Abstract
Objectives
A previously unreported, asymmetrically positioned hypoechoic extra layer (APHEL) in the submucosa of the feline distal jejunum and ileum has been recognised using high-frequency ultrasound. The objectives of this study were to characterise the APHEL histologically, and to describe the prevalence and ultrasonographic features of the APHEL in a population of clinically healthy young cats.
Methods
In an anatomical study, two cats were autopsied and histopathology of the small intestine was performed. An APHEL was detected with ultrasound in the distal jejunum and ileum ante-mortem in the first cat and post mortem in the second cat. Samples for histopathology were obtained from these areas. In the second, prospective part of the study, to document the presence or absence of an APHEL, high-frequency (18 MHz) ultrasound was performed of the intestinal tract in 20 other cats. These cats were client-owned cats aged 6–18 months presented for neutering. The cats were included in the study based on a normal clinical examination, lack of previous or concurrent signs of disease, and having no abnormalities detected at abdominal ultrasound.
Results
Histopathology from the distal jejunum and ileum in the two cats in the anatomical part of the study showed that the APHEL represented asymmetrically positioned normal lymphatic tissue (Peyer’s patches) in the lamina propria and submucosa. In the second part of the study, an APHEL was identified in the submucosa of the distal part of the jejunum and ileum in all 20 cats. Additionally, a similar layer could also be seen further proximally in the jejunum in 10 (50%) of the cats. The thickness of the APHEL was 1.0 mm in both jejunum and ileum.
Conclusions and relevance
Presumed normal lymphatic tissue in the small intestinal submucosa can be seen with high-frequency ultrasound and is a common finding in young cats.
Introduction
During abdominal ultrasound examinations of feline patients at our animal hospital, an asymmetrically positioned hypoechoic extra layer (APHEL) has frequently been observed within the submucosa of the ileum and jejunum. This has been seen using a high-frequency (18 MHz), broad-bandwidth ultrasound probe. APHELs have been seen in cats of different breeds and ages but subjectively more often in young cats. An APHEL has been noted in cats with varying clinical signs, and in several cats with signs not related to the gastrointestinal tract. Our assumption has therefore been that this likely represents normal lymphatic tissue.
The ultrasonographical appearance of the normal feline small intestine has been described, as well as a good correlation between the ultrasonographical and histological appearance of the layers.1–5 However, a layer resembling an APHEL, described in this study, has, to our knowledge, not previously been described in the feline ultrasonographical literature. It has been revealed that hyperplastic Peyer’s patches are detectable with ultrasound of the canine intestine ex vivo; however, there is no information about the equipment used and no images have been published. 4
The normal distribution of Peyer’s patches in the feline intestinal wall is described as 4–6 areas of aggregated lymphoid nodules in the jejunum, measuring 4–30 mm in length. An additional elongated patch is also described in the distal part of the small intestine, close to the ileocaecocolic junction, and this area measures up to 10 cm in length. All Peyer’s patches are described as antimesenteric. 6 Previous anatomical and histopathological studies have not been able to define a clear transition between the jejunum and ileum. The length of the ileocaecal fold has been suggested as an anatomical landmark, as well as the end of the antimesenteric ileal branches of the caecal artery. In dogs, the antimesenteric ileal branches of the caecal artery can be traced further proximally along the intestinal wall than the ileocaecal fold and therefore these anatomical landmarks are unreliable for defining the transition between jejunum and ileum.7–9
The study consisted of two parts. The first part was an anatomical study with the aim of histopathologically characterising the APHEL in the submucosa of the distal jejunum and ileum, and to correlate the histopathology with the ultrasonographical appearance. The aim of the second part of the study, a prospective clinical study, was to describe the prevalence and ultrasonographical features of the APHEL in a sample of clinically healthy young cats.
Materials and methods
All ultrasound examinations in the study were performed with the same 6.7–18.0 MHz linear transducer (L8-18i, GE Medical LOGIQ E9 Ultrasound Imaging System).
Anatomical study
Circumferential, full-thickness specimens from the distal part of the small intestine were obtained from two cats. In these cats an APHEL within the submucosa of the distal jejunum and ileum had been identified with high-frequency (18 MHz) linear ultrasound.
The first cat was a 6-month-old male castrated domestic shorthair cat that was euthanased owing to neurological signs. The reason for the clinical signs could not be established at post-mortem examination. The cat had a complete abdominal ultrasound performed the day before euthanasia and an APHEL was identified in the distal jejunum and ileum. The cat also showed slight generalised lymphadenopathy with normal echogenicity and a mildly hyperechoic liver. Histological specimens were taken from the part of the small intestine where the APHEL had been seen by ultrasound, 1, 3 and 5 cm proximal to the ileocaecocolic junction. Histopathology from abdominal lymph nodes showed reactive hyperplasia. Histopathology from the liver was not obtained.
The second cat was a 3-year-old male castrated Siberian cat that died at home from acute respiratory distress. A post-mortem examination was performed the day after death and the histological diagnosis was a chronic interstitial pneumonia in all lung lobes. At the post-mortem examination, the distal 10 cm of the small intestine and the ileocaecocolic junction were collected and placed in a water bath for subsequent ultrasonographical examination. An APHEL was detected in the submucosa of the distal jejunum and ileum. Two differently coloured needles were placed within the small intestinal wall at positions where the APHEL could be seen, and circumferential, full-thickness samples from these sites were obtained for histopathology.
All tissue samples from both cats were fixed in 10% neutral buffered formalin and embedded in paraffin. Sections (4 µm) were cut and stained with haematoxylin and eosin.
Prospective clinical study
In the second, prospective, part of the study, to document the presence or absence of an APHEL in the small intestine, ultrasonographical examinations of the gastrointestinal tract were performed in clinically healthy cats. All cats were intact male domestic shorthair cats that were brought to the small animal clinic for routine neutering. Informed owner consent was obtained for all cats. Owners were questioned about their cat’s clinical history, and cats with a current or previous history of gastrointestinal disease were excluded. The cats underwent a thorough clinical examination; however, blood or fecal samples could not be collected. All cats were fasted for a minimum of 12 h prior to the ultrasonographical examination. Cats received routine premedication with medetomidine 0.08 mg/kg, buprenorphine 0.009 mg/kg and meloxicam 0.2 mg/kg, according to the hospital’s anaesthetic protocol for the neutering of male cats. Fifteen to 20 mins after premedication, the hair of the ventral abdomen was clipped and acoustic coupling gel applied. Ultrasound was performed with the cats in dorsal recumbency. The probe frequency was set at 18 MHz, maximum depth at 3 cm with one focal zone. Other settings were adjusted for optimal image quality. The entire gastrointestinal tract was scanned from the stomach to the colon, and the associated lymph nodes were evaluated for being normal or abnormal. 10 The liver, spleen, kidneys, gall bladder, urinary bladder and pancreas were also scanned. Cats with any pathology detected at ultrasound were excluded. All ultrasonographical examinations were performed by one of the authors (TN). The scan time was between 10 and 15 mins per cat.
During the ultrasonographical examination it was decided if the cat had an APHEL in the small intestinal submucosa or not. If an APHEL was present, the location within the small intestine was estimated. For the purpose of this study, the APHEL was classified as present in the ileum if any part of it could be seen within the same image as the ileocaecocolic junction. The footprint of the probe was 2.5 cm. Consequently, the APHEL was classified as located within the jejunum if it could not be seen within the same image as the ileocaecocolic junction. To document that the APHEL was present, at least three cross-sectional images and one longitudinal image were saved at each part of the intestine. The images were stored in a picture archive and communication system (PACS, GE Centricity RA 600 v 8.0; General Electric Medical Systems) using the DICOM file format and reviewed at a dedicated workstation.
The distance from the most distal margin of the APHEL to the ileocaecocolic junction was measured, as well as the length of the APHEL. If the APHEL in longitudinal was longer than the footprint of the probe, or if the intestinal segment had a curved position within the abdomen, an approximation of its length was made. In cross-section images, the thickness of the APHEL, the total thickness of the wall containing the APHEL and the total thickness of the wall on the opposite side of the intestinal segment, not containing the APHEL, were measured. All measurements were obtained in three different images of each segment of the small intestine containing the APHEL. An attempt was made to distinguish the mesenteric from the antimesenteric side of the intestinal segment containing the APHEL. Mean and SDs were calculated from the measurements. The two cats in the anatomical part of the study were not included in the statistical analysis.
Results
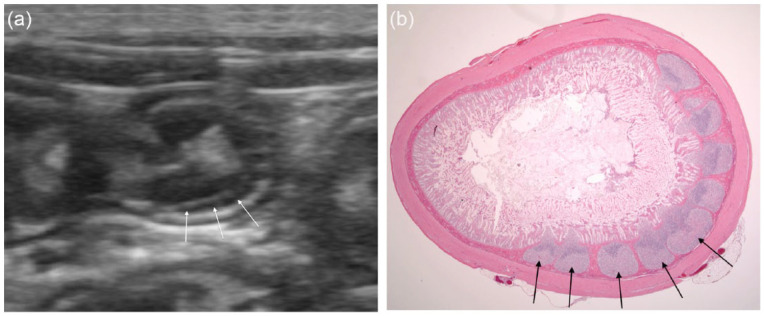
The histopathology of the two cats in the anatomical study confirmed that the APHEL represented aggregates of multiple lymphoid nodules in the lamina propria and submucosa of the distal jejunum and ileum (Figure 1). This is a normal histological appearance of the feline distal small intestine, also known as Peyer’s patches.11,12 When the intestinal segments were collected for histopathology, the mesentery and the ileocaecal fold were removed and it was therefore not possible to identify the mesenteric vs antimesenteric side of the intestine. The small intestine in these cats showed no pathology.
Figure 1.
(a) An asymmetrically positioned hypoechoic extra layer (APHEL) is present within the submucosa of the intestinal circumference to the right, deep side in the ultrasound image (white arrows). (b) Histological specimen of the same area in the same cat as the ultrasonographical image in (a). Note the lymphatic tissue formed as separated aggregates in the intestinal submucosa to the right in the image (black arrows). Haematoxylin and eosin (no magnification)
The owners of 23 cats gave consent to participate in the prospective part of the study. Two of the cats were excluded owing to previous gastrointestinal disease. Twenty-one clinically healthy cats had an ultrasonographical examination of the abdomen. One of these cats showed severe hydronephrosis and was therefore excluded. Twenty intact male domestic shorthair cats aged 6–18 months met the inclusion criteria. An APHEL could be seen in the submucosa of the ileum in all 20 cats. It was not possible, using ultrasound, to identify the exact transition between the distal jejunum and ileum; however, the intestinal segment containing the APHEL could be seen over a distance that should include not only the ileum, but also the distal jejunum in most of the cats. Ten (50%) cats had an additional intestinal segment with an APHEL in a more proximal part of the jejunum. An APHEL could not be seen in the duodenum or in the colon in any of the cats. The mean ± SD length of the intestinal segment containing the APHEL in the more proximal part of the jejunum was 1.5 ± 0.7 cm. In the ileum the mean length of the APHEL was 4.8 cm (range 1.8 to approximately 7 cm). In all cats the APHEL had a similar, hypoechoic crescent-formed shape; however, some variation was noted. In most of the cats, the APHEL was seen in the wall of approximately half the intestinal circumference; however, in some cats it was seen in a smaller part of the intestinal wall in cross section (Figure 2). In some areas, parts of the APHEL could be seen as separate, rounded, hypoechoic structures next to each other, resulting in a lobulated pattern (Figure 2c) resembling the separate aggregates within the submucosa seen in the histological specimen (Figure 1). However, the most common appearance was a homogeneous hypoechoic layer (Figure 2). The border between the APHEL and the mucosa was a thin, hyperechoic line. In the longitudinal plane, the APHEL was most often seen tapering off in both proximal and distal ends, but in some cats, the distal margin of the APHEL in the ileum ended abruptly, creating a step formation in the outer contour of the intestinal segment. The mean ± SD distance between the distal margin of the APHEL and the ileocaecocolic junction was 1.2 ± 0.5 cm (Figure 3).
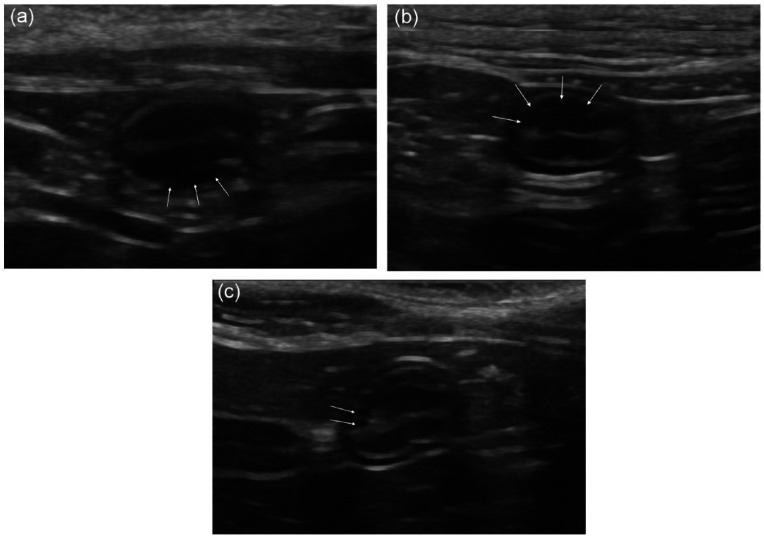
Figure 2.
Cross-sectional ultrasonographical images of the small intestine in three different young, clinically healthy cats with the asymmetrically positioned hypoechoic extra layer (APHEL) occupying a varying degree of the intestinal circumference. (a) APHEL in the deepest part of the intestinal circumference. This APHEL occupies a quarter of the intestinal circumference in cross section (white arrows). (b) Intestinal segment containing an APHEL in the most superficial part of the intestinal circumference (white arrows). The APHEL in this cat follows one-third of the intestinal circumference. (c) This cat has an APHEL in the most superficial part of the small intestine that follows almost half of the intestinal circumference. In this image it is possible to see some of the separate follicles (white arrows)
Figure 3.
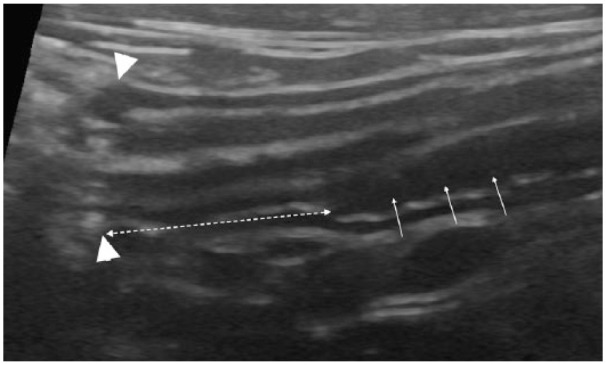
Longitudinal ultrasonographical image of the ileum and ileocolic junction in a clinically healthy, young cat. An asymmetrically positioned hypoechoic extra layer (APHEL) is present in the deepest part of the intestinal wall, in the ileum (white arrows). The ileocolic junction is to the left in the image (arrowheads). The dashed double arrow shows the distance between the ileocolic junction and the most distal margin of the APHEL
The mean ± SD thickness of the APHEL, the total thickness of the wall, including the APHEL, and the total thickness of the wall on the opposite side of the APHEL in the ileum was 1.0 mm ± 0.3 mm, 2.8 ± 0.5 mm and 1.8 ± 0.3 mm, respectively. The corresponding measurements in the jejunum were 1.0 ± 0.1 mm, 2.9 ± 0.3 mm and 2.0 ± 0.4 mm, respectively. Ultrasonographically, it was not possible to distinguish whether the APHEL was mesenteric or antimesenteric, as the mesenteric artery and the opposite antimesenteric site of the ileocolic artery were small and similar in appearance. It was not possible to detect with ultrasound the vessels at either side of the intestine in all cats. All scanned lymph nodes were normal in agreement with previously published data. 10
Three of the 20 healthy cats included in the present study had smooth, tubular, hyperechoic, non-shadowing structures representing roundworms within one or two areas of the small intestinal lumen.
Discussion
This report describes the ultrasonographical appearance of presumed lymphoid tissue in the small intestinal wall in 20 young, clinically healthy cats. The lack of histopathological, biochemical and faecal analyses of these 20 cats is a limitation of the study design; however, these samples were not possible to obtain. As the cats were presumed healthy, ethical approval did not allow us to obtain samples unnecessary for the treatment of the cats.
Feline leukaemia virus (FeLV) and feline immunodeficiency virus (FIV) are infections that could possibly affect the appearance of the lymphoid tissue. However, these are notifiable diseases in Sweden and reported annually to the Swedish Board of Agriculture. In 2013, a total of 21 FIV and 13 FeLV cases were reported, and none of these were in the county of the study population. 13 FIV or FeLV infection in the 20 cats of the present study cannot be excluded, but it seems unlikely that all 20 clinically healthy-appearing cats would have been infected.
Another limitation is that the cats in the anatomical study were diseased and one of them (the first cat) showed ultrasonographical changes within abdominal lymph nodes and liver. The liver was not examined histopathologically; however, lymph nodes showed reactive hyperplasia. These changes could be signs of a more generalised lymphatic stimulation or pathology. However, the ultrasonographical appearance of the APHEL in these cats was similar to the APHEL seen in all cats in the second part of the study. It is unlikely that all 22 cats in this study had a generalised pathological lymphatic stimulation that affected the intestinal lymphatic tissue. Furthermore, histopathology from the areas where the APHEL was seen with ultrasound in the two cats of the anatomical study showed no abnormalities, and therefore we presume that the APHEL represents multiple normal lymphoid nodules – Peyer’s patches. The size and shape of the APHEL detected in the 20 cats in the clinical, prospective part of the study corresponds to what has been published about the normal anatomical distribution and size of Peyer’s patches in cats, despite the fact that fewer areas were detected in the jejunum than previously reported in anatomical studies. 6
The second cat in the anatomical part of the study was examined with ultrasound post mortem with the intestinal segment placed in water. This was done so that we were able to mark the APHEL in the intestinal wall and to ensure that the samples for histopathology were obtained at the correct locations. The higher intracellular concentration of electrolytes causes water to diffuse initially into the intestinal cells, which induces swelling. This could potentially affect measurements done at this intestinal segment but should not affect detection of the APHEL. The examination procedure also prolongs the time before the intestinal segment was fixated in formalin, which increases the post mortem changes of the intestine. Despite this, the histopathological specimens from the second cat were diagnostic.
Three of the 20 healthy cats in the present study had adult roundworms within the small intestine, seen on ultrasonography. As roundworm infection is common in young cats, it is possible that even more of the 20 healthy cats were infected. This is a study limitation. However, studies have not showed any histopathological changes within the small intestines of cats infected with roundworms. 14 Therefore, it was not believed to affect the appearance of the small intestine of the cats significantly in the present study, and the three cats with roundworms were therefore included.
Some variation in the shape of the APHEL was noted. It was most often seen as a homogeneous hypoechoic layer; however, in some of the cats, parts of the APHEL showed separate hypoechoic rounded structures next to each other, which is presumed to represent the individual lymphoid follicles. This difference in appearance is most likely due to the tissue arrangement and the difference in probe angle. When the ultrasound beam is tangential to the borders of the nodules no echo is produced, in contrast to when the beam is perpendicular to the border (Figure 2). The border between the APHEL and the mucosa was a thin, hyperechoic line that likely represents an interphase echo. The width and length of the APHEL varied between cats; however, all cats in the study had an APHEL in the submucosa within the most distal 2 cm of the ileum.
This study shows that it is possible to identify an APHEL in the distal part of the small intestine with high-frequency ultrasound and that it is a common finding in clinically healthy young cats. High frequency improves axial resolution of the ultrasound beam as a result of shorter wavelength. At 18 MHz and with a two-cycle pulse, the axial resolution is 0.09 mm, which facilitates detection and distinction of these 1 mm structures and their borders. In comparison, at 8 MHz ultrasound with the same short two-cycle pulse, the axial resolution is 0.19 mm. The resolution is also improved by using broad-bandwidth transducers and spatial compound imaging.15,16
All cats in the second, prospective part of the study were under 18 months of age, 19/20 cats were younger than 12 months old. It has been described that lymphatic organs such as the thymus and lymph nodes are more prominent in younger animals, and this could also be the case for lymphatic tissue within the intestinal submucosa.17–20 This study did not evaluate possible change in the APHEL over time. It is our experience that the APHEL can be seen with ultrasound in cats of different ages but subjectively less frequently in older cats. It seems to be a common and normal finding in the young cat but the clinical significance of the layer present in older cats needs further investigation.
Conclusions
This study shows that presumed lymphatic tissue, Peyer’s patches, within the submucosa of the feline jejunum and ileum can be seen with high-frequency ultrasound. The layer of lymphatic tissue is asymmetrically positioned in the intestinal circumference and therefore affects the symmetry of the intestinal segment. This should not be mistaken for pathology in the small intestinal wall.
Footnotes
The authors do not have any potential conflicts of interest to declare.
Funding: This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Accepted: 19 March 2015
References
- 1. Winter MD, Londono L, Berry CR, et al. Ultrasonographic evaluation of relative gastrointestinal layer thickness in cats without clinical evidence of gastrointestinal tract disease. J Feline Med Surg 2014; 16: 118–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Di Donato P, Penninck D, Pietra M, et al. Ultrasonographic measurement of the relative thickness of intestinal wall layers in clinically healthy cats. J Feline Med Surg 2014; 16: 333–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Goggin JM, Biller DS, Debey BM, et al. Ultrasonographic measurement of gastrointestinal wall thickness and the ultrasonographic appearance of the ileocolic region in healthy cats. J Am Anim Hosp Assoc 2000; 36: 224–228. [DOI] [PubMed] [Google Scholar]
- 4. Le Roux A, Granger A, Wakamatsu N, et al. Correlation between histologic and ultrasonographic small intestinal layers in dogs [abstract]. Vet Radiol Ultrasound 2013; 54: 691. [Google Scholar]
- 5. Penninck D, d’Anjou MA. Gastrointestinal tract. In: Penninck D, d’Anjou MA. (eds). Atlas of small animal ultrasonography. 1st ed. Ames, IA: Blackwell Publishing, 2008, pp 281–318. [Google Scholar]
- 6. Nickel R, Schummer A, Seiferle E. Mittel- und Enddarm. In: Nickel R, Schummer A, Seiferle E. (eds). Lehrbuch der anatomie der haustiere. Vol 2. 6th ed. Berlin: Paul Parey, 1987, pp 138–144. [Google Scholar]
- 7. Evans HE, de Lahunta A. The digestive apparatus and abdomen. In: Evans HE, de Lahunta A. (eds). Miller’s anatomy of the dog. 4th ed. St Louis, MO: Elsevier Saunders, 2013, p 320. [Google Scholar]
- 8. Pasquini C, Spurgeon T. Digestive system. In: Pasquini C, Spurgeon T. (eds). Anatomy of domestic animals. 5th ed. 1989, p 275. [Google Scholar]
- 9. Dyce KM, Sack WO, Wensing CJG. The digestive apparatus. In: Dyce KM, Sack WO, Wensing CJG. (eds). Textbook of veterinary anatomy. 3rd ed. Philadelphia, PA: Elsevier Saunders, 2002, pp 130. [Google Scholar]
- 10. Taeymans O, Holt N, Penninck DG, et al. Ultrasonographic characterization of feline ileocecocolic abnormalities. Vet Radiol Ultrasound 2011; 52: 335–339. [DOI] [PubMed] [Google Scholar]
- 11. HogenEsch H, Hahn F. The lymphoid organs: anatomy, development, and age-related changes. Pathobiol Aging Dog 2001; 1: 127–135. [Google Scholar]
- 12. Bushberg J, Seibert J, Leidholdt E, et al. Ultrasound. In: Bushberg J, Seiberg J, Leidholdt E. (eds). The essential physics of medical imaging. 3rd ed. Philadelphia, PA: Lippincott Williams & Wilkins, 2012, pp 524–525. [Google Scholar]
- 13. Ziegler L, O’Brien RT. Harmonic ultrasound: a review. Vet Radiol Ultrasound 2002; 43: 501–509. [DOI] [PubMed] [Google Scholar]
- 14. Schreurs E, Vermote K, Barberet V, et al. Ultrasonographic anatomy of abdominal lymph nodes in the normal cat. Vet Radiol Ultrasound 2008; 49: 68–72. [DOI] [PubMed] [Google Scholar]
- 15. Bacha WJ, Jr, Bacha LM. Lymphatic system. In: Bacha WJ, Bacha LM. (eds). Color atlas of veterinary histology. 2nd ed. Philadelphia, PA: Lippincott Williams & Wilkins, 2000, p 73. [Google Scholar]
- 16. Brown CC, Baker DC, Barker IK. Alimentary system. In: Maxie MG. (ed). Jubb, Kennedy & Palmer’s pathology of domestic animals. 5th ed. Edinburgh: Elsevier, 2007, p 73. [Google Scholar]
- 17. Swedish Board of Agriculture. Årsrapport över anmälningspliktiga djursjukdomar 2013. Swedish Board of Agriculture. http://www2.jordbruksverket.se/webdav/files/SJV/trycksaker/Pdf_ovrigt/ovr310.pdf (2013, accessed January 14, 2015)
- 18. Thomson R, McGavin M, Zachary J. Ascariasis. In: Thomson R, McGavin M, Zachary J. (eds). Thomson’s special veterinary pathology. 3rd ed. St Louis, MO: Mosby, 2000, p 73. [Google Scholar]
- 19. Pabst R, Geist M, Rothkotter HJ, et al. Postnatal development and lymphocyte production of jejunal and ileal Peyer’s patches in normal and gnotobiotic pigs. Immunology 1988; 64: 539–544. [PMC free article] [PubMed] [Google Scholar]
- 20. Barberet V, Schreurs E, Rademacher N, et al. Quantification of the effect of various patient and image factors on ultrasonographic detection of select canine abdominal organs. Vet Radiol Ultrasound 2008; 49: 273–276. [DOI] [PubMed] [Google Scholar]