Abstract
Consistent evidence documents powerful effects of social inequality on health, well-being, and academic achievement. Yet research on whether social inequality may also be linked to brain structure and function has, until recently, been rare. Here, we describe three methodological approaches—including single-site, single study; multi-site, single study; and spatial meta-analysis—that can be used to study this question. We review empirical work that, using these approaches, has observed associations between structural measures of social inequality—including structural stigma, community-level prejudice, gender inequality, neighborhood disadvantage, and the generosity of the social safety net for low-income families—and neural outcomes. We evaluate the relative strengths and limitations of these methods, discuss ethical considerations, and outline directions for future research. In doing so, we advocate for a paradigm shift in cognitive neuroscience that explicitly incorporates upstream structural and contextual factors, which we argue holds promise for uncovering the neural correlates of social inequality.
Extensive evidence from numerous disciplines, including sociology, psychology, economics, and public health, demonstrates that various forms of social inequality may exert a powerful influence on human health and wellbeing. This work has examined the role of income inequality,1 racial residential segregation,2,3 exposure to neighborhood violence,4,5 community-level prejudice,6–12 structural racism,13 and institutional policies that restrict the rights of immigrants14–16 and lesbian, gay, bisexual, and transgender (LGBT)17–24 people. The link between such factors and outcomes as diverse as longevity,25,26 educational achievement,27 mental health problems,28 and physical disease prevalence29,30 is well documented. Yet despite the weight of evidence that social inequalities are key risk factors for so many outcomes, there has been much less research on how inequalities may be linked to the structure and function of the human brain.
We believe that one of the main barriers to the study of social impacts on neural outcomes is the fact that most neuroimaging studies are conducted in a single community. In such designs, respondents are ubiquitously exposed to the same macro-social context,31 which precludes the possibility of studying the effects of differences in social context. In this Perspective, we draw on advances in population neuroscience32,33 to present a call to action to the field of cognitive neuroscience to systematically examine associations between social inequalities and neural outcomes, as well as potential causal mechanisms. We first describe how social inequality is operationalized and make the case for why studying social inequalities matters for cognitive neuroscience. We then describe three methodological approaches that can be used to explore associations between social inequalities and neural outcomes. Finally, we highlight recent evidence that has begun to leverage these methods to identify the associations of social inequality with brain structure and function. In doing so, we advocate for a paradigm shift in cognitive neuroscience that explicitly incorporates upstream contextual factors, which we argue holds promise for uncovering the neural correlates of social inequality.
Conceptualizing and Operationalizing Social Inequality
Social inequalities have been defined in various ways across disciplines but generally refer to “the unequal distribution of, and unequal access to, highly valued and desired material and nonmaterial social goods. Social inequalities imply systematic advantages and disadvantages in life chances, living conditions, opportunity structures, and life outcomes of individuals and social groups.”34 As suggested by this conceptualization, scholars have examined different dimensions and forms of social inequality—including economic inequality, health inequality, and inequality related to social position (e.g., based on gender, race, sexuality). Depending on the research question, a dimension of social inequality can reflect either an outcome (e.g., studies examining causes of gender inequality) or a mechanism (e.g., studies examining whether economic inequality causes differences in health status between white and Black Americans).
Social inequality is measured in a variety of ways. To illustrate these differing approaches to operationalization, we draw on illustrative examples from research on two sources/forms of social inequality—stigma and socioeconomic status (SES). Stigma is a social factor that has been conceptualized as existing at individual, interpersonal, and structural levels.35,36 Stigma has been measured: 1) at the individual level, in the form of perceptions and reactions, such as stereotype embodiment37 or identity concealment;38 2) at the interpersonal level, as differential treatment resulting from one’s social position, such as having a criminal record;39 and 3) at the structural level, in the form of social policies that restrict opportunities, resources, and wellbeing, such as state laws denying services to same-sex couples.21 The literature on SES, which can similarly been measured across individual, group, and structural levels, offers another instructive example. SES has been variously measured: 1) at the individual level—as personal income, occupation, educational attainment, or perceptions of one’s subjective social status; 2) at the group level, via household family income or the highest educational level achieved by an adult in the household; and 3) at the structural level, as the median income level of one’s neighborhood, an area-level measure of deprivation, or level of income inequality across countries.40–42 Of course, these three levels are not independent, but rather are mutually constitutive. That is, structural forms of stigma not only shape how individuals perceive and react to stigma43 but also influence how the stigmatized are treated in interpersonal contexts (e.g., employment).44 Similarly, individuals with lower income reside in neighborhoods with greater material deprivation, which in turn shapes individual income through institutional policies and practices, as in the case of racial covenants that restrict Black Americans from purchasing homes in neighborhoods with more economic resources.2
As is evident from these examples, structural measures of social inequalities are those that reflect properties of a particular spatial location at a particular moment in time that are either aggregated across the group of people who inhabit that location (e.g., median household income) or that exist only at a level of aggregation above individuals (e.g., city, county, state, or country-level policies). Consequently, the measurement approaches that are necessary to operationalize these structural constructs differ from those approaches that are used to capture the individual-level experiences (e.g., income or educational attainment) more traditionally explored in the cognitive neuroscience literature (Table 1). Thus, in our paper, we only review articles that have used the kinds of structural measures of social inequality as reflected in Table 1.
Table 1.
Measurement Approaches for Studying Associations between Social Inequalities and Neural Structure/Function: Examples from the Literatures on Stigma and Prejudice
| Measure | Level of Aggregation | Data Sources | Illustrative References* |
|---|---|---|---|
| Individual-, Interpersonal-, and Group-Level Social Factors | |||
| Perceived Discrimination | Individual | Everyday Discrimination Scale | Williams et al.110 |
| Couple-Level Minority Stress | Dyadic | Relationship Timeline | Frost et al.111 |
| Intergroup Conflict | Intergroup | In-group>out-group: e.g., resource allocation, prosocial behaviors | Hewstone et al.112 |
| Structural Factors | |||
|
Attitude Measures (Self-reported) |
County, state | General Social Survey American National Election Survey |
Hatzenbuehler et al.12
Reid et al.113 |
|
Attitude Measures
(Non-Self-Reported) |
County, state | Project Implicit Google Search Terms |
Payne et al.114
Chae et al.7 |
| Social Policies | City, state | Movement Advancement Project Historical records (e.g., presence of Jim Crow laws) Public Health Law Research National Conference of State Legislatures |
Hatzenbuehler et al.115
Krieger et al.116 Burris et al.117 Hatzenbuehler et al.15 |
| Behavioral Measures | Neighborhood, city, state | Federal Bureau of Justice Statistics (Hate Crimes) Federal Bureau of Justice Statistics (Incarceration/Death Row) |
Levy & Levy22 Lukachko et al.30 |
| Media market ad-buy data for exposure to negative political campaigns | Media market | Campaign Media Analysis Group (CMAG) Advertising Data Report and Ad Alerts | Flores et al.118 |
Notes.
References provide examples of studies that have used these measures of structural factors related to stigma and prejudice to examine their influence on a range of outcomes (e.g., psychological distress, social behaviors, etc.), but most have not been linked specifically to neural structure and function.
Why Studying Social Inequalities Matters for Cognitive Neuroscience
Although the importance of studying whether broad macro-social factors are related to brain development has repeatedly been articulated,33,45–47 studies have only recently begun to examine associations of social inequality with brain structure and function. This work has shown, for example, that greater neighborhood-level disadvantage in early childhood is associated with elevated amygdala response to neutral faces in early adulthood,48 that exposure to state-level structural stigma is associated with smaller hippocampal volume among Black and Latinx youth,49 and that the magnitude of the association between SES and brain volume varies significantly across European countries.50
We argue that further systematic investigation into associations between social inequalities and neural outcomes will advance research in cognitive neuroscience in several substantive ways. First, cognitive neuroscience has the potential to reveal the neural mechanisms through which social inequality relates to behavior and school achievement as well as health disparities,51,52 particularly mediating processes that may be difficult to detect via self-report.53 By linking macro-level factors related to social inequality with micro-level neural processes, such findings would complement research on other mechanisms underlying the negative effects of social inequality—such as health behaviors,54 access to medical care,55 and disinvestment of economic resources.2
Second, cognitive neuroscience has provided essential insights into how social factors—such as social rejection,56 exposure to interpersonal violence,57 intergroup prejudice,58 childhood maltreatment,59 and low SES60,61—relate to brain structure and function. To date, however, this work has focused almost exclusively on social factors measured at the level of individual/interpersonal experiences and/or perceptions. Expanding the level of analysis to broader structural factors may shed light onto previously unexamined correlates of neural structure and function.
Third, integrating greater focus on structural factors in neuroimaging research can contribute to efforts to improve reproducibility in cognitive neuroscience by revealing meaningful explanations for replication failures.62–64 For example, the association of SES with brain volume and cognitive ability varies significantly across European countries,50 with the association being weak in some countries and pronounced in others. Thus, depending on where the neuroimaging study is conducted, researchers may come to different conclusions about the significance and magnitude of observed associations. Rather than a failure to replicate, this may instead reflect the fact that social context is a meaningful moderator of associations frequently examined in cognitive neuroscience studies. Although the role of contextual sensitivity has been highlighted in discussions of scientific reproducibility,65 few studies have provided empirical evidence for it, particularly in cognitive neuroscience.
Finally, understanding whether social inequalities are associated with brain structure and function has not only scientific but also societal implications. Debate about the impact of decades of growing income inequality, persistent systemic racism, and policies that restrict the rights of large swaths of the population (e.g., on the basis of sexual orientation, gender identity, or immigration status) is at the forefront of public discourse. Research into the neural correlates of social inequality may inform these debates as well as litigation efforts to address inequality, similar to the role that such evidence has played in other legal domains, including the treatment of minors in the criminal justice system.66
We develop our arguments, first, by reviewing three methodological approaches that can be used to examine the relationship between social inequalities and neural outcomes. After reviewing these three methods, we discuss their relative strengths and limitations (summarized in Table 2) and suggest areas for future inquiry that are necessary to advance this work.
Table 2.
Advantages and Limitations of Different Methodological Approaches for Studying Associations between Social Inequalities and Neural Structure/Function
| Methodology | Advantages | Limitations | Questions to Consider When Using this Methodology |
|---|---|---|---|
| Single-Site, Single Study | Pragmatic (easiest) | When there is only one site, research questions are limited to “objective” measures of social inequalities that vary within that single site, typically neighborhood-level influences. While neighborhood influences are certainly important, social inequalities are often generated by institutional policies and practices that occur at broader geographic scales, including counties, states, and countries, and thus will be missed with this approach. | 1) Does the measure of social inequality exist at the neighborhood level, or at a broader geographic scale? 2) Do you have adequate variation in the measure of social inequality of interest among your study sample? |
|
Multi-Site,
Single Study |
Provides variation in exposure to broad social contexts, such as states and countries, that vary on the dimension of interest related to social inequality. | The resources needed to conduct and coordinate these large team-based efforts are typically prohibitively expensive. Some existing multi-site studies do not provide information that would enable participants to be linked to site locations. |
1) How will you address the substantial resource challenges in conducting this type of design? 2) Among the study sites you have, is there sufficient variation in the measure of social inequality? |
| Multi-Site, Multi-Study (via spatial meta-analyses) | Easier to conduct than the multi-site, single study, while still having adequate structural variation in social inequality. Can examine temporal dimensions (e.g., do the associations between social inequalities and neural outcomes differ across time or across historical changes?). |
Data constraints in terms of where studies were conducted (i.e., spatial clustering, or geospatial autocorrelation), what data are available (e.g., length of exposure to current environment, covariates, mechanisms), and ability to synthesize fMRI data across multiple labs. Often individual studies included in the meta-analysis provide inexact data on where the study occurred. |
1) Are the measures you need (e.g., for confounders and outcome) available across all studies? 2) Where were the studies in the meta-analysis conducted, and do they vary along the dimensions of social inequality of interest? |
Methodological Approach #1: Single Site, Single Study
The most straightforward and frequently employed approach to examining associations between social inequalities and neural outcomes is the single-site, single-study approach. In these studies, structural measures of social inequality are typically assessed at the neighborhood level, because this is the only contextual unit of analysis with variability within a single site (i.e., a metropolitan area and/or its surrounding regions). Most commonly, these studies measure neighborhood-level socioeconomic disadvantage,48,67–71 frequently operationalized via composite scales, such as the Area Deprivation Index (ADI), which includes area-level factors such as income, education, housing quality, and employment. As with all measures, the ADI has strengths and limitations, including a potential over-emphasis on home values in some regions.72 We refer readers to an excellent scoping review of different area-based socioeconomic deprivation indices73 to guide their selection of the appropriate measurement approach.
In an example of this approach, Gard and colleagues48 examined the association of neighborhood-level socioeconomic disadvantage—operationalized with a composite measure (e.g., percent families below the poverty line, percent households on public assistance)74,75—with neural responses to ambiguous (i.e., neutral) faces among participants sampled from the Pittsburgh area. Greater neighborhood disadvantage in early childhood was associated with elevated amygdala response to neutral faces in early adulthood, after adjusting for family-level SES and other forms of adversity including maternal depression and harsh parenting (Figure 1).48 These results suggest that neighborhood-level socioeconomic disadvantage is associated with neural response to ambiguous social cues over and above individual and family-level factors known to be associated with these responses.
Figure 1. Childhood Neighborhood Disadvantage is Associated with Greater Amygdala Reactivity.
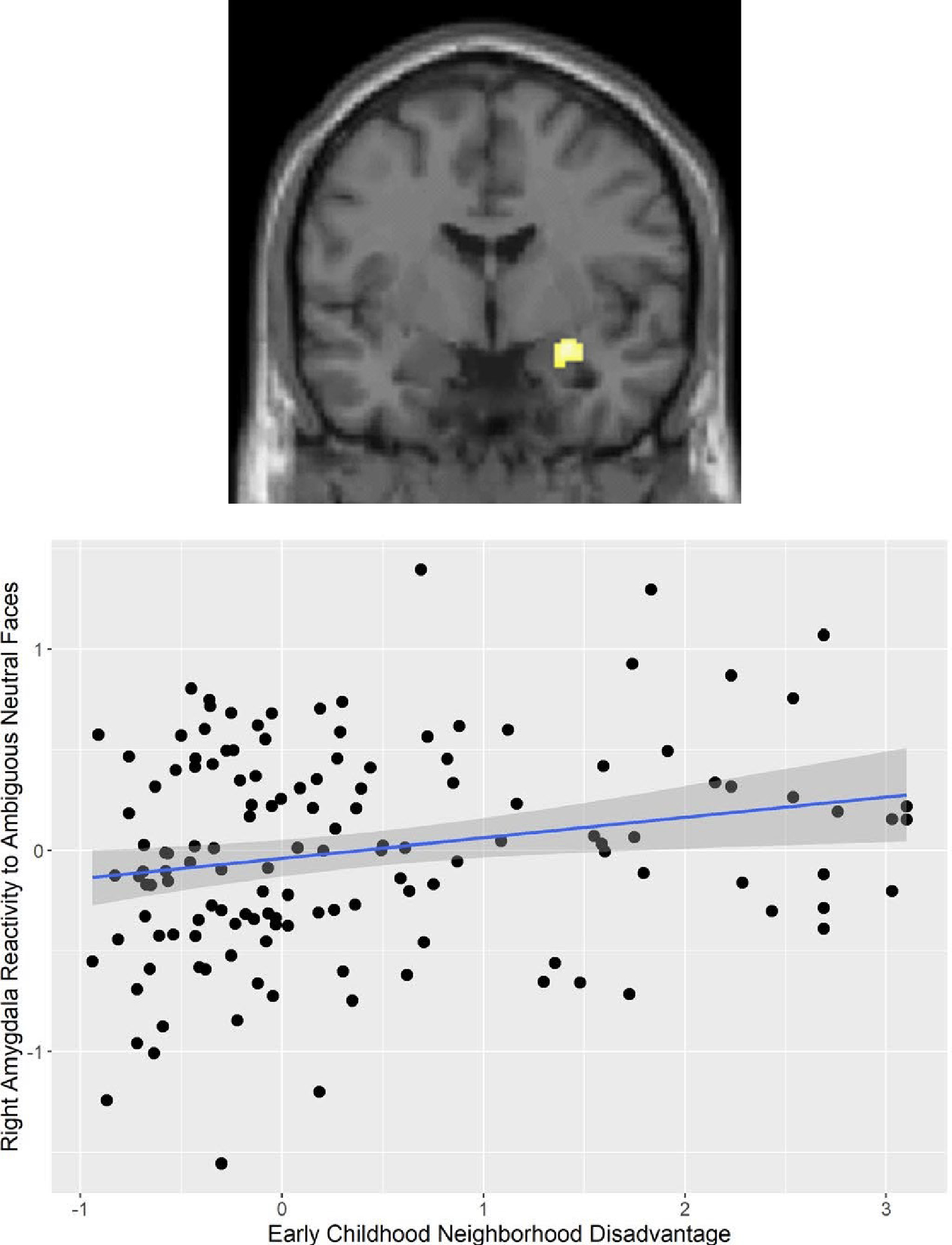
Figure adapted from Gard et al. (2021).48 Greater neighborhood disadvantage in early childhood was associated with elevated amygdala response to neutral faces in early adulthood, after adjusting for family-level SES and other forms of adversity including maternal depression and harsh parenting. These findings were replicated in 2 studies of boys from low-income family backgrounds, (a) at the University of Pittsburgh with participants from Pittsburgh (n=167) and (b) at the University of Michigan with participants from Chicago, Toledo, and Detroit.
Strengths and limitations.
The primary advantage of the single-site, single-study approach is pragmatic—it is easier to obtain neuroimaging data on samples living within a smaller geographic region (i.e., neighborhoods) and on a single scanner. But this advantage also represents the principal limitation of this approach: it is constrained in its ability to examine social inequalities beyond neighborhood-level characteristics. This is an important limitation, given that social inequalities are often generated by norms, attitudes, and institutional policies and practices that occur at broader geographic scales, including counties, states, and countries. Researchers interested in evaluating these broader sources of social inequalities must use one of the two other methods, to which we now turn.
Methodological Approach #2: Multi-Site, Single Study
The second methodological approach involves a single study that includes multiple data collection sites that have harmonized the collection of neuroimaging data. By including multiple sites that provide variation in social inequalities across different geographic scales (e.g., states, countries), this approach overcomes one of the key limitations of the single-site, single-study design. While multi-site studies have examined sources of social inequality across smaller geographic scales like neighborhoods—including racial residential segregation76 and socioeconomic disadvantage77—we focus in this section on studies that have investigated social inequality at broader units of analysis.
Two recent studies leveraged the contextual variability from a multi-site study—the Adolescent Brain and Cognitive Development (ABCD) study, which was conducted at 21 sites across the United States—to examine whether social inequalities, measured at the state level, were associated with neural outcomes among youth. In one study, Hatzenbuehler and colleagues49 operationalized the level of structural stigma related to gender, race, and ethnicity in each state, which was measured separately for each stigmatized group using state-level indicators of social policies (e.g., whether immigrants were granted access to health services) and aggregated prejudicial attitudes (e.g., endorsement of racial stereotypes). Black youth residing in environments characterized by higher structural racism had smaller hippocampal volume than Black youth residing in environments with lower levels of structural racism, controlling for demographics and family SES; the same pattern was observed for Latinx youth residing in contexts involving high structural stigma related to Latinx ethnicity compared to Latinx youth in low-stigma contexts. Further, perceived discrimination was unrelated to hippocampal volume among Black and Latinx youth, suggesting that an objective measure of stigma at the contextual level (i.e., structural stigma) may be more strongly associated with neurodevelopment than subjective perceptions of stigma measured at the individual level.49
In another study, Weissman and colleagues78 examined whether cost of living and the generosity of the social safety net for low-income families moderated the well-replicated association between family income and hippocampal volume in children61,75,79,80 across 21 sites in the ABCD study. Three policies aimed at providing support for low-income families that vary meaningfully across states were examined: the amount of monthly benefits provided by the Temporary Assistance for Needy Families (i.e., welfare: a federal program but operates through state block grants; the generosity of the benefit therefore varies between U.S. states); the amount of the state-level earned income tax credit; and whether the state enacted the expansion of Medicaid benefits made available by the Affordable Care Act, which expanded access to free health insurance through Medicaid to all U.S. citizens with income up to 138% of the federal poverty line, although not all states adopted these expanded benefits. The association between family income and hippocampal volume varied significantly across states, such that the association was stronger in states with higher cost of living. Critically, however, the magnitude of this association also varied as a function of the generosity of state-level policies designed to help low-income families. Among high cost of living states, more generous cash benefits for lower-SES families reduced the association between SES and hippocampal volume by 34% (Figure 2).
Figure 2. Association between Family Income and Hippocampal Volume is Stronger in States with Higher Costs of Living, But Weaker in U.S. States with More Generous Anti-Poverty Policies.
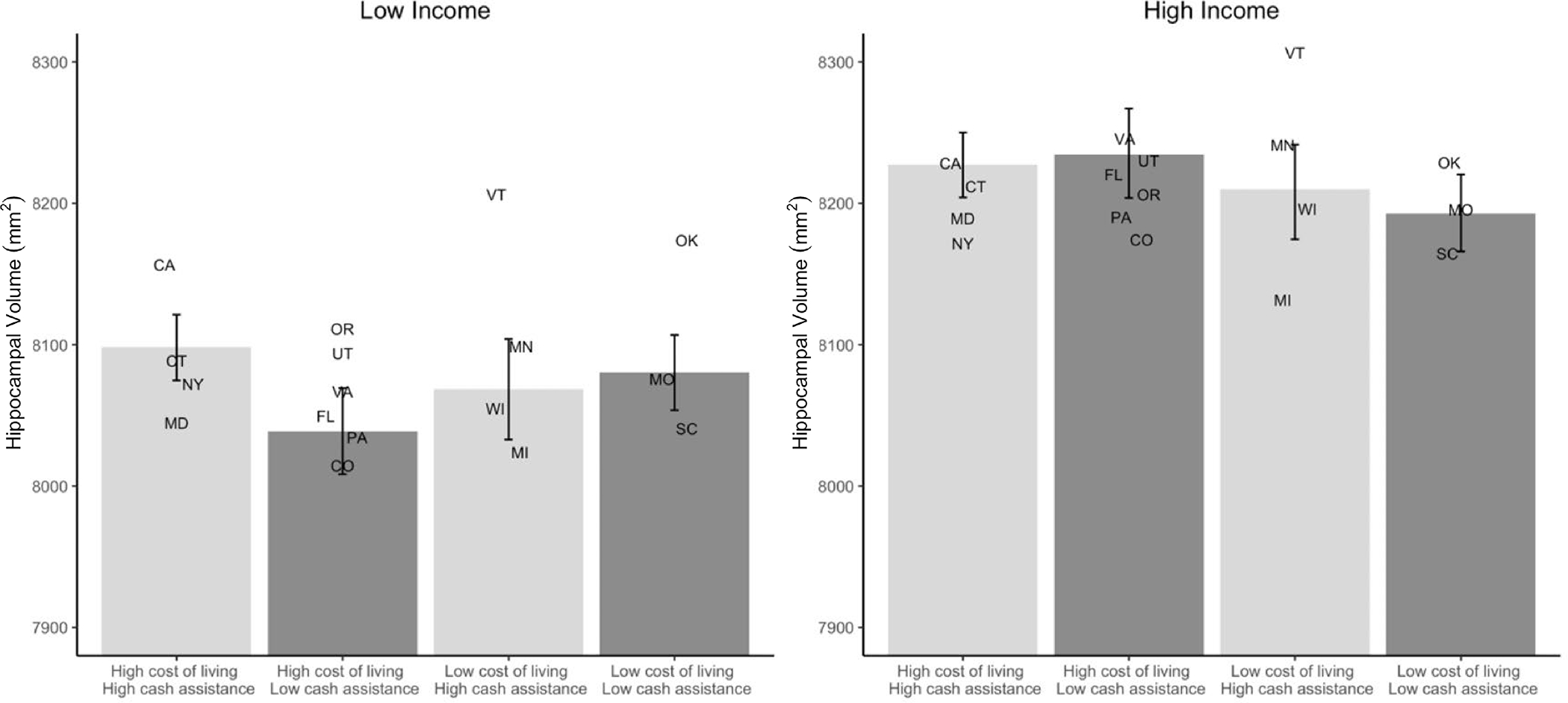
Figure adapted from Weissman et al. (2023).78 3-way interactions between state-level cost of living and generosity of anti-poverty programs and individual family income-to-needs ratio (log-transformed). Cash assistance was based on both monthly Temporary Assistance for Needy Families (TANF) benefits in that state and the average monthly Earned Income Tax Credit (EITC) in that state. Higher cost of living was associated with smaller hippocampal volume among low-income participants, but this was attenuated when states also offered more generous cash benefits. Postal abbreviations for the 17 states in the ABCD study (CA: California; CO: Colorado; CT: Connecticut; FL: Florida; MD: Maryland: MI: Michigan; MN: Minnesota; MO: Missouri; NY: New York; OK: Oklahoma; OR: Oregon; PA: Pennsylvania; SC: South Carolina; UT: Utah; VT: Vermont; VA: Virginia; WI: Wisconsin) are placed along the X-axis in the location corresponding most closely to their cost of living and cash assistance relative to the other states. Hippocampal volume estimates are equivalent to the random intercept of the relation between income and hippocampal volume for that state when family income is 1 SD above (high income) or below (low income) the mean.
Strengths and limitations.
The primary advantage of the multi-site, single-study approach is that it provides variation in exposure to broad social contexts, such as states and countries, that vary on the dimension of interest related to social inequality. In Table 3, we provide details of several multi-site neuroimaging studies that have sufficient variability in social contexts beyond the neighborhood level to examine associations between social inequality and neural outcomes. We also refer interested readers to the Linked External Data source provided by the ABCD study, which includes residential, census, and state-level variables that provide new opportunities for examining how social inequalities relate to neural outcomes.81
Table 3.
Examples of Multi-Site Neuroimaging Studies
| Study Name | Sample Size (N) | Sites |
|---|---|---|
| NIH MRI Study of Normal Brain Development | 505 | Massachusetts, Ohio, Texas, California, Pennsylvania, Missouri |
| Adolescent Brain and Development Study | 11,878 | California, Colorado, Connecticut, Florida, Maryland, Michigan, Minnesota, Missouri, New York, Oklahoma, Oregon, Pennsylvania, South Carolina, Utah, Virginia, Vermont, Wisconsin |
| Human Connectome Project | 1,350 | Massachusetts, California, Minnesota, Missouri |
| Lifespan Human Connectome Project | 1,200 | Massachusetts, California, Minnesota, Missouri |
| Lifebrain Consortium | 5,140 | Spain, Germany, Sweden, Norway, Great Britain, Denmark, Netherlands, Switzerland |
| IMAGEN Study | 2,000 | Great Britain, Ireland, Germany, France |
| UK Biobank (Neuroimaging Subsample) | 46,924 (as of 02/2023) ~100,00 (planned) |
Counties in the United Kingdom |
One limitation of this approach is that some multi-site studies do not provide information about the site where each participant was scanned, precluding the ability to link the dataset to structural measures of social inequality. An additional limitation is that the resources needed to conduct and coordinate large team-based efforts are often prohibitive, which means that researchers must almost always rely on existing multi-site studies, like the ABCD study, where the data have already been collected. Consequently, researchers are constrained by the measures and tasks that were previously collected, which may not always align with the research question and may not include the measures that are needed to improve inferences (e.g., key confounders, plausible alternative explanations, candidate mechanisms). Given these challenges, researchers may need to consider alternative approaches to study whether social inequalities are related to neural outcomes, such as the one we consider next.
Methodological Approach #3: Multi-Site, Multi-Study
Despite the important insights that the methodological approaches reviewed above have produced, they are limited in that single-site studies can only examine variation across neighborhoods, and multi-site studies require massive funding investments and coordination across institutions and researchers. As such, a third approach—a multi-site, multi-study approach known as spatial meta-analysis—circumvents the challenges associated with single- and multi-site single-study designs. This approach retains many aspects of a traditional meta-analysis, with the added step that studies are geo-located, allowing researchers to characterize each included study in terms of the social context in which it was conducted.82 Spatial meta-analyses therefore leverage the contextual variability that naturally exists across neuroimaging studies to examine associations between contextual variables and neural outcomes. This approach allows researchers to utilize data that are already published and generate new insights by linking those results to structural measures of inequality after the fact.
Although meta-analyses of fMRI data are commonplace in cognitive neuroscience, only two recent studies, to our knowledge, have used spatial meta-analyses to examine contextual variation across studies. The first re-analyzed a comprehensive set of studies examining white participants’ neural responses to Black (vs. white) faces within the U.S. to determine whether community-level racial prejudice was associated with the degree of neural activation to Black (vs. white) faces in primarily white participants.83 A substantial body of work in social neuroscience has examined the neural underpinnings of racial prejudice.58 Initial work on this topic centered on the role of threat-related responses in the amygdala to out-group members as a potential neural mechanism underlying racial prejudice.58 Despite decades of research, however, evidence for a stronger amygdala response to racial out-group compared to in-group members has been mixed.58 Hatzenbuehler and colleagues83 examined whether these inconsistencies may be due, in part, to contextual factors typically ignored in cognitive neuroscience, such that observed associations are more (or less) pronounced depending on the structural context in which participants are embedded—specifically, to the varying levels of racial prejudice in these communities. Racial attitudes, obtained from over 10,000 respondents from Project Implicit, were aggregated to the 17 counties in which each study was conducted. Multi-level kernel density analysis demonstrated that significant differences in neural activation to Black (vs. white) faces in two key nodes of the salience network (right amygdala and dorsal anterior cingulate cortex [dACC]) were detected more often in communities with higher (vs. lower) levels of explicit racial prejudice. Sensitivity analyses revealed that this pattern of activation was unrelated to three alternative variables that may serve as common causes or consequences of racial prejudice (i.e., income inequality, community-level racial composition, and community-level education), providing further evidence for specificity of the results to community-level racial prejudice.83
Whereas this spatial meta-analysis measured structural sources of inequality (i.e., area-level prejudice) at the local level (U.S. counties), a second spatial meta-analysis assessed gender inequality at the level of 29 countries, using nation-level data derived from two widely utilized indicators of gender inequality.84,85 The authors then examined associations of gender inequality with sex differences in cortical thickness and surface area in adult men and women. The study found thinner cortices among women (vs. men) in countries with greater gender inequality—especially in regions involved in salience processing (i.e., right caudal anterior cingulate and right medial orbitofrontal) and in left lateral occipital cortex (Figure 3).86 In contrast, there were no sex differences in these regions between men and women in countries with less gender inequality. Analyses remained robust after controlling for other country-level economic characteristics (i.e., per capita gross domestic product).
Figure 3. Spatial Meta-analysis of the Association between Gender Inequality in 29 Countries and Cortical Structure.
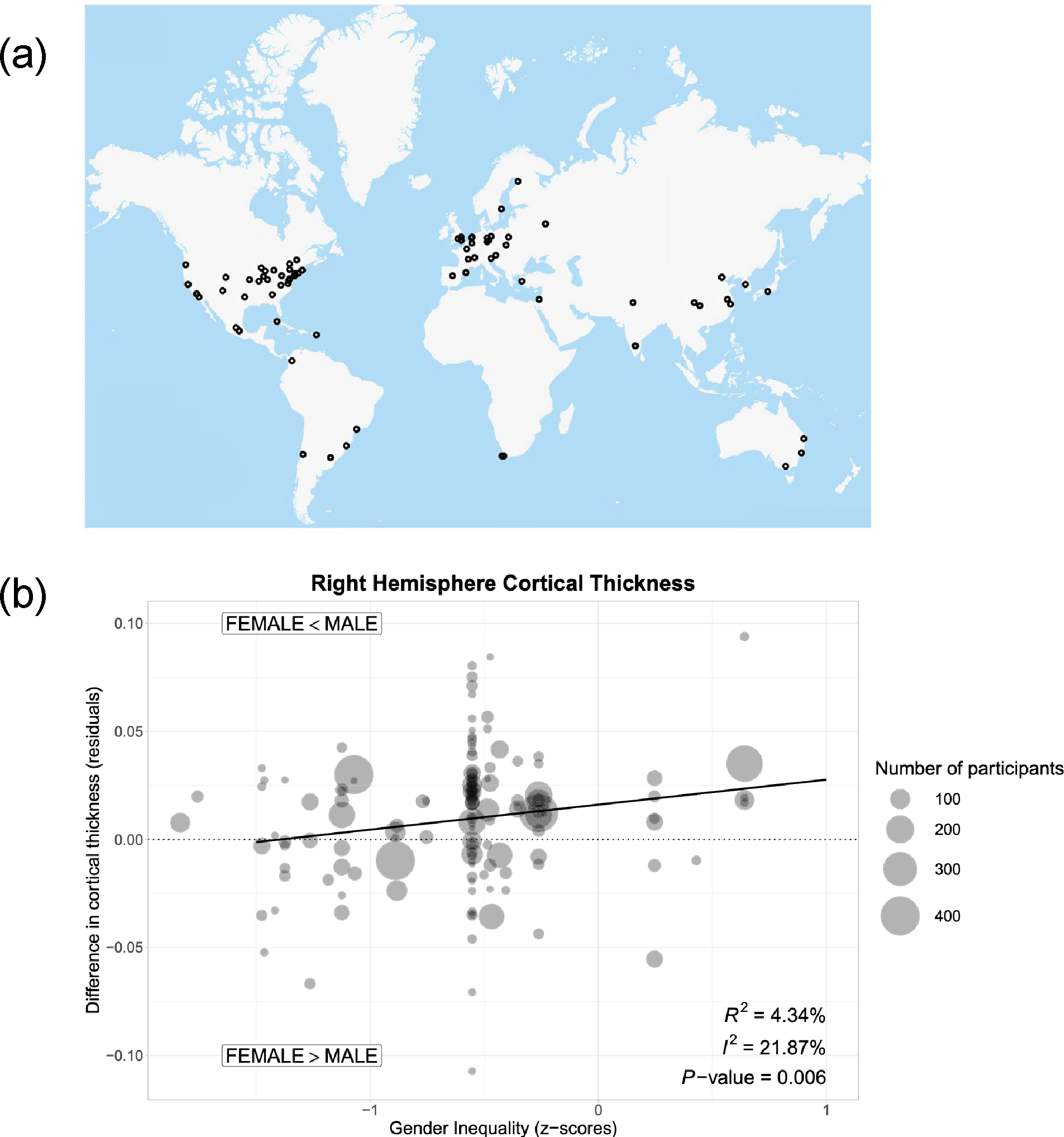
Figure adapted from Zugman et al. (2023).86 (a) The authors identified 139 studies conducted in 29 countries. (b) Using nation-level data from the United Nations and the World Economic Forum, the authors examined the association between gender inequality and sex differences in cortical thickness and surface area. They found that in studies conducted in countries with greater gender inequality, men tended to have greater right hemisphere cortical thickness. Associations between gender inequality and sex differences in cortical thickness in specific regions were also observed.
Collectively, these two sets of findings confirm the feasibility of using spatial meta-analysis to link structural measures of social inequality to neural outcomes, highlight the novel insights it can generate regarding how social inequality relates to brain structure and function, and underscore the utility of this method for reconciling conflicting results in the cognitive neuroscience literature.
Strengths and limitations.
Spatial meta-analysis capitalizes on the substantial heterogeneity in exposure to various forms of social inequality that occur across individual neuroimaging studies. This represents its greatest advantage: the ability to leverage geographic and temporal variation in existing neuroimaging studies to examine relationships between social inequalities and neural outcomes.
At the same time, this approach has limitations. One has to do with data constraints in terms of where studies are conducted, as the social contexts have already been selected based on where the individual studies happened to be conducted. This may not be an issue if these studies are spatially distributed; however, if studies are conducted in a few communities, this could introduce issues related to spatial clustering (e.g., geospatial autocorrelation) or to restricted ranges in the measures of social inequality. A second set of limitations concerns the ability to synthesize fMRI data across multiple labs. These issues include differences in pre-processing, thresholding of whole-brain effects, reporting of parameter estimates, and regions of interest used to extract effects. That said, researchers have developed analytic techniques to overcome these challenges, including in meta-analyses, with notable successes in identifying, for example, the brain bases of emotion and memory.87–93 A third limitation involves the availability of data on the location of the individual studies. Often, this information is not provided, is inexact, or must be inferred based on the institution of the first or senior author. This limitation means that it is often necessary to contact individual researchers to request specific details on study location. One recommendation of our analysis, which others have also called for,82 is to require this type of geographic information to be more systematically reported in reports of neuroimaging studies.
Considerations for Research Linking Social Inequality and Neural Outcomes
In this section, we offer several strategies and considerations to guide programmatic research on the links between social inequality and neural outcomes, and we discuss ethical issues in conducting this work.
Step 1: Identify Form of Social Inequality to be Evaluated
The first step is to identify the form of social inequality that will be the focus of the investigation. We suggest three specific questions to help inform the selection of this variable. First, what theoretical support exists for this factor? Second, what is the empirical evidence for this factor influencing cognitive, affective, and behavioral processes, and are these processes plausibly related to brain structure and function? Third, how strong is this evidence? Has it been established across multiple methods (e.g., observational, quasi-experimental) and measures? In answering these questions, we encourage scholars to consider literatures outside of cognitive neuroscience, given that the topic of social inequality is an inherently interdisciplinary field. For example, scholarship from sociology,35 psychology,36 anthropology,94 and public health2 has revealed that stigma and discrimination are structural causes of population-level inequalities.95 Interdisciplinary collaborations with colleagues from these allied disciplines ensures that cognitive neuroscientists are well-versed in the sources of social inequality that may be most relevant to their question of interest.
Step 2: Identify Appropriate Structural Measures of Social Inequality Across Relevant Levels of Analysis
A second step is to identify reliable and valid measures of the social inequality variable of interest. Structural measures, including social attitudes, have been collected by survey research firms or other agencies (e.g., National Opinion Research Center). However, it is often necessary to apply for restricted access to obtain these measures at certain geographic scales (e.g., states, counties). In other instances, structural measures must be assembled by researchers themselves. In these cases, it is advisable to include collaborators on the research team who possess the necessary expertise in the collection of these data, as in the case of social policies. Scholars have also noted the importance of incorporating the perspectives of communities with lived experience in the development of measures of structural inequality (e.g., structural racism), through methods such as community-based participatory research.96 Doing so ensures that measurement approaches are also ecologically valid.
Another important measurement consideration is the geographic level(s) most relevant for the research question. In the context of social attitudes, it is likely important to obtain them at levels that are most proximate to the respondent (e.g., county).97 In contrast, for other measures, like laws, states or countries may be the most relevant unit of analysis.
Step 3: Identify the Sample
Once researchers have selected the structural measure(s) of social inequality, they must make decisions regarding the study sample(s). Typically, research on the consequences of social inequality is focused on marginalized groups. As other commentators have noted, sample sizes for minoritized individuals are typically quite small in neuroscience research,98 and stratified estimates are frequently not reported for key sociodemographic characteristics (e.g., race).99 To these important points we add that social inequality may influence who is ultimately recruited and retained in research samples, including in neuroscience studies. While such selection factors are often treated as nuisance variables, sociologists have urged scholars to conceptualize selection instead as a social process that is worthy of study in its own right.100
These observations have important implications for identifying the samples in studies that employ the methodological approaches outlined in this paper. For single-site, single-study approaches, in which researchers are typically collecting their own data, a priori power analysis should be used to determine sufficient sample sizes of marginalized groups. For multi-site studies (whether single- or multi-study), cognitive neuroscientists must rely on previously collected data, and thus should be cognizant that selection processes could operate such that marginalized individuals who are most vulnerable to the consequences of social inequality are the least likely to be included in these neuroimaging studies. Critically, this selection bias most likely leads to an underestimate of the association between social inequality and neural outcomes, a point that is important to consider in evaluating findings across studies.
Step 4: Identify Appropriate Research Design
The next step is the identification of the appropriate research design. See Table 2 for a list of questions across each of the three methodological approaches to help guide the selection of study design for a particular research question.
Step 5: Analysis and Addressing Issues of Causal Inference
In many respects, after Steps 1–4 have been completed, the final step in terms of analysis proceeds according to most other research studies. Cognitive neuroscientists are already intimately acquainted with the error of reverse inference in neuroimaging data.101 We highlight two additional issues that deserve particular attention when examining social inequalities as predictor variables. The first is the importance of using mixed-effects models (also known as multi-level models) to appropriately account for clustering, given that individuals will be nested within context. In addition, in multi-site, single study approaches, it is often necessary to include random effects for site.
The second issue concerns causal inference. In experimental studies, individuals are randomly assigned to condition; researchers can therefore be reasonably confident that the independent (manipulated) variable caused the dependent variable (outcome), thereby ruling out alternative explanations. It is neither ethical nor feasible to randomly assign individuals to different social contexts. As such, researchers must rely on observational and quasi-experimental designs, which necessitate the use of different strategies for addressing alternative explanations for the observed association between social inequality and neural outcomes. Here, we briefly highlight two such strategies that have been used in extant studies.
One strategy is addressing alternative explanations through statistical controls. Because other features of the social context co-occur with structural forms of inequality, researchers must examine whether their measure of inequality remains associated with the neural outcome(s) over and above other area-level covariates. For instance, Weissman et al.78 found that state-level policies expanding or restricting the social safety net for low-income families moderated the relationship between family SES and hippocampal volume. In supplementary analyses, they showed that these findings were robust to controls for a wide range of state-level social, economic, and political characteristics (e.g., state preschool enrollment, unemployment). Of course, as with all observational designs, this method cannot rule out the possibility of unmeasured confounding variables, and thus results in such studies can suggest—but not definitively confirm—a causal link.
A second strategy for addressing plausible alternative explanations is the strategic selection of control groups (also known as “negative control analyses”)102 in which researchers examine whether there is an association in a group where it would not be expected to occur. In one example, Hatzenbuehler et al.49 showed that structural forms of stigma (e.g., aggregated social attitudes, social policies) were associated with smaller hippocampal volume among Latinx and Black youth. In contrast, structural stigma was unrelated to hippocampal volume in non-stigmatized youth. This evidence for result specificity supports the hypothesis that results are due to structural stigma itself and not to other macro-social factors associated with it (e.g., area-level SES), which should theoretically affect both stigmatized and non-stigmatized youth in similar ways.
There are many other methodological and analytic strategies for marshaling evidence for causality with observational data—including instrumental variables, regression discontinuity designs, and others. Researchers interested in testing neuroscience models using structural data on social inequality should consider collaborating with scholars from economics, sociology, and social epidemiology who have expertise in these various approaches to causal inference.
Ethical Considerations
There is a long, ignominious history of the (mis)use of scientific data with populations who have borne the brunt of the consequences of social inequality. In light of this history, researchers must be especially attentive to how their study might further contribute to the marginalization of certain social groups—especially in the context of public misunderstandings of neuroscience results, such as biological reductionism.45 Ethical considerations require thoughtful engagement at each step of the research process outlined above—from exploring why researchers are posing their specific questions, to the specific measures they select, to the analytic approaches they employ, to how their results are communicated to the scientific community and broader public. While the harms of historical and contemporary neuroscience practices to marginalized communities have been reviewed recently elsewhere,103 there are potential benefits as well. Indeed, providing evidence that structural sources of inequality predict neural outcomes locates any group difference in brain structure or function within aspects of the broader social context rather than within individuals; such findings may therefore be less likely to be used to perpetuate stereotypes or to justify discrimination. We refer readers to helpful recommendations for how cognitive neuroscience datasets can be used to advance health equity and to minimize harm.104
Recommendations for Future Research
Existing studies that we have reviewed in this paper all use observational data, which cannot establish causality. Future research would therefore benefit from utilizing methods from other fields (e.g., econometrics, sociology, epidemiology) to strengthen causal inferences regarding the relationship between social inequalities and neutral outcomes in order to ensure a more robust evidence base. These methods might include quasi-experimental designs that leverage short-term changes in social inequality (e.g., social policies that differentially target marginalized groups for social exclusion),105 or divergent mobility patterns that naturally occur in longitudinal studies (e.g., movement of respondents to different social contexts, such as moves from higher-to-lower poverty neighborhoods, or higher-to-lower stigmatizing climates). Both types of designs have been effectively used to study biopsychosocial consequences of social inequality, and thus hold promise for cognitive neuroscience (see reviews in the area of stigma and prejudice by Hatzenbuehler;36,106 for an example of mobility studies in economics, see Chetty et al.107).
Several research questions also remain unanswered regarding whether, how, and for whom social inequalities are related to neural outcomes. For instance, our paper examined structural measures of social inequality that have received the most empirical attention in the cognitive neuroscience literature—including structural stigma, community-level prejudice, gender inequality, neighborhood disadvantage, and the generosity of the social safety net for low-income families. Future studies are needed to examine linkages between additional forms of social inequality and neural outcomes, employing the methods that we have outlined in this paper. Examples might include air pollution108 and access to green spaces,109 both of which are socially patterned.108 This research will provide important information regarding potential boundary conditions of the consequences of social inequality for brain structure and function.
In addition, existing studies have focused on direct associations of social inequalities with measures of neural structure and function. Less attention has been paid to identifying the factors that may influence the direction and magnitude of these relationships (i.e., moderators). The identification of moderators at multiple levels of influence—material resources, social, psychological, biological—therefore represents an important area of inquiry. Additional questions for future inquiry include the following: Are the associations between social inequalities and neural outcomes similar across different geographic units of analysis—e.g., city and state—or are these associations stronger at more proximal levels? Do these different units interact to explain variation in neural structure and function, as has been found for various psychological phenomenon, such as identity concealment?43 Are associations between social inequalities and neural outcomes sensitive to particular developmental periods?
Conclusions
We present a call to action for the field of cognitive neuroscience to begin to grapple with the role that social inequality may play in shaping neural outcomes and highlight emerging findings suggesting that structural approaches may yield new insights into whether and how various dimensions of social inequality relate to neural structure and function. We present three methodological approaches that have recently been utilized to study associations between structural measures of social inequalities and neural outcomes. We hope our paper invigorates new research in cognitive neuroscience that explicitly incorporates upstream contextual factors, which holds potential promise for contributing to public discourse on some of the most meaningful social, health, and policy-related questions of our time.
Footnotes
Competing Interests Statement: The authors declare no competing interests.
References
- 1.Pickett KE, James OW & Wilkinson RG Income inequality and the prevalence of mental illness: A preliminary international analysis. J Epidemiol Community Health (1978) 60, (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Williams DR & Collins C Racial residential segregation: A fundamental cause of racial disparities in health. Public Health Reports 116, (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mehra R, Boyd LM & Ickovics JR Racial residential segregation and adverse birth outcomes: A systematic review and meta-analysis. Soc Sci Med 191, (2017). [DOI] [PubMed] [Google Scholar]
- 4.McCoy DC, Raver CC & Sharkey P Children’s Cognitive Performance and Selective Attention Following Recent Community Violence. J Health Soc Behav 56, (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sharkey P, Schwartz AE, Ellen IG & Lacoe J High stakes in the classroom, high stakes on the street: The effects of community violence on students’ standardized test performance. Sociol Sci 1, (2014). [Google Scholar]
- 6.Evans-Lacko S, Brohan E, Mojtabai R & Thornicroft G Association between public views of mental illness and self-stigma among individuals with mental illness in 14 European countries. Psychol Med 42, 1741–1752 (2012). [DOI] [PubMed] [Google Scholar]
- 7.Chae DH et al. Association between an Internet-Based Measure of Area Racism and Black Mortality. PLoS One 10, e0122963- (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chae DH et al. Area racism and birth outcomes among Blacks in the United States. Soc Sci Med 199, 49–55 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miller CT, Grover KW, Bunn JY & Solomon SE Community norms about suppression of AIDS-related prejudice and perceptions of stigma by people with HIV or AIDS. Psychol Sci 22, 579–583 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miller CT, Varni SE, Solomon SE, DeSarno MJ & Bunn JY Macro-level implicit HIV prejudice and the health of community residents with HIV. Health Psychology 35, 807–815 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Perales F & Todd A Structural stigma and the health and wellbeing of Australian LGB populations: Exploiting geographic variation in the results of the 2017 same-sex marriage plebiscite. Soc Sci Med 208, 190–199 (2018). [DOI] [PubMed] [Google Scholar]
- 12.Hatzenbuehler ML, Flores AR & Gates GJ Social attitudes regarding same-sex marriage and LGBT health disparities: Results from a national probability sample. J Soc Issues 73, 508–528 (2017). [Google Scholar]
- 13.Krieger N et al. Structural racism, historical redlining, and risk of preterm birth in New York City, 2013–2017. Am J Public Health (2020) doi: 10.2105/AJPH.2020.305656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huo YJ, Dovidio JF, Jiménez TR & Schildkraut DJ Local policy proposals can bridge Latino and (most) white Americans’ response to immigration. Proc Natl Acad Sci U S A 115, 945–950 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hatzenbuehler ML et al. Immigration policies and mental health morbidity among Latinos: A state-level analysis. Soc Sci Med 174, 169–178 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Samari G, Catalano R, Alcalá HE & Gemmill A The Muslim Ban and preterm birth: Analysis of US Vital statistics data from 2009 to 2018. Soc Sci Med 265, (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hatzenbuehler ML, Keyes KM & Hasin DS State-level policies and psychiatric morbidity in lesbian, gay, and bisexual populations. Am J Public Health 99, 2275–2281 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hatzenbuehler ML, McLaughlin KA, Keyes KM & Hasin DS The impact of institutional discrimination on psychiatric disorders in lesbian, gay, and bisexual populations: A prospective study. Am J Public Health 100, 452–459 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hatzenbuehler ML et al. Effect of same-sex marriage laws on health care use and expenditures in sexual minority men: a quasi-natural experiment. Am J Public Health 102, 285–291 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Raifman J, Moscoe E, Austin SB & McConnell M Difference-in-Differences Analysis of the Association Between State Same-Sex Marriage Policies and Adolescent Suicide Attempts. JAMA Pediatr 171, 350–356 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Raifman J, Moscoe E, Austin B, Hatzenhuehler ML & Galea S Association of State Laws Permitting Denial of Services to Same-Sex Couples With Mental Distress in Sexual Minority Adults A Difference-in-Difference-in-Differences Analysis. JAMA Psych 75, 671–677 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Levy BL & Levy DL When love meets hate: The relationship between state policies on gay and lesbian rights and hate crime incidence. Soc Sci Res 61, 142–159 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Everett BG, Hatzenbuehler ML & Hughes TL The impact of civil union legislation on minority stress, depression, and hazardous drinking in a diverse sample of sexual-minority women: A quasi-natural experiment. Soc Sci Med 169, 180–190 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Blosnich JR et al. Religious freedom restoration acts and sexual minority population health in the United States. Am J Orthopsychiatry (2018) doi: 10.1037/ort0000349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Leitner JB, Hehman E, Ayduk O & Mendoza-Denton, Racial bias is associated with ingroup death rate for Blacks and whites: Insights from Project Implicit. Soc Sci Med 170, 220–227 (2016). [DOI] [PubMed] [Google Scholar]
- 26.Morey BN, Gee GC, Muennig P & Hatzenbuehler ML Community-level prejudice and mortality among immigrant groups. Soc Sci Med 199, 56–66 (2018). [DOI] [PubMed] [Google Scholar]
- 27.Sharkey P The acute effect of local homicides on children’s cognitive performance. Proc Natl Acad Sci U S A 107, (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Patel V et al. Income inequality and depression: a systematic review and meta-analysis of the association and a scoping review of mechanisms. World Psychiatry 17, (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Leitner JB, Hehman E, Ayduk O & Mendoza-Denton R Blacks’ death rate due to circulatory diseases is positively related to whites’ explicit racial bias: A nationwide investigation using project implicit. Psychol Sci 27, 1299–1311 (2016). [DOI] [PubMed] [Google Scholar]
- 30.Lukachko A, Hatzenbuehler ML & Keyes KM Structural racism and myocardial infarction in the United States. Soc Sci Med 103, 42–50 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pearce N Epidemiology in a changing world: Variation, causation and ubiquitous risk factors. Int J Epidemiol 40, (2011). [DOI] [PubMed] [Google Scholar]
- 32.Paus T Population neuroscience: Why and how. Hum Brain Mapp 31, (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Falk EB et al. What is a representative brain? Neuroscience meets population science. Proc Natl Acad Sci U S A 110, (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Suter C Social inequalities. Encyclopedia of quality of life and well-being research 1–6 (2020). [Google Scholar]
- 35.Link BG & Phelan JC Conceptualizing stigma. Annu Rev Sociol 27, 363–385 (2001). [Google Scholar]
- 36.Hatzenbuehler ML Structural stigma: Research evidence and implications for psychological science. Am Psychologist 71, 742–751 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Levy B Stereotype embodiment: A psychosocial approach to aging. Curr Dir Psychol Sci 18, (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pachankis JE The psychological implications of concealing a stigma: A cognitive-affective-behavioral model. Psychol Bull 133, 328–345 (2007). [DOI] [PubMed] [Google Scholar]
- 39.Pager D The Mark of a Criminal Record. Am J Sociology 108, 937–975 (2003). [Google Scholar]
- 40.Galobardes B, Lynch J & Smith GD Measuring socioeconomic position in health research. Br Med Bull 81–82, (2007). [DOI] [PubMed] [Google Scholar]
- 41.Krieger N, Williams DR, & Moss NE Measuring social class in US public health research: Concepts, methodologies and guidelines. Am J Public Health 18, 341–378 (1997). [DOI] [PubMed] [Google Scholar]
- 42.Rehkopf DH et al. Monitoring socioeconomic disparities in death: Comparing individual-level education and area-based socioeconomic measures. Am J Public Health 96, 2135–2138 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lattanner MR et al. A Contextual Approach to the Psychological Study of Identity Concealment: Examining Direct, Interactive, and Indirect Effects of Structural Stigma on Concealment Motivation Across Proximal and Distal Geographic Levels. Psychol Sci 32, 1684–1696 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tilcsik A Pride and prejudice: Employment discrimination against openly gay men in the United States. Am J Sociology 117, 586–626 (2011). [DOI] [PubMed] [Google Scholar]
- 45.Hastings PD, Guyer AE & Parra LA Conceptualizing the Influence of Social and Structural Determinants of Neurobiology and Mental Health: Why and How Biological Psychiatry Can Do Better at Addressing the Consequences of Inequity. Biol Psychiatry Cogn Neurosci Neuroimaging 7, (2022). [DOI] [PubMed] [Google Scholar]
- 46.Lewinn KZ, Sheridan MA, Keyes KM, Hamilton A & McLaughlin KA Sample composition alters associations between age and brain structure. Nat Commun 8, (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hyde LW et al. An ecological approach to understanding the developing brain: Examples linking poverty, parenting, neighborhoods, and the brain. Am Psychologist 75, (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gard AM et al. Beyond family-level adversities: Exploring the developmental timing of neighborhood disadvantage effects on the brain. Dev Sci 24, (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hatzenbuehler ML et al. Smaller hippocampal volume among black and latinx youth living in high-stigma contexts. J Am Acad Child Adolesc Psychiatry 61, 809–819 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Walhovd KB et al. Education and Income Show Heterogeneous Relationships to Lifespan Brain and Cognitive Differences Across European and US Cohorts. Cerebral Cortex 32, (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Adler NE & Newman K Socioeconomic disparities in health: Pathways and policies. Health Aff 21, (2002). [DOI] [PubMed] [Google Scholar]
- 52.Adler NE & Rehkopf DH. U.S. disparities in health: Descriptions, causes, and mechanisms. in Ann Rev Pub Health vol. 29 (2008). [DOI] [PubMed] [Google Scholar]
- 53.Schmader T & Johns M Converging Evidence that Stereotype Threat Reduces Working Memory Capacity. J Pers Soc Psychol 85, (2003). [DOI] [PubMed] [Google Scholar]
- 54.Macintyre S, Maciver S & Sooman A Area, class and health: should we be focusing on places or people? J Soc Policy 22, (1993). [Google Scholar]
- 55.Whiteis DG Hospital and community characteristics in closures of urban hospitals, 1980–87. Public Health Reports 107, (1992). [PMC free article] [PubMed] [Google Scholar]
- 56.Eisenberger NI, Lieberman MD & Williams KD Does rejection hurt? An fMRI study of social exclusion. Science (1979) 302, (2003). [DOI] [PubMed] [Google Scholar]
- 57.McLaughlin KA, Weissman D & Bitrán D Childhood Adversity and Neural Development: A Systematic Review. Annu Rev Dev Psychol 1, (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Amodio DM & Cikara M The Social Neuroscience of Prejudice. Annu Rev Psychol 72, (2021). [DOI] [PubMed] [Google Scholar]
- 59.Hein TC & Monk CS Research Review: Neural response to threat in children, adolescents, and adults after child maltreatment – a quantitative meta-analysis. J Child Psychol Psychiatry 58, (2017). [DOI] [PubMed] [Google Scholar]
- 60.Noble KG et al. Family income, parental education and brain structure in children and adolescents. Nat Neurosci 18, (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Luby J et al. The effects of poverty on childhood brain development: The mediating effect of caregiving and stressful life events. JAMA Pediatr 167, (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gilmore RO, Diaz MT, Wyble BA & Yarkoni T Progress toward openness, transparency, and reproducibility in cognitive neuroscience. Ann N Y Acad Sci 1396, (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Klapwijk ET, van den Bos W, Tamnes CK, Raschle NM & Mills KL Opportunities for increased reproducibility and replicability of developmental neuroimaging. Dev Cogn Neurosci 47, (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Poldrack RA et al. Scanning the horizon: Towards transparent and reproducible neuroimaging research. Nat Rev Neurosci 18, (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Van Bavel JJ, Mende-Siedlecki P, Brady WJ & Reinero DA Contextual sensitivity in scientific reproducibility. Proc Natl Acad Sci U S A 113, (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Casey BJ, Taylor-Thompson K, Rubien-Thomas E, Robbins M & Baskin-Sommers A Healthy development as a human right: Insights from developmental neuroscience for youth justice. Annu Rev Law Soc Sci 16, (2020). [Google Scholar]
- 67.Tomlinson RC et al. Neighborhood poverty predicts altered neural and behavioral response inhibition. Neuroimage 209, (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Tooley UA et al. Associations between Neighborhood SES and Functional Brain Network Development. Cerebral Cortex 30, (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Krishnadas R et al. Socioeconomic deprivation and cortical morphology: Psychological, social, and biological determinants of ill health study. Psychosom Med 75, (2013). [DOI] [PubMed] [Google Scholar]
- 70.Ramphal B et al. Brain connectivity and socioeconomic status at birth and externalizing symptoms at age 2 years. Dev Cogn Neurosci 45, (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Murtha K et al. Associations between neighborhood socioeconomic status, parental education, and executive system activation in youth. Cerebral Cortex 33, (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hannan EL, Wu Y, Cozzens K & Anderson B The Neighborhood Atlas Area Deprivation Index For Measuring Socioeconomic Status: An Overemphasis On Home Value. Health Aff 42, (2023). [DOI] [PubMed] [Google Scholar]
- 73.Trinidad S et al. Use Of Area-Based Socioeconomic Deprivation Indices: A Scoping Review And Qualitative Analysis. Health Aff 41, (2022). [DOI] [PubMed] [Google Scholar]
- 74.Leventhal T & Brooks-Gunn J The Neighborhoods They Live in: The Effects of Neighborhood Residence on Child and Adolescent Outcomes. Psychol Bull 126, (2000). [DOI] [PubMed] [Google Scholar]
- 75.Jenkins LM et al. Subcortical structural variations associated with low socioeconomic status in adolescents. Hum Brain Mapp 41, (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Al Hazzouri AZ et al. Racial Residential Segregation in Young Adulthood and Brain Integrity in Middle Age: Can We Learn From Small Samples? Am J Epidemiol 191, (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Harnett NG et al. Structural inequities contribute to racial/ethnic differences in neurophysiological tone, but not threat reactivity, after trauma exposure. Mol Psychiatry (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Weissman DG, Hatzenbuehler ML, Cikara M, Barch DM & McLaughlin KA State-level macro-economic factors moderate the association of low income with brain structure and mental health in U.S. children. Nat Commun 14 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hanson JL, Chandra A, Wolfe BL & Pollak SD Association between income and the hippocampus. PLoS One 6, (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Decker AL, Duncan K, Finn AS & Mabbott DJ Children’s family income is associated with cognitive function and volume of anterior not posterior hippocampus. Nat Commun 11, (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Fan CC et al. Adolescent Brain Cognitive Development (ABCD) study Linked External Data (LED): Protocol and practices for geocoding and assignment of environmental data. Dev Cogn Neurosci 52, (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Johnson BT, Cromley EK & Marrouch N Spatiotemporal meta-analysis: reviewing health psychology phenomena over space and time. Health Psychol Rev 11, (2017). [DOI] [PubMed] [Google Scholar]
- 83.Hatzenbuehler ML, Mclaughlin KA, Weissman DG & Cikara M Community-level explicit racial prejudice potentiates whites’ neural responses to black faces: A spatial meta-analysis. Soc Neurosci 17, (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gaye A, Klugman J, Kovacevic M, Twigg S & Zambrano E Measuring Key Disparities in Human Development: the Gender Inequality Index. Human Development Research Paper vol. 46 (2010). [Google Scholar]
- 85.Crotti R, Pal KK, Ratcheva V & Zahidi S The global gender gap report 2021. World Economic Forum (2021). [Google Scholar]
- 86.Zugman A et al. Country-level gender inequality is associated with structural differences in the brains of women and men. Proc Natl Acad Sci U S A 120, (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kober H & Wager TD Meta-analysis of neuroimaging data. Wiley Interdiscip Rev Cogn Sci 1, (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lindquist KA, Satpute AB, Wager TD, Weber J & Barrett LF The Brain Basis of Positive and Negative Affect: Evidence from a Meta-Analysis of the Human Neuroimaging Literature. Cerebral Cortex 26, (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Murty VP, Ritchey M, Adcock RA & LaBar KS Reprint of: fMRI studies of successful emotional memory encoding: A quantitative meta-analysis. Neuropsychologia 49, (2011). [DOI] [PubMed] [Google Scholar]
- 90.Turner BO, Paul EJ, Miller MB & Barbey AK Small sample sizes reduce the replicability of task-based fMRI studies. Commun Biol 1, (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wager TD, Lindquist M & Kaplan L Meta-analysis of functional neuroimaging data: Current and future directions. Soc Cogn Affect Neurosci 2, (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lindquist KA, Wager TD, Kober H, Bliss-Moreau E & Barrett LF The brain basis of emotion: A meta-analytic review. Behavioral and Brain Sciences 35, (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Cremers HR, Wager TD & Yarkoni T The relation between statistical power and inference in fMRI. PLoS One 12, (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Parker R & Aggleton P HIV and AIDS-related stigma and discrimination: A conceptual framework and implications for action. Soc Sci Med 57, 13–24 (2003). [DOI] [PubMed] [Google Scholar]
- 95.Hatzenbuehler ML, Phelan JC & Link BG Stigma as a fundamental cause of population health inequalities. Am J Public Health 103, 813–821 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hardeman RR, Homan PA, Chantarat T, Davis BA & Brown TH Improving The Measurement Of Structural Racism To Achieve Antiracist Health Policy. Health Aff 41, (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Cikara M, Fouka V & Tabellini M Hate crime towards minoritized groups increases as they increase in sized-based rank. Nat Hum Behav 6, (2022). [DOI] [PubMed] [Google Scholar]
- 98.Garcini LM et al. Increasing diversity in developmental cognitive neuroscience: A roadmap for increasing representation in pediatric neuroimaging research. Dev Cogn Neurosci 58, (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Keyes KM et al. What Is Not Measured Cannot Be Counted: Sample Characteristics Reported in Studies of Hippocampal Volume and Depression in Neuroimaging Studies. Biol Psychiatry Cogn Neurosci Neuroimaging 8, (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Sampson RJ Moving to inequality: Neighborhood effects and experiments meet social structure. Am J Sociology 114, (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Poldrack RA Inferring mental states from neuroimaging data: From reverse inference to large-scale decoding. Neuron 72, (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lipsitch M, Tchetgen Tchetgen E & Cohen T Negative Controls: A tool for detecting confounding and bias in observational studies. Epidemiology 21, 383–388 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Ricard JA et al. Confronting racially exclusionary practices in the acquisition and analyses of neuroimaging data. Nat Neurosci 26, 4–11 (2023). [DOI] [PubMed] [Google Scholar]
- 104.White EJ et al. Five recommendations for using large-scale publicly available data to advance health among American Indian peoples: the Adolescent Brain and Cognitive Development (ABCD) StudySM as an illustrative case. Neuropsychopharmacology 48, (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Cook TD, Campbell DT & Shadish W Experimental and quasi-experimental designs for generalized causal inference. vol. 1195 (Houghton Mifflin; Boston, MA, 2002). [Google Scholar]
- 106.Hatzenbuehler ML Advancing Research on Structural Stigma and Sexual Orientation Disparities in Mental Health Among Youth. J Clin Child Adol Psychology 46, 463–475 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Chetty R, Hendren N The impacts of neighborhoods on intergenerational mobility I: Childhood exposure effects. Q J Econ 133, 1107–1162 (2017). [Google Scholar]
- 108.Sukumaran K et al. Ambient fine particulate exposure and subcortical gray matter microarchitecture in 9- and 10-year-old children across the United States. iScience 26, (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Berman MG, Stier AJ & Akcelik GN Environmental neuroscience. Am Psychologist 74, (2019). [DOI] [PubMed] [Google Scholar]
- 110.Williams DR, Yu Y, Jackson JS & Anderson NB Racial differences in physical and mental health: Socio-economic status, stress and discrimination. J Health Psychol 2, 335–351 (1997). [DOI] [PubMed] [Google Scholar]
- 111.Frost DM et al. Couple-level Minority Stress: An Examination of Same-sex Couples’ Unique Experiences. J Health Soc Behav 58, 455–472 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Hewstone M, Rubin M & Willis H Intergroup bias. Annu Rev Psychol 53, 575–604 (2002). [DOI] [PubMed] [Google Scholar]
- 113.Reid AE, Dovidio JF, Ballester E & Johnson BT HIV prevention interventions to reduce sexual risk for African Americans: The influence of community-level stigma and psychological processes. Soc Sci Med 103, 118–125 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Payne BK, Vuletich HA & Brown-Iannuzzi JL Historical roots of implicit bias in slavery. Proc Natl Acad Sci U S A 116, (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Hatzenbuehler ML et al. Trends in State Policy Support for Sexual Minorities and HIV-Related Outcomes Among Men Who Have Sex With Men in the United States, 2008–2014. J Acquir Immune Defic Syndr (1988) 85, 39–45 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Krieger N, Chen JT, Coull B, Waterman PD & Beckfield J The unique impact of abolition of Jim Crow laws on reducing inequities in infant death rates and implications for choice of comparison groups in analyzing societal determinants of health. Am J Public Health 103, 2234–2244 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Burris S et al. Making the case for laws that improve health: A framework for public health law research. Milbank Quarterly 88, (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Flores AR, Hatzenbuehler ML & Gates GJ Identifying psychological responses of stigmatized groups to referendums. Proc Natl Acad Sci U S A 115, 3816–3821 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]


