Abstract
Autoimmune disorders include vast and distinct illnesses and are characterized by an immune system-mediated attack on the body’s own tissues. Because of their ability to impact any portion of the body, their clinical symptoms are incredibly varied. The variations in symptoms are normally linked with the release and activation of vasoactive, chemotactic substances and cytokines. Cytokines perform a multitude of vital biological tasks, such as immune response control, inflammation, proliferation, and tissue repair. The reversal of inflammatory cytokines and leukocyte infiltration into the inflamed tissue by natural compounds provides an effective remedy for autoimmune diseases. Here, the oral administration of trans-chalcone (TC) for 28 days was tested with gradually increasing doses (30, 60, and 120 mg/kg) in complete Freund’s adjuvant (CFA)-provoked joint tissue stiffness in rats. Paw edema, arthritic index, joint stiffness, thermal and flexion pain, C-reactive protein, and rheumatoid factor (RF) levels were determined to check the tested drug effectiveness in a chronic inflammatory model. Molecular docking studies revealed strong binding affinity with inflammatory cytokines and mediators such as TNF-α, IL-17, COX-2, and iNOS; further, they were quantified at the mRNA level by RT-PCR and ELISA analysis. Oral administration of TC significantly ameliorated paw edema, thymus and spleen indices, joint stiffness, thermal and flexion pain, C-reactive protein, RF, mobility, and stance of the treated animals. This therapeutic effectiveness was linked with a reduction in the mRNA expression of proinflammatory cytokines such as IL-1β, IL-6, and IL-17. The findings of the reported research confirmed the effectiveness of TC in ameliorating joint stiffness and flexion pain by prominently lowering the inflammatory cytokines.
1. Introduction
Inflammation plays a major pathological role in a broad range of diseases in both humans and animals. Prolonged or untreated inflammatory reactions are the cause of a number of pathological problems that lead to a variety of illnesses, including diabetes, atherosclerosis, rheumatoid arthritis, and several other fatal conditions.1 The autoimmune inflammatory illness known as rheumatoid arthritis (RA) mainly affects the joints and is characterized by severe joint destruction, considerable bone and cartilage deterioration, proliferative synovitis and synovial inflammation, and restricted functioning.2 One crucial step in the pathology of rheumatoid arthritis is the recruitment of leukocytes into the connective tissue and joint area.3 The invasion of several inflammatory cells into the affected and inflamed area releases proinflammatory cytokines TNF-α, IL-1β, IL-6, and interleukin-17.4 Many other inflammatory mediators, including proteases, cyclooxygenases, phospholipases, prostaglandins, reactive oxygen species (ROS), nitric oxide (NO), and leukotrienes are crucial in the destruction of bone and inflammation of the synovial membrane during the progression of rheumatoid arthritis.2
Trans-chalcone (1–3-diphenyl-2-propen-1-one) is a phytochemical having a molecular weight of 208.26 g/moL and is physically present as a yellow powder. It is soluble in chloroform, ether, benzene, and ethanol (slightly). Plants including Aniba riparia, Piper methysticum, and Didymocarpus corchorijolia can produce trans-chalcone.5Trans-chalcone, an open-chain flavonoid, has antifibrotic, antioxidant, antidiabetic, hepatoprotective, and anti-inflammatory impacts.6,7 It targets the expression of TGF-β and ICAM-1, as well as STAT3 and NF-κB modulation, to prevent ischemia-induced retinal neovascularization.8Trans-chalcone reduces hepatic fibrosis and inflammation driven by acetaminophen and carbon tetrachloride by lowering the generation of nitric oxide, TGF-β, and TNF-α, lipid peroxidation, and diminished glutathione deprivation.9 In a research on high-fat-diet-induced pulmonary inflammation, trans-chalcone actively lowered the expressions of IL-6, TNF-α, and IL-1β.10 In an acute gout arthritis model, trans-chalcone decreased pain and inflammation by inhibiting IL-6, TNF-α, IL-1β, and NLRP3 inflammasome activation and reducing inflammatory cell recruitment and infiltration.11 Considering the above-mentioned facts, the objective of this study was to investigate the antirheumatoid activity of trans-chalcone in chronic rodent models of joint inflammation along with its effect on inflammatory mediators.
2. Materials and Methods
2.1. Chemicals, Reagents, and Kits
Trans-Chalcone (Sigma-Aldrich), diclofenac sodium, complete Freund’s adjuvant, formalin, DMSO, chloroform, isopropanol, and ethanol were from Sigma-Aldrich, besides TRI-Reagent RT (BioShop Canada), WizScript cDNA Synthesis Kit (High Capacity) (Wizbiosolutions), GI I EvaGreen qPCR Master Mix (GeneDirex), Rat PGE2 ELISA Kit (Wuhan Zokeyo Biotechnology Co., Ltd.), and nitric oxide (NO) assay kit (Solarbio Life Sciences).
2.2. Animals and Housing Conditions
Sprague–Dawley rats (220 ± 30 g in weight) were utilized in this research work. Rats were retained in plastic cages in a 12-h light/dark cycle environment with a humidity level of 55 ± 5% and a room temperature of 25 ± 2 °C. Rodents were provided with sufficient water and food. Before the experimentation, rats underwent a week-long acclimatization phase. Approvals of standards featuring animals were obtained from the Biosafety and Ethical Review Committee, University of Sargodha, Pakistan, having approval No. SU/ORIC/2860.
2.3. Animal Grouping and Disease Induction by CFA
A complete Freund’s adjuvant (CFA)-prompted arthritis model was adopted to explore the antiarthritis impact of trans-chalcone. Rats were separated into different groups comprising six animals in each group.
Group I and II: Normal control (NC) and disease control (DC) rats were gavaged with distilled water (10 mL/kg).
Group III–V rats: Rats were administered an oral dose of trans-chalcone (TC) (30, 60, and 120 mg/kg accordingly).
Group VI: Rats were given diclofenac 5 mg/kg orally.
All treatments were given to the respective groups for a period of 28 days. Complete Freund’s adjuvant (CFA) was employed for arthritis development in rats according to a previously published study.12 On day 0, baseline parameters were measured, and then arthritis was instigated 30 min after oral dosing on day 1 by intradermal inoculation of 0.1 mL of CFA into the subplanter area on the left rear paw in all animals except normal control rats, to which 0.1 mL of saline was administered.
2.4. Estimation of Inflammation Induction Parameters
The body weight was determined by a digital weight balance, the paw volume was assessed by using a digital plethysmometer, and the arthritic index was calculated by visual scoring as described in ref (13) on days 0, 3, 7, 14, 21, and 28.
2.5. Antinociceptive Effect Analysis
The antinociceptive effect of trans-chalcone was assessed by a tail immersion test in CFA-provoked arthritic rats on days 0, 3, 7, 14, 21, and 28, as described in ref (14), with slight modifications. Heated water maintained at 55 ± 2 °C was poured on the distal portion of the tail. The time (in seconds), recorded with a stopwatch until the rat flicks or withdraws its tail from hot water, is reported as latency. The maximum time was considered as the cutoff time to avoid any thermal damage to the tail.
2.6. Mobility Assessment Parameters
The joint stiffness, mobility score, flexion, and stance score were computed on days 14 and 28 by observing and scoring each animal.15,16
2.7. Blood Cell Count and LFT Analysis
On day 29, rats were anesthetized with pentobarbitone (6 mg/100g), and blood samples were obtained by heart puncture. Hemoglobin, ESR, and blood cells such as WBCs, RBC, and platelets were determined from the whole blood, and ALT, AST, ALP, creatinine, urea, CRP, and Rf factor were estimated from the blood serum.
Thymus & spleen were dissected out and weighed for determination of thymus and spleen indices by dividing respective organ weight in mg to total body weight in grams.
2.8. RNA Extraction from Blood Samples
The total RNA was obtained using TRI-Reagent RT (BioShop Canda) from rat blood. Briefly, 600 μL of TRI-Reagent RT was taken in an Eppendorf tube; 200 μL of blood was added to it and thoroughly mixed with a vortex mixer, and then samples were incubated for 5 min at ambient temperature. After that, 200 μL of chilled chloroform was dissolved and vigorously blended using a vortex mixer. The mixture was centrifuged for 15 min at 12,000 rpm at 4 C after being incubated for 2 min at room temperature. Three distinct layers were formed: (1) a red lower phenol–chloroform phase, (2) an interface, and (3) a colorless upper aqueous phase. The aqueous phase comprising RNA was separated gently into a fresh Eppendorf tube by not disturbing the interface to avoid any DNA contamination. The same volume of isopropanol was vigorously mixed with the aqueous phase later by incubating for 10 min at ambient temperature to precipitate RNA. The resultant mixture was spun for 10 min at 12,000 rpm, and the supernatant was worn out very gently. Next, the RNA was washed by dissolving in 1 mL of 75% ethanol and centrifuged at 7500 rpm for 5 min at 4 C. Later on, gentle removal of ethanol was carried out, and RNA pellets were air-dried. Finally, 20 μL of RNase-free water was used to dissolve the RNA pellets. The extracted RNA was quantified by Nanodrop and further used in the synthesis of cDNA.
2.9. Complementary DNA (cDNA) Synthesis and Real-Time Quantitative Polymerase Chain Reaction (qPCR)
Complementary DNA was synthesized using the reverse transcription method with the WizScript cDNA Synthesis Kit (Wizbiosolutions). Briefly, 10 μL of 2× RT master mix was prepared in microtubes by adding a random hexamer (2 μL), 10× reaction buffer (2 μL), WizScript RTase (1 μL), 20× dNTP mix (1 μL), RNase inhibitor (0.5 μL), and RNase-free water (3.5 μL) on ice. After that, 500 ng of total RNA was incorporated with the 2× RT master mix. A final volume of 20 μL was made up with RNase-free water, and the microtubes were centrifuged to remove any air bubbles. The microtubes were then put in a thermal cycler set at the following settings: the first step of 10 min at 25 °C, the second step of 120 min at 37 °C, the third step of 5 min at 85 °C, and the fourth step of infinite time at 4 °C. The prepared cDNA was preserved at −20 °C, and qPCR was performed to study the gene expression using GI I EvaGreen qPCR Master Mix (GeneDirex). Briefly, 10 μL of the master mix was mixed with 0.5 μL each of the forward and reverse primers, 1 μL of cDNA, and 8 μL of DNase-free water and put into an iQ5 real-time PCR detection system (Bio-Rad) at an annealing temperature of 56 °C. The primer sequence used in this study was reported in an earlier study,17 and HPRT1 was used as an internal control gene. The relative fold change expression was estimated by the 2–ΔΔCt method.18
2.10. PGE2 and Nitric Oxide Determination through ELISA Kits
The serum PGE2 level was determined by sandwich ELISA technique using a Rat PGE2 ELISA Kit (Wuhan Zokeyo Biotechnology Co., Ltd.) according to the manufacturer’s assay procedure.19 The level of nitric oxide (NO) in the serum was also quantified through a nitric oxide (NO) assay kit (Solarbio Life Sciences) using the provided protocol.
2.11. Molecular Docking Analysis
A molecular docking study was conducted to ascertain the binding interaction of trans-chalcone with different target proteins. In the current research, MOE-Dock (Chemical Computing Group Inc.) was used for docking studies. The chemical structure of trans-chalcone was obtained from Pubchem as canonical SMILES and pasted at the MOE interface. After the addition of hydrogen atoms, energy minimization was performed at a gradient of 0.001, following which the compound database was generated and saved as an mdb file. The protein crystal structures were downloaded in PDB format from the protein data bank (PDB ID TNF-α: 2AZ5, IL-17A: 7AMA, COX-2:5KIR, and iNOS: 4CX7). MOE was used to launch the acquired protein structure. The target protein structures were protonated in three dimensions, and the site finder tool was used to identify the protein’s active site.20 Then, energy minimization was performed to decrease collisions, and the MMFF94x force field was used to optimize the structure. After the removal of water molecules from the structure, docking was initiated. Ten different conformations were chosen with thoughtfulness. The MMFF94x force field energy calculation was then used to derive the energy-related parameters and assess the docked interactions at the binding pockets (active site), applying the resultant docked complex model.21 The lowest energy conformation of docked compounds was used for the examination of the binding patterns.
2.12. Histological Assessment
The ankle joint tissue was decalcified, entrenched in paraffin wax, and cut into 5 μm sections; then, it was subjected to hematoxylin and eosin staining. Then, a light microscope was used to observe histopathological changes.22
2.13. Statistical Analysis
The results obtained were expressed in graphs and tables as means with SEM. The interpretation of the results was carried out by subjecting the data to an ANOVA test followed by the Dunnett test for comparison between groups utilizing Graph Pad Prism 9. Impacts were thought to be significant at the level of p < 0.05.
3. Results
3.1. Evaluation of Inflammatory Parameters
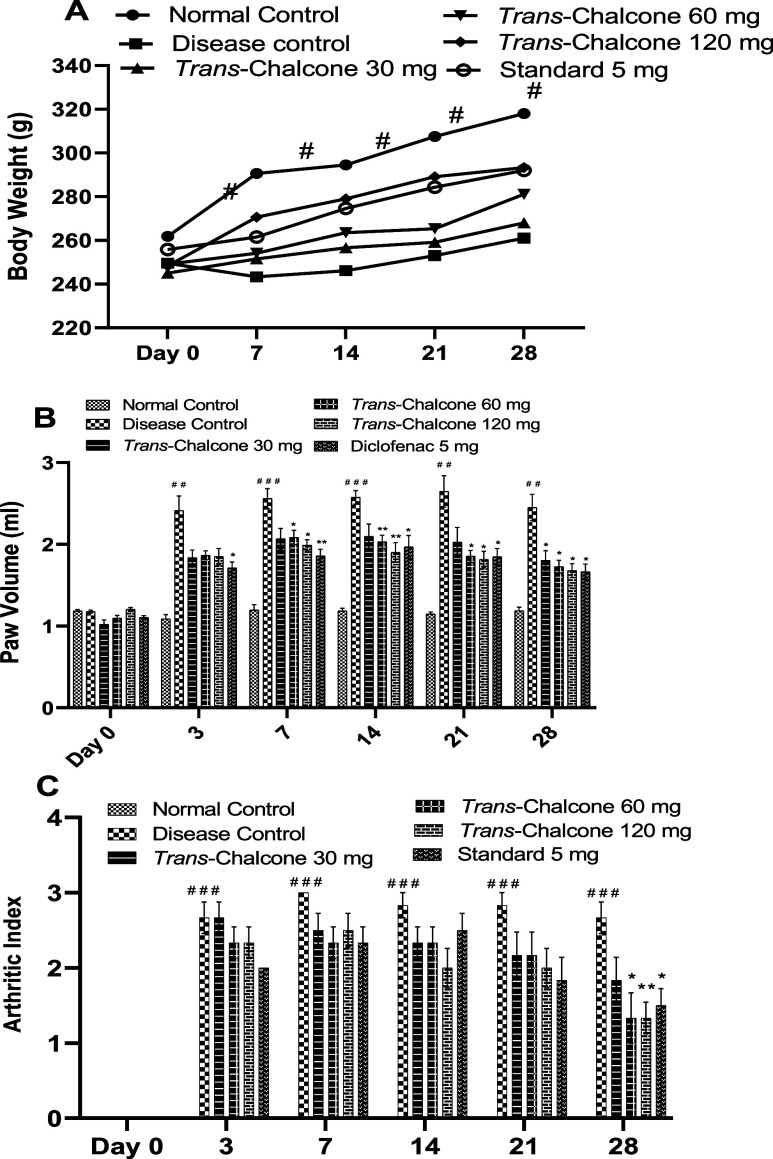
After the injection of CFA, rats significantly lost their body weight on day 3, and later, very slow weight gain was noted in the disease control (DC) group. A continuous sharp increase in body weight was seen in normal animals throughout the study period, which was significantly (p < 0.05) high as opposed to that of the DC group. On the other hand, trans-chalcone 30, 60, and 120 mg/kg increased the body weight of animals in a dose-dependent manner and protected the animals from the deleterious effects of CFA on weight, but these results were nonsignificant with respect to DC (Figure 1A).
Figure 1.
Effects of trans-chalcone on the body weight (A), paw volume (B), and arthritic index (C) of rats in the CFA-induced inflammatory model. Data presented as means with SEM (n = 6). Significance levels: ### indicates p < 0.001 in contrast to the normal control; * denotes p < 0.05 and ** denotes p < 0.01 in contrast to the disease control. The statistical test used was the two-way ANOVA right before the Dunnett test.
Significant inflammation was produced, which was evident from a marked increase (p < 0.01 and p < 0.001) in the paw volume of DC rats when compared with normal rats. Upon oral administration of trans-chalcone and diclofenac, the paw volume decreased during the experiment period of 28 days compared to that of DC rats. Trans-chalcone 30 mg/kg produced significant effects only on day 28 (p < 0.05), while doses of 60 and 120 mg/kg revealed a notable decrease in the paw volume starting from day 7 to day 28 (p < 0.05 and p < 0.01). In a similar way, diclofenac 5 mg/kg had notable (p < 0.05) reduction effects on the footpad volume over 28 days (Figure 1B). The results of the arthritic index were calculated by clinical scoring of the degree of inflammation. A huge increase was seen in the arthritic index of the CFA-injected group when correlated to normal animals (p < 0.001). A nonsignificant reduction of the arthritic index was demonstrated by trans-chalcone at 30, 60, and 120 mg/kg and the standard drug (diclofenac) until 21 days of administration, but on the 28th day, trans-chalcone at doses of 60 and 120 mg/kg and diclofenac caused a significant decrease in the arthritic index (p < 0.05, p < 0.01, and p < 0.05 accordingly), as depicted in Figure 1C.
3.2. Effect of Trans-Chalcone on Nociceptive Stimulus
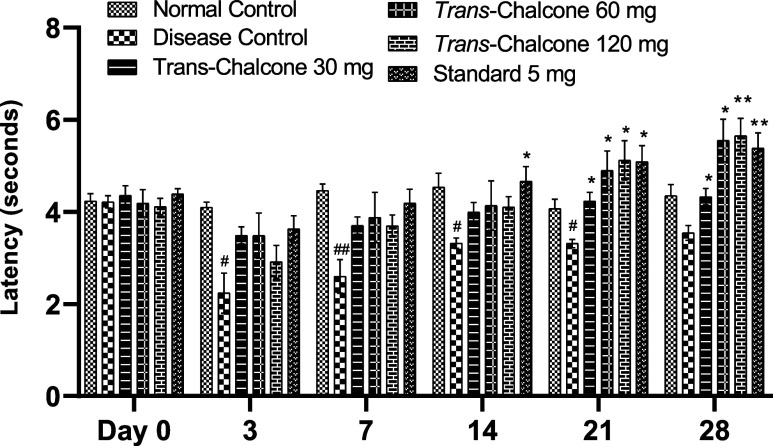
When the tails of CFA-injected arthritic rats were doused in hot water, nociceptive stimulation caused pain, and the rats flicked their tail. A significant decrease in the latency time was seen in the DC group (Figure 2). After 28 days of treatment with trans-chalcone (at 30, 60, and 120 mg/kg doses), a significant increase in the latency time was seen in a dose-dependent manner on days 21 and 28 (p < 0.05, p < 0.01). Likewise, the latency time of the diclofenac-treated group was also significantly increased on days 14, 21, and 28 (p < 0.05, p < 0.01).
Figure 2.
Trans-chalcone effects on the latency time in the tail immersion test in the chronic inflammatory model. Data are given as means with SEM (n = 6). Significance levels: # indicates p < 0.05 and ## indicates p < 0.01 in contrast to the normal control; * denotes p < 0.05 and ** denotes p < 0.01 compared to the disease control. Statistical tests used were the two-way ANOVA and the Dunnett tests.
3.3. Effect of Trans-Chalcone on Joint Stiffness, Flexion Pain, and Mobility and Stance Scores
Results presented in Figure 3 demonstrate that the joint stiffness in DC rats was remarkably elevated on days 14 and 28 (p < 0.001). Early treatment with trans-chalcone 30, 60, and 120 mg/kg and diclofenac 5 mg/kg did not cause any significant decline of joint stiffness at day 14, but later on, a notable (p < 0.05, p < 0.01) ameliorative effect was observed at day 28, and the best results were obtained with trans-chalcone 120 mg/kg (p < 0.01). The flexion pain score of untreated and CFA-injected rats was noticeably high compared to those of untreated and CFA-noninjected rats (p < 0.001). 28 days of treatment with trans-chalcone with gradually increasing doses markedly minimized flexion pain (p < 0.05, p < 0.01, and p < 0.001 accordingly) in a dose-dependent way. Additionally, diclofenac 5 mg/kg exhibited results similar to those of the trans-chalcone 30 mg/kg dose (p < 0.05).
Figure 3.
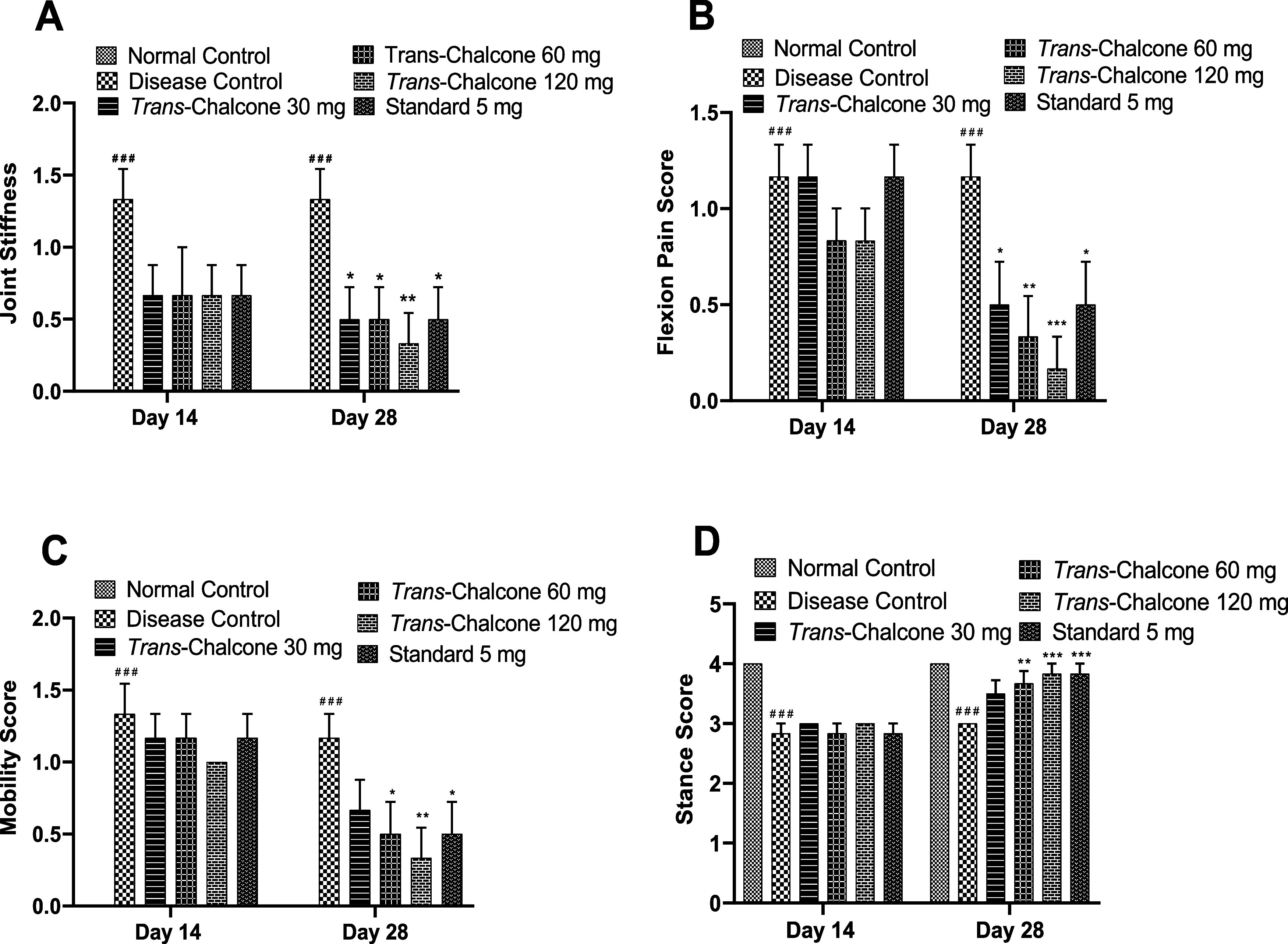
Effects of trans-chalcone treatment on joint stiffness (A), flexion pain (B), mobility (C), and stance score (D) in CFA-injected rats. Values are presented as the mean with SEM (n = 6). Significance levels: ### indicates p < 0.001 in comparison to the normal control. * indicates p < 0.05, ** denotes p < 0.01, and *** represents p < 0.001 in comparison to the arthritis control. The statistical test used was a two-way ANOVA followed by the Dunnett test.
The mobility of diseased control rats was significantly affected (p < 0.001) on days 14 and 28 as the mobility score increased. trans-Chalcone at 120 mg/kg dose produced the maximum (p < 0.01) improvement in mobility, even greater than that of diclofenac 5 mg/kg (p < 0.05). Furthermore, trans-chalcone (60 and 120 mg/kg doses) administration caused a notable increase in the stance score (p < 0.01, p < 0.001), which was limited in DC rats (Figure 3).
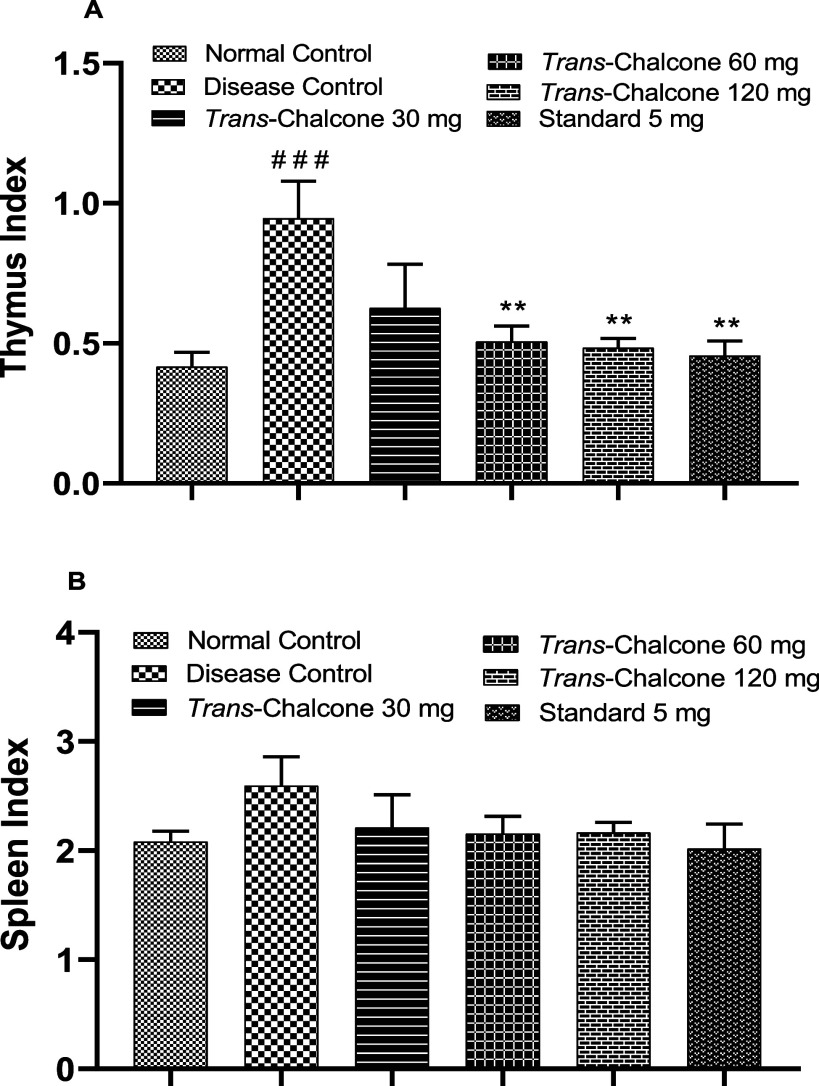
3.4. Effects of Trans-Chalcone on Thymus and Spleen Indices
The thymus index was estimated as the ratio of the thymus weight (mg) to animal weight (g), and results showed that in the DC group, the thymus index was significantly (p < 0.001) increased. In the present experiment, trans-chalcone at 30 mg/kg dose caused a nonsignificant reduction in the thymus index, but 60 and 120 mg/kg doses of trans-chalcone caused a significant (p < 0.01) reduction in the thymus index (Figure 4). Likewise, all tested doses of trans-chalcone (30, 60, and 120 mg/kg) presented a reduction effect on the spleen index in a nonsignificant way when compared to the disease control group (Figure 4).
Figure 4.
Effects of trans-chalcone on the thymus index (A) and spleen index (B) in CFA-mediated arthritic rats. Data are displayed as the mean with SEM (n = 6). Significance levels: ### indicates p < 0.001 in comparison to the normal control; ** denotes p < 0.01 and *** denotes p < 0.001 in comparison to the arthritic control. The statistical test used was one-way ANOVA followed by the Dunnett test.
3.5. Impact of Trans-Chalcone on the Blood Cell Count and LFT Analysis
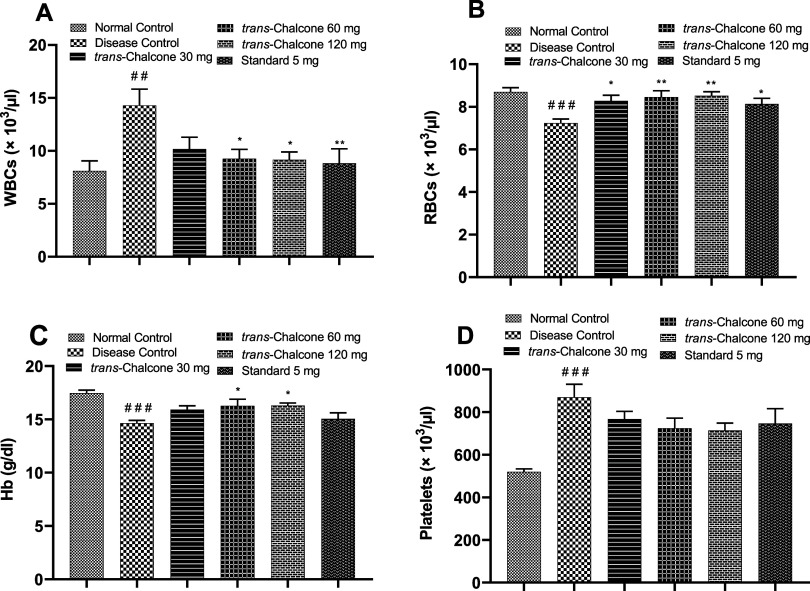
The white blood cell (WBC) count of the arthritic control group was significantly (p < 0.01) greater than that of the normal control group. Trans-chalcone 30 mg/kg did not have a significant WBC lowering effect, but trans–chalcone 60 and 120 mg/kg caused an appreciable decrease in the WBC count (p < 0.05, p < 0.01). The number of RBCs and Hb levels were significantly reduced in the diseased control rats (p < 0.001). After treatment with trans-chalcone 30, 60, and 120 mg/kg for 28 days, a substantial increase was observed in the RBC count and Hb levels in rats (p < 0.05, p < 0.01). The number of platelets was increased to a significant level in CFA-injected nontreated rats in contrast to normal healthy rats (p < 0.001). The administration of trans-chalcone leads to a decrease in the platelet count in a dose-dependent way (nonsignificant), as shown in Figure 5.
Figure 5.
Impacts of trans-chalcone on WBCs (A), RBCs (B), Hb (C), and platelets (D) in the control and treated groups of the CFA model. Values are depicted as the mean with SEM (n = 6). ### indicates p < 0.001 in comparison to the normal control, while * denotes p < 0.05, ** denotes p < 0.01, and *** represents p < 0.001 in comparison to the disease control. Statistical tests used were the one-way ANOVA and the Dunnett test.
The serum creatinine concentration was significantly increased in diseased control rats (p < 0.01), but rats treated with trans-chalcone 30 and 60 mg/kg and diclofenac 5 mg/kg exhibited no decrease in the creatinine level while trans-chalcone 120 mg/kg dose caused a notable decrease in the serum creatinine (p < 0.05). The blood urea level of DC rats was found to be elevated when correlated with normal rats (p < 0.01), and trans-chalcone at doses of 30, 60, and 120 mg/kg showed a slight and nonsignificant reduction in the urea level. The levels of liver enzymes such as ALT, AST, and ALP in DC rats were significantly increased until 28 days after arthritis induction correlated to the normal group (p < 0.05, p < 0.001, and p < 0.01 respectively). Trans-chalcone and standard drug treatment caused a reduction in these enzyme levels, and the 120 mg/kg dose showed the maximum lowering effect (p < 0.01). ESR is one of the key diagnostic parameters of RA, and in the present study, the ESR of DC rats was significantly increased in contrast to normal animals (p < 0.01). An insignificant decrease in the ESR was demonstrated by the 30 mg/kg dose; however, TC at 60 and 120 mg/kg showed a positive decrease in the ESR value (p < 0.05). The C-reactive protein is an inflammatory marker, and its level increases in several diseases, including rheumatoid arthritis. In the present experiment, CFA-induced inflammation caused a significant increase in the CRP in DC rats (p < 0.01), which was nonsignificantly reversed with the 30 mg/kg dose, while the 60 and 120 mg/kg doses produced a notable reduction in the CRP level (p < 0.05). The rheumatoid factor (RF) is a characteristic marker of rheumatoid arthritis, and its amount was upregulated in the disease control group in contrast to normal animals (p < 0.01). All treatments decreased the RF value, and only the 120 mg/kg dose caused a positive reduction in the RF level (p < 0.05), as shown in Table 1.
Table 1. Impact of Trans-Chalcone on the Biochemical Parameters in the CFA-Induced Arthritis Modela.
| parameter | normal control | disease control | trans-chalcone 30 mg | trans-chalcone 60 mg | trans-chalcone 120 mg | standard 5 mg |
|---|---|---|---|---|---|---|
| creatinine (mg/dL) | 0.58 ± 0.031 | 0.83 ± 0.061## | 0.78 ± 0.048 | 0.75 ± 0.043 | 0.62 ± 0.060* | 0.72 ± 0.048 |
| urea (mg/dL) | 23.03 ± 1.294 | 35.67 ± 3.095## | 29.33 ± 1.961 | 30.33 ± 2.929 | 28.91 ± 1.886 | 26.17 ± 2.469* |
| ALT (U/L) | 64.57 ± 5.038 | 85.52 ± 7.801# | 71.68 ± 3.497 | 67.45 ± 1.589* | 66.50 ± 2.964* | 64.68 ± 3.751* |
| AST (U/L) | 70.03 ± 4.654 | 185.20 ± 4.958### | 154.3 ± 11.38* | 152.5 ± 9.586* | 150.0 ± 8.054* | 151.6 ± 5.108* |
| ALP (U/L) | 313.2 ± 35.05 | 566.0 ± 40.13## | 484.8 ± 57.97 | 467.3 ± 48.87 | 333.2 ± 36.38** | 403.7 ± 31.94* |
| ESR (mm/h) | 9.33 ± 0.42 | 13.33 ± 0.61## | 11.00 ± 1.29 | 10.00 ± 0.82* | 9.83 ± 0.65* | 9.50 ± 0.96* |
| CRP (mg/L) | 0.405 ± 0.068 | 0.810 ± 0.115## | 0.557 ± 0.064 | 0.505 ± 0.075* | 0.490 ± 0.061* | 0.507 ± 0.046* |
| RF (IU/mL) | 1.615 ± 0.151 | 2.545 ± 0.174## | 2.045 ± 0.239 | 1.940 ± 0.217 | 1.822 ± 0.154* | 1.832 ± 0.122* |
Results are stated as the mean with SEM (n = 6). Significance levels: # indicates p < 0.05, ## indicates p < 0.01, and ### indicates p < 0.001 in comparison to the normal control’ * denotes p < 0.05 and ** denotes p < 0.01 vs the disease control. The statistical test used was one-way ANOVA followed by the Dunnett test.
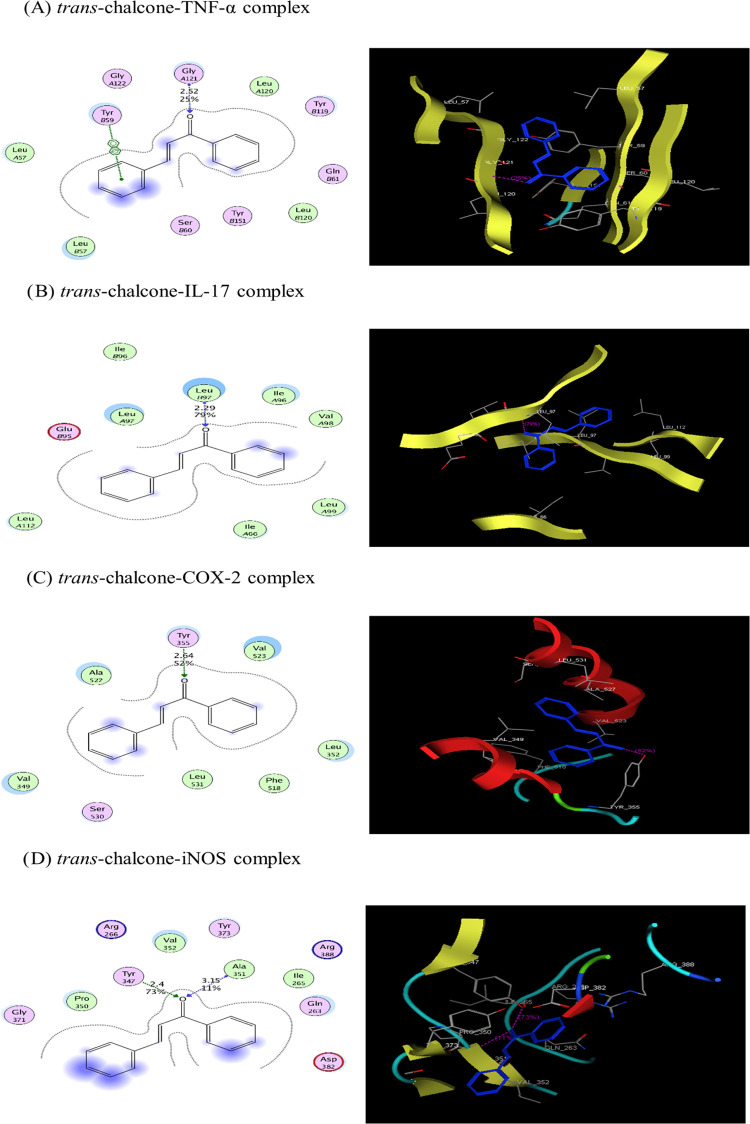
3.6. Trans-Chalcone Docking Results
Trans-chalcone and the cocrystallized ligand were docked against selected protein structures (TNF-α, IL-17, COX-2, iNOS) playing vital roles in inflammatory diseases. The results of docking demonstrated a strong binding interaction of trans-chalcone with target proteins. Trans-chalcone was found to form a stable complex with proteins, as predicted by their negative binding energy values (Table 2). The binding of trans-chalcone and a cocrystallized ligand is presented in 2D and 3D views in Figure 6. Multiple types of bonding interactions, including hydrogen bonds and arene–arene interactions, of trans-chalcone were observed with different active site amino acid residues of TNF-α (Gly 121, Tyr 59), IL-17 (Leu 97), COX-2 (Tyr 355), and iNOS (Ala 351, Tyr 347), as presented in Table 2.
Table 2. Binding Interaction of Trans-Chalcone and the Cocrystallized Ligand with Different Target Proteins Determined through Docking Analysis.
| H-bond interactions |
|||||||
|---|---|---|---|---|---|---|---|
| target protein | phytochemical/ligand | binding energy (kJ/mol) | score (%) | distance (°A) | residue amino acid | arene–arene interactions | arene–cation interactions |
| TNF-α | trans-chalcone | –8.774 | 25 | 2.52 | Gly 121 | Tyr 59 | |
| cocrystallized ligand | –13.410 | 92 | 1.78 | Tyr 119 | Tyr 119 | ||
| IL-17 | trans-chalcone | –9.505 | 79 | 2.29 | Leu 97 | ||
| cocrystallized ligand | –10.825 | 31 | 1.23 | Leu 97 | |||
| COX-2 | trans-chalcone | –9.071 | 52 | 2.64 | Tyr 355 | ||
| cocrystallized ligand | –12.171 | 71 | 1.79 | Ser 530 | |||
| 91 | 2.07 | Ser 353 | |||||
| iNOS | trans-chalcone | –8.718 | 11 | 3.15 | Ala 351 | ||
| 73 | 2.4 | Tyr 347 | |||||
| cocrystallized ligand | –11.819 | 31 | 1.83 | Trp 372 | |||
| 13 | 2.23 | Tyr 347 | |||||
| 14 | 2.67 | Gly 371 | |||||
Figure 6.
2D and 3D views of the interaction of trans-chalcone (A–D) with TNF-α, IL-17, COX-2, and iNOS, respectively.
3.7. Effect of Trans-Chalcone on the mRNA Expression of Proinflammatory and Anti-Inflammatory Mediators
The impact of trans-chalcone on the transcription of different inflammatory and anti-inflammatory mediators was appraised by a real-time PCR experiment. Figure 7A,B shows that the mRNAs of TNF-α and IL-1β were markedly overexpressed in diseased control rats compared to normal healthy animals (p < 0.001). The administration of all three doses of trans-chalcone (30, 60, and 120 mg/kg) for 28 days caused significant suppression of TNF-α and IL-1β expression (p < 0.001), and trans-chalcone 120 mg/kg produced superior results compared to the standard drug. In the present study, the mRNA level of IL-6 was highly increased in CFA-injected control animals (p < 0.001), and the trans-chalcone 30 mg/kg dose showed a nonsignificant decrease while 60 and 120 mg/kg doses significantly (p < 0.001) controlled IL-6 expression, as shown in Figure 7C. Another inflammatory cytokine IL-17 was observed to have high expression in arthritic rats (p < 0.001). Upon treatment with the trans-chalcone 30 mg/kg dose, the IL-17 expression reduced in an insignificant manner, but the administration of 60 and 120 mg/kg doses caused a significant decrease in IL-17 expression (p < 0.05 and p < 0.01), as shown in Figure 7D. The findings presented in Figure 7E demonstrate that there was a notable (p < 0.001) hike in the COX-2 expression in the arthritic group, and trans-chalcone 30 and 60 mg/kg doses did not produce significant lowering effects in contrast to the 120 mg/kg dose, and diclofenac 5 mg/kg revealed a remarkable decline of COX-2 expression. In the ongoing study, the mRNA level of iNOS was significantly increased in arthritis control vs normal rats (p < 0.001). Oral treatment with trans-chalcone (30, 60, and 12 mg/kg) repressed iNOS expression in a dose-dependent way (p < 0.001), as presented in Figure 7F. Results presented in Figure 7G reveal that the expression of the anti-inflammatory mediator IL-10 was positively suppressed in the diseased control animals compared to normal animals (p < 0.05). After 28 days, oral administration with trans-chalone (30, 60, and 120 mg/kg) caused an increase in the IL-10 expression. In this regard, trans-chalcone 30 mg dose showed a nonsignificant increase while trans-chalcone 60 and 120 mg/kg doses showed a notable (p < 0.001) increase in IL-10 expression.
Figure 7.
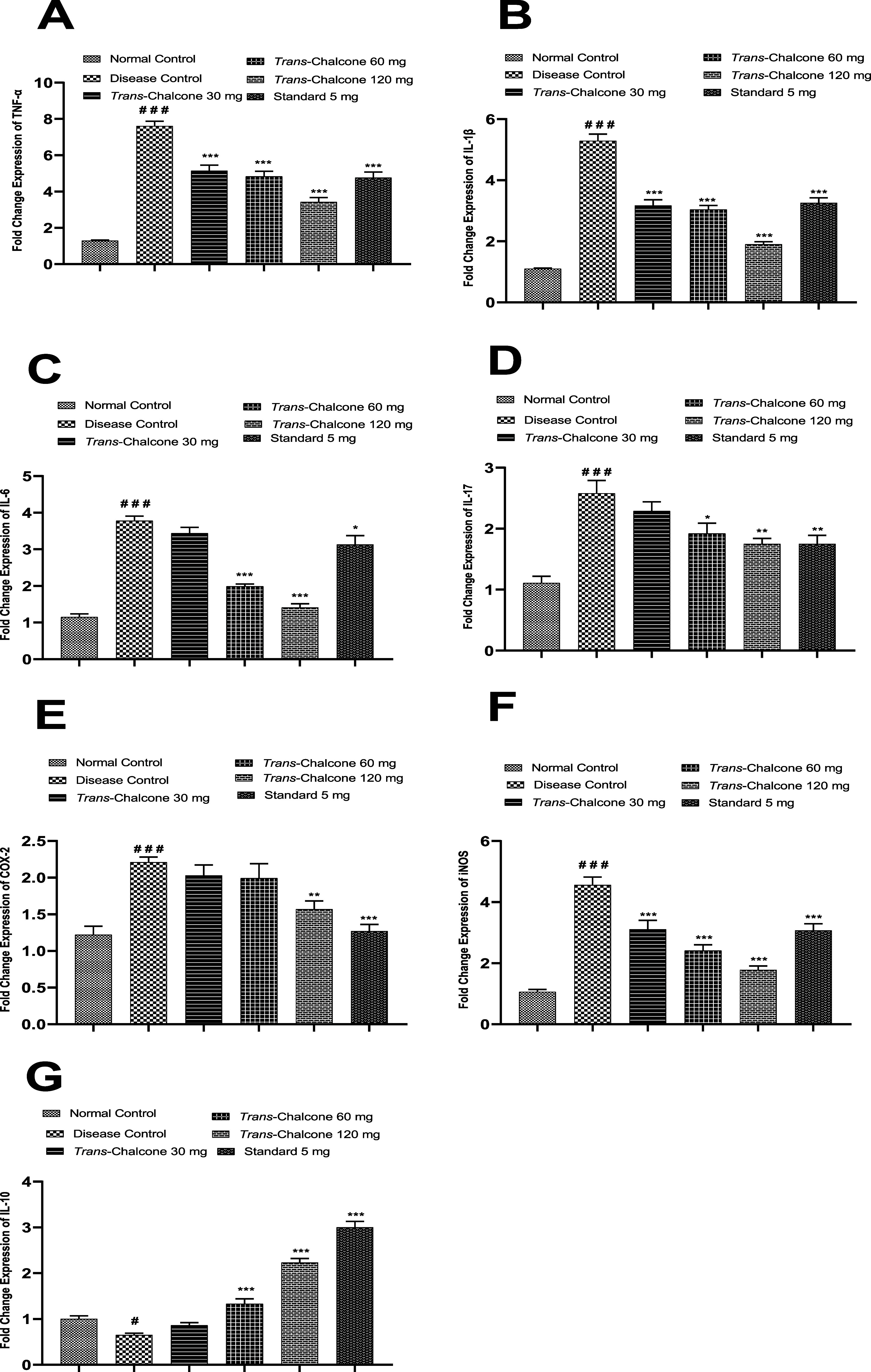
Effect of trans-chalcone treatment on the mRNA values of TNF-α (A), IL-1β (B), IL-6 (C), IL-17 (D), COX-2 (E), iNOS (F), and IL-10 (G) in arthritic rats determined by real-time PCR. Results are given as means with SEM (n = 6). Significance levels: ### indicates p < 0.001 in contrast to the normal control ** denotes p < 0.01 and *** denotes p < 0.001 in comparison to the arthritic control. Statistical tests used were one-way ANOVA and the Dunnett tests.
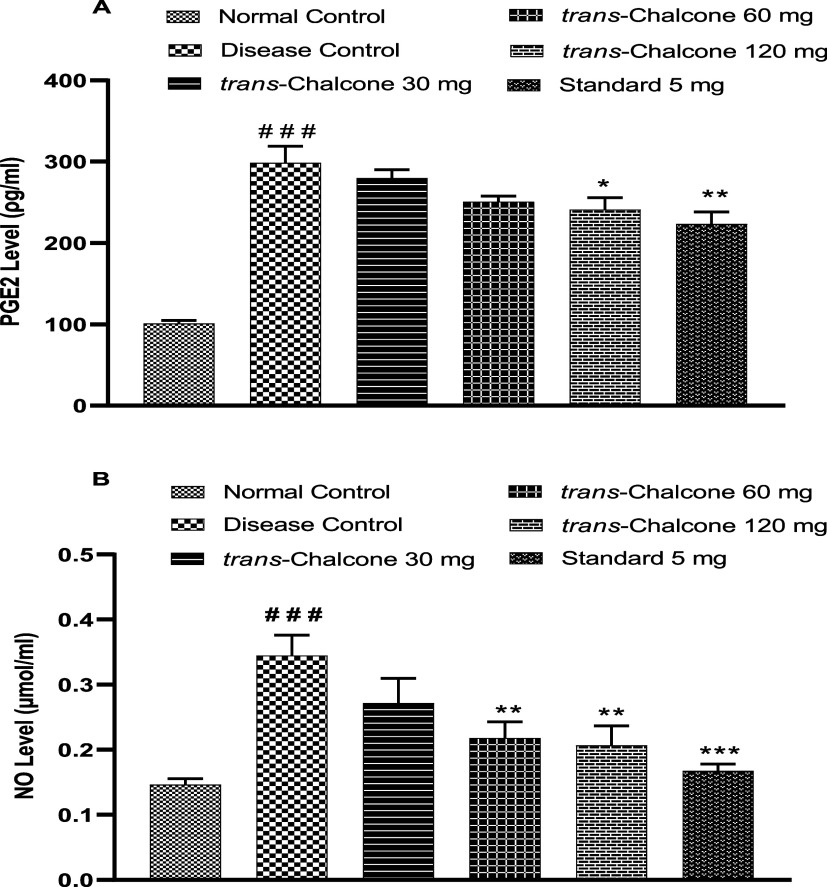
3.8. Effect of Trans-Chalcone on Serum PGE2 and NO Levels
The serum prostaglandin E2 level was estimated by an enzyme-linked immunosorbent assay. Results presented in Figure 8A demonstrate that a significant increase in the PGE2 level took place in DC rats in contrast to the normal group (p < 0.001). On completion of the CFA model (28 days), trans-chalcone 30 and 60 mg/kg doses lowered the PGE2 concentration in a nonsignificant manner. However, trans-chalcone 120 mg/kg and diclofenac 5 mg/kg caused a substantial decline in the PGE2 level in the serum of treated rats (p < 0.05 and p < 0.01 accordingly).
Figure 8.
Effect of trans-chalcone on serum PGE2 (A) and NO (B) levels in the CFA-mediated model. Results are given as mean with SEM (n = 6). Significance levels: ### indicates p < 0.001 in contrast to the normal control; * denotes p < 0.05 and ** denotes p < 0.01 in contrast to the disease control. The statistical test used was one-way ANOVA followed by the Dunnett test.
Furthermore, the disease control group exhibited notable enhancement of NO concentration in contrast to the normal healthy rats (p < 0.001). Treatment of animals with trans-chalcone for 28 days produced a dose-dependent decrease in the NO concentration. Trans-chalcone 30 mg had nonsignificant effects, while 60 and 120 mg/kg doses had significant (p < 0.001) effects (Figure 8B).
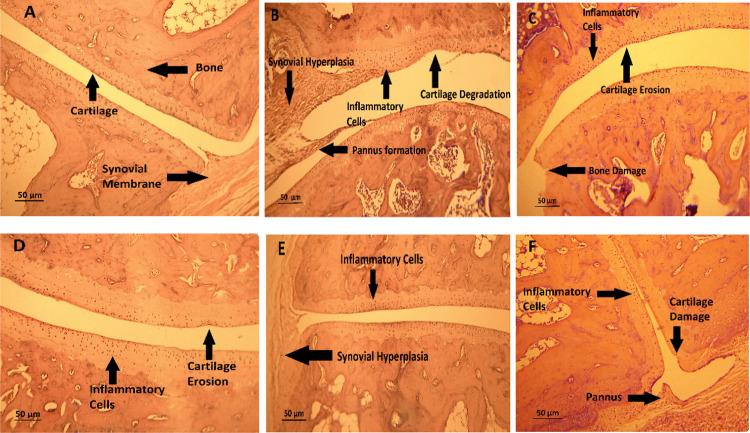
3.9. Effect of Trans-Chalcone on Histological Changes
Histopathology slides of the ankle joint of arthritic control rats unveiled tissue changes such as pannus formation, synovium hypergrowth, cartilage damage, and bone erosion of the ankle joint. Treatments of trans-chalcone with gradually increasing doses predominantly mitigated these abnormalities (Figure 9).
Figure 9.
Effects of different doses of trans-chalcone on the histopathology of ankle joints in the CFA-mediated arthritic rats: (A) normal control, (B) disease control, (C) trans-chalcone 30 mg/kg, (D) trans-chalcone 60 mg/kg, (E) trans-chalcone 120 mg/kg, and (F) standard 5 mg/kg.
4. Discussion
Rheumatoid arthritis is a chronic inflammation of synovial joints caused by an excessive immune system activation against self-antigens. Several extra-articular manifestations including vasculitis, rheumatoid nodules, and systemic comorbidities are also seen in severe cases.23 Although there is no cure for RA, remission is an attainable goal. To date, rheumatoid arthritis is treated with DMARDs, glucocorticoids, and NSAIDs.24 However, several patients are still unable to achieve remission, and additional work is required to provide the benefit of therapeutic success to every patient. Therefore, the present era needs to develop more effective drugs with low cost and fewer adverse effects that have a greater success rate in the remission and cure of rheumatoid arthritis. In the present research, the anti-rheumatoid-arthritis potential of trans-chalcone, a phytochemical, was demonstrated in a complete Freund’s adjuvant-instigated arthritis model in rats.
Rheumatoid cachexia, also known as muscle wasting, is a condition that is linked to RA and can be brought on by a variety of reasons, such as decreased mobility caused by pain, decreased dietary intake, higher energy expenditure, and cytokines, particularly TNF-α, which cause accelerated lipid and protein degradation.25,26 In the current study, CFA-provoked arthritis leads to significant weight loss in rats, while the administration of trans-chalcone improved the body weight of animals over the study duration, which showed its protective effect on cachexia. The decreased expression of TNF-α caused by trans-chalcone, as found in the PCR analysis, might be the possible mechanism of this weight improvement. Paw edema evaluation is used to study the antiarthritic action of multiple compounds. The intensity of arthritis is evaluated clinically using the visible arthritis index and is indicative of secondary lesions. Paw swelling and arthritis index assessment is a rapid, easy, and precise process for determining the level of inflammation along with the curative properties of medications.27 In the current investigation, injection of CFA into the paw caused inflammation, which was defined as a primary lesion; the administration of trans-chalcone prominently alleviated paw edema; thus, a reduction in paw volume was seen throughout the experimental model. Similarly, the arthritic index was found to be increased in CFA-injected rats, while trans-chalcone treatment for 28 days significantly improved the severity of secondary lesions. This visible decline in paw volume and arthritic index indicated the arthritis ameliorative effects of trans-chalcone. Pain is undoubtedly one of the key characteristics of inflammation.28 There are well-established nociceptor circuits that control acute pain in the wake of inflammation. Local immune cells in the outer regions discharge inflammatory substances such as cytokines, which affect nociceptor neuron endings in the peripheral nerves.29 The tail immersion test of the current study showed that in the CFA control group, the pain threshold significantly reduced, causing severe pain at a lower level of thermal stimulus. Twenty-eight days of trans-chalcone administration in rats produced a prominent increase in the threshold level, causing low pain sensation upon thermal stimulus. A previous study also reported that trans-chalcone inhibits IL-1, IL-6, and TNF-α release and prevented mice from developing mechanical hyperalgesia caused by MSU.11 Being a connective tissue ailment, rheumatoid arthritis affects para-articular tissues and causes stiffness, swelling, and pain in the joints. The functional degradation of an animal’s ability to walk is likewise linked to arthritis.30,31 The present research came out with evidence of significant joint stiffness, marked flexion pain, impairment of mobility, and a lower stance score in CFA-injected rats compared to normal animals. However, the administration of trans-chalcone to animals considerably mitigated joint stiffness, relieved flexion pain, and improved mobility and stance. The level of WBCs increases as the RA disease progresses, which causes an increase in granulocyte, colony-stimulating factor, and macrophage production.32 It has been reported that an increase in WBC numbers in the arthritic state is caused by the IL-1β-mediated upregulation of pertinent colony-stimulating factors.12 In the current study, arthritic rats had elevated WBC, platelet, and ESR levels, and further treatment with trans-chalcone reduced the WBC, platelet, and ESR levels, which indicates the antiarthritic potential of this drug. These alterations may be brought on by test medications that suppress the production of IL-1β.
The most common extracellular presentation of rheumatoid arthritis is anemia characterized by a decreased RBC count and Hb level. Arthritis-associated anemia occurs due to gastrointestinal blood loss by medications and alteration in the bone marrow, which prevents iron availability for RBCs.33 The present study demonstrated a significant decrease in the RBC count and Hb level of diseased control rats; moreover, oral administration of trans-chalcone improved the RBC and Hb levels, which confirms its antianemic effects.
The assessment of serum hepatic enzymes, creatinine and urea levels, provides information about the degree of liver and kidney injury, which is also thought to be an important hallmark of adjuvant-induced arthritis.33,34 In the current study, arthritic rats showed a significant increase in the serum ALT, AST, ALP, creatinine, and urea levels; however, animals treated with trans-chalcone had an attenuated level of these enzymes, showing its protective effect on liver and kidney damage in rheumatoid arthritis. Rheumatoid arthritis is thought to be strongly indicated by an abnormal elevation in blood CRP and RF levels.35 Findings of the present research showed that arthritic rats had elevated levels of serum CRP and RF; this demonstrates the inflammatory changes caused by CFA immunization. However, all tested doses of trans-chalcone had a prominent inhibitory impact on these parameters, indicating the resolution of the disease.
The thymus and spleen play a role in immune system control, and RA is frequently worsened by splenitis, splenomegaly, and lymphoid hyperplasia.27,36 It is believed that variations in the weights of the thymus or spleen serve as indicators of the body’s overall immune response.37 The effectiveness of immunomodulatory medications used to treat RA is assessed using the indices of these organs.38 In the present study, the thymus index was elevated significantly, while the spleen index showed a nonsignificant increase in CFA-administered rats, indicating their activated immune function. Treated groups of trans-chalcone showed notably reduced thymus and spleen indices, confirming its immunomodulatory and antiarthritic potential.
Numerous studies have shown that in rheumatoid arthritis, the environment causing the loss of cartilage and bone in arthritic joints is characterized by the production of inflammatory cytokines, including TNF-α, IL-6, IL-1, and IL-17, as well as other mediators in downstream pathways.39 These inflammatory mediators stimulate many different signaling pathways and cause the transcription of genes that are crucial to tissue breakdown and inflammation.40 Together with IL-1β, TNF-α may facilitate angiogenesis, intracellular adhesion, migration, the production of acute-phase proteins and proteolytic enzymes, interleukins (such as IL-6 and IL-12), macrophage inflammatory protein-1, monocyte chemoattractant protein-1, and epithelial-neutrophil activating peptide-78 in the synovium.41
By altering VEGF expression, it has been discovered that IL-6 increases joint inflammation and damage in RA patients. The powerful angiogenic factor recognized as VEGF encourages endothelial cell movement and proliferation. Along with mediating inflammation, VEGF also increases vascular permeability.40 Synovial joints contain Th17 cells that release IL-17, a proinflammatory cytokine that works in conjunction with TNF-α and IL-6 to promote osteoclastogenesis. IL-17 affects both early and late stages of RA disease. It encourages the migration and triggering of neutrophils, macrophages, and B cells, besides the stimulation of osteoclastogenesis by FLS.42 The cytokine IL-10 has opposing regulatory functions and the potential to suppress proinflammatory responses. IL-10 may prevent the synthesis of inflammatory cytokines such as IL-6, IL-1, and TNF-α in RA.40 In the present research, it is found that the mRNA levels of TNF-α, IL-1β, IL-6, and IL-17 were significantly elevated in animals that were injected with CFA, while the mRNA level of IL-10 was lowered compared to that of normal rats, indicating proinflammatory cytokine upregulation and anti-inflammatory cytokine downregulation. Daily treatment of animals with trans-chalcone orally for 28 days showed prominent control of IL-1β, IL-6, TNF-α, and IL-17 expressions at the transcription level in contrast to the upregulation of IL-10 expression, showing a shift of the inflammatory forces toward anti-inflammatory effects. Comparative results were reported in a previous study, wherein trans-chalchone showed a significant reduction of mRNAs of IL-1β, TNF-α, and IL-6 in high-fat-induced pulmonary inflammation in rats.10 Further, protein levels of these inflammatory cytokines were lowered prominently by trans-chalcone in gouty arthritis, measured through ELISA, while in contrast to our findings, the IL-10 level was reduced compared to those of arthritic mice and Leishmania amazonensis-infected macrophages in two separate studies.11,43
People having rheumatoid arthritis (RA) exhibit higher COX-2 production in their synovial tissues. The inflammatory proteins IL-1 and TNF-α induce elevated COX-2 expression. It has been demonstrated that PGE2, the main product of COX-2 in synoviocytes, changes the equilibrium of the matrix metalloproteinase and increases the production of the angiogenic element VEGF.44 Current research showed prominent elevation of COX-2 and PGE2 production in disease control rats, confirming the deleterious role of these mediators in arthritis, while significant inhibition of COX-2 mRNA expression and later the PGE2 level by trans-chalcone was observed. In line with our findings, previous reports conclude that trans-chalcone in topical dosage form prevented skin inflammation and reduced COX-2 mRNA in mice.45 Natural chalcone flavokawain B (28) could possibly prevent LPS-induced murine leukemia macrophage RAW 264.7 cells from producing PGE2 and COX-2 proteins.46
There is mounting evidence that NO plays a role in the pathophysiology of numerous autoimmune disorders, including RA. Preclinical investigations indicated significantly increased NO levels in experimentally generated arthritic rats and restored such levels by selective inhibitors. Additionally, the positive benefits of the inhibition of NO production were inferred indirectly in RA patients using glucocorticoids, salicylates, indomethacin, and methotrexate.30 The present study also demonstrated a significant increase in iNOS expression at the RNA level and nitric oxide (NO) concentration in the blood of experimentally induced arthritic rats. Daily administration of trans-chalcone to arthritic rats for 28 days had a prominent inhibitory effect on the production of iNOS, and the blood nitric oxide level strengthened its protective role in rheumatoid arthritis. In accordance with our results, previous researchers found that trans-chalcone treatment reduced the nitrate production (NO assay) in mice knee in gouty arthritis,11L. amazonensis-infected macrophages,43 and hepatic inflammation.9 Flavokawain B (28), a naturally occurring chalcone, also inhibited the production of the iNOS protein and reduced the NO concentration.46
The noncovalent bonding of molecules, such as a receptor protein and a ligand, is predicted via a computer-based method called molecular docking. Because it produces the conformation and usually the binding affinity of the tiny molecule in its projected least-energy state, this model is used to virtually screen enormous libraries of compounds.47 In the reported research, molecular docking of the ligand (trans-chalcone) against selected target inflammatory proteins was performed to study their ligand–protein interactions. Trans-chalcone showed a strong binding affinity with target proteins at the site of their inhibitory structural ligands. Trans-chalcone interacted with target proteins through hydrogen bonding via the oxygen atom of its structure and arene–arene interactions.
Histological alterations in the joints of rats revealed that the ankle joint of normal control rats has an intact connective tissue structure and an intact synovial lining free of necrosis and inflammation. Rats treated with CFA had notable abnormalities in their joints, including hyperplasia of the synovium, damage to the synovial lining, an inflow of inflammatory cells, the creation of pannus, and damage to the cartilage and bone. Conversely, rats treated with trans-chalcone showed notable protection against these alterations.
5. Conclusions
The present research demonstrated that trans-chalcone prominently reduced paw edema and arthritic index in rats. It showed good analgesic properties in CFA-injected animals. Long-term administration of trans-chalcone caused minimal joint stiffness and joint pain and improved mobility. Thymus and spleen indices were decreased by the trans-chalcone treatment in rats. The molecular mechanism of trans-chalcone involves a decrease in mRNA expression of inflammatory mediators such as TNF-α, IL-1β, IL-6, IL-17, COX-2, and iNOS, respectively.
Acknowledgments
The authors extend their gratitude to King Saud University (Riyadh, Saudi Arabia) for funding this research through the researchers supporting project number RSP-2024-R406.
The authors declare no competing financial interest.
References
- Wu Y.; Li K.; Zeng M.; Qiao B.; Zhou B. Serum metabolomics analysis of the anti-inflammatory effects of gallic acid on rats with acute inflammation. Front. Pharmacol. 2022, 13, 830439 10.3389/fphar.2022.830439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shobier A. H.; Ismail M. M.; Hassan S. W. M. Variation in Anti-inflammatory, Anti-arthritic, and Antimicrobial Activities of Different Extracts of Common Egyptian Seaweeds with an Emphasis on Their Phytochemical and Heavy Metal Contents. Biol. Trace Elem. Res. 2023, 201 (4), 2071–2087. 10.1007/s12011-022-03297-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarrant T. K.; Patel D. D. Chemokines and leukocyte trafficking in rheumatoid arthritis. Pathophysiology 2006, 13 (1), 1–14. 10.1016/j.pathophys.2005.11.001. [DOI] [PubMed] [Google Scholar]
- El-Tanbouly G. S.; Abdelrahman R. S. Novel anti-arthritic mechanisms of trans-cinnamaldehyde against complete Freund’s adjuvant-induced arthritis in mice: involvement of NF-kB/TNF-α and IL-6/IL-23/ IL-17 pathways in the immuno-inflammatory responses. Inflammopharmacology 2022, 30 (5), 1769–1780. 10.1007/s10787-022-01005-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferraz C. R.; Carvalho T. T.; Manchope M. F.; Artero N. A.; Rasquel-Oliveira F. S.; Fattori V.; Casagrande R.; Verri W. A. Jr Therapeutic potential of flavonoids in pain and inflammation: mechanisms of action, pre-clinical and clinical data, and pharmaceutical development. Molecules 2020, 25 (3), 762. 10.3390/molecules25030762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karimi-Sales E.; Jeddi S.; Ghaffari-Nasab A.; Salimi M.; Alipour M. R. Effect of trans-chalcone on hepatic IL-8 through the regulation of miR-451 in male rats. Endocr. Regul. 2018, 52 (1), 1–5. 10.2478/enr-2018-0001. [DOI] [PubMed] [Google Scholar]
- Alipour M. R.; Jeddi S.; Karimi-Sales E. trans-Chalcone inhibits high-fat diet-induced disturbances in FXR/SREBP-1c/FAS and FXR/Smad-3 pathways in the kidney of rats. J. Food Biochem. 2020, 44 (11), e13476 10.1111/jfbc.13476. [DOI] [PubMed] [Google Scholar]
- Lamoke F.; Labazi M.; Montemari A.; Parisi G.; Varano M.; Bartoli M. Trans-Chalcone prevents VEGF expression and retinal neovascularization in the ischemic retina. Exp. Eye Res. 2011, 93 (4), 350–354. 10.1016/j.exer.2011.02.007. [DOI] [PubMed] [Google Scholar]
- Singh H.; Sidhu S.; Chopra K.; Khan M. Hepatoprotective effect of trans-chalcone on experimentally induced hepatic injury in rats: inhibition of hepatic inflammation and fibrosis. Can. J. Physiol. Pharmacol. 2016, 94 (08), 879–887. 10.1139/cjpp-2016-0071. [DOI] [PubMed] [Google Scholar]
- Karimi-Sales E.; Alipour M. R.; Naderi R.; Hosseinzadeh E.; Ghiasi R. Protective effect of trans-chalcone against high-fat diet-induced pulmonary inflammation is associated with changes in miR-146a and pro-inflammatory cytokines expression in male rats. Inflammation 2019, 42 (6), 2048–2055. 10.1007/s10753-019-01067-1. [DOI] [PubMed] [Google Scholar]
- Staurengo-Ferrari L.; Ruiz-Miyazawa K. W.; Pinho-Ribeiro F. A.; Fattori V.; Zaninelli T. H.; Badaro-Garcia S.; Borghi S. M.; Carvalho T. T.; Alves-Filho J. C.; Cunha T. M. Trans-Chalcone attenuates pain and inflammation in experimental acute gout arthritis in mice. Front. Pharmacol. 2018, 9, 1123 10.3389/fphar.2018.01123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahnashi M. H.; Jabbar Z.; Irfan H. M.; Asim M. H.; Akram M.; Saif A.; Alshahrani M. A.; Alshehri M. A.; Asiri S. A. Venlafaxine demonstrated anti-arthritic activity possibly through down regulation of TNF-α, IL-6, IL-1β, and COX-2. Inflammopharmacology 2021, 29, 1413–1425. 10.1007/s10787-021-00849-0. [DOI] [PubMed] [Google Scholar]
- Gohil P.; Patel V.; Deshpande S.; Chorawala M.; Shah G. Anti-arthritic activity of cell wall content of Lactobacillus plantarum in freund’s adjuvant-induced arthritic rats: involvement of cellular inflammatory mediators and other biomarkers. Inflammopharmacology 2018, 26, 171–181. 10.1007/s10787-017-0370-z. [DOI] [PubMed] [Google Scholar]
- Bakare A. O.; Owoyele B. V. Bromelain reduced pro-inflammatory mediators as a common pathway that mediate antinociceptive and anti-anxiety effects in sciatic nerve ligated Wistar rats. Sci. Rep. 2021, 11 (1), 289 10.1038/s41598-020-79421-9. [DOI] [PMC free article] [PubMed] [Google Scholar]; From NLM.
- Nagakura Y.; Okada M.; Kohara A.; Kiso T.; Toya T.; Iwai A.; Wanibuchi F.; Yamaguchi T. Allodynia and hyperalgesia in adjuvant-induced arthritic rats: time course of progression and efficacy of analgesics. J. Pharmacol. Exp. Ther. 2003, 306 (2), 490–497. 10.1124/jpet.103.050781. [DOI] [PubMed] [Google Scholar]
- Patil K. R.; Patil C. R.; Jadhav R. B.; Mahajan V. K.; Patil P. R.; Gaikwad P. S. Anti-Arthritic Activity of Bartogenic Acid Isolated from Fruits of Barringtonia racemosa Roxb. (Lecythidaceae). Evidence-Based Complementary Altern. Med. 2011, 2011, 785245 10.1093/ecam/nep148. [DOI] [PMC free article] [PubMed] [Google Scholar]; From NLM.
- Nawaz S.; Irfan H. M.; Akram M.; Jahan S. Linalool: Monoterpene alcohol effectiveness in chronic synovitis through lowering Interleukin-17, spleen and thymus indices. Int. Immunopharmacol. 2023, 121, 110517 10.1016/j.intimp.2023.110517. [DOI] [PubMed] [Google Scholar]
- Shabbir A.; Shahzad M.; Ali A.; Zia-ur-Rehman M. Discovery of new benzothiazine derivative as modulator of pro-and anti-inflammatory cytokines in rheumatoid arthritis. Inflammation 2016, 39 (6), 1918–1929. 10.1007/s10753-016-0427-y. [DOI] [PubMed] [Google Scholar]
- Nawaz S.; Irfan H. M.; Alamgeer; Arshad L.; Jahan S. Attenuation of CFA-induced chronic inflammation by a bicyclic monoterpene fenchone targeting inducible nitric oxide, prostaglandins, C-reactive protein and urea. Inflammopharmacology 2023, 31, 2479. 10.1007/s10787-023-01333-7. [DOI] [PubMed] [Google Scholar]
- Shafique K.; Farrukh A.; Mahmood Ali T.; Qasim S.; Jafri L.; Abd-Rabboh H. S.; Al-Anazy M. M.; Kalsoom S. Designing Click One-Pot Synthesis and Antidiabetic Studies of 1, 2, 3-Triazole Derivatives. Molecules 2023, 28 (7), 3104. 10.3390/molecules28073104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alzarea S. I.; Qasim S.; Uttra A. M.; Khan Y. H.; Aljoufi F. A.; Ahmed S. R.; Alanazi M.; Malhi T. H. Network Pharmacology and Molecular Docking Based Prediction of Mechanism of Pharmacological Attributes of Glutinol. Processes 2022, 10 (8), 1492. 10.3390/pr10081492. [DOI] [Google Scholar]
- Chen G.; Song Y.; Ma F.; Ma Y. Anti-arthritic activity of D-carvone against complete Freund’s adjuvant-induced arthritis in rats through modulation of inflammatory cytokines. Korean J. Physiol. Pharmacol. 2020, 24 (6), 453–462. 10.4196/kjpp.2020.24.6.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smolen J. S.; Aletaha D.; McInnes I. B. Rheumatoid arthritis. Lancet 2016, 388, 2023–2038. 10.1016/S0140-6736(16)30173-8. [DOI] [PubMed] [Google Scholar]
- Crocetti L.; Vergelli C.; Guerrini G.; Giovannoni M. P.; Kirpotina L. N.; Khlebnikov A. I.; Ghelardini C.; Di Cesare Mannelli L.; Lucarini E.; Schepetkin I. A. Pyridinone Derivatives as Interesting Formyl Peptide Receptor (FPR) Agonists for the Treatment of Rheumatoid Arthritis. Molecules 2021, 26 (21), 6583 10.3390/molecules26216583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qasim S.; Alamgeer; Kalsoom S.; Shahzad M.; Bukhari I. A.; Vohra F.; Afzal S. Rosuvastatin Attenuates Rheumatoid Arthritis-Associated Manifestations via Modulation of the Pro-and Anti-inflammatory Cytokine Network: A Combination of In Vitro and In Vivo Studies. ACS Omega 2021, 6 (3), 2074–2084. 10.1021/acsomega.0c05054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu L.; Zhang Z.; Xia N.; Zhang W.; Wei Y.; Huang J.; Ren Z.; Meng F.; Yang L. Anti-arthritic activity of ferulic acid in complete Freund’s adjuvant (CFA)-induced arthritis in rats: JAK2 inhibition. Inflammopharmacology 2020, 28, 463–473. 10.1007/s10787-019-00642-0. [DOI] [PubMed] [Google Scholar]
- Wang S.; Wang Y.; Liu X.; Guan L.; Yu L.; Zhang X. Anti-inflammatory and anti-arthritic effects of taraxasterol on adjuvant-induced arthritis in rats. J. Ethnopharmacol. 2016, 187, 42–48. 10.1016/j.jep.2016.04.031. [DOI] [PubMed] [Google Scholar]
- Gilroy D. W.; Bishop-Bailey D. Lipid mediators in immune regulation and resolution. Br. J. Pharmacol. 2019, 176 (8), 1009–1023. 10.1111/bph.14587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kathryn B.; Nidhi S.; Hamdy A. M. R.. Understanding the Mechanisms of Pain in Rheumatoid Arthritis. In Rheumatoid Arthritis; IntechOpen, 2020; p 3. [Google Scholar]
- Patel R.; Kadri S.; Gohil P.; Deshpande S.; Shah G. Amelioration of complete Freund’s adjuvant-induced arthritis by Calotropis procera latex in rats. Future J. Pharm. Sci. 2021, 7 (1), 213. 10.1186/s43094-021-00361-w. [DOI] [Google Scholar]
- Nasuti C.; Fedeli D.; Bordoni L.; Piangerelli M.; Servili M.; Selvaggini R.; Gabbianelli R. Anti-inflammatory, anti-arthritic and anti-nociceptive activities of Nigella sativa oil in a rat model of arthritis. Antioxidants 2019, 8 (9), 342. 10.3390/antiox8090342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qasim S.; Kalsoom S.; Shahzad M.; Irfan H. M.; Zafar M. S.; Bukhari I. A.; Vohra F.; Afzal S. Appraisal of disease-modifying potential of amlodipine as an anti-arthritic agent: new indication for an old drug. Inflammopharmacology 2020, 28 (4), 1121–1136. 10.1007/s10787-020-00692-9. [DOI] [PubMed] [Google Scholar]
- Ingawale D. K.; Patel S. S. Hecogenin exhibits anti-arthritic activity in rats through suppression of pro-inflammatory cytokines in Complete Freund’s adjuvant-induced arthritis. Immunopharmacol. Immunotoxicol. 2018, 40 (1), 59–71. 10.1080/08923973.2017.1405439. [DOI] [PubMed] [Google Scholar]
- Uttra A. M.; Shahzad M.; Shabbir A.; Jahan S. Ephedra gerardiana aqueous ethanolic extract and fractions attenuate Freund Complete Adjuvant induced arthritis in Sprague Dawley rats by downregulating PGE2, COX2, IL-1β, IL-6, TNF-α, NF-kB and upregulating IL-4 and IL-10. J. Ethnopharmacol. 2018, 224, 482–496. 10.1016/j.jep.2018.06.018. [DOI] [PubMed] [Google Scholar]
- Mbiantcha M.; Almas J.; Shabana S. U.; Nida D.; Aisha F. Anti-arthritic property of crude extracts of Piptadeniastrum africanum (Mimosaceae) in complete Freund’s adjuvant-induced arthritis in rats. BMC Complementary Altern. Med. 2017, 17 (1), 111 10.1186/s12906-017-1623-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saleem A.; Saleem M.; Akhtar M. F.; Shahzad M.; Jahan S. Moringa rivae leaf extracts attenuate Complete Freund’s adjuvant-induced arthritis in Wistar rats via modulation of inflammatory and oxidative stress biomarkers. Inflammopharmacology 2020, 28 (1), 139–151. 10.1007/s10787-019-00596-3. [DOI] [PubMed] [Google Scholar]; From NLM.
- Li X.; Wu Z.; He B.; Zhong W. Tetrandrine alleviates symptoms of rheumatoid arthritis in rats by regulating the expression of cyclooxygenase-2 and inflammatory factors. Exp. Ther. Med. 2018, 16 (3), 2670–2676. 10.3892/etm.2018.6498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui X.; Wang R.; Bian P.; Wu Q.; Seshadri V. D. D.; Liu L. Evaluation of antiarthritic activity of nimbolide against Freund’s adjuvant induced arthritis in rats. Artif. Cells, Nanomed., Biotechnol. 2019, 47 (1), 3391–3398. 10.1080/21691401.2019.1649269. [DOI] [PubMed] [Google Scholar]
- Alghasham A.; Rasheed Z. Therapeutic targets for rheumatoid arthritis: Progress and promises. Autoimmunity 2014, 47 (2), 77–94. 10.3109/08916934.2013.873413. [DOI] [PubMed] [Google Scholar]
- Zhang C. Flare-up of cytokines in rheumatoid arthritis and their role in triggering depression: Shared common function and their possible applications in treatment. Biomed. Rep. 2020, 14 (1), 16. 10.3892/br.2020.1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noh A. S. M.; Chuan T. D.; Khir N. A. M.; Zin A. A. M.; Ghazali A. K.; Long I.; Ab Aziz C. B.; Ismail C. A. N. Effects of different doses of complete Freund’s adjuvant on nociceptive behaviour and inflammatory parameters in polyarthritic rat model mimicking rheumatoid arthritis. PLoS One 2021, 16 (12), e0260423 10.1371/journal.pone.0260423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo N.; Kuroda T.; Kobayashi D. Cytokine Networks in the Pathogenesis of Rheumatoid Arthritis. Int. J. Mol. Sci. 2021, 22 (20), 10922. 10.3390/ijms222010922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda-Sapla M. M.; Tomiotto-Pellissier F.; Assolini J. P.; Carloto A. C. M.; da Silva Bortoleti B. T.; Goncalves M. D.; Tavares E. R.; da Silva Rodrigues J. H.; Simão A. N. C.; Yamauchi L. M. trans-Chalcone modulates Leishmania amazonensis infection in vitro by Nrf2 overexpression affecting iron availability. Eur. J. Pharmacol. 2019, 853, 275–288. 10.1016/j.ejphar.2019.03.049. [DOI] [PubMed] [Google Scholar]
- Crofford L. J. The role of COX-2 in rheumatoid arthritis synovial tissues. Arthritis Res. Ther. 1999, 1 (Suppl 1), S30 10.1186/ar44. [DOI] [Google Scholar]
- Martinez R. M.; Pinho-Ribeiro F. A.; Vale D. L.; Steffen V. S.; Vicentini F. T.; Vignoli J. A.; Baracat M. M.; Georgetti S. R.; Verri W. A. Jr; Casagrande R. Trans-chalcone added in topical formulation inhibits skin inflammation and oxidative stress in a model of ultraviolet B radiation skin damage in hairless mice. J. Photochem. Photobiol., B 2017, 171, 139–146. 10.1016/j.jphotobiol.2017.05.002. [DOI] [PubMed] [Google Scholar]
- Jasim H. A.; Nahar L.; Jasim M. A.; Moore S. A.; Ritchie K. J.; Sarker S. D. Chalcones: synthetic chemistry follows where nature leads. Biomolecules 2021, 11 (8), 1203. 10.3390/biom11081203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNutt A. T.; Francoeur P.; Aggarwal R.; Masuda T.; Meli R.; Ragoza M.; Sunseri J.; Koes D. R. GNINA 1.0: molecular docking with deep learning. J. Cheminf. 2021, 13 (1), 43. 10.1186/s13321-021-00522-2. [DOI] [PMC free article] [PubMed] [Google Scholar]