Abstract
Transdermal methimazole is suggested as an alternative to oral therapy for hyperthyroid cats that are difficult to pill. However, no information on long-term management with this treatment is available. Our objective was therefore to retrospectively evaluate the efficacy and safety of long-term transdermal methimazole treatment in hyperthyroid cats. Sixty cats with newly diagnosed hyperthyroidism and available long-term follow-up information were included. Methimazole was formulated in a pluronic lecithin organogel-based vehicle and was applied to the pinna of the inner ear. Cats were re-evaluated at regular intervals. Median (range) follow-up was 22.6 months (3.6–88.4 months). Clinical improvement was observed in all cats and side effects were rare (mild transient gastrointestinal signs: n = 3; erythema of the pinna: n = 2, necessitating a switch to oral medication). Despite a significant decrease, with median T4 concentrations within the reference interval during the follow-up period, several cats repeatedly had T4 concentrations in the thyrotoxic and hypothyroid range. Maximal and minimal daily doses during the follow-up period were 15.0 and 1.0 mg, respectively; they were significantly higher than the starting dose after 24–36 months of therapy. Although the majority of owners were highly satisfied with the treatment, several admitted not treating their cat regularly. Transdermal methimazole is a safe option for the long-term management of feline hyperthyroidism. However, it seems difficult to keep the T4 concentrations constantly within the reference interval. Higher doses can be expected after prolonged treatment and, despite the convenience of transdermal application, owner compliance should be assessed regularly.
Introduction
Hyperthyroidism is the most common endocrinopathy of older feline patients. Its diagnosis is based on appropriate clinical signs, palpation of an enlarged thyroid gland and an increase in serum total T4 concentration (T4).1,2 Compared with the diagnosis, the therapy of hyperthyroid cats can be intricate. Radio-iodine has been recommended as the first choice of treatment;3,4 however, limitations are the scarcity of facilities in some countries and the up to 4 week confinement period following the injection of the radioactive compound. Surgical removal of the hyperplastic thyroid gland, although curative, still holds the risk of anaesthesia in often already debilitated cats and the risk of postsurgical hypoparathyroidism. Therefore, medical treatment with methimazole or carbimazole, thyroid hormone synthesis inhibitors, is frequently chosen for the long-term management of hyperthyroid cats. However, an effective treatment response requires daily administration of the medication, which can be difficult, especially in refractory, aggressive or vomiting cats.
Transdermal application of a gel containing methimazole has been suggested as an alternative treatment regimen to the oral form.5–7 Potential advantages of the transdermal treatment, among others, include non-invasiveness and avoidance of the gastric route, reducing the potential for both degradation of the drug and gastric irritation, and, moreover, improved owner compliance with drug administration.8–10 Methimazole is typically formulated in a pluronic lecithin organogel (PLO). Despite bioavailability after administration, a single dose of this product is poor in healthy cats, 11 but several studies have documented a good clinical short-term response in hyperthyroid cats treated with transdermal PLO-based methimazole.5–7 This discrepancy is assumed to be owing to cats licking the gel off their paws which they used to clean their ears with. To our knowledge, there are no long-term follow-up studies available that confirm transdermal methimazole as an acceptable alternative to the oral therapy. However, to determine the efficacy of a particular treatment, long-term follow-up of patients is vital. Treatment response can change over time, calling for a change in therapy. Moreover, the generally assumed better owner compliance with transdermal treatment protocols has not been demonstrated for methimazole.8–10
Therefore, the objectives of the present study were to retrospectively assess the effectiveness, adverse side effects, and owner compliance during the long-term transdermal methimazole management of hyperthyroid cats. To this end, 60 cats with available long-term follow-up information were included and their treatment response to a PLO-formulated methimazole gel assessed. Besides the T4 concentrations before and during therapy, development of body weight (BW), serum blood urea nitrogen (BUN) and creatinine concentrations, and, if available, cause of death or euthanasia were recorded. Moreover, special focus was directed to the ease of administration of the gel and on the satisfaction and compliance of the owners. Side effects occurring during therapy and the number of dose adjustments necessary to keep the T4 concentrations within the reference interval were also recorded.
Materials and methods
Animals
Client-owned cats presented to the Clinic for Small Animal Internal Medicine of the University of Zurich between December 2004 and October 2012 with newly diagnosed, naturally occurring hyperthyroidism treated with a transdermal preparation of methimazole and with a follow-up period of at least 3 months were eligible for the study. Diagnosis was based on clinical signs consistent with hyperthyroidism and a T4 concentration ≥3.5 μg/dl. Cats were excluded if transdermal treatment was changed to oral medication, either at the request of the owners or owing to side effects within the first 3 months.
Methimazole preparation and application
Methimazole was formulated in a PLO-based vehicle and purchased from a commercial veterinary compounding pharmacy (Christoffel Pharmacy). The resulting gel contained methimazole at 5 mg/0.1 ml and was dispensed in 1 ml tuberculin syringes without needles. The PLO gels were applied to the non-haired portion of the pinna of the inner ear at a starting dose of 2.5–5.0 mg/cat administered in one dose (q24h) or two divided doses (q12h). Standardised instructions on drug administration were provided to the owners, including recommendations for protection with gloves while applying the gel.
Procedures and monitoring
Blood was drawn (jugular venepuncture, 2.5 ml whole blood) to determine complete blood count (CBC), serum blood chemistry and serum T4 concentration. After clot retraction at room temperature, serum was harvested by low-speed centrifugation and transferred to tubes for storage at −80°C for later hormone assay. Urine was collected for urinalysis by cystocentesis, as requested by the attending veterinarian. All procedures were conducted in accordance with guidelines established by the Animal Welfare Act of Switzerland.
Follow-up
Cats were re-evaluated after 1–2 weeks (recheck 1), 2–4 weeks (recheck 2), 1–2 months (recheck 3), 2–3 months (recheck 4), 3–6 months (recheck 5), 6–12 months (recheck 6), 12–18 months (recheck 7), 18–24 months (recheck 8), 24–36 months (recheck 9), 36–48 months (recheck 10), 48–60 months (recheck 11), 60–72 months (recheck 12) and 72–84 months (recheck 13).
At the time of each examination the attending veterinarian was instructed to question the owners regarding improvement or resolution of clinical signs, development of side effects (eg, nausea, inappetence or reluctance to eat, lethargy, vomiting and diarrhoea) and the ease of product application, as well as owner compliance and the daily methimazole dosage; however, there was no standardised questionnaire. In addition, clinical examinations were undertaken, with particular attention paid to the development of local cutaneous reactions in the pinna of the ears. At each recheck, serum T4 concentrations, serum BUN and creatinine concentrations were determined. CBC, serum biochemical testing (other than BUN and creatinine) and urinalysis were performed as requested by the attending veterinarian (presence of azotaemia and/or polyuria/polydipsia as complaining sign at presentation).
Renal azotaemia (International Renal Interest Society [IRIS] stage 2) was defined as serum creatinine and/or BUN concentration >140 μmol/l and >12.6 mmol/l, respectively, in conjunction with inadequate urine concentrating ability (urine-specific gravity [USG] <1035) or persistently increased creatinine and/or BUN concentrations for at least 3 months.
Daily transdermal methimazole dose was adjusted after the recheck by 1.0–2.5 mg increments as needed, with the intention of maintaining circulating T4 concentrations within the normal range.
Cats were evaluated until they died, were lost to follow-up or until the study ended (December 2012). Follow-up time was determined as the time from diagnosis of hyperthyroidism to the time of death or the end of the study period.
If available, the causes of euthanasia or death were recorded. If these data were not available, owners were contacted by telephone to request follow-up information. Cats were categorised as lost to follow-up if they had not attended the clinic for 3 months and the owners were not contactable by telephone.
Analytical procedures
Serum T4 concentration was measured by use of a commercially available immunoassay validated for use in cats (Immulite Total T4; Diagnostic Products); intra-assay and inter-assay coefficients of variation were 3.9–10.8% and 5.2–13.8%, respectively. Within-series (intra-assay) precision was determined by performing 10 consecutive tests on two feline serum specimens with low (mean 2.1 µg/dl) and high (mean 8.6 µg/dl) T4 concentrations. Day-to-day precision (inter-assay) was assessed by testing commercially-available control serum of low and high T4 levels once daily over a 10-day period. Afterwards mean, SD and coefficient of variation as a measurement of the random error were calculated. Maximal allowable imprecision for T4 concentration is 8.4%. 12 Coefficients of variations for within-series precision were far below this threshold (3% and 5.7%), indicating excellent precision for this assay in the cat.
Statistical analyses
Data were analysed using non-parametric statistical methods (SPSS, for Windows, Version 18.0 and GraphPad PRISM for Windows, version 5.0). To analyse several time points during the follow-up period, the Friedman’s repeated measures test and Dunn’s multiple comparisons post-test were used. If only two time points were compared, the Wilcoxon matched-pairs signed rank test was used. The Mann–Whitney U-test was used to determine differences between two groups; if more than two groups were analysed, the Kruskal–Wallis and Dunn’s post-test were used. Values of P <0.05 were considered significant.
Results
Sixty-three cats were evaluated for inclusion. Three of them had to be excluded because treatment had to be changed to the oral form during the first 3 months of therapy: one of them developed a local cutaneous reaction, one of the owners could not touch the cat’s ears at all, and one cat had to be changed for logistical reasons because the owner was disabled and therefore unable to handle the syringe with the gel, but was able to administer one pill per day.
A total of 60 cats finally fulfilled the inclusion criteria: 26 male castrated and 34 female spayed cats, including 49 European shorthairs, two Maine Coons, four Persians, one Siamese, one British shorthair, one Norwegian Forest, one Chartreux and one Oriental shorthair, with a median (range) age of 14 years (9–22 years) and a median (range) BW of 3.6 kg (2.0–8.1 kg). Median (range) T4 concentrations before treatment were 7.4 μg/dl (3.5–46.0 μg/dl). Thyroid gland was palpable in 46 cats (26 bilateral, 20 unilateral), was not palpable in 10 cats and was not recorded in four cats.
Follow-up information
The median (range) follow-up period of all 60 cats was 22.6 months (3.6–88.4 months). At the end of the study period, 19 cats were still alive, two were lost to follow-up (after 6.3 and 27.3 months, respectively) and 39 had been euthanased. Median (range) survival time of the euthanased cats was 21.9 months (3.6–76.8 months). Causes for euthanasia were renal failure (end-stage chronic kidney disease) in 10 cats, renal and heart failure combined in two cats, heart failure in four cats (two with pleural effusion) and neoplasia in 12 cats (squamous cell carcinoma of the tongue/mouth, nose, eyelid; adeno carcinoma of the nose, lung, liver, small intestine; transitional cell carcinoma of the urinary bladder; mast cell tumour of the liver and spleen). One cat was euthanased at the age of 22 years owing to senility. Ten cats were euthanased by a private veterinarian where the reason for euthanasia remained obscure.
All except two owners were satisfied with the ease of administration of the topical gel. Two owners reported that the cat was difficult to handle and touch with gloves; however, they did not want to change to oral treatment. According to the owners, all cats showed an improvement in clinical signs (eg, cessation of vomiting and diarrhoea, increase in BW, normalisation of appetite, cessation of vocalisation and nervousness) following the administration of transdermal methimazole within the first two rechecks.
During the follow-up period, 10 owners admitted not having administered the transdermal gel on a regular basis. Reasons for not treating their cat given by the owners were outdoor cat not coming home regularly, absence/vacation of the owner and no worsening of the clinical signs after cessation of treatment. Four other owners admitted that they had completely stopped the anti-thyroid treatment for at least 3 days, 5 days, 3 weeks and 2 months, respectively, before the recheck.
Change of treatment regimen/side effects
Treatment regimen was changed in a total of six cats: four cats were changed to oral medication after 19.4, 25.3, 26.7 and 57.1 months of transdermal treatment, respectively. Two of the four had developed an erythema on the pinna (after 26.7 and 57.1 months, respectively); in the other two cats, the owners had received oral methimazole from the local veterinarian and did not want to switch back to the transdermal form.
In the other two cats, the owners elected to change to an iodine-restricted diet after 26.2 and 33.5 months without giving a medical reason.
Other reported side effects possibly resulting from transdermal treatment were mild gastrointestinal problems (anorexia, vomiting, diarrhoea) in three cats, which resolved without cessation of the methimazole administration.
BW development
For one cat no follow-up of the BW was available and thus it was excluded from analysis. There was a significant increase in BW during therapy with a median (range) increase of 0.46 kg (0.1–2.15 kg), calculated from first examination to the maximum BW reached during the follow-up period (PWilcoxon <0.0001).
At the end of the observation period, 52 cats showed a significant loss of BW compared with the maximum BW during therapy (median [range] 0.47 kg [0.1–2.36 kg]; PWilcoxon <0.0001) and in 33 of the 52 cats, BW at the end of the observation period was below the BW before starting therapy, with a median (range) loss of 0.6 kg (0.1–2.0 kg).
Serum creatinine and BUN concentrations
Renal azotaemia IRIS stage 2 before treatment was documented in 21 of the 60 cats (in 17 USG was <1035; in two of them BUN and creatinine concentrations were persistently increased, and small kidneys on palpation and abdominal ultrasound were recorded). Median (range) follow-up time of these cats was 21.6 months (5.2–53.8 months). At the end of the observation period four cats were still alive (one of which had been changed to oral treatment), one was lost to follow-up and 16 had been euthanased.
Sixteen cats with normal or decreased serum creatinine and/or BUN concentrations before therapy, worsened to IRIS stage 2 during treatment after a median (range) time span of 5.0 months (0.3–56.5 months). Total follow-up (median [range]) of these 16 cats was 21.4 months (6.7–58.7 months). Four cats were still alive at the end of the observation period (two of them were changed to oral therapy); 12 had been euthanased.
In 23 of the 60 cats serum BUN and creatinine concentrations remained within the reference interval during the whole observation period and median (range) follow-up of those cats was 22.6 months (3.6–88.4 months). At the end of the observation period 11 cats were still alive (two of them were changed to a low-iodine diet, one to oral treatment), one had been lost to follow-up and 11 euthanased.
Cats with an azotaemia before starting therapy were significantly older compared with those developing azotaemia during therapy (PMann–Whitney = 0.01) and those that remained non-azotaemic (PMann–Whitney<0.0001). There was no difference in age between cats with normal values before therapy and those developing azotaemia during therapy (PMann–Whitney = 0.1). There was no significant difference in the survival time between non-azotaemic cats and those developing azotaemia during therapy and those that were azotaemic before therapy (PKruskal–Wallis = 0.5).
Serum T4 concentrations
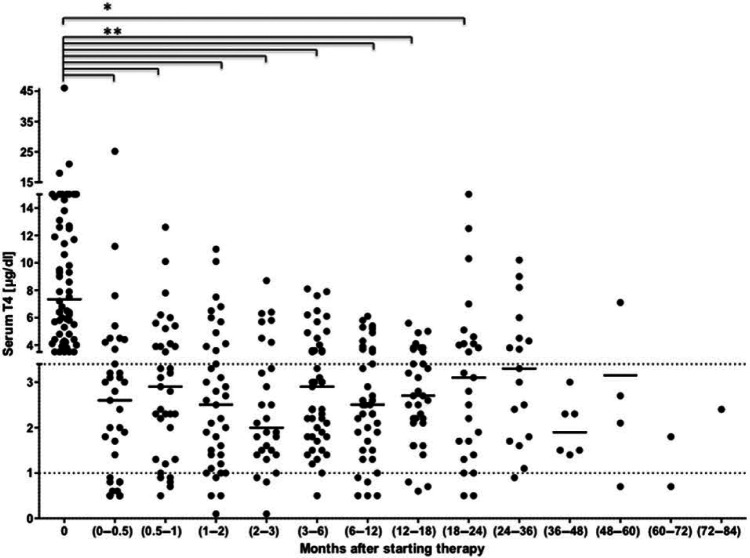
Compared with before therapy, there was a significant decrease in the T4 concentration at the first recheck. Also, from the second recheck up to the eighth recheck T4 concentrations were significantly lower compared with time point 0 (Figure 1). The median serum T4 concentration was within the reference interval on all rechecks. However, 43 cats (71.7%) had T4 concentrations above normal on one to five occasions, and subnormal T4 values were observed in 26 cats (43.3%) on one to three occasions (Figure 1). Increased T4 concentrations on one recheck were recorded in 15 cats (25%), on two occasions in 14 cats (23.3%), on three in 10 cats (16.7%), on four in two cats (3.3%) and on five occasions in two cats (3.3%). Decreased T4 concentrations below the reference interval were observed on one occasion in 19 cats (31.7%), on two occasions in five cats (8.3%) and on three occasions in two cats (3.3%). In only 11 cats (18.3%) were T4 values within the reference interval on all rechecks.
Figure 1.
Scatter plot of serum T4 concentrations before and at different time points during long-term transdermal methimazole therapy. The median is indicated by a horizontal line. The horizontal dotted lines represent the reference T4 concentrations.
*PWilcoxon = 0.0004; **PWilcoxon <0.0001
Dose adjustments – change in treatment regimen
Starting dose in all except two cats was 5 mg daily, either divided into two doses (34 cats) or in one single dose (24 cats). Two cats were started with 2.5 mg daily, in one cat divided into two doses and in the other in one single dose. Median (range) daily methimazole doses at each recheck are presented in Table 1. In 50 cats at least one dose adjustment was necessary, with a first increase in dose in 26 cats and a decrease in 24 cats. The dose of eleven of the 26 cats with a first increase had to be decreased, and 13 of the 24 cats with a first decrease had to have the dose re-increased at a later recheck. In 11, 16, 12, eight and three cats, the dose was adjusted once, twice, three times, four times and six times, respectively, during the follow-up period. Maximal and minimal daily doses during the follow-up period were 15 and 1 mg, respectively. In eight cats the treatment regimen was changed, in five from q12h to q24h (dose had to be decreased owing to iatrogenic hypothyroidism), and in three from q24h to q12h (dose had to be increased owing to persistently high T4 concentrations). Two cats that were changed to q24h had to be changed back to q12h after 4 and 8 weeks, respectively, as the T4 had re-increased to above the reference interval after the dose reduction to q24h. In cats with a follow-up period of >12 months, the daily methimazole dose was significantly higher after 24–36 months compared with the starting dose (PWilcoxon = 0.0034; Table 1).
Table 1.
Dose per cat of transdermal methimazole at the different rechecks
| Months after starting therapy |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Daily dose per cat (mg) | 0* | 0.0–0.5 | 0.5–1.0 | 1–2 | 2–3 | 3–6 | 6–12 | 12–18 | 18–24 | 24–36* |
| Minimum | 2.5 | 1.5 | 1 | 1.5 | 1.5 | 1.5 | 1.5 | 1.5 | 1.5 | 2.5 |
| Median | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 7 |
| Maximum | 5 | 7.5 | 10 | 15 | 10 | 15 | 15 | 15 | 10 | 10 |
Daily dose significantly higher than starting dose (P <0.05)
Discussion
This is the first study to evaluate hyperthyroid cats on long-term transdermal methimazole with a median follow-up time of 22.6 months. Therapy was well tolerated and led to a significant decrease in serum T4 concentrations only shortly after starting therapy in all 60 cats. The majority of owners were highly satisfied with the ease of administration of the PLO-formulated methimazole gel. However, in two of the cats treatment had to be changed to oral medication owing to local reactions (inflammation and erythema of the pinna). Interestingly, however, this side effect did not occur within the first 3 months of treatment, the time span in which most side effects are normally expected, but instead, after prolonged use of the gel for 26.7 and 57.1 months, respectively.
Although median T4 concentrations were within the reference interval at all rechecks, a substantial number of our cats repeatedly had serum T4 concentrations not only in the thyrotoxic but also in the hypothyroid range. This ‘ping-pong’ effect of the T4 concentrations over time might have a negative influence on the kidney function and on the outcome of the treatment. It is important to keep the serum T4 concentration within the mid-normal range. Chronically increased T4 concentrations may contribute to the development or progression of chronic renal disease.13,14 However, it is also important to avoid iatrogenic hypothyroidism because of the deleterious effects on kidney function; 15 therefore, both under- and overtreatment should be avoided. Moreover, increased T4 concentrations are known to negatively influence the cardiac muscle and a hypertrophy of the myocardium is a common finding in these animals. 16 In one study, almost 50% of hyperthyroid cats had increased circulating serum troponin I concentrations, which is a sensitive marker for myocardial damage. 17 Interestingly, after therapy with radioiodine, serum troponin normalised in the majority of the treated cats. We did not fully assess the systemic consequences of the hyperthyroid state and we therefore cannot say whether the transdermal therapy suffices to prevent negative sequelae such as congestive heart failure (CHF) due to hyperthyroid-induced cardiomyopathies. This would be interesting to be considered in the future.
Reasons for increased T4 concentrations during therapy are manifold. The most obvious one is owner compliance, which was extremely variable: several owners admitted not treating the cat on a regular basis or completely stopping the therapy. It is known that serum T4 concentrations will return to hyperthyroid values within 48 h of cessation of methimazole if administered orally, 18 and it is likely that this is also the case with the transdermal treatment. However, T4 variations during therapy were also observed in the cats of owners claiming to medicate their cat steadily. A possible reason for increased T4 concentrations during therapy in these cats might be an increased demand of methimazole over prolonged lengths of time of hyperthyroidism. This assumption is supported by the finding that higher daily doses (compared with the starting dose) were required after 24–36 months of treatment in order to keep T4 within the normal reference interval. A so-called ‘methimazole resistance’ to medication during chronic treatment has been suggested, one explanation being that the thyroid adenoma continues to grow during medical therapy, which might lead to increases in secretion of T4; 19 moreover, it has been suspected that these cats can develop thyroid carcinoma from initial thyroid adenomas over time. 19 No conclusion can be drawn as to whether this was also the case in our cats, as the size of the thyroid gland was not assessed and histopathology was not available. Therefore, this needs further investigation. A further explanation for the ups and downs in the T4 concentration might be a variable concentration of methimazole in the PLO formulation. So far, there is no PLO-based methimazole available as a pharmacologically registered product, and the consistency of the methimazole concentrations within this gel is not officially guaranteed. We think this an unlikely explanation, however, as similar T4 results were also recorded in an earlier study by Peterson et al, 18 showing long-term follow-up data of oral methimazole-treated cats: mean serum T4 concentrations remained within the normal range at all rechecks; however, selected cats had T4 values above normal on several occasions. The timing of blood sampling after methimazole administration has anecdotally been assumed to be a factor when assessing response to methimazole treatment and, clearly, was not standardised in our patients. However, only recently, we were able to show that transdermal methimazole application led to a prolonged T4 suppression during the day. 20 Lastly, euthyroid sick syndrome could have affected the T4 levels as well, especially in the ageing population.
Other treatment options such as radio-iodine therapy lead to more constant T4 concentrations over time and might be preferable for long-term management, as they potentially have a better outcome. It has been shown that the median survival time for hyperthyroid cats treated with oral methimazole alone was significantly shorter than the median survival time for cats treated with radio-iodine. 21 The inconsistent T4 concentrations with extremely high variations between the testing intervals could be a possible explanation for the observed shorter survival time compared with the radio-iodine treated cats.
A possible advantage of the transdermal therapy over the oral therapy is the possibility of more accurately adapting the dose. Adaptations in increments of 1 mg, which are hardly possible with tablets, are more feasible with gel; moreover, according to the manufacturer, tablets should not be divided. Certainly, owing to the above-mentioned poor and variable bioavailability, we can only speculate that our transdermal dosing was truly accurate; nonetheless, we tried to use the lowest possible effective dose of methimazole. This so-called ‘conservative treatment protocol’ to obtain a clinical effect and to maintain serum T4 within the normal range has been recommended, as this approach can lead to a reduced risk of side effects. 18 Compared with previous studies using oral methimazole, the daily dosage we used was, indeed, rather low and hence could explain the low incidence of side effects observed in our cats.
The first increase in BW after starting therapy reflects a normalisation of the catabolic metabolism and hence can be regarded as the sign of an appropriate treatment response. A further decrease in BW during the long-term management reflects, at least in part, poorly controlled hyperthyroidism, commonly observed in our cats, especially after 24 months of treatment. However, in some of the cats, the decrease in BW may also be a sign of euthyroid-sick syndrome triggered by a concurrent progressive disease (eg, neoplasia, heart failure) that finally led to the euthanasia of those cats.
Serum BUN and creatinine concentrations increased above the reference interval during therapy in 28% of the cats, a number that is comparable with that described in the literature during anti-thyroidal treatment.22–24 None of our cats developed overt acute renal decompensation due to transdermal methimazole treatment, and values remained within a stable range over a prolonged period. As the number of animals was too low, no conclusion can be drawn on the influence of kidney function on survival time, which was not significantly different in our population. Another limitation of the study is that the transdermal therapy was not directly compared with oral methimazole through a randomised study; therefore, our results can only be compared with results from the literature. Moreover, improvement in clinical signs was not standardised according to a scoring system and therefore might be rather vague, except for the determination of BW.
Conclusions
Transdermal methimazole is a safe long-term treatment option and can be regarded as a valuable alternative in situations where radio iodine treatment is not available or not an acceptable treatment option. However, one has to bear in mind that it seems difficult to keep the T4 concentrations constantly within the reference interval over the long term and, despite the ease of transdermal application, owner compliance should be monitored carefully.
Acknowledgments
We thank Drs P Laluha, S Quante, N Siebeck, S Schellenberg, F Tschuor, P Kook, C Mueller, S Jenni, M Baumstark, W Burkhardt and E Salesov for their contribution of cases, and all of the cat owners for their willingness to take part in the study.
Footnotes
Funding: This research received no grant from any funding agency in the public, commercial or not-for-profit sectors.
The authors do not have any potential conflicts of interest to declare.
Accepted: 1 October 2013
This paper was presented in part at the 22nd European College of Veterinary Internal Medicine – Companion Animals congress, Maastricht, the Netherlands, September 2012
References
- 1. Peterson ME, Aucoin DP. Comparison of the disposition of carbimazole and methimazole in clinically normal cats. Res Vet Sci 1993; 54: 351–355. [DOI] [PubMed] [Google Scholar]
- 2. Mooney CT. Feline hyperthyroidism. Diagnostics and therapeutics. Vet Clin North Am Small Anim Pract 2001; 31: 963–983. [DOI] [PubMed] [Google Scholar]
- 3. Feldmann EC, Nelson RW. Feline hyperthyroidism (thyrotoxicosis). In: Feldmann EC, Nelson RW. (eds). Canine and feline endocrinology and reproduction. 3rd ed. St Louis, MO: Saunders, 2004, p 162. [Google Scholar]
- 4. Peterson ME. Radioiodine treatment of hyperthyroidism. Clin Tech Small Anim Pract 2006; 21: 34–39. [DOI] [PubMed] [Google Scholar]
- 5. Hoffmann G, Marks SL, Taboada J, et al. Transdermal methimazole treatment in cats with hyperthyroidism. J Feline Med Surg 2003; 5: 77–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lecuyer M, Prini S, Dunn ME, Doucet MY. Clinical efficacy and safety of transdermal methimazole in the treatment of feline hyperthyroidism. Can Vet J 2006; 47: 131–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sartor LL, Trepanier LA, Kroll MM, et al. Efficacy and safety of transdermal methimazole in the treatment of cats with hyperthyroidism. J Vet Intern Med 2004; 18: 651–655. [DOI] [PubMed] [Google Scholar]
- 8. Magnusson BM, Walters KA, Roberts MS. Veterinary drug delivery: potential for skin penetration enhancement. Adv Drug Deliv Rev 2001; 50: 205–227. [DOI] [PubMed] [Google Scholar]
- 9. Mills PC, Cross SE. Transdermal drug delivery: basic principles for the veterinarian. Vet J 2006; 172: 218–233. [DOI] [PubMed] [Google Scholar]
- 10. Trepanier LA. Pharmacologic management of feline hyperthyroidism. Vet Clin North Am Small Anim Pract 2007; 37: 775–788. [DOI] [PubMed] [Google Scholar]
- 11. Hoffman SB, Yoder AR, Trepanier LA. Bioavailability of transdermal methimazole in a pluronic lecithin organogel (PLO) in healthy cats. J Vet Pharmacol Ther 2002; 25: 189–193. [DOI] [PubMed] [Google Scholar]
- 12. Jensen AL, Kjelgaard-Hansen M. Method comparison in the clinical laboratory. Vet Clin Pathol 2006; 35: 276–286. [DOI] [PubMed] [Google Scholar]
- 13. Lapointe C, Belanger MC, Dunn M, et al. N-acetyl-beta-D-glucosaminidase index as an early biomarker for chronic kidney disease in cats with hyperthyroidism. J Vet Intern Med 2008; 22: 1103–1110. [DOI] [PubMed] [Google Scholar]
- 14. van Hoek I, Lefebvre HP, Peremans K, et al. Short- and long-term follow-up of glomerular and tubular renal markers of kidney function in hyperthyroid cats after treatment with radioiodine. Domest Anim Endocrinol 2009; 36: 45–56. [DOI] [PubMed] [Google Scholar]
- 15. Williams TL, Elliott J, Syme HM. Association of iatrogenic hypothyroidism with azotemia and reduced survival time in cats treated for hyperthyroidism. J Vet Intern Med 2010; 24: 1086–1092. [DOI] [PubMed] [Google Scholar]
- 16. Bond BR, Fox PR, Peterson ME, Skavaril RV. Echocardiographic findings in 103 cats with hyperthyroidism. J Am Vet Med Assoc 1988; 192: 1546–1549. [PubMed] [Google Scholar]
- 17. Connolly DJ, Guitian J, Boswood A, Neiger R. Serum troponin I levels in hyperthyroid cats before and after treatment with radioactive iodine. J Feline Med Surg 2005; 7: 289–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Peterson ME, Kintzer PP, Hurvitz AI. Methimazole treatment of 262 cats with hyperthyroidism. J Vet Intern Med. 1988; 2: 150–157. [DOI] [PubMed] [Google Scholar]
- 19. Peterson M, Broome MR. Hyperthyroid cats on long-term medical treatment show a progressive increase in the prevalence of large thyroid tumors, intrathoracic thyroids masses, and suspected thyroid carcinoma. J Vet Intern Med 2012; 26: 1523. [Google Scholar]
- 20. Boretti FS, Sieber-Ruckstuhl NS, Schafer S, et al. Duration of t4 suppression in hyperthyroid cats treated once and twice daily with transdermal methimazole. J Vet Intern Med 2013; 27: 377–381. [DOI] [PubMed] [Google Scholar]
- 21. Milner RJ, Channell CD, Levy JK, Schaer M. Survival times for cats with hyperthyroidism treated with iodine 131, methimazole, or both: 167 cases (1996–2003). J Am Vet Med Assoc 2006; 228: 559–563. [DOI] [PubMed] [Google Scholar]
- 22. Adams WH, Daniel GB, Legendre AM, et al. Changes in renal function in cats following treatment of hyperthyroidism using 131I. Vet Radiol Ultrasound 1997; 38: 231–238. [DOI] [PubMed] [Google Scholar]
- 23. Becker TJ, Graves TK, Kruger JM, et al. Effects of methimazole on renal function in cats with hyperthyroidism. J Am Anim Hosp Assoc 2000; 36: 215–223. [DOI] [PubMed] [Google Scholar]
- 24. Graves TK, Olivier NB, Nachreiner RF, et al. Changes in renal function associated with treatment of hyperthyroidism in cats. Am J Vet Res 1994; 55: 1745–1749. [PubMed] [Google Scholar]