Abstract
CD44, a nonkinase single span transmembrane glycoprotein, is a major cell surface receptor for many other extracellular matrix components as well as classic markers of cancer stem cells and immune cells. Through alternative splicing of CD44 gene, CD44 is divided into two isoforms, the standard isoform of CD44 (CD44s) and the variant isoform of CD44 (CD44v). Different isoforms of CD44 participate in regulating various signaling pathways, modulating cancer proliferation, invasion, metastasis, and drug resistance, with its aberrant expression and dysregulation contributing to tumor initiation and progression. However, CD44s and CD44v play overlapping or contradictory roles in tumor initiation and progression, which is not fully understood. Herein, we discuss the present understanding of the functional and structural roles of CD44 in the pathogenic mechanism of multiple cancers. The regulation functions of CD44 in cancers‐associated signaling pathways is summarized. Moreover, we provide an overview of the anticancer therapeutic strategies that targeting CD44 and preclinical and clinical trials evaluating the pharmacokinetics, efficacy, and drug‐related toxicity about CD44‐targeted therapies. This review provides up‐to‐date information about the roles of CD44 in neoplastic diseases, which may open new perspectives in the field of cancer treatment through targeting CD44.
Keywords: CD44, neoplastic diseases, signaling pathways, therapeutic strategies
The expression of CD44 is associated with stemness in cancer cells and activation in immune cells. In this review, we have mainly discussed the influence of CD44 expressing in cancer cells and therapeutic strategies through targeting CD44.
1. INTRODUCTION
CD44, a nonkinase transmembrane glycoprotein, 1 is expressed in various human cell types, such as immune cells, differentiated cells, cancer cells, and so on. 2 , 3 CD44 has numerous ligands, such as hyaluronic acid (HA), 4 osteopontin (OPN), 5 and serglycin. 6 The extracellular region of CD44 binding to ligands has been found to involve various of signaling pathways associated with physiological and pathological processes, 7 , 8 in particular, pathways related to carcinogenesis and tumor progression including proliferation and migration of cells, drug resistance, as well as epithelial–mesenchymal transition (EMT). 9 , 10 , 11
Alternative splicing of the CD44 gene produces two splice isoforms, CD44s and CD44v. Notably, CD44s and CD44v play an overlapping or distinct role in cancers. In most cases, CD44s is associated with tumor growth 12 and progression, 13 while CD44v, such as CD44v3 and CD44v6, is associated with invasiveness 14 and chemoresistance. 15
Accumulating evidence indicates that CD44 can regulate numerous cancer‐associated signaling pathways to influence cancer cell motility, EMT, and stemness. 16 , 17 , 18 In recent years, a number of novel functions of CD44 and its associations with cancers have been revealed. Recent advances in understanding the complex interactions of CD44 with ligands have led to the development of CD44 not only as an important cancer stem cells (CSCs) marker but also as a potential cancer therapeutic target. 3 , 19 , 20 In neoplastic diseases therapeutic areas, it is very crucial to clarify the mechanism of CD44 in the signaling pathways, thereby delaying, treating, and preventing the development of tumors through targeting CD44. However, there are relatively few clinical studies about CD44‐targeted therapies in cancer treatment. Hence, an updated and comprehensive understanding of CD44 is very crucial, which contributes to the research and development of innovative CD44‐targeted therapeutic strategies.
In this review, we discuss the structure and ligands of CD44 briefly. And we focus on the biological functions of CD44 in different cancers and cancer‐related signaling pathways regulated by CD44 and provide critical assessment of therapeutic strategies and clinical studies through targeting CD44.
2. THE STRUCTURE OF CD44
CD44 is a nonkinase cell surface transmembrane proteoglycan that includes an ectodomain, a stem region, composed of standard region and variable region, a transmembrane region and an intracellular tail. 21 In human, the gene encoding CD44 protein is on chromosome 11, while in mice, the gene is on chromosome 2, which comprises 19 exons and 20 exons. 22 Comparing with mice, CD44v1, includes homolog exon 6, is not expressed in humans. 23 For the reason that the variable exon 6 of the human gene has a stop codon that is normally not expressed in human CD44, 24 CD44s is the most common and smallest CD44 isoform, which includes the constant exons 1−5 and 16−20. Based on the structure of CD44s, CD44v consists of variable exons 6−15 located in the exon 1−5 and 16−20 regions by alternate splicing or insertion. 25 CD44v1–v10 correspond to alternative splicing or insertion of variable exons 6–15, respectively, 26 which have different functions in neoplastic diseases. 27 Moreover, a CD44v isoform could have more than one variable exon. For example, CD44v8–10 is based on CD44s structure with the insertion of v8, v9, and v10, three variable exons. 28 The correspondence between structure and gene arrangement of CD44 in mice is shown in Figure 1.
FIGURE 1.
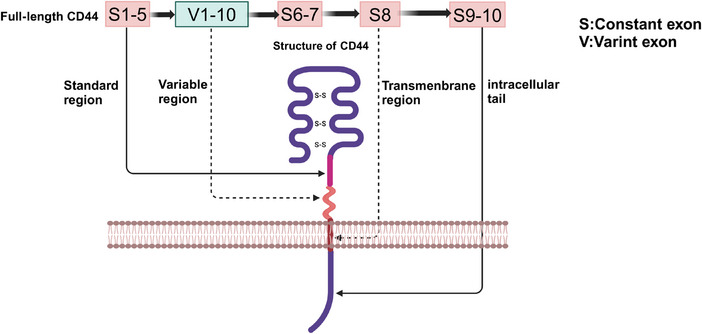
The gene arrangement and structure of CD44 in mice. The full‐length CD44 in mice has 20 exons, including constant exons and variant exons. The exon of S1–5, V1–10, S8, S9–10 corresponding to the standard region, variable region, transmembrane region and intracellular tail of CD44 respectively. This scheme was generated using Biorender.
3. CD44 IN NEOPLASTIC DISEASES
CD44 has been reported as a classic marker of CSCs, which is mainly associated with of iron endocytosis‐mediated cellular plasticity. 29 A recent study has found that in cancer cell lines, through the endocytosis of iron‐bound HA regulated by CD44, the EMT was enhanced. 30 The effects of the expressing of CD44 on immune cells and cancer cells in neoplastic diseases are summarized in Figure 2. Moreover, previous studies have found that the expression of CD44 isoforms in tumors plays crucial roles. 31 Some researchers have demonstrated that part of CD44v is associated with aggressive tumor progression and drug resistance, 32 whereas CD44s is involved in tumorigenesis and tumor growth. 13 However, recent studies have shown the inverse result. For instance, CD44s has been shown to promote migratory, invasive, and lung metastatic potential in breast cancer. 33 In 3D cultures, CD44 switching from the standard isoform to the variant 6 isoform was found to be associated with EMT in gastric cells. 34 The different or overlapping functions of CD44s and CD44v may depend on the difference in tumor types. In addition, different induction conditions, accompanied by alternative splicing of CD44 isoforms, may lead to different expression of features in tumor cells. The correlation between different cancers and CD44 are summarized in Table 1. Moreover, CD44 could also play its biological roles by interacting with ligands and messenger molecules, such as HA, 35 OPN, 36 chondroitin sulfate (CS) 37 and growth factors 38 mainly found in the tumor microenvironment. In this part, we have not only made a discussion in terms of different kinds of cancers but also discussed the effects of CD44 interacting with its different ligands on cancers.
FIGURE 2.
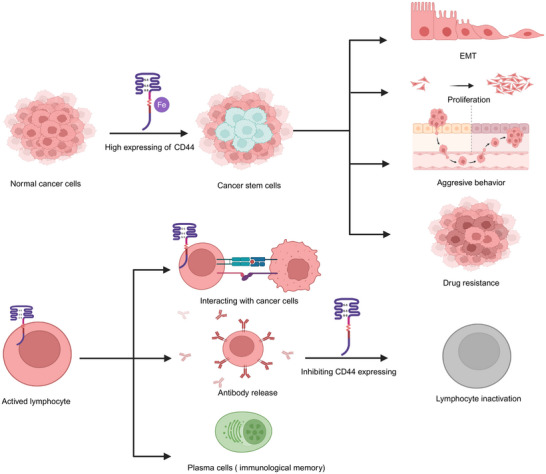
The role of CD44 expressing cancer stem cells and immune cells in neoplastic disease. The expression of CD44 on cancer cells contributes to stemness mediated by iron, which is associated with EMT, proliferation, aggressive behavior, and drug resistance. On top of that, CD44 expressing immune cells is associated with adhesion, migration, and the activation of immune cells and immunological memory. Inhabiting the expression of CD44 could cause immune cells inactivation and thus tumor immune tolerance. This scheme was generated using Biorender.
TABLE 1.
The correlation between different cancers and CD44.
| Cancer type | Role of CD44 in cancer progress | References |
|---|---|---|
| Brain cancer | Poor prognosis, tumor progression, aggressive behavior | 39 , 40 , 41 |
| Head and neck cancer | Stemness, tumor progression | 48 , 49 |
| Breast cancer | Aggressive behavior, tumor progression, stemness, poor prognosis | 56 , 57 , 58 , 59 , 60 |
| Kidney cancer | Poor prognosis, aggressive behavior, tumor growth | 65 , 66 , 67 |
| Liver cancer | Cancer initiation, poor prognosis, stemness | 72 , 73 , 74 |
| Pancreatic cancer | Tumorigenicity, clinicopathological features, aggressive behavior | 80 , 81 , 82 |
| Gallbladder cancer | Stemness, aggressive behavior, tumor progression, poor prognosis | 91 , 92 , 93 , 94 |
| Esophageal cancer | Tumor progression, stemness, poor prognosis, aggressive behavior | 97 , 98 , 99 , 101 , 102 |
| Prostate cancer | Tumor progression, stemness, aggressive behavior | 104 , 105 , 106 , 109 |
| Gastrointestinal cancer | Tumor progression, stemness, aggressive behavior, CSCs self‐renewal characteristic tumorigenesis | 114 , 115 , 116 , 117 , 119 |
| Melanoma | Stemness, tumorigenesis, aggressive behavior | 122 , 123 , 125 |
| Squamous cell carcinoma | Aggressive behavior, stemness, tumorigenesis, tumor progression | 128 , 129 , 130 |
| Sarcoma | Tumor progression, stemness, aggressive behavior, poor prognosis | 133 , 134 , 135 , 138 , 139 |
3.1. Brain cancer
It has been shown that overexpression of CD44 is linked to poor prognosis, 39 tumor progression, 40 and aggressive behavior 41 of tumors. For instance, CD44 is a possible CSCs marker in meningioma; the high expression of CD44 was associated with a shorter progression‐free survival. 42 Furthermore, in meningioma, the level of CD44 expression is correlated with the grade of the tumor and its invasiveness. 43 Moreover, inhibiting CD44 dimerization via verbascoside has been reported to suppress stem cell‐like cell properties and tumor cell growth in glioblastoma. 44 Recent studies have found that tumor cells with high expression of CD44 is associated with brain metastases. It has been revealed that in lung, melanoma, and breast cancer patients, the circulating tumor cells expressing CD44 was a prognostic marker for brain metastases. 45 Moreover, in lung adenocarcinoma, GPR124‐enhanced trans‐endothelial migration mediated brain metastases caused by lung CSCs with high expression of CD44. 46 Furthermore, in breast cancer brain metastases patients, a retrospective transversal study revealed that CD44 was associated with worse overall survival. 47 In general, CD44 is a promising marker associated with brain metastases in cancer patients, which provides us with the new insight into stratification of patients and therapy clinically.
3.2. Head and neck cancer
It is found that CD44 seems to be a classic CSCs marker, 48 which is also associated with tumorigenesis in head and neck cancer. 49 In head and neck squamous cell carcinoma (HNSCC) CSCs mouse models, targeting CD44 was shown to inhibit PI3K–4EBP1–SOX2 signaling and tumor growth and decrease the number of CSCs. 50 Moreover, it is also suggested that the switch from CD44s to CD44v8–10 was associated with tumorigenic phenotypes. 52 In addition, CD44+ cells were reported to stimulate tumor angiogenesis in HNSCC. 53 In the invasion zone of HNSCC, Odenthal et al. 54 found that CD44v6 could express constitutively. This suggests that targeting CD44v6 could be used to trustworthy near‐infrared detection. Furthermore, Choi et al. 55 has found that the interaction of COL1A1 and CD44 between fibroblasts and malignant cells was associated with HNSCC progression. Therefore, CD44 could be a promising biomarker for clinical diagnosis, and targeting CD44 could be a treatment strategy for drug resistance and tumor metastasis.
3.3. Breast cancer
In breast cancer, CD44 plays key roles in aggressive tumor behavior, 56 tumor progression, 57 CSCs trait induction, 58 , 59 and prognosis. 60 Rokana et al. 51 found that aggregated tumor cells with highly expressed CD44 could promote tumorigenesis and polyclonal metastasis. Moreover, depletion of CD44 has been found to effectively prevent the aggregation of tumor cell and decrease the levels of PAK2. 51 Interestingly, CD44s was reported to inhibit breast cancer stemness, while the cleaved product of CD44 was reported to contribute to breast cancer stemness. 61 However, bioinformatics analysis of breast cancer patients showed the reverse result. Notably, multiple studies have reported inconsistent results. For instance, CD44s being positively associated with CSCs gene signatures, while CD44v exhibited an inverse association. 62 Compelling evidence further suggests that the splicing switch of CD44 may play inverse roles in breast cancer. For instance, Yang et al. 63 found that enhanced transformation of CD44high to CD44low cancer cells induced migration and invasion behavior. Additionally, CD44 isoform switching from CD44s to CD44v resulted in an increase in the stemness of triple‐negative breast cancer. 64 Targeting different CD44 isoforms or inducing CD44 alternative splicing may be a favorable treatment strategy for breast cancer. The specific effects of CD44v and CD44s need to be further studied.
3.4. Kidney cancer
It is well documented that CD44 could serve as biomarkers that reflect poor prognosis 65 and cancer risk in kidney cancer. 66 , 67 In transformed cells within multilayered epithelia, CD44 and collagen XVII were reported to play a key role in the clonal expansion. 68 Moreover, the ferroptosis‐related gene CHAC1 was shown to contribute to poor prognosis in kidney renal clear cell carcinoma associated with the expression of the checkpoint gene CD44. 69 Notably, it was reported that silencing CD44, PLOD1, and PLOD2 genes could inhibit the proliferative and invasive potential of renal cancer cells, which suggested that these genes may serve as renal cell carcinoma oncogenes. 70 Interestingly, unlike the role of CD44 in other cancer, it was found to display the opposite effect. In renal carcinoma, CD44− cancer cells display stem‐like properties and show a higher level of invasiveness. 71 All the above results show that targeting CD44 or inhabiting CD44 expression could be a promising therapeutic strategy to suppress kidney progression.
3.5. Liver cancer
CD44 is clearly associated with liver cancer initiation, 72 poor prognosis, 73 and cancer cells stemness. 74 CD44 is expressed in carcinogen‐exposed hepatocytes in a STAT3‐dependent manner. 75 Accordingly, CD44v6 was also found to be a promising biomarker. In patients with grade 1 intrahepatic carcinomas and grade 1 hepatocellular carcinomas (HCCs), TIPRL/LC3/CD133/CD44 also play a key role in prognosis. 76 Furthermore, compelling evidence suggests that CD44 is related to stemness in liver cancer. CD44‐positive HCC patient‐derived organoids were shown to be obviously resistant to sorafenib via Hedgehog signaling. 77 Besides, in CD24+/CD44+ cells, knocking down the signature gene CTSE was found to significantly inhibit the self‐renewal potential of HCC cells. 78 CD44 surface markers were found in cancer cells with stemness properties, which indicate that, in HCC, targeting CD44 expressed in tumorigenic cells through JAK/STAT pathway is a promising therapeutic strategy 79
3.6. Pancreatic cancer
In pancreatic cancer, CD44 is involved in tumorigenicity, 80 clinicopathological features, 81 and invasiveness. 82 In pancreatic ductal adenocarcinoma (PDAC) patients with liver metastasis or poor prognosis, the expression of CD44v6 and complement C1q binding protein was higher. 83 Moreover, CD44+ PDAC cells were reduced with nimbolide treatment. 84 A meta‐analysis showed that CD44 overexpression contribute to a poor 5‐year overall survival rate and lymph node invasion. 85 On top of that, CD44+ stem cells from the Panc‐1 cell line have been found to be involved in multiresistance and metastasis. 86 In addition, more recent research has demonstrated that the interaction of HA and CD44 could be used for drug delivery in pancreatic cancer. HA‐based carriers have been shown to target tumors via interaction with CD44 in pancreatic cell lines. 87 HA‐based nanomicelles loaded with 3,4‐difluorobenzylidene curcumin could also kill CD44+ stem‐like pancreatic cancer cells. 88 Additionally, HA‐conjugated polyamidoamine dendrimers were revealed to deliver 3,4‐difluorobenzylidene curcumin to pancreatic cancer cells overexpressed CD44. 89 Furthermore, Kang et al. 90 reported a nanoparticle loaded with anticancer drug consists of HA, which could target CD44 in pancreatic cancer cells to eliminating tumor‐resident intracellular bacteria. Hence, targeting CD44 drug delivery systems in cancer cells provide an avenue for pancreatic cancer treatment. Moreover, decreasing the expression of CD44 in pancreatic cancer is also a promising therapeutic strategy.
3.7. Gallbladder cancer
There are few studies on CD44 in gallbladder cancer (GBC), which mainly focus on the different functions of CD44 isoforms and the stemness of cancer cells expressing CD44. 91 , 92 CD44v9 and CD44s cells were found to play key parts in the progression and metastasis of GBC respectively, and the isoform switch triggered EMT. 93 Interestingly, a research suggested that CD44v8–10 expression was closely associated with perineural invasion, venous invasion, and lymph node metastasis, and in the clinic, the patients with CD44v8–10+ tumors showed poor prognosis. 94 In addition, the high expression of CD44 as a stem cell marker in sphere clones of the human GBC cell line GBC‐SD was further explored. 95 Interestingly, in general, the stemness of cancer cells is positively correlated with drug resistance, but the opposite effect is shown in GBC. 96 All the studies indicate that the characteristics of CD44 in GBC provide some new thought in diagnosis and treatment of GBC.
3.8. Esophageal cancer
High expression of CD44 plays roles in tumor progression and stemness in esophageal squamous cell carcinoma (ESCC). 97 , 98 , 99 CD44 is a novel stem cell marker in ESCC that has been reported to be eliminated by inhibiting the canonical NOTCH pathway. 100 Moreover, CD44v9 was shown to be strongly associated with EMT and poor prognosis in patients with ESCC. 101 Furthermore, microRNA (miR)‐34a suppressed invasion and metastasis in ESCC by regulating CD44. 102 In addition, the latest research has found that the alternative splicing of CD44 isoform from CD44s to CD44v8–10 is associated with ESCC metastasis and poor prognosis in clinic. 103 The CD44v isoforms has similar functions in ESCC, which provide useful insights for the development of ESCC treatment and prognostic biomarkers.
3.9. Prostate cancer
CD44 is involved in prostate cancer, and different CD44 isoforms play different roles in tumor progression and stemness. 104 , 105 , 106 A recent study showed that TGF‐β1‐mediated alternative splicing could switch CD44v to CD44s, which enhanced EMT and stemness in human prostate cancer cells. 107 In DU145 and PC3 prostate cancer cells, the high expression of CD44v4, v5, and v7 mediated by sulforaphane contributed to tumor cell growth and proliferative activity. 108 Furthermore, CD44high stem cell‐like cells have been found to be involved in drug resistance and invasive phenotypes in DU145 and PC3 cell populations. 109 In addition, high expression of CD44 was reported to be associated with prostate cancer cell migration and proliferation. 110 Accumulating evidence suggests that CD44 is a stem cell marker in prostate cancer. Translationally controlled tumor protein (TCTP) was closely associated with survival factor of stem cells, and the TCTP inhibitor sertraline highly downregulated the expression of CD44. 111 Enzalutamide has been found to induce stem‐like characteristics to acquire resistance, and CD44 has been found in enzalutamide‐resistant cells. 112 Additionally, STAT3 also contributes to the cell stemness and activation of the CSCs marker CD44 in PC cells. 113 In general, CD44v isoform is associated with cancer stemness. However, only some studies have identified the specific CD44v isoform. The specific CD44v isoforms in other studies are unclear, which need further study.
3.10. Gastrointestinal cancer
Abundant evidence indicates that CD44 contributes to tumor progression 114 and stemness 115 in gastrointestinal cancer. It was revealed that MUC5AC interacting with CD44 promoted cell invasive and migrative potential and decreased apoptosis of colorectal cancer (CRC) cells via Src signaling. 116 In addition, PD‐L1 was found to increase the population sizes and the tumorspheres forming ability in CD133+CD44+ cell, which resulted in colorectal CSCs self‐renewal. 117 A previous study revealed that CD44, a stem cell marker in gastric cancer (GC) lines, could be suppressed by DAXX. 118 Interestingly, CD44 was also found to play either different or overlapping roles in different gastrointestinal cancers, which may be related to the species of CD44 and its isoforms. A previous study revealed that CD44v8–10, but not CD44s, expressing could restore the tumor‐initiating potential of GC cells reduced by silencing total CD44. 119 Moreover, CD44v8–10 also plays an important part in regulation of ROS defense and tumor growth by p38 (MAPK) and p21 (CIP1/WAF1) in human gastrointestinal cancer cells. 120 CD44v6 was found to be a stem cell marker, and its overexpression promoted migration and metastasis in colorectal CSCs by activating Wnt/β‐catenin. 14 In addition, accumulating evidence suggests that miRs binding to CD44 plays key roles in drug resistance, tumor growth, and stemness. For instance, miR‐302a binding to CD44 was shown to suppress CSCs‐like properties and restore cetuximab (CTX) responsiveness in CRC. 121
3.11. Melanoma
CD44 is a CSCs marker 122 in melanoma cancer and participates in tumor initiation progression. 123 Wei et al. 124 found that downregulated RNF128‐activated Wnt signaling induced cellular EMT and stemness by ubiquitinating and degrading CD44/cortactin. Additionally, the depalmitoylation of the prometastatic cell adhesion molecule CD44 was shown to result in increased melanoma invasion via Wnt5a. 125 Compelling evidence suggests that drugs targeting CD44 in melanoma is a promising strategy for melanoma treatment. For instance, expressing BMP4/7‐dependent Id1/3 protein was reported to decrease the survival rate in melanoma patients promoted by HA–CD44 interactions with BMPR. 126 Moreover, nanodrug based on CS could target CD44 to treat melanoma by inducing mitochondrial apoptosis. 127
3.12. Squamous cell carcinoma
CD44 plays a role in various squamous cell carcinomas and has been shown to be mainly involved in EMT 128 and cancer stemness. 129 In HNSCC, HA binding to CD44 was found to increase CSCs numbers by PI3K–4EBP1–SOX2, whereas CD44 binding to VCAM‐1 contribute to invasiveness by ezrin/PI3K. Moreover, the switch from CD44v8–10 to CD44s was reported to promote EMT and participate in tumor invasion, 52 which is contrary to the results in ESCC of Yu et al. 102 In mouse and human squamous cell carcinoma, it has been demonstrated that induction of a hybrid EMT state contributes to tumor initiation, progression, invasiveness, stemness, and metastasis by activating the CAMK2–CD44–SRC axis. 130 Moreover, in ESCC patients, SOCS6 has been shown to significantly decrease the population of CSCs expressing the surface biomarker CD24low/CD44high to overcome radioresistance. 131 Furthermore, in CD44high OSCC cells, TGF‐β1 was found to induce amoeboid‐to‐mesenchymal transition (AMT) via activating ERK and phosphorylating Cofilin‐1. 132 In summary, the role of CD44 in squamous cell carcinoma could serve as a vital area for drug development and tumor marker.
3.13. Sarcoma
Compelling evidence suggests that the overexpression of CD44 in most sarcomas participates in tumor progression, 133 , 134 stemness, 135 and dissemination. 134 CD44 was found to increase the resistance of osteosarcoma cells to doxorubicin by upregulating multidrug resistance 1 protein expression. 136 The human osteosarcoma cell lines MNNG/HOS and 143B were both highly metastatic, and CD44 was reported to be knocked out by CRISPR/Cas9. Additionally, inhibiting cell proliferation and tumor sphere formation cultured in 3D environment was found to depend on CD44 inactivation. 137 Furthermore, in a mouse fibrosarcoma model, the increased expression of human CD44s was shown to promote micrometastasis events. 138 Moreover, in osteosarcoma, CD44v6 is an important prognostic factor of patient prognosis. 139 In sarcomas, research on the roles of CD44 is conductive to understanding the pathogenesis of these rare cancers.
3.14. Effects of CD44 interacting with HA on cancers
HA, a linear glycosaminoglycan (GAG), 140 binds to the N‐terminus of the extracellular domain of all CD44 isoforms. 141 Aruffo et al. 142 first proposed the relationship between CD44 and HA. Through endocytosis mediated by iron, 143 , 144 the interaction between CD44 and HA plays crucial role in the progression, 145 invasion, 146 and chemoresistance 147 of cancer cells. As reported, patients with CD44s‐positive tumors may gain a survival benefit from HA–irinotecan, which is a formulation of HA and irinotecan. 148 In tumors with increased expression of CD44, the using of HA‐based nanocarriers was reported to have the benefits in the enhancement of drug delivery, the increase therapeutic efficacy with low cytotoxicity, the inhibition of tumor growth, as well as the high potential for targeted chemotherapy. 149 Moreover, kynureninase associated with the onset and development of breast cancer was found to be upregulated by CD44, which was induced and activated by HA. 141 In addition, CD44 isoforms also regulate the uptake and expression of HA. It has been revealed that the expression of cancer cells with CD44v can negatively influence the uptake of HA, whereas cells expressing CD44s has positive correlations with the uptake and expression of HA. Moreover, the ability of HA uptaking varies across the cell lines. CD44shigh cancer cells could uptake HA more efficiently compared with CD44slow human dermal fibroblasts. 87 In summary, the interaction of CD44 with HA and different CD44 isoforms regulates the ability of HA uptaking and expression have effect on cancer, which provides important advances with respect to cancer treatment strategies based on HA and the of selection of different CD44 isoforms as a target.
3.15. Effects of CD44 interacting with OPN on cancers
OPN, a phosphorylated glycoprotein, which expressed in the mineralized extracellular matrix (ECM) of bone in normal tissues cells such as fibroblasts, osteoblasts, and osteocytes. 150 , 151 As one of the classical and important ligands of CD44, OPN affects the proliferation and invasion of tumor cells as well as inflammation in normal cells, by regulating related signaling pathways. 150 , 152 Activation of the JUN N‐terminal kinase pathway has been shown to contribute to the promotion of clonogenicity and tumor growth in a xenograft model via OPN secreted by tumor‐associated macrophages. 153 In PC3 cells, OPN binds to CD44 receptors that could inhibit the c‐RaF and ERK1/2 by activating the AKT pathway, thereby inhibiting cell cycle arrest. 154 Additionally, Yang et al. 155 found that the interaction of CD44 with OPN could promote tumor‐associated mesenchymal stem cells formation, leading to lung cancer cells invasion and migration. Moreover, decreased expression of interferon regulatory factor 8 in colon carcinoma was reported to increase the expression of OPN, which is associated with lower patient survival rate. This phenomenon is associated with the interaction of OPN with CD44 leading to immune escape. 156 Taking together, high expression of OPN could promote immune escape of tumor cells, which may be related to regulate T cells activation through OPN interacting with its receptor CD44 and compensating for the function of PD‐L1. 157
3.16. Effects of CD44 interacting with serglycin on cancers
Serglycin has been shown to be a ligand for CD44. Previous studies have found that the binding of CD44 to serglycin is implicated in tumorigenesis and prognosis. 158 The attachment of GAGs to serglycin could facilitate this binding. 159 For instance, the proteoglycan serglycin was reported to promote the migration of non‐small cell lung cancer cells via the binding of its GAG motif to CD44. 160 Moreover, serglycin binding to CD44 was shown to regulate the expression of CD44 in a β catenin‐dependent manner, a finding that might improve the poor prognosis in triple‐negative breast cancer. 161 In addition, the interaction of serglycin with CD44 was revealed to promote tumorigenesis in giant cell tumors of bone via activating focal adhesion kinase. 162 There are few studies on serglycin and CD44 in tumors, but for the important role of serglycin binding to CD44 in tumorigenesis, it is believed that there will be more therapeutic strategies based on the regulating the binding of the two in future studies.
3.17. Effects of CD44 interacting with CS on cancers
In recent years, the studies on CD44 and CS mainly focused on the nanodelivery systems based on the specific binding between CD44 and CS. 163 Accumulating evidence suggests that CS modifies the drug delivery of nanoparticles that target CD44 into tumor cells with low cytotoxicity. 164 Nanoparticles composed of CS, doxorubicin, and bovine serum albumin targeting CD44 were reported to suppress 4T1 tumor growth. 165 Additionally, a nanosystem targeting CD44, composed of d‐α‐tocopherol polyethylene 1000 glycol succinate and CS dual‐modified lipid‐albumin, was found to deliver paclitaxel into multidrug‐resistant tumor cells, thereby overcoming drug resistance. 166 Moreover, Chu et al. 167 found that suppressing the expression of CD44 and integrin β1 could reduce the invasiveness of glioma cells by suppressing CS synthase 1. All the evidence shows that CD44 plays an important role in cancer treatments by nanoparticles modified by CS and other drugs.
3.18. Effects of CD44 interacting with matrix metalloproteinases on cancers
Matrix metalloproteinases (MMPs) is a large family of zinc endopeptidases whose members can interact with CD44. The binding of CD44 to MMPs has also been reported to mediate tumor growth, stemness, as well as the aggressive behavior. 168 , 169 It has been shown that hybrid nanoparticles targeting CD44 or MMPs have antitumor efficacy in 4T1 breast tumor, 170 MCF‐7, and MDA‐MB‐231 cells 171 by inhibiting the expression and activity of MMPs. Also, polyphenols extracted from Artemisia annua L. showed anticancer effects by suppressing CD44 and MMP9 in radio‐resistant MDA‐MB‐231 human breast cancer cells. 172 Interestingly, CD44 has been shown to be both positively and negatively correlated with the expression of MMPs. 173 For instance, the overexpression of CD44 was reported to reduce the levels of MMPs for the maintenance of ECM homeostasis. 174 In Papadopoulou's study, 175 the increase in MMP1 and MMP13 expression followed by the decreased expression levels of CD44 was found to promote tumor growth. Another study also showed that MMP2 activation contributed to decrease the number of CD44+/CD24− cells in MDA‐MB‐231 cells after normothermic microwave treatment. 176 In contrast, inhibiting the expression of MMPs also inhibited the expression of CD44, for instance, DSPP/MMP20 gene silencing resulted in downregulation of CD44, a marker of oral squamous cell carcinoma (OSCC), and increased sensitivity to cisplatin. 177 Moreover, CD44 was shown to promote lung ADC cell invasion by regulating MMP‐2 expression. 178 Also, in PC3 cells and breast carcinoma cells, the intracellular domain of CD44 was found to form a complex with the RUNX2 protein, which mediated cancer metastasis, migration, and progression through activation of MMP‐9. 179 In encapsulated papillary carcinoma (EPC), the high expression of CD44s induced high expression of MMP2 associated with invasion. 180 Overall, the complex relationship between CD44 and MMPs expression provides us with more ideas for selecting different targets in cancer therapeutic strategies.
3.19. Effects of CD44 interacting with fibronectin on cancers
It was revealed that fibronectin (FN) in the ECM could interact with CD44, which is involved in cell metastasis, 181 migration, 182 adhesion, 183 and survival. 184 In endothelial cells, space microgravity was found to modulate the expression of CD44 and restrain collagen I and FN deposition, which involved cellular adhesion. 185 Additionally, by using a CD44 antibody to partially inhibit extracellular FN signaling, a significantly decrease in adhesion and survival of melanoma cells was obversed. 186 Furthermore, FN type III domain‐containing protein 3B circular RNA increased migration and invasion in GC with a decline in CD44. 187 Although there are few studies on regulating the interaction of CD44 with FN to treat cancer, owing to the key role of FN in cell migration and adhesion, this aspect may be a promising therapeutic strategy in cancer metastasis.
The relationship in neoplastic diseases between CD44 and its ligands is complex, and the expression of the two may be positively or negatively correlated. The main studies is about CD44 rather than a specific CD44 isoform interacts with its ligands. Together, CD44 interacts with its ligands plays key roles in cancer progression. All the abovementioned studies provide new insights into cancer therapy by interfering the interaction of CD44 with its ligands.
4. CANCER‐ASSOCIATED PATHWAYS REGULATED BY CD44
Activation and deactivation of CD44 isoforms regulate the activities of the components of signaling pathways, including enzymes, 188 protein kinase pathways, 189 , 190 and transcription factors, 191 which have been found to be associated with tumor initiation progression and aggressive behavior. 192 , 193 Moreover, CD44 is also found to be a component in some cancer‐associated signaling pathways. 194 Some signaling pathways associated other cancers regulated by CD44 are summarized in Figure 3. Moreover, the biological functions of different CD44 isoforms in cancer are summarized in Table 2
FIGURE 3.
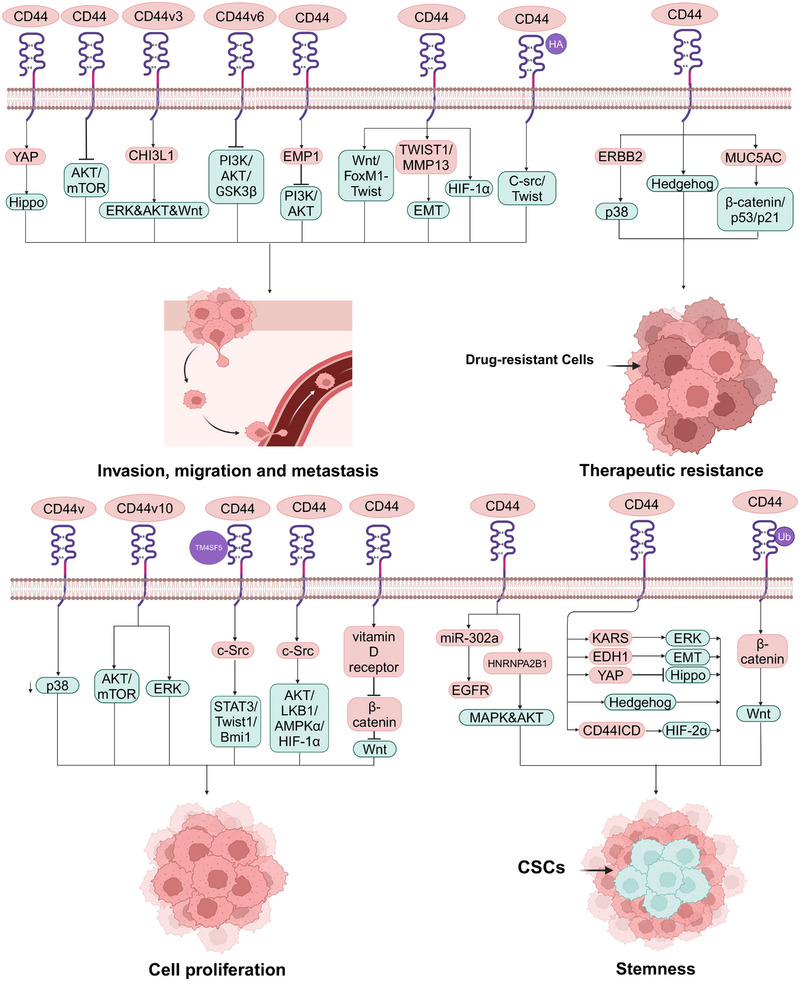
CD44‐mediated cancer‐associated signaling pathways. CD44 can contribute to cancer invasion, migration, and metastasis through Hippo–YAP, HIF‐1α, and Wnt–FoxM1–Twist signaling. CD44v3 binds to CHI3L1, resulting in metastasis of GC via the ERK–AKT–Wnt pathway. Silencing of CD44v6 blocks PI3K/AKT/GSK3β signaling pathway, EMP1‐affected CD44 expression inhibits the PI3K/AKT signaling pathways, CD44 inhabits the phosphorylation of AKT/mTOR, HA‐induced CD44 interaction with C‐Src‐activated Twist can result in inhibiting cancer aggressive behavior. The TWIST1–CD44–MMP13 axis involves in tumor aggressiveness through EMT. MiR‐302a binds to CD44, resulting in suppressing CSCs‐like properties via EGFR‐mediated MAPK and AKT signaling. CD44high CSCs activate the AKT and MAPK pathways via the expression of HNRNPA2B1, CD44 activates KRAS/ERK signaling, EDH1 interaction with CD44 inhabiting Hippo pathway, the upregulation of CD44 activates YAP through inactivation of Hippo signaling pathway, CD44ICD binding to HIF‐2α via activation of HIF‐targeting genes, and the ubiquitination and degradation of CD44/cortactin via activating Wnt/β‐catenin signaling can result in cancer stemness and EMT features. Ablation of CD44v via activation of p38 signaling and Vitamin D receptor‐induced inhibition of CD44 via Wnt/β‐catenin signaling can result in inhabiting tumor growth and cell proliferation. CD44v10 activates ERK/p38 MAPK and AKT/mTOR signaling, the TM4SF5/CD44 interaction via activating c‐Src/STAT3/Twist1/Bmi1 signaling can result in facilitating cell proliferation. Reducing the glycolytic phenotype of cancer cells through the c‐Src/AKT/LKB1/AMPKα/HIF‐1α signaling pathway can silence CD44 to regulate cell proliferation. CD44 collaborates with ERBB2 to promote the radioresistance of cancer cells via p38 phosphorylation. CD44+ cancer cells reverse sorafenib resistance via suppressing Hedgehog signaling. Upregulation of CD44 confers chemoresistance via β‐catenin/p53/p21, which is associated with the secretory mucin MUC5AC. This scheme was generated using Biorender.
TABLE 2.
Biological functions of different CD44 isoforms in cancer.
| CD44 isoforms | Biological functions | References |
|---|---|---|
| CD44s | Tumorigenesis, tumor growth, aggressive behavior, micrometastasis events, inhabiting stemness, CSCs characteristics, EMT | 13 , 52 , 61 , 62 , 93 , 107 , 138 , 180 |
| CD44v | Invasiveness, stemness, tumor growth | 62 , 64 , 120 |
| CD44v3 | Metastasis, EMT | 221 |
| CD44v4, v5, and v7 | Growth and proliferative activity | 108 |
| CD44v6 | EMT, aggressive behavior, poor prognosis, | 34 , 54 , 139 , 223 |
| CD44v8–v10 | Tumorigenic phenotypes, aggressive behavior, poor prognosis, restoration of tumor‐initiating potential, tumor growth | 52 , 76 , 83 , 94 , 103 , 119 , 120 |
| CD44v9 | Progression and metastasis, poor prognosis, EMT | 14 , 93 , 101 |
| CD44v10 | Cell proliferation | 199 |
4.1. MAPK signaling pathway
The activity of the MAPK signaling pathway by CD44 has been associated with the growth of cancer cells. 195 In CRC patients, miR‐302a binding to CD44 was shown to restore CTX responsiveness by suppressing CSCs‐like properties via EGFR‐mediated MAPK and AKT signaling. 121 Furthermore, MCF‐7‐A2B1 cells with a high proportion of CD44+/CD24−/low CSCs were reported to activate the AKT and MAPK pathways via the expression of HNRNPA2B1. 196 Moreover, although there are few studies on the regulation of CD44 on p38 in cancer, it is well documented that CD44 regulates cancer progression via activation of p38. 197 , 198 In a transgenic mouse model of GC, ablation of CD44v was reported to suppress tumor growth via activation of p38 signaling. 120 In prostate cancer cells, CD44 collaborates with ERBB2 to contribute to the radioresistance of cancer cells via p38 phosphorylation. In breast cancer cells, CD44v10 was found to facilitate cell proliferation via activating ERK/p38 MAPK and AKT/mTOR signaling. 199 Cell cycle arrest and inhibition of the survival, proliferation, and migration of PC3 cells expressing CD44 and CD133 isolated from prostate cancer by inhibiting p‐p38, p‐ERK, NF‐κB, and PARP. 200 Furthermore, CD44‐regulated ERK signaling is mostly described as stemness and aggressive behavior of tumors. 201 , 202 Yokoyama et al. 132 found that OSCC cells highly expressing CD44 had been shown to be associated with AMT via activation of ERK. In glioblastoma multiforme cells, high expression of CD44 was found to induce cancer stemness and EMT features via KRAS/ERK signaling activation. 203 In addition, the high expression of CD44 was capable of inducing ERK phosphorylation, which affects the migratory and invasive potential of lung cancer cells. 204
4.2. Hippo signaling pathway
In the Hippo pathway, CD44 plays key role in cancer stemness and aggressive behavior. 205 , 206 , 207 In lung adenocarcinoma, EDH1 interaction with CD44 was shown to promote CSCs‐like traits, EMT, and metastasis via inhibition of the Hippo pathway. 208 CD44 upregulation can also confer CSCs‐like properties to malignant mesothelioma cells by activating YAP via Hippo signaling pathway inactivation. 209 In addition, in docetaxel‐resistant prostate cancer cells, CD44 was reported to promote cell migration and invasion via induction of Hippo–YAP pathways. 210 Moreover, knockdown YAP partially abolished the stemness of CD44+ retinoblastoma stem‐like cells. 211
4.3. Hedgehog signaling pathway
CD44+ CSCs is a more appreciated subset which plays key roles in conventional stemness‐related Hedgehog signaling activation. 212 , 213 In CD44+ HCC, suppressing Hedgehog signaling was reported to reverse sorafenib resistance. 77 On top of that, in patients with CRC, CD44‐high tumors have been shown to be enriched for the Wnt/β‐catenin and Hedgehog signaling pathways. 214 Additionally, a bladder CSCs subpopulation with the stem cell‐like marker CD44 was responsive to inhibition of Sonic Hedgehog signaling. 215 Overall, regulation of the Hedgehog signaling pathway is a promising therapeutic target in CD44+ CSCs. CD44+ CSCs is associated with activation of Hedgehog signaling. 216
4.4. PI3K‐AKT signaling pathway
A number of studies have also highlighted the effect of CD44 on AKT in relation to cancer cell growth, motility, invasion and stemness. 217 , 218 , 219 Thanee et al. 220 found that silencing CD44 inhibited cholangiocarcinoma progression and aggressiveness, as well as AKT and mTOR phosphorylation. As demonstrated by GC cell lines and an experimental animal model, chitinase‐like protein CHI3L1 binding to CD44v3 was a specific inducer of activation of ERK, AKT, and β‐catenin signaling and enhancement of GC transfer. 221 Moreover, studies of in vitro and in vivo found that CD44 promoted HCC migration and extrahepatic metastases mediated by the AKT/ERK signaling CXCR4 axis. 222 In addition, more recent researches have demonstrated that activation of the PI3K signaling pathway is positively associated with tumor growth and aggressive behavior. In HNSCC, targeting CD44 was found to decrease tumor growth and CSCs by inhibiting PI3K–4EBP1–SOX2 signaling. 52 Furthermore, in OSCC, silencing CD44v6 diminished invasion/metastasis potential by blocking the PI3K/AKT/GSK3β pathway. 223 Finally, inhibiting the PI3K–AKT signaling pathway contribute to inhabit invasion and proliferation of glioma cell mediated by epithelial membrane protein 1 (EMP1), which was affected by the expression of CD44. 224
4.5. Twist signaling pathway
Twist is mainly related to EMT in cancer cells, 225 and CD44 is found to play a key part in the adaptive plasticity of cancer cells by regulating Twist signaling. 226 CD44 was reported to promote lung CSCs metastasis through Wnt–FoxM1–Twist signaling. 227 In addition, in colon CSCs with CD133+ and CD44+ markers, triptolide inhibited cell death, apoptosis and altered cell cycle distribution by inhibiting snail slug and Twist expression, which has been reported to be associated with EMT. 228 Moreover, in ESCC, the TWIST1–CD44–MMP13 axis has been shown to be involved in tumor aggressiveness and EMT. 229 On top of that, T mutant allele of CD44 (rs8193C>T) could trigger PKC–Twist, PKC–Nanog, and Nanog–Stat signaling pathways through binding to PUM 2, which is related to prediction of prostate neoplasms and prognosis factor in prostate neoplasms. 230
4.6. HIF signaling pathway
Both HIF‐1α and HIF‐2α play roles in tumor stemness, 231 , 232 progression, 233 and metabolism. 234 Researches found the expression of CD44 could affect HIF signaling pathway. In glioma, CD44ICD binding to HIF‐2α (but not HIF‐1α) induced stemness via activation of HIF‐targeting genes. 235 Interestingly, Yang et al. 236 found HIF‐1α is also associated with MDA‐MB‐231 and 468 cells stemness through Coenzyme Q0 treatment to decrease the expression of CD44. Moreover, in TE10 and TE11 cells, the expression of SET domain‐containing 5 could increase the expressing level of cancer stemness‐related protein HIF‐1α and CD68 expression, which is associated with the coexpression of CD44 and SET domain‐containing 5. 237 The multiple effects of HIF may associated with the type of cancers, which expressing different interacting factors affected by CD44. In the human gastric cell lines SGC‐7901 and BGC‐823, hypoxia‐increased expression of CD44 was found to increase cell viability and invasion associated with high expression of HIF‐1α. 238 Additionally, in human breast cancer cells, silencing CD44 was also shown to decrease the glycolytic phenotype of cancer cells via regulating c‐Src/AKT/LKB1/AMPKα/HIF‐1α signaling. 239
4.7. c‐Src signaling pathway
In recent years, although few studies have investigated the c‐Src signaling pathway in cancer regulated by CD44, accumulating evidence suggests that activation of c‐Src plays a key part in tumor progression. 240 , 241 In human breast cancer cells, CD44 knockdown suppressed both the mRNA and protein expressing of c‐Src and its downstream MAPK signal. 242 Moreover, in breast cancer cells, HA‐induced CD44 interacting with c‐Src‐activated Twist resulted in downregulation of tumor suppressor protein, Rho GTPase ROK activation and tumor cell invasion, which are critical prerequisite steps for obtaining metastasis. 243 Additionally, the TM4SF5/CD44 interaction of metastatic HCC cells activated c‐Src/STAT3/Twist1/Bmi1 signaling, which caused spheroid formation. 244
4.8. Wnt signaling pathway
CD44 is a target gene in the Wnt signaling pathway 245 and plays a key part in activation of the Wnt pathway mediating chemoresistance, 246 EMT, 247 and tumor progression. 248 In CRC cells, upregulation of CD44, positively associated with the secretory mucin MUC5AC, was reported to confer chemoresistance via β‐catenin/p53/p21. 116 Moreover, downregulation of RNF128 led to the ubiquitination and degradation of CD44/cortactin, inducing cellular EMT and stemness via activating Wnt/β‐catenin signaling. 124 Furthermore, in human GC cells MKN45 and KATO III, vitamin D receptor‐induced suppression of CD44 was shown to suppress cell growth, probably via inhibition of Wnt/β‐catenin signaling. 249
5. ANTICANCER THERAPEUTIC STRATEGIES BASED ON TARGETING CD44
5.1. Antibodies, peptides, and aptamers
It has been shown that suppressing the expression of CD44 250 or blocking its interaction with other ligands 251 , 252 is a promising strategy in cancer therapy. Antibody against CD44 has been reported to be used in this way in CD44‐positive cancer. For instance, in the human bladder cancer cell line EJ, a novel mouse monoclonal antibody (mAb) KMP1 inhibited proliferation, migration, and adhesion as well as suppressed the xenograft tumor growth in nude mice by blocking CD44. 253 In OSCC, a defucosylated anti‐CD44 mab 5‐mG2a‐f was found to have antitumor effects both in vitro and in vivo. 254 Apart from antibodies, another antitumor strategy is to take advantage of synthetic peptides to selectively bind CD44 to inhibit its functions or block the interaction of CD44 and its ligands. In mice harboring tumors, intravenously administered CNLNTIDTC and CNEWQLKSC peptides targeted tumors and inhibited metastasis by binding to CD44v6. 255 Moreover, it has been demonstrated that a synthetic IGFBP‐3 peptide (215‐KKGFYKKKQCRPSKGRKR‐232) can inhibit the viability of A549 cells as a result of CD44 competing with the HA receptor. 256 Indeed, a research suggested that CD44 aptamers exert antitumor effects by targeting CD44. In ovarian cancer, an RNA‐based bispecific CD44–EpCAM aptamer was shown to inhibit cell growth and to induce apoptosis by blocking CD44 and EpCAM simultaneously. 257 Moreover, follow‐up experiments identified the inhibition of orthotopic glioma growth by AS1411 aptamer coloading shikonin and docetaxel targeting CD44‐overexpressing glioma. 258 Of note, the anticancer effect of antibodies, peptides, and aptamers depends on their binding affinity and specificity to CD44. This suggests a direction for targeted therapy targeting CD44 isoforms and highlights the different roles of the stroma in tumors.
5.2. Pharmacological inhibition
Other than directly targeting CD44, several natural compounds and chemotherapeutic agents are also important anticancer agents in cancer cells and CSCs expressing CD44. 259 , 260 , 261 In glioma cells, galangin was found to inhibit EMT and angiogenesis by suppressing the expression of CD44. 262 Similar results are further supported by Jobani et al., 263 who demonstrated that combination treatment with allicin and all‐trans retinoic acid significantly reduced the IC50 value in CD44 expressing melanoma cells CD44. Additionally, in the breast cancer cell line MDA‐MB‐231, ivermectin was shown to preferentially inhibit the CD44+/CD24− CSCs subpopulation. Overall, these data suggest that pharmacological therapy that suppresses CD44 expressing in cancer cells is a promising strategy.
5.3. Gene therapies
MiRNAs can directly bind to CD44 to silence gene expression at the RNA level, which has been shown to regulate CSCs characteristics and tumor progression. 264 , 265 , 266 For example, in colon cancer cells, miR‐145 and antagomir‐21 were found to suppress CSCs proliferation by inhibiting the expression of CD44. 267 In addition, another study found that in CRC cells, CTX responsiveness could restored by miR‐302a by inhibiting CD44‐induced CSCs‐like properties. 121 Moreover, Liu et al. 268 found miR‐34a could directly target CD44 and miR‐34a inhibited prostate CSCs and metastasis by negatively regulating the expression of CD44. On top of that, Feng et al. 269 found that miR‐373 and miR‐520s could affect the growth and invasiveness of glioblastoma cells through interacting with CD44 to decrease its expression. In papillary thyroid cancer, miR‐205‐5p/GGCT was found to inhabit its growth and metastasis through regulating the expression of CD44. 270
On the other hand, it is worth noting that short interfering RNAs (siRNAs) have been shown to knockdown the expression of CD44 via repression of translation to suppress EMT, drug resistance, and growth in tumors. 271 , 272 , 273 In colorectal CSCs, siRNA inhibited EMT‐induced proliferation and invasion by silencing the expression of CD44. 274 In addition, in the human breast cancer cell line MDA‐MB468, siRNA‐mediated silencing of CD44 was shown to enhance doxorubicin chemosensitivity. 275 In addition, in the EGFR wild‐type non‐small cell lung cancer cell line H460, knocking down CD44 by siRNA has been found to reduce cell growth and induce cell apoptosis. 276
Overall, recent developments support the implementation of gene therapy as a new component in therapeutic agents targeting CD44.
5.4. Cell therapies
Recently, CD44 isoforms have been found as an promising target for chimeric antigen receptor T cells (CAR T cells) to eliminate CD44 expressing cancer, which is a novel and specific treatment. 277 , 278 CD44v6 is found to be associated with tumor progression and aggressive behavior. 279 Hence, CD44v6 CAR T cells is an attractive therapy to control tumor growth. For instance, CD44v6–CAR T cells were found to specifically lyse CD44v6+ acute myeloid leukemia cells associated with cytokine release. 280 Moreover, Haist et al. 281 showed a positive link between CD44v6 expression levels and the cytotoxicity of CAR T cells and found that CD44v6–CAR T cells specifically eliminated CD44v6+ HNSCC. Furthermore, minicircle DNA‐mediated CD44–CAR T cells were shown to suppress HCC. 282 Token together, little research has been done on CAR T cells targeting CD44 expressing cancers cells, but it is being rolled out and is a novel therapeutic strategy. CAR T cells targeting CD44s or other CD44v expressing cancer cells require further study, for the reason that highly customized CAR T treatments could focus on the differences of CD44 isoforms expressing in cancer cells to improve their specificity and off‐target effect.
5.5. Biological materials
Robust evidence supports that antitumor agents conjugated by biomaterials show good antitumor effects and targeting activity through a ligand–receptor‐mediated targeting mechanism. 283 , 284 , 285 For instance, CS‐conjugated doxorubicin poly(lactic‐co‐glycolic acid) (PLGA) nanoparticles were found to directly target CD44 with low cardiac toxicity and strong antitumor ability. 286 Moreover, an HA‐labeled PLGA nanoparticle encapsulating both paclitaxel and focal adhesion kinase siRNA was reported to bind to CD44‐positive epithelial ovarian cancer cells to overcome chemoresistance. 287 In addition, novel HA cross‐linked zein nanogels were developed to deliver curcumin into CT26 cells expressing CD44, which showed high anticancer activity. 288 In conclusion, biomaterial coupling of antitumor agents shows good target activity and low toxicity, which is a promising therapeutic strategy in cancer treatment. Moreover, a recent research has found a self‐crosslinkable chitosan–HA dialdehyde nanoparticles could target the delivery of siRNA to T24 bladder cancer cells, which could affect bladder cancer through the interaction of CD44 and HA. 289
6. CLINICAL STUDIES
Although accumulating evidence suggests that CD44 plays a key role in the clinicopathological features of numerous cancers, 290 , 291 there are relatively few clinical studies designed to evaluate the efficacy of CD44‐targeted therapies in cancer treatment.
To date, 16 clinical studies have been registered on ClinicalTrials. Gov to assess the anticancer effects of targeting CD44. The summary content is shown in Table 3. Among them, three trials are designed to evaluate CD44v6‐specific CAR T‐cell therapies; two trials are using humanized mAb drugs for the treatment of CD44+ cancer; two trials are aimed at inhibiting the expression of CD44 in cancer; and one trial is about a drug designed to bind to CD44 for the treatment of chronic lymphocytic leukemia and small lymphocytic lymphoma. However, a phase I trial of an anti‐CD44 humanized antibody, RG7356, in CD44 expressing solid tumor patients was failed. 292 The study was terminated early because RG7356 does not have a relationship with clinical and/or pharmacodynamic dose–response. On the one hand, in plasma, the RG7536 was converted to a binding‐impaired molecule that cannot result in sufficient antibody levels due to the deamidation of asparaginases in the complementarity determining region of intact antibody. One the other hand, the expression of CD44s or CD44v in patient tumor is crucial. Some CD44s expressing tumor patients (four out of nine) showed tumor shrinkage which was not observed in CD44v expressing tumor patients. This phenomenon may be associated with the iron endocytosis is recompensated by transferrin receptor, resulting in the reducing of drug specific uptake of tumor cells. 30 , 293 , 294
TABLE 3.
Clinical studies using CD44‐targeting therapies for cancer treatment.
| Form of drug | Cancer applications | Interventions | Phase | NCT No. |
|---|---|---|---|---|
| Gene‐engineered T cells | CD44v6+ cancer | CD44v6 CAR T cells | I/II | NCT04427449 |
| Breast cancer | Her2, GD2, and CD44v6 CAR T cells | I/II | NCT04430595 | |
| CD44v6+ acute myeloid leukemia and multiple myeloma | Drug: MLM‐CAR44.1 T cells | I/II | NCT04097301 | |
| Humanized mAb | CD44v6+ breast neoplasms | Drug: Bivatuzumab mertansine | I | |
| CD44+ malignant solid tumors | Drug: RO5429083 | I | NCT01358903 | |
| CD44v6 inhibitor | Malignant solid tumor | Drug: AMC303 | I | NCT03009214 |
| CD44+ ovarian epithelial cancer | Drug: SPL‐108 | I | NCT03078400 | |
| CD44 binding peptide | Chronic lymphocytic leukemia and small lymphocytic lymphoma | Drug: A6 | I | NCT02046928 |
Data sources: ClinicalTrials. Gov.
Together, treatments targeting CD44 are promising, and it is expected that more translational studies will be concentrated on targeting different kinds of CD44 isoforms. Moreover, due to the dominant advantage of combination regimens, 295 , 296 more clinic trials about combination regimens should be considered to overcome potential tumor escape or drug resistance.
7. CONCLUSIONS
In recent years, there has been an increasing awareness of the complex functions of CD44. A systematic review of landmark CD44‐related studies could facilitate a better understanding of the functional role of CD44 in cancer development and progression and thus will contribute to the development of novel strategies to circumvent their disadvantage and acquire optimal clinical efficacy. Here, we encapsulate the present understanding of the structural and functional roles of CD44 in neoplastic diseases as well as CD44‐regulated signaling pathways. We also discuss current targeting CD44 therapeutic strategies as well as prospective directions for future expansion and highlight existing clinical data supporting its use.
Overall, high expression of CD44, regardless of isoforms, is mainly positively associated with the development of neoplastic disease. Extensive studies have revealed that CD44 mediates cancer initiation and progression through interactions with its ligands, including but not limited to HA, OPN, serglycin, CS, the MMPs family, and FN. As one of the important functions of CD44, its regulation of cancer‐related pathways is pleiotropic, including cancer initiation, aggressive behavior, and stemness. CD44 promotes activation of MAPK, p38, and so on, which is associated with cancer cell stemness, growth, radiation resistance, and cell proliferation. Moreover, CD44 interacting with proteins such as YAP in the Hippo signaling pathway can regulate the stemness and aggressive behavior of cancer. On top of that, as a classical CSCs marker, CD44 regulates cell plasticity through iron mediation.
Expansive evidence indicates that anticancer therapeutic strategies based on targeting CD44 are effective methods, including antibodies, peptides, aptamers, natural and synthetic inhibitors, gene and cell therapy and biomaterials, depending on the interaction of CD44 with its ligands. Among these methods, targeting CD44–CAR T cells is promising. Although it has few applied clinically, its good targeting and durability in CD44 expressing patients show CAR T treatment is worthy of further study. However, clinical trials on CD44 in the treatment of cancer are limited, concentrating on targeting CD44v6, an isoform associated with metastasis and invasiveness. Further studies may focus on other isoforms. On top of that, it is expectable that more translational studies will be conducted to focus on chelating iron or regulate iron concentration to depleting CSCs through regulating the expression of CD44. In addition, clinical evidence suggests that highly expressing CD44 is a potential biomarker, including poor prognosis, tumor grade, and potential malignancy. However, the expression of CD44 was also found to be negatively associated with tumor invasiveness and Gleason grades, which is mainly related to the switch between CD44s and CD44v on the cancer cells through alternative splicing of CD44 isoforms. In conclusion, the conflicting data reports indicate that further study calls for exploring the specific functions of different CD44 isoforms in different kinds of cancers.
AUTHOR CONTRIBUTIONS
Chenying Fu and Ping Cheng designed this study. Yiming Xu drafted the manuscript. Yiming Xu, Ziyi Bai, and Tianxia Lan revised the manuscript. All authors read and approved the final manuscript.
CONFLICT OF INTEREST STATEMENT
The authors declare that they have no conflict of interests.
ETHICS STATEMENT
No ethics approval was required for this review that did not involve patients or patient data.
ACKNOWLEDGMENTS
This study was supported by grant from the National Science and Technology Major Projects of New Drugs (2018ZX09201018−013).
Xu Y, Bai Z, Lan T, Fu C, Cheng P. CD44 and its implication in neoplastic diseases. MedComm. 2024;5:e554. 10.1002/mco2.554
Contributor Information
Chenying Fu, Email: fcying_2004@163.com.
Ping Cheng, Email: ping.cheng@foxmail.com.
DATA AVAILABILITY STATEMENT
Not applicable.
REFERENCES
- 1. Gomari MM, Farsimadan M, Rostami N, et al. CD44 polymorphisms and its variants, as an inconsistent marker in cancer investigations. Mutat Res Rev Mutat Res. 2021;787:108374. [DOI] [PubMed] [Google Scholar]
- 2. Ponta H, Wainwright D, Herrlich P. The CD44 protein family. Int J Biochem Cell Biol. 1998;30(3):299‐305. [DOI] [PubMed] [Google Scholar]
- 3. Liu S, Liu Z, Shang A, et al. CD44 is a potential immunotherapeutic target and affects macrophage infiltration leading to poor prognosis. Sci Rep. 2023;13(1):9657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gupta RC, Lall R, Srivastava A, Sinha A. Hyaluronic acid: molecular mechanisms and therapeutic trajectory. Front Vet Sci. 2019;6:192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chen S, Zhang M, Li J, et al. β‐catenin‐controlled tubular cell‐derived exosomes play a key role in fibroblast activation via the OPN‐CD44 axis. J Extracell Vesicles. 2022;11(3):e12203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chu Q, Huang H, Huang T, et al. Extracellular serglycin upregulates the CD44 receptor in an autocrine manner to maintain self‐renewal in nasopharyngeal carcinoma cells by reciprocally activating the MAPK/β‐catenin axis. Cell Death Dis. 2016;7(11):e2456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Amorim S, Reis CA, Reis RL, Pires RA. Extracellular matrix mimics using hyaluronan‐based biomaterials. Trends Biotechnol. 2021;39(1):90‐104. [DOI] [PubMed] [Google Scholar]
- 8. Chaudhry G‐ES, Akim A, Naveed Zafar M, Safdar N, Sung YY, Muhammad TST. Understanding hyaluronan receptor (CD44) interaction, HA‐CD44 activated potential targets in cancer therapeutics. Adv Pharm Bull. 2021;11(3):426‐438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chen C, Zhao S, Karnad A, Freeman JW. The biology and role of CD44 in cancer progression: therapeutic implications. J Hematol Oncol. 2018;11(1):1‐23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yang C, Sheng Y, Shi X, et al. CD44/HA signaling mediates acquired resistance to a PI3Kα inhibitor. Cell Death Dis. 2020;11(10):831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Farahani DB, Akrami H, Moradi B, Mehdizadeh K, Fattahi MR. The effect of hsa‐miR‐451b knockdown on biological functions of gastric cancer stem‐like cells. Biochem Genet. 2021;59(5):1203‐1224. [DOI] [PubMed] [Google Scholar]
- 12. Li L, Hao X, Qin J, et al. Antibody against CD44s inhibits pancreatic tumor initiation and postradiation recurrence in mice. Gastroenterology. 2014;146(4):1108‐1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Brown RL, Reinke LM, Damerow MS, et al. CD44 splice isoform switching in human and mouse epithelium is essential for epithelial‐mesenchymal transition and breast cancer progression. J Clin Invest. 2011;121(3):1064‐1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Todaro M, Gaggianesi M, Catalano V, et al. CD44v6 is a marker of constitutive and reprogrammed cancer stem cells driving colon cancer metastasis. Cell Stem Cell. 2014;14(3):342‐356. [DOI] [PubMed] [Google Scholar]
- 15. Wang SJ, Wreesmann VB, Bourguignon LYW. Association of CD44 V3‐containing isoforms with tumor cell growth, migration, matrix metalloproteinase expression, and lymph node metastasis in head and neck cancer. Head Neck. 2007;29(6):550‐558. [DOI] [PubMed] [Google Scholar]
- 16. Kolliopoulos C, Lin C‐Y, Heldin C‐H, Moustakas A, Heldin P. Has2 natural antisense RNA and Hmga2 promote Has2 expression during TGFβ‐induced EMT in breast cancer. Matrix Biol. 2019;80:29‐45. [DOI] [PubMed] [Google Scholar]
- 17. Huang C, Yoon C, Zhou X‐H, et al. ERK1/2‐Nanog signaling pathway enhances CD44(+) cancer stem‐like cell phenotypes and epithelial‐to‐mesenchymal transition in head and neck squamous cell carcinomas. Cell Death Dis. 2020;11(4):266. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 18. Ji H, Kong L, Wang Y, et al. CD44 expression is correlated with osteosarcoma cell progression and immune infiltration and affects the Wnt/β‐catenin signaling pathway. J Bone Oncol. 2023;41:100487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ahmad SMS, Nazar H, Rahman MM, Rusyniak RS, Ouhtit A. ITGB1BP1, a novel transcriptional target of cd44‐downstream signaling promoting cancer cell invasion. Breast Cancer (Dove Med Press). 2023;15:373‐380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Li D, Wang X, Han K, et al. Hypoxia and CD44 receptors dual‐targeted nano‐micelles with AGT‐inhibitory activity for the targeting delivery of carmustine. Int J Biol Macromol. 2023;246:125657. [DOI] [PubMed] [Google Scholar]
- 21. Chen K‐L, Li D, Lu T‐X, Chang S‐W. Structural characterization of the CD44 stem region for standard and cancer‐associated isoforms. Int J Mol Sci. 2020;21(1):336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Karousou E, Misra S, Ghatak S, et al. Roles and targeting of the HAS/hyaluronan/CD44 molecular system in cancer. Matrix Biol. 2017;59:3‐22. [DOI] [PubMed] [Google Scholar]
- 23. Hassn Mesrati M, Syafruddin SE, Mohtar MA, Syahir A. CD44: a multifunctional mediator of cancer progression. Biomolecules. 2021;11(12):1850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tölg C, Hofmann M, Herrlich P, Ponta H. Splicing choice from ten variant exons establishes CD44 variability. Nucleic Acids Res. 1993;21(5):1225‐1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mishra MN, Chandavarkar V, Sharma R, Bhargava D. Structure, function and role of CD44 in neoplasia. J Oral Maxillofac Pathol. 2019;23(2):267‐272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Sneath RJ, Mangham DC. The normal structure and function of CD44 and its role in neoplasia. Mol Pathol. 1998;51(4):191‐200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Muys BR, Anastasakis DG, Claypool D, et al. The p53‐induced RNA‐binding protein ZMAT3 is a splicing regulator that inhibits the splicing of oncogenic CD44 variants in colorectal carcinoma. Genes Dev. 2021;35(1‐2):102‐116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bennett KL, Jackson DG, Simon JC, et al. CD44 isoforms containing exon V3 are responsible for the presentation of heparin‐binding growth factor. J Cell Biol. 1995;128(4):687‐698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Basuli D, Tesfay L, Deng Z, et al. Iron addiction: a novel therapeutic target in ovarian cancer. Oncogene. 2017;36(29):4089‐4099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Müller S, Sindikubwabo F, Cañeque T, et al. CD44 regulates epigenetic plasticity by mediating iron endocytosis. Nat Chem. 2020;12(10):929‐938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Guo Q, Yang C, Gao F. The state of CD44 activation in cancer progression and therapeutic targeting. FEBS J. 2022;289(24):7970‐7986. [DOI] [PubMed] [Google Scholar]
- 32. Piselli P, Vendetti S, Vismara D, et al. Different expression of CD44, ICAM‐1, and HSP60 on primary tumor and metastases of a human pancreatic carcinoma growing in scid mice. Anticancer Res. 2000;20(2A):825‐831. [PubMed] [Google Scholar]
- 33. Zhang F‐L, Cao J‐L, Xie H‐Y, et al. Cancer‐associated MORC2‐mutant M276I regulates an hnRNPM‐mediated CD44 splicing switch to promote invasion and metastasis in triple‐negative breast cancer. Cancer Res. 2018;78(20):5780‐5792. [DOI] [PubMed] [Google Scholar]
- 34. da Cunha CB, Klumpers DD, Koshy ST, et al. CD44 alternative splicing in gastric cancer cells is regulated by culture dimensionality and matrix stiffness. Biomaterials. 2016;98:152‐162. [DOI] [PubMed] [Google Scholar]
- 35. Espejo‐Román JM, Rubio‐Ruiz B, Chayah‐Ghaddab M, et al. N‐aryltetrahydroisoquinoline derivatives as HA‐CD44 interaction inhibitors: design, synthesis, computational studies, and antitumor effect. Eur J Med Chem. 2023;258:115570. [DOI] [PubMed] [Google Scholar]
- 36. Chen L, Huan X, Xiao G‐H, et al. Osteopontin and its downstream carcinogenic molecules: regulatory mechanisms and prognostic value in cancer progression. Neoplasma. 2022;69(6):1253‐1269. [DOI] [PubMed] [Google Scholar]
- 37. Liang S, Duan Y, Xing Z, et al. Inhibition of cell proliferation and migration by chondroitin sulfate‐g‐polyethylenimine‐mediated miR‐34a delivery. Colloids Surf B Biointerfaces. 2015;136:577‐584. [DOI] [PubMed] [Google Scholar]
- 38. Morath I, Hartmann TN, Orian‐Rousseau V. CD44: More than a mere stem cell marker. Int J Biochem Cell Biol. 2016;81(Pt A):166‐173. [DOI] [PubMed] [Google Scholar]
- 39. Wu G, Song X, Liu J, et al. Expression of CD44 and the survival in glioma: a meta‐analysis. Biosci Rep. 2020;40(4):BSR20200520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kolliopoulos C, Ali MM, Castillejo‐Lopez C, Heldin C‐H, Heldin P. CD44 depletion in glioblastoma cells suppresses growth and stemness and induces senescence. Cancers (Basel). 2022;14(15):3747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Zhang H, Cao H, Luo H, et al. RUNX1/CD44 axis regulates the proliferation, migration, and immunotherapy of gliomas: a single‐cell sequencing analysis. Front Immunol. 2023;14:1086280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kamamoto D, Saga I, Ohara K, Yoshida K, Sasaki H. Association between CD133, CD44, and nestin expression and prognostic factors in high‐grade meningioma. World Neurosurg. 2018; S1878‐8750 (18) 32890‐0. [DOI] [PubMed] [Google Scholar]
- 43. Li HZ, Gong HD, Wang C, Li JK. The role of osteopontin and its receptor in meningioma development and progression. J Biol Regul Homeost Agents. 2018;32(1):69‐74. [PubMed] [Google Scholar]
- 44. Wang C, Wang Z, Chen C, et al. A low MW inhibitor of CD44 dimerization for the treatment of glioblastoma. Br J Pharmacol. 2020;177(13):3009‐3023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Loreth D, Schuette M, Zinke J, et al. CD74 and CD44 expression on CTCs in cancer patients with brain metastasis. Int J Mol Sci. 2021;22(13):6993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Huang Q, Liu L, Xiao D, et al. CD44+ lung cancer stem cell‐derived pericyte‐like cells cause brain metastases through GPR124‐enhanced trans‐endothelial migration. Cancer Cell. 2023;41(9):1621‐1636.e8. [DOI] [PubMed] [Google Scholar]
- 47. Martins Gama J, Caetano Oliveira R, Teixeira P, et al. An immunohistochemical study of breast cancer brain metastases: the role of CD44 and AKT in the prognosis. Appl Immunohistochem Mol Morphol. 2023;31(5):318‐323. [DOI] [PubMed] [Google Scholar]
- 48. Heft Neal ME, Brenner JC, Prince MEP, Chinn SB. Advancement in cancer stem cell biology and precision medicine‐review article head and neck cancer stem cell plasticity and the tumor microenvironment. Front Cell Dev Biol. 2021;9:660210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Leinung M, Ernst B, Döring C, et al. Expression of ALDH1A1 and CD44 in primary head and neck squamous cell carcinoma and their value for carcinogenesis, tumor progression and cancer stem cell identification. Oncol Lett. 2015;10(4):2289‐2294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Gomez KE, Wu F, Keysar SB, et al. Cancer Cell CD44 Mediates Macrophage/Monocyte‐Driven Regulation of Head and Neck Cancer Stem CellsCD44 and Macrophages Regulate HNSCC Stem Cells. Cancer research. 2020;80(19):4185‐4198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Liu H, Rokana T, Kawaguchi M, et al. Homophilic CD44 interactions mediate tumor cell aggregation and polyclonal metastasis in patient‐derived breast cancer models. Cancer Discov. 2019;9(1):96‐113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Gomez KE, Wu F, Keysar SB, et al. Cancer cell CD44 mediates macrophage/monocyte‐driven regulation of head and neck cancer stem cells CD44 and macrophages regulate HNSCC stem cells. Cancer Res. 2020;80(19):4185‐4198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Ludwig N, Szczepanski MJ, Gluszko A, et al. CD44 (+) tumor cells promote early angiogenesis in head and neck squamous cell carcinoma. Cancer Lett. 2019;467:85‐95. [DOI] [PubMed] [Google Scholar]
- 54. Odenthal J, Rijpkema M, Bos D, et al. Targeting CD44v6 for fluorescence‐guided surgery in head and neck squamous cell carcinoma. Sci Rep. 2018;8(1):10467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Choi J‐H, Lee B‐S, Jang JY, et al. Single‐cell transcriptome profiling of the stepwise progression of head and neck cancer. Nat Commun. 2023;14(1):1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Escudero Mendez L, Srinivasan M, Hamouda RK, et al. Evaluation of CD44+/CD24‐ and aldehyde dehydrogenase enzyme markers in cancer stem cells as prognostic indicators for triple‐negative breast cancer. Cureus. 2022;14(8):e28056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Alateyah N, Gupta I, Rusyniak RS, Ouhtit A. SOD2, a potential transcriptional target underpinning CD44‐promoted breast cancer progression. molecules. 2022;27(3):811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Stevens LE, Peluffo G, Qiu X, et al. JAK‐STAT signaling in inflammatory breast cancer enables chemotherapy‐resistant cell states. Cancer Res. 2023;83(2):264‐284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Dashzeveg NK, Jia Y, Zhang Y, et al. Dynamic glycoprotein hyposialylation promotes chemotherapy evasion and metastatic seeding of quiescent circulating tumor cell clusters in breast cancer. Cancer Discov. 2023;13(9):2050‐2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Gu J, Chen D, Li Z, Yang Y, Ma Z, Huang G. Prognosis assessment of CD44+/CD24‐ in breast cancer patients: a systematic review and meta‐analysis. Arch Gynecol Obstet. 2022;306(4):1147‐1160. [DOI] [PubMed] [Google Scholar]
- 61. Gao R, Li D, Xun J, et al. CD44ICD promotes breast cancer stemness via PFKFB4‐mediated glucose metabolism. Theranostics. 2018;8(22):6248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Zhang H, Brown RL, Wei Y, et al. CD44 splice isoform switching determines breast cancer stem cell state. Genes Dev. 2019;33(3‐4):166‐179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Yang C, Cao M, Liu Y, et al. Inducible formation of leader cells driven by CD44 switching gives rise to collective invasion and metastases in luminal breast carcinomas. Oncogene. 2019;38(46):7113‐7132. [DOI] [PubMed] [Google Scholar]
- 64. Bei Y, Cheng N, Chen T, et al. CDK5 inhibition abrogates TNBC stem‐cell property and enhances anti‐PD‐1 therapy. Adv Sci. 2020;7(22):2001417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Ma J, Wu R, Chen Z, et al. CD44 is a prognostic biomarker correlated with immune infiltrates and metastasis in clear cell renal cell carcinoma. Anticancer Res. 2023;43(8):3493‐3506. [DOI] [PubMed] [Google Scholar]
- 66. Ma J, Li M, Chai J, et al. Expression of RSK4, CD44 and MMP‐9 is upregulated and positively correlated in metastatic ccRCC. Diagn Pathol. 2020;15(1):1‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Sekino Y, Han X, Kobayashi G, et al. BUB1B overexpression is an independent prognostic marker and associated with CD44, p53, and PD‐L1 in renal cell carcinoma. Oncology. 2021;99(4):240‐250. [DOI] [PubMed] [Google Scholar]
- 68. Kozawa K, Sekai M, Ohba K, et al. The CD44/COL17A1 pathway promotes the formation of multilayered, transformed epithelia. Curr Biol. 2021;31(14):3086‐3097.e7. [DOI] [PubMed] [Google Scholar]
- 69. Li D, Liu S, Xu J, et al. Ferroptosis‐related gene CHAC1 is a valid indicator for the poor prognosis of kidney renal clear cell carcinoma. J Cell Mol Med. 2021;25(7):3610‐3621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Xu F, Guan Y, Xue L, et al. The effect of a novel glycolysis‐related gene signature on progression, prognosis and immune microenvironment of renal cell carcinoma. BMC Cancer. 2020;20(1):1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Fiedorowicz M, Khan MI, Strzemecki D, et al. Renal carcinoma CD105‐/CD44‐ cells display stem‐like properties in vitro and form aggressive tumors in vivo. Sci Rep. 2020;10(1):5379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Liu Y‐C, Yeh C‐T, Lin K‐H. Cancer stem cell functions in hepatocellular carcinoma and comprehensive therapeutic strategies. Cells. 2020;9(6):1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Zarębska I, Gzil A, Durślewicz J, et al. The clinical, prognostic and therapeutic significance of liver cancer stem cells and their markers. Clin Res Hepatol Gastroenterol. 2021;45(3):101664. [DOI] [PubMed] [Google Scholar]
- 74. Asai R, Tsuchiya H, Amisaki M, et al. CD44 standard isoform is involved in maintenance of cancer stem cells of a hepatocellular carcinoma cell line. Cancer Med. 2019;8(2):773‐782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Dhar D, Antonucci L, Nakagawa H, et al. Liver cancer initiation requires p53 inhibition by CD44‐enhanced growth factor signaling. Cancer Cell. 2018;33(6):1061‐1077.e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Jun SY, Yoon HR, Yoon J‐Y, et al. The human TOR signaling regulator is the key indicator of liver cancer patients' overall survival: TIPRL/LC3/CD133/CD44 as potential biomarkers for early liver cancers. Cancers (Basel). 2021;13(12):2925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Wang S, Wang Y, Xun X, et al. Hedgehog signaling promotes sorafenib resistance in hepatocellular carcinoma patient‐derived organoids. J Exp Clin Cancer Res. 2020;39(1):22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Ho DW‐H, Tsui Y‐M, Sze KM‐F, et al. Single‐cell transcriptomics reveals the landscape of intra‐tumoral heterogeneity and stemness‐related subpopulations in liver cancer. Cancer Lett. 2019;459:176‐185. [DOI] [PubMed] [Google Scholar]
- 79. Toh TB, Lim JJ, Hooi L, Rashid MBMA, Chow EK‐H. Targeting Jak/Stat pathway as a therapeutic strategy against SP/CD44+ tumorigenic cells in Akt/β‐catenin‐driven hepatocellular carcinoma. J Hepatol. 2020;72(1):104‐118. [DOI] [PubMed] [Google Scholar]
- 80. Bishnupuri KS, Sainathan SK, Ciorba MA, Houchen CW, Dieckgraefe BK. Reg4 interacts with CD44 to regulate proliferation and stemness of colorectal and pancreatic cancer cells. Mol Cancer Res. 2022;20(3):387‐399. [DOI] [PubMed] [Google Scholar]
- 81. Gzil A, Zarębska I, Bursiewicz W, Antosik P, Grzanka D, Szylberg Ł. Markers of pancreatic cancer stem cells and their clinical and therapeutic implications. Mol Biol Rep. 2019;46(6):6629‐6645. [DOI] [PubMed] [Google Scholar]
- 82. Leon F, Seshacharyulu P, Nimmakayala RK, et al. Reduction in O‐glycome induces differentially glycosylated CD44 to promote stemness and metastasis in pancreatic cancer. Oncogene. 2022;41(1):57‐71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Xie Z, Gao Y, Ho C, et al. Exosome‐delivered CD44v6/C1QBP complex drives pancreatic cancer liver metastasis by promoting fibrotic liver microenvironment. Gut. 2022;71(3):568‐579. [DOI] [PubMed] [Google Scholar]
- 84. Kumar S, Inigo JR, Kumar R, et al. Nimbolide reduces CD44 positive cell population and induces mitochondrial apoptosis in pancreatic cancer cells. Cancer Lett. 2018;413:82‐93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Liu Y, Wu T, Lu D, Zhen J, Zhang L. CD44 overexpression related to lymph node metastasis and poor prognosis of pancreatic cancer. Int J Biol Markers. 2018;33(3):308‐313. [DOI] [PubMed] [Google Scholar]
- 86. Wang D, Zhu H, Zhu Y, et al. Retraction notice to “CD133+/CD44+/Oct4+/Nestin+ stem‐like cells isolated from Panc‐1 cell line may contribute to multi‐resistance and metastasis of pancreatic cancer” [Acta Histochemica 115 (2013) 349‐356]. Acta Histochem. 2018;120(3):302. [DOI] [PubMed] [Google Scholar]
- 87. Spadea A, de la Rosa JMR, Tirella A, et al. Evaluating the efficiency of hyaluronic acid for tumor targeting via CD44. Mol Pharm. 2019;16(6):2481‐2493. [DOI] [PubMed] [Google Scholar]
- 88. Kesharwani P, Banerjee S, Padhye S, Sarkar FH, Iyer AK. Hyaluronic acid engineered nanomicelles loaded with 3,4‐difluorobenzylidene curcumin for targeted killing of CD44+ stem‐like pancreatic cancer cells. Biomacromolecules. 2015;16(9):3042‐3053. [DOI] [PubMed] [Google Scholar]
- 89. Kesharwani P, Xie L, Banerjee S, et al. Hyaluronic acid‐conjugated polyamidoamine dendrimers for targeted delivery of 3, 4‐difluorobenzylidene curcumin to CD44 overexpressing pancreatic cancer cells. Colloids Surf B. 2015;136:413‐423. [DOI] [PubMed] [Google Scholar]
- 90. Kang X, Bu F, Feng W, et al. Dual‐cascade responsive nanoparticles enhance pancreatic cancer therapy by eliminating tumor‐resident intracellular bacteria. Adv Mater. 2022;34(49):e2206765. [DOI] [PubMed] [Google Scholar]
- 91. Qian L, Su H, Wang G, Li B, Shen G, Gao Q. Anti‐tumor activity of bufalin by inhibiting c‐MET mediated MEK/ERK and PI3K/AKT signaling pathways in gallbladder cancer. J Cancer. 2020;11(11):3114‐3123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Kalekou H, Miliaras D. Immunohistochemical study of microvessel density, CD44 (standard form), p53 protein and c‐erbB2 in gallbladder carcinoma. J Gastroenterol Hepatol. 2004;19(7):812‐818. [DOI] [PubMed] [Google Scholar]
- 93. Miwa T, Nagata T, Kojima H, Sekine S, Okumura T. Isoform switch of CD44 induces different chemotactic and tumorigenic ability in gallbladder cancer. Int J Oncol. 2017;51(3):771‐780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Yamaguchi A, Zhang M, Goi T, et al. Expression of variant CD44 containing variant exon v8‐10 in gallbladder cancer. Oncol Rep. 2000;7(3):541‐544. [DOI] [PubMed] [Google Scholar]
- 95. Yin B‐B, Wu S‐J, Zong H‐J, Ma B‐J, Cai D. Preliminary screening and identification of stem cell‐like sphere clones in a gallbladder cancer cell line GBC‐SD. J Zhejiang Univ Sci B. 2011;12(4):256‐263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Lai J, Yang S, Lin Z, et al. Update on chemoresistance mechanisms to first‐line chemotherapy for gallbladder cancer and potential reversal strategies. Am J Clin Oncol. 2023;46(4):131‐141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Khales SA, Mozaffari‐Jovin S, Geerts D, Abbaszadegan MR. TWIST1 activates cancer stem cell marker genes to promote epithelial‐mesenchymal transition and tumorigenesis in esophageal squamous cell carcinoma. BMC Cancer. 2022;22(1):1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Tsuchihashi K, Hirata Y, Yamasaki J, et al. Presence of spontaneous epithelial‐mesenchymal plasticity in esophageal cancer. Biochem Biophys Rep. 2022;30:101246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Horitani S, Fukui T, Tanimura Y, et al. Specific Smad2/3 linker phosphorylation indicates esophageal non‐neoplastic and neoplastic stem‐like cells and neoplastic development. Dig Dis Sci. 2021;66(6):1862‐1874. [DOI] [PubMed] [Google Scholar]
- 100. Moghbeli M, Mosannen Mozaffari H, Memar B, Forghanifard MM, Gholamin M, Abbaszadegan MR. Role of MAML1 in targeted therapy against the esophageal cancer stem cells. J Transl Med. 2019;17(1):1‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Taniguchi D, Saeki H, Nakashima Y, et al. CD44v9 is associated with epithelial‐mesenchymal transition and poor outcomes in esophageal squamous cell carcinoma. Cancer Med. 2018;7(12):6258‐6268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Zuo J, Zhu K, Wang Y, Yu Z. MicroRNA‐34a suppresses invasion and metastatic in esophageal squamous cell carcinoma by regulating CD44. Mol Cell Biochem. 2018;443(1‐2):139‐149. [DOI] [PubMed] [Google Scholar]
- 103. Yu X‐M, Li S‐J, Yao Z‐T, et al. N4‐acetylcytidine modification of lncRNA CTC‐490G23.2 promotes cancer metastasis through interacting with PTBP1 to increase CD44 alternative splicing. Oncogene. 2023;42(14):1101‐1116. [DOI] [PubMed] [Google Scholar]
- 104. Li K, Sun X, Zha R, et al. Counterintuitive production of tumor‐suppressive secretomes from Oct4‐ and c‐Myc‐overexpressing tumor cells and MSCs. Theranostics. 2022;12(7):3084‐3103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Wang X, Cai J, Zhao L, et al. NUMB suppression by miR‐9‐5P enhances CD44+ prostate cancer stem cell growth and metastasis. Sci Rep. 2021;11(1):11210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Koukourakis IM, Platoni K, Kouloulias V, Arelaki S, Zygogianni A. Prostate cancer stem cells: biology and treatment implications. Int J Mol Sci. 2023;24(19):14890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Chen Q, Gu M, Cai Z‐K, et al. TGF‐β1 promotes epithelial‐to‐mesenchymal transition and stemness of prostate cancer cells by inducing PCBP1 degradation and alternative splicing of CD44. Cell Mol Life Sci. 2021;78(3):949‐962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Rutz J, Thaler S, Maxeiner S, Chun FKH, Blaheta RA. Sulforaphane reduces prostate cancer cell growth and proliferation in vitro by modulating the Cdk‐cyclin axis and expression of the CD44 variants 4, 5, and 7. Int J Mol Sci. 2020;21(22):8724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Wróbel T, Luty M, Catapano J, et al. CD44+ cells determine fenofibrate‐induced microevolution of drug‐resistance in prostate cancer cell populations. Stem Cells. 2020;38(12):1544‐1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Liu Z, Wang L, Xu H, et al. Heterogeneous responses to mechanical force of prostate cancer cells inducing different metastasis patterns. Adv Sci. 2020;7(15):1903583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Chinnapaka S, Bakthavachalam V, Munirathinam G. Repurposing antidepressant sertraline as a pharmacological drug to target prostate cancer stem cells: dual activation of apoptosis and autophagy signaling by deregulating redox balance. Am J Cancer Res. 2020;10(7):2043‐2065. [PMC free article] [PubMed] [Google Scholar]
- 112. Verma S, Shankar E, Kalayci FNC, et al. Androgen deprivation induces transcriptional reprogramming in prostate cancer cells to develop stem cell‐like characteristics. Int J Mol Sci. 2020;21(24):9568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Li J, Pu T, Yin L, Li Q, Liao C‐P, Wu BJ. MAOA‐mediated reprogramming of stromal fibroblasts promotes prostate tumorigenesis and cancer stemness. Oncogene. 2020;39(16):3305‐3321. [DOI] [PubMed] [Google Scholar]
- 114. Hou W, Kong L, Hou Z, Ji H. CD44 is a prognostic biomarker and correlated with immune infiltrates in gastric cancer. BMC Med Genomics. 2022;15(1):225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Fujiwara‐Tani R, Sasaki T, Ohmori H, et al. Concurrent expression of CD47 and CD44 in colorectal cancer promotes malignancy. Pathobiology. 2019;86(4):182‐189. [DOI] [PubMed] [Google Scholar]
- 116. Pothuraju R, Rachagani S, Krishn SR, et al. Molecular implications of MUC5AC‐CD44 axis in colorectal cancer progression and chemoresistance. Mol Cancer. 2020;19(1):37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Wei F, Zhang T, Deng S‐C, et al. PD‐L1 promotes colorectal cancer stem cell expansion by activating HMGA1‐dependent signaling pathways. Cancer Lett. 2019;450:1‐13. [DOI] [PubMed] [Google Scholar]
- 118. Wu C, Ding H, Wang S, et al. DAXX inhibits cancer stemness and epithelial–mesenchymal transition in gastric cancer. Br J Cancer. 2020;122(10):1477‐1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Lau WM, Teng E, Chong HS, et al. CD44v8‐10 is a cancer‐specific marker for gastric cancer stem cells. Cancer Res. 2014;74(9):2630‐2641. [DOI] [PubMed] [Google Scholar]
- 120. Ishimoto T, Nagano O, Yae T, et al. CD44 variant regulates redox status in cancer cells by stabilizing the xCT subunit of system xc(‐) and thereby promotes tumor growth. Cancer Cell. 2011;19(3):387‐400. [DOI] [PubMed] [Google Scholar]
- 121. Sun L, Fang Y, Wang X, et al. miR‐302a inhibits metastasis and cetuximab resistance in colorectal cancer by targeting NFIB and CD44. Theranostics. 2019;9(26):8409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Weitzenböck HP, Gschwendtner A, Wiesner C, et al. Proteome analysis of NRF2 inhibition in melanoma reveals CD44 up‐regulation and increased apoptosis resistance upon vemurafenib treatment. Cancer Med. 2022;11(4):956‐967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Mohammadi A, Najafi S, Amini M, Baradaran B, Firouzamandi M. B7H6 silencing increases chemosensitivity to dacarbazine and suppresses cell survival and migration in cutaneous melanoma. Melanoma Res. 2023;33(3):173‐183. [DOI] [PubMed] [Google Scholar]
- 124. Wei C‐Y, Zhu M‐X, Yang Y‐W, et al. Downregulation of RNF128 activates Wnt/β‐catenin signaling to induce cellular EMT and stemness via CD44 and CTTN ubiquitination in melanoma. J Hematol Oncol. 2019;12(1):21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Sadeghi RS, Kulej K, Kathayat RS, et al. Wnt5a signaling induced phosphorylation increases APT1 activity and promotes melanoma metastatic behavior. eLife. 2018;7:e34362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126. Wu R‐L, Sedlmeier G, Kyjacova L, et al. Hyaluronic acid‐CD44 interactions promote BMP4/7‐dependent Id1/3 expression in melanoma cells. Sci Rep. 2018;8(1):14913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127. Li Y, Hou H, Liu Z, et al. CD44 targeting nanodrug based on chondroitin sulfate for melanoma therapy by inducing mitochondrial apoptosis pathways. Carbohydr Polym. 2023;320:121255. [DOI] [PubMed] [Google Scholar]
- 128. Hu W, Zhao Y, Su L, et al. Silencing the lncRNA NORAD inhibits EMT of head and neck squamous cell carcinoma stem cells via miR‑26a‑5p. Mol Med Rep. 2021;24(5):743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Yuan S‐SF, Hung AC, Hsu C‐W, et al. CD44 mediates oral squamous cell carcinoma‐promoting activity of MRE11 via AKT signaling. J Pers Med. 2022;12(5):841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Pastushenko I, Mauri F, Song Y, et al. Fat1 deletion promotes hybrid EMT state, tumour stemness and metastasis. Nature. 2021;589(7842):448‐455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Sun X, Sun Y, Li J, et al. SOCS6 promotes radiosensitivity and decreases cancer cell stemness in esophageal squamous cell carcinoma by regulating c‐Kit ubiquitylation. Cancer Cell Int. 2021;21(1):1‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Yokoyama S, Shigeishi H, Murodumi H, et al. TGF‐β1 induces amoeboid‐to‐mesenchymal transition of CD44high oral squamous cell carcinoma cells via miR‐422a downregulation through ERK activation and Cofilin‐1 phosphorylation. J Oral Pathol Med. 2021;50(2):155‐164. [DOI] [PubMed] [Google Scholar]
- 133. Fernández‐Tabanera E, García‐García L, Rodríguez‐Martín C, et al. CD44 modulates cell migration and invasion in Ewing sarcoma cells. Int J Mol Sci. 2023;24(14):11774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Fernández‐Tabanera E, Melero‐Fernández de Mera RM, Alonso J. CD44 in sarcomas: a comprehensive review and future perspectives. Front Oncol. 2022;12:909450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Skubitz KM, Wilson JD, Cheng EY, Lindgren BR, Boylan KLM, Skubitz APN. Effect of chemotherapy on cancer stem cells and tumor‐associated macrophages in a prospective study of preoperative chemotherapy in soft tissue sarcoma. J Transl Med. 2019;17(1):130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Gerardo‐Ramírez M, Keggenhoff FL, Giam V, et al. CD44 contributes to the regulation of MDR1 protein and doxorubicin chemoresistance in osteosarcoma. Int J Mol Sci. 2022;23(15):8616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Liu T, Yan Z, Liu Y, et al. CRISPR‐Cas9‐mediated silencing of CD44 in human highly metastatic osteosarcoma cells. Cell Physiol Biochem. 2018;46(3):1218‐1230. [DOI] [PubMed] [Google Scholar]
- 138. Kogerman P, Sy M‐S, Culp LA. Counter‐selection for over‐expressed human CD44s in primary tumors versus lung metastases in a mouse fibrosarcoma model. Oncogene. 1997;15(12):1407‐1416. [DOI] [PubMed] [Google Scholar]
- 139. Kuryu M, Ozaki T, Nishida K, Shibahara M, Kawai A, Inoue H. Expression of CD44 variants in osteosarcoma. J Cancer Res Clin Oncol. 1999;125(11):646‐652. [DOI] [PubMed] [Google Scholar]
- 140. Toole BP. Hyaluronan: from extracellular glue to pericellular cue. Nat Rev Cancer. 2004;4(7):528‐539. [DOI] [PubMed] [Google Scholar]
- 141. Al‐Mansoob M, Gupta I, Stefan Rusyniak R, Ouhtit A. KYNU, a novel potential target that underpins CD44‐promoted breast tumour cell invasion. J Cell Mol Med. 2021;25(5):2309‐2314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Aruffo A, Stamenkovic I, Melnick M, Underhill CB, Seed B. CD44 is the principal cell surface receptor for hyaluronate. Cell. 1990;61(7):1303‐1313. [DOI] [PubMed] [Google Scholar]
- 143. Hua Q, Knudson CB, Knudson W. Internalization of hyaluronan by chondrocytes occurs via receptor‐mediated endocytosis. J Cell Sci. 1993;106(Pt 1):365‐375. [DOI] [PubMed] [Google Scholar]
- 144. Soleymani M, Velashjerdi M, Shaterabadi Z, Barati A. One‐pot preparation of hyaluronic acid‐coated iron oxide nanoparticles for magnetic hyperthermia therapy and targeting CD44‐overexpressing cancer cells. Carbohydr Polym. 2020;237:116130. [DOI] [PubMed] [Google Scholar]
- 145. Bourguignon LY, Earle C, Shiina M. Activation of matrix hyaluronan‐mediated CD44 signaling, epigenetic regulation and chemoresistance in head and neck cancer stem cells. Int J Mol Sci. 2017;18(9):1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. Golshani R, Lopez L, Estrella V, Kramer M, Iida N, Lokeshwar VB. Hyaluronic acid synthase‐1 expression regulates bladder cancer growth, invasion, and angiogenesis through CD44. Cancer Res. 2008;68(2):483‐491. [DOI] [PubMed] [Google Scholar]
- 147. Bourguignon LY, Xia W, Wong G. Hyaluronan‐mediated CD44 interaction with p300 and SIRT1 regulates β‐catenin signaling and NFκB‐specific transcription activity leading to MDR1 and Bcl‐xL gene expression and chemoresistance in breast tumor cells. J Biol Chem. 2009;284(5):2657‐2671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Alamgeer M, Neil Watkins D, Banakh I, et al. A phase IIa study of HA‐irinotecan, formulation of hyaluronic acid and irinotecan targeting CD44 in extensive‐stage small cell lung cancer. Invest New Drugs. 2018;36(2):288‐298. [DOI] [PubMed] [Google Scholar]
- 149. Salari N, Mansouri K, Valipour E, et al. Hyaluronic acid‐based drug nanocarriers as a novel drug delivery system for cancer chemotherapy: a systematic review. DARU. 2021;29(2):439‐447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Ahmed M, Sottnik JL, Dancik GM, et al. An osteopontin/CD44 axis in RhoGDI2‐mediated metastasis suppression. Cancer Cell. 2016;30(3):432‐443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Ji J, Zheng S, Liu Y, et al. Increased expression of OPN contributes to idiopathic pulmonary fibrosis and indicates a poor prognosis. J Transl Med. 2023;21(1):640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Pang X, Gong K, Zhang X, Wu S, Cui Y, Qian B‐Z. Osteopontin as a multifaceted driver of bone metastasis and drug resistance. Pharmacol Res. 2019;144:235‐244. [DOI] [PubMed] [Google Scholar]
- 153. Rao G, Wang H, Li B, et al. Reciprocal interactions between tumor‐associated macrophages and CD44‐positive cancer cells via osteopontin/CD44 promote tumorigenicity in colorectal cancer the interaction of OPN and CD44 in colorectal cancer. Clin Cancer Res. 2013;19(4):785‐797. [DOI] [PubMed] [Google Scholar]
- 154. Robertson BW, Bonsal L, Chellaiah MA. Regulation of Erk1/2 activation by osteopontin in PC3 human prostate cancer cells. Mol Cancer. 2010;9:260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. Jiang Y‐J, Chao C‐C, Chang A‐C, et al. Cigarette smoke‐promoted increases in osteopontin expression attract mesenchymal stem cell recruitment and facilitate lung cancer metastasis. J Adv Res. 2022;41:77‐87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156. Klement JD, Paschall AV, Redd PS, et al. An osteopontin/CD44 immune checkpoint controls CD8+ T cell activation and tumor immune evasion. J Clin Invest. 2018;128(12):5549‐5560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Lu C, Liu Z, Klement JD, et al. WDR5‐H3K4me3 epigenetic axis regulates OPN expression to compensate PD‐L1 function to promote pancreatic cancer immune escape. J Immunother Cancer. 2021;9(7):e002624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Manou D, Karamanos NK, Theocharis AD. Tumorigenic functions of serglycin: Regulatory roles in epithelial to mesenchymal transition and oncogenic signaling. Semin Cancer Biol. 2020;62:108‐115. [DOI] [PubMed] [Google Scholar]
- 159. Pejler G, Åbrink M, Wernersson S. Serglycin proteoglycan: regulating the storage and activities of hematopoietic proteases. Biofactors. 2009;35(1):61‐68. [DOI] [PubMed] [Google Scholar]
- 160. Guo J‐Y, Chiu C‐H, Wang M‐J, Li F‐A, Chen J‐Y. Proteoglycan serglycin promotes non‐small cell lung cancer cell migration through the interaction of its glycosaminoglycans with CD44. J Biomed Sci. 2020;27(1):1‐18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161. Cao L, Luo FF, Huang HB, et al. The autoregulatory serglycin/CD44 axis drives stemness‐like phenotypes in TNBC in a β‐catenin‐dependent manner. Clin Transl Med. 2021;11(2):e311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162. He Y, Cheng D, Lian C, et al. Serglycin induces osteoclastogenesis and promotes tumor growth in giant cell tumor of bone. Cell Death Dis. 2021;12(10):868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163. Liang X, Liang X. Chondroitin sulfate modified and adriamycin preloaded hybrid nanoparticles for tumor‐targeted chemotherapy of lung cancer. Kaohsiung J Med Sci. 2021;37(5):411‐418. [DOI] [PubMed] [Google Scholar]
- 164. Lin WJ, Lee WC. Polysaccharide‐modified nanoparticles with intelligent CD44 receptor targeting ability for gene delivery. Int J Nanomed. 2018;13:3989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165. Tan T, Yang Q, Chen D, et al. Chondroitin sulfate‐mediated albumin corona nanoparticles for the treatment of breast cancer. Asian J Pharm Sci. 2021;16(4):508‐518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166. Luo K, Xu F, Yao T, et al. TPGS and chondroitin sulfate dual‐modified lipid‐albumin nanosystem for targeted delivery of chemotherapeutic agent against multidrug‐resistant cancer. Int J Biol Macromol. 2021;183:1270‐1282. [DOI] [PubMed] [Google Scholar]
- 167. Chu Y‐H, Liao W‐C, Ho Y‐J, Huang C‐H, Tseng T‐J, Liu C‐H. Targeting chondroitin sulfate reduces invasiveness of glioma cells by suppressing CD44 and integrin β1 expression. Cells. 2021;10(12):3594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Chrabańska M, Rynkiewicz M, Kiczmer P, Drozdzowska B. Immunohistochemical expression of CD44, MMP‐2, MMP‐9, and Ki‐67 as the prognostic markers in non‐clear cell renal cell carcinomas: a prospective cohort study. J Clin Med. 2022;11(17):5196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169. Song X, Ding F, Luo W, et al. Knockdown of CD44 inhibits proliferation, migration, and invasiveness in hepatocellular carcinoma cells by modulating CXCR4/Wnt/β‐Catenin Axis. Acta Biochim Pol. 2023;70(1):117‐122. [DOI] [PubMed] [Google Scholar]
- 170. Lv Y, Zhao X, Zhu L, et al. Targeting intracellular MMPs efficiently inhibits tumor metastasis and angiogenesis. Theranostics. 2018;8(10):2830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171. Shi J, Ren Y, Ma J, et al. Novel CD44‐targeting and pH/redox‐dual‐stimuli‐responsive core–shell nanoparticles loading triptolide combats breast cancer growth and lung metastasis. J Nanobiotechnol. 2021;19(1):1‐22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172. Ko YS, Jung EJ, Go S‐I, et al. Polyphenols extracted from Artemisia annua L. exhibit anti‐cancer effects on radio‐resistant MDA‐MB‐231 human breast cancer cells by suppressing stem cell phenotype, β‐catenin, and MMP‐9. Molecules. 2020;25(8):1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173. Lee Y‐M, Kim JM, Lee HJ, Seong I‐O, Kim K‐H. Immunohistochemical Expression of CD44, Matrix Metalloproteinase2 and Matrix Metalloproteinase9 in Renal Cell Carcinomas. Elsevier; 2019:742‐748. [DOI] [PubMed] [Google Scholar]
- 174. Chen S‐Y, Jou I‐M, Ko P‐Y, et al. Amelioration of experimental tendinopathy by lentiviral CD44 gene therapy targeting senescence‐associated secretory phenotypes. Mol Ther Methods Clin Dev. 2022;26:157‐168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Papadopoulou A, Kalodimou VE, Mavrogonatou E, et al. Decreased differentiation capacity and altered expression of extracellular matrix components in irradiation‐mediated senescent human breast adipose‐derived stem cells. IUBMB Life. 2022;74(10):969‐981. [DOI] [PubMed] [Google Scholar]
- 176. Asano M, Tanaka S, Sakaguchi M. Effects of normothermic microwave irradiation on CD44+/CD24‒in breast cancer MDA‐MB‐231 and MCF‐7 cell lines. Biosci Biotechnol Biochem. 2020;84(1):103‐110. [DOI] [PubMed] [Google Scholar]
- 177. Nikitakis NG, Gkouveris I, Aseervatham J, Barahona K, Ogbureke KU. DSPP‐MMP20 gene silencing downregulates cancer stem cell markers in human oral cancer cells. Cell Mol Biol Lett. 2018;23(1):1‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178. Li L, Qi L, Qu T, et al. Epithelial splicing regulatory protein 1 inhibits the invasion and metastasis of lung adenocarcinoma. Am J Pathol. 2018;188(8):1882‐1894. [DOI] [PubMed] [Google Scholar]
- 179. Senbanjo LT, AlJohani H, Majumdar S, Chellaiah MA. Characterization of CD44 intracellular domain interaction with RUNX2 in PC3 human prostate cancer cells. Cell Commun Signal. 2019;17(1):1‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Kato H, Naiki‐Ito A, Yamada T, et al. The standard form of CD44 as a marker for invasion of encapsulated papillary carcinoma of the breast. Pathol Int. 2020;70(11):835‐843. [DOI] [PubMed] [Google Scholar]
- 181. Sun X, Li K, Hase M, et al. Suppression of breast cancer‐associated bone loss with osteoblast proteomes via Hsp90ab1/moesin‐mediated inhibition of TGFβ/FN1/CD44 signaling. Theranostics. 2022;12(2):929‐943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182. Choi S, Yu J, Park A, et al. BMP‐4 enhances epithelial mesenchymal transition and cancer stem cell properties of breast cancer cells via Notch signaling. Sci Rep. 2019;9(1):11724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183. Frahs SM, Reeck JC, Yocham KM, et al. Prechondrogenic ATDC5 cell attachment and differentiation on graphene foam; modulation by surface functionalization with fibronectin. ACS Appl Mater Interfaces. 2019;11(45):41906‐41924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Hu C, Li M, Guo T, et al. Anti‐metastasis activity of curcumin against breast cancer via the inhibition of stem cell‐like properties and EMT. Phytomedicine. 2019;58:152740. [DOI] [PubMed] [Google Scholar]
- 185. Li N, Wang C, Sun S, et al. Microgravity‐induced alterations of inflammation‐related mechanotransduction in endothelial cells on Board SJ‐10 satellite. Front Physiol. 2018;9:1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186. Huang G‐X, Qi M‐F, Li X‐L, Tang F, Zhu L. Involvement of upregulation of fibronectin in the pro‑adhesive and pro‑survival effects of glucocorticoid on melanoma cells. Mol Med Rep. 2018;17(2):3380‐3387. [DOI] [PubMed] [Google Scholar]
- 187. Hong Y, Qin H, Li Y, et al. FNDC3B circular RNA promotes the migration and invasion of gastric cancer cells via the regulation of E‐cadherin and CD44 expression. J Cell Physiol. 2019;234(11):19895‐19910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188. Figiel I, Kruk PK, Zaręba‐Kozioł M, et al. MMP‐9 signaling pathways that engage Rho GTPases in brain plasticity. Cells. 2021;10(1):166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189. Tata P, Gondaliya P, Sunkaria A, Srivastava A, Kalia K. Modulation of CD44, EGFR and RAC pathway genes (WAVE complex) in epithelial cancers. Curr Pharm Des. 2019;25(8):833‐848. [DOI] [PubMed] [Google Scholar]
- 190. Bai R‐J, Liu D, Li Y‐S, et al. OPN inhibits autophagy through CD44, integrin and the MAPK pathway in osteoarthritic chondrocytes. Front Endocrinol (Lausanne). 2022;13:919366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191. Bourguignon LYW, Earle C, Wong G, Spevak CC, Krueger K. Stem cell marker (Nanog) and Stat‐3 signaling promote MicroRNA‐21 expression and chemoresistance in hyaluronan/CD44‐activated head and neck squamous cell carcinoma cells. Oncogene. 2012;31(2):149‐160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. Medrano‐González PA, Rivera‐Ramírez O, Montaño LF, Rendón‐Huerta EP. Proteolytic processing of CD44 and its implications in cancer. Stem Cells Int. 2021;2021:6667735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193. Primeaux M, Gowrikumar S, Dhawan P. Role of CD44 isoforms in epithelial‐mesenchymal plasticity and metastasis. Clin Exp Metastasis. 2022;39(3):391‐406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194. Roy R, Mandal S, Chakrabarti J, Saha P, Panda CK. Downregulation of hyaluronic acid‐CD44 signaling pathway in cervical cancer cell by natural polyphenols Plumbagin, Pongapin and Karanjin. Mol Cell Biochem. 2021;476(10):3701‐3709. [DOI] [PubMed] [Google Scholar]
- 195. Jiang P, Li F, Liu Z, Hao S, Gao J, Li S. BTB and CNC homology 1 (Bach1) induces lung cancer stem cell phenotypes by stimulating CD44 expression. Respir Res. 2021;22(1):320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196. Petri BJ, Piell KM, Whitt GCS, et al. HNRNPA2B1 regulates tamoxifen‐and fulvestrant‐sensitivity and hallmarks of endocrine resistance in breast cancer cells. Cancer Lett. 2021;518:152‐168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Erdogan S, Turkekul K. Neferine inhibits proliferation and migration of human prostate cancer stem cells through p38 MAPK/JNK activation. J Food Biochem. 2020;44(7):e13253. [DOI] [PubMed] [Google Scholar]
- 198. Lin C, Yuan H, Wang W, et al. Importance of PNO1 for growth and survival of urinary bladder carcinoma: role in core‐regulatory circuitry. J Cell Mol Med. 2020;24(2):1504‐1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199. Guo Q, Liu Y, He Y, et al. CD44 activation state regulated by the CD44v10 isoform determines breast cancer proliferation. Oncol Rep. 2021;45(4):7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Erdogan S, Turkekul K, Dibirdik I, Doganlar ZB, Doganlar O, Bilir A. Midkine silencing enhances the anti‐prostate cancer stem cell activity of the flavone apigenin: cooperation on signaling pathways regulated by ERK, p38, PTEN, PARP, and NF‐κB. Invest New Drugs. 2020;38(2):246‐263. [DOI] [PubMed] [Google Scholar]
- 201. Jang T‐H, Huang W‐C, Tung S‐L, et al. MicroRNA‐485‐5p targets keratin 17 to regulate oral cancer stemness and chemoresistance via the integrin/FAK/Src/ERK/β‐catenin pathway. J Biomed Sci. 2022;29(1):42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. Sun J, Xu Y, Liu J, Cui H, Cao H, Ren J. PDRG1 promotes the proliferation and migration of GBM cells by the MEK/ERK/CD44 pathway. Cancer Sci. 2022;113(2):500‐516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203. Zhao Y, Kang J‐H, Yoo K‐C, Kang S‐G, Lee H‐J, Lee S‐J. K‐RAS acts as a critical regulator of CD44 to promote the invasiveness and stemness of GBM in response to ionizing radiation. Int J Mol Sci. 2021;22(20):10923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204. Wang Y‐Y, Vadhan A, Chen P‐H, et al. Cd44 promotes lung cancer cell metastasis through erk–zeb1 signaling. Cancers (Basel). 2021;13(16):4057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205. Thirusangu P, Ray U, Sarkar Bhattacharya S, et al. PFKFB3 regulates cancer stemness through the hippo pathway in small cell lung carcinoma. Oncogene. 2022;41(33):4003‐4017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206. Zhang J, He X, Wan Y, et al. CD44 promotes hepatocellular carcinoma progression via upregulation of YAP. Exp Hematol Oncol. 2021;10(1):54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207. Giraud J, Molina‐Castro S, Seeneevassen L, et al. Verteporfin targeting YAP1/TAZ‐TEAD transcriptional activity inhibits the tumorigenic properties of gastric cancer stem cells. Int J Cancer. 2020;146(8):2255‐2267. [DOI] [PubMed] [Google Scholar]
- 208. Liu Y, Song Y, Cao M, et al. A novel EHD1/CD44/Hippo/SP1 positive feedback loop potentiates stemness and metastasis in lung adenocarcinoma. Clin Transl Med. 2022;12(4):e836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209. Tanaka K, Osada H, Murakami‐Tonami Y, Horio Y, Hida T, Sekido Y. Statin suppresses Hippo pathway‐inactivated malignant mesothelioma cells and blocks the YAP/CD44 growth stimulatory axis. Cancer Lett. 2017;385:215‐224. [DOI] [PubMed] [Google Scholar]
- 210. Lai C‐J, Lin C‐Y, Liao W‐Y, Hour T‐C, Wang H‐D, Chuu C‐P. CD44 promotes migration and invasion of docetaxel‐resistant prostate cancer cells likely via induction of hippo‐yap signaling. Cells. 2019;8(4):295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211. Zhao N, Zhou L, Lu Q, et al. SOX2 maintains the stemness of retinoblastoma stem‐like cells through Hippo/YAP signaling pathway. Exp Eye Res. 2022;214:108887. [DOI] [PubMed] [Google Scholar]
- 212. Zhou Y, Qiu S, Kim JT, et al. Garcinone C suppresses tumorsphere formation and invasiveness by hedgehog/Gli1 signaling in colorectal cancer stem‐like cells. J Agric Food Chem. 2022;70(26):7941‐7952. [DOI] [PubMed] [Google Scholar]
- 213. Lall SP, Alsafwani ZW, Batra SK, Seshacharyulu P. ASPORIN: A root of the matter in tumors and their host environment. Biochim Biophys Acta Rev Cancer. 2023;1879(1):189029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214. Huang JL, Oshi M, Endo I, Takabe K. Clinical relevance of stem cell surface markers CD133, CD24, and CD44 in colorectal cancer. Am J Cancer Res. 2021;11(10):5141‐5154. [PMC free article] [PubMed] [Google Scholar]
- 215. Li C, Du Y, Yang Z, et al. GALNT1‐mediated glycosylation and activation of sonic hedgehog signaling maintains the self‐renewal and tumor‐initiating capacity of bladder cancer stem cells. Cancer Res. 2016;76(5):1273‐1283. [DOI] [PubMed] [Google Scholar]
- 216. Tsao A‐N, Chuang Y‐S, Lin Y‐C, Su Y, Chao T‐C. Dinaciclib inhibits the stemness of two subtypes of human breast cancer cells by targeting the FoxM1 and Hedgehog signaling pathway. Oncol Rep. 2022;47(5):105. [DOI] [PubMed] [Google Scholar]
- 217. Zhou D, He Y, Li H, Huang W. KLK6 mediates stemness and metabolism of gastric carcinoma cells via the PI3K/AKT/mTOR signaling pathway. Oncol Lett. 2021;22(6):824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218. Fan L, Peng C, Zhu X, et al. Dihydrotanshinone I enhances cell adhesion and inhibits cell migration in osteosarcoma U‐2 OS cells through CD44 and chemokine signaling. Molecules. 2022;27(12):3714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219. Coleman K‐L, Chiaramonti M, Haddad B, et al. Phosphorylation of IGFBP‐3 by casein kinase 2 blocks its interaction with hyaluronan, enabling HA‐CD44 signaling leading to increased NSCLC cell survival and cisplatin resistance. Cells. 2023;12(3):405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220. Thanee M, Dokduang H, Kittirat Y, et al. CD44 modulates metabolic pathways and altered ROS‐mediated Akt signal promoting cholangiocarcinoma progression. PLoS One. 2021;16(3):e0245871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221. Geng B, Pan J, Zhao T, et al. Chitinase 3‐like 208‐CD44 interaction promotes metastasis and epithelial‐to‐mesenchymal transition through β‐catenin/Erk/Akt signaling in gastric cancer. J Exp Clin Cancer Res. 2018;37(1):1‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222. Xie P, Yan J, Wu M, et al. CD44 potentiates hepatocellular carcinoma migration and extrahepatic metastases via the AKT/ERK signaling CXCR4 axis. Ann Transl Med. 2022;10(12):689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223. Kashyap T, Pramanik KK, Nath N, et al. Crosstalk between Raf‐MEK‐ERK and PI3K‐Akt‐GSK3β signaling networks promotes chemoresistance, invasion/migration and stemness via expression of CD44 variants (v4 and v6) in oral cancer. Oral Oncol. 2018;86:234‐243. [DOI] [PubMed] [Google Scholar]
- 224. Wang J, Li X, Wu H, et al. EMP1 regulates cell proliferation, migration, and stemness in gliomas through PI3K‐AKT signaling and CD44. J Cell Biochem. 2019;120(10):17142‐17150. [DOI] [PubMed] [Google Scholar]
- 225. Koh YW, Han J‐H, Haam S. Expression of PD‐L1, cancer stem cell and epithelial‐mesenchymal transition phenotype in non‐small cell lung cancer. Pathology (Phila). 2021;53(2):239‐246. [DOI] [PubMed] [Google Scholar]
- 226. Chen J‐T, Hsu Y‐L, Hsu Y‐C, et al. Id2 exerts tumor suppressor properties in lung cancer through its effects on cancer cell invasion and migration. Front Oncol. 2022;12:801300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227. Su J, Wu S, Wu H, Li L, Guo T. CD44 is functionally crucial for driving lung cancer stem cells metastasis through Wnt/β‐catenin‐FoxM1‐Twist signaling. Mol Carcinog. 2016;55(12):1962‐1973. [DOI] [PubMed] [Google Scholar]
- 228. Acikgoz E, Tatar C, Oktem G. Triptolide inhibits CD133+ /CD44+ colon cancer stem cell growth and migration through triggering apoptosis and represses epithelial‐mesenchymal transition via downregulating expressions of snail, slug, and twist. J Cell Biochem. 2020;121(5‐6):3313‐3324. [DOI] [PubMed] [Google Scholar]
- 229. Mahmoudian RA, Gharaie ML, Abbaszadegan MR, et al. Crosstalk between MMP‐13, CD44, and TWIST1 and its role in regulation of EMT in patients with esophageal squamous cell carcinoma. Mol Cell Biochem. 2021;476(6):2465‐2478. [DOI] [PubMed] [Google Scholar]
- 230. Heydari M, Hosseinzadeh Colagar A, Moudi E. Mutant Allele of CD44 (rs8193C>T) and Pum2 regulatory element as a prognosis factor of prostate neoplasms: a case‐control and in silico studies. Cell J. 2022;24(12):723‐731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231. Byun J‐Y, Huang K, Lee JS, et al. Targeting HIF‐1α/NOTCH1 pathway eliminates CD44+ cancer stem‐like cell phenotypes, malignancy, and resistance to therapy in head and neck squamous cell carcinoma. Oncogene. 2022;41(9):1352‐1363. [DOI] [PubMed] [Google Scholar]
- 232. Bai J, Chen W‐B, Zhang X‐Y, et al. HIF‐2α regulates CD44 to promote cancer stem cell activation in triple‐negative breast cancer via PI3K/AKT/mTOR signaling. World J Stem Cells. 2020;12(1):87‐99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233. Gu X, Zhang J, Shi Y, et al. ESM1/HIF‑1α pathway modulates chronic intermittent hypoxia‑induced non‑small‑cell lung cancer proliferation, stemness and epithelial‑mesenchymal transition. Oncol Rep. 2021;45(3):1226‐1234. [DOI] [PubMed] [Google Scholar]
- 234. Inoue A, Ohnishi T, Nishikawa M, et al. A narrative review on CD44's role in glioblastoma invasion, proliferation, and tumor recurrence. Cancers (Basel). 2023;15(19):4898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235. Johansson E, Grassi ES, Pantazopoulou V, et al. CD44 Interacts with HIF‐2α to modulate the hypoxic phenotype of perinecrotic and perivascular glioma cells. Cell Rep. 2017;20(7):1641‐1653. [DOI] [PubMed] [Google Scholar]
- 236. Yang H‐L, Lin P‐Y, Vadivalagan C, Lin Y‐A, Lin K‐Y, Hseu Y‐C. Coenzyme Q0 defeats NLRP3‐mediated inflammation, EMT/metastasis, and Warburg effects by inhibiting HIF‐1α expression in human triple‐negative breast cancer cells. Arch Toxicol. 2023;97(4):1047‐1068. [DOI] [PubMed] [Google Scholar]
- 237. Piao L, Li H, Feng Y, Yang Z, Kim S, Xuan Y. SET domain‐containing 5 is a potential prognostic biomarker that promotes esophageal squamous cell carcinoma stemness. Exp Cell Res. 2020;389(1):111861. [DOI] [PubMed] [Google Scholar]
- 238. Liang G, Li S, Du W, Ke Q, Cai J, Yang J. Hypoxia regulates CD44 expression via hypoxia‐inducible factor‐1α in human gastric cancer cells. Oncol Lett. 2017;13(2):967‐972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239. Nam K, Oh S, Shin I. Ablation of CD44 induces glycolysis‐to‐oxidative phosphorylation transition via modulation of the c‐Src‐Akt‐LKB1‐AMPKα pathway. Biochem J. 2016;473(19):3013‐3030. [DOI] [PubMed] [Google Scholar]
- 240. Kim S, Cho CY, Lee D, et al. CD133‐induced TM4SF5 expression promotes sphere growth via recruitment and blocking of protein tyrosine phosphatase receptor type F (PTPRF). Cancer Lett. 2018;438:219‐231. [DOI] [PubMed] [Google Scholar]
- 241. Liu J, Chen X, Ward T, et al. Niclosamide inhibits epithelial‐mesenchymal transition and tumor growth in lapatinib‐resistant human epidermal growth factor receptor 2‐positive breast cancer. Int J Biochem Cell Biol. 2016;71:12‐23. [DOI] [PubMed] [Google Scholar]
- 242. Nam K, Oh S, Lee K‐M, Yoo S‐A, Shin I. CD44 regulates cell proliferation, migration, and invasion via modulation of c‐Src transcription in human breast cancer cells. Cell Signal. 2015;27(9):1882‐1894. [DOI] [PubMed] [Google Scholar]
- 243. Bourguignon LYW, Wong G, Earle C, Krueger K, Spevak CC. Hyaluronan‐CD44 interaction promotes c‐Src‐mediated twist signaling, microRNA‐10b expression, and RhoA/RhoC up‐regulation, leading to Rho‐kinase‐associated cytoskeleton activation and breast tumor cell invasion. J Biol Chem. 2010;285(47):36721‐36735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244. Lee D, Na J, Ryu J, et al. Interaction of tetraspan (in) TM4SF5 with CD44 promotes self‐renewal and circulating capacities of hepatocarcinoma cells. Hepatology. 2015;61(6):1978‐1997. [DOI] [PubMed] [Google Scholar]
- 245. Katoh M. Multi‑layered prevention and treatment of chronic inflammation, organ fibrosis and cancer associated with canonical WNT/β‑catenin signaling activation (Review). Int J Mol Med. 2018;42(2):713‐725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246. Roy S, Kar M, Roy S, et al. Inhibition of CD44 sensitizes cisplatin‐resistance and affects Wnt/β‐catenin signaling in HNSCC cells. Int J Biol Macromol. 2020;149:501‐512. [DOI] [PubMed] [Google Scholar]
- 247. Guo K, Duan J, Lu J, et al. Tumor necrosis factor‐α‐inducing protein of Helicobacter pylori promotes epithelial‐mesenchymal transition and cancer stem‐like cells properties via activation of Wnt/β‐catenin signaling pathway in gastric cancer cells. Pathog Dis. 2022;80(1):ftac025. [DOI] [PubMed] [Google Scholar]
- 248. Zhang M, Wang X, Xia X, Fang X, Zhang T, Huang F. Endometrial epithelial cells‐derived exosomes deliver microRNA‐30c to block the BCL9/Wnt/CD44 signaling and inhibit cell invasion and migration in ovarian endometriosis. Cell Death Discov. 2022;8(1):151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249. Li Q, Li Y, Jiang H, et al. Vitamin D suppressed gastric cancer cell growth through downregulating CD44 expression in vitro and in vivo. Nutrition. 2021;91:111413. [DOI] [PubMed] [Google Scholar]
- 250. Fan Y, Cheng H, Liu Y, et al. Metformin anticancer: Reverses tumor hypoxia induced by bevacizumab and reduces the expression of cancer stem cell markers CD44/CD117 in human ovarian cancer SKOV3 cells. Front Pharmacol. 2022;13:955984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251. Kim M, Lee JS, Kim W, et al. Aptamer‐conjugated nano‐liposome for immunogenic chemotherapy with reversal of immunosuppression. J Control Release. 2022;348:893‐910. [DOI] [PubMed] [Google Scholar]
- 252. Espejo‐Román JM, Rubio‐Ruiz B, Cano‐Cortés V, et al. Selective anticancer therapy based on a HA‐CD44 interaction inhibitor loaded on polymeric nanoparticles. Pharmaceutics. 2022;14(4):788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253. Chen Y, Wang H, Zuo Y, Li N, Ding M, Li C. A novel monoclonal antibody KMP1 has potential antitumor activity of bladder cancer by blocking CD44 in vivo and in vitro. Cancer Med. 2018;7(5):2064‐2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254. Takei J, Kaneko MK, Ohishi T, et al. A defucosylated anti‑CD44 monoclonal antibody 5‑mG2a‑f exerts antitumor effects in mouse xenograft models of oral squamous cell carcinoma. Oncol Rep. 2020;44(5):1949‐1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255. Khan F, Gurung S, Gunassekaran GR, et al. Identification of novel CD44v6‐binding peptides that block CD44v6 and deliver a pro‐apoptotic peptide to tumors to inhibit tumor growth and metastasis in mice. Theranostics. 2021;11(3):1326‐1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256. Price D, Muterspaugh R, Clegg B, et al. IGFBP‐3 blocks hyaluronan‐CD44 signaling, leading to increased acetylcholinesterase levels in A549 cell media and apoptosis in a p53‐dependent manner. Sci Rep. 2020;10(1):5083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257. Zheng J, Zhao S, Yu X, Huang S, Liu HY. Simultaneous targeting of CD44 and EpCAM with a bispecific aptamer effectively inhibits intraperitoneal ovarian cancer growth. Theranostics. 2017;7(5):1373‐1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258. Wang H, Zhu Z, Zhang G, et al. AS1411 aptamer/hyaluronic acid‐bifunctionalized microemulsion co‐loading shikonin and docetaxel for enhanced antiglioma therapy. J Pharm Sci. 2019;108(11):3684‐3694. [DOI] [PubMed] [Google Scholar]
- 259. Zhu Y, Fu F, Wang Z, et al. Polyphyllin VII is a potential drug targeting CD44 positive colon cancer cells. Curr Cancer Drug Targets. 2022;22(5):426‐435. [DOI] [PubMed] [Google Scholar]
- 260. Li J, Li M, Tian L, et al. Facile strategy by hyaluronic acid functional carbon dot‐doxorubicin nanoparticles for CD44 targeted drug delivery and enhanced breast cancer therapy. Int J Pharm. 2020;578:119122. [DOI] [PubMed] [Google Scholar]
- 261. Yilmaz Ç, Köksoy S, Çeker T, Aslan M. Diclofenac down‐regulates COX‐2 induced expression of CD44 and ICAM‐1 in human HT29 colorectal cancer cells. Naunyn Schmiedebergs Arch Pharmacol. 2021;394(11):2259‐2272. [DOI] [PubMed] [Google Scholar]
- 262. Chen D, Li D, Xu X‐B, et al. Galangin inhibits epithelial‐mesenchymal transition and angiogenesis by downregulating CD44 in glioma. J Cancer. 2019;10(19):4499‐4508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263. Jobani BM, Najafzadeh N, Mazani M, Arzanlou M, Vardin MM. Molecular mechanism and cytotoxicity of allicin and all‐trans retinoic acid against CD44+ versus CD117+ melanoma cells. Phytomedicine. 2018;48:161‐169. [DOI] [PubMed] [Google Scholar]
- 264. Lee H‐J, Lim SM, Jang HY, Kim YR, Hong J‐S, Kim GJ. miR‐373‐3p regulates invasion and migration abilities of trophoblast cells via targeted CD44 and radixin. Int J Mol Sci. 2021;22(12):6260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265. Gao Z, Ye X, Bordeaux A, et al. miR‐26b regulates cell proliferation and apoptosis of CD117+CD44+ ovarian cancer stem cells by targeting PTEN. Eur J Histochem. 2021;65(1):3186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266. Li WJ, Wang Y, Liu R, et al. MicroRNA‐34a: potent tumor suppressor, cancer stem cell inhibitor, and potential anticancer therapeutic. Front Cell Dev Biol. 2021;9:640587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267. Yu Y, Nangia‐Makker P, Farhana L, Rajendra SG, Levi E, Majumdar APN. miR‐21 and miR‐145 cooperation in regulation of colon cancer stem cells. Mol Cancer. 2015;14:98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268. Liu C, Kelnar K, Liu B, et al. The microRNA miR‐34a inhibits prostate cancer stem cells and metastasis by directly repressing CD44. Nat Med. 2011;17(2):211‐215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269. Feng S, Wang K, Shao Z, Lin Q, Li B, Liu P. MiR‐373/miR‐520s‐CD44 axis significantly inhibits the growth and invasion of human glioblastoma cells. Arch Med Res. 2022;53(6):550‐561. [DOI] [PubMed] [Google Scholar]
- 270. Li H‐N, Zhang H‐M, Li X‐R, et al. MiR‐205‐5p/GGCT attenuates growth and metastasis of papillary thyroid cancer by regulating CD44. Endocrinology. 2022;163(4):bqac022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271. Yao H, Sun L, Li J, et al. A novel therapeutic siRNA nanoparticle designed for dual‐targeting CD44 and Gli1 of gastric cancer stem cells. Int J Nanomed. 2020;15:7013‐7034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272. Mahinfar P, Mokhtarzadeh A, Baradaran B, Siasi Torbati E. Antiproliferative activity of CD44 siRNA‐PEI‐PEG nanoparticles in glioblastoma: involvement of AKT signaling. Res Pharm Sci. 2022;17(1):78‐85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273. Alemohammad H, Motafakkerazad R, Asadzadeh Z, et al. siRNA‐mediated silencing of Nanog reduces stemness properties and increases the sensitivity of HepG2 cells to cisplatin. Gene. 2022;821:146333. [DOI] [PubMed] [Google Scholar]
- 274. Zou W, Zhang Y, Bai G, et al. siRNA‐induced CD44 knockdown suppresses the proliferation and invasion of colorectal cancer stem cells through inhibiting epithelial‐mesenchymal transition. J Cell Mol Med. 2022;26(7):1969‐1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275. Vahidian F, Safarzadeh E, Mohammadi A, et al. siRNA‐mediated silencing of CD44 delivered by Jet Pei enhanced Doxorubicin chemo sensitivity and altered miRNA expression in human breast cancer cell line (MDA‐MB468). Mol Biol Rep. 2020;47(12):9541‐9551. [DOI] [PubMed] [Google Scholar]
- 276. Yin J, Zhang H, Wu X, et al. CD44 inhibition attenuates EGFR signaling and enhances cisplatin sensitivity in human EGFR wild‑type non‑small‑cell lung cancer cells. Int J Mol Med. 2020;45(6):1783‐1792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277. Porcellini S, Asperti C, Corna S, et al. CAR T cells redirected to CD44v6 control tumor growth in lung and ovary adenocarcinoma bearing mice. Front Immunol. 2020;11:99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278. Cao Y, Efetov SK, He M, et al. Updated clinical perspectives and challenges of chimeric antigen receptor‐T cell therapy in colorectal cancer and invasive breast cancer. Arch Immunol Ther Exp (Warsz). 2023;71(1):19. [DOI] [PubMed] [Google Scholar]
- 279. Grunewald CM, Haist C, König C, et al. Epigenetic priming of bladder cancer cells with decitabine increases cytotoxicity of human EGFR and CD44v6 CAR engineered T‐cells. Front Immunol. 2021;12:782448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280. Tang L, Huang H, Tang Y, et al. CD44v6 chimeric antigen receptor T cell specificity towards AML with FLT3 or DNMT3A mutations. Clin Transl Med. 2022;12(9):e1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281. Haist C, Schulte E, Bartels N, et al. CD44v6‐targeted CAR T‐cells specifically eliminate CD44 isoform 6 expressing head/neck squamous cell carcinoma cells. Oral Oncol. 2021;116:105259. [DOI] [PubMed] [Google Scholar]
- 282. Wang H, Ye X, Ju Y, et al. Minicircle DNA‐mediated CAR T cells targeting CD44 suppressed hepatocellular carcinoma both in vitro and in vivo. Onco Targets Ther. 2020;13:3703‐3716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283. Xia H, Hao M, Li K, et al. CD44 and HAP‐conjugated hADSCs as living materials for targeted tumor therapy and bone regeneration. Adv Sci (Weinh). 2023;10(20):e2206393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284. Gonzalez‐Valdivieso J, Vallejo R, Rodriguez‐Rojo S, et al. CD44‐targeted nanoparticles for co‐delivery of docetaxel and an Akt inhibitor against colorectal cancer. Biomater Adv. 2023;154:213595. [DOI] [PubMed] [Google Scholar]
- 285. Uma Maheswari RT, Ajithkumar V, Varalakshmi P, Rajan M. CD44 tagged hyaluronic acid ‐ chitosan liposome carrier for the delivery of berberine and doxorubicin into lung cancer cells. Int J Biol Macromol. 2023;253(Pt 2):126599. [DOI] [PubMed] [Google Scholar]
- 286. Liu P, Chen N, Yan L, et al. Preparation, characterisation and in vitro and in vivo evaluation of CD44‐targeted chondroitin sulphate‐conjugated doxorubicin PLGA nanoparticles. Carbohydr Polym. 2019;213:17‐26. [DOI] [PubMed] [Google Scholar]
- 287. Byeon Y, Lee J‐W, Choi WS, et al. CD44‐targeting PLGA nanoparticles incorporating paclitaxel and FAK siRNA overcome chemoresistance in epithelial ovarian cancer overcoming chemoresistance by HA‐PLGA‐NP in ovarian cancer. Cancer Res. 2018;78(21):6247‐6256. [DOI] [PubMed] [Google Scholar]
- 288. Seok H‐Y, Rejinold NS, Lekshmi KM, Cherukula K, Park I‐K, Kim Y‐C. CD44 targeting biocompatible and biodegradable hyaluronic acid cross‐linked zein nanogels for curcumin delivery to cancer cells: in vitro and in vivo evaluation. J Control Release. 2018;280:20‐30. [DOI] [PubMed] [Google Scholar]
- 289. Liang Y, Wang Y, Wang L, et al. Self‐crosslinkable chitosan‐hyaluronic acid dialdehyde nanoparticles for CD44‐targeted siRNA delivery to treat bladder caner. Bioact Mater. 2021;6(2):433‐446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290. Wang Y, Guo Y, Lin H, et al. Expression of CD44 in tumor tissue and serum of small cell lung cancer and its clinical prognostic significance. Zhongguo Fei Ai Za Zhi. 2021;24(8):583‐590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291. Agrawal A, Datta C, Panda CK, Pal DK. Association of beta‐catenin and CD44 in the development of renal cell carcinoma. Urologia. 2021;88(2):125‐129. [DOI] [PubMed] [Google Scholar]
- 292. Menke‐van der Houven van Oordt CW, Gomez‐Roca C, van Herpen C, et al. First‐in‐human phase I clinical trial of RG7356, an anti‐CD44 humanized antibody, in patients with advanced, CD44‐expressing solid tumors. Oncotarget. 2016;7(48):80046‐80058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293. Gong L, Zhou H, Zhang S, et al. CD44‐targeting drug delivery system of exosomes loading forsythiaside a combats liver fibrosis via regulating NLRP3‐mediated pyroptosis. Adv Healthc Mater. 2023;12(11):e2202228. [DOI] [PubMed] [Google Scholar]
- 294. Schonberg DL, Miller TE, Wu Q, et al. Preferential iron trafficking characterizes glioblastoma stem‐like cells. Cancer Cell. 2015;28(4):441‐455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295. Mace TA, Shakya R, Pitarresi JR, et al. IL‐6 and PD‐L1 antibody blockade combination therapy reduces tumour progression in murine models of pancreatic cancer. Gut. 2018;67(2):320‐332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296. Nanbu T, Umemura N, Ohkoshi E, Nanbu K, Sakagami H, Shimada J. Combined SN‐38 and gefitinib treatment promotes CD44 degradation in head and neck squamous cell carcinoma cells. Oncol Rep. 2018;39(1):367‐375. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.


