Abstract
Background
Blood levels of N-terminal pro-B-type natriuretic peptide (NT-proBNP) has been suggested as a future guidance tool for the selection of patients for aortic valve replacement. This study aimed to examine how levels of NT-proBNP pre-transcatheter aortic valve implantation (TAVI) is associated with one-year rates of heart failure (HF) admission and mortality following TAVI.
Methods
With Danish nationwide registries, we identified all patients undergoing TAVI from 2014 to 2021 who had at least one recorded NT-pro-BNP measurement within one year before TAVI. Patients were compared by quartiles of pre-TAVI NT-proBNP: quartile 4 (high NT-proBNP group) vs quartile 1–3 (low NT-proBNP group). Comparisons of all-cause mortality and HF-admissions were conducted using Kaplan-Meier analysis, cumulative incidence, and Cox analysis, as appropriate.
Results
We identified 1,140 patients undergoing first-time TAVI with a recorded NT-pro-BNP; 846 (74.2 %) with a low NT-proBNP (<420 pmol/L) (55.0 % male, median age 81 year) and 294 (25.8 %) with a high NT-proBNP (≥420 pmol/L) (53.1 % male, median age 82 year). A high versus low NT-proBNP was associated with increased one-year cumulative incidence of HF-admissions (9.1 % vs. 23.1 %, adjusted HR 2.00 [95 % CI, 1.40–2.85]) and all-cause mortality (6.0 % vs. 14.6 %, adjusted HR 1.95 [95 % CI: 1.24–3.07]). A high NT-proBNP was associated with higher rates of outcomes irrespective of previously known atrial fibrillation, HF, chronic kidney disease, and hypertension.
Conclusion
In patients undergoing TAVI, a baseline NT-proBNP ≥ 420 pmol/L was associated with increased one-year rates of HF-admission and mortality post-TAVI and may be utilized to identify a high-risk population.
Keywords: N-Terminal Pro-B-Type Natriuretic Peptide, NT-proBNP, Transcatheter Aortic Valve, Implantation, TAVI, Aortic Valve Replacement, Aortic Stenosis
1. Introduction
Transcatheter aortic valve implantation (TAVI) has become widely used for the treatment of severe, symptomatic aortic stenosis, and the use of TAVI has expanded over the last years – now exceeding surgical aortic valve intervention in numbers in the US [1], [2], [3]. Still we continue to lack specific biomarkers to help identify patients at high postoperative risk, especially a biomarker that can divide the patients depending on the longitudinal risk. In severe aortic stenosis, the afterload is elevated, propagating to the left atrium and subsequently in the pulmonary circulation, potentially resulting in congestion [4]. ProBNP levels gradually increase along this continuum, and this biomarker, and especially its two fragments C-terminal B-type natriuretic peptide (BNP) and N-terminal Pro-B-Type Natriuretic Peptide (NT-proBNP), could potentially identify patients at particularly high risk following TAVI [5], [6]. There are reports on NT-proBNP and TAVI outcomes, but data on longitudinal rates of heart failure (HF) and death are warranted.
Studies have shown a correlation between high pre-operative NT-proBNP and increased risk of morbidity and mortality [7], [8], [9], [10], [11], [12], [13], [14]. However these studies have been limited by small study populations, single-center studies, and lack of data from the follow-up period (e.g. rehospitalization).
In this study we examined the pre-TAVI distribution of NT-proBNP and how this was related to a one-year risk of HF-admission and death. The study has the potential to provide an overview of the association between NT-proBNP and TAVI-outcomes and help clarify whether NT-proBNP may be utilized to identify a high-risk population.
2. Methods
2.1. Data sources
In Denmark a unique personal Central Person Register (CPR) number allows linkage between administrative registries on a nationwide basis [15], [16].
In this study we retrieved data from The National Population Registry, The Danish National Patient Registry (DNPR), The National Prescription Registry, and Statistics Denmark. From the National Population Registry [17], we obtained data on sex, vital status, migration, birth date, and information on dates of deaths. From the DNPR we retrieved information regarding all hospital admissions from Danish hospitals with discharge diagnosis based on the International Classification of Diseases (ICD)-10 codes. In addition surgical procedures classified according to The Nordic Medico-Statistical Committee [16] were obtained from DNPR as well. From Statistics Denmark we retrieved data on blood samples including NT-proBNP [18].
2.2. Study population
We identified all patients undergoing first-time TAVI between January 1, 2014, and December 31, 2021. The first-time TAVI procedure was defined by the following procedure codes: KFMD11, KMFD12, and KMFD14. Patients who underwent TAVI and had a recorded NT-proBNP blood sample within 1 year prior to TAVI were available for study inclusion (Supplementary Table 2 for NT-proBNP NPU-codes). If more than one NT-pro-BNP-sample were available within 1 year prior to TAVI, the sample closest to TAVI was used.
According to the NT-proBNP levels, patients were classified according to quartiles and separated into two groups: quartile 1–3 (i.e., NT-proBNP < 420 pmol/L) and quartile 4 (i.e., NT-proBNP ≥ 420 pmol/L). This was done to identify a population at particularly high risk with a clinically relevant cutoff. In the supplementary materials, a presentation of the baseline characteristics for each quartile is provided (Supplementary Table 3). Further the associated rate of outcomes was assessed by looking at the NT-proBNP level at baseline as a logarithmic scale.
2.3. Covariates
Comorbidities recorded at any time prior to the admission date were identified through the utilization of International Classification of Diseases − 10th edition (ICD-10) codes, with the following three exceptions: First, hypertension was defined as a diagnosis of hypertension or use of two or more blood pressure lowering drugs as described previously [19]. Second, diabetes was defined as a diagnosis of diabetes or the use of anti-diabetic drugs [20]. Third, chronic kidney disease (CKD) was defined as an eGFR < 60 ml/min/1.73 m2 and was based on the last measured creatinine prior to baseline [21].
Medication was defined as prescription drugs dispensed within 180 days prior to date of the TAVI.
2.4. Outcome and follow-up
We studied three outcomes: HF admission after discharge from TAVI, admission for any cause after discharge from TAVI, and all-cause mortality. HF admission was defined as admission after TAVI discharge with a primary or secondary diagnosis for HF. Further, patients discharged after TAVI were followed from the date of discharge until HF admission, death, end of study period (31 December 2021), or a maximum of 1 year of follow-up, whichever came first.
2.5. Statistics
Baseline characteristics were compared by the two study groups. Continuous variables were presented as medians with interquartile range (IQR) and categorical variables were presented as counts and percentage. Differences in baseline characteristics between the two groups were examined by chi-square or Fisher exact test for categorical variables and the Wilcoxon test for the continuous variables. The one-year cumulative incidence of HF-admission and admission for any cause were assessed with Aalen Johansen estimator considering death as a competing risk, and the crude differences between the two groups were assed with Gray’s test. For patients discharged after TAVI we used the Kaplan-Meier estimator to assess the one-year cumulative incidence of all-cause mortality, and crude differences between the two groups were assessed with the log-rank test. The rate of outcomes was compared between study groups with multivariable Cox proportional hazard analysis, with the group with NT-proBNP < 420 pmol/L serving as a reference. The model examining HF admission included the following variables: sex, age, calendar period (2014–2016, 2017–2019, 2020–2021), and known comorbidities at baseline: atrial fibrillation, chronic HF, ischemic heart disease, pacemaker pre-TAVI, hypertension, and chronic kidney disease. The model examining mortality included the following variables: sex, age, calendar period (2014–2016, 2017–2019, 2020–2021), and known comorbidities at admission (baseline): atrial fibrillation, stroke, chronic HF, ischemic heart disease, treatment with statins, chronic obstructive lung disease, liver disease, chronic kidney disease, and malignancy. To test the robustness of our findings across important subgroups we examined the 1-year rates of outcomes stratified by presence of atrial fibrillation, chronic HF, chronic kidney disease, and hypertension and tested for significant effect modification.
All statistical analyses were performed using the SAS statistical software (version 9.4, Cary, NC, USA) and R (version 3.6.1 The R Foundation) [22]. Level of statistical significance was defined by ad P-value < 0.05.
2.6. Supplemmentary analyses
We performed several sensitivity analyses of both HF-admission after discharge and all-cause mortality, excluding patients with a HF-diagnosis pre-TAVI. The cumulative incidence of first-time HF-admission and cumulative mortality was shown for patients discharged after TAVI. Furthermore, we examined baseline characteristics for all patients undergoing TAVI within the study period regardless of an available NT-proBNP. This was done to illuminate a potential selection bias.
3. Results
3.1. Study population and baseline characteristics
A total of 5,832 patients underwent first-time TAVI during the study period from 2014 to 2021. Among these, 1,161 (19.9 %) had a recorded NT-proBNP within 1-year pre-TAVI. Of these, 14 patients (1.7 %) with a NT-proBNP < 420 pmol/L and 7 patients (2.4 %) with a NT-proBNP ≥ 420 pmol/L died during admission and were therefore excluded, leaving 1,140 TAVI-patients for further analysis (Fig. 1). Of the 1,140 patients, 846 (74.2 %) had a NT-proBNP < 420 pmol/L (55.0 % male, median age 81 years) and 294 (25.8 %) had a NT-proBNP ≥ 420 pmol/L (53.1 % male, median age 82 years). Table 1 displays the patient characteristics. The median time between the recorded NT-proBNP and the TAVI procedure was 1 day, with IQR as presented in Table 1. Transfemoral TAVI access was performed in 97 % of patients with NT-proBNP < 420 pmol/L and 98 % in patients with NT-proBNP ≥ 420 pmol/L. Briefly, compared with patients with NT-proBNP < 420 pmol/L, patients with NT-proBNP ≥ 420 pmol/L presented with higher proportions of comorbidity including hypertension (66.4 % vs 75.2 %), chronic kidney disease (40.2 % vs 64.6 %), atrial fibrillation (32.6 % vs. 41.5 %), and chronic HF (26.1 % vs 54.1 %).
Fig. 1.
Inclusion of patients. This flowchart shows the inclusion of patients in the study.
Table 1.
Baseline characteristics of patients undergoing TAVI with an available NT-proBNP.
|
NT-proBNP < 420 N = 846 |
NT-proBNP ≥ 420 N = 294 |
P-values | |||
|---|---|---|---|---|---|
| Male, N (%) | 465 | (55.0) | 156 | (53.1) | 0.59 |
| Age, median years [IQR] | 80.8 | [75.8, 84.7] | 82.2 | [77.4, 85.8] | < 0.001 |
| Calendar period, N (%) | |||||
| 2014–2016 | 342 | (40.4) | 137 | (46.6) | 0.12 |
| 2017–2021 | 504 | (59.6) | 157 | (53.4) | |
| TAVI modality, N (%) | |||||
| Transapical | 4 | (0.47) | 4 | (1.4) | 0.48 |
| Ministernotomy | 26 | (3.1) | 4 | (1.4) | |
| Transfemoral | 816 | (96.5) | 289 | (98.3) | |
| Pacemaker implantation, N (%) | 105 | (12.4) | 42 | (14.3) | 0.42 |
| Admission time, median days [IQR] | 5.0 | [3.0, 7.0] | 6.0 | [4.0, 12.0] | < 0.001 |
| Time between NT-proBNP blood sample to TAVI, median days [IQR] | 1.0 | [1.0, 4.0] | 1.0 | [1.0, 3.0] | 0.24 |
| Coronary artery revascularization, N (%) | |||||
| CABG | 75 | (8.9) | 26 | (8.8) | 1.00 |
| PCI | 209 | (24.7) | 73 | (24.8) | 1.00 |
| Comorbidities, N (%) | |||||
| Hypertension | 562 | (66.4) | 221 | (75.2) | 0.01 |
| Acute myocardial infarction (AMI) | 150 | (17.7) | 70 | (23.8) | 0.03 |
| Ischemic heart disease | 448 | (53.0) | 168 | (57.1) | 0.22 |
| Chronic heart failure | 221 | (26.1) | 159 | (54.1) | < 0.001 |
| Atrial fibrillation | 276 | (32.6) | 122 | (41.5) | 0.01 |
| Permanent pacemaker | 73 | (8.6) | 31 | (10.5) | 0.35 |
| Stroke (ischemic/hemorrhagic)/ TIA | 184 | (21.7) | 74 | (25.2) | 0.24 |
| Diabetes | 177 | (20.9) | 50 | (17.0) | 0.17 |
| Peripheral artery disease | 56 | (6.6) | 35 | (11.9) | 0.01 |
| Chronic kidney disease (eGFR < 61) | 340 | (40.2) | 190 | (64.6) | < 0.001 |
| Malignancy | 201 | (23.8) | 73 | (24.8) | 0.75 |
| Liver disease | 23 | (2.7) | 9 | (3.1) | 0.84 |
| COPD | 157 | (18.6) | 53 | (18.0) | 0.86 |
| Medication within prior to TAVI admission, N (%) | |||||
| Statins | 545 | (64.4) | 163 | (55.4) | 0.01 |
| Beta blockers | 381 | (45.0) | 152 | (51.7) | 0.05 |
| Loop diuretics | 365 | (43.1) | 207 | (70.4) | < 0.001 |
| Spiron | 64 | (7.6) | 38 | (12.9) | 0.01 |
| Thiazid | 147 | (17.4) | 36 | (12.2) | 0.04 |
| Calcium channel blockers | 259 | (30.6) | 68 | (23.1) | 0.02 |
| RAAS inhibitors | 456 | (53.9) | 143 | (48.6) | 0.14 |
| ASA | 369 | (43.6) | 111 | (37.8) | 0.09 |
| ADP-inhibitors | 226 | (26.7) | 80 | (27.2) | 0.88 |
| OAC | 257 | (30.4) | 112 | (38.1) | 0.02 |
3.2. HF-admission
The one-year cumulative incidence of HF-admission post-TAVI was 9.1 % and 23.1 % for patients with a NT-proBNP < 420 pmol/L and ≥ 420 pmol/L, respectively (p < 0.001 for difference), Fig. 2. This corresponded to an adjusted hazard ratio of 2.00 (95 % CI: 1.40–2.85). When NT-proBNP increase was assessed as logarithmic scale each one-point increase was associated with an increased rate of HF-admission (adjusted hazard ratio 1.36, 95 % CI: 0.95–1.94).
Fig. 2.
One-year cumulative incidence of HF admission in patients undergoing TAVI. This figure shows the one-year cumulative incidence of HF admission post discharge in patients undergoing a first-time TAVI-procedure.
The one-year cumulative incidence of HF-admission post-TAVI for each quartile of NT-pro-BNP was 5.9 % for Q1 (NT-proBNP < 74.2 pmol/L), 10.8 % for Q2 (74.2 ≤ NT-proBNP < 185 pmol/L), 10.8 % for Q3 (185 ≤ NT-proBNP < 420 pmol/L), and 23.1 % for Q4 (NT-proBNP ≥ 420 pmol/L) (Supplementary Fig. 1) (p < 0.001 for difference). Compared with Q1, the adjusted hazard ratio was 1.55 (95 % CI: 0.87 – 2.77) for Q2, 1.19 (95 % CI: 0.63 – 2.25) for Q3, and 2.23 (95 % CI: 1.41 – 4.53) for Q4.
3.3. Readmission for any cause
Total one-year cumulative incidence of readmission post-TAVI for any cause was 58.0 % for the patients with a NT-proBNP < 420 pmol/L and 71.1 % for the patients with a NT-proBNP ≥ 420 pmol/L, (p < 0.001 for difference), Supplementary Fig. 2A. This corresponded with a hazard ratio of 1.28 (95 % CI: 1.06 – 1.51). When NT-proBNP increase was assessed as logarithmic scale each one-point increase was associated with an increased risk of readmission for any cause (adjusted hazard ratio 1.09, 95 % CI: 1.03 – 1.17).
The one-year cumulative incidence of readmission post-TAVI for any cause for each quartile of NT-pro-BNP was 52.8 % for Q1 (NT-proBNP < 74.2 pmol/L), 60.3 % for Q2 (74.2 ≤ NT-proBNP < 185 pmol/L), 61.4 % for Q3 (185 ≤ NT-proBNP < 420 pmol/L), and 71.1 % for Q4 (NT-proBNP ≥ 420 pmol/L) (Supplementary Fig. 2B) (p < 0.001 for difference). Compared with Q1, the adjusted hazard ratio was 1.08 (95 % CI: 0.87–1.35) for Q2, 1.07 (95 % CI: 0.84–1.36) for Q3, and 1.35 (95 % CI: 1.08–1.69) for Q4.
3.4. Mortality
The one-year cumulative incidence of all-cause mortality was 6.0 % and 14.6 % for patients with a NT-proBNP < 420 pmol/L and ≥ 420 pmol/L, respectively (p < 0.001 for difference) Fig. 3. This corresponded to an adjusted hazard ratio of 1.95 (95 % CI: 1.24–3.07). When NT-proBNP increase was assessed as logarithmic scale each one-point increase was associated with an increased risk of all-cause mortality (adjusted hazard ratio 1.28, 95 % CI: 1.08 – 1.53).
Fig. 3.
One-year cumulative incidence of all-cause mortality in patients undergoing TAVI. This figure shows the one-year cumulative incidence of all-cause mortality in patients undergoing a first-time TAVI procedure.
The one-year cumulative incidence of all-cause mortality for each quartile of NT-pro-BNP was 3.8 % for Q1 (NT-proBNP < 74.2 pmol/L), 6.1 % for Q2 (74.2 ≤ NT-proBNP < 185 pmol/L), 8.5 % for Q3 (185 ≤ NT-proBNP < 420 pmol/L), and 14.6 % for Q4 (NT-proBNP ≥ 420 pmol/L) (Supplementary Fig. 1) (p < 0.001 for difference). Compared with Q1, the adjusted hazard ratio was 1.40 (95 % CI: 0.64–3.06) for Q2, 1.79 (95 % CI: 0.78–4.09) for Q3, and 2.83 (95 % CI: 1.32–6.07) for Q4.
3.5. Subgroup analyses
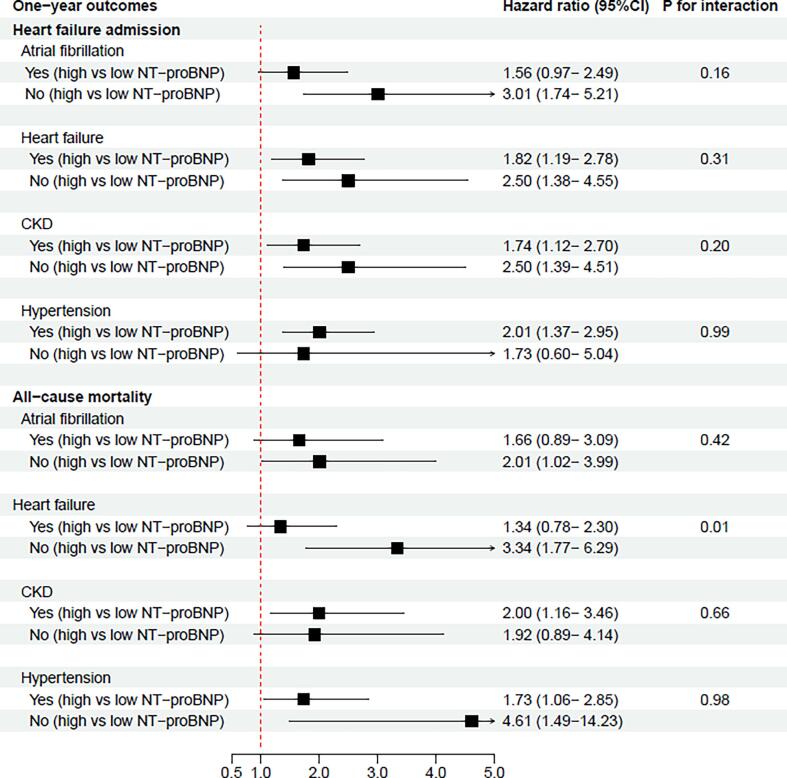
Further, we examined the adjusted rates of HF-admission and all-cause mortality, stratified by the comorbidities atrial fibrillation, chronic HF, chronic kidney disease, and hypertension. According to patients with and without atrial fibrillation, chronic HF, chronic kidney disease and hypertension, Fig. 4 demonstrates the adjusted one-year rates of HF-admission and all-cause mortality. A NT-proBNP ≥ 420 pmol/L was associated higher one-year rates of HF-admissions irrespective of atrial fibrillation (p = 0.16 for interaction), chronic HF (p = 0.31 for interaction), chronic kidney disease (p = 0.20 for interaction), and hypertension (p = 0.99 for interaction). For the outcome of mortality previous HF served as an effect modifier (p = 0.01 for interaction) while no interaction was identified for the other covariates.
Fig. 4.
Forrest plot of one-year HF admissions rates and one-year mortality rates according to pre-specified subgroups. This figure shows the adjusted hazard ratios associated with high versus low NT-proBNP (≥420 pmol/L versus < 420 pmol/L) and one-year HF admission and all-cause mortality in patients with and without the following comorbidities: atrial fibrillation, HF, chronic kidney disease, and hypertension. For the adjusted hazard ratios, the group with NT-proBNP < 420 pmol/L served as a reference.
3.6. Supplementary analyses
Two supplementary analyses were conducted. First when examining the outcomes in HF-naïve patients the one-year cumulative incidence of HF-admission post-TAVI was 5.3 % and 13.3 % for patients with a NT-proBNP < 420 pmol/L and ≥ 420 pmol/L, respectively (p < 0.001), Supplementary Fig. 3. The result corresponded to an adjusted hazard ratio of 2.50 (95 % CI: 1.38 – 4.55). The one-year cumulative incidence of all-cause mortality was 3.8 % and 13.3 % for HF-naïve patients with a NT-proBNP < 420 pmol/L and ≥ 420 pmol/L, respectively (p < 0.001), Supplementary Fig. 4, this corresponded to an adjusted hazard ratio of 3.33 (95 % CI: 1.77–6.29). Second, when comparing the baseline characteristics between the included patients and the group of patients who were not included in the study due to the unavailability of NT-proBNP (Supplementary Table 1), no major differences were revealed. The majority of patients in the excluded group underwent the procedure during the most recent calendar period (2020–2021), this differed from our study population where most patient underwent the procedure between 2017 and 2019. Chronic HF as a comorbidity were more common in the main population, compared with the excluded group of patients.
4. Discussion
In this nationwide cohort study we examined the association between the measured level of NT-proBNP before TAVI and the longitudinal outcomes post-TAVI. The major findings of this study were threefold: First, patients with a NT-proBNP ≥ 420 pmol/L were slightly older, had more comorbidities and had a higher associated rate of both HF-admissions and death following the TAVI procedure. Second, although being less pronounced, these associations were shown to be consistent in a HF naïve population. Third, we found no important effect modifiers and the association was similar across subgroups with and without atrial fibrillation, chronic kidney disease, and hypertension.
Among patients undergoing a first-time TAVI procedure, those with a NT-proBNP ≥ 420 pmol/L were a few years older, had higher prevalence of atrial fibrillation, peripheral artery disease, chronic kidney disease, and chronic heart faillure. This corresponded well with the baseline characteristics in similar studies.[8], [10], [11], [23] A common characteristic observed in the majority of prior studies was the median and mean age of the study population (81–83 years). Further the prevalence of atrial fibrillation in the simillar studies was between 20 and 55 %, which corresponds well with our results.
The baseline NT-proBNP levels were measured with a median time of 1 day prior to TAVI in both groups. The cutoff value for NT-proBNP that separated the two groups (420 pmol/L) was based on the fourth quartile’s cutoff value, and when comparing this value to other similar studies the baseline NT-proBNP value associated with worse outcomes was within the same range (e.g. 236 pmol/L and 554 pmol/L).[9], [10] Hence, our categorization of NT-proBNP is comparable to other studies. In Elhmidi et al.’s prospective study from 2013 they included 373 TAVI-patients and divided the patients into tertiles based on the baseline NT-proBNP-level. The upper tertile was defined by a NT-proBNP ≥ 4,991 ng/L (≈ 556.7 pmol/L). They found that baseline NT-proBNP was associated with a patient risk profile at baseline and that patients in the third tertile had significantly higher 1-year mortality (29.2 %) than patients in the first (14.9 %) and second (20.2 %) tertiles (p < 0.05).[10] These results show a similar trend as well, although the data are from a very different era of TAVI. Our results are mainly based on transfemoral TAVI and baseline characteristics in selection for TAVI have developed significantly over the past 10 years.[24].
Compared with NT-proBNP below 420 pmol/L, patients with NT-proBNP ≥ 420 pmol/L were associated with a higher risk of admission with HF and all-cause mortality following the TAVI procedure. This association persisted after multivariable adjustments for patient characteristics known to be associated with adverse outcomes and remained stable even after excluding patients with a previous history of HF. In subgroup analyses, the associations were consistent irrespective of atrial fibrillation, HF, chronic kidney disease, and hypertension. In the mortality subgroup analysis however, the risk of death was more pronounced in patients with high NT-proBNP and no known HF-diagnosis relative to patients with high NT-proBNP and a known HF-diagnosis. Prior studies have reported similar [8], [9], [13], [14], [23], [25], but also dissimilar [26], [27] results for the association between pre-TAVI NT-proBNP and longitudinal risks. In a prospective study from 2014 on 333 TAVI patients with a 4-year follow-up, Ribeiro et al. found that a baseline NT-proBNP cut-off value off 2,200 pg/ml (≈ 236 pmol/L) identified patients at greater risk of both cardiac death and rehospitalization due to HF, with a HF-admission rate of 22.9 % for patients in the group with a high NT-proBNP and 10.8 % for patients in the low NT-proBNP group. Further their results on mortality for the patients with a high and a low NT-proBNP were 28.9 % and 16.7 % respectively.[9] The association between a high baseline NT-proBNP and mortality was furthermore confirmed in the retrospective study on 504 patients with a normal left ventricular ejection fraction undergoing TAVI from 2010 to 2018. Here they found a mortality rate around 21 % and 10 % for Q4 (median NT-proBNP: 425 pmol/L) and Q1-3 (median NT-proBNP: 82 pmol/L), respectively.[11] Although these associations were more pronounced compared with our results, these studies show a similar trend. On the other hand, dissimilar results were found in a retrospective study from 2014, including 845 patients undergoing TAVI.[26] The study aim was to develop a pre-procedural risk evaluation scheme beyond the current surgical risk scores and pre-procedural NT-proBNP was included as a variable. However, the pre-procedural NT-proBNP in their sample did not reach statistical significance in their relation to mortality.
The association between a high baseline NT-proBNP and one-year outcomes could partially be explained by the higher rate of comorbidities such as HF, atrial fibrillation, and chronic kidney disease in the group with NT-proBNP ≥ 420 pmol/L. In addition, the higher rate of both HF-admission and all-cause mortality could be explained by the higher NT-proBNP as a surrogate for chronic HF. NT-proBNP is already well implemented in the screening of HF.[28] Further NT-proBNP has been linked to severity of aortic stenosis [29], [30] and therefore an increased risk of serious remodeling of the left ventricle.[31] Thus, NT-proBNP can be an additional way of risk stratifying the patients both pre- and post-TAVI. In a smaller study from 2003, Gerber et al. found that natriuretic peptide levels were better at distinguishing between symptomatic and asymptomatic patients compared to other used measures of aortic stenosis severity such as peak aortic velocity and the aortic valve area.[30] Subsequent studies suggested that NT-proBNP was included in a risk stratification strategy for TAVI patients [9], [11] and it was further suggested that improving the hemodynamic status, thus reducing the pro-BNP levels could improve the peri-procedural and long-term risk following TAVI.[32] Based on the results from our study, we believe that alternative parameters to echocardiography, which moreover are easily accessible, can assist in risk stratification of patients undergoing TAVI. For future studies it would be of interest to investigate whether potential therapeutic interventions such as standard HF-treatment could help TAVI-patients by reducing the burden of HF-admission and mortality following TAVI.
4.1. Limitation
The results of this study should be viewed in the context of some limitations. Only 20 % of the eligible TAVI patients had a recorded NT-proBNP—however, those with and without recorded NT-proBNP were not significantly different. The volume of NT-proBNP samples is decreasing from the start of our inclusion period to the end of the inclusion period, most likely due to the cost of the blood sample. Another limitation is that all registry studies are dependent on the underlying validity of the diagnosis codes used. While the registries exhibit a high level of data completeness, important covariates are not available, including frailty score, smoking status, echocardiography measurements, body mass index (BMI), and procedure-related complications. Finally, the observational design of the study presents a limitation, particularly due to the risk of cause-effect relationships. Despite our efforts to adjust for potential confounders, we cannot guarantee complete elimination of confounding.
5. Conclusion
In patients undergoing TAVI, a baseline NT-proBNP ≥ 420 pmol/L was associated with an almost two-fold increased one-year rate of HF-admissions and mortality post-TAVI. These associations held true even after excluding patients with previously known HF. The results suggest that NT-proBNP is associated with adverse outcomes and that this may be utilized to identify a high-risk population, however further investigation is warranted.
Funding
Dr. Sørensen has received funding from the Foundation Rigshospitalet outside this work, no further funding was obtained.
CRediT authorship contribution statement
Louise Marqvard Sørensen: Writing – review & editing, Writing – original draft, Visualization, Project administration, Methodology, Investigation, Funding acquisition, Formal analysis, Data curation, Conceptualization. Jeppe Kofoed Petersen: Writing – review & editing, Formal analysis, Data curation. Jarl Emanuel Strange: Writing – review & editing, Formal analysis. Lauge Østergaard: Writing – review & editing, Formal analysis. Jacob Eifer Møller: Writing – review & editing, Formal analysis. Morten Schou: Writing – review & editing, Formal analysis. Lars Køber: Writing – review & editing, Formal analysis. Ole de Backer: Writing – review & editing. Emil Fosbøl: Writing – review & editing, Writing – original draft, Visualization, Project administration, Methodology, Investigation, Funding acquisition, Formal analysis, Data curation, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
None
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijcha.2024.101423.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
Supplementary figure 1: One-year cumulative incidence of first time HF admission and all-cause mortality in patients undergoing TAVI (quartiles). This figure shows the one-year cumulative incidence of HF admission (A) and all-cause mortality (B) for each quartile in patients undergoing a first-time TAVI procedure.
Supplementary figure 2A+B: One-year cumulative incidence of admission for any cause in patients undergoing TAVI (A: groups, B: quartiles). This figure shows the one-year cumulative incidence of admission for any cause post-discharge in patients undergoing a first-time TAVI procedure. Part A shows the one-cumulative incidence for the two groups and part B shows the one-year cumulative incidence for each quartile.
Supplementary figure 3A+B: One-year cumulative incidence of first time HF admission in patients undergoing TAVI (A: HF patients excluded, B: HF patients included). This figure shows the one-year cumulative incidence of HF admission post discharge in both HF-naïve patients (part A) and for all included patients (part B) undergoing a first-time TAVI-procedure.
Supplementary figure 4A+B. One-year cumulative incidence of all-cause mortality in patients undergoing TAVI (A: HF patients excluded, B: HF patients included). This figure shows the one-year cumulative incidence of all-cause mortality post discharge in both HF-naïve patients (part A) and for all included patients (part B) undergoing a first-time TAVI-procedure.
References
- 1.Carroll J.D., et al. STS-ACC TVT Registry of Transcatheter Aortic Valve Replacement. J Am Coll Cardiol. 2020;76(21):2492–2516. doi: 10.1016/j.jacc.2020.09.595. [DOI] [PubMed] [Google Scholar]
- 2.Paradies V., Mamas M.A. Aortic valve treatment: from the first aortic valve replacement to the last decade of revolution. Heart. 2023;109(7):502–503. doi: 10.1136/heartjnl-2022-321933. [DOI] [PubMed] [Google Scholar]
- 3.Siontis G.C.M., et al. Transcatheter aortic valve implantation vs. surgical aortic valve replacement for treatment of symptomatic severe aortic stenosis: an updated meta-analysis. Eur Heart J. 2019;40(38):3143–3153. doi: 10.1093/eurheartj/ehz275. [DOI] [PubMed] [Google Scholar]
- 4.Qi W., et al. Natriuretic peptides in patients with aortic stenosis. Am Heart J. 2001;142(4):725–732. doi: 10.1067/mhj.2001.117131. [DOI] [PubMed] [Google Scholar]
- 5.Logeart D., et al. Predischarge B-type natriuretic peptide assay for identifying patients at high risk of re-admission after decompensated heart failure. J Am Coll Cardiol. 2004;43(4):635–641. doi: 10.1016/j.jacc.2003.09.044. [DOI] [PubMed] [Google Scholar]
- 6.Weber M., et al. Relation of N-terminal pro B-type natriuretic peptide to progression of aortic valve disease. Eur Heart J. 2005;26(10):1023–1030. doi: 10.1093/eurheartj/ehi236. [DOI] [PubMed] [Google Scholar]
- 7.Kefer J., et al. Usefulness of B-type natriuretic peptide to predict outcome of patients treated by transcatheter aortic valve implantation. Am J Cardiol. 2010;106(12):1782–1786. doi: 10.1016/j.amjcard.2010.07.051. [DOI] [PubMed] [Google Scholar]
- 8.Spargias K., et al. The predictive value and evolution of N-terminal pro-B-type natriuretic peptide levels following transcutaneous aortic valve implantation. J Interv Cardiol. 2011;24(5):462–469. doi: 10.1111/j.1540-8183.2011.00654.x. [DOI] [PubMed] [Google Scholar]
- 9.Ribeiro H.B., et al. Long-term prognostic value and serial changes of plasma N-terminal prohormone B-type natriuretic peptide in patients undergoing transcatheter aortic valve implantation. Am J Cardiol. 2014;113(5):851–859. doi: 10.1016/j.amjcard.2013.11.038. [DOI] [PubMed] [Google Scholar]
- 10.Elhmidi Y., et al. The evolution and prognostic value of N-terminal brain natriuretic peptide in predicting 1-year mortality in patients following transcatheter aortic valve implantation. J Invasive Cardiol. 2013;25(1):38–44. [PubMed] [Google Scholar]
- 11.Seoudy H., et al. Prognostic implications of N-terminal pro-B-type natriuretic peptide in patients with normal left ventricular ejection fraction undergoing transcatheter aortic valve implantation. Int J Cardiol. 2020;301:195–199. doi: 10.1016/j.ijcard.2019.11.101. [DOI] [PubMed] [Google Scholar]
- 12.Kaneko H., et al. Impact of N-terminal pro-B-type natriuretic peptide response on long-term prognosis after transcatheter aortic valve implantation for severe aortic stenosis and heart failure. Heart Vessels. 2019;34(5):777–783. doi: 10.1007/s00380-018-1297-z. [DOI] [PubMed] [Google Scholar]
- 13.Allen C.J., et al. Baseline NT-proBNP Accurately Predicts Symptom Response to Transcatheter Aortic Valve Implantation. J Am Heart Assoc. 2020;9(23):e017574. doi: 10.1161/JAHA.120.017574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abramowitz Y., et al. Impact of Preprocedural B-Type Natriuretic Peptide Levels on the Outcomes After Transcatheter Aortic Valve Implantation. Am J Cardiol. 2015;116(12):1904–1909. doi: 10.1016/j.amjcard.2015.09.031. [DOI] [PubMed] [Google Scholar]
- 15.Mainz J., Hess M.H., Johnsen S.P. The Danish unique personal identifier and the Danish Civil Registration System as a tool for research and quality improvement. Int J Qual Health Care. 2019;31(9):717–720. doi: 10.1093/intqhc/mzz008. [DOI] [PubMed] [Google Scholar]
- 16.Schmidt M., et al. The Danish National Patient Registry: a review of content, data quality, and research potential. Clin Epidemiol. 2015;7:449–490. doi: 10.2147/CLEP.S91125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kildemoes H.W., Sørensen H.T., Hallas J. The Danish National Prescription Registry. Scand J Public Health. 2011;39(7 Suppl):38–41. doi: 10.1177/1403494810394717. [DOI] [PubMed] [Google Scholar]
- 18.Arendt J.F.H., et al. Existing Data Sources in Clinical Epidemiology: Laboratory Information System Databases in Denmark. Clin Epidemiol. 2020;12:469–475. doi: 10.2147/CLEP.S245060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Olesen J.B., et al. Validation of risk stratification schemes for predicting stroke and thromboembolism in patients with atrial fibrillation: nationwide cohort study. Bmj. 2011;342 doi: 10.1136/bmj.d124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schramm T.K., et al. Diabetes patients requiring glucose-lowering therapy and nondiabetics with a prior myocardial infarction carry the same cardiovascular risk: a population study of 3.3 million people. Circulation. 2008;117(15):1945–1954. doi: 10.1161/CIRCULATIONAHA.107.720847. [DOI] [PubMed] [Google Scholar]
- 21.Stevens P.E., Levin A. Evaluation and management of chronic kidney disease: synopsis of the kidney disease: improving global outcomes 2012 clinical practice guideline. Ann Intern Med. 2013;158(11):825–830. doi: 10.7326/0003-4819-158-11-201306040-00007. [DOI] [PubMed] [Google Scholar]
- 22.R Core Team. R: a language and environment for statistical computing 2018; Available from: https://www.R-project.org/.
- 23.Koskinas K.C., et al. Effect of B-type natriuretic peptides on long-term outcomes after transcatheter aortic valve implantation. Am J Cardiol. 2015;116(10):1560–1565. doi: 10.1016/j.amjcard.2015.08.016. [DOI] [PubMed] [Google Scholar]
- 24.Strange J.E., et al. Temporal trends in utilization of transcatheter aortic valve replacement and patient characteristics: A nationwide study. Am Heart J. 2022;243:140–146. doi: 10.1016/j.ahj.2021.09.010. [DOI] [PubMed] [Google Scholar]
- 25.Chen S., et al. Low and elevated B-type natriuretic peptide levels are associated with increased mortality in patients with preserved ejection fraction undergoing transcatheter aortic valve replacement: an analysis of the PARTNER II trial and registry. Eur Heart J. 2020;41(8):958–969. doi: 10.1093/eurheartj/ehz892. [DOI] [PubMed] [Google Scholar]
- 26.Seiffert M., et al. Development of a risk score for outcome after transcatheter aortic valve implantation. Clin Res Cardiol. 2014;103(8):631–640. doi: 10.1007/s00392-014-0692-4. [DOI] [PubMed] [Google Scholar]
- 27.Hultkvist H., et al. Rise and fall of NT-proBNP in aortic valve intervention. Open Heart. 2018;5(1):e000739. doi: 10.1136/openhrt-2017-000739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ponikowski P., et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;37(27):2129–2200. doi: 10.1093/eurheartj/ehw128. [DOI] [PubMed] [Google Scholar]
- 29.Weber M., et al. Relation of N-terminal pro-B-type natriuretic peptide to severity of valvular aortic stenosis. Am J Cardiol. 2004;94(6):740–745. doi: 10.1016/j.amjcard.2004.05.055. [DOI] [PubMed] [Google Scholar]
- 30.Gerber I.L., et al. Increased plasma natriuretic peptide levels reflect symptom onset in aortic stenosis. Circulation. 2003;107(14):1884–1890. doi: 10.1161/01.CIR.0000060533.79248.0C. [DOI] [PubMed] [Google Scholar]
- 31.Rader F., et al. Left ventricular hypertrophy in valvular aortic stenosis: mechanisms and clinical implications. Am J Med. 2015;128(4):344–352. doi: 10.1016/j.amjmed.2014.10.054. [DOI] [PubMed] [Google Scholar]
- 32.López-Otero D., et al. Pro B-type natriuretic peptide plasma value: a new criterion for the prediction of short- and long-term outcomes after transcatheter aortic valve implantation. Int J Cardiol. 2013;168(2):1264–1268. doi: 10.1016/j.ijcard.2012.11.116. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary figure 1: One-year cumulative incidence of first time HF admission and all-cause mortality in patients undergoing TAVI (quartiles). This figure shows the one-year cumulative incidence of HF admission (A) and all-cause mortality (B) for each quartile in patients undergoing a first-time TAVI procedure.
Supplementary figure 2A+B: One-year cumulative incidence of admission for any cause in patients undergoing TAVI (A: groups, B: quartiles). This figure shows the one-year cumulative incidence of admission for any cause post-discharge in patients undergoing a first-time TAVI procedure. Part A shows the one-cumulative incidence for the two groups and part B shows the one-year cumulative incidence for each quartile.
Supplementary figure 3A+B: One-year cumulative incidence of first time HF admission in patients undergoing TAVI (A: HF patients excluded, B: HF patients included). This figure shows the one-year cumulative incidence of HF admission post discharge in both HF-naïve patients (part A) and for all included patients (part B) undergoing a first-time TAVI-procedure.
Supplementary figure 4A+B. One-year cumulative incidence of all-cause mortality in patients undergoing TAVI (A: HF patients excluded, B: HF patients included). This figure shows the one-year cumulative incidence of all-cause mortality post discharge in both HF-naïve patients (part A) and for all included patients (part B) undergoing a first-time TAVI-procedure.