Abstract
BACKGROUND
Poliomyelitis is a global disabling disease affecting 12-20 million of people. Post poliomyelitis syndrome (PPS) may affect up to 80% of polio survivors: increased muscle weakness, pain, fatigue, functional decline. It relies on aging of an impaired neuro-muscular system with ongoing denervation processes. A late involvement of humoral or cellular pro-inflammatory phenomena is also suspected.
AIM
To assess the dysimmune hypothesis of PPS by comparing lymphocyte subpopulations and humoral immune factors between PPS patients and controls.
DESIGN
Cross-sectional study.
SETTING
Montpellier University Hospital.
POPULATION
Forty-seven PPS and 27 healthy controls.
METHODS
PPS patients and controls were compared on their lymphocyte subpopulations and humoral immune factors (IL-1β, IL-6, IL-8, IL-17, IL-21, IL-22, IL-23, IFN-γ, TNF-α, GM-CSF, RANTES, MCP1, MIP-3a, IL-10, TGF-β, IL4, IL13). Patients were further compared according to their dominant clinical symptoms. Sample size guaranteed a power >90% for all comparisons.
RESULTS
PPS patients and controls were comparable in gender, age and corpulence. Most patients had lower limb motor sequelae (N.=45, 95.7%), a minority had upper limb motor impairment (N.=16, 34.0%). Forty-five were able to walk (94%), 35/45 with technical aids. The median of the two-minute walking test was 110 meters (interquartile range 55; 132). Eighteen (38%) required help in their daily life. Their quality of life was low (SF36). All described an increased muscular weakness, 40 (85%) a general fatigue, and 39 (83%) muscular or joint pain. Blood count, serum electrolytes, T and B lymphocyte subpopulations and cytokines were comparable between patients and controls, except for creatine phospho kinase that was significantly higher in PPS patients. None of these variables differed between the 20/47 patients whose late main symptoms were pain or fatigue, and other patients.
CONCLUSIONS
Our results suggest that PPS is not a dysimmune disease.
CLINICAL REHABILITATION IMPACT
Our results do not sustain immunotherapy for PPS. Our work suggest that PPS may be mostly linked to physiological age-related phenomena in a disabled neuromuscular condition. Thus, our results emphasize the role of prevention and elimination of aggravating factors to avoid late functional worsening, and the importance of rehabilitation programs that should be adapted to patients’ specific conditions.
Key words: Postpoliomyelitis syndrome, Physiopathology, Immunity, Lymphocytes, Cytokines, Muscle weakness
Poliomyelitis is a global disabling disease affecting 12-20 million of people.1, 2 Currently, thanks to the preventive vaccination campaigns, the Eastern Mediterranean region (Afghanistan and Pakistan) remains the main part of the world that is not free of wild poliovirus.3
At the chronic phase, a recent epidemiological review suggests an average worldwide prevalence of 295/100,000, that may be over-estimated.4 Although the prevalence of poliomyelitis sequelae is decreasing in Europe and in the USA, it remains very high in developing countries5 which constitutes a global concern for the next decades. Indeed, among polio survivors, post poliomyelitis syndrome (PPS) resulting in functional decline may affect up to 80% of them.6 PPS diagnosis relies on Halstead criteria.7 The main symptom is the gradual appearance of muscles weakness or fatigability after a period of stable neuromuscular function in patients with prior paralytic poliomyelitis. Patients can also experience various symptoms affecting different motor or non-motor functions, especially pain or fatigue. PPS is a clinical diagnosis, requiring excluding any medical or orthopedic conditions as causes of symptoms. The severity and the time course evolution of the symptoms may vary among patients.8 These impairments can lead to severe decrease in functioning with important consequences on quality of life.
The prevalence of PPS may increase in the world in the next few decades, rendering its treatment very important, especially in emerging countries were poliomyelitis has been lately eradicated. Symptomatic and rehabilitative treatments are efficient but could be improved by specific treatments targeting the pathological mechanisms underlying PPS.
PPS pathophysiological explanations are still unclear and several hypotheses have been formulated.5 First, PPS results from the aging of an impaired neuro-muscular system with ongoing denervation/reinnervation processes and stress-induced degeneration of surviving neurons. In this case, PPS is considered as a consequence of a gradual motor unit failure due to multiple factors like physiological aging, metabolic exhaustion, and overuse.6 This motor unit failure is superimposed with age-related sarcopenia and changes in contractile properties of muscular fibers contributing to muscular fatigue and myalgia. Another hypothesis that could explain part of the symptoms of PPS relies on the persistence or reactivation of the polio virus in the central nervous system9-12 leading to a late increase in humoral and/or cellular pro-inflammatory phenomena.13, 14 This dysimmune hypothesis is sustained by some studies showing an abnormal increased level of pro-inflammatory cytokines and molecules both in blood15-17 and cerebral spinal fluid,18 and inflammatory changes in central nervous system and muscles biopsies.19, 20 It is currently admitted that the increase in pro-inflammatory cytokines may contribute to damage neurons through the release of oxidative agents and glutamate.17 In that context, intravenous immunoglobulins have been tested to improve patients with PPS,21 with inconclusive results. This hypothesis of an immune-mediated disease could also open up key therapeutic perspectives based on drugs affecting the immune-response such as monoclonal antibodies. This hypothesis remains highly controversial to date.22
Therefore, this study aimed at comparing lymphocyte sub-populations and humoral immunity between PPS patients and controls, expecting to provide evidence of the dysimmune hypothesis of PPS. Based on previous studies suggesting a link between clinical presentation of patients and level of pro-inflammatory cytokines in the blood,17 we also studied subpopulations of PPS patients to compare the same variables according to the dominant symptoms (pain and/or fatigue versus other symptoms).
Materials and methods
Study design
This monocentric prospective study was carried out in Montpellier university hospital, France, from December 2017 to March 2020. We included adult patients with PPS and healthy controls. Recruitment of controls was stratified by gender and age to match the gender and age distribution of patients with PPS. The study protocol was approved by the Comité de Protection des Personnes Nord-Ouest III (chairperson: Mme Charlotte Gourio) on 13/05/2017, before the experiment was started (protocol number: 2017-13). It was registered on ClinicalTrials.gov (NCT03396783). The study has been conducted in accordance with the declaration of Helsinki, and all participants signed a written informed consent form.
Participants
Eligible patients were those above 18 years with a diagnosis of PPS made by a Physical and Rehabilitation Medicine (PRM) physician, according to Halstead et al. criteria: 1) prior infection with poliovirus with initial motor impairment confirmed by medical history, residual motor deficit and muscle atrophy on clinical examination, and possibly signs of denervation on electromyography; 2) a full or partial recovery period after the initial acute phase, with neurological and functional stability for at least 15 years; 3) a subsequent gradual or rapid loss of muscle strength and/or endurance with or without new recent muscle atrophy, generalized fatigue, muscle or joint pain; other rarer symptoms may also be noted (sleep disorders, breathing difficulties, dysphagia, dysarthria, etc.); 4) these symptoms are unusual and long-lasting, evolving over more than one year; 5) alternative medical causes that may be responsible for the various symptoms have been ruled out.
Control subjects were eligible if they were above 18 years and had no history of acute poliomyelitis. They were recruited among family members of other inpatients from the PRM department.
Non-inclusion criteria for both patients and controls were all pathologies or treatments that could distort the clinical and immunological profile: intercurrent neurological pathology; uncontrolled cardiovascular risk factors (unbalanced diabetes with HbA1c >7%, obesity with Body Mass Index >35 kg/m2, hypertriglyceridemia or hypercholesterolemia, heart failure); pulmonary comorbidity (Chronic obstructive pulmonary disease or asthma requiring disease-modifying treatment); previous endocrine disorders such as thyroid damage; anemia; systemic inflammatory pathology, autoimmune disease or sicca syndrome; renal failure (creatinine clearance < 60ml/min); anti-inflammatory treatment in progress or in the previous month, immunoregulatory treatment whatever its nature; patients with PPS who received polyvalent IV immunoglobulins in the previous 3 years; vaccination in the previous month.
Clinical assessment
Clinical assessment was made in the group of patients with PPS by the PRM physician. Main symptoms (muscular weakness, general fatigue or muscular/joint pain), ability to walk and autonomy for daily activities were assessed through clinical examination and patient interview. The patients also answered self-questionnaires about pain, fatigue and quality of life.
Further clinical assessment was carried out by a physiotherapist. It included a 2-minute walking test23 to measure the distance covered in 2 minutes at a comfortable speed. Muscle strength was assessed using the Manual Muscle Testing described by Mendell et al.,24 scoring each muscle from 0 (no contraction) to 5 (normal force against strong resistance applied by the evaluator) for six lower limb muscles (gluteus maximus, hip flexors, quadriceps, tibialis anterior, hamstrings, triceps surae) and four upper limb muscles (shoulder abductors, elbow flexors, elbow extensor, wrist extensors) on both sides. We calculated a score summing the results of the 12 lower limb muscles (therefore ranging from 0 to 60) and a score summing the eight upper limb muscles (ranging from 0 to 40). A higher score indicated stronger muscles. In addition, grip strength was assessed on both sides with a Jamar Test.25
Biological dosages
In both groups, the immunological profile was assessed by peripheral blood sampling. An EDTA tube (3 mL) was used for lymphocyte phenotyping in order to quantify the T lymphocyte subpopulations (Th1, Th2, Th17, regulatory T cells), as well as B and NK lymphocytes. Phenotyping was performed by flow cytometry (Navios-Beckman Coulter cytometer). The results were expressed in percent of total lymphocytes and in absolute values. In addition, a dry tube (5 mL) was first centrifuged and the sera obtained were stored at -80°C for further analysis. Measurement of the serum concentration of an extended profile of blood cytokines and chemokines including proinflammatory cytokines (IL-1β, IL-6, IL-8, IL-17, IL-21, IL-22, IL-23, IFN-γ, TNF-α, GM-CSF, RANTES, MCP1, MIP-3a), anti-inflammatory cytokines (IL-10, TGF-β), and others (IL-4, IL-13), was carried out remotely with assay by Luminex® technique (Fidis™-Theradiag). Lastly, a peripheral blood sample (7 mL in total) on EDTA tube and heparinized tube was performed for complete blood count (hematology laboratory), CRP and CPK measurement (biochemistry laboratory).
The primary endpoint was defined as the immunological profile of participants, consisting of the blood concentrations of the cytokines studied, as well as the rate and absolute number of the different peripheral blood lymphocyte populations (T-lymphocyte subpopulations, B and NK lymphocytes).
Self-questionnaires
Patients filled in the following self-questionnaires.
Self-Reported Impairments in Persons with Late Effects of Polio (SIPP)26
Patients rated the impact of thirteen PPS-related symptoms (muscle fatigue, muscle weakness, pain, etc.) over the past two weeks on a scale ranging from 1 (not at all) to 4 (extremely): maximum score 52.
Visual Analogue Scale (VAS) for pain
Patients assessed their general level of pain during the last week on a scale ranging from 0 (no pain) to 100 (maximal pain).
Fatigue Severity Scale (FSS)27
Patients rated nine items ranging from 1 (minimum) to 7 (maximum) to assess their level of fatigue. The total score ranged from 9 to 63.
Fatigue Impact Scale (FIS)
This self-questionnaire assesses the effect of fatigue on activities of daily living over the last month. Patients rated 40 items from 0 to 4 corresponding to three subscales: physical, cognitive and social. A high score corresponds to greater fatigue, with a maximum possible score of 160. This scale is validated in French in multiple sclerosis.28, 29
Short-Form 36 (SF-36)
This 36-item generic scale measures health-related quality of life in eight dimensions, each ranging from 0 to 100. Two composite scores can be calculated: a physical component summary (mean 50 in the general population) and a mental component summary score (mean 50 in the general population). A higher score indicates a higher quality of life. It has been validated in various diseases.30
Statistical analysis
In previous studies comparing PPS patients and healthy controls, the smallest significant difference was the TNF-α concentration: 18.2 vs. 12.2 pg/mL, with a common standard deviation of 3.3 pg/mL.15, 31 We applied the Bonferroni correction to test 24 biological values, and calculated that we needed 14 patients per group to have a 90% power to show a significant difference (with a corrected alpha risk of 0.002) in any of these biological values. We decided a priori to increase this number to 50 PPS patients and 40 controls, to obtain more reliable descriptive data.
We described patients with PPS and controls using mean and standard deviation or median and interquartile range (IQR) for quantitative variables, and number and percentages for qualitative variables. Comparisons of quantitative variables between patients and controls were based on Student’s t-tests or Mann-Whitney Tests, and comparisons of qualitative variables were based on chi square tests or Fisher’s Exact Test. The same methods were used to compare patients according to their dominant clinical symptoms (pain or fatigue versus other symptoms). Missing data were not replaced. Statistical analyses were performed using SAS Enterprise Guide, version 4.3 (SAS Institute, Cary, NC, USA) and Stata, SE 15.0 (StataCorp LCC, College Station, TX, USA).
Data availability
Individual participant data that underlie the results reported in this article can be made available upon reasonable request to the corresponding author.
Results
Patients and clinical description
We recruited 74 participants, including 47 patients and 27 controls. Patients and controls were comparable in gender (64% female vs. 56% respectively, P=0.48), age (mean±SD: 61±9 vs. 60±10, P=0.64) and corpulence (Body Mass Index 25.3±4.3 kg/m2 vs. 25.9±5.0, P=0.72). Clinical characteristics and questionnaires’ results of the 47 patients with PPS are described in Table I.
Table I. —Characteristics of the patients with post-poliomyelitis syndrome (PPS).
| Variable | N. | PPS patients (N.=47) |
|---|---|---|
| Symptoms | ||
| Muscular weakness | 47 | 47 (100%) |
| General fatigue | 47 | 40 (85%) |
| Muscular/joint pain | 47 | 39 (83%) |
| Time since new symptoms onset (years) | 46 | 9.5 (3; 15) |
| Able to walk | 47 | 44 (94%) |
| 2-min walking test, distance (meters) | 43 | 110 (55; 132) |
| Grip strength, right hand (Jamar) | 46 | 22 (18; 32) |
| Grip strength, left hand (Jamar) | 46 | 24 (17; 29) |
| Motor testing, upper limbs (max 40) | 45 | 38 (31; 39) |
| Motor testing, lower limbs (max 60) | 46 | 23 (17; 31) |
| SIPP (max 52) | 38 | 34 (30; 42) |
| VAS for pain (max 100) | 46 | 43 (20; 70) |
| Fatigue Impact Scale (FIS) | ||
| Cognitive dimension | 43 | 23 (16; 30) |
| Physical dimension | 42 | 41 (37; 44) |
| Social dimension | 41 | 33 (23; 38) |
| Psychological dimension | 42 | 11 (8; 12) |
| Total score (Max 160) | 39 | 108 (86; 124) |
| Fatigue Severity Scale (FSS) | ||
| Total score (min 9, max 63) | 39 | 47 (37; 55) |
| Quality of life (SF-36) | ||
| Physical Component Summary (mean 50 in the general population) | 38 | 31.1 (27.7; 36.7) |
| Mental Component Summary (mean 50 in the general population) | 38 | 40.1 (33.6; 50.1) |
Values are median (interquartile range) unless otherwise stated. SIPP: Self-Reported Impairments in Persons with Late Effects of Polio; VAS: Visual Analogue Scale.
Most patients had lower limbs motor sequels (45, 95.7%), a minority upper limb motor impairment (16, 34.0%). Forty-five were able to walk (94%), of which 35/45 with technical aids. The median of the two-minutes walking test was 110 meters (IQR 55; 132). Eighteen (38%) required help in their daily life. Their quality of life assessed with the SF-36 questionnaire was low. All described an increase in muscular weakness, 40 (85%) a general fatigue, and 39 (83%) muscular or joint pain.
Biology
Blood counts and serum electrolytes were comparable between patients and controls, except for creatine phospho kinase that was higher in PPS patients (Table II).
Table II. —Blood count and serum electrolytes in patients with post-poliomyelitis syndrome (PPS) and controls.
| Variable | PPS patients (N.=46) | Controls (N.=27) | P value |
|---|---|---|---|
| Hemoglobin (g/dL) | 14.5 (13.6; 15.1) | 14.8 (14.0; 15.5) | 0.42 |
| MCV (fl) | 92 (89; 95) | 94 (91; 97) | 0.25 |
| Platelet count (109/L) | 252 (215; 298) | 238 (206; 278) | 0.60 |
| Leucocytes (109/L) | 6.43 (5.48; 6.96) | 6.68 (5.78; 8.15) | 0.19 |
| Neutrophils (%) | 58.5 (51.0; 63.0) | 57.0 (51.0; 62.0) | 0.87 |
| Eosinophils (%) | 3.5 (2.0; 5.0) | 3.0 (2.0; 4.0) | 0.39 |
| Basophils (%) | 1.0 (1.0; 1.0) | 1.0 (1.0; 1.0) | 0.67 |
| Lymphocytes (%) | 28.5 (25.0; 37.0) | 31.0 (26.0; 38.0) | 0.79 |
| Monocytes (%) | 8.0 (6.0; 9.0) | 7.0 (6.0; 9.0) | 0.77 |
| CRP (mg/L) | 2.0 (0.9; 3.2) | 1.4 (0.8; 2.3) | 0.37 |
| CPK (UI/L) | 139 (79; 196) | 93 (61; 140) | 0.01 |
MCV: mean corpuscular volume; CRP: C-reactive protein; CPK: creatine phospho kinase. Values are median (interquartile range). Blood sample analysis was missing for one PPS patient.
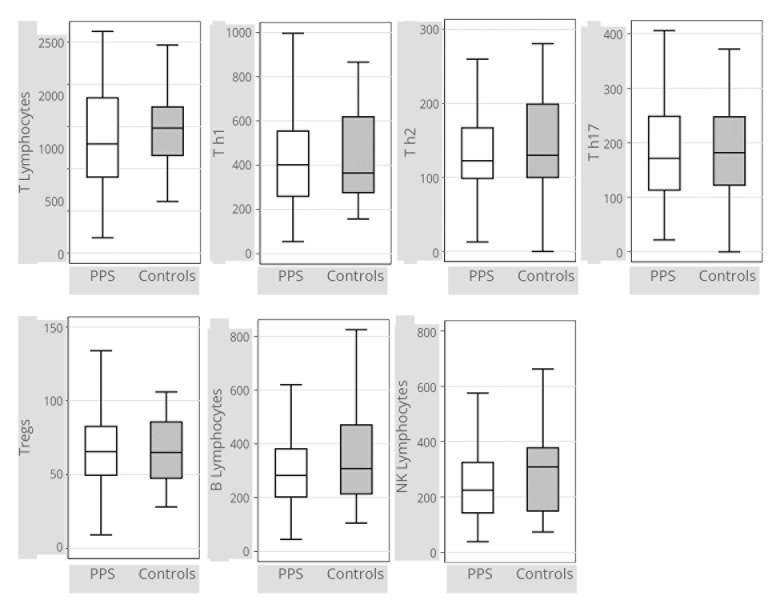
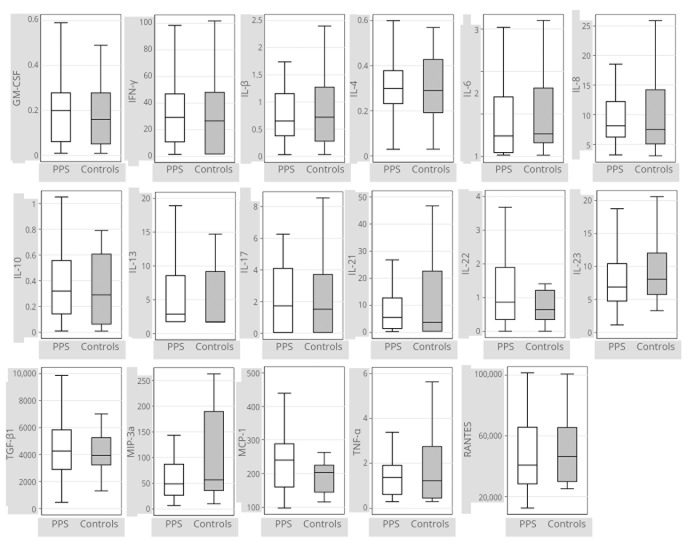
Lymphocyte counts, comprising a fine analysis of the subpopulations of T lymphocytes (Figure 1) (Supplementary Digital Material 1: Supplementary Table I), and cytokine and peptides dosages (Figure 2) (Supplementary Digital Material 2: Supplementary Table II) were comparable between the two groups.
Figure 1.
—Box plots representing lymphocyte levels in patients with PPS (N.=47) and controls (N.=25). Tregs: Regulatory T lymphocytes. Lymphocytes counts are expressed in N/mm3. Data were missing for 2 out of 27 subjects in the control group. Box plot interpretation: the medium line represents the median value, the box represents the first and third quartiles, and whiskers represent minimum and maximum adjacent values. Outliers are not plotted. Exact values are given in Supplementary Digital Material 1.
Figure 2.
— Box plots representing cytokine levels in patients with PPS (N.=47) and controls (N.=25). Cytokine levels are expressed in pg/mL. Data were missing for two out of 27 subjects in the control group. Box plot interpretation: the medium line represents the median value, the box represents the first and third quartiles, and whiskers represent minimum and maximum adjacent values. Outliers are not plotted. Exact values are given in Supplementary Digital Material 2.
Blood counts, T lymphocytes and cytokine or peptide levels were also compared between patients whose late main symptoms were pain or fatigue (N.=20) and other PPS patients (N.=27). None of the variables differed clinically between the two groups (Supplementary Digital Material 3: Supplementary Table III and IV).
Discussion
Summary of the results
Our study aimed to explore the dysimmune hypothesis of PPS in the scope of developing specific treatments in the context of an epidemiological global emergency. Our results did not show any difference in cellular nor humoral immunity between 47 PPS patients and 27 age-matched controls, based on blood analysis of lymphocyte counts and on dosage of pro- and anti-inflammatory cytokines and peptides. Subgroup analysis failed to demonstrate different immunological profiles of patients depending on their clinical presentation.
These results are conflicting with the results from Fordyce17 who conducted the main similar study in 2008 on 56 PPS patients and 26 controls. They concluded that TNFα levels, as well as IL-6 and leptin were significantly increased in patients compared to controls. They also demonstrated that the elevated TNFα levels in PPS were associated with increased muscle pain. Since their method was very similar to ours, such differences could be linked to other bias – inclusion of patients with confusing other diseases leading to the increase of immunological markers, differences in dosage kits and reliability of their results – or to chance.
Medical literature sustaining the dysimmune origin of the PPS is questionable
Over the past 30 years, some studies have shown an abnormally high level of pro-inflammatory cytokines or peptides in the blood or in the cerebrospinal fluid (CSF) of PPS patients (Table III).13, 15-18, 31-35 These results are controversial, mostly because of the small sample size of the populations studied, and because some of them reported conflicting negative results.
Table III. —Studies on blood and CSF dosages of pro-inflammatory and anti-inflammatory cytokines and peptides in PPS patients13, 15-18, 31-35 and on muscle histological or biological changes in PPS.19, 20, 32, 36.
| Reference | Method | Patients | Controls | Results | Conclusion |
|---|---|---|---|---|---|
| Blood and CSF dosages of cytokines and peptides in PPS patients | |||||
| Dalakas 198632 | CSF immunologic dosages | 27 PPS | Oligoclonal bands (IgG) were found in the cerebrospinal fluid of 7 of 13 patients studied. | Controversial | |
| Sharief 199118 | CSF | 36 PPS | 13 stable poliomyelitic patients + 18 ALS + 36 other neuromuscular disease | Oligoclonal IgM were found in 58% of PPS and none of controls, and were poliovirus-specific. CSF levels of IL-2 and soluble IL-2 receptors were higher in PPS patients than in controls. Results support intrathecal immune response to poliovirus, suggesting new or persistent poliovirus infection in the CNS of PPS. | In favor |
| Roivainen 199435 | CSF | 21 PPS | No poliovirus-specific IgM Antibodies in the CSF of PPS patients | Negative | |
| Gonzalez 200216 | CSF and blood | 13 PPS | 8 non-inflammatory controls + 7 multiple sclerosis | Increased expression of inflammatory cytokines (TNF-α, IFN-γ, IL-4, IL-10) in CSF (but not in peripheral blood) of PPS compared to non-inflammatory controls. The increase was comparable to that of multiple sclerosis, a well-known neuroinflammatory disease. | In favor |
| Gonzalez 200433 | CSF and Blood | 16 PPS before and after IVIG treatment | 26 patients with non-inflammatory other neurological diseases | TNF-alpha, IFN-gamma and IL-10 CSF mRNA levels were elevated in untreated persons with PPS compared to other neurological diseases. Upon IVIG treatment, IFN-gamma and TNF-alpha mRNA levels were reduced, while IL-10 remained unchanged. | In favor |
| Fiorini 200713 | CSF | 16 PPS | 3 stable poliomyelitic patients | Inflammatory changes occur in both stable polio and PPS: 14-3-3 increased in both groups; Tau was within normal range; Cystatin C was non interpretable. | Controversial |
| Fordyce 200817 | Blood | 51 PPS patients | 26 healthy controls | TNFα levels, as well as IL-6 and leptin were significantly increased compared to controls. The elevated TNFα levels in PPS were associated with increased muscle pain. | In favor |
| Gonzales 201231 | Blood + CSF | 20 with IVIG | 30 with other neurological diseases | At baseline (before IVIG), TNF and IFN-γ in CSF and peripheral blood were higher in PPS patients than in controls. One year after IVIG, PPS patients had beneficial changes in cytokine profiles with decreased IFN-γ and IL23 + increased anti-inflammatory IL-13 in CSF | In favor |
| Melin 201434 | Blood | 20 PPS | 95 healthy controls | No increase in circulating immune complex or in TNF-inducing effects of circulating immune complex | Negative |
| Bickerstaffe 201515 | Blood | 45 PPS | 18 healthy controls | IL-6, IL-8, TNF-α and leptin increased in PPS patients compared to controls but no evidence for an association between inflammation and clinical deterioration was found. Other inflammatory mediators (IL-10, IL-18 and IL-13) did not differ. | Controversial |
| Inflammatory infiltrates in muscles of PPS patients | |||||
| Dalakas 198632 | Muscle biopsies | 27 PPS | The newly affected muscles evaluated longitudinally showed chronic and new denervation. | Negative | |
| Dalakas 198820 | Muscle | 27 PPS | 5 stable poliomyelitic patients | Perivascular or interstitial inflammatory cells (predominantly lymphocytes unrelated to phagocytosis) were noted in 40% of all PPS patients. The newly weakened muscles show signs of recent denervation. | Controversial |
| Borg 198836 | Muscle | 19 PPS | 4 patients with radicular lesions | Non-specific changes in fiber type composition with transition of type 2 to type 1 muscle fibers with marked hypertrophy. | Negative |
| Melin 201419 | Muscle | 8 PPS | 6 healthy controls | Higher expression of enzymes of the prostaglandin E2 synthetic pathway, in muscle from PPS patients, compared with controls. Evidence for an inflammatory process of the muscle, which could be secondary to systemic inflammation. | Controversial |
ALS: amyothrophic lateral sclerosis; CNS: central nervous system; CSF: cerebrospinal fluid; IVIG: intravenous immunoglobulin.
Other studies reported the existence of inflammatory infiltrates in the skeletal muscles of PPS patients, none of them being able to conclude if the results were due to a general inflammatory process or to muscles overuse (Table III).19, 20, 32, 36
Medical literature sustaining the persistence or reactivation of the poliovirus in the CNS is controversial
Initially, these dysimmune hypotheses were based on previous works suggesting persistence or reactivation of polio virus (PV) in the CNS of patients with PPS (Table IV).10-12, 18, 32, 37-39
Table IV. —Studies searching for the persistence or reactivation of the poliovirus in the CNS.10-12, 18, 32, 37-39.
| Reference | Patients | Controls | Method | Results | Conclusion |
|---|---|---|---|---|---|
| Dalakas 198632 | 27 PPS | CSF virologic dosages | No elevation of antibodies to poliovirus was observed in the CSF | Negative | |
| Sharief 199118 | 36 PPS | 13 stable poliomyelitic patients + 18 ALS + 36 other neuromuscular diseases | CSF dosages | Oligoclonal IgM were found in 58% of PPS and 0 of controls, and were poliovirus-specific. CSF levels of IL-2 and soluble IL-2 receptors were higher in PPS patients than in controls. Results support intrathecal immune response to poliovirus, suggesting new or persistent poliovirus infection in the CNS of PPS | In favor |
| Melchers 199239 | 16 PPS | 25 other neurological diseases | PCR and IgM antibody-capture enzyme-linked immunosorbent assay. Blood, CSF and muscles biopsies. | Poliovirus RNA or a poliovirus type-specific IgM response was detected in none of the specimens. | Negative |
| Dalakas 199537 | Unknown | Histopathology, histochemistry, Immunocytochemistry, PCR, lymphocytes counts, virological searches. | Presence of poliovirus in the spinal fluid of 4/40 PPS patients, in the peripheral blood lymphocytes of 7/37 PPS patients, and not in muscle. Their role in the pathogenesis of PPS is unknown | Controversial | |
| Muir 199512 | 24 PPS | 36 stable poliomyelitic patients + 36 other neurologic conditions | PCR in CSF (Viral RNA) | 3/24 ongoing PPS patients and 0 control patient had positive PCR | Negative |
| Jubelt 199538 | 146 PPS patients from 7 studies | Review of previous poliovirus antibody studies in PPS | 1 positive study (21/36 patients) and 6 negative studies | Negative | |
| Leparc 199611 | 10 PPS | 10 ALS + 10 other neurological disease + 3 stable poliomyelitic patients | Genomic sequences CSF / reverse transcription PCR | Poliovirus-specific genomic sequences in the 5* untranslated region and in the capsid region (VP1) were detected in 5/10 PPS patients but in 0/23 control patient. | Controversial |
| Julien 199910 | 20 PPS | 20 unrelated neurological diseases + 7 stable poliomyelitic patients | RT-PCR | Poliovirus genomic sequences were detected in the CSF of 11/20 PPS patients and in none of the control group | Controversial |
ALS: amyothrophic lateral sclerosis; CSF: cerebrospinal fluid.
Starting with an old experimental animal study showing that poliovirus may cause persistent infection and paralysis upon immunosuppression in mice,40 the possible pathogenic role of a persistent PV infection and its related chronic inflammation due to the upregulation of pro-inflammatory cytokines and chemokines has been suggested. Currently, studies in this field are sparse and weak.9
Arguments sustaining CNS morphological or biological changes in PPS patients suggesting an evolutive process
Some authors conducted biochemical, histological or anatomical studies in PPS patients that could indirectly sustain the hypothesis of a potentially age-related, neurodegenerative, or dysimmune evolving pathology of the CNS (Table V).13, 41-43 These studies are scarce and non convincing.
Table V. —Review of studies exploring CNS morphological or biological changes in PPS patients.13, 41-43.
| Reference | Patients | Controls | Method | Results |
|---|---|---|---|---|
| Pezeshkpour 198843 | 3 PPS patients | 10 ALS + 5 spino-cerebellar degeneration patients + 5 stable polio | Sections of spinal cord histology | Atrophy of motor neurons, severe reactive gliosis, and mild to moderate perivascular and interparenchymal inflammation. No difference between PPS patients and stable ones. |
| Fiorini 200713 | 16 PPS patients | 3 stable polio | CSF proteins: 14-3-3, cystatin C, and tau | Inflammatory changes occur in both stable polio and PPS: 14-3-3 increased in both groups; Tau was within normal range; Cystatin C was non interpretable. |
| Gonzales 200941 | 15 PPS patients | 9 healthy + 34 other non-inflammatory diseases + 17 secondary progressive multiple sclerosis | CSF protein biomarker | Differential expression of gelsolin, hemopexin, and kallikrein 6 in PPS patients compared to controls. |
| Li Hi Shing 202142 | 36 polio survivors | 88 ALS + 117 healthy | Cortical and white matter reorganisation in poliomyelitis survivors which may be interpreted as compensatory, change in response to severe lower motor neuron injury in infancy. |
ALS: amyothrophic lateral sclerosis; CSF: cerebrospinal fluid.
Strengths and limitations of the study
Our study recruited one of the largest samples described in this research field. We carefully selected patients with confirmed PPS, and without alternative inflammatory disease. Controls were healthy subjects, frequency matched on age and sex. A large subset of humoral and cellular inflammatory markers was analyzed. However, our study may present limitations. This was a monocentric study – but we are a regional reference center. We recruited prevalent cases – but as this syndrome does not spontaneously heals, nor leads to death, we do not feel that this would select a particular subgroup of patients; and as the PPS tends to worsen over years, it would be surprising that an initial inflammatory syndrom had disappeared, which explains our negative results. Lastly, we did not reach the intended sample size because of recruitment difficulties. However, the statistical power was guaranteed because we included 47 and 27 patients, and both groups were comparable in age and sex.
Conclusions
Our study does not confirm the dysimmune hypothesis of the post-poliomyelitis syndrome. It reinforces the idea that these clinical manifestations may be linked to neurological aging phenomena that could be explored by the dosages in blood or CSF of specific biomarkers or abnormal proteins known to be involved in different pathways associated with tissue damage and apoptosis.41 If confirmed, they would open the door for biological or drug neuroprotective treatments. These results also reinforce the importance of PRM care and cure with preventive and curative strategies, whose effectiveness has been widely documented in the literature:8 prevention of secondary complications, rehabilitation, adjustments to living conditions.
Supplementary Digital Material 1
Supplementary Table I
Lymphocytes levels in patients with post-poliomyelitis syndrome (PPS) and controls.
Supplementary Digital Material 2
Supplementary Table II
Cytokines levels in patients with post-poliomyelitis syndrome (PPS) and controls.
Supplementary Digital Material 3
Supplementary Table III
Blood count and serum electrolytes in patients whose late main symptoms were pain or fatigue as compared with other PPS patients.
Supplementary Table IV
Cytokines levels in patients whose late main symptoms were pain or fatigue as compared with other PPS patients.
Acknowledgements
The authors wish to acknowledge William Camu for his great experience in neuro-muscular diseases, Claire Belloc for careful data-management, and patients and controls involved in this study.
Footnotes
Conflicts of interest: The authors certify that there is no conflict of interest with any financial organization regarding the material discussed in the manuscript.
Funding: The authors report no involvement in the research by the sponsor that could have influenced the outcome of this work.
Congresses: This paper was presented as oral communication at the ISPRM/ESPRM Congress that was held in Lisbon on July 2022 and at the SOFMER Congress that was held in Rennes in December 2022.
References
- 1.Groce NE, Banks LM, Stein MA. Surviving polio in a post-polio world. Soc Sci Med 2014;107:171–8. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=24607679&dopt=Abstract 10.1016/j.socscimed.2014.02.024 [DOI] [PubMed] [Google Scholar]
- 2.Li Hi Shing S, Chipika RH, Finegan E, Murray D, Hardiman O, Bede P. Post-polio Syndrome: More Than Just a Lower Motor Neuron Disease. Front Neurol 2019;10:773. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=31379723&dopt=Abstract 10.3389/fneur.2019.00773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization. Global polio eradication initiative applauds WHO African region for wild polio-free certification; 2020 [Internet]. Available from: https://www.who.int/news/item/25-08-2020-global-polio-eradication-initiative-applauds-who-african-region-for-wild-polio-free-certification [cited 2023, Jan 8].
- 4.Jones KM, Balalla S, Theadom A, Jackman G, Feigin VL. A systematic review of the worldwide prevalence of survivors of poliomyelitis reported in 31 studies. BMJ Open 2017;7:e015470. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=28694346&dopt=Abstract 10.1136/bmjopen-2016-015470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gonzalez H, Olsson T, Borg K. Management of postpolio syndrome. Lancet Neurol 2010;9:634–42. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=20494327&dopt=Abstract 10.1016/S1474-4422(10)70095-8 [DOI] [PubMed] [Google Scholar]
- 6.Yelnik A, Laffont I. The psychological aspects of polio survivors through their life experience. Ann Phys Rehabil Med 2010;53:60–7. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=20022578&dopt=Abstract 10.1016/j.rehab.2009.11.006 [DOI] [PubMed] [Google Scholar]
- 7.Halstead LS. Post-polio syndrome. Sci Am 1998;278:42–7. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=9532770&dopt=Abstract 10.1038/scientificamerican0498-42 [DOI] [PubMed] [Google Scholar]
- 8.Lo JK, Robinson LR. Postpolio syndrome and the late effects of poliomyelitis. Part 1. pathogenesis, biomechanical considerations, diagnosis, and investigations. Muscle Nerve 2018;58:751–9. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=29752819&dopt=Abstract 10.1002/mus.26168 [DOI] [PubMed] [Google Scholar]
- 9.Baj A, Colombo M, Headley JL, McFarlane JR, Liethof MA, Toniolo A. Post-poliomyelitis syndrome as a possible viral disease. Int J Infect Dis 2015;35:107–16. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=25939306&dopt=Abstract 10.1016/j.ijid.2015.04.018 [DOI] [PubMed] [Google Scholar]
- 10.Julien J, Leparc-Goffart I, Lina B, Fuchs F, Foray S, Janatova I, et al. Postpolio syndrome: poliovirus persistence is involved in the pathogenesis. J Neurol 1999;246:472–6. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=10431774&dopt=Abstract 10.1007/s004150050386 [DOI] [PubMed] [Google Scholar]
- 11.Leparc-Goffart I, Julien J, Fuchs F, Janatova I, Aymard M, Kopecka H. Evidence of presence of poliovirus genomic sequences in cerebrospinal fluid from patients with postpolio syndrome. J Clin Microbiol 1996;34:2023–6. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=8818905&dopt=Abstract 10.1128/jcm.34.8.2023-2026.1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Muir P, Nicholson F, Sharief MK, Thompson EJ, Cairns NJ, Lantos P, et al. Evidence for persistent enterovirus infection of the central nervous system in patients with previous paralytic poliomyelitis. Ann N Y Acad Sci 1995;753:219–32. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=7611631&dopt=Abstract 10.1111/j.1749-6632.1995.tb27548.x [DOI] [PubMed] [Google Scholar]
- 13.Fiorini M, Zanusso G, Baj A, Bertolasi L, Toniolo A, Monaco S. Post-polio syndrome: clinical manifestations and cerebrospinal fluid markers. Future Neurol 2007;2:451–63. 10.2217/14796708.2.4.451 [DOI] [Google Scholar]
- 14.Ostlund G, Broman L, Werhagen L, Borg K. IVIG treatment in post-polio patients: evaluation of responders. J Neurol 2012;259:2571–8. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=22592288&dopt=Abstract 10.1007/s00415-012-6538-y [DOI] [PubMed] [Google Scholar]
- 15.Bickerstaffe A, Beelen A, Lutter R, Nollet F. Elevated plasma inflammatory mediators in post-polio syndrome: no association with long-term functional decline. J Neuroimmunol 2015;289:162–7. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=26616886&dopt=Abstract 10.1016/j.jneuroim.2015.10.019 [DOI] [PubMed] [Google Scholar]
- 16.Gonzalez H, Khademi M, Andersson M, Wallström E, Borg K, Olsson T. Prior poliomyelitis-evidence of cytokine production in the central nervous system. J Neurol Sci 2002;205:9–13. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=12409177&dopt=Abstract 10.1016/S0022-510X(02)00316-7 [DOI] [PubMed] [Google Scholar]
- 17.Fordyce CB, Gagne D, Jalili F, Alatab S, Arnold DL, Da Costa D, et al. Elevated serum inflammatory markers in post-poliomyelitis syndrome. J Neurol Sci 2008;271:80–6. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=18474371&dopt=Abstract 10.1016/j.jns.2008.03.015 [DOI] [PubMed] [Google Scholar]
- 18.Sharief MK, Hentges R, Ciardi M. Intrathecal immune response in patients with the post-polio syndrome. N Engl J Med 1991;325:749–55. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=1651456&dopt=Abstract 10.1056/NEJM199109123251101 [DOI] [PubMed] [Google Scholar]
- 19.Melin E, Lindroos E, Lundberg IE, Borg K, Korotkova M. Elevated expression of prostaglandin E2 synthetic pathway in skeletal muscle of prior polio patients. J Rehabil Med 2014;46:67–72. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=24162727&dopt=Abstract 10.2340/16501977-1230 [DOI] [PubMed] [Google Scholar]
- 20.Dalakas MC. Morphologic changes in the muscles of patients with postpoliomyelitis neuromuscular symptoms. Neurology 1988;38:99–104. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=3336469&dopt=Abstract 10.1212/WNL.38.1.99 [DOI] [PubMed] [Google Scholar]
- 21.Huang YH, Chen HC, Huang KW, Chen PC, Hu CJ, Tsai CP, et al. Intravenous immunoglobulin for postpolio syndrome: a systematic review and meta-analysis. BMC Neurol 2015;15:39. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=25886512&dopt=Abstract 10.1186/s12883-015-0301-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dalakas MC. Pro-inflammatory cytokines and motor neuron dysfunction: is there a connection in post-polio syndrome? J Neurol Sci 2002;205:5–8. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=12409176&dopt=Abstract 10.1016/S0022-510X(02)00314-3 [DOI] [PubMed] [Google Scholar]
- 23.Stolwijk-Swüste JM, Beelen A, Lankhorst GJ, Nollet F, CARPA study group . SF36 physical functioning scale and 2-minute walk test advocated as core qualifiers to evaluate physical functioning in patients with late-onset sequelae of poliomyelitis. J Rehabil Med 2008;40:387–94. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=18461265&dopt=Abstract 10.2340/16501977-0188 [DOI] [PubMed] [Google Scholar]
- 24.Mendell JR, Florence J. Manual muscle testing. Muscle Nerve 1990;13(Suppl):S16–20. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=2233877&dopt=Abstract 10.1002/mus.880131307 [DOI] [PubMed] [Google Scholar]
- 25.Mathiowetz V, Weber K, Volland G, Kashman N. Reliability and validity of grip and pinch strength evaluations. J Hand Surg Am 1984;9:222–6. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=6715829&dopt=Abstract 10.1016/S0363-5023(84)80146-X [DOI] [PubMed] [Google Scholar]
- 26.Brogårdh C, Lexell J, Lundgren-Nilsson A. Construct validity of a new rating scale for self-reported impairments in persons with late effects of polio. PM R 2013;5:176–81, quiz 181. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=22939237&dopt=Abstract 10.1016/j.pmrj.2012.07.007 [DOI] [PubMed] [Google Scholar]
- 27.Vasconcelos OM, Jr, Prokhorenko OA, Kelley KF, Vo AH, Olsen CH, Dalakas MC, et al. A comparison of fatigue scales in postpoliomyelitis syndrome. Arch Phys Med Rehabil 2006;87:1213–7. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=16935057&dopt=Abstract 10.1016/j.apmr.2006.06.009 [DOI] [PubMed] [Google Scholar]
- 28.Frith J, Newton J. Fatigue Impact Scale. Occup Med (Lond) 2010;60:159. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=20157190&dopt=Abstract 10.1093/occmed/kqp180 [DOI] [PubMed] [Google Scholar]
- 29.Debouverie M, Pittion-Vouyovitch S, Louis S, Guillemin F. Validity of a French version of the fatigue impact scale in multiple sclerosis. Mult Scler 2007;13:1026–32. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=17895294&dopt=Abstract 10.1177/1352458507077942 [DOI] [PubMed] [Google Scholar]
- 30.McHorney CA, Ware JE, Jr, Raczek AE. The MOS 36-Item Short-Form Health Survey (SF-36): II. Psychometric and clinical tests of validity in measuring physical and mental health constructs. Med Care 1993;31:247–63. Available from: https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=8450681&dopt=Abstract 10.1097/00005650-199303000-00006 [DOI] [PubMed] [Google Scholar]
- 31.Gonzalez H, Khademi M, Borg K, Olsson T. Intravenous immunoglobulin treatment of the post-polio syndrome: sustained effects on quality of life variables and cytokine expression after one year follow up. J Neuroinflammation 2012;9:167. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=22776106&dopt=Abstract 10.1186/1742-2094-9-167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dalakas MC, Elder G, Hallett M, Ravits J, Baker M, Papadopoulos N, et al. A long-term follow-up study of patients with post-poliomyelitis neuromuscular symptoms. N Engl J Med 1986;314:959–63. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=3007983&dopt=Abstract 10.1056/NEJM198604103141505 [DOI] [PubMed] [Google Scholar]
- 33.Gonzalez H, Khademi M, Andersson M, Piehl F, Wallström E, Borg K, et al. Prior poliomyelitis-IVIg treatment reduces proinflammatory cytokine production. J Neuroimmunol 2004;150:139–44. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=15081258&dopt=Abstract 10.1016/j.jneuroim.2004.01.010 [DOI] [PubMed] [Google Scholar]
- 34.Melin E, Sohrabian A, Rönnelid J, Borg K. Normal serum levels of immune complexes in postpolio patients. Results Immunol 2014;4:54–7. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=25009767&dopt=Abstract 10.1016/j.rinim.2014.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Roivainen M, Kinnunen E, Hovi T. Twenty-one patients with strictly defined postpoliomyelitis syndrome: no poliovirus-specific IgM antibodies in the cerebrospinal fluid. Ann Neurol 1994;36:115–6. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=8024252&dopt=Abstract 10.1002/ana.410360125 [DOI] [PubMed] [Google Scholar]
- 36.Borg K, Borg J, Edström L, Grimby L. Effects of excessive use of remaining muscle fibers in prior polio and LV lesion. Muscle Nerve 1988;11:1219–30. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=3237237&dopt=Abstract 10.1002/mus.880111206 [DOI] [PubMed] [Google Scholar]
- 37.Dalakas MC. Pathogenetic mechanisms of post-polio syndrome: morphological, electrophysiological, virological, and immunological correlations. Ann N Y Acad Sci 1995;753:167–85. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=7611626&dopt=Abstract 10.1111/j.1749-6632.1995.tb27543.x [DOI] [PubMed] [Google Scholar]
- 38.Jubelt B, Salazar-Grueso EF, Roos RP, Cashman NR. Antibody titer to the poliovirus in blood and cerebrospinal fluid of patients with post-polio syndrome. Ann N Y Acad Sci 1995;753:201–7. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=7611629&dopt=Abstract 10.1111/j.1749-6632.1995.tb27546.x [DOI] [PubMed] [Google Scholar]
- 39.Melchers W, de Visser M, Jongen P, van Loon A, Nibbeling R, Oostvogel P, et al. The postpolio syndrome: no evidence for poliovirus persistence. Ann Neurol 1992;32:728–32. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=1335224&dopt=Abstract 10.1002/ana.410320605 [DOI] [PubMed] [Google Scholar]
- 40.Jubelt B, Meagher JB. Poliovirus infection of cyclophosphamide-treated mice results in persistence and late paralysis: II. Virologic studies. Neurology 1984;34:494–9. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=6322052&dopt=Abstract 10.1212/WNL.34.4.494 [DOI] [PubMed] [Google Scholar]
- 41.Gonzalez H, Ottervald J, Nilsson KC, Sjögren N, Miliotis T, Von Bahr H, et al. Identification of novel candidate protein biomarkers for the post-polio syndrome - implications for diagnosis, neurodegeneration and neuroinflammation. J Proteomics 2009;71:670–81. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=19100873&dopt=Abstract 10.1016/j.jprot.2008.11.014 [DOI] [PubMed] [Google Scholar]
- 42.Li Hi Shing S, Lope J, McKenna MC, Chipika RH, Hardiman O, Bede P. Increased cerebral integrity metrics in poliomyelitis survivors: putative adaptation to longstanding lower motor neuron degeneration. J Neurol Sci 2021;424:117361. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=33773768&dopt=Abstract 10.1016/j.jns.2021.117361 [DOI] [PubMed] [Google Scholar]
- 43.Pezeshkpour GH, Dalakas MC. Long-term changes in the spinal cords of patients with old poliomyelitis. Signs of continuous disease activity. Arch Neurol 1988;45:505–8. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=3358701&dopt=Abstract 10.1001/archneur.1988.00520290033010 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table I
Lymphocytes levels in patients with post-poliomyelitis syndrome (PPS) and controls.
Supplementary Table II
Cytokines levels in patients with post-poliomyelitis syndrome (PPS) and controls.
Supplementary Table III
Blood count and serum electrolytes in patients whose late main symptoms were pain or fatigue as compared with other PPS patients.
Supplementary Table IV
Cytokines levels in patients whose late main symptoms were pain or fatigue as compared with other PPS patients.
Data Availability Statement
Individual participant data that underlie the results reported in this article can be made available upon reasonable request to the corresponding author.