Abstract
This prospective study evaluated a novel immunochromatographic (IC) blood typing test for the AB blood group system. Typing was conducted comparatively on ethylenediamine tetra-acetic acid-anticoagulated blood samples from 89 sick and 16 healthy cats with the IC test, as well as two tests as reference methods, a tube agglutination and a gel column test. The samples were between 0 and 10 days old (median 3 days) and were tested for haemolysis and agglutination; the packed cell volume ranged from 0.07 to 0.57 l/l (median 0.40 l/l). The reference methods agreed with each other in 100% of the test runs. Of the 85 samples tested as blood type A by the two reference methods, 80 were correctly identified by the IC test, four were misidentified as AB and one was rated inconclusive. All B samples were correctly typed. Two of the three AB samples were correctly identified by the IC test and one was rated inconclusive. The sample quality had no influence on test performance. Of 30 repeats, 28 were readable and showed agreement in 27 cases. The agreement of the IC test with the control methods was 96.1% for the 103 conclusive tests, and it showed high sensitivity and specificity for A and B antigen detection. It is suggested that AB results be reconfirmed with a laboratory method and that a ‘back-typing’ be performed with plasma from B samples to detect the presence of alloantibodies. Given its very good performance and ease of use, the IC test can be recommended for clinical settings.
Introduction
The feline AB blood group system was first described over three decades ago 1 and is associated with two erythrocyte antigens. Blood type A is linked to N-glycolylneuraminic acid and, to a much lesser extent, to N-acetylneuraminic acid. While the latter is linked to type B, cats with blood type AB carry both erythrocyte antigens.2–4 Identifying these blood types has become the standard in feline transfusion medicine as it plays a significant role in reducing the incidence of transfusion reactions.5–9 Blood typing also plays an important role in breeding programmes, as mating type B queens with type A or AB toms is linked to the rare, but potentially fatal, feline neonatal isoerythrolysis.10–13 To this end, both laboratory and point-of-care testing methods for the AB blood group system are available to veterinary laboratories and practices.
The available blood typing methods for the feline AB blood group system have been evaluated in a number of studies.14–18 The slide and tube agglutination methods are mainly used in specialised laboratories and clinics; the tube method is often relied on as the standard method.15,18 One commercially available point-of-care test is supplied in a card format with lyophilised anti-A and anti-B reagent wells in addition to a well that screens for autoagglutination. A newer point-of-care test kit is based on immunochromatographic (IC) sample migration along a single membrane that contains bands with monoclonal antibodies for antigens A and B, as well as a control band. A multicolumn cartridge system with gel matrices (GEL, ID-Gel Test Feline Anti A+B Typing; Diamed) into which monoclonal anti-A and anti-B antibodies are embedded was a reliable method, 15 but is no longer on the market at the time of writing.
This study was performed to assess the agreement with the reference methods and to evaluate the ease of use in everyday practice of a novel feline AB blood typing device, the RapidVet-H IC Feline test (DMS Laboratories). This point-of-care test was recently developed as an all-in-one kit and is based on IC sample migration technology. Here, the IC test is compared with a tube agglutination assay and the GEL test as reference methods.
Materials and methods
Blood samples
Ethylenediamine tetra-acetic acid (EDTA)-anticoagulated blood samples are routinely collected for diagnostic purposes at the Small Animal Clinic of Freie Universität Berlin from feline patients and blood donors. Unused remnants of these blood samples were included in this prospective study. Owner consent to use these blood samples for scientific purposes is routinely given at the intake examination every time a patient is first seen at the clinic, therefore no further approval for this study was needed. Eighty-nine such samples were used in this study and 16 samples were provided by outside laboratories. In order to determine sample quality, each sample’s packed cell volume (PCV) was measured using microcapillaries that were centrifuged for 5 mins at 14,926 g. The same microcapillary was used to test for haemolysis on the following scale: no haemolysis visible to the human eye (0) to haemolysis that does not allow for visual differentiation of red blood cells (RBC) from plasma (4+). A drop of the sample was placed on a slide to detect the presence of agglutination and was assessed on the following scale: no agglutinates (0) to 1–2 large agglutinates with clear plasma (4+). Both scales were adapted from a previous study. 15 The same investigator tested all of the samples, including quality assessment and typing, and another conducted a blind analysis of the IC test results. A total of 30 repeats were performed as part of the evaluation process, 22 and eight of which were same day and next day repeats, respectively.
Typing methods and equipment
Single-use, sterile laboratory equipment (tubes, slides, pipettes, etc) was used throughout. This study used 135 IC blood-typing kits. The device was compared with tests that are or have been in regular use at the Small Animal Clinic of Freie Universität Berlin.
In a modified version of the Pennsylvania tube test (TUBE), 15 a specific amount of an EDTA whole blood sample is tested for an agglutination reaction when added to a standardised amount of both an anti-A serum and an anti-B solution containing Triticum vulgaris lectin. The whole blood samples are washed before being added to the reagents, and a standardised 3–5% RBC suspension is used for the test. The reactions take place in tubes during an incubation period of 15 mins at room temperature, after which the tubes are centrifuged. The supernatant is assessed macroscopically, as is the sediment in the process of gentle re-suspension. Microscopic evaluation of the re-suspended RBCs allows for more precise results in this method, when no agglutination is detected macroscopically. The TUBE method mandates ‘back-typing’ to confirm B or AB test results, that is, patient plasma is tested for the presence or absence of alloantibodies by incubating with a 3–5% type A RBC suspension. The GEL test used to be available to veterinary laboratories large enough to accommodate a special, single-use centrifuge required for the multicolumn gel cartridges. Two of the columns contain a gel matrix laced with monoclonal antibodies for A and B; the third serves as a negative control, containing only the gel matrix. The typing methods in this study were used and interpreted according to established in-house protocols and manufacturer’s instructions, as well as according to the most recent published guidelines.15,18,19
The IC test is a cell-capture assay, the mechanism of action of which is based on sample migration along three intersecting membranes arranged as an inverted T in the cartridge: the vertical control membrane contains a substance in a specific area that captures all cells; the horizontal membrane on the left-hand side contains monoclonal antibodies for type A blood and the horizontal membrane on the other side contains antibodies for type B blood (Figure 1). The test is marketed as a kit containing a dropper bottle of diluent and optionally five or 10 92 × 57 × 6 mm semicircular cartridges (in sealed pouches) with a microtube prefilled with 600 µl of a diluent, as well as twodisposable pipettes for each cartridge. The cartridge has three viewing ports that are arranged in a circular fashion around the sample port, directly above the portion of each membrane that contains the cell-capturing substance and the antibodies, respectively. This means there is a control port, as well as a port each for the A positive and the B positive readings (Figures 2–5). The manufacturer recommends storage at room temperature.
Figure 1.

RapidVet-H immunochromatographic feline blood typing cartridge, opened
Figure 2.
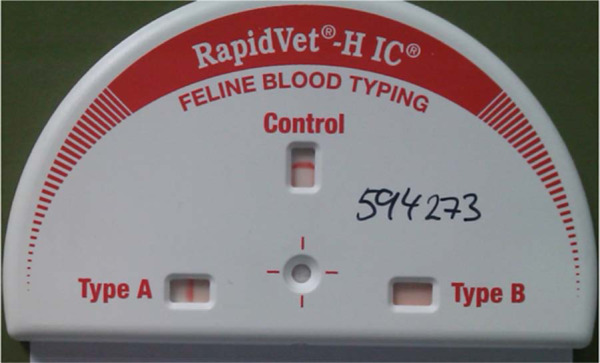
RapidVet-H immunochromatographic feline blood typing cartridge showing an A result
Figure 3.
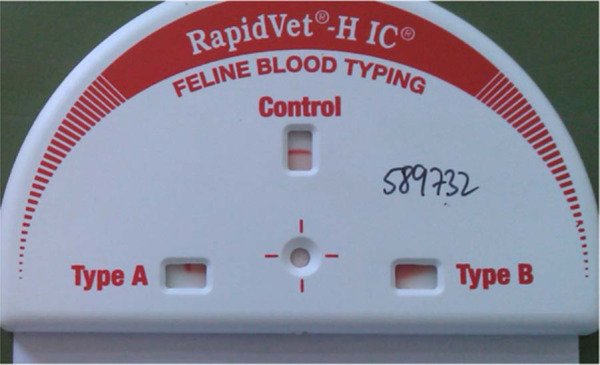
RapidVet-H immunochromatographic feline blood typing cartridge showing the test of an A sample with 25% of the indicator line visible
Figure 4.
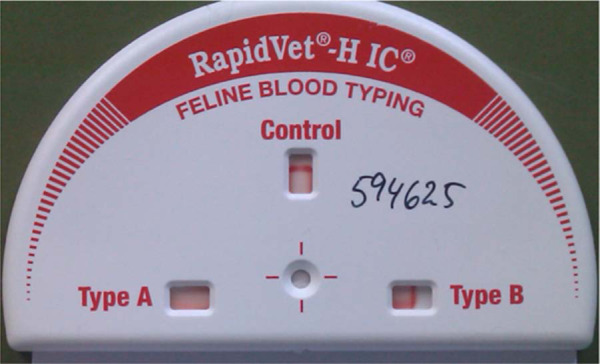
RapidVet-H immunochromatographic feline blood typing cartridge showing the test of a B sample
Figure 5.
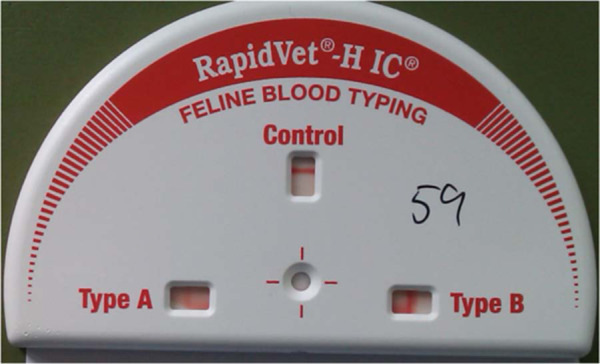
RapidVet-H IC feline blood typing cartridge showing the test of an AB sample
The cartridges were used exactly as prescribed by the manufacturer: they were first labelled, then one drop (30 µl) of a feline EDTA whole blood sample was added to the tube with the help of one of the included pipettes. The next three steps had to follow immediately: inverting the tube several times to mix properly, placing two drops (60 µl) of the now diluted blood into the cartridge sample port with the other pipette and adding to it two drops (80 µl) from the dropper bottle. A conclusive reading was defined in this study’s protocol as the appearance of a clearly visible red vertical indicator line filling at least 25% of one (or both) of the A or B viewing ports within 10 mins of starting the test, along with the appearance of the horizontal indicator line in the control viewing port. If those criteria were not met, the result was rated inconclusive; if no line appeared at all, then it was deemed not readable (Figures 1–5). For the majority of test runs (59 tests in total), the IC assay was used before all other tests in order to minimise bias. For the purposes of this study, detailed notes were taken in order to monitor any deviation from the device’s expected performance. Times were noted for the following events: first time any line appears; time at which line appears at 25, 50, 75 and 100% of its full length from top to bottom of the A/B viewing ports. Photographs were taken at predetermined stages of the device’s operational process.
Statistical analysis
Results were obtained and analysed according to the recommendations set forth elsewhere for method comparison studies.20–22 Sensitivity for A and B antigen detection was calculated as the number of true positives for each antigen determined by the IC test divided by the number of positives determined by the reference methods. Conversely, specificity was calculated as the number of true negatives for each antigen determined by the IC test divided by the number of negatives determined by the reference methods. Overall agreement with the reference methods was determined via contingency tabulation and the Cohen’s kappa coefficient was used to assess the robustness of the test’s performance results. 22
Results
Blood samples
Clinical data were available for 89/105 of the sample population wherein the following breeds were represented: domestic shorthair (n = 70), Persian mix (n = 4), British Shorthair (n = 3), Maine Coon mix (n = 3), Norwegian Forest cat (n = 3), Siamese (n = 2), Birman (n =1), Chartreux (n = 1), Persian (n = 1) and Siberian (n = 1). Disease distribution was as follows: gastrointestinal disorders (n = 16), wounds/trauma (n = 15), disorders of the urogenital tract (n = 13), neoplasia (n = 10), respiratory (n = 8), neuromuscular/orthopaedic (n = 8), endocrine (n = 4), ophthalmic (n = 4) or infectious disease (n = 3), immune-mediated haemolytic anaemia (n = 3), postoperative haemorrhage (n = 2), fever of unknown origin (n = 1), dental disease (n = 1) and routine surgery (n = 1). One of 15 tested cats was feline leukaemia virus (FeLV)-positive. The samples were between 0 and 10 days old (mean 3 days) and were stored between 2 and 4°C. The PCV ranged from 0.07 to 0.57 l/l (median 0.40 l/l), 16/105 samples had a PCV <0.30 l/l (median 0.26 l/l, range 0.07–0.29 l/l), six samples had a PCV <0.20 l/l. Very weak to weak slide agglutination occurred in 9/105 samples, and the plasma of 79/105 samples showed very weak to very strong haemolysis.
TUBE and GEL test
The TUBE and GEL tests, which yielded exactly the same testing results on all 105 samples (which corresponds to 100% agreement) were used as the reference methods against which the IC test’s performance was evaluated. According to the reference methods, 85 samples were A positive, 17 samples B positive and three samples AB positive.
IC test
Eighty of the A samples were correctly identified by the IC method, four were misidentified as AB and one test was inconclusive (very weak indicator line). All of the 17 B samples and two of the three AB samples were correctly identified by the IC method (one AB test was inconclusive owing to a very weak indicator line in the A viewing port). The seven samples that were over six days old did not lead to any problematic test results. The same applied to the 16 anaemic samples. Hence, the misidentified A samples did not come from anaemic cats. The first of the four misidentified A samples had a high PCV of 0.52 l/l and showed very weak RBC agglutination, as well as very weak haemolysis. The second showed weak RBC agglutination and haemolysis. The third showed very weak haemolysis and the fourth, from a cat with previously diagnosed immune-mediated haemolytic anaemia, had a PCV of 0.33 l/l and showed no quality divergence at all. The one sample that had tested positive for FeLV caused no problems with blood typing (blood type A). The 10 samples with intermediate to very strong haemolysis tested concordantly with the IC device. The IC test showed an overall agreement of 96.1% with the control methods for blood types A, B and AB in the 103 conclusive samples (Table 1). In addition, a statistical analysis of the results reported here using Cohen’s kappa shows a coefficient of 0.89 for the reported 96.1% agreement of the IC test results with the standards. Its sensitivity was 100% for both A and B antigen detection, and it showed a specificity of 100% for A antigen detection, as well as a 95% specificity for B antigen detection.
Table 1.
Blood typing results of the TUBE/GEL and first run immunochromatographic (IC) tests. The numbers in parentheses are percentages
| TUBE/GEL tests |
||||
|---|---|---|---|---|
| IC test | A (n = 85) | B (n = 17 ) | AB (n = 3 ) | Total (n = 105) |
| A | 80 | 0 | 0 | 80 (76.2) |
| B | 0 | 17 | 0 | 17 (16.2) |
| AB | 4 | 0 | 2 | 6 (5.7) |
| Inconclusive | 1 | 0 | 1 | 2 (1.9) |
| Total | 85 (80.9) | 17 (16.2) | 3 (2.9) | 105 (100) |
Of the 30 repeats, two that belonged to A samples were not readable (no indicator line appeared within 10 mins). One of those was a same day, the other a next day repeat. Twenty-eight were readable and showed agreement in 27/28 cases (96.4%); the one divergent sample was from a type A cat misidentified in the first run as AB and in the repeat run as B. The sample had a PCV of0.42 l/l, and showed weak haemolysis (1+) and weak agglutination (1+). This cat had chronic malaise after blunt trauma to the jaw (not tested for FeLV).
Of 131 readable test runs, 107 results were obtained in ≤5 mins (81.7%), the rest (24) were recorded in 10 mins or less (18.3%). In three of the 135 utilised cartridges the indicator line only reached 25% of the viewing port, in five cases only 50%, while in the remaining 127 cases the full indicator line was visible by the 10 min cut-off.
Discussion
The IC test examined in this study is designed to be a quick and reliable patient-side device for everyday use by the heterogeneous group of users in a veterinary practice. It is important that such devices are quick to use because of the often acute nature of cases in transfusion medicine; they must be reliable as a prerequisite for helping avoid haemolytic transfusion reactions; and veterinary staff of various training levels should be able to use them in order to participate in the care of patients in the area of transfusion medicine. The test was quick, as in most cases results were obtained in ≤5 mins, and in most cases it could be used and interpreted easily by following the enclosed step-by-step instructions. The manufacturer indicates that an incomplete indicator line in the viewing port is not a sign of a failed test. However, the occurrence of such incomplete lines in eight cases, along with that of a weak indicator line in two cases, particularly in AB results, presents potential for difficulties in interpreting this test by inexperienced users and, we suggest, ought to be a consideration in revising the package insert.
The IC test’s agreement of 96.1% with the reference methods compares well with other point-of-care tests described elsewhere: 91% and 95% for the card test and 95% for the other immunochromatographic test.17,18
Of 135 performed tests, two were inconclusive and two were unreadable (corresponding to 1.5% each), which attests to a high reliability of the test kit. The IC test’s high specificity and sensitivity for the individual blood types also demonstrates a test performance that is comparable to tests examined in other studies. 18 The 100% sensitivity for the B antigen is particularly important, as type B cats, with their high titre of anti-A alloantibodies, transfused with type A or AB blood, can exhibit especially severe transfusion reactions. 23 The Cohen’s kappa coefficient of 0.89 corresponds to a score of ‘very good’, and is considered a more robust measure of agreement between two methods of clinical measurement than a simple percentage alone. 22
Severe haemolysis or agglutination can cause problems both in determining blood type and in cross-matching. 8 Potential issues with sample quality, such as degrees of erythrocyte agglutination, anaemia and haemolysis, were determined in this study and the numbers indicate that, overall, the sample quality had no influence on test results. However, the agglutination in this study did not measure above weak, and true autoagglutination did not occur; therefore, it could not be determined as to which degree agglutination affects test performance, as it has been shown with the card test (see the manufacturer’s recommendations). 14
A patient’s disease status has likewise been suspected in previous studies to cause discrepancies in blood testing results, as has the possibility of antigenic mimicry with RBC surface antigens or a change in the activity of the cytidine monophospho-N-acetylneuraminic acid hydroxylase, which is responsible for converting N-acetylneuraminic acid to N-glycolylneuraminic acid. 18 Anaemia caused by FeLV, in particular, has been named in the context of diseases that can impede routine blood testing.6,18 There was no conclusive evidence that the divergent results in this study can be explained by patient disease status, level of anaemia or other sample quality impediment.
Generally, in a clinical setting, when result interpretation is difficult, seeking a second opinion can lead to a more confident decision-making process. This is easiest, in turn, when results can be documented as part of the permanent patient record. Currently available laboratory blood testing methods, for example, the tube test, do not all allow for easy documentation of test results without the availability of microscopic photography. Owing to the ability to photograph the results of the IC test, and because results can be read for hours following initial testing, these documentation needs are met.
As with other typing methods, it is suggested that rare blood type results, such as blood type AB in general and B in breeds where B is not usually seen, should be reconfirmed with a standard laboratory method. In general, it is recommended that for B samples a ‘back-typing’ be performed to detect the presence of anti-A alloantibodies in their serum.15,18
It must be pointed out, however, that no matter how high the agreement of a test with the reference methods it cannot afford complete protection against haemolytic transfusion reactions. The identification of the Mik antigen is a case in point.24,25 No testing methods other than cross-matching can be used to identify RBC incompatibilities, as the possibility of there being more unknown antigens exists. Thus, any type of blood testing, including point-of-care testing, still caries this caveat, despite very good performance results. 7 In fact, it could be argued that no transfusion should be administered without performing a cross-match. 26 Transfusion reactions directed at blood components other than RBCs, such as white blood cells and plasma proteins, cannot be tested for, but tend to be self-limiting. Lastly, screening for infectious diseases in blood donors and proper handling of blood products are integral to safe transfusion protocols. 25 Therefore, there still remains an uncalculable risk to every transfusion.
Conclusions
Given its high agreement rate and ease of use, as well as its convenient storage requirements, the RapidVet-H IC Feline test can be recommended for clinical settings.
Footnotes
The authors do not have any potential conflicts of interest to declare.
Funding: Dr Kohn received research support in the form of test kits and financial reimbursement for laboratory materials from DMS Laboratories, USA.
Accepted: 7 January 2014
This work is part of research towards a doctoral thesis and has been presented as an abstract at the following conferences: 21st annual conference of the German Veterinary Association (DVG) on Internal Medicine and Laboratory Diagnostics (InnLab), 1–2 February 2013, Munich, Germany; 2013 American College of Veterinary Internal Medicine Forum, 12–15 June 2013, Seattle, WA, USA
References
- 1. Auer L, Bell K. The AB blood group system of cats. Anim Blood Groups Bi 1981; 12: 287–297. [DOI] [PubMed] [Google Scholar]
- 2. Andrews GA, Chavey PS, Smith JE, et al. N-glycolylneuraminic acid and N-acetylneuraminic acid define feline blood group A and B antigens. Blood 1992; 79: 2485–2491. [PubMed] [Google Scholar]
- 3. Griot-Wenk ME, Callan MB, Casal ML, et al. Blood type AB in the feline AB blood group system. Am J Vet Res 1996; 57: 1438–1442. [PubMed] [Google Scholar]
- 4. Bighignoli B, Niini T, Grahn RA, et al. Cytidine monophospho-N-acetylneuraminic acid hydroxylase (CMAH) mutations associated with the domestic cat AB blood group. BMC Genetics 2007; 8: 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Giger U, Akol KG. Acute hemolytic transfusion reaction in an Abyssinian cat with blood type B. J Vet Intern Med 1990; 4: 315–316. [DOI] [PubMed] [Google Scholar]
- 6. Griot-Wenk ME, Giger U. Feline transfusion medicine. Blood types and their clinical importance. Vet Clin North Am Small Anim Pract 1995; 25: 1305–1322. [DOI] [PubMed] [Google Scholar]
- 7. Weingart C, Giger U, Kohn B. Whole blood transfusions in 91 cats: a clinical evaluation. J Feline Med Surg 2004; 6: 139–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tocci LJ, Ewing PJ. Increasing patient safety in veterinary transfusion medicine: an overview of pretransfusion testing. J Vet Emerg Crit Care 2009; 19: 66–73. [DOI] [PubMed] [Google Scholar]
- 9. Barfield D, Adamantos S. Feline blood transfusions: a pinker shade of pale. J Feline Med Surg 2011; 13: 11–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Giger U, Casal ML. Feline colostrum – friend or foe: maternal antibodies in queens and kittens. J Reprod Fertil Suppl 1997; 51: 313–316. [PubMed] [Google Scholar]
- 11. Bücheler J. Fading kitten syndrome and neonatal isoerythrolysis. Vet Clin North Am Small Anim Pract 1999; 29: 853–870. [PubMed] [Google Scholar]
- 12. Weingart C, Arndt G, Kohn B. The prevalence of blood types A, B and AB in domestic and pure bred cats in the Berlin-Brandenburg Metropolitan Region. Kleintierprax 2006; 51: 189–197. [Google Scholar]
- 13. Silvestre-Ferreira AC, Pastor J. Feline neonatal isoerythrolysis and the importance of feline blood types. Vet Med Int 2010: 753726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kohn B, Niggemeier A, Reitemeyer S, et al. Feline blood typing with a new card based test kit. Kleintierprax 1997; 42: 941–950. [Google Scholar]
- 15. Stieger K, Palos H, Giger U. Comparison of various blood-typing methods for the feline AB blood group system. Am J Vet Res 2005; 66: 1393–1399. [DOI] [PubMed] [Google Scholar]
- 16. Proverbio D, Spada E, Baggiani L, et al. Assessment of a gel column technique for feline blood typing. Vet Res Commun 2009; 33 Suppl 1: 201–203. [DOI] [PubMed] [Google Scholar]
- 17. Proverbio D, Spada E, Baggiani L, et al. Comparison of gel column agglutination with monoclonal antibodies and card agglutination methods for assessing the feline AB group system and a frequency study of feline blood types in northern Italy. Vet Clin Pathol 2011; 40: 32–39. [DOI] [PubMed] [Google Scholar]
- 18. Seth M, Jackson KV, Giger U. Comparison of five blood-typing methods for the feline AB blood group system. Am J Vet Res 2011; 72: 203–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Vap LM, Harr KE, Arnold JE, et al. ASVCP quality assurance guidelines: control of preanalytical and analytical factors for hematology for mammalian and nonmammalian species, hemostasis, and crossmatching in veterinary laboratories. Vet Clin Pathol 2012; 41: 8–17. [DOI] [PubMed] [Google Scholar]
- 20. Flatland B, Freeman KP, Friedrichs KR, et al. ASVCP quality assurance guidelines: control of general analytical factors in veterinary laboratories. Vet Clin Pathol 2010; 39: 264–277. [DOI] [PubMed] [Google Scholar]
- 21. Jensen AL, Kjelgaard-Hansen M. Method comparison in the clinical laboratory. Vet Clin Pathol 2006; 35: 276–286. [DOI] [PubMed] [Google Scholar]
- 22. Kwiecien R, Kopp-Schneider A, Blettner M. Concordance analysis – part 16 of a series on evaluation of scientific publications. Dtsch Arztebl Int 2011; 108: 515–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Auer L, Bell K. Transfusion reactions in cats due to AB blood group incompatibility. Res Vet Sci 1983; 35: 145–152. [PubMed] [Google Scholar]
- 24. Weinstein NM, Blais MC, Harris K, et al. A newly recognized blood group in domestic shorthair cats: the Mik red cell antigen. J Vet Intern Med 2007; 21: 287–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kohn B, Weingart C. Feline transfusion medicine. In: Day MJ, Kohn B. (eds). BSAVA manual of canine and feline haematology and transfusion medicine. 2nd ed. Quedgeley: BSAVA, 2012, pp 308–318. [Google Scholar]
- 26. Giger U. Blood typing and crossmatching. In: Bonagura JD, Twedt DC. (eds). Kirk’s current veterinary therapy XIV. 14th ed. St Louis, MO: Saunders Elsevier, 2009, pp 260–265. [Google Scholar]


