Abstract
Objectives
The aim of the study was to evaluate the tolerability, sedative and analgesic effects of methadone in combination with medetomidine for premedication prior to neutering in healthy cats.
Methods
This was an assessor-blinded, randomised, clinical research study. Forty-five cats were recruited and divided into three treatment groups of 15. Following premedication with medetomidine (20 µg/kg) and one of the three test drugs – methadone 0.5 mg/kg, buprenorphine 20 µg/kg or butorphanol 0.4 mg/kg intramuscularly – anaesthesia was induced with propofol and maintained with isoflurane, and neutering was carried out. Sedation and physiological parameters were assessed before premedication, after premedication before induction of anaesthesia, and at 90 mins and 2, 3, 4, 6, 7, 8 and 24 h after premedication. Pain and mechanical nociceptive threshold were assessed at similar time points.
Results
There were no differences between groups with respect to age, sex, duration of anaesthesia or surgery. Most cats had low pain scores in the postoperative period, with small differences in pain scores between groups at individual time points only. Five, two and no cats required additional rescue analgesia in the postoperative period in the butorphanol, methadone and buprenorphine groups, respectively, representing no significant difference between groups.
Conclusions and relevance
Medetomidine combined with methadone for premedication prior to neutering in healthy cats provided adequate analgesia for the first 6 h after administration with no adverse effects; effects overall were comparable with medetomidine combined with buprenorphine or butorphanol. Administration of further analgesia with methadone at 6 h and a non-steroidal anti-inflammatory drug at 8 h provided adequate analgesia for the first 24 h after surgery.
Introduction
Methadone is a µ opioid agonist drug that is widely used to provide analgesia in cats.1–3 It is a racemic mixture, with both enantiomers contributing to its analgesic effects, 4 although the analgesic action of the racemic mixture is primarily attributed to levo-methadone, which is considered to have 10–50-fold greater affinity for the µ opioid receptor than the dextrorotatory enantiomer. 5 Unlike morphine, which acts primarily at the µ opioid receptor, 6 methadone acts at a number of different receptor systems, including variable affinity at N-methyl-D-aspartate (NMDA) and α2 adrenergic receptors. 7 Some preclinical animal studies have investigated the non-opioid effects of methadone on pain and hyperalgesia and have shown a reduction in central sensitisation after tissue injury, mediated by NMDA receptor antagonism.8,9 The NMDA antagonist effects of methadone may confer additional benefits to choosing methadone for analgesia over other opioid drugs by preventing the development of central sensitisation after tissue injury.
A number of clinical and experimental studies have investigated the analgesic effects of methadone administered to cats.2,3,10,11 A range of doses has been investigated in these studies, varying from 0.2–0.6 mg/kg subcutaneously (SC).2,11 In a very similar study, Bortolami et al 3 investigated analgesia provided by 0.5 mg/kg methadone intramuscularly (IM) in cats undergoing ovariohysterectomy and castration. The aim of the present investigation was to evaluate, prior to neutering in healthy cats, the tolerability, and sedative and analgesic effects of methadone in combination with medetomidine for premedication compared with butorphanol as a positive control for sedation and buprenorphine as a positive control for analgesia. We hypothesised that methadone, a full µ agonist with NMDA receptor antagonist effects, would provide better postoperative analgesia and reduced mechanical hyperalgesia than buprenorphine and butorphanol.
Materials and methods
Animals and housing
The study protocol received institutional ethical approval and statutory ethical, animal and human safety approval via an Animal Test Certificate from the Veterinary Medicines Directorate. Forty-five cats entering the clinic for routine neutering surgery (ovariohysterectomy or castration) were recruited to the study. All owners or their agents provided signed consent prior to their cat entering the study. All cats were examined prior to premedication, only those falling in the American Society of Anesthesiologists’ categories of 1 or 2 were included. Exclusion criteria were cats that had been treated with any analgesic, sedative or anaesthetic drug in the previous 7 days. The study was conducted between July 2010 and May 2011.
Treatments
After initial assessments, cats were administered premedication consisting of intramuscular medetomidine (20 µg/kg) (Sedator 1.0 mg/ml; Eurovet Animal Health) and one of the three test drugs – methadone (0.5 mg/kg) (Comfortan 10 mg/ml; Eurovet Animal Health), buprenorphine (20 µg/kg) (Vetergesic 0.3 mg/ml; Alstoe Animal Health) or butorphanol (0.4 mg/kg) (Dolorex 10 mg/ml; MSD Animal Health). The premedicants were administered into the lumbar epaxial muscles and the time of premedication was defined as T = 0 (mins). The person administering the premedicants was not involved in any assessments at any time points to ensure that the assessor was blinded to treatment group. After premedication, animals were kept in a quiet environment for 20 mins. Following assessments (see below) each cat was then placed in a specialised cat restraint bag (Feline Restraint Bag; Medi-Vet Animal Health) to facilitate placement of a 22 G intravenous (IV) catheter in the cephalic vein. Anaesthesia was induced with propofol (PropoFlo 10 mg/ml; Abbott Animal Health) to effect and the dose of propofol was recorded. The larynx was desensitised using lidocaine spray (Intubeaze; Dechra Pharmaceuticals) and the trachea was intubated with an uncuffed endotracheal tube (Portex tracheal tube; Smiths Medical International). Anaesthesia was maintained with isoflurane (Isoflurane-Vet; Merial Animal Health) in oxygen delivered via a T-piece breathing system. The vaporiser setting was adjusted to maintain an adequate depth of anaesthesia for surgery, based on assessment of clinical signs. During anaesthesia, a multi-parameter monitor (Passport 2; Datascope) was used to monitor continuously end-tidal CO2 concentration (PE’CO2), the inspired and expired fraction of isoflurane (Fi’Iso, FE’Iso) and SpO2; measurements were recorded every 5 mins. Non-invasive blood pressure was measured and recorded every 5 mins during surgery in female cats only using the Doppler method (Doppler flow detector; Parks Medical Electronics), with a size 1 or 2 cuff (Critikon Soft-Cuff; GE Healthcare) and sphygmanometer (DuraShock Welch Allyn) with the probe placed on the shaved skin over the palmar digital artery.
All surgeries were carried out by a single experienced veterinary surgeon who was blinded to treatment group. Ovariohysterectomy was carried out through a flank incision, which was approximately 2 cm in length.
Assessments
A single investigator (LS) who was unaware of which opioid was used for premedication carried out all of the assessments.
Sedation and physiological parameters
Sedation was assessed by observation of behaviour and scored using both a visual analogue scale (VAS) and a four-point simple descriptive scale (SDS) (see Appendix 1 in the Supplementary material). The VAS was a 100 mm scale, with 0 mm being no sedation and 100 mm being complete sedation, where the cat could not be roused by noise or physical stimulation. Assessments were made at the following time points: before premedication; every 5 mins after premedication for 20 mins; immediately before IV catheterisation; and at 90 mins and 2, 3, 4, 6, 7 and 8 h after premedication. The last assessment was carried out at the time of discharge between 20 and 24 h after premedication. At the same time points rectal temperature was measured using a lubricated digital thermometer, and pulse and respiratory rate were measured by auscultation of the chest and counting the number of breaths and heart beats over 15 s.
Ease of restraint for catheter placement
Ease of restraint for catheter placement was assessed using a four-point SDS (see Appendix 2 in the Supplementary material).
Pain
The degree of pain at the surgical site was assessed before premedication, at the time of IV catheterisation, at 90 mins, 2, 3, 4, 5, 6, 7 and 8 h after premedication, and at the time of discharge between 20 and 24 h after premedication.
Pain was assessed using an interactive visual analogue scale (IVAS) for cats,3,12 which involved observation of the animal from outside of the cage, observation of response to opening the cage door, speaking to the animal and subsequent handling. A 100 mm IVAS was used where 0 mm was considered no pain at all and 100 mm the worst possible pain. If the IVAS score was ⩾50 mm in the first 6 h after premedication ‘rescue analgesia’ was administered. Immediately after IVAS assessment mechanical nociceptive threshold (MNT) was measured using a Pressure Rate Onset Device (PRoD; TopCat Metrology Ltd). This is a mechanical testing device for use in clinical cases and consists of an 8 mm hemispherical probe that is applied perpendicular to the test area (which was either the site of flank ovariohysterectomy or skin of the scrotum). The probe is applied at a pressure that increases by 1 Newton (N)/s. The force at which the cat responded with a clear escape behaviour was recorded as the MNT. Preoperatively the MNT was measured at the test site three times at 15 min intervals and the mean of these three measurements was calculated to give the baseline MNT. Postoperatively, MNT was measured at the surgical site immediately adjacent to the surgical incision but not directly over the incision, one measurement being taken at each of the pain assessment time points. MNT change from baseline was calculated for each postoperative time point and taken as the MNT minus the baseline MNT. Further analgesia was provided to all cats using methadone (0.5 mg/kg) IM 6 h after premedication and carprofen (4 mg/kg) (Rimadyl 50 mg/ml; Pfizer) SC 8 h after premedication. The two analgesic drugs were administered contemporaneously once if rescue analgesia was deemed necessary.
Recovery
Time from the end of anaesthesia to the first head lift, first achieving sternal recumbency and first standing was recorded. Recovery quality was scored on a four-point simple descriptive scale (see Appendix 3 in the Supplementary material).
Adverse events
Any adverse events that occurred during the course of the study were noted.
Statistical analysis
Sample size calculations were performed before starting the study. Using historical data, for a two-sample, two-sided t-test with a difference between mean IVAS scores of 20 mm, SD of 15 mm, β of 0.8 and α of 0.05, a minimum of 10 animals per group were required. A block randomisation was performed with cats divided into male and females and then into three groups, so that there were eight females and seven males per group. Repeated measures comparisons (pain scores, sedation scores and MNTs) between groups were made using two-way repeated measures (RM) ANOVA (Graph Pad Prism 5; Graph Pad Software). Within-groups comparisons were performed comparing post-treatment MNTs with baseline MNTs using one-way RM ANOVA with Tukey’s multiple comparison test. Data regarding analgesia in female cats were analysed in two ways. Data were analysed both uncorrected for rescue analgesia (that is, SDS and IVAS pain scores were not altered or ‘held’ after administration of rescue analgesia at any time point), and corrected for rescue analgesia. IVAS scores were analysed up to and including the 6 h time point only when ‘held’ at the score at which rescue analgesia was administered because after 6 h all cats received a further dose of methadone. IVAS scores in male cats were not subject to statistical analysis.
Single comparisons for normally distributed data (eg, dose of induction agent) were made with ANOVA and single comparisons for non-normally distributed data (eg, ease of IV catheterisation score) were performed with a Kruskall–Wallis test. Rescue analgesia required was analysed with a Fisher’s exact test. P values <0.05 were deemed significant. Parametric data are presented as mean ± SD and non-parametric data are presented as median (range).
Results
Ages and weight
Overall the mean age and weight of the cats were 8.7 ± 5.1 months and 3.1 ± 0.6 kg, respectively. There were no significant differences in age and weight between groups.
Timing
Overall mean time from premedication to induction of anaesthesia was 28 ± 4 mins, and mean duration of surgery for male and female cats was 4.0 ± 3.0 and 28.3 ± 11.7 mins, respectively, with no significant differences between groups. The other times assessed during anaesthesia and surgery were time from induction to end of volatile agent administration, and induction to disconnection from the breathing circuit, and these times were not significantly different between groups.
Ease of restraint for catheter placement
Overall, the median score for catheter placement was 3 (range 1–3) (ie, very easy), with no statistical difference between treatment groups. No cat was awarded a score of 0 (ie, very difficult) but a few cats received a score of 1; therefore, premedication failed to achieve one of its goals in these few animals.
Dose of induction agent
When data from male and female cats were pooled the mean dose of propofol required to induce anaesthesia was 2.3 ± 1.3 mg/kg, with no significant difference between treatment groups.
Recovery
Recovery score was good to excellent for most animals, with no significant differences between treatment groups. When male and female cats were grouped together, median recovery score for the buprenorphine group was 3 (range 1–3), for the butorphanol group was 3 (range 0–3) and for the methadone group was 2 (range 0–3).
Intraoperative variables
Induction of anaesthesia was uneventful in all animals. One female cat in the butorphanol group was administered 10 ml Hartmann’s solution (Vetivex 11; Dechra Animal Health) during surgery as a result of greater than expected blood loss, but heart rate and systolic arterial blood pressure remained within normal limits in this cat. In one female cat and one male cat in the methadone group it was difficult to maintain an adequate depth of anaesthesia for surgery, and incremental doses of propofol were administered during the maintenance phase of anaesthesia. Intraoperative variables for female and male cats are shown in Tables 1 and 2, respectively. Data were not collected for all variables from all animals at all time points; missing values after induction of anaesthesia were attributed to the time taken to instrument the cat, and missing data at later time points occurred because of differences in the duration of anaesthesia in individual animals. In male cats data were only analysed up to 15 mins; in female cats data were analysed from either 5 or 10 mins after the induction of anaesthesia to 30 mins after induction of anaesthesia. Physiological variables remained within clinically acceptable limits in all cats during anaesthesia and there were no significant differences in variables between treatment groups for either male or female animals. The inspired and expired fraction of isoflurane was also not significantly different between treatment groups in either male or female cats.
Table 1.
Intraoperative variables during the first 25 mins of anaesthesia in female cats
| Time | 5 mins |
10 mins |
15 mins |
20 mins |
25 mins |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean | SD | n | Mean | SD | n | Mean | SD | n | Mean | SD | n | Mean | SD | ||
| Heart rate (beats/min) | Buprenorphine | 8 | 98 | 24 | 7 | 104 | 11 | 8 | 106 | 14 | 8 | 136 | 27 | 8 | 140 | 19 |
| Butorphanol | 8 | 90 | 11 | 8 | 99 | 12 | 8 | 108 | 17 | 8 | 136 | 24 | 8 | 168 | 101 | |
| Methadone | 8 | 111 | 18 | 8 | 113 | 16 | 8 | 122 | 16 | 8 | 133 | 24 | 8 | 150 | 33 | |
| Respiratory rate (breaths/min) | Buprenorphine | 4 | 29 | 5 | 6 | 21 | 2 | 8 | 22 | 6 | 8 | 24 | 9 | 8 | 24 | 8 |
| Butorphanol | 7 | 24 | 9 | 7 | 20 | 6 | 8 | 22 | 8 | 8 | 34 | 27 | 8 | 25 | 7 | |
| Methadone | 7 | 24 | 13 | 7 | 20 | 6 | 8 | 23 | 17 | 8 | 17 | 9 | 7 | 14 | 7 | |
| Blood pressure (mmHg) | Buprenorphine | 0 | – | – | 6 | 102 | 20 | 6 | 94 | 10 | 7 | 107 | 15 | 7 | 102 | 25 |
| Butorphanol | 5 | 121 | 29 | 7 | 119 | 29 | 7 | 111 | 25 | 8 | 114 | 29 | 8 | 114 | 43 | |
| Methadone | 3 | 104 | 31 | 8 | 113 | 22 | 8 | 107 | 22 | 8 | 105 | 24 | 8 | 105 | 26 | |
| Expired (isoflurane) (%) | Buprenorphine | 5 | 1.6 | 0.2 | 5 | 1.6 | 0.2 | 6 | 1.6 | 0.3 | 6 | 1.6 | 0.3 | 6 | 1.6 | 0.3 |
| Butorphanol | 6 | 1.4 | 0.4 | 6 | 1.4 | 0.4 | 6 | 1.4 | 0.3 | 6 | 1.4 | 0.3 | 6 | 1.4 | 0.3 | |
| Methadone | 4 | 1.4 | 0.1 | 4 | 1.3 | 0.3 | 4 | 1.3 | 0.3 | 5 | 1.3 | 0.3 | 4 | 1.3 | 0 | |
| End-tidal CO2 (kPa) | Buprenorphine | 2 | 1.4 | 0.4 | 3 | 1.4 | 0.3 | 4 | 1.4 | 0.2 | 4 | 1.3 | 0.3 | 4 | 1.5 | 0.5 |
| Butorphanol | 2 | 1.3 | 0.1 | 5 | 1.3 | 0.1 | 5 | 1.3 | 0.1 | 5 | 1.3 | 0.2 | 5 | 1.3 | 0.2 | |
| Methadone | 3 | 1.7 | 0.6 | 4 | 1.5 | 0.3 | 3 | 1.8 | 0.3 | 5 | 2.3 | 1.3 | 4 | 1.8 | 0.3 | |
Table 2.
Intraoperative variables during the first 25 mins of anaesthesia in male cats
| Time | 5 mins |
10 mins |
15 mins |
20 mins |
25 mins |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean | SD | n | Mean | SD | n | Mean | SD | n | Mean | SD | n | Mean | SD | ||
| Heart rate (beats/min) | Buprenorphine | 7 | 94 | 27 | 7 | 93 | 24 | 7 | 107 | 22 | 6 | 113 | 23 | 3 | 111 | 21 |
| Butorphanol | 6 | 106 | 25 | 7 | 111 | 27 | 7 | 123 | 21 | 5 | 124 | 8 | 3 | 115 | 8 | |
| Methadone | 7 | 101 | 33 | 7 | 100 | 34 | 6 | 99 | 33 | 3 | 97 | 27 | 3 | 98 | 24 | |
| Respiratory rate (breaths/min) | Buprenorphine | 7 | 31 | 11 | 7 | 30 | 11 | 7 | 30 | 15 | 6 | 39 | 23 | 3 | 33 | 12 |
| Butorphanol | 6 | 27 | 11 | 7 | 25 | 11 | 7 | 28 | 11 | 5 | 23 | 8 | 3 | 30 | 3 | |
| Methadone | 7 | 38 | 18 | 6 | 30 | 11 | 6 | 30 | 12 | 3 | 29 | 10 | 3 | 28 | 11 | |
| Expired (isoflurane) (%) | Buprenorphine | 3 | 1.6 | 0.4 | 3 | 1.6 | 0.4 | 3 | 1.7 | 0.4 | 2 | 0.7 | 1.0 | 0 | – | – |
| Butorphanol | 2 | 1.5 | 0.1 | 3 | 1.5 | 0.1 | 3 | 1.0 | 0.8 | 2 | 0.8 | 1.1 | 0 | – | – | |
| Methadone | 2 | 1.4 | 0.0 | 2 | 1.5 | 0.0 | 2 | 1.6 | 0.1 | 0 | – | – | 0 | – | – | |
Sedation
All sedation scores were 0 mm (VAS) and 0 (SDS) prior to administration of medetomidine and the test opioid drugs. As expected following the administration of medetomidine, sedation scores increased significantly in the 20 mins after premedication, with no significant differences between the treatment groups at any time point (as measured by the VAS [Figure 1] or SDS [data not shown]). In female cats, the 90 mins time point was excluded from the analysis of postoperative sedation scores because some cats were still anaesthetised. Sedation decreased significantly from the end of the anaesthetic but there were no significant effects of treatment from 2–6 h from premedication (VAS sedation scores; Figure 2). Once 6 h had elapsed since administration of premedicants, few cats demonstrated clinical signs of sedation. Male cats were still sedated at 90 mins after premedication and sedation decreased significantly over time from 90 mins to 6 h after premedication (VAS sedation scores; Figure 3). There were no significant differences between treatment groups.
Figure 1.
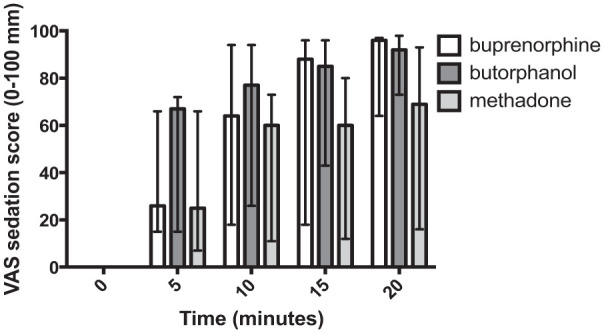
Preoperative visual analogue scale (VAS) sedation scores (median and interquartile range), combined male and female data (n = 15 cats per group, eight females and seven males); the x-axis is the time from premedication (T = 0) in mins and the y-axis is the sedation score (0–100 mm). There were no significant differences in sedation scores between treatment groups
Figure 2.
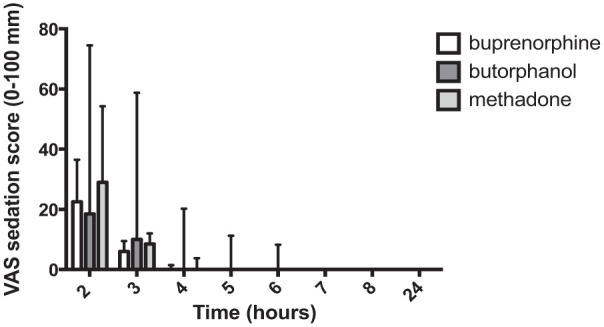
Postoperative visual analogue scale (VAS) sedation scores (median and interquartile range) in female cats (n = 8 per group); the x-axis is the time from premedication (T = 0) in h and the y-axis is the sedation score (0–100 mm). There were no significant differences in sedation scores between treatment groups
Figure 3.
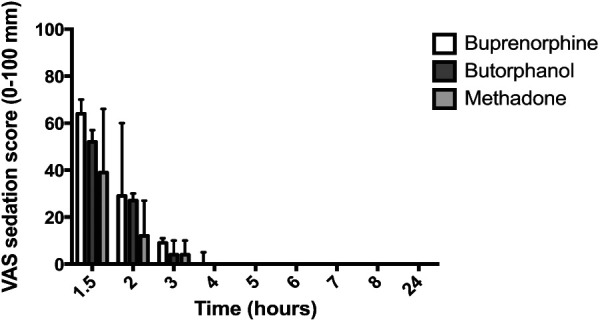
Postoperative visual analogue scale (VAS) sedation scores (median and interquartile range) in male cats (n = 7 per group); the x-axis is the time from premedication (T = 0) in h and the y-axis is the sedation score (0–100 mm). There were no significant differences in sedation scores between treatment groups
Analgesia
All cats were pain free at the start of the study and immediately before induction of anaesthesia (IVAS = 0 mm). Five cats in the butorphanol group required rescue analgesia (one at 2 h and four at 3 h), and two cats in the methadone-treated group received rescue analgesia (one cat at 2 h and one cat at 3 h after premedication). No cats in the buprenorphine group received rescue analgesia. There was no significant difference between treatment groups in requirement for rescue analgesia.
The IVAS pain scores for female cats are presented in Figure 4a (uncorrected for rescue analgesia) and 4b (corrected for rescue analgesia). Four cats were anaesthetised at the 90 mins assessment time point and were awarded a score of 0 mm. With the exception of the cats that were treated with rescue analgesia, pain scores were generally low in all cats throughout the assessment time period. When scores were uncorrected for rescue analgesia there was no significant main effect for treatment (P = 0.058); there was a significant main effect for time (P <0.001), but the interaction between time and treatment was significant (P = 0.0086). At the 3 h time point IVAS scores were significantly higher in the butorphanol group than the buprenorphine (P <0.0001) and methadone group (P <0.05), and significantly lower in the buprenorphine group than the methadone group (P <0.05). Pain scores were significantly lower in the butorphanol group than the methadone group 6 h after premedication (P <0.05). When scores were corrected for rescue analgesia there was a significant effect of treatment (P = 0.0082), time (P <0.0001) and a significant treatment × time interaction (P <0.0001). IVAS scores were significantly higher in the butorphanol group than the buprenorphine group at time points +3 h, +4 h, +5 h and +6 h. IVAS scores were significantly higher in the methadone group than the buprenorphine group at +3, +4 and +6 h, and scores were significantly higher in the butorphanol than the methadone group at +4 h.
Figure 4.
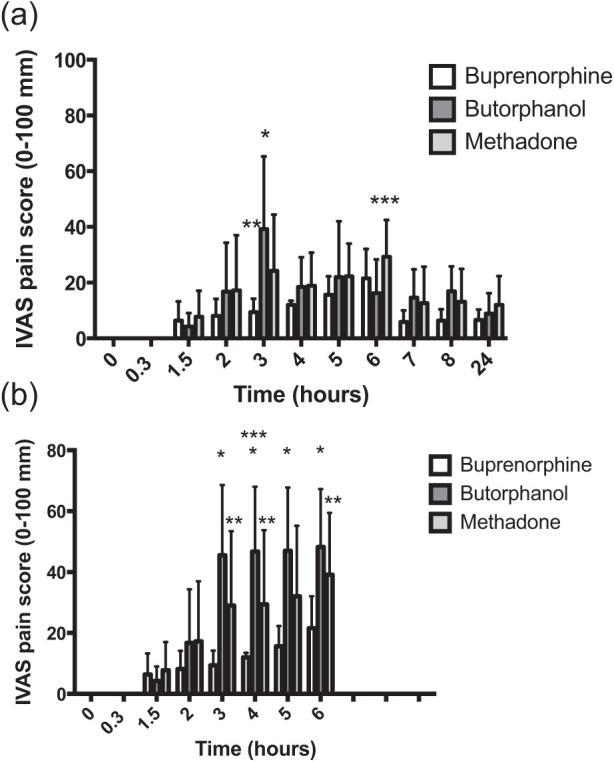
(a) Perioperative interactive visual analogue scale (IVAS) pain scores in females (mean ± SD) (n = 8 per group) when scores were uncorrected for rescue analgesia; the x-axis is the time from premedication and the y-axis is the IVAS pain score (0–100 mm). At the 3 h time point pain scores were significantly higher in the butorphanol group than the buprenorphine and methadone group (*P <0.05) and significantly lower in the buprenorphine group than the methadone group (**P <0.05). Pain scores were significantly lower in the methadone group than the butorphanol group 6 h after premedication (***P <0.05). (b) Perioperative IVAS pain scores in females (mean ± SD) (n = 8 per group) when scores were corrected (ie, held) after rescue analgesia; the x-axis is the time from premedication and the y-axis is the IVAS pain score (0–100 mm). Data are shown for the first 6 h after test drug administration only, as all cats received methadone after this time point. IVAS scores were significantly higher in the butorphanol group than the buprenorphine group (*) at time points +3 h, +4 h, +5 h (all P <0.0001) and +6 h (P <0.001). IVAS scores were significantly higher in the methadone group than the buprenorphine group (**) at +3, +4 and +6 h (P <0.05) and scores were significantly higher in the butorphanol than the methadone group (***) at +4 h (P <0.05)
The IVAS pain scores were low (<20 mm) throughout the observation period in all male cats (Figure 5); therefore, owing to the difficulty of discriminating meaningfully between groups when pain scores are very low, no statistical analyses were carried on IVAS scores in male cats.
Figure 5.
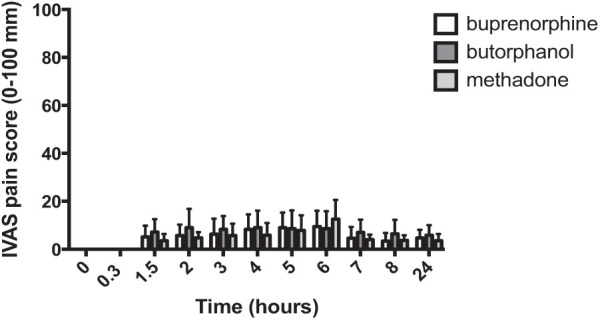
Perioperative interactive visual analogue scale (IVAS) pain scores in males (mean ± SD) (n = 7 per group); the x-axis is the time from premedication and the y-axis is the IVAS pain score (0–100 mm). No statistical analyses were carried out on these data because pain scores were low in all treatment groups after surgery
Mechanical nociceptive threshold
There were no significant differences between groups in baseline values, and the overall mean baseline in female cats was 3.3 ± 0.8 N and 1.7 ± 0.8 N in male cats. In female cats MNT increased after premedication and then decreased (Figure 6), indicative of hyperalgesia, for the 24 h after surgery, with no significant differences between treatment groups (P = 0.97), but a significant effect of time (P <0.001). MNT was statistically significantly lower than baseline in all treatment groups at all time points in the first 24 h after surgery (P <0.0001 for all comparisons). There was no significant interaction between treatment and time.
Figure 6.
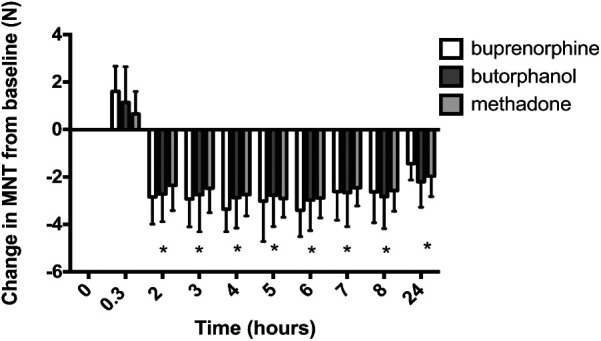
Mechanical nociceptive threshold (MNT) change in female cats (mean ± SD) (n = 8 per group), at baseline and postoperatively. The x-axis is the time from premedication (baseline) and the y-axis is the change in MNT, expressed in Newtons (N). MNT was significantly different to baseline (*) at all time points after surgery in all treatment groups with no significant differences between groups (P <0.0001 for all comparisons within treatment group compared to baseline)
The pattern of change of MNT in male cats appeared similar to female cats but changes within group relative to baseline were not significant after surgery (rather than after premedication) (Figure 7). There was no significant main effect for treatment (P = 0.23); there was a significant main effect for time (P <0.0001), but the interaction between time and treatment was significant (P = 0.04). At the 1.5 h time point MNT was significantly higher (positive) in the methadone group than the buprenorphine group (P <0.01), and at 2 h the MNT was significantly higher (positive) in the methadone group than in both the butorphanol and buprenorphine groups (P <0.05).
Figure 7.
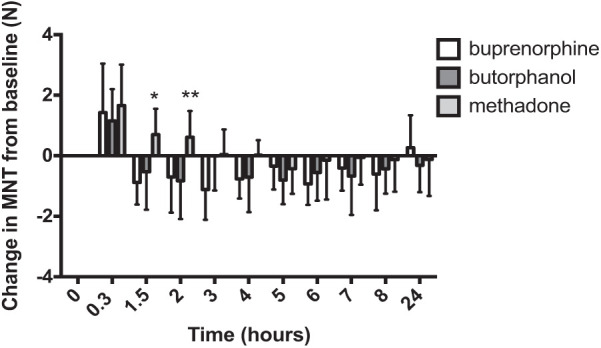
Mechanical nociceptive threshold (MNT) change in male cats (mean ± SD) (n = 7 per group), at baseline and postoperatively. The x-axis is the time from premedication (baseline) and the y-axis is the change in MNT, expressed in Newtons (N). There was no significant main effect for treatment. There was a significant main effect for time (P <0.0001), and the interaction between time and treatment was significant (P = 0.04). At the 1.5 h time point MNT was significantly higher (positive) in the methadone group than the buprenorphine group (*P <0.01) and at 2 h the MNT was significantly higher (positive) in the methadone group than in both the butorphanol and buprenorphine groups (**P <0.05). Within-group changes over time after surgery were not statistically significantly different compared with baseline
Adverse events
No adverse events such as salivation, vomiting or other gastrointestinal disturbances were noticed in any cats throughout the study.
Discussion
This is the first study to report the analgesic efficacy of methadone in combination with medetomidine for premedication prior to neutering in healthy cats. We hypothesised that cats treated with methadone would have lower pain scores and decreased mechanical hyperalgesia than cats treated with either buprenorphine or butorphanol. However, pain scores were low in the majority of female and male cats, suggesting that all opioids provided adequate analgesia after surgery. Pain scores in female cats were analysed in two different ways, with scores either uncorrected for rescue analgesia or corrected (held) after rescue analgesia, with differing results depending on the analysis. ‘Holding’ scores in individual cats after administration of rescue analgesia prevents ‘artificial’ lowering of the pain score by administration of the rescue treatment, and can increase the sensitivity of data analysis to discriminate between treatment groups, as evidenced in the present study. When scores were ‘held’, buprenorphine provided superior analgesia to butorphanol at all time points between 3 and 6 h after test drug administration, and superior analgesia to methadone at 3, 4 and 6 h after test drug administration. Conversely, when pain scores were not ‘held’ there were few statistically significant differences detected between groups. The exception was the early recovery period in female cats; at 3 h post-test drug administration, pain scores were highest in the butorphanol group and lowest in the buprenorphine group, with differences in pain score between cats receiving butorphanol, methadone and buprenorphine found to be statistically significant.
One possible explanation for the superiority of buprenorphine in the present study is the longer duration of action of buprenorphine compared with butorphanol and methadone. In experimental studies in cats, butorphanol is reported to provide a short duration of antinociception of up to 2 h.13–15 Data are conflicting about the duration of action of methadone in cats, dependent on dose and route of administration,10,11 but clinical studies suggest a duration of action of methadone of approximately 4–6 h.2,3,16 The duration of action of buprenorphine 20 µg/kg administered IM is considered to be 6–8 h.14,17,18 The postoperative analgesic effects of different opioids have been compared previously in cats undergoing neutering. In a very similar study, Bortolami et al 3 found no difference between postoperative analgesia provided by identical doses of methadone, butorphanol or buprenorphine to those used in the present study, administered with acepromazine, to cats undergoing neutering. Polson et al 12 also found no difference in postoperative analgesia provided by either butorphanol or buprenorphine administered in combination with midazolam, medetomidine and ketamine to cats under ovariohysterectomy. However, unlike in the present study when a non-steroidal anti-inflammatory drug (NSAID) was administered 8 h after surgery, Polson et al 12 administered an NSAID preoperatively to all cats, which may have reduced the likelihood of detecting differences in postoperative pain score, dependent on opioid administration. 19 In contrast, Warne et al 2 investigated postoperative analgesia in cats premedicated with acepromazine combined with either methadone or butorphanol and showed that significantly fewer cats treated with methadone required rescue analgesia than cats treated with butorphanol. Taylor et al 20 also detected a clinical difference in analgesic efficacy between buprenorphine and butorphanol in cats undergoing ovariohysterectomy, with buprenorphine providing better and longer lasting analgesia than butorphanol. Pharmacologically, it would be predicted that methadone, a full µ opioid agonist, 5 would provide better analgesia than buprenorphine, a partial µ opioid agonist, 21 which would provide better analgesia than butorphanol, a µ opioid antagonist and k opioid agonist. 22
The ability to discriminate between the analgesic efficacies of different opioids for management of postoperative pain depends on both the severity of the pain challenge and the sensitivity of the scoring system used to detect and quantify clinical pain, which may explain the disparity between the aforementioned studies. Although these studies all used ovariohysterectomy as a clinical pain model, it is likely that there were differences between studies in the skill of the surgeon and the technical approach used to carry out the surgery; therefore, the severity of pain challenge was unlikely to be consistent between them. Furthermore, each study used different assessors and different scoring tools to recognise and quantify pain.
In the present study a power analysis was conducted in order to calculate minimum group sizes necessary to detect a statistically significant difference, should one be present, in analgesic efficacy between treatment groups. The minimum number of 10 animals calculated per group was exceeded in the study but it must be acknowledged that half of the animals in each group were male cats undergoing castration. The low pain scores and absence of hyperalgesia after surgery in male cats indicates that castration is a poor surgical model to discriminate between the efficacy of different analgesic drugs and should be avoided in future studies. The number of cats undergoing ovariohysterectomy was less than 10 in each group, suggesting that the study may have been underpowered to detect differences between treatment groups when pain scores were uncorrected for the administration of rescue analgesia.
The preparation of methadone used in the present investigation was a racemic mixture of the L and D isomers of methadone. The D isomer exerts an antagonist action at the NMDA receptor and therefore methadone might be predicted to prevent or limit secondary mechanical hyperalgesia following tissue injury caused by surgery.23,24 Secondary mechanical hyperalgesia developed in all female cats and was not significantly different between groups. Although there were differences in MNT between treatment groups in male cats after surgery, changes in MNT relative to baseline were not significantly different within each treatment group, indicating that mechanical hyperalgesia did not develop in any treatment group in male cats after castration. Secondary mechanical hyperalgesia manifests clinically as increased sensitivity to pressure applied around a wound, and the fact that methadone did not prevent secondary mechanical hyperalgesia in female cats indicates the magnitude of any effect to prevent hyperalgesia is not clinically significant. Racemic methadone is ranked as the most potent NMDA receptor inhibiting opioid; 24 however, it is debatable whether any modulation of secondary hyperalgesia by methadone in the present investigation is attributable to NMDA receptor antagonist or µ receptor agonist effects. 25 Recent data suggest that methadone-induced antinociception is mediated only by µ receptor agonism in intact naïve rats and in rats with inflammatory pain induced by intraplantar injection of carrageenan.26,27 In contrast, in the formalin model, where there is abundant evidence to suggest that nociceptive responses involve NMDA receptors,28,29 intrathecal methadone dose dependently reduced flinching behaviour in phase 2 of the test and these effects were not blocked by spinal naloxone. 8 Although there is very good evidence that tissue trauma caused by surgery results in activation of NMDA receptors and that this is a key mechanism underpinning central sensitisation (for a review see Latremoliere and Woolf 30 ), the relative contribution of NMDA receptor antagonism and µ receptor agonism effects to the antinociceptive effects of methadone in the present study are unknown.
Medetomidine combined with all of the test opioids produced reliable sedation, so that IV catheter placement was easily achieved in the majority of cats, with no significant differences between treatment groups. This is in contrast to the poor sedation provided by acepromazine combined with identical doses of the test opioids in healthy cats prior to neutering in a similar study, and likely reflects the reliable sedation provided by 20 µg/kg medetomidine IM in cats. 31 Synergism is reported between opioids and alpha 2 agonists with respect to both sedation and analgesia. 32 Anecdotally, butorphanol is considered to provide better sedation when combined with medetomidine than buprenorphine, but there are no published data to support this contention in cats. The absence of control groups that received medetomidine or the test opioid only, and the likely profound sedative effects of medetomidine 20 µg/kg alone precluded detection of any differences in sedation between the three test opioids and medetomidine, 31 or determination of whether clinically significant synergism in the sedative effects occurred.
Conclusions
Medetomidine combined with methadone for premed-ication prior to neutering in healthy cats provided adeq-uate analgesia for the first 6 h after administration, with no adverse effects; effects overall were comparable with medetomidine combined with buprenorphine or butorp-hanol. Administration of further analgesia with meth-adone at 6 h and an NSAID at 8 h provided adequate analgesia for the first 24 h after surgery.
Supplemental Material
Appendix 1: Simple descriptive scale for sedation Appendix 2: Simple descriptive scale for ease of intravenous catheter placement after premedication Appendix 3: Simple descriptive scale for recovery after general anaesthesia
Footnotes
Supplementary material: Appendix 1: Simple descriptive scale for sedation
Appendix 2: Simple descriptive scale for ease of intravenous catheter placement after premedication
Appendix 3: Simple descriptive scale for recovery after general anaesthesia
The authors do not have any potential conflicts of interest to declare.
Funding: Funding for the study was provided by Eurovet Animal Health.
Accepted: 16 October 2014
References
- 1. Möllenhoff A, Nolte I, Kramer S. Anti-nociceptive efficacy of carprofen, levomethadone and buprenorphine for pain relief in cats following major orthopaedic surgery. J Vet Med A Physiol Pathol Clin Med 2005; 52: 186–198. [DOI] [PubMed] [Google Scholar]
- 2. Warne LN, Beths T, Holm M, et al. Comparison of perioperative analgesic efficacy between methadone and butorphanol in cats. J Am Vet Med Assoc 2013; 243: 844–850. [DOI] [PubMed] [Google Scholar]
- 3. Bortolami E, Murrell JC, Slingsby LS. Methadone in combination with acepromazine as premedication prior to neutering in the cat. Vet Anaesth Analg 2013; 40: 181–193. [DOI] [PubMed] [Google Scholar]
- 4. Holtman JR, Jr, Wala EP. Characterization of the antinociceptive and pronociceptive effects of methadone in rats. Anesthesiology 2007; 106: 563–571. [DOI] [PubMed] [Google Scholar]
- 5. Kristensen K, Christensen CB, Christrup LL. The mu1, mu2, delta, kappa opioid receptor binding profiles of methadone stereoisomers and morphine. Life Sci 1995; 56: PL45–PL50. [DOI] [PubMed] [Google Scholar]
- 6. Pollock AB, Tegeler ML, Morgan V, et al. Morphine to methadone conversion: an interpretation of published data. Am J Hosp Palliat Care 2011; 28: 135–140. [DOI] [PubMed] [Google Scholar]
- 7. Codd EE, Shank RP, Schupsky JJ, et al. Serotonin and norepinephrine uptake inhibiting activity of centrally acting analgesics: structural determinants and role in antinociception. J Pharmacol Exp Ther 1995; 274: 1263–1270. [PubMed] [Google Scholar]
- 8. Shimoyama N, Shimoyama M, Elliott KJ, et al. d-Methadone is antinociceptive in the rat formalin test. J Pharmacol Exp Ther 1997; 283: 648–652. [PubMed] [Google Scholar]
- 9. Davis AM, Inturrisi CE. d-Methadone blocks morphine tolerance and N-methyl-D-aspartate-induced hyperalgesia. J Pharmacol Exp Ther 1999; 289: 1048–1053. [PubMed] [Google Scholar]
- 10. Steagall PV, Carnicelli P, Taylor PM, et al. Effects of subcutaneous methadone, morphine, buprenorphine or saline on thermal and pressure thresholds in cats. J Vet Pharmacol Ther 2006; 29: 531–537. [DOI] [PubMed] [Google Scholar]
- 11. Ferreira TH, Rezende ML, Mama KR, et al. Plasma concentrations and behavioral, antinociceptive, and physiologic effects of methadone after intravenous and oral transmucosal administration in cats. Am J Vet Res 2011; 72: 764–771. [DOI] [PubMed] [Google Scholar]
- 12. Polson S, Taylor PM, Yates D. Analgesia after feline ovariohysterectomy under midazolam-medetomidine-ketamine anaesthesia with buprenorphine or butorphanol, and carprofen or meloxicam: a prospective, randomised clinical trial. J Feline Med Surg 2012; 14: 553–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sawyer DC, Rech RH. Analgesia and behavioral effects of butorphanol, nalbuphine, and pentazocine in the cat. J Am Animal Hosp Assoc 1987; 23: 438–446. [Google Scholar]
- 14. Robertson SA, Taylor PM, Lascelles BD, et al. Changes in thermal threshold response in eight cats after administration of buprenorphine, butorphanol and morphine. Vet Rec 2003; 153: 462–465. [DOI] [PubMed] [Google Scholar]
- 15. Lascelles BD, Robertson SA. Use of thermal threshold response to evaluate the antinociceptive effects of butorphanol in cats. Am J Vet Res 2004; 65: 1085–1089. [DOI] [PubMed] [Google Scholar]
- 16. Rohrer Bley C, Neiger-Aeschbacher G, Busato A, et al. Comparison of perioperative racemic methadone, levo-methadone and dextromoramide in cats using indicators of post-operative pain. Vet Anaesth Analg 2004; 31: 175–182. [DOI] [PubMed] [Google Scholar]
- 17. Robertson SA, Lascelles BD, Taylor PM, et al. PK-PD modeling of buprenorphine in cats: intravenous and oral transmucosal administration. J Vet Pharmacol Ther 2005; 28: 453–460. [DOI] [PubMed] [Google Scholar]
- 18. Steagall PV, Taylor PM, Brondani JT, et al. Effects of buprenorphine, carprofen and saline on thermal and mechanical nociceptive thresholds in cats. Vet Anaesth Analg 2007; 34: 344–350. [DOI] [PubMed] [Google Scholar]
- 19. Staffieri F, Centonze P, Gigante G, et al. Comparison of the analgesic effects of robenacoxib, buprenorphine and their combination in cats after ovariohysterectomy. Vet J 2013; 197: 363–367. [DOI] [PubMed] [Google Scholar]
- 20. Taylor PM, Kirby JJ, Robinson C, et al. A prospective multi-centre clinical trial to compare buprenorphine and butorphanol for postoperative analgesia in cats. J Feline Med Surg 2010; 12: 247–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Raffa RB, Ding Z. Examination of the preclinical antinociceptive efficacy of buprenorphine and its designation as full- or partial-agonist. Acute Pain 2007; 9: 145–152. [Google Scholar]
- 22. Hoskin PJ, Hanks GW. Opioid agonist-antagonist drugs in acute and chronic pain states. Drugs 1991; 41: 326–344. [DOI] [PubMed] [Google Scholar]
- 23. Callahan RJ, Au JD, Paul M, et al. Functional inhibition by methadone of N-methyl-D-aspartate receptors expressed in Xenopus oocytes: stereospecific and subunit effects. Anesth Analg 2004; 98: 653–659. [DOI] [PubMed] [Google Scholar]
- 24. Inturrisi CE. Pharmacology of methadone and its isomers. Minerva Anestesiol 2005; 71: 435–437. [PubMed] [Google Scholar]
- 25. Sotgiu ML, Valente M, Storchi R, et al. Cooperative N-methyl-D-aspartate (NMDA) receptor antagonism and mu-opioid receptor agonism mediate the methadone inhibition of the spinal neuron pain-related hyperactivity in a rat model of neuropathic pain. Pharmacol Res 2009; 60: 284–290. [DOI] [PubMed] [Google Scholar]
- 26. Carpenter KJ, Chapman V, Dickenson AH. Neuronal inhibitory effects of methadone are predominantly opioid receptor mediated in the rat spinal cord in vivo. Eur J Pain 2000; 4: 19–26. [DOI] [PubMed] [Google Scholar]
- 27. Chizh BA, Schlütz H, Scheede M, et al. The N-methyl-D-aspartate antagonistic and opioid components of d-methadone antinociception in the rat spinal cord. Neurosci Lett 2000; 296: 117–120. [DOI] [PubMed] [Google Scholar]
- 28. Coderre TJ, Melzack R. The role of NMDA receptor-operated calcium channels in persistent nociception after formalin-induced tissue injury. J Neurosci 1992; 12: 3671–3675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Yamamoto T, Yaksh TL. Comparison of the antinociceptive effects of pre- and posttreatment with intrathecal morphine and MK801, an NMDA antagonist, on the formalin test in the rat. Anesthesiology 1992; 77: 757–763. [DOI] [PubMed] [Google Scholar]
- 30. Latremoliere A, Woolf CJ. Central sensitization: a generator of pain hypersensitivity by central neural plasticity. J Pain 2009; 10: 895–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lamont LA, Bulmer BJ, Grimm KA, et al. Cardiopulmonary evaluation of the use of medetomidine hydrochloride in cats. Am J Vet Res 2001; 62: 1745–1749. [DOI] [PubMed] [Google Scholar]
- 32. Ossipov MH, Suarez LJ, Spaulding TC. Antinociceptive interactions between alpha 2-adrenergic and opiate agonists at the spinal level in rodents. Anesth Analg 1989; 68: 194–200. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix 1: Simple descriptive scale for sedation Appendix 2: Simple descriptive scale for ease of intravenous catheter placement after premedication Appendix 3: Simple descriptive scale for recovery after general anaesthesia


