Abstract
Objectives
The aim of the study was to investigate if the feline vomeronasal organ (VNO) can be affected by inflammatory lesions and if these changes are associated with behavioural alterations.
Methods
VNOs from 20 cats were sampled during necropsy, submitted for routine tissue processing and stained with haematoxylin and eosin for histopathological evaluation. For the 20 cats, data on the presence of aggressive behaviours towards cats or humans were collected by questionnaire survey at the point of death. Inflammatory lesions were classified depending on the duration of the process as acute or chronic, both in vomeronasal sensory epithelium (VNSE) and in non-sensory epithelium (NSE). Fisher’s exact test was used to compare VNO inflammation with behavioural data.
Results
The VNSE was inflamed in 11/20 VNOs (55%) while the NSE was inflamed in 13/20 (65%). Overall, the VNO was affected by inflammation in 14/20 (70%) cats, and all the lesions were classified as chronic. Five out of 20 cats (25%) had documented intraspecific aggressive behaviours and 8/20 (40%) had shown aggression towards humans. Fisher’s exact test showed a statistically significant correlation between inflammation of the VNSE and intraspecific aggression (P = 0.038). No statistically correlations were observed between VNSE inflammation and aggression towards humans and between NSE inflammation and aggression towards cats or humans.
Conclusions and relevance
Our results show, for the first time, the existence of vomeronasalitis in animals and its possible association with intraspecific aggressive behaviours. The inflammatory microenvironment could impair VNSE functionality, causing intraspecific communication alterations, probably through a reduction in chemical communication action and perception. Owing to the pivotal role of the VNO in the social life of cats and other species, this report provides a rationale to further investigate this disease in relation to a variety of behavioural disorders.
Introduction
The vomeronasal organ (VNO) is a peripheral chemosensory structure involved in the detection of pheromones in vertebrates. 1 In cats, this organ has been widely studied, and its key role in feline behaviour and communication has been clearly demonstrated.2,3 Salazar et al showed that the anatomical and histological morphology of the feline VNO does not differ from other species. 4 The feline VNO is surrounded by the vomeronasal cartilage and is located in the nasal cavity in contact with the vomer, the palatine process of the palatine bone and the incisive bone. 4 Laterally, it is in contact with the nasal mucosa; ventrally it opens into the oral cavity through the palatine fissure. 4 From a histological point of view, VNO lumen is delimited by two types of epithelia: the non-sensory epithelium (NSE) and the vomeronasal sensory epithelium (VNSE). 4 The NSE closely resembles the respiratory epithelium of the nasal cavity,4,5 while the VNSE bears some similarity to the sensorial epithelium of the main olfactory system.4,5
To date, evidence of VNO alteration has been rarely reported in literature. In 1974, Loo and Chin described the presence of lymphatic nodules in the VNO of tree shrews, but this finding was not considered a pathological change. 6 More recently, microabscesses were observed in the VNO of rabbits after the experimental instillation of chemical solutions into the nasal cavity. 7 No reports have described spontaneous pathological changes of the VNO in other animals. In fact, only induced VNO alterations have been reported, with several studies finding that surgical removal of the VNO leads to a marked increase in deficits in social and reproductive behaviour in various species.8–10
Pheromones are involved in territorial marking behaviour in cats and in complex social exchanges. 11 Chemical messages emitted by means of marking behaviours are a more permanent form of communication than either postures or vocalisations. 12 Individuals can space chemical messages to prevent meetings, recognise territory, control reproduction and organise their social life in general. 12 Alterations in marking behaviours and impairments in chemical communication understanding could lead to aggression related to perturbations of the social and spatial environment. 12 The VNO is the organ that receives chemical messages and is pivotal in mediating a specific response. 13
Considering the lack of literature about VNO diseases and the pivotal role of the VNO in the social life and communication of the cat, the aim of this study was to evaluate if the VNO can be affected by inflammatory alterations, and if these conditions can be associated with aggressive feline behaviours.
Materials and methods
Twenty cats (10 males, 10 females) from multi-cat households (range two to five cats per home) aged between 1 and 13 years (mean ± SD 7.8 ± 3.9 years) were included in this study. Six subjects were humanely euthanased (0.7 ml/kg pentobarbital sodium IV) owing to the persistence of severe aggressive behaviours, 14 while 14 died for different organic causes and were routinely necropsied to exclude the presence of pathologies that could have influenced their behaviour. During necropsy, the VNOs of all the cats were sampled, fixed in 10% buffered formalin (pH 7.4) for 48 h and processed by routine methods for histological analysis. VNOs were cut transversely along, transversely along their length in order to observe both the NSE and the VNSE at histological examination. Sections 4 µm thick were stained with haematoxylin and eosin for histopathologicalanalysis. Owing to the different roles played by the NSE and the VNSE in the VNO,4,5 the NSE and VNSE were considered as separate entities for histopathological evaluation and statistical analysis. Inflammation was classified as acute or chronic, depending on the type of cellular infiltrate and the duration of the process.15,16
Data regarding the presence of aggressive behaviours towards humans or towards other cats were collected for all subjects by means of a questionnaire completed by the owners at the point of their cat’s death.
Statistical analysis was performed using 9.4 SAS software (SAS Institute). Fisher’s exact test was used to investigate the significance of the relationship between VNSE and NSE inflammation and the following parameters: sex, age, presence of intraspecific aggression and presence of aggression towards humans, using the FREQ procedure. Statistical significance was based on a 5% (0.05) significance level.
Results
Histological analysis
Fourteen of the 20 cats (70%) had inflammation in the VNO, with 10/20 (50%) presenting concurrent inflammation of the two epithelia. Of the four cats with inflammation in only one of the epithelia, the NSE was inflamed in three cats and the VNSE was inflamed in one. In total, the VNSE was affected by inflammation in 11/20 samples (55%) included in the study, while the NSE was affected in 13/20 (65%). Data regarding VNO inflammation are presented in Table 1.
Table 1.
Histopathological findings in the vomeronasal organ (VNO) of the 20 cats included in this study
| VNO |
VNSE |
NSE |
||||
|---|---|---|---|---|---|---|
| Histopathological finding | n | % | n | % | n | % |
| No alterations | 6 | 30 | 9 | 45 | 7 | 35 |
| Inflammation | 14 | 70 | 11 | 55 | 13 | 65 |
| Chronic | 5 | 25 | 3 | 15 | 5 | 25 |
| Chronic–active | 6 | 30 | 5 | 25 | 5 | 25 |
| Pyogranulomatous | 2 | 10 | 2 | 10 | 2 | 10 |
| Vasculitis | 1 | 5 | 1 | 5 | 1 | 5 |
VNSE = vomeronasal sensory epithelium; NSE = non-sensory epithelium
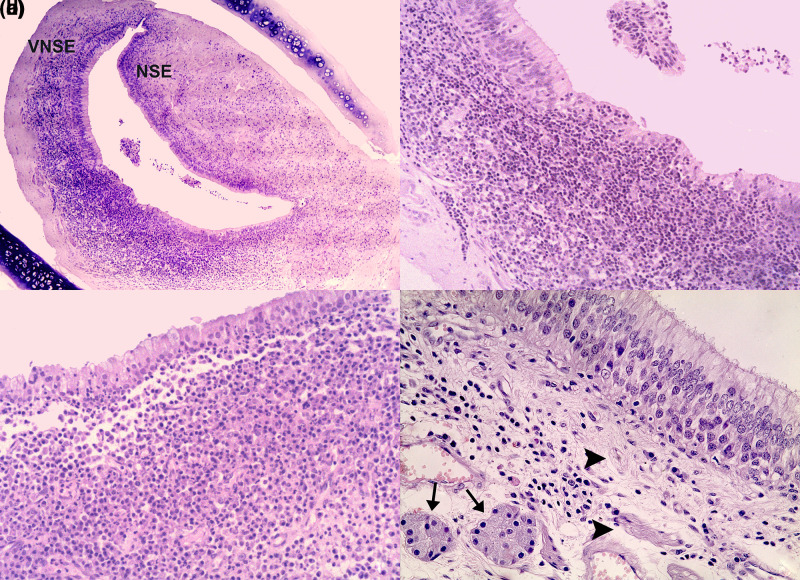
All 14 cases of vomeronasalitis were classified as chronic inflammation. Nine of these lesions were also subclassified as follows: 6/20 (30%) were chronic–active processes, 2/20 (10%) were pyogranulomatous inflammation and 1/20 (5%) was VNO vasculitis. The last affected the VNO of a cat that had died due to an effusive form of feline infectious peritonitis, and the vasculitis was characterised by the infiltration of inflammatory cells around blood vessels and vascular congestion. In the 5/20 (25%) VNOs affected by a classic chronic process, the inflammatory infiltrate was mainly composed of small lymphocytes, with minor involvement of plasma cells and macrophages (Figure 1a,b). The six cases of chronic–active vomeronasalitis were characterised by the same cellular infiltrate as the chronic cases, but with the concurrent involvement of mature neutrophils, signalling the restarting of the inflammatory process. In the two VNOs presenting pyogranulomatous inflammation, the cellular infiltrate was composed of macrophages and neutrophils, often affected by degenerative processes (Figure 1c). In chronic, chronic–active and pyogranulomatous lesions, inflammatory cells mainly infiltrated the VNO soft tissue under the NSE and VNSE, and among the epithelial layers (Figure 1). In four VNOs there was only mild infiltration of vomeronasal glands and nerves by inflammatory cells. Necrosis of the VNO epithelia was rarely observed and commonly associated with detachment of the necrotic cells into the lumen (Figure 1b,d). Furthermore, the inflammation of VNSE and NSE was often accompanied by hydropic degeneration of the epithelial cells (Figure 1c).
Figure 1.
Feline vomeronasal organ (VNO). (a) Feline chronic vomeronasalitis affecting the vomeronasal sensory epithelium (VNSE) and the non-sensory epithelium (NSE) (haematoxylin and eosin, × 40). (b) Feline chronic vomeronasalitis affecting the VNSE. The inflammatory infiltrate is mainly composed of lymphocytes; macrophages and plasma cells are also present. The VNSE is totally disrupted by the presence of inflammatory cells, and clusters of necrotic epithelial cells are observed extending into the VNO lumen (haematoxylin and eosin, × 200). (c) Feline pyogranulomatous vomeronasalitis affecting the VNSE. Macrophages and neutrophils massively infiltrate the soft tissue under the VNSE, which is reduced in thickness and partially necrotic (haematoxylin and eosin, × 200). (d) Feline chronic vomeronasalitis affecting the VNSE. Lymphocytes and plasma cells are interspersed within nerves (arrowheads) and vomeronasal glands (arrows) (haematoxylin and eosin, × 400)
Behavioural data
Of the 20 cats, five (25%) presented aggressive behaviours towards the other cats and eight (40%) towards humans. Two of the 20 cats (10%) presented aggression towards both cats and humans, while 9/20 subjects (45%) were not aggressive at all. Data regarding aggressive behaviours are reported in Table 2.
Table 2.
Presence of aggressive behaviours in the 20 cats included in this study
| Aggression towards cats |
Aggression towards humans |
|||
|---|---|---|---|---|
| n | % | n | % | |
| Positive | 5 | 25 | 8 | 40 |
| Negative | 15 | 75 | 12 | 60 |
Statistical analysis
Fisher’s exact test showed a statistically significant correlation between VNSE inflammation and the presence of aggressive behaviours towards cats (P = 0.038). No correlation was observed between VNSE inflammation and sex, age and aggression towards humans (P >0.05). However, the test showed a statistical trend between VNSE inflammation and female cats (P = 0.065). No statistically significant correlation or trend was observed between NSE inflammation and the other parameters included in the statistical analysis.
Discussion
This study describes, for the first time, feline vomeronasalitis and its possible association with aggression in cats. Our data showed that a large percentage of feline VNOs can be affected by chronic inflammation, which can involve both the NSE and VNSE. Statistical analysis showed that only inflammation of the VNSE could be associated with inter-cat aggression perceived by the owners.
The importance of the VNO in several aspects of animal behaviour has been widely demonstrated by the surgical removal of this structure. 8 In fact, after VNO ablation or rendering the VNO inaccessible, animals showed marked deficits in social and reproductive behaviour.9,10,17–19 However, there are no studies in the literature focused on VNO diseases, and VNO alterations have been rarely reported and only as a secondary finding in experimental studies.6,7 The presence oflymphatic nodules under the vomeronasal epithelium has been described in tree shrews, 6 while in rabbits the formation of VNO microabscesses as a consequence of experimental instillation of chemical solutions into the nasal cavity has been observed. 7
Owing to the absence of literature about vomeronasalitis, we can draw a parallel between our findings and feline chronic rhinitis, considering the similar position and histomorphology of the two structures. 4 Chronic rhinitis is a common finding in cats of any age, and is mainly composed of a lymphoplasmacytic infiltrate.15,16 A number of different causes can be responsible for the onset of this condition, but a viral aetiology is most commonly involved. 16 Bacterial infections are also commonly identified during feline chronic rhinitis, but this finding is normally considered a secondary consequence of the viral infection. 16 The similar anatomical position suggests that feline chronic vomeronasalitis may be caused by the same aetiologies as chronic rhinitis. A peculiar finding of our study is that the feline VNO was affected only by chronic processes, while neutrophils were involved only in association with a pre-existing lymphocytic or macrophagic infiltrate. In feline rhinitis, neutrophilic infiltrate is considered to be twice as common as the lymphocytic type, and is associated with acute and suppurative processes. 16 In our study, we did not observe acute suppurative vomeronasalitis, and this finding could suggest some differences in the immunity function between nasal and vomeronasal epithelium or in the entry pathway of the aetiological cause. In fact, the entry of air into the VNO is regulated by a pumping/suction mechanism that makes the VNO inaccessible the majority of the time, which is different from what happens in the nasal cavity. 12
Further studies involving larger numbers of subjects are required to verify the cause–effect relationship between vomeronasalitis and intraspecific aggression. Nonetheless, our data showed that inflammation of the VNSE was statistically correlated with intraspecific aggression, while NSE inflammation was not correlated. As previously reported, the VNSE closely resembles the sensorial epithelium of the main olfactory system of the nasal cavity.4,5 In humans, chronic inflammation of the olfactory mucosa has been associated with the reduction of odour perception as a probable consequence of inflammation mediators and cytokines on receptor neurons and nerves. 20 In mice, chronic exposure to tumour necrosis factor (TNF)-α leads to olfactory dysfunction through a reduction in the number of neurons, olfactory epithelium thickness and nervous function.21,22 TNF-α is a cytokine mainly produced by lymphocytes and macrophages, 22 cells that were predominant in the inflammatory infiltrate of the feline VNOs analysed in this study. Also, another macrophagic and lymphocytic cytokine, interleukin-6, has been associated with human hyposmia. 23 Thus, it is our opinion that the inflammatory microenvironment proposed as a cause for human and mouse olfactory loss may also impair the feline VNO during spontaneous vomeronasalitis. Our own data confirm that only the VNSE is responsible for the detection of pheromones, as widely reported in the literature.4,5,8 VNSE and NSE inflammation was not correlated with aggression towards humans, suggesting that it is probable that in cat–human interactions other factors (eg, postural communication, vocalisations, owners’ characteristics) play a more crucial role. 24 Our findings suggest that chemical communication plays a possibly stronger role in inter-cat relationships than those factors involved in human–cat interactions.
From a clinical point of view, cats with vomeronasalitis did not present manifest signs of this disease. The VNO is enclosed between the oral and nasal cavities, 4 and its internal position makes finding inflammation difficult. Further studies are warranted to determine alternative tools to diagnose vomeronasalitis in living animals by means of techniques other than histology, in order to aid clinicians during the clinical examination.
Intraspecific aggression is the most common feline behavioural disorder.25,26 This condition can be fear-related, status- or conflict-related, play-related, redirected or caused by the arrival of a new cat into a pre-existing group of cats. 26 However, to date, VNO diseases have never been proposed as a potential factor in inter-cat aggression. Our findings seem to suggest that inflammation of this organ and its ensuing dysfunction could lead to intraspecific communication alterations, causing inter-cat aggression. This opens a new avenue of investigation that links pathology to behavioural medicine. Moreover, future studies should investigate the possible correlation between vomeronasalitis and different forms of aggression, in order to evaluate possible relationships between them or the subjects involved in the social interactions (familiar vs unfamiliar subjects).
Conclusions
To our knowledge, this is the first study to describe VNO diseases in cats or indeed any species. Owing to the pivotal role of this organ in animal behaviour, this first report reveals several clinical and behavioural implications of VNO pathological changes. A wider number of VNO samples are needed to draw firmer conclusions, but our data suggest that vomeronasalitis could be a cofactor in the onset of inter-cat aggression.
Footnotes
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Accepted: 24 August 2015
References
- 1. Baxi KN, Dorries KM, Eisthen HL. Is the vomeronasal system really specialized for detecting pheromones? Trends Neurosci 2006; 29: 1–7. [DOI] [PubMed] [Google Scholar]
- 2. Verberne G. Chemocommunication among domestic cats, mediated by the olfactory and vomeronasal senses. II. The relation between the function of Jacobson’s organ (vomeronasal organ) and Flehmen behaviour. Z Tierpsychol 1976; 42: 113–128. [DOI] [PubMed] [Google Scholar]
- 3. Verberne G, de Boer J. Chemocommunication among domestic cats, mediated by the olfactory and vomeronasal senses. I. Chemocommunication. Z Tierpsychol 1976; 42: 86–109. [PubMed] [Google Scholar]
- 4. Salazar I, Sanchez Quinteiro P, Cifuentes JM. The vomeronasal organ of the cat. J Anat 1996; 188: 445–454. [PMC free article] [PubMed] [Google Scholar]
- 5. Zancanaro C. Vomeronasal organ: a short history of discovery and an account of development and morphology in the mouse. In: Mucignat-Caretta C. (ed). Neurobiology of chemical communication. 1st ed. Boca Raton, FL: CRC Press, 2014, pp 285–296. [PubMed] [Google Scholar]
- 6. Loo SK, Chin KN. Lymphoid tissue in the nasal mucosa of primates, with particular reference to intraepithelial lymphocytes. J Anat 1974; 117: 249–259. [PMC free article] [PubMed] [Google Scholar]
- 7. Pereira ME, Macri NP, Creasy DM. Evaluation of the rabbit nasal cavity in inhalation studies and a comparison with other common laboratory species and man. Toxicol Pathol 2011; 39: 893–900. [DOI] [PubMed] [Google Scholar]
- 8. Francia S, Pifferi S, Menini A. Vomeronasal receptors and signal transduction in the vomeronasal organ of mammals. In: Mucignat-Caretta C (ed). Neurobiology of chemical communication. 1st ed. Boca Raton, FL: CRC Press, 2014, pp 297–324. [PubMed] [Google Scholar]
- 9. Kiyokawa Y, Kikusui T, Takeuchi Y, et al. Removal of the vomeronasal organ blocks the stress-induced hyperthermia response to alarm pheromone in male rats. Chem Senses 2007; 32: 57–64. [DOI] [PubMed] [Google Scholar]
- 10. Wysocki CJ, Lepri JJ. Consequences of removing the vomeronasal organ. J Steroid Biochem Mol Biol 1991; 39: 661–669. [DOI] [PubMed] [Google Scholar]
- 11. Pageat P, Gaultier E. Current research in canine and feline pheromones. Vet Clin North Am Small Anim Pract 2003; 33: 187–211. [DOI] [PubMed] [Google Scholar]
- 12. Beaver BV. Feline behavior: a guide for veterinarians. 2nd ed. St Louis, MO: WB Saunders, 2003. [Google Scholar]
- 13. Wyatt TD. Pheromones and animal behavior: chemical signals and signatures. 2nd ed. Cambridge: Cambridge University Press, 2014, pp 173–222. [Google Scholar]
- 14. Fatjó J, Ruiz-de-la-Torre JL, Manteca X. The epidemiology of behavioural problems in dogs and cats: a survey of veterinary practitioners. Anim Welf 2006; 15: 179–185. [Google Scholar]
- 15. Henderson SM, Bradley K, Day MJ, et al. Investigation of nasal disease in the cat – a retrospective study of 77 cases. J Feline Med Surg 2004; 6: 245–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Reed N. Chronic rhinitis in the cat. Vet Clin North Am Small Anim Pract 2014; 44: 33–50. [DOI] [PubMed] [Google Scholar]
- 17. Booth KK, Katz LS. Role of the vomeronasal organ in neonatal offspring recognition in sheep. Biol Reprod 2000; 63: 953–958. [DOI] [PubMed] [Google Scholar]
- 18. Booth KK, Webb EC. Effect of blockage of the ducts of the vomeronasal organ on LH plasma levels during the “Whitten effect” in does. Vet Med Int 2010; 2010: 305468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pankevich DE, Cherry JA, Baum MJ. Effect of vomeronasal organ removal from male mice on their preference for and neural Fos responses to female urinary odors. Behav Neurosci 2006; 120: 925–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kern RC. Chronic sinusitis and anosmia: pathologic changes in the olfactory mucosa. Laryngoscope 2000; 110: 1071–1077. [DOI] [PubMed] [Google Scholar]
- 21. Lane AP, Turner J, May L, et al. A genetic model ofchronic rhinosinusitis-associated olfactory inflammation reveals reversible functional impairment and dramatic neuroepithelial reorganization. J Neurosci 2010; 30: 2324–2329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Turner JH, May L, Reed RR, et al. Reversible loss ofneuronal marker protein expression in a transgenic mouse model for sinusitis-associated olfactory dysfunction. Am J Rhinol Allergy 2010; 24: 192–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Henkin RI, Schmidt L, Velicu I. Interleukin 6 in hyposmia. JAMA Otolaryngol Head Neck Surg 2013; 139: 728–734. [DOI] [PubMed] [Google Scholar]
- 24. Casey RA, Bradshaw JWS. The effects of additional socialisation for kittens in a rescue centre on their behaviour and suitability as a pet. Appl Anim Behav Sci 2008; 114: 196–205. [Google Scholar]
- 25. Amat M, de la Torre JLR, Fatjo J, et al. Potential risk factors associated with feline behaviour problems. Appl Anim Behav Sci 2009; 121: 134–139. [Google Scholar]
- 26. Moesta A, Crowell-Davis S. Intercat aggression – general considerations, prevention and treatment. Tierarztl Prax Ausg K Kleintiere Heimtiere 2011; 39: 97–104. [PubMed] [Google Scholar]