Abstract
Objectives
For the purpose of applying a barometric whole-body plethysmography (BWBP) device as a routine clinical tool in client-owned cats, the objective of this study was to evaluate the methodological importance of simultaneous visual inspection (SVI) of graphic tracing.
Methods
To investigate the effect of SVI on the results obtained, 50 client-owned cats were included. Breath-by-breath analysis was conducted with BWBP software, and a commonly used rejection setting was chosen for automatic elimination (AE) of non-breath artefactual waveforms, according to tidal volume (TV), inspiratory and expiratory time, and the difference between inspiratory and expiratory volumes. During 10 mins of data recording, SVI for BWBP waveforms was performed to record manually time periods that were free of any artefacts. The two datasets derived from AE alone (AEA method) and AE plus SVI (SVI-AE method) were compared. The inter-observer effect on the process of SVI was evaluated on six cats.
Results
There were statistically significant differences (P <0.001) between the AEA and SVI-AE datasets for most BWBP parameters. Bland–Altman analysis of the parameter-enhanced pause (Penh) showed heterogeneous variances, indicating less agreement when the Penh values were large. Intra-individual coefficients of variation of Penh were significantly higher with the AEA method than with the SVI-AE method (61.1% vs 34.7%, respectively; P <0.001). Inter-observer agreement on the SVI process was excellent, and no statistically significant differences between the two observers were found for any BWBP parameters obtained by the SVI-AE method (P >0.05).
Conclusions and relevance
Visual inspection for BWBP waveforms in real time can reliably identify stable breathing signals in client-owned cats. The obtained results were significantly different when the SVI method was used in addition to AE. In the interpretation of BWBP parameters or comparison of measurements among studies, whether an SVI methodology was applied should be considered.
Introduction
Pulmonary function testing (PFT) can provide important information regarding the presence of an obstructive airway problem, disease progression and therapeutic response in various respiratory diseases. Multiple techniques can to be used for different purposes in evaluating lung function, and active coaching is usually a necessary part of most tests in human medicine to obtain acceptable and meaningful data. 1 However, manoeuvres that require cooperation and maximal effort cannot be applied to veterinary patients. Alternative methodologies have been developed for respiratory function assessment in small animals, 2 and barometric whole-body plethysmography (BWBP) has, over the past decade, become a popular non-invasive system for feline pulmonary function studies.3–13
A BWBP system is a non-invasive tool that allows dynamic assessment of ventilation. In a BWBP system, a cat is placed into a standardised BWBP chamber and breathes normally. The airflow at the nasal opening and thoracic movement of the cat cause pressure changes inside the chamber, and a difference between the volume of nasal airflow and thoracic displacement is generated when the air is warmed and humidified in the chest.2,3,14 The net result of the pressure change creates the BWBP signal. As this signal is not simply a measure of direct airflow at the nasal opening, the term ‘box flow’ or ‘pseudoflow’ is commonly used. 15 With the use of a pneumotachograph on the chamber wall and a differential pressure transducer, BWBP signals are detected, amplified and recorded electronically. Conventional BWBP parameters under tidal breathing, including pseudoflow, pseudovolume and time-related variables, can be obtained using commercial software and used to assess pulmonary function.2,15 Airway reactivity assessment with bronchoprovocative stimulation can also be measured with a BWBP system in conscious cats, using a BWBP parameter-enhanced pause (Penh) as the surrogate index for bronchoconstriction.3,4,6,7,9,12
The BWBP system might be especially suitable for use in cats because no manipulation is needed while the cats are in the test chamber, allowing the cats to be non-restrained and relaxed during the process. However, artefactual waveforms caused by non-breath movements or vocalisations of the cats could affect the analysis of true breathing. Non-breath artefactual waveforms are usually reported to be eliminated by the rejection setting of the software when the tidal volume (TV), the difference between inspiratory and expiratory volume, or the inspiratory or expiratory time (Ti or Te) is smaller or greater than a certain value.3–8
In a human clinical pulmonary laboratory it is essential to visualise the graphical tracing in real time for inspection of manoeuvre acceptance and data adequacy.16,17 However, the importance of visual inspection has not been extensively emphasised in feline pulmonary function studies with the BWBP method. Simultaneous visual inspection (SVI) of BWBP waveforms, and the recognition and manual elimination of artefactual waveforms that are not automatically detected by the software, have been described in one study with healthy dogs, 18 while many studies in cats have reported that disturbed waveforms were automatically eliminated by the software under similar rejection criteria (when TV <5–10 ml, when Ti <0.15 s or >10 s, or when the difference between inspiratory and expiratory volumes exceeds 20%).5,6,8,19,20 Although the BWBP system has been applied in clinical studies using client-owned cats,9–13,21 it is not known whether the commonly used rejection criteria are sufficient for excluding most non-breath artefacts, which may affect the final outcome in these clinical cases. The present study was designed to evaluate the effect of SVI on BWBP waveforms on the results obtained from the examinations. We hypothesised that as the automatic elimination (AE) setting of the BWBP software (AEA method) could identify most artefactual waveforms in client-owned cat populations, the datasets produced with the AEA method and the SVI-AE method (AE plus SVI for waveforms) should not be significantly different.
Materials and methods
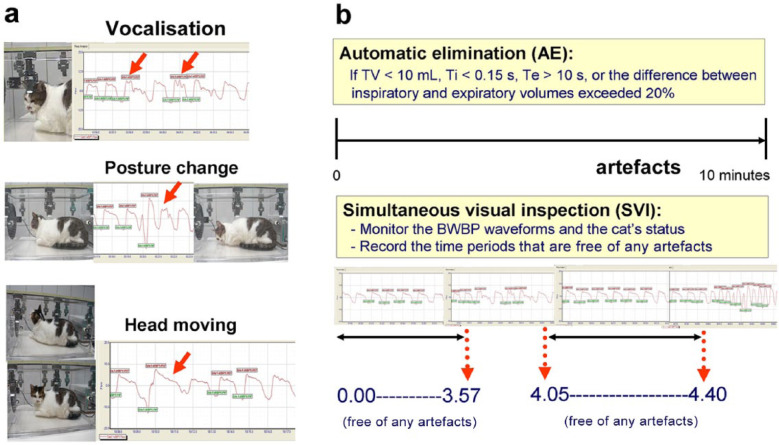
Client-owned cats undergoing PFT with the BWBP method for any reason were included in the study. The transparent Plexiglas chamber of the BWBP system (Buxco Electronics) has a height of 25 cm, a length of 51 cm and a width of 30 cm. The system was calibrated according to the manufacturer’s instructions before use by injecting 50 ml of air into the chamber. The cat was placed in the chamber, which was in a quiet room with the owner for company, and an acclimation period of 1–10 mins was allowed for cats that were not immediately stable upon placement, depending on each cat’s condition. A bias flow of 6 l/min was provided throughout the examination. The analogue signals from a differential pressure transducer were amplified and digitised, with a sampling rate of 100 Hz. Box flow data were collected for 10 mins for each cat. Breath-by-breath analysis and conventional parameters were calculated and reported by the BWBP software (BioSystem XA 2.11.0 software; Buxco Electronics) as previously described,3–5 including respiratory rate (RR [breaths per minute]), TV (ml), minute volume (MV [ml]), Ti and Te (s), peak inspiratory (PIF) and expiratory flow (PEF [ml/s]), relaxation time (RT [s]; the time point when 65% of TV is expired), pause (PAU [unitless]; [Te – RT]/RT), and Penh (unitless; [PEF/PIF] × PAU]. AE of non-breath artefactual waveforms was processed by the rejection setting of the BWBP software. Waveforms were rejected if TV was <10 ml, Ti was <0.15 s, Te was >10 s, or the difference between inspiratory and expiratory volumes exceeded 20%. BWBP parameters were obtained by directly exporting data to a Microsoft Excel file, representing the dataset generated by the AEA method. SVI was performed by the same observer for the entire recording period. The time scale was zeroed before each recording, and multiple time periods that were free of any artefactual waveforms (eg, vocalisations, posture changes, sniffing, body movements, etc) were recorded manually (Figure 1). A second dataset was formed by manually excluding results from the first dataset that did not fall in the manually recorded free-of-artefacts time periods, representing the dataset generated by the SVI-AE method. The two datasets were then stored for subsequent analysis. Extremely uncooperative individuals were excluded; ie, if the cat could not tolerate being placed in the chamber for 10 mins or if <50 acceptable breaths could be obtained in 10 mins.
Figure 1.
(a) How non-breath movements affect the barometric whole-body plethysmography (BWBP) waveforms. The little boxes displayed around the graphic tracing are set in the software to indicate the parameters that the BWBP software calculates. Red and green boxes represent the parameters of peak expiratory and inspiratory flow being calculated, respectively. It was noted that waveforms disturbed by vocalisations and movement (red arrows) were still included in the calculation of outcome variables. (b) The simultaneous visual inspection (SVI) methodology. With real-time visual inspection for signals and the cat’s status, multiple time periods that were free of artefacts were manually recorded. TV = tidal volume
Six cats were used to test the inter-observer effect on the process of visual inspection. During data recording for each case, two observers were responsible for SVI at the same time and separately recorded time periods that were free of artefactual waveforms. The datasets generated by the two observers (C-H L and P-Y L) using the SVI-AE method were used for evaluating inter-observer variation.
Statistical analysis
The Shapiro–Wilk test was used to examine Gaussian distribution. Descriptive statistics were used to present the data as mean ± SD or median with interquartile range (IQR) for normally distributed or non-parametric data, respectively. The paired t-test or Wilcoxon signed rank test was used to compare the differences between datasets generated by the AEA and SVI-AE methods. The relationships of the results of the two datasets were further examined with Bland–Altman plots. Intra-individual coefficients of variation (CV) between the AEA and SVI-AE methods were compared with the Wilcoxon signed rank test. Inter-observer agreement of the SVI process was estimated with the intraclass correlation coefficient (ICC). 10 Differences between data processed by the two observers were tested with the Wilcoxon signed rank test. Commercial software (SPSS Statistics 19.0.0; IBM) was used for statistical analyses, and the level of significance was defined as P <0.05, except when Bonferroni correction was applied on multiple comparisons.
Results
Fifty client-owned cats were included in the final analysis. The 50 cats included 35 domestic shorthairs, four Persians, three American Shorthair, two domestic longhairs, two Russian Blues, two Persian crosses, one Himalayan and one Siamese-crossed cat. There were 27 male and 23 female cats, with a mean ± SD age of 7.0 ± 3.8 years. Mean body weight and median nine-point body condition score were 4.9 ± 1.1 kg and 6 (IQR 5–7), respectively. Thirty-four cats had untreated various respiratory diseases, 10 had treated and stable respiratory diseases and six healthy cats presented for routine health check. Most of the cats (45/50) received PFT with the BWBP method for the first time as part of this study.
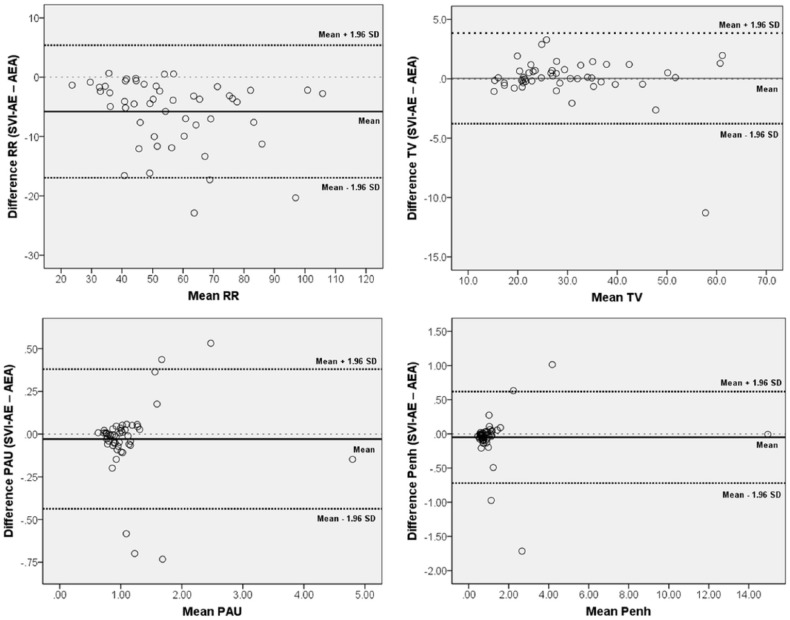
There were significant differences between the datasets obtained for the AEA and SVI-AE methods with regard to the results of the BWBP parameters. With the use of Bonferroni correction, most parameters were statistically different between the two datasets, including RR (P <0.001), MV (P <0.001), Ti (P <0.001), Te (P <0.001), PIF (P <0.001), PEF (P <0.001) and RT (P <0.001) (Table 1). On Bland–Altman analysis, the mean biases (and limits of agreement) of PAU and Penh between the two datasets were −0.029 (0.38–0.44) and −0.050 (0.62–0.72), respectively. The Bland–Altman plot for PAU and Penh revealed heterogeneous variances (Figure 2). Intra-individual CVs were significantly lower with the SVI-AE method than with the AEA method (P <0.001) (Table 2).
Table 1.
Results of barometric whole-body plethysmography (BWBP) measurements with automatic elimination (AE) alone (AEA method) and with AE plus simultaneous visual inspection (SVI) (SVI-AE method)
| BWBP parameters | AEA method | SVI-AE method |
|---|---|---|
| RR (breaths per minute)* | 56 (44–73) | 50 (40–62) |
| TV (ml) | 26.7 (21.2–35.0) | 27.2 (21.1–35.1) |
| MV (ml)* | 1473.7 (1281.7–1658.9) | 1401.5 (1191.8–1599.9) |
| Ti (s)* | 0.47 (0.37–0.62) | 0.51 (0.40–0.63) |
| Te (s)* | 0.72 (0.52–0.85) | 0.74 (0.57–0.94) |
| PIF (ml/s)* | 82.4 (76.2–100.3) | 79.7 (70.3–94.1) |
| PEF (ml/s)* | 71.2 (61.5–93.0) | 66.2 (55.6–80.7) |
| RT (s)* | 0.35 ± 0.11 | 0.37 ± 0.11 |
| PAU | 1.00 (0.85–1.18) | 0.93 (0.80–1.13) |
| Penh † | 0.81 (0.68–1.09) | 0.77 (0.63–1.09) |
Data are presented as mean ± SD or median with interquartile range (IQR)
Statistically significant differences:
P <0.05
P <0.005 (based on Bonferroni correction)
RR = respiratory rate; TV = tidal volume; MV = minute volume; Ti = inspiratory time; Te = expiratory time; PIF = peak inspiratory flow; PEF = peak expiratory flow; RT = relaxation time (the time point when 65% of tidal volume is expired); PAU = pause, a unitless parameter ([Te – RT/RT]); Penh = enhanced pause, a unitless parameter [PEF/PIF] × PAU)
Figure 2.
Bland–Altman plots of respiratory rate (RR), tidal volume (TV), pause (PAU) and enhanced pause (Penh). The mean differences between data obtained from the simultaneous visual inspection plus automatic elimination (SVI-AE) and automatic elimination alone (AEA) methods are shown in each plot. Limits of agreement are the mean ± 1.96 SD
Table 2.
Intra-individual coefficients of variation (CV) with the automatic elimination (AEA) and simultaneous visual inspection plus automatic elimination (SVI-AE) methods. Data are presented as median (interquartile range)
| BWBP parameters | Intra-individual CV of the AEA method (%) | Intra-individual CV of the SVI-AE method (%) |
|---|---|---|
| RR* | 24.9 (20.1–34.5) | 12.6 (9.7–15.2) |
| TV* | 24.7 (18.5–34.8) | 13.9 (10.8–15.4) |
| Penh* | 61.1 (43.5–89.9) | 34.7 (28.2–44.9) |
P <0.001
BWBP = barometric whole-body plethysmography; RR = respiratory rate; TV = tidal volume; Penh = enhanced pause, a unitless parameter ([peak expiratory flow/peak inspiratory flow] × pause)
All ICC values were >0.8, indicating excellent agreement for inter-observer variation on the SVI process, ranging from 0.858 (PAU) to 0.998 (Ti). There were no statistical differences with regard to any BWBP parameters obtained with the SVI-AE method by the two observers (P >0.05).
Discussion
The null hypothesis of the present study was rejected. The results of the BWBP measurements obtained with the AEA method were significantly different from those obtained with the SVI-AE method. The commonly used rejection setting in previous feline BWBP studies was unable to eliminate all artefactual waveforms during data recording. Visual inspection of the waveforms facilitated the identification of artefacts not excluded by the software, allowed the recording of stable tidal breathing signals, and showed good inter-observer agreement. The results of this study suggest that whether SVI is applied or not could result in significantly different measured outcomes in client-owned cats, a factor that should be kept in mind when comparing studies using BWBP measurements.
Cats are usually allowed to acclimate to the BWBP chamber for several minutes before each recording.3,11,12,19 However, these efforts may not prevent cats from moving during data recording; an unrestrained cat will naturally change postures, stretch or even groom, especially when not under stress. In a previous study, cats were familiarised with the chamber for a period of time prior to recording trials, 5 which is possible with experimental cats but less practical for most client-owned cats. For the purpose of applying the BWBP device as a routine clinical tool to highly variable client-owned cats, it is important to consider the methodology used to eliminate non-breath artefacts.
The differences in the results obtained with the two methodologies, one with and one without SVI, were significant for most of the BWBP parameters. In the current study, RR and MV tended to be higher in the AEA dataset than in the SVI-AE dataset. It is postulated that non-breath waveforms were included in the analysis. Because artefacts cannot always be identified by the currently used rejection setting of the BWBP software, the RR calculation and subsequent values of MV are inflated. Lower Ti, Te and RT were noted in the AEA dataset, possibly because the body movements that were falsely included in the parameter calculations were mostly short in duration. Visual inspection revealed that non-breath body movements frequently caused large amplitudes on BWBP box flow waveforms. These larger amplitudes may explain why parameters such as PIF and PEF had more extreme values. However, the results of TV were not significantly different between the two datasets, and Bland–Altman analysis showed acceptable agreement. In an evaluation of different curve selection methods of tidal breathing analysis in human children, TV was the only parameter that lacked significant differences between methods of unselected curves vs selection by eye. 22 The measured values of TV do not appear to be significantly influenced by the methodology used.
BWBP measurements in feline PFT studies are usually reported as averaged values of breath-by-breath data obtained over 5–12 mins of recording;3,7,11,12 however, the inclusion of artefactual waveforms of large amplitude could dramatically shift the results obtained. On Bland–Altman analysis, most of the mean biases were not severe, but the limits of agreement were often too large to be acceptable. This indicates that the failure to recognise artefactual waveforms with the AEA method leads to the inclusion of extreme outliers in the obtained data, which significantly change the outcome. This finding is also supported by the significantly higher intra-individual CVs obtained with the AEA method than with the SVI-AE method. Furthermore, heterogeneous variance of Penh was noted on the Bland–Altman plot. When the Penh values were large, less agreement would be expected between the two methods. This finding implies that care should be taken during the evaluation of cats with severe bronchoconstriction or while performing a bronchoprovocation test when a high Penh value is expected under such conditions.
Real-time visual inspection for signals in addition to the use of a computer program could be easily and reliably applied to client-owned cats receiving PFT with the BWBP method. Although our results might suggest that the criteria for computed rejection setting should be modified, the development of stricter criteria was not a focus of this study. It is impractical to expect that a single setting could be appropriate for use in patients with various respiratory conditions or levels of stability, particularly because tidal breathing itself is a complex phenomenon and affected by many factors. 16 Although a computer program can help to reduce selection bias during analysis, 22 visual detection of signals to ensure a stable and regular respiratory pattern is essential when tidal breathing analysis is applied as a measure of respiratory function in human children.16,22 Moreover, it was shown that selection bias did not contribute to the methodological differences in the present study – the agreement on inter-observer variation on the SVI process was excellent. There were no statistically significant differences between the data obtained from the two observers.
The limitations of this study should be noted. Because no gold-standard method was available for comparison of the accuracy of the data generated by the AEA and SVI-AE methods, the aim of the present study was not to conclude that one method had superior accuracy, but to focus on the important effect of visual inspection on the results obtained. For functional studies relying on absolute values of BWBP parameters, especially in non-experimental cats, it should be taken into consideration that the methodology used in an individual study may change the outcome measurements. Another limitation was that the minimum duration of BWBP data recording necessary in order to obtain representative data was not clear, but it is certainly an interesting issue deserving a further study. Finally, only a few healthy cats were included in this study. It was unknown whether the difference between both the AEA and SVI-AE method impacted more or less on BWBP results in healthy or diseased cats.
Conclusions
Visual inspection of waveforms in real time can reliably identify stable tidal breathing signals in client-owned cats evaluated with the BWBP method. Measurements obtained with and without the SVI method could be significantly different. Whether a visual inspection methodology was used should be taken into consideration during the interpretation of BWBP results or comparison of measurements among studies. SVI should be routinely performed on all cats receiving PFT with the BWBP method in order to achieve methodological consistency and to reduce variability caused by non-breath artefacts.
Footnotes
Part of the study was presented in the format of an oral presentation at the 31st Veterinary Comparative Respiratory Society symposium, July 15–17, 2013, Calgary, Alberta, Canada.
The authors do not have any potential conflicts of interest to declare.
Funding: This research received no specific grant from any funding agency in the public, commercial or non-for-profit sectors.
Accepted: 1 June 2015
References
- 1. Culver BH. Pulmonary function testing. In: Spiro SG, Silvestri GA, Agustí A. (eds). Clinical respiratory medicine. 4th ed. Philadelphia, PA: WB Saunders, 2012, pp 133–142. [Google Scholar]
- 2. Rozanski EA, Hoffman AM. Pulmonary function testing in small animals. Clin Tech Small Anim Pract 1999; 14: 237–241. [DOI] [PubMed] [Google Scholar]
- 3. Hoffman AM, Dhupa N, Cimetti L. Airway reactivity measured by barometric whole-body plethysmography in healthy cats. Am J Vet Res 1999; 60: 1487–1492. [PubMed] [Google Scholar]
- 4. Hirt RA, Dederichs D, Boehler A, et al. Relationship of age, sex, body weight, and hematologic and respiratory variables with airway reactivity in adult cats. Am J Vet Res 2003; 64: 26–31. [DOI] [PubMed] [Google Scholar]
- 5. Kirschvink N, Leemans J, Delvaux F, et al. Non-invasive assessment of growth, gender and time of day related changes of respiratory pattern in healthy cats by use of barometric whole body plethysmography. Vet J 2006; 172: 446–454. [DOI] [PubMed] [Google Scholar]
- 6. Kirschvink N, Leemans J, Delvaux F, et al. Inhaled fluticasone reduces bronchial responsiveness and airway inflammation in cats with mild chronic bronchitis. J Feline Med Surg 2006; 8: 45–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kirschvink N, Leemans J, Delvaux F, et al. Non-invasive assessment of airway responsiveness in healthy and allergen-sensitised cats by use of barometric whole body plethysmography. Vet J 2007; 173: 343–352. [DOI] [PubMed] [Google Scholar]
- 8. Kirschvink N, Leemans J, Delvaux F, et al. Bronchodilators in bronchoscopy-induced airflow limitation in allergen-sensitized cats. J Vet Intern Med 2005; 19: 161–167. [DOI] [PubMed] [Google Scholar]
- 9. Allerton FJ, Leemans J, Tual C, et al. Correlation of bronchoalveolar eosinophilic percentage with airway responsiveness in cats with chronic bronchial disease. J Small Anim Pract 2013; 54: 258–264. [DOI] [PubMed] [Google Scholar]
- 10. Lin CH, Lee JJ, Liu CH. Functional assessment of expiratory flow pattern in feline lower airway disease. J Feline Med Surg 2014; 16: 616–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Garcia-Guasch L, Caro-Vadillo A, Manubens-Grau J, et al. Pulmonary function in obese vs non-obese cats. J Feline Med Surg 2015; 17: 494–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hirt RA, Galler A, Shibly S, et al. Airway hyperresponsiveness to adenosine 5’-monophosphate in feline chronic inflammatory lower airway disease. Vet J 2011; 187: 54–59. [DOI] [PubMed] [Google Scholar]
- 13. Lin CH, Wu HD, Lee JJ, et al. Functional phenotype and its correlation with therapeutic response and inflammatory type of bronchoalveolar lavage fluid in feline lower airway disease. J Vet Intern Med 2015; 29: 88–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lomask M. Further exploration of the Penh parameter. Exp Toxicol Pathol 2006; 57: 13–20. [DOI] [PubMed] [Google Scholar]
- 15. Kirschvink N. Barometric whole body plethysmography and enhanced pause (PENH): how relevant are they? Vet J 2008; 176: 125–126. [DOI] [PubMed] [Google Scholar]
- 16. Beydon N, Davis SD, Lombardi E, et al. An official American Thoracic Society/European Respiratory Society statement: pulmonary function testing in preschool children. Am J Respir Crit Care Med 2007; 175: 1304–1345. [DOI] [PubMed] [Google Scholar]
- 17. Miller MR, Crapo R, Hankinson J, et al. General considerations for lung function testing. Eur Respir J 2005; 26: 153–161. [DOI] [PubMed] [Google Scholar]
- 18. Talavera J, Kirschvink N, Schuller S, et al. Evaluation of respiratory function by barometric whole-body plethysmography in healthy dogs. Vet J 2006; 172: 67–77. [DOI] [PubMed] [Google Scholar]
- 19. Leemans J, Kirschvink N, Bernaerts F, et al. A pilot study comparing the antispasmodic effects of inhaled salmeterol, salbutamol and ipratropium bromide using different aerosol devices on muscarinic bronchoconstriction in healthy cats. Vet J 2009; 180: 236–245. [DOI] [PubMed] [Google Scholar]
- 20. Leemans J, Kirschvink N, Clercx C, et al. Functional response to inhaled salbutamol and/or ipratropium bromide in Ascaris suum-sensitised cats with allergen-induced bronchospasms. Vet J 2010; 186: 76–83. [DOI] [PubMed] [Google Scholar]
- 21. Galler A, Shibly S, Bilek A, et al. Inhaled budesonide therapy in cats with naturally occurring chronic bronchial disease (feline asthma and chronic bronchitis). J Small Anim Pract 2013; 54: 531–536. [DOI] [PubMed] [Google Scholar]
- 22. van der Ent CK, Brackel HJ, Mulder P, et al. Improvement of tidal breathing pattern analysis in children with asthma by on-line automatic data processing. Eur Respir J 1996; 9: 1306–1313. [DOI] [PubMed] [Google Scholar]