Abstract
Five cats presented with acute-onset neurological signs. Magnetic resonance imaging in four cats showed a T2-weighted hyperintense spinal cord lesion that was mildly contrast-enhancing in three cats. Owing to inflammatory cerebrospinal fluid changes three cats were treated with immunosuppression. One cat was treated with antibiotics. All cats improved initially, but were eventually euthanased owing to the recurrence of neurological signs. Histopathology in all cats showed hyaline degeneration of the ventral spinal artery, basilar artery or associated branches with aneurysmal dilation, thrombosis and ischemic degeneration and necrosis of the spinal cord and brain. Two cats also had similar vascular changes in meningeal vessels. Vascular hyaline degeneration resulting in vascular aneurysmal dilation and thrombosis should be a differential diagnosis in cats presenting with acute central nervous system signs.
Ischemic myelopathy (IM) is a consequence of spinal cord vascular occlusion and resultant ischemic necrosis of the territory supplied. 1 Fibrocartilaginous embolic myelopathy (FCEM) is the most common cause of IM in dogs and cats,2,3 but vascular occlusion can also result from thromboembolization due to parasites, sepsis, vasculopathy or hypercoagulable states.4,5 IM has characteristic clinical and magnetic resonance imaging (MRI) features, but histopathology is required for a definitive diagnosis.2,5,6 This report describes five cats with IM and encephalopathy resulting from thrombosis secondary to hyaline arteriopathy.
Clinical presentation is shown in Table 1.
Table 1.
Summary of clinical findings in five cats with hyaline degeneration
| Signalment | Neuroanatomic diagnosis | MRI findings | CSF | Treatment | Outcome | Underlying disease | |
|---|---|---|---|---|---|---|---|
| Cat 1 | 15-year-old FS DSH | C1–C5 myelopathy | T2W hyperintense T1 isointense Non-contrast-enhancing |
TP 67 mg/dl TNCC 60/µl |
Prednisone Cytarabine (intrathecal) Lomustine Methotextrate (intrathecal) |
Euthanasia after 14 weeks | No |
| Cat 2 | 11-year-old MN DSH | Multifocal CNS | T2W, FLAIR hyperintense Mild contrast-enhancing lesion C2 |
TP 49 mg/dl TNCC 10/µl |
Prednisone cyclosporine | Euthanasia after 11 months | Mild HCM |
| Cat 3 | 12-year-old DSH | C1–C5 myelopathy | C2 T2W hyperintense poorly contrast-enhancing C4 T2W hyperintense non-contrast-enhancing |
TP 76 mg/dl TNCC 1179/µl |
Dexamethasone | Euthanasia after 22 months | No |
| Cat 4 | 16-year-old FS Maine Coon | C1–C5 myelopathy | C3 T2W hyperintense poorly contrast-enhancing | TP 184 mg/dl TNCC 150/µl |
Marbofloxacin furosemide | Euthanasia after 18 months | HCM hyperthyroidism |
| Cat 5 | 18-year-old MN Persian | T3–L3 myelopathy | N/A | N/A | N/A | Euthanased | HCM Moderate chronic renal disease |
CSF = cerebrospinal fluid; FS = female spayed; DSH = domestic shorthair; MN = male neutered; CNS = central nervous system; T2W = T2-weighted; FLAIR = fluid-attenuated inversion recovery; N/A = not applicable; TP = total protein; TNCC = total nucleated cell count; HCM = hypertrophic cardiomyopathy
Cat 1
A 15-year-old female spayed domestic shorthair (DSH) cat presented for acute, non-ambulatory tetraparesis. Physical examination was unremarkable. Neurological examination suggested C1–C5 myelopathy.
Complete blood count (CBC) and serum biochemistry results were unremarkable. Abdominal ultrasonography revealed small kidneys displaying reduced corticomedullary definition. Urine specific gravity (USG) was 1.033. MRI (0.4T; Hitachi Medical Systems) revealed C2–C3 spinal cord swelling with diffuse hyperintensity on T2-weighted (T2W) and fluid-attenuated inversion recovery (FLAIR) sequences, isointense T1-weighted (T1W) sequences and mild contrast enhancement (Dotarem, gadoteric acid 0.5 mmol/ml) (Figure 1). Cisternal cerebrospinal fluid (CSF) analysis revealed increased protein concentration (67, reference interval [RI] <25 mg/dl) and total nucleated cell count (TNCC) (60, RI <3/µl) with neutrophilic pleocytosis (67% neutrophils). Real-time reverse transcriptase polymerase chain reaction (qRT-PCR) on CSF for infectious agents (Scanelis) (coronavirus, feline leukemia virus, feline immunodeficiency virus, feline herpes virus, calicivirus, Bordetella speceis, Chlamydophilia species, Toxoplasma species and Leishmania species) and serological testing for Toxoplasma gondii immunoglobulin (Ig)M/IgG were negative.
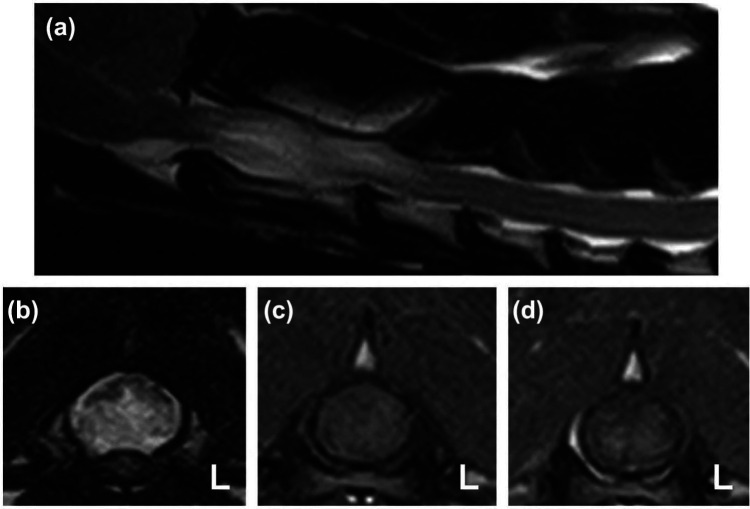
Figure 1.
T2-weighted (T2W) sagittal (a) and transverse (b) images of the cranial cervical spinal cord of cat 1 showing marked spinal cord swelling at the level of C2. The transverse T2W image reveals the ventral half of the spinal cord to be diffusely hyperintense with a relatively sharp distinction to the dorsal spinal cord. T1-weighted pre-contrast (c) and post-contrast (d) transverse images at the level of C2 reveal faint contrast enhancement in the ventral spinal cord predominantly affecting the gray matter
Immunosuppressive treatment was started with prednisolone (Prednidale, 1 mg/kg PO q12h; Dechra Veterinary Products), cytarabine (Cytarabine, 50 mg/m2 SC q12h for two consecutive days; Hospira) and lomustine (CeeNU, 10 mg PO once; Bristol–Myers Squibb). Clindamycin (Antirobe, 10 mg/kg PO q12h; Pfizer) was administered pending qRT-PCR and serology results. Twelve days later the cat was ambulatory with mild generalized ataxia and was discharged to receive prednisolone (1 mg/kg PO q12h).
Immunosuppressive treatment was started with prednisolone (Prednidale, 1 mg/kg PO q12h; Dechra Veterinary Products), cytarabine (Cytarabine, 50 mg/m2 SC q12h for two consecutive days; Hospira) and lomustine (CeeNU, 10 mg PO once; Bristol–Myers Squibb). Clindamycin (Antirobe, 10 mg/kg PO q12h; Pfizer) was administered pending qRT-PCR and serology results. Twelve days later the cat was ambulatory with mild generalized ataxia and was discharged to receive prednisolone (1 mg/kg PO q12h).
Six weeks later the cat had reoccurrence of clinical signs. Repeat cisternal CSF analysis showed elevated TNCC (10/µl) with neutrophilic pleocytosis and increased protein concentration (52 mg/dl). Treatment with lomustine (10 mg PO once), dexamethasone (Dexamethasone, 0.2 mg/kg PO q24h; Auden Mckenzie) and intrathecal cytarabine (50 mg once) and methotrexate (Methotrexate, 2.5 mg once; Hospira) was followed by rapid clinical improvement. Dexamethasone (0.2 mg/kg PO q24h) was prescribed and reduced following neurological improvement 6 weeks later (0.2 mg/kg PO q48h).
Two months later the cat became acutely tetraparetic with severe, generalized ataxia and circling to the right. Neurological localization was multifocal central nervous system (CNS). Treatment with lomustine (10 mg PO once) and an increased dexamethasone dosage (0.2 mg/kg PO q24h) did not result in improvement and the owner elected euthanasia. The brain and cervical spinal cord were submitted for pathological evaluation.
Cat 2
An 11-year-old male castrated DSH cat presented with a 1 day history of ataxia. Pre-referral CBC, serum biochemistry, total T4 concentration and urinalysis were unremarkable. Indirect systolic blood pressure was 160 mmHg (N <160 mmHg).
Physical examination detected a grade III/VI left, systolic heart murmur. On neurological examination the cat was mildly obtunded with a right-sided head turn and spontaneous rotary nystagmus when placed in dorsal recumbency. The cat was ambulatory with severe generalized ataxia and tetraparesis, with postural reaction deficits in all four limbs. No pain was elicited on spinal palpation. The neurological examination was consistent with multifocal CNS disease. Thoracic radiography and abdominal ultrasound examination were unremarkable. An MRI (Signa Advantage 1.0 T; GE Medical Systems) of the head and neck showed a ventral intramedullary lesion at the level of the C2 vertebra that was T2W and FLAIR hyperintense, T1W hypointense with mild contrast enhancement (Omniscan, gadodiamide 287 mg/ml; GE Healthcare) (Figure 2). Fluid was present in the right tympanic cavity. Cisternal CSF analysis revealed increased protein concentration (49 mg/dl), and increased TNCC (10/µl) with a mixed pleocytosis. Treatment with prednisone (0.5 mg/kg PO q12h) was started.
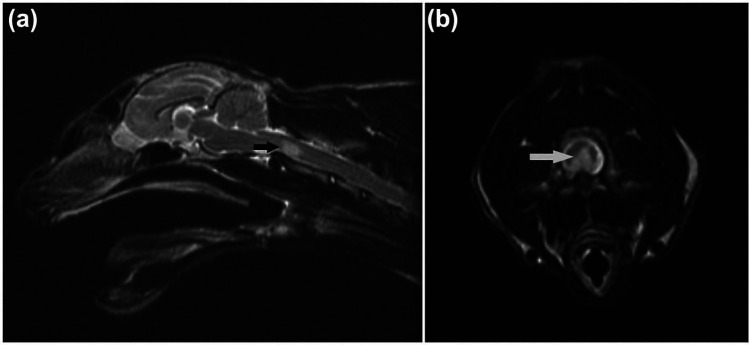
Figure 2.
(a) Sagittal T2-weighted (T2W) image of the head and neck of cat 2. Note the sharply demarcated hyperintense area at the level of C2 vertebra (arrow). (b) Transverse T2W image at the level of cranial C2 vertebra in cat 2. Note the ventral hyperintense area of the spinal cord (arrow)
Two months later only proprioceptive deficits in the right limbs were detected. Repeat CSF analysis 2, 5 and 7 months after initial examination showed a persistent mild increase in protein with mixed pleocytosis. Prednisone was continued (0.5 mg/kg PO q12h).
Ten months later the cat became acutely non-ambulatory. Increased prednisone dose (1 mg/kg PO q12h 2 days) was ineffective. Neurological examination revealed non-ambulatory tetraparesis with increased muscle tone and proprioceptive deficits in all limbs. There was an absent corneal reflex, facial paralysis and hypoalgesia on the left side. Neuroanatomical localization was multifocal CNS. Cyclosporine (2 mg/kg PO q12h) was added. The cat improved, but declined 1 month later and was euthanased. A post-mortem examination was performed.
Cat 3
A 12-year-old DSH cat presented for acute-onset non-ambulatory tetraparesis. Physical examination was unremarkable. Neurological examination was suggestive of C1–C5 myelopathy. No pain was elicited on spinal palpation. Hematology and serum biochemistry profile prior to referral were unremarkable.
MRI (0.4T; Hitachi Medical Systems) showed a ventral intramedullary lesion at the level of C2 that was T2W hyperintense, poorly contrast-enhancing, and a dorsal intramedullary triangular lesion at the level of C4 that was T2W hyperintense, non-contrast enhancing. Cisternal CSF analysis showed increased protein (76 mg/dl) and TNCC (1179/µl) with neutrophilic pleocytosis. qRT-PCR for infectious diseases was negative. USG (while on intravenous [IV] fluid therapy) was 1.010. A urine culture was negative.
The cat improved on dexamethasone (0.2mg/kg, q24h, initially IV then PO) but was non-ambulatory when discharged on dexamethasone (0.1 mg/kg, PO, q24h) 8 days later.
Three weeks after initial presentation the cat was ambulatory tetraparetic. Dexamethasone was tapered over the next months to 0.5 mg three times a week.
Twenty-two months after initial examination the cat deteriorated and the owner requested euthanasia. The brain and spinal cord were submitted for pathological evaluation.
Cat 4
A 16-year-old female spayed Maine Coon cat presented for an acute episode of collapse, left hemiparesis, and increased respiratory rate and effort. Neurological examination suggested C1–C5 myelopathy. Hematology and serum biochemistry profile showed an elevated creatine kinase (1024, RI 64–440 IU/l). Thoracic radiographs showed interstitial parenchymal pulmonary disease. Abdominal ultrasound examination was unremarkable. MRI (1.5 T Philips Achieva) showed an ill-defined lesion in the left ventrolateral portion of the C3 spinal cord segment that was T2W hyperintense and poorly contrast-enhancing. Cisternal CSF analysis revealed increased protein (184 mg/dl) and TNCC (150/µl) with a mixed pleocytosis. The cat improved the following day and was discharged with marbofloxacin (Zeniquin, 25 mg q24h; Pfizer).
Hemiparesis began to slowly progress 1 year later, and 18 months from initial evaluation the cat presented for acute weakness, dyspnea and vocalization with intermittent vomiting, polyuria and polydipsia. Physical examination revealed a grade II/VI parasternal, systolic murmur, increased respiratory rate and effort, and ascultatory crackles. Thoracic radiographs revealed mild-to-moderate generalized cardiomegaly with pulmonary changes consistent with left-sided congestive heart failure. An echocardiogram showed mild left ventricular hypertrophy. Indirect systolic blood pressure was 200 mmHg. Furosemide, 2 mg/kg IV and supplemental oxygen therapy was started. Total T4 was elevated (5.0, RI 0.8–4.7 μg/dl), and there was mild azotemia (blood urea nitrogen [BUN] 51, RI 14–36 mg/dl; creatinine 2.0, RI 0.6–2.4 mg/dl). The owner elected euthanasia and a postmortem examination was performed.
Cat 5
An 18-year-old, male castrated Persian cat presented for 1 week of progressive pelvic limb weakness. Treatment with dexamethasone and acupuncture had not resulted in clinical improvement. Pre-referral bloodwork revealed mild azotemia (BUN 48, RI 14–36 mg/dl), and elevated serum cholesterol (296, RI 75–220 mg/dl) and amylase (2122, RI 100–1200 U/l). Total T4 concentration was normal (1.7, RI 0.8–4.0 μg/dl). CBC revealed lymphopenia (602, RI 1200–8000) and monocytosis (774, RI 0–600). USG was 1.018, with proteinuria. Systolic blood pressure was 140 mmHg. Electrocardiography showed third-degree atrioventricular block with accelerated idioventricular rhythm. Physical examination revealed hypothermia (97.5ºF) and bradycardia (140 beats per minute). Neurological examination findings were consistent with a T3–L3 myelopathy. The owners elected euthanasia, and a post-mortem examination was performed.
Post-mortem examination
Table 2 summarizes the post-mortem findings in five cats with hyaline degeneration.
Table 2.
Summary of findings on post-mortem examination in five cats with hyaline degeneration
| Macroscopic findings | Microscopic findings: brain | Microscopic findings: spinal cord | |
|---|---|---|---|
| Cat 1 | Hemorrhage – ventral brainstem/cervical SC | AD, HyD (BA), thrombosis, WM degeneration (caudal medulla) | AD (VSA) C3-C5, thrombosis, ventral funiculus WM degeneration |
| Cat 2 | Hemorrhage – ventral brainstem/cervical SC (Figure 3) | HyD (meninges), thrombosis (BA branch), leptomeningeal fibrosis, acute, chronic hemorrhage (medulla) (Figure 5c). WM degeneration cerebellar peduncle hemorrhage | Cranial cervical SC, bilateral WM/GM necrosis |
| Cat 3 | Red nodule (vascular ectasia [aneurysm]) – ventral C2 | HyD (meninges), mixed perivascular/vascular inflammation | AD (VSA), chronic perivascular hemorrhage, GM degeneration, hemorrhage, gliosis, HyD (C3) |
| Cat 4 | Red nodules (vascular ectasia [aneurysm]) – ventral C3–C5, L3–L4, medulla (Figure 4) |
AD, HyD (BA branch), compression, midline shift, regional WM degeneration, necrosis, chromatolysis | AD, HyD (VSA) C3–C5, L3, L4, thrombosis, compression (Figures 5a,b), WM degeneration, occasional necrosis, chronic hemorrhage, inflammation |
| Cat 5 | Red nodules (vascular ectasia [aneurysm]) – C7–T1, L3 | HyD (BA branches), mineralization, meningeal chronic inflammation and hemorrhage, focal cerebellar necrosis | AD, HyD (VSA) C7–T1, L3, thrombosis, compression, ventral funiculi WM degeneration, gliosis, chromatolysis |
SC = spinal cord; AD = aneurysmal dilation; HyD = hyaline degeneration; BA = basilar artery; WM = white matter; VSA = ventral spinal artery; GM = gray matter
Macroscopic examination revealed hemorrhage on the ventral surface of the brainstem and cervical spinal cord in cats 1 and 2 (Figure 3). Cat 3 had a red mass ventral to the C2 spinal cord segment. Cats 4 and 5 had several dark red, raised nodules at the ventral aspect of C3–C5 (cat 4; Figure 4a), L3–L4 (cat 4), and at C7–T1 and L3 (cat 5), suggestive of multifocal, severe vascular ectasia (aneurysm). When sectioned, these were homogeneously dark red, and extended into the cervical and lumbar spinal cord parenchyma (Figure 4b). In the brain of cat 4 there was a 6 × 5 × 7 mm, dark red, firm mass at the ventral medulla adjacent to a basilar artery branch.
Figure 3.
Ventral aspect of the brain of cat 2. Hemorrhage is visible in the leptomeninges extending from the midbrain to the cervical spinal cord
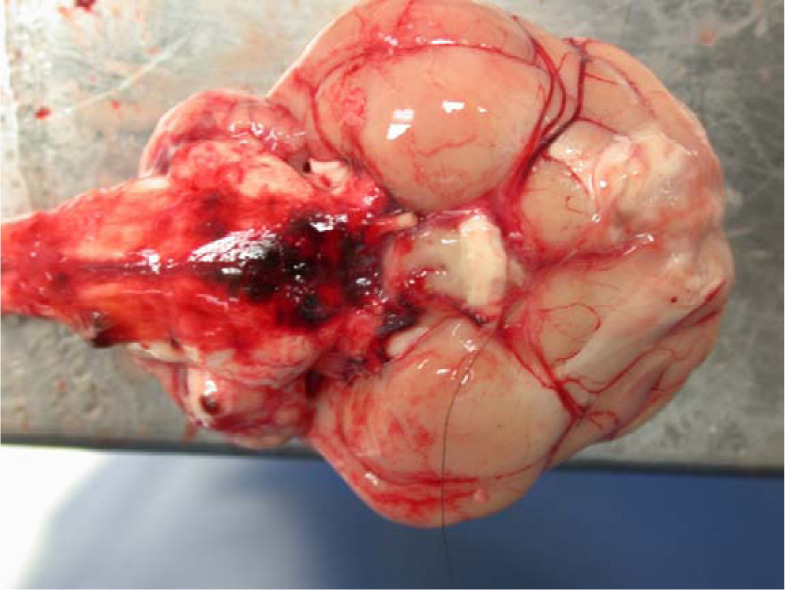
Figure 4.
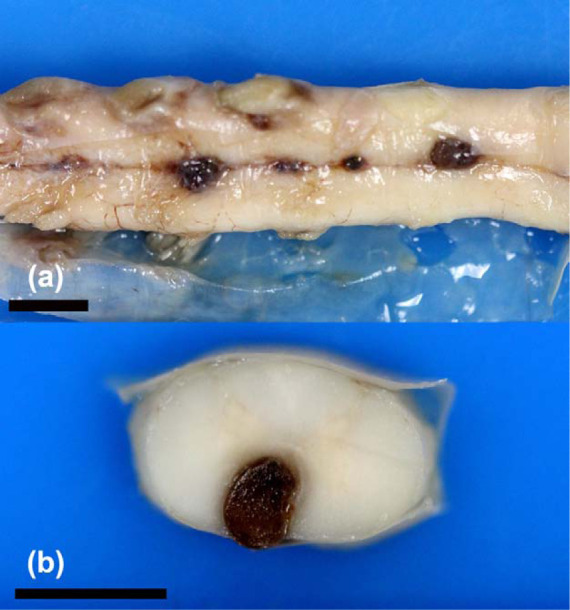
(a) Several dark raised nodules are evident on the ventral aspect of the cervical spinal cord in cat 4 from C3 to C5 at the level of the ventral spinal artery, consistent with multifocal vascular ectasia (aneurysm). Formalin-fixed tissue. (b) Cross-section of the cervical spinal cord. The nodule has compressed the regional ventral spinal cord parenchyma. Formalin-fixed tissue. Bars = 5 mm
Histopathological examination of the brain of cat 1 revealed thrombosis and aneurysmal dilation of the basilar artery within the caudal medulla and mild degeneration of the ventral white matter (WM). The arteriolar wall was thickened with hyaline degeneration in the tunica intima and parts of the tunica media. At the level of C3–C5 the ventral spinal artery (VSA) was dilated and thrombosed, and there was vacuolation and cavitation of the ventral funiculus of the spinal cord.
Cat 2 showed chronic and acute leptomeningeal hemorrhage and irregular areas of fibrous tissue proliferation on the ventral surface of the brainstem from the level of the medulla to C1. Focal areas of myelin swelling and hemorrhage were apparent in the WM of the cerebellar peduncle. At the level of the medulla an artery, presumably the communicating branch of the basilar artery, was thrombosed (see Figure 5c). There was hyalinization of several meningeal arteries. In the cranial cervical spinal cord there was necrosis of the ventral WM and gray matter bilaterally.
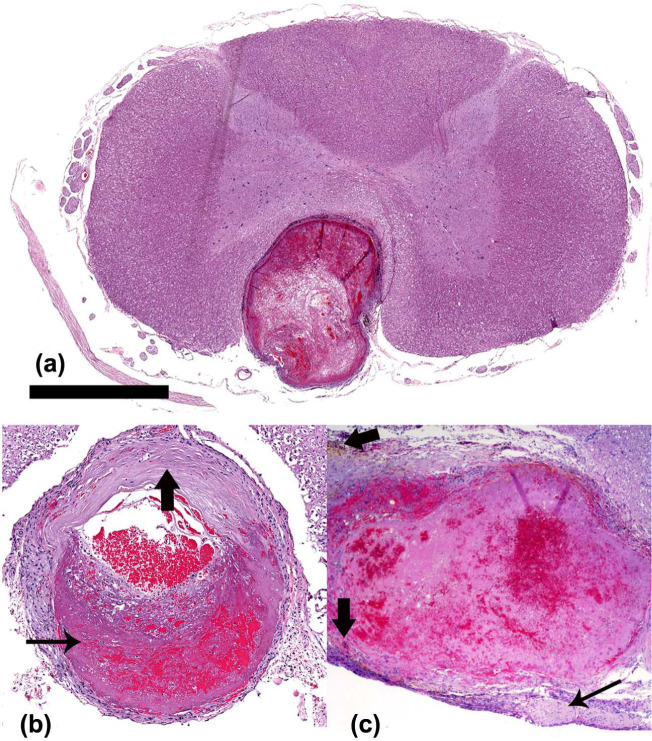
Figure 5.
(a) Low magnification view of a cross-section of the cervical spinal cord at the same level as Figure 4b, cat 4. There is aneurysmal dilation of the thrombosed ventral spinal artery. Bar = 2 mm. Hematoxylin and eosin ([HE] staining). (b) Ventral spinal artery, cervical spinal cord segment, cat 4. There is aneurysmal dilation of the artery, with thrombosis (thin arrow) and expansion of the vessel wall by hyaline degeneration (thick arrow). Magnification: × 5.9. HE staining. (c) Basilar artery communicating branch, cat 2. Aneurysmal dilation and thrombosis, which fills the artery lumen. There is fibrous tissue proliferation in the leptomeninges (thin arrow). Hemosiderin-laden macrophages surround the thrombosed vessel (thick arrows). Magnification: × 20. HE staining
Cat 3 had vascular changes affecting meningeal arterioles throughout the brain and at the C3 spinal cord segment. There was hyaline degeneration of the vessel wall with perivascular mixed inflammatory cells infiltrates and vasculitis. At C2, the grossly evident red mass was a swollen, degenerate and thrombosed VSA with chronic perivascular hemorrhage. The intermediate and ventral gray matters were degenerate with chronic hemorrhage and gliosis.
Cat 4 had multifocal aneurysmal dilation of the VSA at C3–C5, L3 and L4 with areas of thickening and attenuation of the vessel wall. There was hyaline degeneration of the tunica intima and media with mildly increased populations of fibrocytes typically within the tunica adventitia and tunica media, thrombosis and chronic periarteriolar hemorrhage with few lymphocytes and plasma cells. The vessels compressed the adjacent parenchyma (Figures 5a,b). In the ventral funiculi throughout the spinal cord there were various degrees of WM vacuolation, spheroids, gliosis, hemorrhage and edema, with occasional malacia. The medullary mass was a dilated, thrombosed artery, presumably the communicating branch of the basilar artery, which showed similar changes to the VSA and was causing compression of the brain parenchyma and midline shift at the level of the olivary nucleus and deep arcuate fibers. Similar changes were observed in the surrounding ventral brainstem arteries and arterioles. There was WM vacuolation, spheroid formation and necrosis, with occasional neuronal chromatolysis and necrosis.
In cat 5, aneurysmal dilation of the VSA was present at C7–T1 and L3. The VSA showed the same changes as in cat 4, also with thrombosis and compression of the spinal cord. The spinal cord ventral funiculi exhibited myelin vacuolation with spheroids, gliosis with gemistocytic astrocytosis, occasional chromatolysis of ventral gray matter neurons and Rosenthal fibers. Similar arterial lesions were found within vessels of the ventral brainstem, including branches of the basilar artery, as well as regions of vascular mineralization, lymphocytes and chronic hemorrhage within the meninges. In the cerebellum and medulla, a locally extensive region of Purkinje and granular cell necrosis were present.
Congo red staining of the material in the vessel walls was negative in all cats.
Evaluation of the heart revealed mild changes consistent with hypertrophic cardiomyopathy (HCM) in cats 2, 4 and 5. Hyaline arteriopathy was noted in the heart, and hyperplastic arteriolosclerosis were found in the lungs and heart in cat 4.
The kidneys in cat 5 showed signs of moderate chronic renal disease. Hyperplastic arteriolosclerosis was observed in the kidney and stomach.
Discussion
Feline IM may affect any spinal cord segment.3,7 In the present study, MRI findings correlated with clinical neuroanatomical localization in all cats. Lesions were documented in all cats to involve the ventral cervical spinal cord, particularly the vascular region of the VSA. Two cats had additional lesions in the thoracolumbar and lumbar spinal cord segments. The symmetry of neurological signs in four cats was consistent with obstruction of a larger vessel supplying a wider region of spinal cord. This is in contrast to the asymmetrical signs reported in the majority of histologically confirmed FCEM cases where the occlusion often affects a regional, smaller vessel.5–9 Histopathological examination in all cats revealed symmetrical ischemic degeneration of the ventral spinal cord WM, with hyalinization and thrombosis of the VSA, the basilar artery or the communicating branch of the basilar artery. The aneurysmal dilation of the arteries observed in 3/5 cats was believed to have developed secondary to hyaline degeneration of the vessel walls and vascular thrombosis. Histological findings were not consistent with a primary vasculitis in any of the cats. Recurrence of neurological signs in four cats was attributed to mobilization of VSA thrombo-emboli resulting in new areas of ischemic necrosis or new thrombus formation. In three cats pathological lesions in the brain were mild or absent. This might be explained by vascular anatomy, given that the basilar artery carries blood away from the brain and the vascular supply to the feline brain originates only from the maxillary arteries. 10 Brain and spinal cord lesions in cats 4 and 5 appeared to be associated with compression from the hyalinized vessels and thrombotic ischemia.
The MRI abnormalities were similar to those previously described for IM and are not pathognomic for a certain etiology.3,7,9,11,12 A definitive diagnosis can only be made after histopathology.
Increased CSF protein concentrations and TNCC detected in four cats in the present study was consistent with prior studies of IM.8,9 CSF pleocytosis in all previously reported feline cases was neutrophilic; however, in some canine cases it has been mixed.3,12 Four cats in the present study improved after immunosuppressive drug therapy or antibiotics, but neurological signs recurred within 6–24 months. Recurrence of CNS abnormalities and persistently increased CSF cell counts in two cats in our study has not previously been described in IM. Inflammation suggested on CSF analysis was most likely secondary to an acute vascular event. One cat temporarily improved without any immunosuppressive or anti-inflammatory treatment, supporting this theory.
Chronic renal disease, mild hypertension or mild HCM were diagnosed in four cats in the present study. In one retrospective study, 12/19 cats had IM localized to C1–T2 spinal cord segments. 3 Four of these were diagnosed with concurrent medical conditions and two had a history of trauma. A study investigating canine cerebrovascular accidents (CVA) identified concurrent medical conditions in over 50% of dogs. 13 A retrospective study on histologically confirmed feline spinal cord diseases in 205 cats identified 19 cases of ischemia or malacia of unknown cause. 14 Hypertension has been linked to arteriolosclerosis in cats, reported in association with chronic renal disease, hyperthyroidism, primary hyperaldosteronism and chronic anemia.15,16 Cerebral amyloid angiopathy, a common cause of CVA in humans, has been reported in geriatric cats.17,18 Congophilic β-amyloid protein was not, however, detected in any of our cases. All cats had hyalinization of the basilar artery or VSA, two also of meningeal arteries and one of visceral arteries. The cause of hyalinization of vessels is unknown, but may have been a predisposing factor to the thrombotic event.19,20 In humans, arteriolar hyalinosis occurs with benign hypertension and is associated with impaired autoregulation.20,21 It can also be seen with aging, diabetes mellitus and focal segmental glomerulosclerosis. 21 All cats in this case series were older cats; however, without any age control group it is unclear if arterial hyalinosis is a common finding in older cats in general.
Conclusions
Hyalinization of the basilar artery, VSA and meningeal arteries may predispose older cats to infarction of brain and spinal cord. Recurrence of neurological signs may be an indication of underlying vascular pathology resulting in repeat infarcts. The MRI findings are not specific for any cause of IM and further diagnostic testing is recommended to find underlying predisposing factors to an ischemic embolus.
Footnotes
The authors do not have any potential conflicts of interest to declare.
Funding: The authors received no specific grant from any funding agency in the public, commercial or not-for-profit sectors for the preparation of this case series.
Accepted: 23 December 2013
Case 1 was presented as an abstract at the 25th annual European Society of Veterinary Neurology/European College of Veterinary Neurology symposium in Ghent, Belgium, September 2012
References
- 1. Cummings JF, De Lahunta A, Summers BA. Degenerative diseases of the central nervous system. Veterinary neuropathology, 1st ed. St Louis, MO: Mosby-Year Book Inc, 2005, pp 237–249. [Google Scholar]
- 2. Cauzinille L, Kornegay JN. Fibrocartilaginous embolism of the spinal cord in dogs: review of 36 histologically confirmed cases and retrospective study of 26 suspected cases. J Vet Intern Med 1996; 10: 241–245. [DOI] [PubMed] [Google Scholar]
- 3. Theobald A, Volk H, Dennis R, et al. Clinical outcome in 19 cats with clinical and magnetic resonance imaging diagnosis of ischaemic myelopathy (2000–2011). J Feline Med Surg 2012; 15: 132–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dyce J, Houlton JEF. Fibrocartilaginous embolism in the dog. J Small Anim Pract 1993; 34: 332–336. [Google Scholar]
- 5. Turner PV, Percy DH, Allyson K. Fibrocartilaginous embolic myelopathy in a cat. Can Vet J 1995; 36: 712–713. [PMC free article] [PubMed] [Google Scholar]
- 6. Coradini M, Johnstone I, Filippich L, et al. Suspected fibrocartilaginous embolism in a cat. Aust Vet J 2005; 83: 550–551. [DOI] [PubMed] [Google Scholar]
- 7. MacKay AD, Rusbridge C, Sparkes AH, et al. MRI characteristics of suspected acute spinal cord infarction in two cats, and a review of the literature. J Feline Med Surg 2005; 7: 101–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Abramson CJ, Platt SR, Stedman NL. Tetraparesis in a cat with fibrocartilaginous emboli. J Am Anim Hosp Assoc 2002; 38: 153–156. [DOI] [PubMed] [Google Scholar]
- 9. Mikszewski JS, Van Winkle TJ, Troxel MT. Fibrocartilaginous embolic myelopathy in five cats. J Am Anim Hosp Assoc 2006; 42: 226–233. [DOI] [PubMed] [Google Scholar]
- 10. King AS. Arterial supply to the central nervous system. In: King AS. (ed). Physiological and clinical anatomy of the domestic mammals. Oxford: Blackwell Science, 1987. [Google Scholar]
- 11. Nakamoto Y, Ozawa T, Mashita T, et al. Clinical outcomes of suspected ischaemic myelopathy in cats. Vet Med Sci 2010; 72: 1657–1660. [DOI] [PubMed] [Google Scholar]
- 12. De Riso L, Adams V, Dennis R, et al. Magnetic resonance imaging findings and clinical associations in 52 dogs with suspected ischemic myelopathy. J Vet Intern Med 2007; 21: 1290–1298. [DOI] [PubMed] [Google Scholar]
- 13. Garosi L, McConnell J, Platt S, et al. Results of diagnostic investigations and long-term outcome of 33 dogs with brain infarction (2000–2004). J Vet Intern Med 2005; 19: 725–731. [DOI] [PubMed] [Google Scholar]
- 14. Marioni-Henry K, Vite CH, Newton AL, et al. Prevalence of diseases of the spinal cord of cats. J Vet Intern Med 2004; 18: 851–858. [DOI] [PubMed] [Google Scholar]
- 15. Jepson R. Feline systemic hypertension, classification and pathogenesis. J Feline Med Surg 2011; 13: 25–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Littman MP. Spontaneous systemic hypertension in 24 cats. J Vet Intern Med 1994; 8: 79–86. [DOI] [PubMed] [Google Scholar]
- 17. Vonsattel J, Myers R, Hedley-White E, et al. Cerebral amyloid angiopathy without and with cerebral hemorrhages. A comparative histological study. Ann Neurol 1991; 30: 637–649. [DOI] [PubMed] [Google Scholar]
- 18. Nakamura S, Nakayama H, Kiatipattanasalu W. Senile plaques in very aged cats. Acta Neuropathol 1996; 91: 437–439. [DOI] [PubMed] [Google Scholar]
- 19. Garosi LS. Cerebrovascular disease in dogs and cats. Vet Clin North Am Small Anim Pract 2010; 40: 65–79. [DOI] [PubMed] [Google Scholar]
- 20. Brown CA, Munday JS, Mathur S, et al. Hypertensive encephalopathy in cats with reduced renal function. Vet Pathol 2005; 42: 642–649. [DOI] [PubMed] [Google Scholar]
- 21. Olsen JL. Hyaline arteriolosclerosis: new meaning for an old lesion. Kidney Int 2003; 63: 1162–1163. [DOI] [PubMed] [Google Scholar]