Abstract
Objectives
To evaluate the potential benefits of high-dose buprenorphine formulations for analgesia in cats, serial and crossover studies were undertaken to investigate their pharmacokinetics and thermal antinociceptive effects.
Methods
Twelve healthy adult domestic shorthair cats (6.0 ± 1.1 kg body weight) were studied. Aqueous solutions of buprenorphine hydrochloride at 0, 0.02, 0.06, 0.12 and 0.24 mg/kg body weight and formulations containing 0, 0.3, 0.6 and 1.2 mg/ml with and without preservatives were given subcutaneously. Blood samples were taken and thermal threshold (TT) measured prior to and at regular time points up to 72 h after dosing. Descriptive statistics and analyses of variance were applied as appropriate.
Results
Baseline TT was 47.6 ± 4.1°C, which increased in all groups treated with all buprenorphine dosages and formulations. After doses of 0.12 mg/kg and above, TT was significantly higher than baseline at most time points from 1–30 h post-treatment. The time to maximum effect (Tmax) ranged between 0.25 and 2.00 h; and plasma concentrations associated with maximum antinociceptive effect (Cmax) were 1.01–1.72 ng/ml after the 0.02 mg/kg dose, 1.4–4.9 ng/ml after the 0.06 mg/kg dose, 4.6–51.4 ng/ml after the 0.12 mg/kg dose and 5.3–22.3 ng/ml after the 0.24 mg/kg dose. The range of estimates for the buprenorphine elimination half-life were as follows: 0.02 mg/kg = 1.35–5.33 h; 0.06 mg/kg = 16.1–31.2 h; 0.12 mg/kg = 10.1–34.0 h; and 0.24 = mg/kg 16.1–31.6 h. The mean ‘plasma concentration for the offset of analgesia’ was 2.3 ± 2.0 ng/ml. No adverse effects were seen. The addition of preservatives to a high-concentration buprenorphine formulation had no impact on antinociception nor any side effects.
Conclusions and relevance
Aqueous high-concentration buprenorphine formulations administered at 0.12 or 0.24 mg/kg have potential for clinical use in cats, providing prolonged antinociception in a single subcutaneous injection of minimal dose volume.
Introduction
In recent years the benefits of pain relief during clinical treatment have become better appreciated in both medical and veterinary care. Although initially progress in the cat was slower than in the dog, there are now numerous reports underlining the need for good pain management in this species, especially during the postoperative period.1,2 Cats are the most popular pet in many countries, and as most are neutered, many thousands will undergo at least one surgery during their life; good postoperative analgesia will clearly benefit a large number of patients.
Opioids are still the best recognised and most effective analgesics, and they have the added benefit of contributing to the injectable anaesthetic protocols often used for neutering surgery in cats. 3 Buprenorphine has been widely used in cats for perioperative analgesia, and has an exemplary track record in this species, providing analgesia of several hours’ duration with remarkably few unwanted side effects.4–6
Cats are often difficult to dose, whether by injection or oral administration, so there is considerable potential for a preparation providing prolonged analgesia from a single injection. A recent investigation reports that postoperative analgesia for 72 h after a single dose of slow-release formulation of buprenorphine administered subcutaneously was similar to that after oral transmucosal administration of the standard aqueous solution given every 12 h. 7 However, during early pilot studies, aqueous solutions given subcutaneously demonstrated the potential of using a simple aqueous formulation, given by an appropriately slow uptake route, for providing prolonged analgesia from a single injection.
This report describes investigations into the potential for high-dose buprenorphine to provide prolonged analgesia in cats following a single injection. The pharmacokinetics and antinociceptive effects of a number of dosages and formulations of aqueous buprenorphine were evaluated using a thermal model of nociception.
Materials and methods
Ethical approval
All studies were conducted at the Sinclair Research Center Inc, Auxvasse, MO, USA, and were approved by the local institutional animal care and use committee.
Animals
Twelve adult (six male, six female) domestic shorthair cats were studied. The cats had a mean ± SD body weight of 6.0 ± 1.1 kg (range 4.7–8.3 kg). They were housed individually and provided with dry feline diet (Purina Cat Chow) and water ad libitum. One day prior to any testing they were weighed and underwent a full clinical examination to confirm normal health. All cats had been treated with routine vaccination prior to the start of the investigation. Cats 1–6 were used in all five studies. Cats 7–12 took part in study V only. Studies I–IV were serial investigations of one to two treatments, and each cat received only one treatment. Study V was a partial crossover study investigating three treatments: cats 1–6 received one of three treatments and cats 7–12 received two of three treatments.
Study protocol
Five separate crossover or serial studies were conducted to evaluate eight variations in buprenorphine formulation and dose (Table 1). Five different aqueous formulations were studied. Formulation A was a standard formulation containing 0.3 mg/ml buprenorphine hydrochloride (Buprenex; Reckitt Benckiser Healthcare). Formulations B and C were high concentration, unpreserved formulations containing 0.6 and 1.2 mg/ml buprenorphine hydrochloride, respectively. Formulation D was a high-concentration formulation containing 1.2 mg/ml buprenorphine hydrochloride, preserved with methylparaben (2.3 mg/ml) and propylparaben (0.3 mg/ml) (pH 5.2). Formulation E was a negative control formulation (0.5% dextrose; Hospira). Doses of 0.02 (standard/low), 0.06 (medium), 0.12 (high) and 0.24 (very high) mg buprenorphine hydrochloride/kg body weight were given by subcutaneous (SC) injection, in volumes ranging from 0.05–0.4 ml/kg. All buprenorphine solutions, except A, were formulated in-house and supplied by Abbott Animal Health.
Table 1.
Five studies in adult cats conducted to evaluate the duration and degree of antinociception after treatment with subcutaneous buprenorphine at doses of 0.02, 0.06, 0.12 and 0.24 mg/kg body weight and using formulations containing 0.3, 0.6 and 1.2 mg/kg with and without preservative
| Study | Dose (mg/kg) | Formulation |
Dose volume (ml/kg) | n | |
|---|---|---|---|---|---|
| ID* | Concentration of buprenorphine (mg/ml) | ||||
| I | 0.02 (standard/low) | A | 0.3 | 0.067 | 3 |
| 0.12 (high) | A | 0.3 | 0.400 | 3 | |
| II | 0.12 (high) | A | 0.3 | 0.400 | 3 |
| III | 0.12 (high) | B | 0.6 | 0.200 | 3 |
| 0.12 (high) | C | 1.2 | 0.100 | 3 | |
| IV | 0.12 (high) | D | 1.2 | 0.100 | 6 |
| V | 0 (control) | E | 0 | 0.100 | 6 |
| 0.06 (medium) | D | 1.2 | 0.050 | 6 | |
| 0.24 (very high) | D | 1.2 | 0.200 | 6 | |
Formulation ID: A = standard (low) concentration formulation; B and C = medium and high concentration, unpreserved formulations, respectively; D = high concentration, preserved formulation; E = negative control formulation, 5% dextrose
Thermal nociceptive threshold testing
A small probe containing a heating element and a temperature sensor was held against the shaved thorax using an elasticated band with a small, lightly inflated bladder behind the probe to ensure consistent contact between probe and skin. 8 During testing the probe was connected to the control unit with light ribbon cable. When activated, the probe heated at 0.6°C/s with an automatic cut-out at 55°C if not stopped earlier. To record thermal threshold (TT), the starting skin temperature was recorded and then the heater was activated; heating was switched off immediately after the cat reacted. The response was usually a skin flick, a jump forward, a turn to bite the band or, rarely, vocalisation. The probe temperature at the reaction point was recorded as TT. The temperature probe was calibrated prior to each study. 9 Baseline TT was recorded as the mean of five tests taken at 15 min intervals prior to drug administration. Following buprenorphine administration, TT was measured at 0.5, 1, 2, 3, 4, 5, 6, 8, 12, 16, 20, 24, 30, 36, 48, 60 and 72 h.
Clinical observation
Throughout each testing session the cats were observed for any abnormal clinical signs or behaviour, and record was made of attitude, general condition, mydriasis and euphoria before and at 1, 4, 8, 12, 24, 36 and 48 h after dosing. The testing site was examined daily for several days after thermal testing, and topical antibiotic ointment was available in the event of skin damage or hyperalgesia being observed.
Blood sampling
Blood samples were collected by direct jugular venepuncture into a Vacutainer (BD Biosciences) containing lithium heparin. Approximately 1.5 ml was withdrawn prior to buprenorphine administration and at 0.25, 0.5, 1, 2, 4, 6, 8, 12, 20, 24, 36, 48 and 72 h following dosing. Blood samples were placed immediately on ice before plasma was extracted by centrifugation at 4°C (1100 × g). Plasma was frozen immediately and stored on dry ice prior to storage at −70°C until assayed. The maximum storage period was 60 days, and studies showed no effect of storage on drug concentrations.
Buprenorphine assay
Samples were coded and shipped on dry ice to AAI Pharma Labs (Shawnee, KS, USA) for analysis of buprenorphine and its nor-metabolite by liquid chromatography mass spectrometry. The coefficients of variation for buprenorphine and norbuprenorphine were ⩽15% for both inter- and intra-assays coefficients at 1.5 ng/ml, 38.4 ng/ml and 64.0 ng/ml, respectively. At the lower limit of quantitation (LLOQ), which was 0.5 ng/ml for both analytes, the inter- and intra-assay coefficients of variation for buprenorphine and norbuprenorphine were ⩽20%. All samples were measured individually.
Data analysis
Data from groups (AG, Table 2) that were treated with the same dosage and formulation were amalgamated for the purposes of statistical and pharmacokinetic analysis. In addition, data from animals receiving the same dosage and concentration with and without preservative were analysed together and separately to evaluate the effect of including preservative (see Table 2).
Table 2.
Identification of groups (AG) for statistical and pharmacokinetic analysis
| Group | Treatment | Dose mg/kg | Formulation |
Dose volume (ml/kg) | Study | Total (n) | |
|---|---|---|---|---|---|---|---|
| ID* | mg/ml | ||||||
| AG1 | Control | 0 | E | 0.0 | 0.1 | V | 6 |
| AG2 | High dose | 0.12 | A | 0.3 | 0.4 | I (n = 3) | 6 |
| Standard (low) concentration | II (n = 3) | ||||||
| AG3 | High dose | 0.12 | C | 1.2 | 0.1 | III (n = 3) | 9 |
| High concentration | D | IV (n = 6) | |||||
| AG4 | Medium dose | 0.06 | D | 1.2 | 0.05 | V | 6 |
| High concentration | |||||||
| AG5 | Very high dose | 0.24 | D | 1.2 | 0.2 | V | 6 |
| High concentration | |||||||
| AG6 | Standard (low) dose | 0.02 | A | 0.3 | 0.07 | I | 3 |
| Standard concentration | |||||||
| AG7 | High dose | 0.12 | B | 0.6 | 0.2 | III | 3 |
| Medium concentration | |||||||
See footnote to Table 1 for formulation ID
Pharmacokinetic analysis
Data from individual cats were used to derive the following kinetic parameters: peak concentration (Cmax) and time to peak concentration (Tmax). The area under the concentration time curve (AUC) was calculated using the linear trapezoidal rule to the final concentration time point. An estimate of the elimination rate constant was made using data from the maximum concentration. From this rate constant, the extrapolated AUC from the final concentration time points to infinity were calculated as C/K(el). The same method was used for both buprenorphine and its nor-metabolite. In those animals where significant concentrations of norbuprenorphine were measured, the ratio of AUC norbuprenorphine to AUC buprenorphine was calculated. To allow for any incomplete bioavailability of the drug after SC dosing, the estimates for clearance (Clp) and apparent volume of distribution (Vdbeta) are expressed as values Clp/F and Vdbeta/F, where F is the unknown bioavailability after SC dosing.
Pharmacokinetic–pharmacodynamic analysis
A preliminary analysis of the pharmacokinetic–pharmacodynamic relationship was carried out to relate the time course of drug concentration and the time course of the TT. Two indices were identified: the time to peak plasma drug concentration and the time to the first appearance of the maximum TT in individual animals. Mean ± 2SD were used to define clinically relevant antinociception. 8 Onset and offset was defined as the plasma drug concentrations where the TT exceeded +2SD (or, if it was between sampling points, it was the extrapolated concentration).
Statistical analysis
TT data were analysed using one and two-way repeated measures ANOVA to compare AGs and assess changes over time (GraphPad Prism version 6.0b). Post-hoc analysis was with Dunnett’s or Tukey’s multiple comparisons tests, as appropriate. P <0.05 was considered significant. Data are given as mean ± SD unless otherwise stated.
Results
Thermal threshold
Pretreatment skin temperature was 35.1 ± 0.8°C in all groups, and did not change significantly during any of the studies. Baseline TT in the control group (5% dextrose, AG1) was 47.6 ± 4.1°C, and did not change significantly throughout the duration of testing.
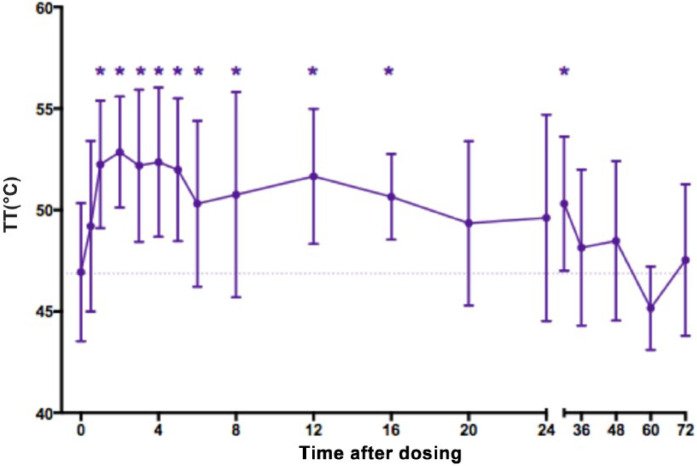
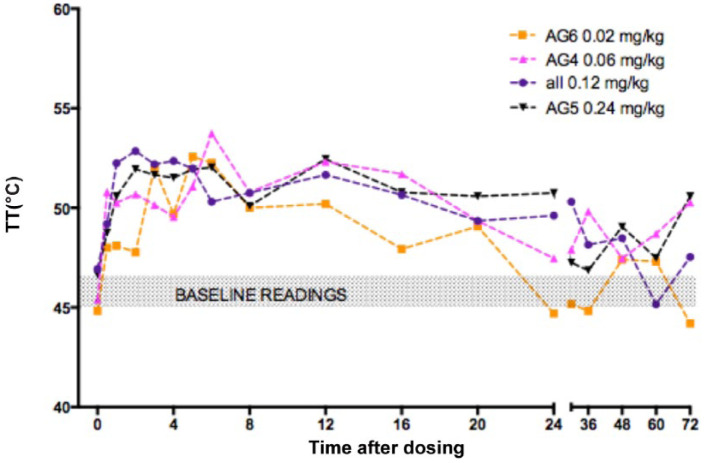
In all groups treated with any dose or formulation of buprenorphine TT increased above baseline at some point post-treatment (Table 3). The cut-out temperature of 55°C was reached in some animals; however, as this was in considerably fewer than half of the post-treatment measurements, cut-out readings were simply recorded as 55°C. In groups with six or more cats, the increase in TT was always statistically significant (P <0.05) (Table 3). After 0.12 mg/kg (AGs 2, 3 and 7) TT was significantly higher than baseline at most time points from 1–30 h post-treatment (Figure 1). There were no statistically significant differences between any of the treatment groups, although the duration of effect appeared shorter after 0.02 mg/kg (AG 6) than with any of the higher doses (Figure 2).
Table 3.
Timing of statistically significant antinociceptive effect of subcutaneous buprenorphine in 33 cat dosings. Repeated measures ANOVA (P <0.05)
| Group | Treatment |
Baseline TT (°C)* | Time points post-treatment: TT significantly higher than baseline | |
|---|---|---|---|---|
| Dose (mg/kg) | Concentration of buprenorphine (mg/ml) | |||
| AG2 (n = 6) | 0.12 (high) | 0.3 | 46.9 ± 2.4 | 1–5 h after treatment |
| AG3 (n = 9) | 0.12 (high) | 1.2 | 47.4 ± 4.5 | 2 h |
| AG4 (n = 6) | 0.06 (medium) | 1.2 | 45.4 ± 2.8 | 6–16 h |
| AG5 (n = 6) | 0.24 (very high) | 1.2 | 46.7 ± 1.2 | 2 and 12 h |
| AG6 (n = 3) | 0.02 (standard/low) | 0.3 | 44.8 ± 0.8 | ND |
| AG7 (n = 3) | 0.12 (high) | 0.6 | 45.7 ± 0.5 | ND |
Mean ± SD; TT = thermal threshold; ND = not determined (insufficient group members for statistical evaluation)
Figure 1.
Mean ± SD thermal threshold (TT) in cats (n = 18) after subcutaneous injection of 0.12 mg/kg buprenorphine. Injection at time 0. Baseline TT at 0 is mean of five measurements taken before treatment. Dotted line is baseline TT for all groups for visualisation. *Significant difference from 0
Figure 2.
Mean thermal threshold (TT) in cats after subcutaneous injection of buprenorphine 0.02 mg/kg (AG6, orange, n = 3), 0.06 mg/kg (AG4, pink, n = 6), 0.12 mg/kg (AGs 2, 3 and 7, purple, n = 18) and 0.24 mg/kg (AG5, black, n = 6). Injection at time 0. Baseline TT at 0 is mean of five measurements taken before treatment. Shaded area is baseline TT in each group for visualisation. Error bars omitted for clarity; no statistical difference between groups – see text
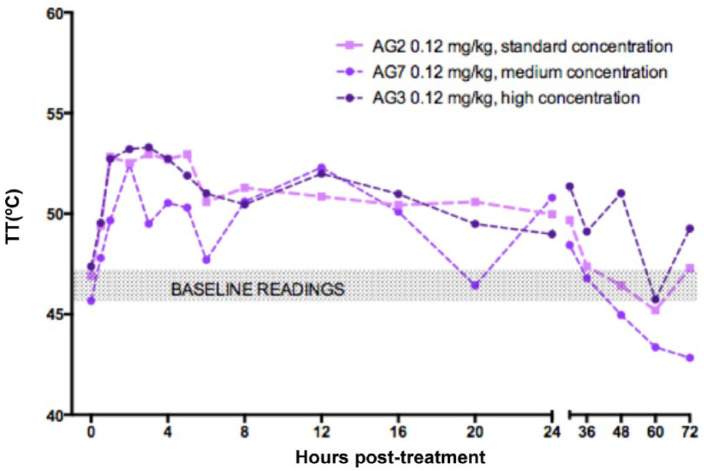
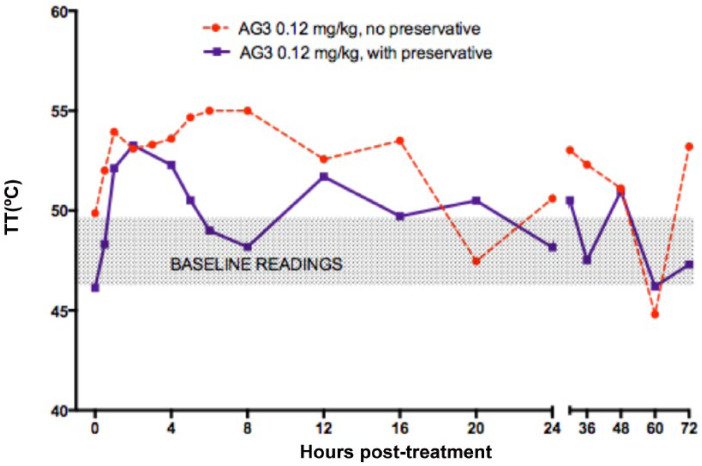
Neither the dose (Figure 2), the concentration (Figure 3) nor the addition of preservative (Figure 4) significantly affected TT.
Figure 3.
Effect of concentration: mean thermal threshold (TT) in cats after subcutaneous injection of 0.12 mg/kg buprenorphine: 0.3 mg/ml (AG2, mauve, n = 6), 0.6 mg/ml (AG7, purple, n = 6) and 1.2 mg/ml (AG3, dark purple, n = 9). Injection at time 0. Baseline TT at 0 is mean of five measurements taken before treatment. Shaded box is baseline TT from all groups for visualisation. Error bars omitted for clarity; no statistical difference between groups – see text
Figure 4.
Effect of preservative: mean thermal threshold (TT) in cats after subcutaneous injection of 0.12 mg/kg buprenorphine (AG3, 1.2 mg/ml) (dotted red, no preservative, n = 3; solid purple, with preservative, n = 6). Injection at time 0. Baseline TT at 0 is mean of five measurements taken before treatment. Shaded box is baseline TT from both groups for visualization. Error bars omitted for clarity; no statistical difference between groups – see text
Kinetics
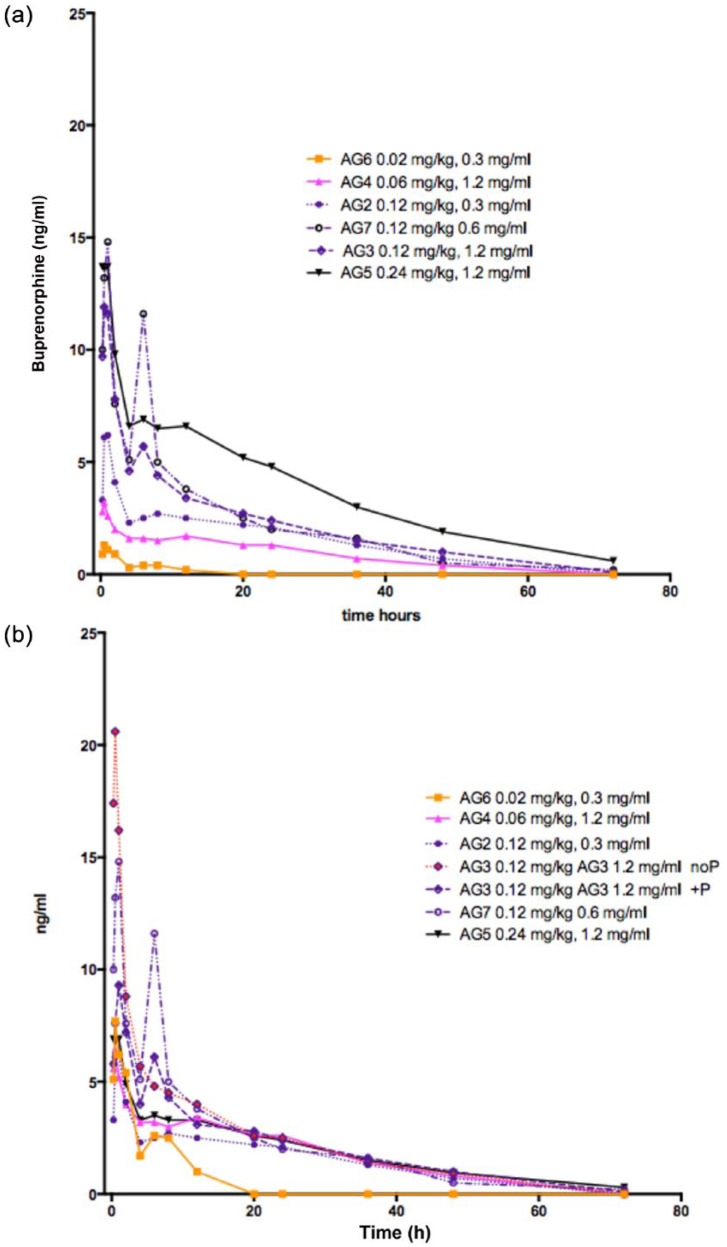
All pharmacokinetic data are shown in Tables 4 and 5. Estimates of Cmax and Tmax for buprenorphine were obtained in 33 treated cats. The Tmax ranged between 0.25 and 2.00 h; Cmax was 1.01–1.72 ng/ml for the 0.02 mg/kg dose (AG6), 1.4–4.9 ng/ml for the 0.06 mg/kg dose (AG4), 4.6–51.4 ng/ml for the 0.12 mg/kg dose (AGs 2, 3 and 7) and 5.3–22.3 ng/ml for the 0.24 mg/kg dose (AG5). Actual plasma concentrations and drug concentrations when corrected to a standard 0.12 mg/kg dose are shown in Figure 5.
Table 4.
Pharmacokinetic parameters from cats after treatment with subcutaneous buprenorphine at 0.02, 0.06, 0.12 and 0.24 mg/kg body weight and using formulations containing 0.3, 0.6 and 1.2 mg/kg with and without preservatives
| Buprenorphine | |||||
|---|---|---|---|---|---|
| Group dose (formulation ID)* | Cmax (ng/ml) † | Tmax (h) † | AUC ng/ml/h ‡ | k(el)/h † | Ratio norbuprenorphine/ buprenorphine † |
| AG6 0.02 mg/kg (standard/low) (A) | 1.3 (1.0–1.7) | 0.42 (0.25–0.50) | 6.43 ± 3.90 | 0.37570 (0.1300–0.5144) | 0 |
| AG4 0.06 mg/kg (medium) (D) | 3.4 (1.4–4.9) | 0.70 (0.25–2.00) | 61.8 ± 13.2 | 0.0324 (0.0222–0.0431) | – |
| AG2 0.12 mg/kg (high) (A) | 6.5 (5.7–7.7) | 0.75 (0.5–1.00) | 115.6 ± 32.1 | 0.03394 (0.0221–0.0471) | 0.45 (0.17–0.34) |
| AG3 0.12 mg/kg (high) (C) | 20.8 (4.5–51.4) | 0.67 (0.50–1.00) | 163.55 ± 64.40 | 0.04152 (0.0310–0.05063) | 0.5 (0.21–0.99) |
| AG3 0.12 mg/kg (high) (D) | 10.3 (6.1–22.6) | 0.7 (0.5–1.0) | 139.6 ± 11.7 | 0.0371 (0.0204–0.0584) | – |
| AG7 0.12 mg/kg (high) (B) | 14.9 (6.6–29.5) | 0.83 (0.50–1.00) | 160.74 ± 19.80 | 0.04851 (0.0348–0.0688) | 0.32 (0.21–0.53) |
| AG5 0.24 mg/kg (very high) (D) | 15.6 (5.3–22.4) | 0.63 (0.25–1.00) | 265.07 ± 53.60 | 0.04206 (0.0302–0.0497) | 0.40 (0.18–0.61) |
See footnote to Table 1 for formulation IDs
Mean (range)
Mean ± SD
Cmax = peak concentration; Tmax = time to peak concentration; AUC = area under the concentration time curve
Table 5.
Pharmacokinetic parameters from cats after treatment with subcutaneous buprenorphine at 0.02, 0.06, 0.12 and 0.24 mg/kg body weight and using formulations containing 0.3, 0.6 and 1.2 mg/kg with and without preservatives
| Norbuprenorphine | |||
|---|---|---|---|
| Group dose formulation (ID)* | Cmax (ng/ml) † | Tmax (h) † | AUC ng/ml/h ‡ |
| AG6 0.02 mg/kg (standard/low) (A) | None measured | None measured | None measured |
| AG4 0.06 mg/kg (medium) (D) | (0.6–1.0) | ||
| AG2 0.12 mg/kg (high) (A) | 1.1 (0.6–2.7) | 31.3 (20–48) | 54.4 ± 50.8 |
| AG3 0.12 mg/kg (high) (C) | 1.6 (0.8–2.5) | 24 (12–36) | 69.9 ± 38.8 |
| AG3 0.12 mg/kg (high) (D) | 0.8 (0.5–1.1) | 32 (24–48) | – |
| AG7 0.12 mg/kg (high) (B) | 1.0 (0.8–1.2) | 21.3 (20–24) | 53.6 ± 33.9 |
| AG5 0.24 mg/kg (very high) (D) | 1.98 (1.1–2.6) | 38 (24–48) | 103.59 ± 48.83 |
See footnote to Table 1 for formulation IDs
Mean (range)
Mean ± SD
Cmax = peak concentration; Tmax = time to peak concentration; AUC = area under the concentration time curve
Figure 5.
(a) Plasma buprenorphine concentrations measured in 33 cats after subcutaneous (SC) injection of buprenorphine 0.02 mg/kg (AG6, orange, n = 3), 0.06 mg/kg (AG4, pink, n = 6), 0.12 mg/kg (AGs 2, 7 and 3, purple, n = 18) and 0.24 mg/kg (AG5, black, n = 6). Injection at time 0. (b) Plasma buprenorphine concentrations corrected to a standard 0.12 mg/kg dose measured in 33 cats after SC injection of buprenorphine 0.02 mg/kg (AG6, orange, n = 3), 0.06 mg/kg (AG4, pink, n = 6), 0.12 mg/kg (AGs 2, 7 and 3, purple, n = 18), 0.24 mg/kg (AG5, black, n = 6). Injection at time 0. NoP = no preservative;+P = with preservatives
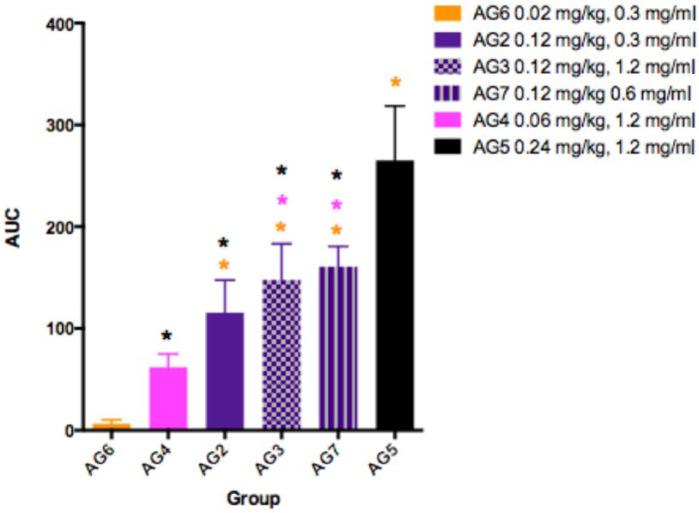
Estimates for the elimination half-life of buprenorphine showed similar variability: 1.35–5.33 h for 0.02 mg/kg (AG6); 16.1–31.2 h for 0.06 mg/kg (AG4); 10.1–34.0 h for 0.12 mg/kg (AGs 2, 3 and 7); and 16.1–31.6 h for 0.24 mg/kg (AG5). The estimates for buprenorphine and norbuprenorphine AUCs, together with average kinetic values for clearance and apparent volume of distribution are shown in Tables 4–6 and Figure 6. The accuracy of the elimination half-life and clearance in the lowest dose group (0.02 mg/kg, AG6) was most likely limited by the sensitivity of the assay. For doses of 0.06–0.24 mg/kg (AGs 2, 3, 4, 5 and 7), there were no significant differences between groups for clearance, apparent volume of distribution or elimination half-life.
Table 6.
Summary of buprenorphine kinetics (mean ± SD) irrespective of formulation, concentration or dose injected where F = unknown bioavailability after subcutaneous dosing
| Dose | AUC ng/ml/h | Clp/F ml/kg/min | Vdbeta/F l/ml | T1/2el h |
|---|---|---|---|---|
| 0.02 mg/kg (standard/low), n = 3 | 6.43 ± 3.89 | ND | ND | 2.71 ± 2.28 |
| 0.06 mg/kg (medium), n = 6 | 61.80 ± 13.18 | 17.07 ± 5.06 | 0.58 ± 0.31 | 22.44 ± 5.56 |
| 0.12 mg/kg (high), n = 18 | 139.10 ± 35.52 | 15.30 ± 4.03 | 0.59 ± 0.21 | 19.77 ± 6.55 |
| 0.24 mg/kg (very high), n = 6 | 265.56 ± 53.58 | 15.62 ± 3.13 | 0.66 ± 0.20 | 17.18 ± 3.43 |
ND = not determined; AUC = area under the curve; Clp = clearance; Vdbeta = apparent volume of distribution; T1/2el = elimination half-life
Figure 6.
Buprenorphine area under the time concentration curve (AUC) in cats after subcutaneous injection of 0.02 mg/kg (AG6, orange, n = 3), 0.06 mg/kg (AG4, pink, n = 6), 0.12 mg/kg buprenorphine (AG2, 0.3 mg/ml, purple plain, n = 6; AG3, 0.12 mg/ml purple checks, n = 9; AG7, 0.6 mg/ml, purple stripes, n = 3) and 0.24 mg/kg (AG5, black, n = 6)
There were detectable plasma concentrations of norbuprenorphine on 25 occasions. After 0.06 mg/kg, norbuprenorphine was detectable in only 2/6 cats, with Cmax concentrations of 0.7 and 1.0 ng/ml. The Cmax concentrations ranged between 0.5 and 2.7 ng/ml (median 0.9 ng/ml) for animals receiving the 0.12 mg/kg dose (n = 17), and 1.1 and 2.6 ng/ml (median 2.2 ng/ml) after the 0.24 mg/kg dose (n = 6). No norbuprenorphine was detected in the plasma following dosing with 0.02 mg/kg; however, the sensitivity of the assay was probably insufficient to detect plasma norbuprenorphine in these cats. Tmax occurred 24 h (4–48 h) and 42 h (24–48 h) after the 0.12 and 0.24 mg/kg dosing, respectively.
Estimates for the norbuprenorphine AUC to 72 h were obtained in 16 cats: in these, the ratio of norbuprenorphine/buprenorphine AUCs ranged between 0.17 and 0.99 (mean ± SD 0.42 ± 0.27) (Tables 4–6).
Pharmacokinetic–pharmacodynamic relationship
The range of normal TT was calculated as the mean ± 2SD of all data collected in the control group AG1 (5% dextrose, n = 6): mean ± SD was 47.8±1.2°C (95% confidence interval 46.9–48.7). An analgesic (antinociceptive) effect was therefore defined as TT >50.2°C. The time point after dosing in each study group where the threshold exceeded this value, and the time to offset, are shown in Table 7, together with the associated plasma buprenorphine concentrations.
Table 7.
Onset and offset of antinociception (analgesia)
| Dose, group (AG), formulation ID* | Cat | Time on (h) | Time off (h) | Associated plasma concentration (ng/ml) |
Time of peak concentration (h) | |
|---|---|---|---|---|---|---|
| On | Off | |||||
| 0.02 mg/kg (standard/low) | 4 | 3.0 | 6 | 11.00 | 0.77 | 1.00 |
| AG6 | 5 | 0.5 | 12 | 1.17 | NQ | 0.50 |
| A | 6 | 4.0 | 12 | NQ | 0.52 | 1.00 |
| 0.06 mg/kg (medium) | 9 | 0.5 | 72 | 4.50 | NQ | 0.25 |
| AG4 | 10 | 3.0 | 30 | 2.35 | 0.60 | 0.25 |
| D | 2 | 2.0 | 72 | 1.20 | NQ | 0.50 |
| 5 | 0.5 | 16 | 4.10 | 1.85 | 2.00 | |
| 11 | 0.5 | 20 | 3.30 | 1.60 | 0.50 | |
| 12 | 12.0 | 36 | 1.50 | 1.10 | 0.75 | |
| 0.12 mg/kg (high) | 1 | 1.0 | 24 | 6.68 | 3.01 | 1.00 |
| AG2 | 2 | 3.0 | 30 | 3.51 | 2.51 | 0.50 |
| A | 3 | 1.0 | 24 | 6.37 | 1.33 | 1.00 |
| 7 | 2.0 | 24 | 4.40 | 2.10 | 1.00 | |
| 8 | 0.5 | 5 | 4.30 | 1.55 | 0.50 | |
| 9 | 1.0 | 36 | 6.80 | 0.70 | 0.50 | |
| AG3 | 1 | 1.0 | 36 | 4.51 | 1.76 | 1.00 |
| C | 2 | 0.5 | 48 | 31.4 | 0.78 | 0.50 |
| 3 | 0.5 | 48 | 6.45 | 0.98 | 0.50 | |
| D | 1 | 1.0 | 48 | 6.16 | 0.90 | 1.00 |
| 2 | 2.0 | 36 | 1.97 | 2.17 | 0.50 | |
| 3 | 0.5 | 48 | 8.75 | 0.98 | 1.00 | |
| 4 | 0.5 | 48 | 7.41 | 0.87 | 0.50 | |
| 5 | 1.0 | 48 | 6.10 | 1.16 | 1.00 | |
| 6 | 1.0 | 20 | 11.30 | 2.62 | 0.50 | |
| AG7 | 4 | 1.0 | 4 | 29.50 | 6.83 | 1.00 |
| B | 5 | 0.5 | 36 | 8.70 | 1.40 | 0.50 |
| 6 | 5.0 | 30 | 6.49 | 1.75 | 1.00 | |
| 0.24 mg/kg (very high) | 8 | 2.0 | 24 | 4.80 | 5.20 | 1.00 |
| AG5 | 11 | 3.0 | 24 | 9.25 | 5.60 | 0.25 |
| D | 1 | 12.0 | 16 | 8.90 | 8.30 | 1.00 |
| 6 | 1.0 | 20 | 15.10 | 5.50 | 0.25 | |
| 7 | 0.5 | 30 | 10.00 | 3.50 | 1.00 | |
| 9 | 0.5 | 72 | 21.30 | NQ | 0.25 | |
Antinociception taken as temperature threshold (TT) >control (AG1) TT ± 2SD: 50.2°C. The best estimate for the ED50 plasma ‘analgesic concentration’ is taken as the ‘offset concentration’
n = 29 (cats with buprenorphine concentration <limit of assay quantitation excluded)
Mean ± SD ‘offset plasma concentration for analgesia’: 2.3 ± 2.0 ng/ml
See footnote to Table 1 for formulation IDs
NQ = not quantified (ie, <0.5 ng/ml)
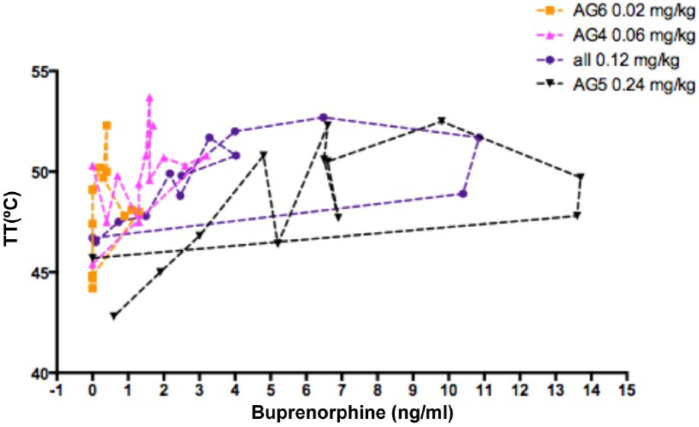
The time of the peak concentration (examining all animals together) was 0.73 ± 0.38 h (range 0.25–2.00 h) and the first time of peak antinociceptive effect was 2.06 ± 2.81 h (range 0.5–12.0 h) (Table 7 and Figure 7). Because of this hysteresis between blood (plasma) and brain effect site concentrations of buprenorphine the plasma concentrations associated with the onset of analgesia will tend to be greater than that at the effect site. As a result, a better estimate for the ED50 plasma ‘analgesic concentration’ is probably reflected as the ‘offset concentration’. When data from 29 occasions are taken together (after exclusion of those cats where the buprenorphine concentration was below the limit of assay quantitation), the mean ‘offset plasma concentration for analgesia’ was 2.3 ± 2.0 ng/ml (Table 7 and Figure 7).
Figure 7.
Plasma buprenorphine concentration effect (thermal threshold [TT]) hysteresis loops from 33 cats after subcutaneous injection of buprenorphine 0.02 mg/kg (AG6, orange, n = 3), 0.06 mg/kg (AG4, pink, n = 6), 0.12 mg/kg (AGs 2, 3 and 7, purple, n = 18), 0.24 mg/kg (AG5, black, n = 6). All loops are anticlockwise
Behaviour and adverse effects
In all groups treated with buprenorphine (AGs 2, 3, 4, 5, 6 and 7) mydriasis developed in most animals and lasted a few hours. The extent and duration of mydriasis was not clearly associated with dose or the increase in TT. No other side effects were noted; cats remained bright, alert and responsive with no obvious behavioural effects. There were no adverse effects that might be considered even remotely life threatening. Small (<2 mm diameter) superficial scaly lesions on the thermal testing site were seen in some cats 24 h after testing. These resolved completely within a few days and did not appear to cause any discomfort; no treatment was required.
Discussion
Nociceptive threshold testing is widely used for laboratory investigation into appropriate dosage and likely duration of clinical analgesic effect. This approach has limitations as clinical pain is not the same as the nociception induced by acute stimuli. However, a number of laboratory investigations have predicted clinical efficacy and duration quite closely, making thermal TT a valuable screening tool for drug development and registration.5,10–14
The data reported here are in general agreement with numerous other studies using thermal TT in cats as a measure of the analgesic effect of buprenorphine.6,13,15–21 All the doses used in this study increased TT above baseline at some point post-treatment. However, the number of animals in several of the groups investigated was too small for robust statistical analysis; previous investigations have reported considerable inter-cat variation,6,13,15–21 thereby reducing the power of small group studies.
In spite of the small numbers, the data strongly suggest that higher doses lead to a longer period of antinociception (see Figure 2) where the effect of the widely used ‘standard’ data sheet dose (0.02 mg/kg) lasted 6–12 h, and the two highest doses led to at least 24 h antinociception in most cases, and up to 48 h in several (Table 5). There was, however, no clear difference between the three higher doses (0.06 mg/kg, 0.12 mg/kg and 0.24 mg/kg). Moreover, the dose appeared to have little effect on the time of onset of action; all started at around 1 h. This is associated with the short time to Tmax in most animals (15–120 mins), although for all formulations there was marked variability in the concentration at Tmax, even after correcting for drug dosage. The estimated elimination half-lives for buprenorphine at doses of 0.06–0.24 mg/kg were between 20 and 30 h, reflecting the long sampling period for which concentrations could be detected, as well as differences in assay sensitivity compared with previously published data for the cat.6,15 Shorter half-lives were seen with the 0.02 mg/kg dose at 1–5 h.
Most cats clearly dislike drug administration by injection, and enabling the dosing frequency to be decreased to once per day has considerable potential for improving feline pain management. By increasing the concentration of buprenorphine in the formulation, a higher dose can be administered in a smaller dose volume compared with formulations currently available. The addition of a preservative to the formulation allows for multiple doses to be withdrawn from a single vial. These formulation changes had no apparent effect on the efficacy or side effects observed in this study.
A further benefit of the dosing reported in this investigation is the apparent effectiveness of the SC route, as this is less unpleasant for the cat and easier for the handler than either the intramuscular (IM) or intravenous (IV) routes. Steagall et al have shown that uptake of standard doses (0.01–0.02 mg/kg) of buprenorphine is poor after SC administration, 15 and this is reflected in both minimal increases in TT and in limited clinical analgesic effect. 12 This has led to strong recommendation that this route should not be used when buprenorphine is used for clinical analgesia in cats. 15 The data in the present report, however, suggest that the slower uptake from the SC route can be used to advantage in allowing a much higher dose to be given, enabling the high dose to produce an adequate plasma concentration, thereby producing prolonged analgesia. The effect is particularly notable for the ability to give a very high dose without causing any adverse effects that might be expected from overdose. The limited analgesic effect of both SC and transdermal buprenorphine previously reported led the authors to surmise that the concentration gradient from the site of administration to effector site was inadequate.12,17,22
A dose relationship for buprenorphine AUC was evident over the complete 0.02–0.24 mg/kg dose range (see Figure 6). However, the smaller-than-predicted AUC for the 0.02 mg/kg dose may reflect an inadequate concentration gradient for the buprenorphine to leave the SC tissues. Alternatively, the LLOQ of the assay and the timing of the blood samples may not have permitted an accurate determination of the AUC at this dosage. For doses in excess of 0.06 mg/kg there appeared to be no effect of formulation concentration. Studies I and III (AGs 2, 6 and 7) examined the effects of 0.3, 0.6 and 1.2 mg/ml formulations, and found no difference in either antinociception or pharmacokinetics, albeit with very small group sizes (see Figures 3 and 5). In study IV (AG3, 0.12 mg/kg), the addition of preservative did not appear to affect antinociception (see Figure 4); nevertheless despite the high plasma buprenorphine concentration, there was less than expected norbuprenorphine when the preserved formulation (D) was given at 0.12 mg/kg. However, with the highest buprenorphine dose in AG5 (0.24 mg/kg dose, formulation D) there was good evidence of drug metabolism. This might suggest that the preservatives in the formulation reduce metabolism of buprenorphine and that a higher dose overrides any postulated inhibition. Methyl- and propylparabens, the preservatives present in formulation D, are widely used in food, cosmetic and pharmaceutical products, and inhibition of drug-metabolising enzymes has not previously been documented for these compounds. 23 An explanation for the variation in buprenorphine metabolism in the presence of the preservatives remains to be investigated.
For those animals receiving a dose of 0.12 mg/kg, the ratio of norbuprenorphine to buprenorphine was 0.35 ± 0.33, while for the 0.24 mg/kg dose it was 0.40 ± 0.18. At the medium dose of 0.06 mg/kg norbuprenorphine was only detected in 2/6 animals, and after 0.02 mg/kg none was detected. These data compare with rhesus macaques, where the ratio was 0.13, dogs, where the ratio was 0.09, and humans, where it was 2.73.23–25 26, 27 Thus, the cat appears to be able to metabolise buprenorphine to norbuprenorphine similarly to dogs and monkeys but probably to a less extent than humans.
Norbuprenophine was detected in most cats treated at 0.12 mg/kg and above. Variation among cats in norbuprenorphine concentration and its ratio to buprenorphine may reflect individual animal differences in their metabolic capability. Buprenorphine metabolites may have intrinsic analgesic properties and contribute to the overall analgesic effect. For instance, norbuprenorphine had mild antinociceptive effects in mice and rats.28,29 Its potential analgesic effect in cats is unknown, and this remains a further unexplored aspect of buprenorphine’s effect in this species. The estimate for the analgesic concentration of buprenorphine may be influenced by the AUC for norbuprenorphine. As there are various routes for the degradation of buprenorphine in different animal species, it is therefore likely that there will be differences in the ‘ED50 plasma analgesic concentrations’ between the species.
Robertson et al reported effective analgesic concentrations to a thermal stimulus of 1.41–4.15 ng/ml. 6 This is comparable with the mean value of 2.3 ng/ml for the offset of analgesia in the present study. However, radioimmunoassay was used for buprenorphine analysis in that study and, as it does not distinguish between buprenorphine and its close metabolites, may have overestimated parent buprenorphine concentration. Taking into account both the present data and that of Robertson et al, 6 an appropriate dose and formulation for clinical use should provide plasma concentrations in excess of 5 ng/ml for 24 h. However, buprenorphine plasma concentrations do not reflect the effect site concentration because of the considerable hysteresis seen in all species. 6 Hence a plasma concentration above 1.41 ng/ml (lower limit reported by Robertson et al 6 ) may be sufficient. This was achieved for up to 2 h after the standard 0.02 mg/kg dose (AG6), for 12–20 h after 0.06 mg/kg (AG4), for 24–36 h after 0.12 mg/kg using the standard 0.3 mg/ml solution (formulation A; AG2), for 24–36 h after 0.12 mg/kg using the 0.6 mg/ml solution (formulation B; AG7), for 24–36 h after 0.12 mg/kg using the 1.2 mg/ml solution (formulation C; AG3) and for 48–71 h after 0.24 mg/kg (AG5) using the 1.2 mg/ml solution (formulation D). These data suggest that any of the formulations given at doses of 0.12 mg/kg or above would be suitable for development. There appears to be no effect on duration of analgesia of preservative or formulation concentration. Despite these doses being considerably in excess of those normally administered to cats (around 0.02 mg/kg) there were no adverse effects other than mild mydriasis, which is usually seen in cats treated with buprenorphine at low doses anyway.13,15,17,19,20
Conclusions
In summary, based on defining TT ± 2SD as antinociception, 0.12 and 0.24 mg/kg doses of aqueous buprenorphine given subcutaneously appear to provide at least 24 h antinociception with no side effects other than mydriasis. The actual dose and concentration appear not to be critical to the antinociceptive effects. Addition of preservatives to a high-concentration buprenorphine formulation had no impact on its antinociceptive properties or side effects profile. High concentration formulations of buprenorphine have potential for clinical use, providing prolonged analgesia by a single SC injection in a minimal dose volume.
Acknowledgments
We thank the staff at Sinclair Research Centre Inc for their care and expert handling of the cats. We thank Liz Cozzi of Abbott Animal Health for much support and encouragement in preparing the manuscript.
Footnotes
PM Taylor and JW Sear are independent scientists and clinicians who have, from time to time, acted as consultants to Abbott Animal Health. PM Taylor is a director of Topcat Metrology Ltd, which developed the thermal threshold testing equipment.
Funding: The investigation was funded by Abbott Animal Health.
Accepted: 19 March 2015
References
- 1. Taylor PM, Robertson SA. Pain management in cats — past, present and future. Part 1. The cat is unique. J Feline Med Surg 2004; 6: 313–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Robertson SA. Managing pain in feline patients. Vet Clin North Am Small Anim Pract 2008; 38: 1267–1290. [DOI] [PubMed] [Google Scholar]
- 3. Polson S, Taylor PM, Yates D. Analgesia after feline ovariohysterectomy under midazolam-medetomidine-ketamine anaesthesia with buprenorphine or butorphanol, and carprofen or meloxicam: a prospective, randomised clinical trial. J Feline Med Surg 2012; 14: 553–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Taylor PM, Kirby JJ, Robinson C, et al. A prospective multi-centre clinical trial to compare buprenorphine and butorphanol for postoperative analgesia in cats. J Feline Med Surg 2010; 12: 247–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Steagall PV, Taylor PM, Rodrigues LC, et al. Analgesia for cats after ovariohysterectomy with either buprenorphine or carprofen alone or in combination. Vet Rec 2009; 164: 359–363. [DOI] [PubMed] [Google Scholar]
- 6. Robertson SA, Lascelles BD, Taylor PM, et al. PK-PD modeling of buprenorphine in cats: intravenous and oral transmucosal administration. J Vet Pharmacol Ther 2005; 28: 453–460. [DOI] [PubMed] [Google Scholar]
- 7. Catbagan DL, Quimby JM, Mama KR, et al. Comparison of the efficacy and adverse effects of sustained-release buprenorphine hydrochloride following subcutaneous administration and buprenorphine hydrochloride following oral transmucosal administration in cats undergoing ovariohysterectomy. Am J Vet Res 2011; 72: 461–466. [DOI] [PubMed] [Google Scholar]
- 8. Dixon MJ, Robertson SA, Taylor PM. A thermal threshold testing device for evaluation of analgesics in cats. Res Vet Sci 2002; 72: 205–210. [DOI] [PubMed] [Google Scholar]
- 9. Dixon M, Taylor PM. Refinement of a thermal threshold probe to prevent burns. 10th World Congress of Veterinary Anaesthesia; Glasgow, UK. August, 2009, p 138. [Google Scholar]
- 10. Robertson SA, Taylor PM. Pain management in cats — past, present and future. Part 2. Treatment of pain — clinical pharmacology. J Feline Med Surg 2004; 6: 321–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Taylor PM, Steagall PV, Dixon MJ, et al. Carprofen and buprenorphine prevent hyperalgesia in a model of inflammatory pain in cats. Res Vet Sci 2007; 83: 369–375. [DOI] [PubMed] [Google Scholar]
- 12. Giordano T, Steagall PV, Ferreira TH, et al. Postoperative analgesic effects of intravenous, intramuscular, subcutaneous or oral transmucosal buprenorphine administered to cats undergoing ovariohysterectomy. Vet Anaesth Analg 2010; 37: 357–366. [DOI] [PubMed] [Google Scholar]
- 13. Steagall PV, Mantovani FB, Taylor PM, et al. Dose-related antinociceptive effects of intravenous buprenorphine in cats. Vet J 2009; 182: 203–209. [DOI] [PubMed] [Google Scholar]
- 14. Slingsby LS, Taylor PM. Thermal antinociception after dexmedetomidine administration in cats: a dose-finding study. J Vet Pharmacol Ther 2008; 31: 135–142. [DOI] [PubMed] [Google Scholar]
- 15. Steagall PV, Pelligand L, Giordano T, et al. Pharmacokinetic and pharmacodynamic modelling of intravenous, intramuscular and subcutaneous buprenorphine in conscious cats. Vet Anaesth Analg 2013; 40: 83–95. [DOI] [PubMed] [Google Scholar]
- 16. Steagall PV, Taylor PM, Brondani JT, et al. Effects of buprenorphine, carprofen and saline on thermal and mechanical nociceptive thresholds in cats. Vet Anaesth Analg 2007; 34: 344–350. [DOI] [PubMed] [Google Scholar]
- 17. Steagall PV, Carnicelli P, Taylor PM, et al. Effects of subcutaneous methadone, morphine, buprenorphine or saline on thermal and pressure thresholds in cats. J Vet Pharmacol Ther 2006; 29: 531–537. [DOI] [PubMed] [Google Scholar]
- 18. Slingsby LS, Murrell JC, Taylor PM. Buprenorphine in combination with naloxone at a ratio of 15:1 does not enhance antinociception from buprenorphine in healthy cats. Vet J 2012; 192: 523–524. [DOI] [PubMed] [Google Scholar]
- 19. Slingsby LS, Murrell JC, Taylor PM. Combination of dexmedetomidine with buprenorphine enhances the antinociceptive effect to a thermal stimulus in the cat compared with either agent alone. Vet Anaesth Analg 2010; 37: 162–170. [DOI] [PubMed] [Google Scholar]
- 20. Slingsby LS, Taylor PM. Pilot dose response study for intravenous buprenorphine using thermal nociceptive threshold testing in cats. AVA-ECVA Spring 2008 meeting. Vet Anaesth Analg 2009; 39: 9–10. [Google Scholar]
- 21. Robertson SA, Taylor PM, Lascelles BD, et al. Changes in thermal threshold response in eight cats after administration of buprenorphine, butorphanol and morphine. Vet Rec 2003; 153: 462–465. [DOI] [PubMed] [Google Scholar]
- 22. Murrell JC, Robertson SA, Taylor PM, et al. Use of a transdermal matrix patch of buprenorphine in cats: preliminary pharmacokinetic and pharmacodynamic data. Vet Rec 2007; 160: 578–583. [DOI] [PubMed] [Google Scholar]
- 23. Huang W, Moody DE, McCance-Katz EF. The in vivo glucuronidation of buprenorphine and norbuprenorphine determined by liquid chromatography-electrospray ionization-tandem mass spectrometry. Ther Drug Monit 2006; 28: 245–251. [DOI] [PubMed] [Google Scholar]
- 24. Nunamaker EA, Halliday LC, Moody DE, et al. Pharmacokinetics of 2 formulations of buprenorphine in macaques (Macaca mulatta and Macaca fascicularis). J Am Assoc Lab Anim Sci 2013; 52: 48–56. [PMC free article] [PubMed] [Google Scholar]
- 25. Abbo LA, Ko JC, Maxwell LK, et al. Pharmacokinetics of buprenorphine following intravenous and oral transmucosal administration in dogs. Vet Ther 2008; 9: 83–93. [PubMed] [Google Scholar]
- 26. Andaluz A, Moll X, Abellan R, et al. Pharmacokinetics of buprenorphine after intravenous administration of clinical doses to dogs. Vet J 2009; 181: 299–304. [DOI] [PubMed] [Google Scholar]
- 27. Andaluz A, Moll X, Ventura R, et al. Plasma buprenorphine concentrations after the application of a 70 microg/h transdermal patch in dogs. Preliminary report. J Vet Pharmacol Ther 2009; 32: 503–505. [DOI] [PubMed] [Google Scholar]
- 28. Brown SM, Holtzman M, Kim, et al. Buprenorphine metabolites, buprenorphine-3-glucuronide and norbuprenorphine-3-glucuronide, are biologically active. Anesthesiol 2011; 115: 1251–1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ohtani M, Kotaki H, Sawada Y, et al. Comparative analysis of buprenorphine- and norbuprenorphine-induced analgesic effects based on pharmacokinetic-pharmacodynamic modeling. J Pharmacol Exp Ther 1995; 272: 505–510. [PubMed] [Google Scholar]