Abstract
Infective endocarditis caused by Gemella species is increasingly recognized as an emerging clinical entity. Gemella species are fastidious gram-positive cocci that are typically commensal organisms but can become opportunistic pathogens. This systematic review aimed to provide a comprehensive overview of endocarditis due to Gemella species by synthesizing existing evidence. A total of 52 case reports were identified through a rigorous search and selection process. The most prevalent causative species were G. morbillorum (46.3%) and G. haemolysans (25.9%), with a striking male predominance (79.6%). The clinical presentation was largely nonspecific, mirroring typical infective endocarditis. However, the indolent nature of the illness and fastidious growth requirements of Gemella species often led to diagnostic delays. Echocardiography, particularly transesophageal echocardiography, played a crucial role in the diagnosis, enabling the detection of valvular vegetation and the assessment of complications. Management posed significant challenges, including the need for broad-spectrum empirical antibiotic therapy and increasing antimicrobial resistance among Gemella isolates. Surgical intervention was frequently required for severe valvular dysfunction, persistent infection, or embolic complications. Despite advances in diagnosis and treatment, endocarditis due to Gemella species remains associated with significant morbidity and mortality, underscoring the importance of early recognition and multidisciplinary management. This review highlights the emerging clinical significance of Gemella species as causative agents of infective endocarditis and identifies areas for further research.
Keywords: systematic review, review, tricuspid, aortic, mitral, infection, valvular heart diseases, echocardiography, gemella, endocarditis
Introduction and background
Infective endocarditis (IE) is a serious and potentially life-threatening condition characterized by microbial infection of the endocardium, most commonly affecting the heart valves [1]. Historically, IE has been predominantly associated with well-known pathogens such as Streptococcus and Staphylococcus species [2]. However, there is growing recognition of the role of fastidious bacteria in the etiology of this condition. Among these fastidious organisms, Gemella species have emerged as noteworthy contributors to infective endocarditis [3-7]. Gemella species belong to the genus Gemella, a group of gram-positive cocci that are facultative anaerobes. These organisms are part of the normal flora of humans [8]. While they typically exist as commensals, Gemella species possess virulence factors that enable them to cause disease under certain conditions. In recent years, Gemella species, including G. haemolysans, G. morbillorum, and G. sanguinis, have been increasingly implicated in cases of infective endocarditis, highlighting the importance of understanding their role in this clinical context.
Endocarditis due to Gemella species presents several unique challenges compared to endocarditis caused by more commonly recognized pathogens. The clinical presentation of endocarditis due to Gemella species often mirrors that of typical IE, with symptoms such as fever, malaise, new-onset murmurs, and signs of systemic embolization. However, the nonspecific nature of these symptoms can complicate diagnosis, leading to delays in appropriate management [9]. Furthermore, Gemella species are known for their fastidious growth requirements, making their isolation and identification challenging in routine microbiological cultures. The diagnosis of endocarditis due to Gemella species relies on a combination of clinical evaluation, echocardiography, and microbiological studies. Echocardiography, including transthoracic echocardiography (TTE) and transesophageal echocardiography (TEE), plays a crucial role in detecting valvular vegetation, assessing valvular function, and identifying complications such as abscess formation or valvular regurgitation. Microbiological confirmation of endocarditis due to Gemella species is often based on peripheral blood culture [9].
The management of endocarditis due to Gemella species poses several therapeutic challenges. Empirical antibiotic therapy must be carefully chosen to cover the spectrum of potential pathogens, including fastidious organisms like Gemella species. However, the increasing prevalence of antimicrobial resistance among Gemella isolates underscores the importance of tailored antibiotic regimens based on susceptibility testing and clinical response [10]. Surgical intervention, such as valve repair or replacement, may be necessary in cases of severe valvular dysfunction, persistent infection, or embolic complications. Despite advances in diagnostic techniques and treatment modalities, endocarditis due to Gemella species remains associated with significant morbidity and mortality. Therefore, there is a critical need for enhanced awareness, early recognition, and multidisciplinary management of this condition. This systematic review aims to provide a comprehensive overview of Gemella-associated endocarditis, including its clinical presentation, diagnostic approach, treatment strategies, and outcomes. By synthesizing existing evidence and identifying areas for further research, this review seeks to inform clinical practice, guide therapeutic decision-making, and improve patient outcomes in the management of endocarditis due to Gemella species.
Review
Materials and methods
Search Strategy
This systematic review adheres to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines to ensure transparency and rigor in the review process. A comprehensive search of relevant literature was conducted across prominent databases renowned for their extensive coverage of medical and scientific literature, including PubMed, Embase, Web of Science, and Scopus. These databases were selected for their comprehensive collection of peer-reviewed articles, providing a robust foundation for our systematic review of endocarditis caused by Gemella species. The search strategy employed a meticulously curated set of keywords and phrases aligned with the objectives of the study. These included terms such as "Gemella" and "Endocarditis". Boolean operators "AND" and "OR" were strategically utilized to construct the search algorithm. For instance, the string "Gemella AND Endocarditis" focused specifically on studies addressing endocarditis caused by Gemella species, while the use of "OR" facilitated the inclusion of broader terms associated with the topic. To ensure the inclusion of contemporary and relevant literature, the search was limited to studies published from the inception of each database to January 2024. This timeframe allowed for the incorporation of both historical and current research, offering a comprehensive overview of endocarditis caused by Gemella species. Filters were applied to include studies published in the English language and those involving human subjects, in line with the objectives of our review. Additionally, manual searches of the reference lists of included studies and relevant reviews were conducted to supplement the electronic database search.
Eligibility Criteria
The eligibility criteria for this systematic review were established to ensure precision and relevance in selecting studies for inclusion. Peer-reviewed research articles, observational studies, case reports, and clinical trials were considered eligible for inclusion, reflecting a commitment to evidence-based knowledge. To maintain methodological rigor, only studies published in the English language were included, acknowledging English as the predominant language of scientific communication. The inclusion timeframe spanned from the inception of the respective databases to the present date, allowing for the synthesis of contemporary research on endocarditis caused by Gemella species. Conversely, exclusion criteria were carefully tailored to maintain focus and rigor. Studies not directly addressing endocarditis caused by Gemella species, as well as those lacking relevant outcome measures, were excluded. Non-English language publications, unpublished works, and gray literature such as conference abstracts were also excluded. Furthermore, studies presenting insufficient data on endocarditis caused by Gemella species were excluded to ensure the integrity of the review's findings.
Data Extraction
The data extraction process was conducted in a meticulous and structured manner to ensure the accuracy and completeness of the review findings. This process involved two stages, emphasizing thoroughness and reliability. In the initial stage, articles were screened based on the relevance indicated by titles and abstracts. Two independent reviewers assessed each article's abstract to determine its relevance to the review's focus. Articles deemed relevant or probably relevant underwent a detailed examination in the second stage. In the second stage, full-text articles meeting the inclusion criteria underwent a detailed data extraction process. Two independent reviewers utilized a standardized data extraction template within Microsoft Excel to capture and organize critical information from each study. Any discrepancies between reviewers were resolved through the adjudication of a third independent reviewer.
Results
Study Selection Process
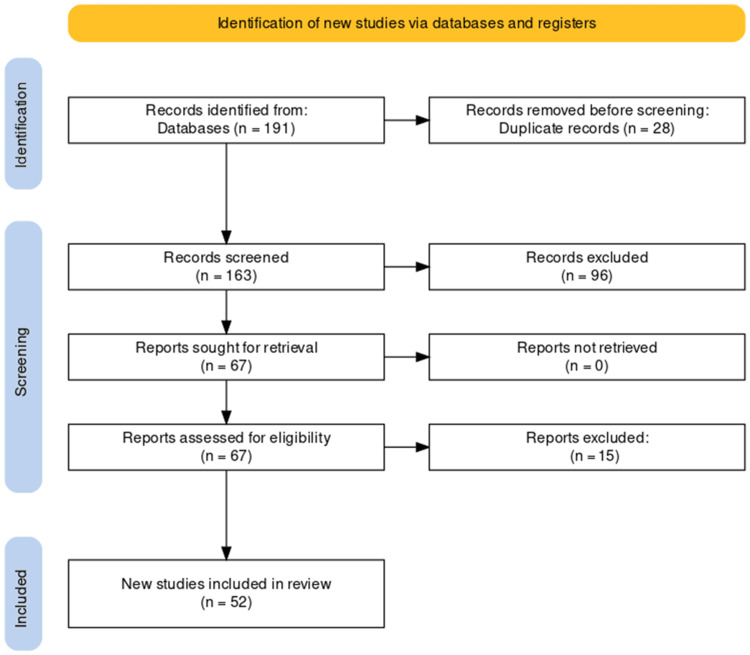
A comprehensive search initially yielded 191 studies, from which duplicates were removed, resulting in a refined pool of 163 unique studies. Subsequent screening of titles and abstracts led to the exclusion of 96 records that did not meet predefined relevance criteria. Full-text evaluation of the remaining 67 articles resulted in the exclusion of 15 reports that did not align with stringent inclusion criteria. The culmination of this rigorous selection process identified 52 studies suitable for inclusion in the systematic review, providing a focused and robust source of evidence for the analysis of endocarditis caused by Gemella species. The PRISMA flowchart detailing the study selection process is presented below (Figure 1).
Figure 1. PRISMA flow diagram of selection of studies for inclusion in the systematic review.
Study Characteristics
This systematic review included a total of 52 case reports on endocarditis caused by Gemella species. Among the reported cases, 43 (79.6%) were male, while 10 (18.5%) were female, indicating a male predominance. Patient ages ranged from 4 to 87 years, with a mean age of 48 years. The most prevalent causative species were G. morbillorum in 25 (46.3%) cases and G. haemolysans in 14 (25.9%) cases, followed by G. sanguinis in 7 (13%) cases and G. bergeri in 4 (74%) cases. There was one case each of infection by both G. haemolysans and G. morbillorum. In terms of valve involvement, isolated mitral and aortic valves were found to have the same incidence of involvement, with 15 (43.4%) cases each. Isolated tricuspid valve was involved in 3 (5.5%) cases. In 10 (18.5%) cases, bivalvular (aortic and mitral) involvement was observed, followed by trivalvular (mitral, pulmonary, and aortic) and panvalvular involvement in 1 case each. In prosthetic valve endocarditis, the prosthetic aortic valve (9.2%) was more commonly involved followed by the prosthetic mitral valve (1.8%). These findings underscore the diversity of Gemella species implicated in endocarditis and the variability in valve involvement among affected individuals (Table 1).
Table 1. Characteristics of the studies included in this systematic review.
| Author | Year | Country | Sample size | Age | Gender | Gemella species | Valve(s) involved |
| Brack et al. [11] | 1991 | United Kingdom | 1 | 74 | Male | G. haemolysans | Mitral |
| Terada et al. [12] | 1994 | Japan | 1 | 64 | Male | G. morbillorum | Mitral and aortic |
| Kerr et al. [13] | 1994 | Ireland | 1 | 29 | Female | G. morbillorum | Mitral |
| Samuel et al. [14] | 1995 | United Kingdom | 1 | 34 | Male | G. haemolysans | Prosthetic aortic |
| Martin et al. [15] | 1995 | United Kingdom | 1 | 75 | Male | G. morbillorum | Mitral |
| Ukimura et al. [16] | 1998 | Japan | 1 | 57 | Male | G. haemolysans, G. morbillorum | Prosthetic aortic |
| La Scola and Raoult [17] | 1998 | France | 3 | (01) 63, (02), 74, (03) N/A | (01) Male, (02) male, (03) male | (01) G. haemolysans, (02) G. morbillorum, (03) G. morbillorum | (01) Mitral, (02) aortic, (03) aortic |
| Mosquera et al. [18] | 2000 | Spain | 1 | 77 | Male | G. haemolysans | Aortic |
| Akiyama et al. [19] | 2001 | Japan | 1 | 55 | Male | G. morbillorum | Aortic |
| Purcell et al. [20] | 2001 | Canada | 1 | 12 | Female | Not specified | Mitral |
| Zakir et al. [21] | 2004 | USA | 1 | 44 | Male | G. morbillorum | Prosthetic mitral |
| Elsayed et al. [22] | 2004 | Canada | 1 | 32 | Male | G. bergeri | Aortic |
| Zheng et al. [23] | 2008 | Singapore | 1 | 67 | Male | G. morbillorum | Mitral and aortic |
| Al Chekakie et al. [24] | 2009 | USA | 1 | 44 | Male | G. morbillorum | Prosthetic aortic |
| Godinho et al. [25] | 2013 | Portugal | 1 | 72 | Male | G. morbillorum | Pulmonary, mitral, tricuspid, and aortic |
| Ural et al. [26] | 2014 | Turkey | 1 | 67 | Male | G. morbillorum | Aortic |
| Shahani et al. [27] | 2014 | USA | 1 | 73 | Male | G. morbillorum | Prosthetic aortic |
| Yang and Tsai [28] | 2014 | Taiwan | 1 | 67 | Male | G. sanguinis | Aortic |
| Virgilio and Chieco [29] | 2014 | Italy | 1 | 50 | Male | G. bergeri | Aortic |
| Hussain et al. [30] | 2014 | UAE | 1 | 24 | Male | G. bergeri | Mitral and aortic |
| Kolhari et al. [31] | 2014 | India | 1 | 34 | Female | G. morbillorum | Mitral |
| Agrawal et al. [32] | 2014 | India | 1 | Middle-aged (exact age not mentioned) | N/A | Pulmonary | |
| Ramchandani et al. [33] | 2014 | USA | 1 | 40 | Female | G. haemolysans | Prosthetic aortic |
| Pachirat et al. [34] | 2015 | Thailand | 1 | 37 | Male | G. bergeri | Tricuspid |
| Rosa et al. [35] | 2015 | Brazil | 1 | 72 | Male | G. morbillorum | Mitral |
| Liu et al. [36] | 2016 | USA | 1 | 87 | Female | G. haemolysans | Aortic |
| Winkler et al. [37] | 2016 | USA | 1 | 67 | Male | - | Mitral |
| Ando et al. [38] | 2016 | USA | 1 | 24 | Male | G. haemolysans | Mitral |
| M et al. [39] | 2016 | India | 1 | 4 | Male | G. sanguinis | Tricuspid |
| Shinha [40] | 2017 | USA | 1 | 37 | Male | G. morbillorum | Aortic |
| Li et al. [41] | 2017 | China | 1 | 28 | Male | G. morbillorum | Pulmonary |
| Maraki et al. [42] | 2019 | Greece | 1 | 21 | Male | G. sanguinis | Bicuspid aortic |
| Youssef et al. [43] | 2019 | USA | 1 | 81 | Male | G. haemolysans | Mitral |
| Agrawal et al. [44] | 2019 | USA | 1 | 38 | Male | G. haemolysans | Mitral and aortic |
| Emmanouilidou et al. [45] | 2019 | Greece | 1 | 85 | Female | G. sanguinis | Mitral and aortic |
| Kobayashi et al. [46] | 2020 | Japan | 1 | 54 | Male | G. morbillorum | Mitral |
| Sideris et al. [47] | 2020 | USA | 1 | 53 | Male | G. sanguinis | Mitral |
| Desai and Bonura [48] | 2021 | USA | 1 | 72 | Male | G. morbillorum | Mitral, pulmonary, and aortic |
| Singer et al. [49] | 2021 | Canada | 1 | 49 | Male | G. morbillorum | Aortic |
| Dogan et al. [50] | 2021 | Turkey | 1 | 37 | Male | G. morbillorum | Mitral and aortic |
| Patel et al. [51] | 2021 | USA | 1 | 56 | Female | G. morbillorum | Aortic |
| Tanveer et al. [52] | 2021 | USA | 1 | 48 | Male | G. morbillorum | Mitral |
| Eslinger and Ahmed [53] | 2022 | USA | 1 | 63 | Male | G. haemolysans | Aortic |
| Rabah et al. [54] | 2022 | USA | 1 | 56 | Male | G. haemolysans | Mitral |
| Shah et al. [3] | 2022 | USA | 1 | 53 | Male | G. sanguinis | Mitral and aortic |
| Filip et al. [4] | 2023 | Romania | 1 | 8 | Male | G. sanguinis | Mitral and aortic |
| Cao and Yuan [6] | 2023 | China | 1 | 37 | Male | G. morbillorum | Mitral and aortic |
| Lim et al. [55] | 2023 | Phillipines | 1 | 40 | Male | G. morbillorum | Mitral and aortic |
| Dini et al. [7] | 2023 | Italy | 1 | 14 | Male | G. haemolysans | Aortic |
| Gonzalez et al. [56] | 2023 | USA | 1 | 30 | Female | G. haemolysans | Tricuspid |
| Taimur et al. [5] | 2023 | Pakistan | 1 | 31 | Female | G. morbillorum | Aortic |
| Kassab et al. [9] | 2024 | USA | 1 | 77 | Female | G. haemolysans | Mitral |
The main findings of the included case reports are summarized in Table 2.
Table 2. Summary of the studies included in this systematic review.
| Author | Year | Clinical presentation | Diagnostic findings | Treatment | Outcome/prognosis |
| Brack et al. [11] | 1991 | Myalgia, headache, rigors, and night sweats | Echocardiography showed prolapse of the posterior leaflet of the mitral valve with a small mass attached suggestive of a vegetation. Blood cultures were positive for Gemella haemolysans | IV Benzyl penicillin and gentamicin daily. After 2 weeks, the penicillin was replaced by oral amoxicillin for 4 further weeks and the gentamicin was given IM for 2 further weeks | Following cessation of antibiotics, he continued to remain apyrexial, with negative blood cultures |
| Terada et al. [12] | 1994 | Low-grade fever and nocturnal dyspnea with a history of previous dental caries | Vegetation and rupture of chorda were observed in the anterior mitral leaflet, and vegetation in the right coronary cusps. Abnormal CBC report (Leukocytosis), elevated CRP and ESR | Treatment with penicillin G (PCG) was then started by giving IV drip injection at a daily dose of 18 million units. Diuretics and cardiotonic agents were administered simultaneously. Dental caries were treated and afterwards, GSF was administered to cope with granulocytopenia. Replacement surgery of aortic and mitral valves was performed | The patient recovered completely and was discharged on the 101st hospital day |
| Kerr et al. [13] | 1994 | Persistent lethargy, flu-like illness with night sweats and dry cough three weeks ago accompanied by swollen left knee a week ago. History of two wisdom teeth extractions three months ago | Electrocardiography showed marked left ventricular hypertrophy, and a transthoracic echocardiogram showed severe hypertrophic obstructive cardiomyopathy. No vegetations were seen. Blood cultures were positive for Gemella morbillorum. Abnormal CBC profile | Empirical treatment with IV benzylpenicillin and gentamicin. Toward the end of the third week of treatment, antibiotics were changed to oral erythromycin and oral rifampicin due to the development of resistance. Chlorpheniramine was given for rash resolution | Treatment continued for a total of 6 weeks after which she was discharged. She remains clinically well to date |
| Samuel et al. [14] | 1995 | Myalgia, fever, and general malaise | Blood cultures were positive for G. haemolysans. Transoesophageal echocardiography showed a lesion on his prosthetic aortic valve suggestive of a vegetation | IV cefuroxime and tobramycin; the latter was stopped after 14 days and oral ciprofloxacin was substituted. Cefuroxime was also stopped after another 5 days due to induced neutropenia | The patient made a good recovery and remains well with no deterioration in his prosthetic valve function |
| Martin et al. [15] | 1995 | Weight loss and lassitude of two months duration and mild ankle edema. He had a history of rheumatic fever as a child | An echocardiogram revealed mitral valve vegetations with regurgitation. Blood cultures were positive for G. morbillorum. Altered complete blood count profile | He was started on benzylpenicillin 1.2 g IV 4 hourly and gentamicin 80 mg IV 12 hourly. After further cultures were taken, he was given teicoplanin. Then he was given IV hydrocortisone, chlorpheniramine, and salbutamol. Then he was given rifampicin 600 mg orally 12 hourly and erythromycin 500 mg orally 6 hourly due to their sensitivity report and the side effects of previous medications | The patient received a total of four weeks of treatment and made a good recovery. He remains well six months later |
| Ukimura et al. [16] | 1998 | Abdominal pain, fever, and oliguria suggestive of renal arterial embolism. | Deranged CBC (increased WBCs and C-reactive protein). A transesophageal echocardiogram showed vegetations on the prosthetic valve during hospitalization. Blood culture was positive for Gemella species | Treated with IV ampicillin and astromycin and underwent valve replacement | The post-operative clinical course was excellent and the patient was stable |
| La Scola and Raoult [17] | 1998 | Patient 01: intermittent fever, loss of weight, and anorexia. Patient 02: intermittent fever, sweating, loss of weight, and basithoracic pain. Patient 03: intermittent fever and a weight loss of 12 kg over a period of several months | (01) Altered CBC Report. Blood cultures were positive for G. haemolysans. A transesophageal echocardiogram demonstrated the presence of vegetation on the mitral valve with moderate mitral valve regurgitation. (02) Deranged CBC Report. Blood cultures were positive for Gmella morbillorum. A transesophageal echocardiogram demonstrated aortic valve incompetence but failed to show any vegetation. (03) Blood cultures were positive for Gemella morbillorum. A transesophageal echocardiogram demonstrated aortic valve vegetation | (01) Treatment with amoxicillin (4 g intravenously at 6-h intervals) and amikacin (5 mg/kg of body weight intravenously at 8-h intervals) was begun. His condition improved rapidly, and after 2 weeks of this regimen, he underwent cardiac surgery to remove the motile vegetation. (02) The patient’s treatment began the day after his admission and comprised amoxicillin (4 g intravenously at 6-hour intervals) and gentamicin (1 mg/kg intravenously at 8-hour intervals). The aortic valve was successfully replaced with a prosthetic device. (03) N/A | (01) One week after surgery antibiotic therapy was discontinued. After 2 years of follow-up, he remains well. (02) Gentamicin was discontinued 1 week later, and amoxicillin was discontinued 3 weeks later. After 1 year of follow-up, he remains well. (03) N/A |
| Mosquera et al. [18] | 2000 | A 77-year-old man with a long history of hemochromatosis with chronic liver disease and arterial hypertension was admitted to the hospital because of malaise, anorexia, weight loss, and progressive dyspnea over the previous 4 months | Echocardiography revealed a vegetation on the aortic valve with moderate valvular insufficiency. G. haemolysans was isolated in the three blood cultures taken on admission | The patient was treated with penicillin G (24 MU IV daily, divided into six doses) and tobramycin (100 mg twice a day IV) for 2 weeks | The patient showed improvement in his clinical status as well as sterilization of the blood cultures. In a follow-up visit 30 days after discharge, the echocardiogram did not show any vegetation |
| Akiyama et al. [19] | 2001 | A 55-year-old man with persistent fever and nocturnal dyspnea was referred to hospital. He had a history of noninsulin-dependent diabetes mellitus and type B hepatitis | Echocardiography demonstrated massive aortic regurgitation with a vegetation-like high-density echo on the right coronary cusp and moderate mitral regurgitation, but the tricuspid and pulmonary valves did not show any abnormality | The patient was treated surgically. Postoperatively, the patient was given IV tobramycin (120 mg/day), cefmetazole sodium (3 g/day), and fosfomycin (3 g/day) for a 6-week period, which resulted in a bacteriologic cure | After medical therapy, the patient remains well without fever or cardiac symptoms and is followed up as an outpatient |
| Purcell et al. [20] | 2001 | A 12-year-old girl with congenital heart disease (mitral stenosis, ventricular septal defect, and patent ductus arteriosus) repaired at six months of age presented to her community pediatrician with a cough, decreased appetite, and a two-week history of fatigue | An echocardiogram revealed marked mitral insufficiency with flail posterior mitral leaflet, thickened mitral leaflets with vegetations, and an enlarged left atrium and ventricle. A diagnosis of infective endocarditis and ruptured chordae tendinae of the posterior mitral leaflet was made | The patient was started with vancomycin. Gentamicin 70 mg given intravenously every 8 h was added on the third day for synergism after consultation with the infectious diseases service. Twelve days after the initial diagnosis, when the organism was identified as a Gemella species sensitive to penicillin, the vancomycin was discontinued, and penicillin 2,200,000 IU was given intravenously every 6 h | The patient's hospital course was uneventful. She remained afebrile and her fatigue resolved. She was discharged home after two weeks in hospital on six weeks of penicillin (2,200,000 IU intravenously every 6 h) and gentamicin (70 mg intravenously every 8 h). At an eight-week follow-up visit with the IWK Grace Health Centre (Halifax, Nova Scotia), she was doing well with normal strength and activity levels |
| Zakir et al. [21] | 2004 | A 44-year-old human immunodeficiency virus-positive man with a history of active IV drug use and a mitral valve replacement only 9 months earlier for native-valve endocarditis due to Staphylococcus aureus was admitted complaining of pleuritic chest pain, shortness of breath, fever, and chills | A TTR revealed severe regurgitation through incompetent leaflets of the bioprosthetic mitral valve. The leaflets of the mitral bioprosthesis were thickened and studded with globular echo densities consistent with vegetations | Ceftriaxone for 4 weeks, with gentamicin added during the first 2 weeks of therapy | The patient was stable 6 months after discharge although he received no surgical therapy |
| Elsayed et al. [22] | 2004 | A 32-year-old Western European man visiting Canada, with a known history of asthma, hypercholesterolemia, and gastroesophageal reflux disease, presented at the hospital with a 1-month history of intermittent low-grade fever, chills, diaphoresis, fatigue, myalgia, lightheadedness, and anorexia and a 1-week history of worsening dyspnea and discomfort on the left side of his chest | A transesophageal echocardiogram was performed, demonstrating the presence of severe aortic regurgitation on a bicuspid valve, an aortic valve ring abscess, and a torn noncoronary cusp harboring multiple vegetations | Ampicillin and gentamicin. Twenty-four hours later, the patient underwent ring abscess curettage and replacement of the aortic valve with a mechanical prosthesis | The patient was discharged from the hospital and sent home on antibiotics. The planned duration of antibiotic therapy was 4 to 6 weeks. Before discharge, he had developed a second-degree heart block requiring temporary pacing |
| Zheng et al. [23] | 2008 | Shortness of breath associated with chills and rigors for three days with a history of left lobar pneumonia, end-stage renal disease mild to moderate aortic regurgitation, and treated syphilis | TTE showed concentric left ventricular hypertrophy, Multiple vegetations on aortic and mitral valves with thickening of the mitral valve, and culture positive for G. morbillorum | Ampicillin and gentamycin administration and surgery were planned | He died due to acute myocardial infarction from worsening aortic incompetence before the surgery |
| Al Chekakie et al. [24] | 2009 | Increased shortness of breath and decreased urine output for the past 4 days and a history of bicuspid aortic valve replacement | A TTE showed a stable aortic prosthetic valve with mild aortic regurgitation, mild mitral regurgitation, and a left ventricular ejection fraction of 15%, Cultures obtained from the aortic valve grew G. morbillorum | Vancomycin, gentamicin, and rifampin later changed to ampicillin and gentamicin, aortic valve replacement with a 22-mm homograft, LVAD was placed | The patient progressively worsened and developed multiorgan failure. He exsanguinated (presumably from a left ventricular assist device cannulation site) and died on postoperative day 14 |
| Godinho et al. [25] | 2013 | Fever, abdominal pain, long-standing dyspnea on exertion, and petechiae on lower limbs | Thickening of all four valves with vegetations on mitral and tricuspid valves, isolation of G. morbillorum in three different blood cultures | Antibiotic therapy with vancomycin and gentamycin, mitral and aortic valve replacement surgery with biological prostheses, and also tricuspid valve annuloplasty | He was discharged from the hospital and continues to do well |
| Ural et al. [26] | 2014 | Fever, fatigue, deterioration of the overall status | Decreased hemoglobin, elevated ESR, CRP, and procalcitonin. Increased spleen size at USG and CT abdomen. Left occipital lobe infarction on cerebral MRI. TTE revealed aortic valve vegetation. G. morbillorum identified on hemocultures | Treatment with ampicillin-sulbactam and gentamicin was started, which was then replaced by a broader-spectrum meropenem and vancomycin | The patient denied early surgical treatment. After 4 weeks of antibiotic therapy, he developed left hemiplegia. Was then referred to an external site for operation by his own will for valve surgery |
| Shahani et al. [27] | 2014 | Fever, chills, fatigue, and bilateral lower extremity swelling with a history of coronary artery bypass graft with an aortic valve replacement for aortic stenosis. Dental caries in bilateral lower molars were also found | Leukocytosis, anemia, and elevated CRP. TTE demonstrated a significant decrease in the ejection fraction along with a mobile vegetation attached to the bioprosthetic aortic valve associated with a perivalvular abscess. Blood culture and later gene sequencing revealed G. morbillorum | 6-week regimen with penicillin G along with gentamicin for the first 2 weeks. Dental extractions were performed to minimize the risk of reinfection. Subsequently, cardiac surgery was conducted, involving debridement of the perivalvular abscess and bioprosthetic aortic valve replacement | The patient completed his 6-week regimen of IV antibiotics and had a successful outcome with his infective endocarditis |
| Yang and Tsai [28] | 2014 | Fever, chills, and generalized weakness and malaise with a history of rheumatic heart disease and gouty arthritis | Leukocytosis, anemia, elevated CRP, fibrinogen and D-dimer, and hypoalbuminemia were found. TTE showed aortic stenosis and aortic regurgitation with suspected paravalvular abscess. Mild mitral regurgitation, atrial fibrillation, and small pericardial effusion were noted. Blood cultures positive for G. sanguinis | Treatment was started with moxifloxacin and ceftriaxone. Switched after 1 day to vancomycin and gentamicin. Shortly thereafter, was adjusted to penicillin and the surgeon was consulted. The patient subsequently underwent surgery with a successful aortic prosthetic valve replacement | He continued to receive IV penicillin treatment for 6 weeks and was discharged with a stable condition. No evidence of recurrence after 3 months of evaluation |
| Virgilio and Chieco [29] | 2014 | Fever, shivering, refractoriness to common oral antibiotics, and past history of bicuspid aortic valve | Leukocytosis, elevated CRP, and ESR. TTE revealed mobile vegetation attached to the anterior cusp of the aortic valve. Blood cultures positive for G. bergeri | Commenced on IV amoxicillin-clavulanate associated with amikacin. Therapy switched to ceftriaxone plus gentamicin according to antibiogram results | The patient became apyrexial on this antibiotic therapy, which was prescribed for 1 month |
| Hussain et al. [30] | 2014 | Headache and left hemiparesis, with a past history of anemia | Leukocytosis, anemia, elevated platelet count, CRP, and serum procalcitonin. Hematuria was also seen. CT brain revealed hypodensities in the right parietal cortex and left posterior frontal cortex, indicating ischemic infarcts. The echocardiogram showed thickened aortic cusps with vegetations causing severe aortic regurgitation. The anterior leaflet of the mitral valve thickened with multiple vegetations and mild mitral regurgitation. The left atrium and left ventricle dilated. Blood cultures positive for G. bergeri | Started empirically on IV ceftriaxone and gentamicin. Later, GCS dropped to 8/15 and CT revealed right fronto-temporoparietal intra-axial hematoma for which right-sided decompression craniotomy and insertion of intracerebral pressure monitoring catheter was done. He then received all brain protective measures as per ICU protocol for high ICP. Based on the susceptibility test of the organism, the same antibiotics were continued | ICP remained persistently high despite brain protective measures. A follow-up CT scan revealed a large hemorrhagic infarct in the right MCA territory with brain swelling and mass effect. The patient remained hypotensive, needing full inotropic support. Lost brain stem reflexes and deceased after 20 days |
| Kolhari et al. [31] | 2014 | low-grade fever, swelling of lower limbs, facial puffiness, breathlessness, tachycardia, Pallor, pedal edema, elevated jugular venous pulse, hyperdynamic apex and ejection systolic murmur, basal crepitations and a palpable spleen | ECG showed major left ventricular hypertrophy and abnormal repolarization in the lateral ECG leads. Two-dimensional echocardiography showed localized septal hypertrophy (2.2 cm). There was a large (22×12 mm), mobile vegetation on the anterior mitral leaflet, and mitral regurgitation (MR) was quantified as mild. Mild pericardial effusion was also seen | IV crystalline penicillin and levofloxacin and mitral valve replacement | Postoperatively the patient improved symptomatically and was discharged after completion of 6 weeks of parenteral antibiotic treatment. At 6 months of follow-up, the patient is asymptomatic |
| Agrawal et al. [32] | 2014 | progressively worsening exertional fatigue and dyspnea; a history of being treated for infective endocarditis by G. morbillorum | severe pulmonary regurgitation, the deformed valve appeared echocardiographically convoluted like a ‘cauliflower’ | This patient underwent successful replacement of the pulmonary valve with a bioprosthetic valve and patch closure of the ASD | The patient has been stable and asymptomatic after 6 months of follow-up |
| Ramchandani et al. [33] | 2014 | progressive shortness of breath, lower extremity edema, fevers with temperatures to 39°C, myalgias and night sweats, and a history of congenital aortic coarctation, repaired at age 4, and a bicuspid aortic valve with subsequent symptomatic aortic stenosis requiring bioprosthetic aortic valve replacement and root enlargement at age 32 | a transthoracic echocardiogram was suggestive of endocarditis with a paravalvular aortic root abscess, 16s rRNA PCR identified G. haemolysans in all tissue specimens. Pathology of her explanted heart showed both ischemic and embolic infarcts of varying ages | vancomycin and ceftriaxone later changed to ampicillin and gentamycin, extensive debridement mandating reconstruction with a TAH, and then a heart transplant 6 months later | She continues to do well seven months afterward |
| Pachirat et al. [34] | 2015 | Prolonged fever, weight loss, dyspnea on exertion | TTE revealed tricuspid valve vegetation with moderate regurgitation. Routine blood cultures were negative, but PCR/sequencing of valve tissue identified G. bergeri | Tricuspid valve repair and vegetectomy | Discharged with good outcome after one month |
| Rosa et al. [35] | 2015 | Dry cough, fever, anorexia, loss of body weight, dyspnea, hypotension, tachycardia, fever, oliguria and signs of poor peripheral perfusion | CXR showed pulmonary congestion and cardiomegaly. CBC showed leukocytosis and normocytic anemia. Elevated ESR and CRP were seen. TEE revealed a thickened mitral valve, anterior leaflet flail, and regurgitation. Lung ultrasound showed diffuse bilateral B-lines. G. morbillorum isolated in blood cultures | Initial empirical antimicrobial therapy with ceftriaxone plus gentamicin. Hemodynamic instability treated with norepinephrine and intra-aortic balloon pump counterpulsation implant. Emergency mitral valve replacement surgery was done. After antimicrobial susceptibility tests, the regimen was changed to penicillin G plus gentamicin | Bioprosthetic mitral valve replacement led to improved cardiogenic shock, followed by recurrence on Day 27, requiring mechanical ventilation. Emergency valve replacement was attempted again, but the patient died during the procedure |
| Liu et al. [36] | 2016 | Back pain, weight gain, jugular venous distention, rales, edema in lower extremities | TTE showed vegetation on non-coronary aortic leaflet and mild aortic stenosis. Blood cultures grew G. haemolysans | Treated with ampicillin and gentamicin | The patient died unexpectedly one month later |
| Winkler et al. [37] | 2016 | Acute chest pain, nausea, and diaphoresis, suggesting embolic acute ST-segment-elevation myocardial infarction | The electrocardiogram showed a right bundle branch block with anterolateral ST-segment elevations and reciprocal inferior ST-segment depressions. TTE revealed mitral valve vegetation | Milrinone, diuretics, aspirin, clopidogrel, heparin | The patient died within 2 days due to severe heart failure and septic shock secondary to a central-line infection |
| Ando et al. [38] | 2016 | Malaise, headache, fever, rigors after treatment for parotitis and G. haemolysans bacteremia | Elevated white cell count, chest radiograph showed enlarged cardiac silhouette. Echocardiography revealed aortic and mitral valve vegetations with severe regurgitation | Urgent mechanical aortic valve replacement and mitral valve repair, IV penicillin G, and vancomycin | Prolonged hospitalization, discharged with lifelong warfarin and IV ceftriaxone for 7 weeks |
| M et al. [39] | 2016 | High-grade fever, chills, rigor, cough, weight loss, abdominal distension | Echocardiography showed tricuspid valve vegetation with mild regurgitation. Gram-positive cocci in pairs identified as G. sanguinis | Initially ceftriaxone and amikacin, then vancomycin and gentamicin for 6 weeks based on susceptibility testing | Improved and discharged after 8 weeks with regular follow-up |
| Shinha [40] | 2017 | Fever, chills, fatigue in a patient with IV drug abuse history | Systolic ejection murmur at apex. TTE revealed aortic valve vegetation. Multiple blood cultures positive for Gram-negative coccus | Not mentioned | Not mentioned |
| Li et al. [41] | 2017 | Fever, shortness of breath, exertional fatigue, dyspnea, weight loss for 3 months | TTE showed pulmonary valve vegetation with moderate regurgitation, ventricular septal defect, atrial septal defect, and double-chambered right ventricle. Blood cultures grew G. morbillorum | Aggressive antibiotic therapy followed by pulmonary valve replacement with aortic bioprosthetic valve, repair of ventricular and atrial septal defects, reconstruction of the right ventricular outflow tract, and excision of vegetations | Uneventful postoperative recovery, asymptomatic at 3-month follow-up |
| Maraki et al. [42] | 2019 | Fever, poor appetite, weakness, fatigue, marked weight loss, arthralgias after the dental procedure | TTE and TEE revealed a bicuspid aortic valve with vegetation on the posterior leaflet, moderate to severe aortic regurgitation | Empiric IV ceftriaxone, gentamicin, and daptomycin initially, then ceftriaxone for 6 weeks and gentamicin for 2 weeks. Aortic valve replacement after 6 weeks | Successful treatment with valve replacement |
| Youssef et al. [43] | 2019 | Dyspnea, weakness, exertional dyspnea | New systolic murmur, echocardiography showed mitral valve vegetation with moderate regurgitation. Blood cultures grew G. haemolysans | Initially vancomycin and ceftriaxone, then penicillin and gentamicin. Advised surgical mitral valve replacement but opted for conservative management | Developed cardiogenic shock and severe pulmonary edema, passed away |
| Agrawal et al. [44] | 2019 | Chronic mastoiditis with exertional dyspnea for 1 month | TTE showed aortic regurgitation and aortic valve abscess. TEE revealed a bicuspid aortic valve with vegetations. Blood cultures grew G. haemolysans | IV ampicillin and gentamicin followed by mechanical aortic valve replacement and bovine reconstruction of the left ventricular outflow tract | Discharged after 3 weeks of IV antibiotics after negative blood cultures. Removal of periapical abscess identified as the source of infection |
| Emmanouilidou et al. [45] | 2019 | Fever, right hemiparesis, and strabismus | TTE showed mitral valve vegetation. A brain CT scan revealed ischemic damage. Blood culture positive for G. sanguinis | Empiric treatment with ceftriaxone, clindamycin, and acetylsalicylic acid followed by vancomycin and gentamicin | Discharged after 6 weeks of antibiotic therapy with no definite vegetation or perivalvular abscess |
| Kobayashi et al. [46] | 2020 | Fever, leg edema, and dyspnea | Roth spots, Janeway lesions, Osler nodules, and purpura on lower limbs. Infective endocarditis and infection-related pauci-immune necrotizing crescentic glomerulonephritis. Blood culture positive for G. morbillorum | Initial treatment with penicillin G and gentamicin, followed by ceftriaxone, daptomycin, and vancomycin. Steroid pulse therapy was administered | Surgical mitral valve replacement performed after recurrent bacteremia. Complete resolution of glomerulonephritis post-steroid therapy |
| Sideris et al. [47] | 2020 | Malaise, thigh and finger pain, and new pansystolic murmur | TTE and TEE revealed severe mitral insufficiency with vegetations. Blood culture positive for G. sanguinis | Empirical treatment with vancomycin and ceftriaxone followed by mechanical mitral valve replacement | Successful recovery post-operative, with office follow-up showing appropriate recovery |
| Desai and Bonura [48] | 2021 | Chronic anemia, worsening renal function, and unintentional weight loss | Renal dysfunction, severe anemia, elevated inflammatory markers, and atrial fibrillation. Imaging was initially negative, but later abdominal imaging identified splenic infarcts prompting suspicion of possible cardiac vegetation on re-evaluated echocardiogram. Blood culture positive for G. morbillorum | Empirical treatment with vancomycin, then cefazolin. Valve replacement surgery was deferred initially due to high surgical risk | Discharged after 6-week antibiotic course with marked clinical improvement. A follow-up echocardiogram showed no need for surgical correction |
| Singer et al. [49] | 2021 | Orthopnea, dyspnea, and weight loss | TEE revealed vegetation on the aortic and mitral valves. Blood culture positive for G. morbillorum | Empirical treatment with piperacillin-tazobactam, then ceftriaxone and gentamicin | Discharged on ceftriaxone with full recovery at 2-year follow-up. |
| Dogan et al. [50] | 2021 | Fever, weight loss, and right upper quadrant pain | TTE revealed vegetations on the bicuspid aortic and mitral valves. Blood culture positive for G. morbillorum | Initial treatment with ceftriaxone. Valve replacement surgery was performed due to persistent vegetation | Successful medical-surgical management with complete recovery |
| Patel et al. [51] | 2021 | Fevers, abdominal pain, and diarrhea | TTE showed aortic valve thickening. Blood culture positive for Actinomyces odontolyticus and G. morbillorum | Empirical treatment with ceftriaxone and metronidazole. Discharged on ceftriaxone and apixaban | Resolution of symptoms post-antibiotic therapy. Discharged for a dental follow-up |
| Tanveer et al. [52] | 2021 | Weight loss, fatigue, night sweats, and shortness of breath | An echocardiogram revealed mitral valve vegetations. CT showed splenomegaly with old infarcts. Blood cultures identified G. morbillorum | Vancomycin and ceftriaxone initially, followed by ceftriaxone alone post-mitral valve replacement | Improvement in symptoms, negative blood cultures post-therapy |
| Eslinger and Ahmed [53] | 2022 | Poor dental hygiene with fatigue, malaise, and acute dyspnea | TTE is consistent with aortic valve vegetation. Panorex and TTE revealed no dental abscess. Blood cultures positive for G. haemolysans | Vancomycin and cefepime, then penicillin G with gentamycin for 4 weeks | Cleared infection, required intermittent transfusions post-discharge |
| Rabah et al. [54] | 2022 | History of root canal presented with low-grade fever | TEE showed mitral valve vegetation. Blood cultures positive for G. haemolysans | Vancomycin and ceftriaxone initially. Scheduled for mitral valve replacement post-treatment completion | Successful treatment without complications |
| Shah et al. [3] | 2022 | Chest pain and palpitations | TEE revealed severe aortic insufficiency and MR | Urgent double valve replacement surgery (aortic and mitral valves) | The patient died due to complications post-surgery. |
| Filip et al. [4] | 2023 | Abdominal pain and fever | Admission echocardiography showed paravalvular aortic abscess and mitral valve vegetation | IV antibiotics followed by complex surgical intervention (Ross operation and coarctectomy) | Improved condition post-surgery, discharged in good clinical state |
| Cao and Yuan [6] | 2023 | Intermittent fever and aortic valve malformation | TTE indicated aortic valve vegetation. Blood cultures grew G. morbillorum | Empirical antibiotic therapy with ceftriaxone and netilmicin | Stable condition upon discharge for further cardiac surgery |
| Lim et al. [55] | 2023 | Left leg weakness and diastolic murmur | TTE and TEE revealed aortic and mitral valve vegetations. MRI showed cerebral infarct | Empirical antibiotics followed by open-heart surgery for valve replacement | Discharged to a rehabilitation center post-surgery |
| Dini et al. [7] | 2023 | Congenital aortic stenosis with fever | TEE revealed a pseudoaneurysm of mitral-aortic intervalvular fibrosa. Blood cultures positive for G. haemolysans | IV antibiotics followed by aortic valve replacement | Symptom-free post-recovery |
| Gonzalez et al. [56] | 2023 | Hepatitis C and IV drugs with weakness and fever | TTE showed tricuspid valve vegetation. Blood cultures positive for P. aeruginosa and G. haemolysans | Urgent tricuspid valve replacement with pacemaker implantation | Improved cardiac function post-surgery |
| Taimur et al. [5] | 2023 | Fever, exertional dyspnea, myalgias, edema, and anorexia | TTE showed aortic valve regurgitation, mild to moderate MR, left ventricular diastolic dysfunction grade II, and tricuspid regurgitation | Empirical treatment with ceftriaxone and gentamycin, based on sensitivity results | Responded well to medical treatment, evidenced by reduced vegetation size and symptom improvement; no surgical intervention required |
| Kassab et al. [9] | 2024 | End-stage renal disease, paroxysmal atrial fibrillation, and recent shoulder injury with malaise, dizziness, poor appetite, fever, and leukocytosis | TTE showed mildly thickened mitral valve leaflets, and trace MR. TEE showed large mobile vegetation on the anterior mitral leaflet. MRI revealed septic emboli with acute infarcts. Blood cultures positive for G. haemolysans | Empirical treatment with IV cefepime and vancomycin later switched to IV ceftriaxone based on the sensitivity | The patient's encephalopathy worsened, not suitable for surgery; transitioned to comfort measures and deceased shortly after |
Discussion
This systematic review aimed to provide a comprehensive overview of infective endocarditis caused by Gemella species, a group of fastidious gram-positive cocci that are typically commensal organisms but can be opportunistic pathogens. The findings of this review underscore the emerging recognition of Gemella species as causative agents of endocarditis, a condition that has been historically associated with more commonly known pathogens. The review identified 52 case reports of endocarditis due to Gemella species, highlighting the diversity of Gemella species implicated, with G. morbillorum (46.3%) and G. haemolysans (25.9%) being the most prevalent species. The male predominance (79.6%) observed in these cases aligns with the general epidemiological pattern of infective endocarditis, although the underlying reasons for this predilection warrant further investigation [57,58].
The clinical presentation of endocarditis due to Gemella species varies widely, ranging from nonspecific symptoms such as fever, malaise, and fatigue to more specific manifestations like myalgia, dyspnea, and focal neurological deficits [3,5,9,30,45]. Additionally, predisposing factors such as dental procedures, congenital heart disease, and immunocompromised states were frequently noted among the patients [5,12,27,42]. However, the indolent and insidious nature of the illness, coupled with the fastidious growth requirements of Gemella species, often led to delays in diagnosis and appropriate management [9]. This underscores the importance of maintaining a high index of suspicion, particularly in patients with predisposing risk factors or persistent unexplained clinical manifestations. Diagnostic modalities, primarily echocardiography and blood cultures, played crucial roles in confirming the diagnosis of endocarditis and identifying the causative organism. Echocardiography, particularly TEE, enabled the detection of valvular vegetations, assessment of valvular function, and identification of complications. Blood cultures were instrumental in isolating Gemella species, facilitating targeted antibiotic therapy. The review revealed a wide range of valve involvement, with both native and prosthetic valves being affected, further emphasizing the need for thorough evaluation in suspected cases.
The management of endocarditis due to Gemella species posed several challenges. Empirical antibiotic regimens typically included broad-spectrum agents such as penicillin and gentamicin followed by tailored regimens based on susceptibility testing and clinical response [4,45,48,53]. The review highlighted the increasing prevalence of antimicrobial resistance among Gemella isolates, underscoring the importance of judicious antibiotic use and the potential need for combination therapy or alternative treatment strategies. Surgical intervention, including valve replacement or repair, was pursued in cases of severe valvular damage, persistent infection, or complications such as abscess formation and embolic events [3,50]. Overall outcomes were generally favorable, with most patients showing clinical improvement and resolution of infection following appropriate medical and/or surgical management. However, a small proportion of cases experienced complications or succumbed to the illness, underscoring the importance of timely diagnosis and comprehensive treatment.
This systematic review has several strengths, including the comprehensive search strategy, rigorous study selection process, and detailed data extraction. However, it is essential to acknowledge the limitations inherent in the inclusion of case reports, which may introduce potential selection bias and limit the generalizability of the findings. Additionally, the heterogeneity in reporting and variability in clinical management approaches across the included cases may have influenced the interpretation of the results.
Conclusions
This systematic review highlights the emerging role of Gemella species as causative agents of infective endocarditis, a condition with significant morbidity and mortality. The findings underscore the importance of maintaining a high index of suspicion, particularly in patients with predisposing risk factors or persistent, unexplained clinical manifestations. Prompt diagnosis, tailored antimicrobial therapy, and timely surgical intervention, when indicated, are crucial for optimal patient outcomes. Future research should focus on elucidating the virulence mechanisms of Gemella species, identifying risk factors for infection, and developing evidence-based guidelines for the management of endocarditis due to Gemella species.
The authors have declared that no competing interests exist.
Author Contributions
Acquisition, analysis, or interpretation of data: Danyal Bakht, Carlos D. Franco, Gina N. Gonzalez, Emilia I. Ramos, Tanya Sinha, Maaz Amir, Muhammad Arsham Javed, Khawar Ali, Nailet Pineda Renté
Drafting of the manuscript: Danyal Bakht, Carlos D. Franco, Gina N. Gonzalez, Emilia I. Ramos, Tanya Sinha, Maaz Amir, Muhammad Arsham Javed, Khawar Ali
Concept and design: Syed Faqeer Hussain Bokhari, Carlos D. Franco, Gina N. Gonzalez
Critical review of the manuscript for important intellectual content: Syed Faqeer Hussain Bokhari, Tanya Sinha, Nailet Pineda Renté
Supervision: Syed Faqeer Hussain Bokhari
References
- 1.Infective endocarditis. Sheppard MN. Diagn Histopathol. 2022;28:199–208. [Google Scholar]
- 2.Infective endocarditis in high-income countries. Nappi F, Martuscelli G, Bellomo F, Avtaar Singh SS, Moon MR. Metabolites. 2022;12 doi: 10.3390/metabo12080682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aortic and mitral valve infective endocarditis caused by Gemella sanguinis. Shah N, SInnatamby Moon D, Wehman B. Cureus. 2022;14:0. doi: 10.7759/cureus.28099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gemella sanguinis infective endocarditis-challenging management of an 8-year-old with Duchenne dystrophy and undiagnosed congenital heart disease: a case report. Filip C, Vasile CM, Nicolae G, et al. Antibiotics (Basel) 2023;12 doi: 10.3390/antibiotics12040706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gemella morbillorum endocarditis in a patient with a bicuspid aortic valve. Taimur S, Madiha R, Samar F, Bushra J. https://pubmed.ncbi.nlm.nih.gov/20378524/ Hellenic J Cardiol. 2010;51:183–186. [PubMed] [Google Scholar]
- 6.Gemella morbillorum infective endocarditis: a case report and literature review. Cao X, Yuan L. Open Life Sci. 2023;18:20220599. doi: 10.1515/biol-2022-0599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Infective endocarditis caused by Gemella haemolysans in a patient with bicuspid aortic valve: a case report. Dini G, Verrotti A, Girella E, De Angelis F, Sardone M, Gorello P, Arcioni F. SAGE Open Med Case Rep. 2023;11:2050313. doi: 10.1177/2050313X231211707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gemella species bacteremia and stroke in an elderly patient with respiratory tract infection. Jayananda S, Gollol-Raju NS, Fadul N. Case Rep Med. 2017;2017:1098527. doi: 10.1155/2017/1098527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.A rare case of Gemella haemolysans endocarditis: a challenging diagnosis. Kassab NS, Alsammarraie N, Sarsam T, Al-Sammarraie M, Watt J. Cureus. 2024;16:0. doi: 10.7759/cureus.52030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Antimicrobial susceptibility to 27 drugs and the molecular mechanisms of macrolide, tetracycline, and quinolone resistance in Gemella SP. Furugaito M, Arai Y, Uzawa Y, Kamisako T, Ogura K, Okamoto S, Kikuchi K. Antibiotics (Basel) 2023;12 doi: 10.3390/antibiotics12101538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gemella haemolysans: a rare and unusual cause of infective endocarditis. Brack MJ, Avery PG, Hubner PJ, Swann RA. Postgrad Med J. 1991;67:210. doi: 10.1136/pgmj.67.784.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Infective endocarditis caused by an indigenous bacterium (Gemella morbillorum) Terada H, Miyahara K, Sohara H, Sonoda M, Uenomachi H, Sanada J, Arima T. Intern Med. 1994;33:628–631. doi: 10.2169/internalmedicine.33.628. [DOI] [PubMed] [Google Scholar]
- 13.Infective endocarditis due to Gemella morbillorum complicating hypertrophic obstructive cardiomyopathy. Kerr JR, Webb CH, McGimpsey JG, Campbell NP. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2449104/ Ulster Med J. 1994;63:108–110. [PMC free article] [PubMed] [Google Scholar]
- 14.Gemella haemolysans prosthetic valve endocarditis. Samuel L, Bloomfield P, Ross P. Postgrad Med J. 1995;71:188. doi: 10.1136/pgmj.71.833.188-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.A case of Gemella morbillorum endocarditis. Martin MJ, Wright DA, Jones AR. Postgrad Med J. 1995;71:188. doi: 10.1136/pgmj.71.833.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Prosthetic ball valve endocarditis due to Gemella species. Ukimura A, Nishihara S, Suwa M, Hirota Y, Kitaura Y, Kawamura K, Sasaki S. Jpn Circ J. 1998;62:626–628. doi: 10.1253/jcj.62.626. [DOI] [PubMed] [Google Scholar]
- 17.Molecular identification of Gemella species from three patients with endocarditis. La Scola B, Raoult D. J Clin Microbiol. 1998;36:866–871. doi: 10.1128/jcm.36.4.866-871.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Endocarditis due to Gemella haemolysans in a patient with hemochromatosis. Mosquera JD, Zabalza M, Lantero M, Blanco JR. Clin Microbiol Infect. 2000;6:566–568. doi: 10.1046/j.1469-0691.2000.00136.x. [DOI] [PubMed] [Google Scholar]
- 19.Recurrent aortic valve endocarditis caused by Gemella morbillorum--report of a case and review of the literature. Akiyama K, Taniyasu N, Hirota J, Iba Y, Maisawa K. Jpn Circ J. 2001;65:997–1000. doi: 10.1253/jcj.65.997. [DOI] [PubMed] [Google Scholar]
- 20.Gemella species endocarditis in a child. Purcell LK, Finley JP, Chen R, Lovgren M, Halperin SA. Can J Infect Dis. 2001;12:317–320. doi: 10.1155/2001/960734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mitral bioprosthetic valve endocarditis caused by an unusual microorganism, Gemella morbillorum, in an intravenous drug user. Zakir RM, Al-Dehneh A, Dabu L, Kapila R, Saric M. J Clin Microbiol. 2004;42:4893–4896. doi: 10.1128/JCM.42.10.4893-4896.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gemella bergeriae endocarditis diagnosed by sequencing of rRNA genes in heart valve tissue. Elsayed S, Zhang K. J Clin Microbiol. 2004;42:4897–4900. doi: 10.1128/JCM.42.10.4897-4900.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aortic and mitral valve endocarditis caused by Gemella morbillorum in a haemodialysis patient. Zheng M, Ng OT, Teo BW. https://pubmed.ncbi.nlm.nih.gov/19122942/ Singapore Med J. 2008;49:0–7. [PubMed] [Google Scholar]
- 24.Gemella morbillorum prosthetic valve endocarditis. Al Chekakie MO, Heroux A, Montpetit M, Nemeh H. Congest Heart Fail. 2009;15:291–292. doi: 10.1111/j.1751-7133.2009.00061.x. [DOI] [PubMed] [Google Scholar]
- 25.Gemella endocarditis: an aggressive entity (Article in Portuguese) Godinho AR, Tomé E, Vaz A, Gomes A, Vaz P. Rev Port Cardiol. 2013;32:1027–1030. doi: 10.1016/j.repc.2013.06.005. [DOI] [PubMed] [Google Scholar]
- 26.Gemella morbillorum Endocarditis. Ural S, Gul Yurtsever S, Ormen B, et al. Case Rep Infect Dis. 2014;2014:456471. doi: 10.1155/2014/456471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gemella morbillorum prosthetic aortic valve endocarditis. Shahani L. BMJ Case Rep. 2014;2014 doi: 10.1136/bcr-2014-207304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gemella sanguinis endocarditis: first case report in Taiwan and review of the literature. Yang CH, Tsai KT. J Formos Med Assoc. 2014;113:562–565. doi: 10.1016/j.jfma.2012.02.012. [DOI] [PubMed] [Google Scholar]
- 29.Sixth case of infective endocarditis caused by Gemella bergeri. Virgilio E, Chieco PA. Braz J Infect Dis. 2014;18:467. doi: 10.1016/j.bjid.2014.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Unreported neurological complications of Gemella bergeriae infective endocarditis. Hussain K, Abubaker J, Al Deesi ZO, Ahmed R. BMJ Case Rep. 2014;2014 doi: 10.1136/bcr-2014-204405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gemella morbillorum endocarditis in hypertrophic cardiomyopathy: a rare organism causing a large vegetation and abscess in an uncommon setting. Kolhari VB, Kumar VV, Agrawal N, Prakash SS. BMJ Case Rep. 2014;2014 doi: 10.1136/bcr-2014-203951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cauliflower-like deformation of pulmonary valve in a case of infective endocarditis by a rare organism: Gemella morbillorum. Agrawal N, Kariyappa M, Kolhari VB, Manjunath CN. BMJ Case Rep. 2014;2014 doi: 10.1136/bcr-2014-204726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Total artificial heart as bridge to transplantation for severe culture-negative prosthetic valve endocarditis due to Gemella haemolysans. Ramchandani MS, Rakita RM, Freeman RV, Levy WC, Von Homeyer P, Mokadam NA. ASAIO J. 2014;60:479–481. doi: 10.1097/MAT.0000000000000080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.First case of tricuspid valve endocarditis caused by Gemella bergeri. Pachirat O, Watt G, Pussadhamma B. Case Rep Med. 2015;2015:704785. doi: 10.1155/2015/704785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cardiogenic shock due to Gemella morbillorum native mitral valve endocarditis. Rosa RG, Rosa MD, Ascoli AM, Mattioni M, Barth JH, Teixeira C. Clin Case Rep. 2015;3:342–344. doi: 10.1002/ccr3.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Endocarditis due to Gemella haemolysans in a newly diagnosed multiple myeloma patient. Liu D, Bateman T, Carr E, Foster P. J Community Hosp Intern Med Perspect. 2016;6:32357. doi: 10.3402/jchimp.v6.32357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gemella endocarditis presenting as an ST-segment-elevation myocardial infarction. Winkler J, Chaudhry SP, Stockwell PH. Tex Heart Inst J. 2016;43:258–260. doi: 10.14503/THIJ-15-5170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Native bivalvular endocarditis by Gemella haemolysans requiring venovenous extracorporeal membrane oxygenation. Ando A, Kagihara J, Chung H, Bolger DT Jr. BMJ Case Rep. 2016;2016 doi: 10.1136/bcr-2016-216029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gemella sanguinis: a rare cause of native valve endocarditis in a child. M M, Bhalla S, Shete V, Grover N. Med J Armed Forces India. 2016;72:0–6. doi: 10.1016/j.mjafi.2015.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Endocarditis due to Gemella morbillorum. Shinha T. Intern Med. 2017;56:1751. doi: 10.2169/internalmedicine.56.8253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gemella morbillorum endocarditis of pulmonary valve:a case report. Li D, Zhu Z, Zheng X, Wang W, Xu R, Liu K. J Cardiothorac Surg. 2017;12:16. doi: 10.1186/s13019-017-0579-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bicuspid aortic valve endocarditis caused by Gemella sanguinis: case report and literature review. Maraki S, Plevritaki A, Kofteridis D, Scoulica E, Eskitzis A, Gikas A, Panagiotakis SH. J Infect Public Health. 2019;12:304–308. doi: 10.1016/j.jiph.2019.01.001. [DOI] [PubMed] [Google Scholar]
- 43.Gemella endocarditis: a case report and a review of the literature. Youssef D, Youssef I, Marroush TS, Sharma M. Avicenna J Med. 2019;9:164–168. doi: 10.4103/ajm.AJM_3_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.A rare case of infective endocarditis caused by Gemella haemolysans. Agrawal T, Irani M, Fuentes Rojas S, Jeroudi O, Janjua E. Cureus. 2019;11:0. doi: 10.7759/cureus.6234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.A case report of successful conservative treatment for infective endocarditis caused by Gemella sanguinis. Emmanouilidou G, Voukelatou P, Vrettos I, et al. Case Rep Infect Dis. 2019;2019:9382395. doi: 10.1155/2019/9382395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Clinical implications of steroid therapy for crescentic glomerulonephritis and Gemella morbillorum-associated infective endocarditis. Kobayashi S, Kakeshita K, Imamura T, Fujioka H, Yamazaki H, Koike T, Kinugawa K. Intern Med. 2021;60:299–303. doi: 10.2169/internalmedicine.5319-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.A rare case of isolated mitral valve endocarditis by Gemella sanguinis: case report and review of the literature. Sideris AC, Zimmermann E, Ogami T, Avgerinos DV. Int J Surg Case Rep. 2020;69:51–54. doi: 10.1016/j.ijscr.2020.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Multi-valvular infective endocarditis from Gemella morbillorum. Desai AK, Bonura EM. BMJ Case Rep. 2021;14 doi: 10.1136/bcr-2021-242093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gemella morbillorum endocarditis and osteomyelitis in a patient with ankylosing spondylitis. Singer Z, Leis B, Nosib S, Kogilwaimath S. J Assoc Med Microbiol Infect Dis Can. 2021;6:69–72. doi: 10.3138/jammi-2020-0032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gemella morbillorum endocarditis in a patient with a bicuspid aortic valve. Dogan M, Eren Topkaya A, Alpsoy S, Gur O, Erdem I. North Clin Istanb. 2021;8:190–192. doi: 10.14744/nci.2020.39206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Aortic valve endocarditis by Actinomyces odontolyticus and Gemella morbillorum oral pathogens. Patel K, MacDonald M, Hmoud H, Czinn E, Wutawunashe C, Fisher P. IDCases. 2021;24:0. doi: 10.1016/j.idcr.2021.e01079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.A case of Gemella morbillorum native valve endocarditis and results of in vitro susceptibility testing. Tanveer F, Pawlak J, Youssef D, Saravolatz LD. IDCases. 2021;23:0. doi: 10.1016/j.idcr.2021.e01045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gemella haemolysans infective endocarditis in a patient with febrile neutropenia. Eslinger LJ, Ahmed T. Cureus. 2022;14:0. doi: 10.7759/cureus.24076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gemella endocarditis. Rabah H, El Gharib K, Assaad M, Kassem A, Mobarakai N. IDCases. 2022;29:0. doi: 10.1016/j.idcr.2022.e01597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.We found a gem in your heart: valvular heart disease and infective endocarditis discovered. Lim R Jr, Barimayena J, Mita KA, Denney B, Coz RM. Cureus. 2023;15:0. doi: 10.7759/cureus.42176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Triple threat: triple pathogen endocarditis. Gonzalez JM, Lowenhaar G, Ramgopal M, Chalasani P. Cureus. 2023;15:0. doi: 10.7759/cureus.47860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.The global, regional, and national burden and trends of infective endocarditis from 1990 to 2019: results from the Global Burden of Disease Study 2019. Chen H, Zhan Y, Zhang K, et al. Front Med (Lausanne) 2022;9:774224. doi: 10.3389/fmed.2022.774224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Epidemiology, microbiology, and outcomes of infective endocarditis in a tertiary center in Jordan. Al-Makhamreh HK, Al Bakri FG, Shaf'ei M, et al. Wien Med Wochenschr. 2024;174:126–132. doi: 10.1007/s10354-023-01004-w. [DOI] [PMC free article] [PubMed] [Google Scholar]