Abstract
A presumed primary pericardial lymphoma was diagnosed in seven cats. Clinical findings at presentation included poor body condition, dehydration and dyspnoea. Thoracic diagnostic imaging was performed in six cases and revealed pleural effusion and a diffuse thickening of the pericardium. A cytological diagnosis of lymphoma was obtained in six cases; in four cases the diagnosis was confirmed at necropsy. Immunophenotyping was performed in six cases: three cases were classified as T-cell and three as B-cell lymphoma. Four cats did not receive any treatment. One cat received only prednisone and two cats received chemotherapy. Six cats lived 7–11 days, except for one cat that received a multi-drug chemotherapy protocol and was still alive at the time of writing (750 days after diagnosis). Primary pericardial lymphoma is a rare extranodal feline lymphoma that has never been described previously.
Introduction
Lymphomas, formerly referred also as malignant lymphoma or lymphosarcoma, are a group of neoplasms that originate from lymphoreticular cells, which are primarily found in lymphoid tissues (eg, lymph nodes, spleen and bone marrow), but they are also present in all other tissues in the body. Lymphoma is one of the most commonly diagnosed neoplasms in cats. 1 Many lymphomas in cats have been associated with feline leukaemia virus (FeLV) infection. However, despite a decreasing frequency of FeLV infection in recent years, the incidence of feline lymphoma has increased, becoming especially relevant in veterinary oncology.1,2 At present, fewer than a quarter of cats with lymphoma are associated with FeLV antigenicity. 1
Lymphomas in cats can be grouped into lymph-nodal and extra-nodal forms, the latter being the most common. The extra-nodal forms are classified, according to anatomic location, in, for example, mediastinic, alimentary, renal, ocular, retrobulbar, nervous, nasal and cutaneous lymphoma tissue, with the gastrointestinal form being the most common.3,4 Over time, the median age of cats diagnosed with each anatomic tumour group has remained the same: the anatomical forms traditionally associated with FeLV infection, such as the mediastinal form, tend to occur in younger, FeLV-positive cats; the alimentary form occurs most often in older, FeLV-negative cats.2,3,5,6 In this article, we describe seven cases of feline extra-nodal lymphoma involving primarily the pericardium, a location that has not been previously described in cats.
Materials and methods
Seven cases of feline pericardial lymphoma were diagnosed at the Veterinary Hospital of the City of Pavia and at the Veterinary University of Milan between 1995 and 2012. All cases were retrospectively identified and underwent full clinical examination, except for one cat (cat 7), which was diagnosed only at necropsy. This cat came from a colony of stray cats and did not undergo extensive diagnostic investigation. Routine haematology and clinical biochemistry were performed in 5/7 cats. A serum FeLV antigenic test [Snap Test (Idexx) or Wittness Test (Symbiotics)] was performed in 6/7 cats. Three cats underwent a feline immunodeficiency virus (FIV) antibody test (Snap Test; Idexx). Thoracic radiography and ultrasonography (US) were performed in all but one cat (the cat diagnosed at necropsy). Antemortem presumptive diagnosis was based on a combination of the following: US thoracic findings (six cases), pleural effusion cytology (four cases), fine-needle cytology of the pericardium (three cases) and pericardial effusion cytology (one case). Complete necropsy and histopathology were available in four cases. Immunophenotyping was available in six cases and was performed by immunohistochemistry in three cases, by immunocytochemistry in two cases and by both methods in one case. For the purpose of phenotypic evaluation, histological cases were stained with anti-CD3-ϵ (clone CD3-12 Rat IgG1, human cross reactive with cat; Serotec) for T-cells and with anti-CD79a (clone HM-57 Mouse IgG1, human cross reactive with cat; Dako) for B-cells. Immunocytochemistry was performed utilizing primary antibodies anti-CD3-ϵ (clone CD3-12 Rat IgG1, human cross reactive with cat; Serotec), CD8α [clone Fe1.10E9, feline-specific (Leukocyte Antigen Biology Laboratory, UC Davis, CA, USA) available only for cat 1] and CD21 (Ca2.1D6 anti-canine cross reacting with cat; Leukocyte Antigen Biology Laboratory).
In three cases, FeLV antigen expression was also assessed by immunohistochemistry utilizing primary monoclonal antibodies recognizing the FeLV gp70 envelope protein (clone C11D8; Custom Monoclonal International) and FeLV p27 core protein (clone PF12J-10A; Custom Monoclonal International).
Results
Signalment and clinical presentation
All the cats described in this report were domestic shorthairs. Age at presentation ranged from 2 to 10 years, with a median age of 5 years. Two cats were neutered males and five were spayed females.
History and clinical presentation were identical for the six cases evaluated clinically, and included weight loss, poor appetite and reluctance to move (Table 1). Physical examination showed poor body condition, dyspnoea, pale mucous membranes, dehydration, weak femoral pulse, and dull heart and lung sounds on thoracic auscultation. The remainder of physical examination was unremarkable.
Table 1.
Signalment, and main clinical and pathological findings in seven cats with pericardial lymphoma
| Cat | Signalment | Age (y) | FeLV status | Thoracic US | Cytology | Histology | Immunophenotype | Treatment | Follow-up* |
|---|---|---|---|---|---|---|---|---|---|
| 1 | DSH Mn | 7 | Negative | Yes | Yes (pericardium) Large-cell lymphoma | Yes Large-cell lymphoma | IHC and ICC | None | Died 7 days |
| CD3+ | |||||||||
| CD8+ | |||||||||
| CD79a- | |||||||||
| T-cell | |||||||||
| 2 | DSH Fs | 3 | Positive | Yes | NP | Yes Intermediate-cell lymphoma | IHC | None | Died 10 days |
| CD79a+ | |||||||||
| CD3- | |||||||||
| B-cell | |||||||||
| FeLV p27+ | |||||||||
| FeLVgp70+ | |||||||||
| 3 | DSH Fs | 4 | Positive | Yes | Yes (pleural effusion and pericardic effusion and pericardium) Medium-size cell lymphoma | NP | NP | Modified UWM | Alive (750 days on complete remission) |
| 4 | DSH Fs | 5 | Positive | Yes | Yes (pleural effusion) Large-cell lymphoma | NP | ICC | Doxorubicin | Died 10 days |
| CD3+ | |||||||||
| CD79a- | |||||||||
| CD20- | |||||||||
| T-cell | |||||||||
| 5 | DSH Mn | 2 | Positive | Yes | Yes (pleural effusion and pericardium) Large-cell lymphoma | NP | ICC | Prednisone | Died 11 days |
| CD3+ | |||||||||
| CD79a- | |||||||||
| CD20- | |||||||||
| T-cell | |||||||||
| 6 | DSH Fs | 5 | Positive | Yes | Yes (pleural and pericardial effusion) Small-cell lymphoma | Yes Small-cell lymphocytic lymphoma | IHC | None | Died 9 days |
| CD79a+ | |||||||||
| CD3- | |||||||||
| B-cell | |||||||||
| FeLV p27+ | |||||||||
| FeLV gp70- | |||||||||
| 7 | DSH Fs | 10 | Positive (performed only on IHC) | No | Yes (pleural effusion) Intermediate-cell lymphoma | Yes Lymphoblastic intermediate-cell lymphoma | IHC | None | Unknown |
| CD79a+ | |||||||||
| CD3- | |||||||||
| B-cell | |||||||||
| FeLV p27+ | |||||||||
| FeLV gp70+ |
FeLV = feline leukaemia virus; US = ultrasonography; DSH = domestic shorthair; Mn = male neutered; Fs = female spayed; IHC = immunohistochemistry; NP = not performed; ICC = immunocytochemistry; UWM = University of Wisconsin–Madison chemotherapy protocol
The survival time is defined as the time from diagnosis to death
Haematology, biochemistry and serology
Complete blood cell counts showed lymphopenia and neutrophilia in two cases (cats 3 and 5) and mild anaemia in one case (cat 1). Blood chemistry showed an increased alanine transaminase activity in two cats (cats 1 and 5). FIV antibody test results were available for three cats (cats 3, 4 and 5); the results were negative.
Diagnostic imaging
Thoracic lateral radiographs were performed in 6/7 cases and revealed the presence of pleural effusion. In these six cases, thoracentesis yielded 70–160 ml fluid. Repeated thoracic radiographs after thoracentesis did not allow for clear identification of the heart silhouette as the section between the cranial part of the thorax and the caudal part of cardiac silhouette was radiopaque. Vessels and pulmonary parenchyma were obscured by a radiopaque mass, causing the trachea to be severely dorsally displaced – almost coming in contact with thoracic spine (Figure 1).
Figure 1.
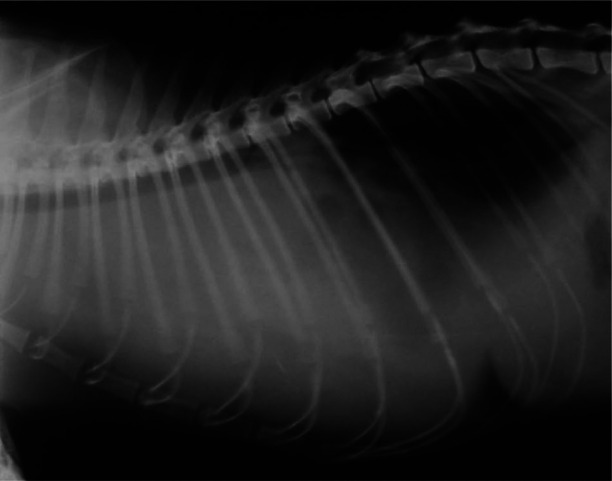
Right lateral thoracic radiograph of a cat with pericardial lymphoma, showing pleural fluid accumulation and dorsal displacement of the trachea
Ecocardiography revealed pleural effusion and an extremely thickened pericardium in six cases and no cranial mediastinal mass was in evidence. The pericardium was 1–1.8 cm thick, with a non-homogeneous echogenicity (‘brain-like’ aspect) (Figure 2). Abdominal US was performed in all but one case, and no lymph node enlargement or echostructure changes of intra-abdominal organs were detected. Mild pericardial effusion was observed in three cases.
Figure 2.
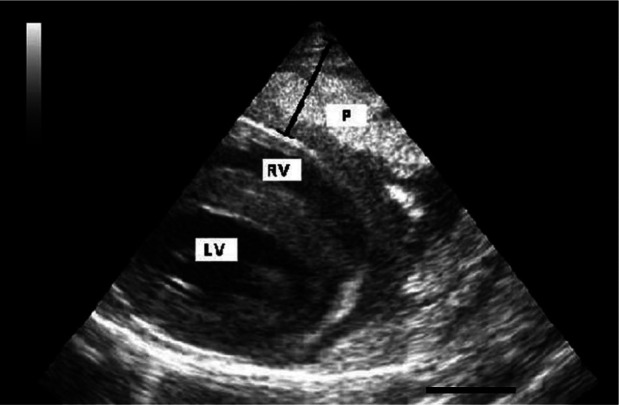
Echocardiographic appearance of a cat with pericardial lymphoma. Marked pericardial thickening is evident. The black bar in the bottom right corresponds to 1 cm. P = pericardium; RV = right ventricle; LV = left ventricle
Cytology
Pleural effusion analysis was performed in five cases. In 4/5 cases (cats 4, 5, 6 and 7), it was consistent with a neoplastic lymphoid effusion (Figure 3), while in cat 3 the effusion analysis was consistent with a protein-rich transudate. When performed (cats 1, 3 and 5), US-guided fine-needle aspiration cytology of the thickened pericardium showed a single population of immature lymphoid cells of medium-to-large size (>2–3 red blood cells), with scant basophilic cytoplasm, and round-to-pleomorphic nuclei with evident nucleoli.
Figure 3.
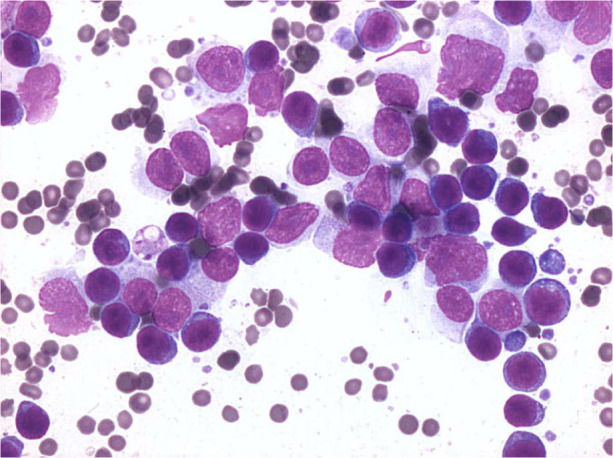
Cytology from a pleural effusion in a cat with pericardial lymphoma. Middle-size (2–3 red blood cells [RBC] in size) to large immature (>3 RBC) lymphoid cells are present (May–Grünwald–Giemsa stain, × 1000)
A few millilitres of pericardial effusion were collected and analysed in two cases (cats 3 and 6): in cat 3 it was consistent with a protein-rich transudate, whereas in cat 6 it was consistent with a neoplastic lymphoid effusion.
Treatment and follow-up
Four cats (cats 1, 2, 6 and 7) did not receive any medication. Survival time of cats 1, 2 and 6 ranged from 7 to 10 days, with a median survival time of 9 days. Survival time of cat 7 was not recorded.
Two cats were administered chemotherapy: one received a modified University of Wisconsin–Madison chemoterapy protocol (UWM) (cat 3), which included cyclophosphamide, doxorubicin, vincristine and prednisone, but no L-asparaginase. 1 The other cat (cat 4) received doxorubicin as a single-agent therapy at 25 mg/m2. One cat received only prednisone at the dosage of 2 mg/kg per day subcutaneously (cat 5).
The cat that underwent UWM protocol is still alive at the time of writing and has been in complete remission for 750 days, with complete remission being intended as the resolution of all clinically detectable diseases confirmed by thoracic radiology and US. The cat that was administered only doxorubicin survived 10 days and did not respond to the treatment.
The cat that received prednisone had a survival time of 11 days, with no response to treatment.
Gross findings, histology and immunophenotyping
Necropsy was performed in four cases. In these cats, the pericardium appeared markedly and diffusely thickened by a solid tissue proliferation (Figure 4). Other thoracic and abdominal organs appeared macroscopically not involved by the tumour. Pleural effusion was present in all cases, although the volume of fluid was not recorded. Pericardial effusion was mild in all cases.
Figure 4.
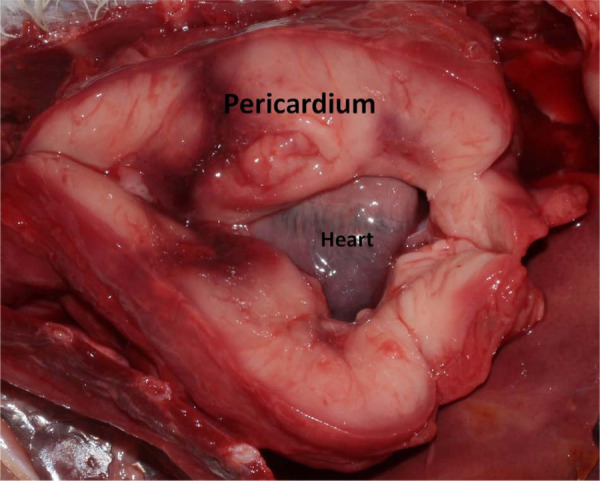
Gross aspect of pericardial lymphoma in a cat at necropsy. Marked diffuse pericardial thickening is evident
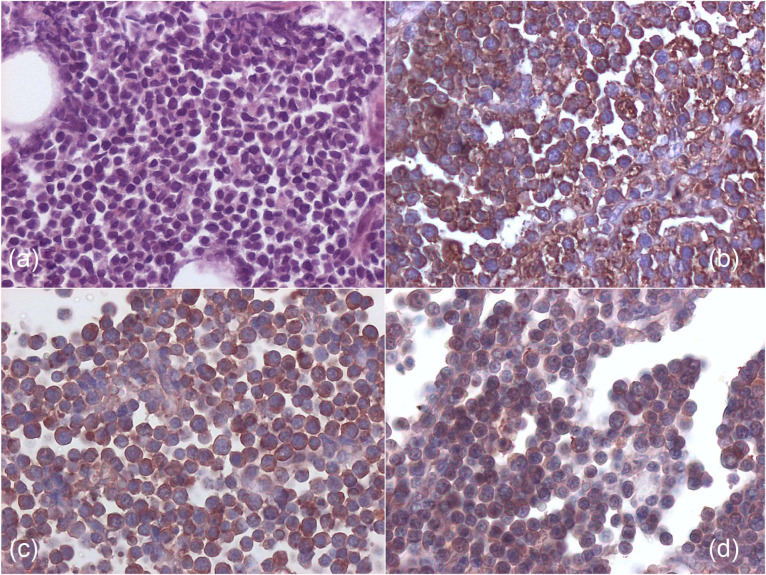
A combination of histology and immunohistochemistry was available in four cases (cats 1, 2, 6 and 7). In all cases examined, severe diffuse expansion of the pericardium (up to 2 cm) by a population of uniform, round cells was detected (Figure 5a). Of these cases, three were classified as small or medium B-cell lymphoma (cats 2, 6 and 7) (Figure 5b) and one was a large T-cell lymphoma (cat 1). Case 1 tested also positive for CD8 on immunocytochemistry. In two cases, histology was not available, but immunophenotype was obtained by immunocytochemistry (cats 4 and 5) from pleural effusion; both were diagnosed as large T-cell lymphoma.
Figure 5.
(a) Histology from medium-size B-cell pericardial lymphoma of cat 2, showing a monomorphic population of round cells (haematoxylin and eosin, × 200). (b) Immunohistochemistry from cat 2, showing a diffuse strong cytoplasmic positivity for CD79a (B-cell phenotype) (avidin–biotin peroxidase method, × 400). (c,d) Immunohistochemistry from cat 2 showing positive reactivity for feline leukaemia virus antigen p27 (c) and gp70 (d) (avidin–biotin peroxidase method, × 400)
In cats 2,6 and 7, myocardium from the different cardiac sectors was examined microscopically and found not to be infiltrated by neoplastic cells. In cat 2, intravascular neoplastic emboli of lymphoid cells were observed in the myocardium, but never in the interstitium.
FeLV status
Only cat 1 was FeLV-negative; all the others were FeLV-positive: three on serology (cats 3, 4 and 5), one on immunohistochemistry (cat 7), and two on both serology and immunohistochemistry (cats 2 and 6) (Figure 5c, d).
Discussion
In this article we have described a small series of presumptive primary pericardial lymphoma in cats. The pericardium was presumed to be the primary site of origin as it was the only organ grossly affected by the tumour. To our knowledge, this study represents the first description of primary lymphoma of the pericardium in cats, even if this case series is based on a retrospective case retrieval, and complete clinical, laboratory and pathological findings were not available for all seven cats. Pericardial lymphoma should therefore be considered a rare extranodal feline lymphoma
Pericardium can be involved in primary or metastatic tumours. In terminally ill human patients, lymphoma, leukaemia, breast or lung tumours can secondarily affect pericardium with microscopic metastasis or small effusion without clinical relevance. 7 In the group of cats described herein, the pericardium appeared markedly and uniformly thickened by the tumour’s growth. This thickening was clearly visible by thoracic US. US findings were always strongly suggestive of pericardial malignancy, and all tested cats showed similar US pericardial abnormalities. Thoracic US examination and findings should therefore be considered the first strong sign of pericardial lymphoma in cats.
The median age of affected cats in this study (5 years) is similar to the median age of FeLV-positive cats affected by other forms of lymphoma. 8 FeLV status was positive in all but one case. The FeLV-negative cat was tested only by serum antigenic test, but immunohistochemistry and polymerase chain reaction were not performed on affected tissues, so we cannot exclude the presence of the retrovirus. Dehydration and weight loss were present in all cases, and might have been caused by severe dyspnoea. Lymphopenia and neutrophilia were present in 2/7 cases and might be considered stress-induced.
Pleural effusion could be caused by impaired cardiac diastolic function due to pericardial thickening, to reduced preload from compressed intra-thoracic vessels or to a combination of both mechanisms. Direct exfoliation of neoplastic cells and fluid transudation from the tumour could also have contributed to fluid accumulation.
Cytology from effusion or pericardium was indicative of lymphoma in six cases. Cytology is a simple, but powerful, diagnostic technique, and the cytological diagnosis of lymphoma is quite straightforward in the presence of a single population of immature lymphoid cells.
Staging of lymphoma was not performed in these cats owing to the owners’ financial constraints and to the severity of the cats’ condition, which did not allow for extensive imaging and biopsy procedures. However, in the four cases that underwent necropsy, gross involvement of other thoracic or abdominal organs was not documented. Microscopic myocardial emboli were found on histopathology only in one case (cat 2). We cannot exclude involvement of other tissues in the remaining three cases; therefore, the diagnosis of primary pericardial lymphoma in these three cats should be considered presumptive.
Immunophenotype was B-cell in three cases and T-cell in three cases. Although the number of cats in our study is very small, this prevalence is different to the higher prevalence of the T-cell phenotype reported in cats with mediastinal lymphoma. 1
The survival time of the cat that was administered prednisone did not differ significantly from that of the cats that were not treated. The cat that received only doxorubicin did not respond to the treatment and survived comparably to the ones that were administered prednisone or no treatment. The lack of response to doxorubicin is consistent with the literature, showing it to be a poor induction agent when used alone.9,10 The cat that underwent the modified UWM protocol went into complete remission 2 days after the first administration of treatment and remained in complete remission until the time of writing (750 days). This is consistent with studies showing that response to therapy is the strongest reported prognostic factor for cats treated for lymphoma.5,11
Conclusions
The presence of pleural effusion, an enlarged globoid cardiac silhouette with dorsally displaced trachea in association with clinical signs and the animal’s age can be indicative of cardiac or pericardial disease. Echocardiography can be used to confirm that the displacement of the trachea is caused by thickening of the pericardium. Cytology of the pericardium or of the thoracic effusion can be useful in reaching the diagnosis of lymphoma.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
The authors do not have any potential conflicts of interest to declare.
Accepted: 22 August 2013
References
- 1. Vail DM. Feline lymphoma and leukemia. In: Withrow SJ, Vail D. (eds). Withrow & MacEwen’s small animal clinical oncology. 4th ed. St Louis, MO: Saunders-Elsevier, 2007, pp 733–756. [Google Scholar]
- 2. Lowerens M, London CA, Pedersen NC, Lyons LA. Feline lymphoma in the post-feline leukaemia virus era. J Vet Intern Med 2005; 19: 329–335. [DOI] [PubMed] [Google Scholar]
- 3. Court EA, Watson ADJ, Peaston AE. Retrospective study of 60 cases of feline lymphosarcoma. Aust Vet J 1997; 75: 424–427. [DOI] [PubMed] [Google Scholar]
- 4. Marconato L, Rossi F, Bettini G, Comazzi S, Bonfanti U, Buchholz J. Linfoma. In: Marconato L, Amadori D. (eds). Oncologia medica veterinaria e comparata. 1st ed. Milan: Poletto editore, 2012, pp 759–817. [Google Scholar]
- 5. Vail DM, Moore AS, Oglilvie GK, Volk LM. Feline lymphoma (145 cases): proliferation indices, CD3 immunoreactivity and their association with prognosis in 90 cats receiving therapy. J Vet Intern Med 1998; 12: 349–354. [DOI] [PubMed] [Google Scholar]
- 6. Gabor LJ, Canfiled PG, Malik R. Immunophenotypic and histological characterization of 109 cases of feline lymphosarcoma. Aust Vet J 1999; 77: 436–441. [DOI] [PubMed] [Google Scholar]
- 7. Lestuzzi C. Neoplastic pericardial disease: old and current strategies for diagnosis and management. World J Cardiol 2010; 26: 270–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mooney SC, Hayes AA, Matus RE, Geary A, Shurgot BA. Treatment and prognostic factors in lymphoma in cats: 103 cases (1977–1981). J Am Vet Med Assoc 1989; 194: 696–699. [PubMed] [Google Scholar]
- 9. Peaston AE, Maddison JE. Efficacy of doxorubicin as an induction agent for cats with lymphosarcoma. Aust Vet J 1999; 77: 442–444. [DOI] [PubMed] [Google Scholar]
- 10. Kristal O, Lana SE, Ogilvie GK, Cotter SM, Moore AS. Single agent chemotherapy with doxorubicin for feline lymphoma. J Vet Intern Med 2001; 15: 125–130. [DOI] [PubMed] [Google Scholar]
- 11. Teske E, van Straten G, van Noort R, Rutteman GR. Chemotherapy with cyclophosphamide, vincristine, and prednisolone (COP) in cats with malignant lymphoma: new results with an old protocol. J Vet Intern Med 2002; 16: 179–186. [DOI] [PubMed] [Google Scholar]