of insult can result from hypotensive events (eg, general anesthesia), hypovolemia (eg, shock, hemorrhage, severe dehydration/volume depletion) and thromboembolic disease. The kidneys normally receive about 20–25% of cardiac output and, as such, are highly susceptible to ischemic insults. Autoregulation allows maintenance of renal perfusion even as mean arterial pressure decreases to as low as 80 mmHg. 28 This maintenance of perfusion occurs through a drop in afferent glomerular arteriolar resistance mediated mostly by prostaglandins to sustain glomerular filtration. Angiotensin II also causes constriction of the efferent arteriole, which aids in the maintenance of glomerular filtration.
If systemic blood pressure continues to decline, endogenous vasoconstrictors will eventually result in increased afferent arteriolar resistance with subsequent reduction in GFR and post-glomerular blood flow perfusing the tubules. Eventually, tubular damage results in cell sloughing and tubular obstruction, impaired sodium reabsorption, backleak of luminal contents, cell swelling and cast formation. In addition to the injury set up by reductions in blood flow to the kidneys, reperfusion can also contribute to AKI through oxidant/free radical damage. 28
Obstructive nephropathy
Urinary tract obstruction can lead to AKI through occlusion of the urethra, ureters or renal pelvises (Figure 3). Potential causes of obstruction include calculi (most commonly calcium oxalate), dried solidified blood calculi, blood clots, mucous plugs, strictures or neoplastic masses occluding or compressing the urinary tract lumen. When urine flow is obstructed, there is a rapid compensatory reduction in GFR and development of inflammation and edema. 29 If this persists, tubular atrophy, fibrosis and apoptosis will eventually develop. If the obstruction affects only one kidney, and the other is functioning normally, the contralateral kidney will hypertrophy to compensate and the process may go undetected.
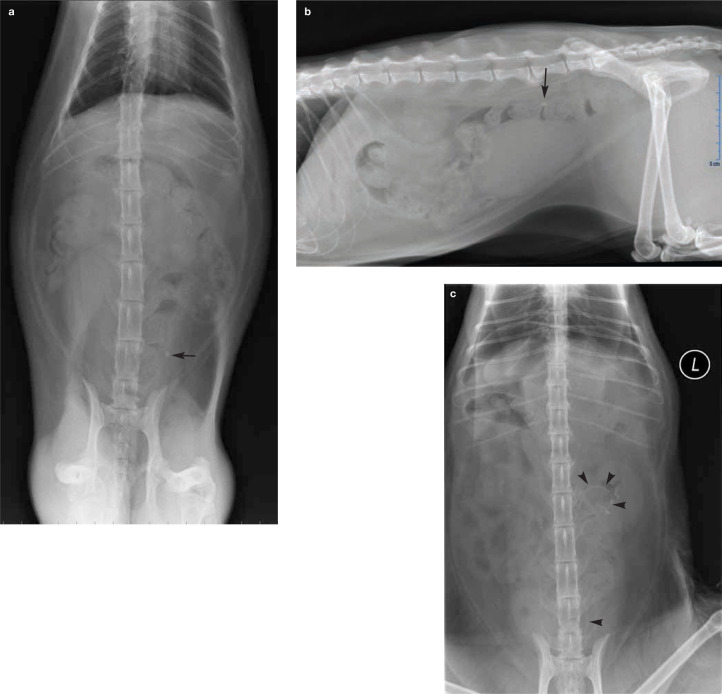
Figure 3.
(a,b) Orthogonal radiographs from a 9-yearold spayed female domestic shorthair cat that presented for azotemia that was unresponsive to fluid therapy. There is a ureterolith on the left side with concurrent renomegaly (arrow). (c) The cat underwent surgical ureteral stent placement to relieve the obstruction; the stent is visible spanning the renal pelvis to the bladder(arrowheads)
Experimentally, complete ureteral obstruction in dogs resulted in only a temporary drop in GFR if the obstruction was relieved within 7 days. 29 If the obstruction was present for 14 days, about 70% of GFR returned after 6 months; if present for 28 days, only 25–30% of function returned; and if present for greater than 42 days, there was no return of GFR. 29 While this guideline provides us with some idea of the rapidity of kidney function decline, it is important to realize that in most cats presenting with ureteral obstruction the clinical duration is unknown and prediction of outcome may be difficult.
In addition to standard therapies for AKI, the ureteral obstruction itself will require specific intervention. Medical management for ureteral obstructions includes diuresis with intravenous fluid therapy and possibly treatment with anti-spasmodic medications such as tamsulosin, amitriptyline or glucagon. 30 Prazosin has also been evaluated in dog ureters but was shown to have little effect and may even contribute to increased ureteral contractions at high levels. 31 If no movement of an obstructive ureterolith is seen on serial imaging performed over 48–72 h, or if azotemia and electrolyte disturbances worsen in the face of therapy, more aggressive treatment is indicated. Such interventions include RRT, placement of nephrostomy tubes, ureteral stenting (Figure 3c) and/or ureterotomy or ureteroneocystotomy for relief of obstruction. 30
Neoplastic nephropathy
Lymphoma is the most common tumor of the feline kidney, which represents the second most frequent location for extranodal lymphoma in cats. 32 However, adenocarcinoma is the most common primary renal tumor found. 33 Other tumors that have been identified less frequently include transitional cell carcinoma, nephroblastoma, hemangiosarcoma and adenoma. 33
Many of these primary tumors will form mass lesions (often unilateral), whereas lymphoma may cause non-specific changes to the kidney(s) visible on ultrasound as hyperechoic cortices, hypoechoic nodules, renomegaly and subcapsular effusion. The presence of hypoechoic subcapsular thickening is considered fairly suggestive of renal lymphoma and, in one study, 80% of cats with this ultrasonographic finding were diagnosed with lymphoma. 34 A suspicion of neoplasia can thus be investigated using ultrasonographic imaging and, if necessary, fine needle aspiration or biopsy of the renal cortex.
Cats with pyelonephritis do not always have a positive urine culture and prolonged antibiotic therapy may still be indicated if there is a positive response to therapy.
If a neoplastic process is identified, therapy should be aimed not only at providing supportive care but also treatment of the under lying malignancy. In cases of lymphoma, the response to chemotherapy may be dramatic enough to result in rapid improvement in renal function.
Infectious nephropathy
Pyelonephritis is a frequent cause of acute-on-chronic kidney disease in cats because of the higher risk of urinary tract infection found in cats with CKD. As such, urine culture should be performed in all patients presenting with AKI or acute-on-chronic kidney disease and anti biotics should be initiated pending the results. However, given that occult infection is possible, a positive response to therapy may be an indication to continue treatment for 6–8 weeks in cases returning a negative urine culture. Pyelonephritis most commonly develops secondarily to ascending lower urinary tract infections; however, hematogenous spread of bacteria may also result in renal colonization.
Although data specifically related to feline pyelonephritis is not available, Escherichia coli and Enterococcus species are the most commonly isolated organisms in cats with bac terial urinary tract infections. 35 Ultrasound findings may be normal or may include changes such as renal pelvic dilation, proximal ureteral dilation, hyperechoic renal cortex, and focal alterations in echogenicity in the cortex and/or medulla (Figure 4). 36
Figure 4.
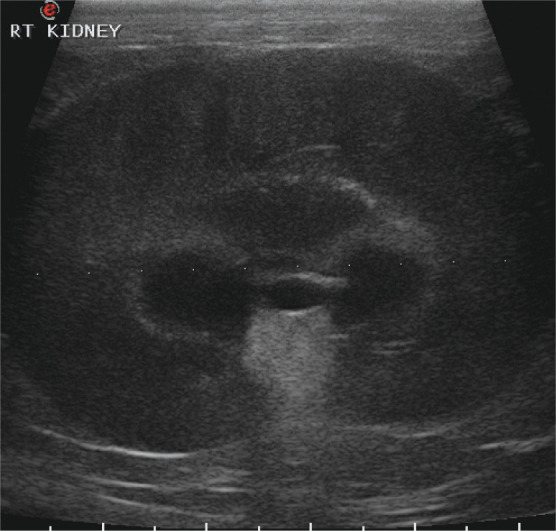
Abdominal ultrasound scan from an 8-year-old castrated domestic shorthair cat that presented with acute lethargy, anorexia and severe azotemia. The image shows renomegaly (length 5.6 cm) and pyelectasia. Although mineral foci were present in both kidneys, no evidence of obstruction was found. Escherichia coli and Enterococcus species were isolated on urine culture and the patient was diagnosed with pyelonephritis
Septic nephropathy
Sepsis is a key contributor to the development of AKI in humans in the ICU setting. One study documented 42% of patients with a primary diagnosis of sepsis as having concomitant AKI, while 32% of AKI patients had sepsis as a contributing factor. 37
The pathophysiology of AKI in sepsis is complex and still under investigation. Potential mechanisms contributing to injury include intrarenal hemodynamic changes such as reduced glomerular capillary pressure, endothelial dysfunction, the effects of the inflammatory cascade, coagulopathy and obstruction of renal tubules. 38
It is likely that sepsis is under-recognized as an etiology of AKI in cats, and it is prudent to diligently monitor urine output and changes in serum creatinine in critically ill patients such that interventions can be made as quickly as possible. An increase of as little as 0.3 mg/dl over a 48 h period is considered significant in human medicine even if the creatinine is still within the reference interval. Improved diligence and investigation of novel urine and serum biomarkers may aid in earlier detection of AKI in these at-risk patients in the future.
Footnotes
Funding: The authors received no specific grant from any funding agency in the public, commercial or not-for-profit sectors for the preparation of this article.
The authors do not have any potential conflicts of interest to declare.
Contributor Information
Kelly Monaghan, Department of Medical Sciences, University of Wisconsin–Madison, School of Veterinary Medicine, Madison, Wisconsin, USA.
Benjamin Nolan, Veterinary Specialty Center, Middleton, Wisconsin, USA.
Mary Labato, Department of Clinical Sciences, Tufts Cummings School of Veterinary Medicine, North Grafton, Massachusetts, USA.
Key Points
AKI (acute kidney injury) is a term that replaces ARF (acute renal failure) in order to describe a wider range of insults to the kidney and to encourage earlier recognition and treatment of these events.
The pathophysiology of AKI is complex, and occurs in four stages: initiation, extension, maintenance and recovery. If AKI can be detected and treated in the early stages this may result in a more favorable outcome.
Research in recent years has produced a great deal of knowledge regarding the etiologies of feline AKI. The most commonly encountered etiology is toxic injury, with important toxins including lily plants, ethylene glycol, NSAIDs and aminoglycosides. Other causes of AKI in cats include infection, neoplasia, obstruction, ischemia and sepsis.
This knowledge has, in turn, facilitated the development of therapies that are tailored for specific conditions. It is thus important to be familiar with the most common causes of AKI in cats, as prompt recognition will allow the most effective treatments to be administered in a timely manner.
References
- 1. Devarajan P. Update on mechanisms of ischemic acute renal failure. J Am Soc Nephrol 2006; 17: 1503–1520. [DOI] [PubMed] [Google Scholar]
- 2. Siegel NJ, Devarajan P, Van Why S. Renal cell injury: metabolic and structural alterations. Pediatr Res 1994; 36: 129–136. [DOI] [PubMed] [Google Scholar]
- 3. Bagshaw SM, George C, Bellomo R. A comparison of the RIFLE and AKIN criteria for acute kidney injury in critically ill patients. Nephrol Dial Transplant 2008; 23: 1569–1574. [DOI] [PubMed] [Google Scholar]
- 4. Cowgill LD, Langston C. Acute kidney insufficiency. In: Bartges J, Polzin DJ. (eds). Nephrology and urology of small animals. Ames: Wiley-Blackwell, 2011, pp 472–523. [Google Scholar]
- 5. Sutton TA, Fisher CJ, Molitoris BA. Microvascular endothelial cell injury and dysfunction during ischemic acute renal failure. Kidney Int 2002; 62: 1539–1549. [DOI] [PubMed] [Google Scholar]
- 6. Molitoris BA, Marrs J. The role of cell adhesion molecules in ischemic acute renal failure. Am J Med 1999; 106: 583–592. [DOI] [PubMed] [Google Scholar]
- 7. Worwag S, Langston CE. Acute intrinsic renal failure in cats: 32 cases (1997–2004). J Am Vet Med Assoc 2008; 232: 728–732. [DOI] [PubMed] [Google Scholar]
- 8. Hall JO. Nephrotoxicity of Easter lily (Lilium longiflorum) when ingested by the cat [abstract]. J Vet Intern Med 1992; 6: 121. [Google Scholar]
- 9. Hadley RM, Richardson JA, Gwaltney-Brant SM. A retrospective study of daylily toxicosis in cats. Vet Hum Toxicol 2003; 45: 38–39. [PubMed] [Google Scholar]
- 10. Rumbeiha WK, Francis JA, Fitzgerald SD, Nair MG, Holan K, Bugyei KA, et al. A comprehensive study of Easter lily poisoning in cats. J Vet Diagn Invest 2004; 16: 527–541. [DOI] [PubMed] [Google Scholar]
- 11. Langston CE. Acute renal failure caused by lily ingestion in six cats. J Am Vet Med Assoc 2002; 220: 49–52. [DOI] [PubMed] [Google Scholar]
- 12. Khan KNM, Venturini CM, Bunch RT, Brassard JA, Koki AT, Morris DL, et al. Interspecies differences in renal localization of cyclooxy genase isoforms: implications in non-steroidal anti-inflammatory drug-related nephrotoxicity. Toxicol Pathol 1998; 26: 612–620. [DOI] [PubMed] [Google Scholar]
- 13. Goodman LA, Brown SA, Torres BT, Reynolds LR, Budsberg SC. Effects of meloxicam on plasma iohexol clearance as a marker of glomerular filtration rate in conscious healthy cats. Am J Vet Res 2009; 70: 826–830. [DOI] [PubMed] [Google Scholar]
- 14. US Food and Drug Administration. FDA announces addition of a boxed warning to Metacam (meloxicam) labels. October 27, 2010. http://www.fda.gov/AnimalVeterinary/NewsEvents/CVMUpdates/ucm231254.htm
- 15. Gowan RA, Lingard AE, Johnston L, Stansen W, Brown SA, Malik R. Retrospective case-control study of the effects of long-term dosing with meloxicam on renal function in aged cats with degenerative joint disease. J Feline Med Surg 2011; 13: 752–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sparkes AH, Heiene R, Lascelles BD, Malik R, Sampietro LR, Robertson S, et al. ISFM and AAFP consensus guidelines: long-term use of NSAIDs in cats. J Feline Med Surg 2010; 12: 521–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Richardson JA. Management of acetaminophen and ibuprofen toxicoses in dogs and cats. J Vet Emerg Crit Care 2000; 10: 285–291. [Google Scholar]
- 18. Connally HE, Thrall MA, Hamar DW. Safety and efficacy of high-dose fomepizole compared with ethanol as therapy for ethylene glycol intoxication in cats. J Vet Emerg Crit Care 2010; 20: 191–206. [DOI] [PubMed] [Google Scholar]
- 19. Tart KM, Powell LL. 4-Methylpyrazole as a treatment in naturally occurring ethylene glycol intoxication in cats. J Vet Emerg Crit Care 2011; 21: 268–272. [DOI] [PubMed] [Google Scholar]
- 20. Acierno MJ, Serra VF, Johnson ME, Mitchell MA. Preliminary validation of a point-of-care ethylene glycol test for cats. J Vet Emerg Crit Care 2008; 18: 477–479. [Google Scholar]
- 21. Connally HE, Hamar DW, Thrall MA. Inhibition of canine and feline alcohol dehydrogenase activity by fomepizole. Am J Vet Res 2000; 61: 450–455. [DOI] [PubMed] [Google Scholar]
- 22. Nagai J, Tanaka H, Nakanishi N, Murakami T, Takano M. Role of megalin in renal handling of aminoglycosides. Am J Physiol Renal Physiol 2001; 281: F337–344. [DOI] [PubMed] [Google Scholar]
- 23. Bertino JS, Booker LA, Franck PA, Jenkins PL, Granck KR, Nafziger AN. Incidence of, and significant risk factors for, aminoglycoside-associated nephrotoxicity in patients dosed by using individualized pharmacokinetic monitoring. J Infect Dis 1993; 167: 173–179. [DOI] [PubMed] [Google Scholar]
- 24. Gookin JL, Riviere JE, Gilger BC, Papich MG. Acute renal failure in four cats treated with paromomycin. J Am Vet Med Assoc 1999; 215: 1821–1823. [PubMed] [Google Scholar]
- 25. Mealey KL, Boothe DM. Nephrotoxicosis associated with topical administration of gentamicin in a cat. J Am Vet Med Assoc 1994; 204: 1919–1921. [PubMed] [Google Scholar]
- 26. Clark CH. Toxicity of aminoglycoside anti biotics. Mod Vet Pract 1977; 58: 594–598. [PubMed] [Google Scholar]
- 27. Greco DS, Turnwald GH, Adams R, Gossett KA, Kearney M, Casey H. Urinary gamma-glutamyl transpeptidase activity in dogs with gentamicin-induced nephrotoxicity. Am J Vet Res 1985; 46: 2332–2335. [PubMed] [Google Scholar]
- 28. Abuelo JG. Normotensive ischemic acute renal failure. N Engl J Med 2007; 357: 797–805. [DOI] [PubMed] [Google Scholar]
- 29. Vaughan ED, Gillenwater JY. Recovery following complete chronic unilateral ureteral occlusion: functional, radiographic and pathologic alterations. J Urol 1971; 106: 27–35. [DOI] [PubMed] [Google Scholar]
- 30. Berent AC. Ureteral obstructions in dogs and cats: a review of traditional and new interventional diagnostic and therapeutic options. J Vet Emerg Crit Care 2011; 21: 86–103. [DOI] [PubMed] [Google Scholar]
- 31. Wanajo I, Tomiyama Y, Tadachi M, Kobayashi M, Yamazaki Y, Kojima M, et al. The potency of KUL-7211, a selective ureteral relaxant, in isolated canine ureter: comparison with various spasmolytics. Urol Res 2005; 33: 409–414. [DOI] [PubMed] [Google Scholar]
- 32. Taylor SS, Goodfellow MR, Browne WJ, Walding B, Murphy S, Tzannes S, et al. Feline extranodal lymphoma: response to chemotherapy and survival in 110 cats. J Small Anim Pract 2009; 50: 584–592. [DOI] [PubMed] [Google Scholar]
- 33. Henry CJ, Turnquist SE, Smith A, Graham JC, Thamm DH, O’Brien M, et al. Primary renal tumours in cats: 19 cases (1992–1998). J Feline Med Surg 1999; 1: 165–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Valdes-Martinez A, Cianciolo R, Mai W. Association between renal hypoechoic subcapsular thickening and lymphosarcoma in cats. Vet Radiol Ultrasound 2007; 48: 357–360. [DOI] [PubMed] [Google Scholar]
- 35. Litster A, Thompson M, Moss S, Trott D. Feline bacterial urinary tract infections: an update on an evolving clinical problem. Vet J 2011; 187: 18–22. [DOI] [PubMed] [Google Scholar]
- 36. Neuwirth L, Mahaffey M, Crowell W, Selcer B, Barsanti J, Cooper R, et al. Comparison of excretory urography and ultrasonography for detection of experimentally induced pyelonephritis in dogs. Am J Vet Res 1993; 54: 660–669. [PubMed] [Google Scholar]
- 37. Bagshaw SM, George C, Bellomo R. Early acute kidney injury and sepsis: a multicenter evaluation. Crit Care 2008; 12: R47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Zarjou A, Agarwal A. Sepsis and acute kidney injury. J Am Soc Nephrol 2011; 22: 999–1006. [DOI] [PubMed] [Google Scholar]