Abstract
The precise temporal-spatial regulation of the p21-activated serine-threonine kinase PAK at the plasma membrane is required for proper cytoskeletal reorganization and cell motility. However, the mechanism by which PAK localizes to focal adhesions has not yet been elucidated. Indirect binding of PAK to the focal adhesion protein paxillin via the Arf-GAP protein paxillin kinase linker (PKL) and PIX/Cool suggested a mechanism. In this report, we demonstrate an essential role for a paxillin–PKL interaction in the recruitment of activated PAK to focal adhesions. Similar to PAK, expression of activated Cdc42 and Rac1, but not RhoA, stimulated the translocation of PKL from a generally diffuse localization to focal adhesions. Expression of the PAK regulatory domain (PAK1–329) or the autoinhibitory domain (AID 83–149) induced PKL, PIX, and PAK localization to focal adhesions, indicating a role for PAK scaffold activation. We show PIX, but not NCK, binding to PAK is necessary for efficient focal adhesion localization of PAK and PKL, consistent with a PAK–PIX–PKL linkage. Although PAK activation is required, it is not sufficient for localization. The PKL amino terminus, containing the PIX-binding site, but lacking paxillin-binding subdomain 2 (PBS2), was unable to localize to focal adhesions and also abrogated PAK localization. An identical result was obtained after PKLΔPBS2 expression. Finally, neither PAK nor PKL was capable of localizing to focal adhesions in cells overexpressing paxillinΔLD4, confirming a requirement for this motif in recruitment of the PAK–PIX–PKL complex to focal adhesions. These results suggest a GTP-Cdc42/GTP-Rac triggered multistep activation cascade leading to the stimulation of the adaptor function of PAK, which through interaction with PIX provokes a functional PKL PBS2–paxillin LD4 association and consequent recruitment to focal adhesions. This mechanism is probably critical for the correct subcellular positioning of PAK, thereby influencing the ability of PAK to coordinate cytoskeletal reorganization associated with changes in cell shape and motility.
INTRODUCTION
Controlled cell adhesion is a process fundamental to normal physiological cell, tissue, organ, and organism development; maintenance; and repair and function, whereas dysregulation contributes to pathophysiological conditions, including inflammation, hypertrophy, tumorigenesis, and metastasis (Schwartz et al., 1995; Aplin et al., 1999). Integrins are the primary mediators of cell adhesion to the extracellular matrix and functionally couple to and transmit signals bidirectionally through the actin cytoskeleton via specialized but dynamic assemblies of proteins called focal complexes, contacts, or adhesions (Jockusch et al., 1995; Sastry and Burridge, 2000).
Growth factor receptors and integrins cooperate to provide cues that direct and adjust many complex cell behaviors, primarily through the regulation of the discrete architecture of the focal contact (Rozengurt, 1995; Schwartz and Baron, 1999). This process is governed largely by the precise temporal and spatial modulation of small GTPases of the Rho family (Hotchin and Hall, 1995; Nobes and Hall, 1995, 1999; Van Aelst and D'Souza-Schorey, 1997; Hall, 1998). Several downstream effectors of Rho family members have been identified and networks of protein interaction characterized, providing insight into the fine control cells have developed to regulate the fundamental processes of adhesion and motility in response to environmental signals (Bishop and Hall, 2000; Schmitz et al., 2000).
Paxillin is a cytoskeletal adaptor protein that functions as a molecular scaffold for protein recruitment to focal adhesions and thereby facilitates protein networking and efficient signal transmission (Turner, 1998, 2000a,b). Through the multiple SH2- and SH3-binding domains, LIM, and LD motifs that comprise paxillin, this molecule is known to interact with the signaling proteins Crk, Src, Csk, FAK, Pyk2, ILK, and PTP-PEST, as well as the structural proteins vinculin, actopaxin, and tubulin (Turner, 2000a,b). Cell adhesion, motility, and differentiation are influenced by the phosphorylation status of paxillin (Brown et al., 1998; Sastry et al., 1999; Turner et al., 1999; Nikolopoulos and Turner, 2000; Petit et al., 2000). In addition, we recently identified a GIT2 Arf-GAP family member, paxillin kinase linker (PKL), that interacts with the paxillin LD4 motif and is involved in cytoskeletal remodeling events associated with matrix and growth factor engagement (Turner et al., 1999). PKL is a member of a large family of Arf-GAP–containing proteins, including GIT1/CAT/APP1, ASAP, ACAP, PAP, and the centaurins (Donaldson and Jackson, 2000; Jackson et al., 2000a,b; Premont et al., 2000; Turner, 2000a; Turner et al., 2001). PKL was originally defined as a functional link between paxillin and the p21-activated kinase (PAK) through the intermediary Cool/PIX Cdc42/Rac guanine nucleotide exchange factor (GEF) (Turner et al., 1999).
PAK has been implicated as a major translation point in Cdc42 and Rac signaling to the cytoskeleton and nucleus (Manser and Lim, 1999). Structurally, PAK is comprised of an amino-terminal scaffold domain containing the hallmark Cdc42/Rac p21-binding domain (PBD) and several proline-rich SH3-binding motifs that mediate binding to the SH3-SH2 adaptor NCK (Bokoch et al., 1996; Galisteo et al., 1996) as well as the Cool/PIX family (Bagrodia et al., 1998; Manser et al., 1998). The PAK carboxyl terminus contains the serine/threonine kinase domain (Knaus and Bokoch, 1998; Bagrodia and Cerione, 1999). Functionally, constitutive PAK kinase activity causes disassembly of RhoA focal adhesions and actin stress fibers (Manser et al., 1997; Frost et al., 1998; Zhao et al., 1998), whereas the amino-terminal scaffold region is involved in focal complex formation and membrane ruffling (Sells et al., 1997; Daniels et al., 1998; Obermeier et al., 1998). Recent crystallographic data reveal that PAK probably exists as an autoinhibited inactive dimer with a kinase inhibitory segment obscuring the catalytic domain (Lei et al., 2000; Buchwald et al., 2001). On GTP-Cdc42 or Rac binding to the PBD, a multistage activation switch is triggered, resulting in dimer dissociation, conformational opening of the molecule, autophosphorylation, and full kinase activation (Lei et al., 2000; Buchwald et al., 2001).
Proper regulation of PAK activity and compartmentalization are essential to effect appropriate signaling in response to cell activation, thus it is not surprising that PAK activation is complex (Manser and Lim, 1999). NCK and PIX binding have been shown to regulate transient PAK localization to the membrane (Lu et al., 1997; Sells et al., 1997), with PIX binding being necessary for PAK localization to Cdc42 focal complexes (Manser et al., 1998). The PKL-related protein GIT1 has recently been suggested to participate in PAK recruitment to focal complexes to facilitate focal adhesion disassembly (Zhao et al., 2000b). However, the precise mode of PAK delivery to focal adhesions is as-yet unknown. The identification of PKL suggested a mechanism for the recruitment of PAK and PIX to focal adhesions (Turner et al., 1999). We have observed that expression of paxillin lacking the LD4 motif, and thereby unable to recruit a PAK–PIX–PKL complex to focal contacts, results in increased membrane protrusion, cell spreading, and random motility associated with persistent Rac activation (West et al., 2001). In the present study, we identify and characterize a paxillin-dependent mechanism of PKL and PAK localization to focal adhesions. Furthermore, this localization is dependent upon Cdc42/Rac activation of the adaptor function of PAK, which through PIX interaction leads to an “unmasking” of the PKL paxillin-binding subdomain 2 (PBS2) and consequent recruitment of the PAK–PIX–PKL complex to focal adhesions through a paxillin LD4 motif association. The recruitment of this complex to focal adhesions may be required for both the PAK kinase-dependent role in directional motility as well as a transition from a Rac to a Rho phenotype that is associated with normal cell spreading.
MATERIALS AND METHODS
Plasmids and Antibodies
Plasmids encoding avian PKL WT (aa 1–757), N-term (aa 1–576), C-term (aa 448–757), ΔPBS2 (aa 643–679) cloned into pEGFPC1 (CLONTECH, Palo Alto, CA), and pcDNA3 WT avian paxillin-α were generated as described previously (Turner and Miller, 1994; Turner et al., 1999). Murine WT Pak3 subcloned into pJ3H and myc-tagged human Cool-1 (β-PIX) and Cool-2 (α-PIX) pcDNA3 vectors were generous gifts from Rick Cerione (Cornell University, Ithaca, NY). The myc-tagged human Pak1 WT and Pak1 T423E in pCMV6 M, pEGFPC1-Pak1 83–149 (autoinhibitory domain, AID) and pEGFPC1-Pak1 83–149 L107F were generous gifts of Jonathan Chernoff (Fox Chase Cancer Center, Philadelphia, PA). pCMV6 M Pak1 1–329, 1–329 P13A, and 1–329 P191G/R192A were generated using the QuikChange mutagenesis kit and sequenced in their entirety at the Cornell BioResource Center (Ithaca, NY). The myc-tagged V12 Cdc42hs pCMV, WT and V12 Rac1 and V14 RhoA pEXV vectors were provided by Marc Symons (Picower Institute for Medical Research, Manhasset, NY) and subcloned into pcDNA3.
Primary antibodies used in this study include paxillin-specific monoclonal antibody 165 and polyclonal avian-specific paxillin antiserum Pax1 (Turner and Miller, 1994); anti-PKL (developed in collaboration with Transduction Laboratories, Lexington KY); anti-Crk (Transduction Laboratories); and anti-myc 9E10 (developed by J. Michael Bishop and maintained at the Developmental Studies Hybridoma Bank, Department of Biological Sciences, University of Iowa, Iowa City, IA) monoclonal antibodies; goat polyclonal anti-β-PIX (L-17; Santa Cruz Biotechnology, Santa Cruz, CA); and anti-green fluorescent protein (GFP) generously provided by Pam Silver (Dana-Farber Cancer Institute, Boston, MA).
Secondary antibodies for immunofluorescence were rhodamine (tetramethylrhodamine B isothiocyanate)-conjugated AffiniPure donkey anti-rabbit (711-025-152) or anti-mouse (715-025-150) IgG (H+L) (Jackson Immunoresearch Laboratories, West Grove, PA); or for triple labeling, Alexa Fluor 350 goat anti-mouse IgG (H+L) (A-21049; Molecular Probes, Eugene, OR). For Western immunoblotting, affinity-isolated horseradish peroxidase-conjugated goat anti-rabbit IgG whole molecule (A6154) or goat anti-mouse IgG whole molecule (A4416) was from Sigma-Aldrich (St. Louis, MO), and donkey anti-goat IgG horseradish peroxidase was from Santa Cruz Biotechnology.
Cell Culture and Transfection
CHO.K1 cells were cultured in modified Ham's F-12 (Mediatech, Herndon, VA) supplemented with 10% (vol/vol) heat-inactivated, certified fetal bovine serum (Atlanta Biologicals, Norcross, GA), 50 U/ml penicillin, and 50 μg/ml streptomycin (complete medium) at 37°C in a humidified chamber with 5% CO2. CHO.K1 paxillinΔLD4 cell cultures were supplemented with 500 μg/ml G418 (Mediatech). Cells were transfected using FuGENE 6 (Roche Applied Science, Indianapolis, IN). Briefly, cells at a density of 4 × 105 cells/100-mm dish were plated in complete medium on ethanol-washed glass coverslips coated with 10 μg/ml fibronectin and bovine serum albumin blocked (Brown et al., 1996). After 12–15 h, a 100-μl mixture of 5 μl of FuGENE 6 and 2 μg of total plasmid DNA (when necessary, pcDNA3.1 HisLacZ was used to bring total DNA to 2 μg) in antibiotic-free/serum-free Ham's F-12 was added to coverslips in six-well dishes containing 2 ml of complete medium. After a 12–15-h incubation coverslips were removed and processed for microscopy.
Immunofluorescence Microscopy
Cells on glass coverslips (Assistant-brand 12 mm; Carolina Biological Supply, Burlington, NC) were fixed for 8 min with 3.7% formaldehyde in phosphate-buffered saline, washed for 10 min with Tris-buffered saline (TBS), and permeablized for 2 min in 0.2% Triton X-100 in TBS followed by washing for 10 min in TBS. Coverslips were incubated for 2 h at 37°C with primary antibody that had been diluted in TBS containing 3% bovine serum albumin and 0.05% Tween 20. After a 10-min wash in TBS, coverslips were incubated for 45 min with secondary antibody or rhodamine-phalloidin diluted into TBS.
Indirect immunofluorescence photomicrographs were generated with a Spot RT-slider charge-coupled device camera (Diagnostic Imaging, Livingston, Scotland) attached to a Zeiss Axiophot microscope (Carl Zeiss, Thornwood, NY) fitted with a 63× Neo-fluar oil immersion objective and a 50-W mercury lamp. Images were processed using Spot V3.0 software and Adobe Photoshop (Adobe Systems, San Jose, CA). For quantitation of PKL and PAK focal contact localization, 150–200 cotransfected cells were counted in at least three independent experiments.
Western Immunoblotting
CHO.K1 transfectants, grown on 100-mm dishes in complete medium, were lysed in 1 ml of lysis buffer (10 mM Tris-HCl pH 7.6, 100 mM NaCl, 1% Triton X-100, 0.1% deoxycholate, 1 mM EDTA). After a 4°C 14,500 × g centrifugation step, ectopically expressed GFP-PKL was immunoprecipitated on a Labquake rotator for 1 h at 4°C from 400 μg of protein by using anti-GFP antibody and protein A-G agarose (Santa Cruz Biotechnology). The immunoprecipitates were boiled in dithiothreitol-based 2× SDS-PAGE buffer, the proteins separated on 12.5% SDS-PAGE mini-gels, transferred to 0.45-μm Immobilon-NC (Millipore, Bedford, MA), and blotted with appropriate antibodies and protein signals detected using the ECL system (Amersham Biosciences, Piscataway, NJ). To characterize partitioning between Triton X-100–soluble and –insoluble fractions, lysates were prepared as described above in a volume of 500 μl. For the soluble fraction, 50 μl of the supernatant was made to 1× SDS-PAGE sample buffer. The pellet was resuspended in 500 μl of 1× SDS-PAGE and sheared with a 22-gauge needle to generate the insoluble fraction. Equivalent proportions of the soluble and insoluble fractions were processed for immunoblotting as described above.
RESULTS
Cdc42 and Rac1, but Not RhoA, Stimulate PKL Focal Adhesion Localization
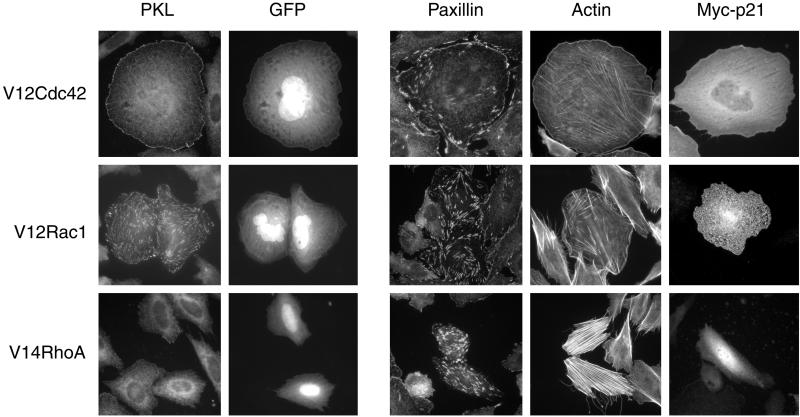
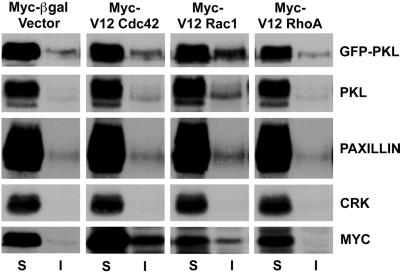
We have previously suggested that PKL may mediate the localization of PAK and PIX to focal adhesions through PIX binding to PKL and subsequent PKL binding to paxillin LD4 (Turner et al., 1999; West et al., 2001). However, in contrast to paxillin, which is generally considered to be a resident focal adhesion protein, PAK is enriched in Cdc42 focal complexes and Rac focal contacts, but is absent from Rho focal adhesions (Manser et al., 1997). We examined the capacity of PKL localization to focal adhesions to be similarly regulated at the level of Rho family p21 activity. Nontransfected CHO.K1 cells showed primarily a diffuse cytoplasmic PKL staining, whereas cotransfection of constitutively active myc-Cdc42Hs with GFP (to identify transfectants) resulted in robust localization of endogenous PKL to peripheral focal complexes (Figure 1, top). In contrast, paxillin was localized to focal adhesions in all cells, as well as enriched at Cdc42-stimulated peripheral complexes (Figure 1, top). Rhodamine phalloidin and myc staining of active Cdc42/GFP transfectants confirmed phenotypic effects on the organization of the actin cytoskeleton and expression of the active p21 GTPases, respectively (Figure 1, top). Expression of constitutively active myc-Rac1 caused significant membrane ruffling and translocation of endogenous PKL to focal adhesions (Figure 1, middle). However, constitutively active myc-RhoA was unable to support the localization of PKL to focal adhesions, whereas paxillin was clearly enriched in the more numerous and larger focal adhesions at the ends of robust actin stress fibers generated in the active RhoA transfectants (Figure 1, bottom). Examination of Triton X-100–soluble vs. –insoluble CHO.K1 fractions revealed that expression of active Rac1 and to a lesser extent active Cdc42, but not RhoA or β-gal control, increased the amount of endogenous PKL, ectopic GFP-PKL, and paxillin in the insoluble fraction (Figure 2). Crk distribution served as a negative control. These data are consistent with PKL translocation to focal adhesions as shown by immunofluorescence (Figure 1). We conclude that the temporal and spatial localization of PKL to focal adhesions requires an identical pattern of p21 GTPase activation as described previously for PAK (Manser et al., 1997).
Figure 1.
Induction of PKL focal adhesion localization by activated Cdc42 and Rac1, but not RhoA. CHO.K1 cells were cotransfected with myc-tagged V12Cdc42 (top), V12Rac1 (middle), or V14RhoA (bottom) and GFP. Immunofluorescence analysis was performed to examine the effects of active p21 expression on endogenous PKL subcellular localization. GFP was cotransfected to identify transfectants. In addition, paxillin localization, actin organization, and ectopic myc-p21 expression was examined in GFP and p21 cotransfectants; 75 ± 8% (n = 3) of active Cdc42 and 90 ± 6% (n = 3) of active Rac1 transfected cells revealed PKL in focal adhesions, whereas PKL within active RhoA transfected cells was primarily diffuse. Transfectants were determined by GFP expression; colabeling is only shown for PKL immunostaining.
Figure 2.
Cdc42 and Rac1 stimulate PKL transition to a Triton X-100–insoluble fraction. The subcellular distribution of PKL was examined in cells expressing active Cdc42, Rac1, or RhoA. Overexpression of Cdc42 and Rac1 but not RhoA stimulated an increase in both endogenous PKL and ectopic GFP-PKL detected within Triton X-100 detergent-insoluble (I) cell fractions consistent with a translocation of PKL to focal adhesions in these cells (Figure 1). Similarly, a shift in paxillin, Cdc42, and Rac1, but not RhoA or the SH2-SH3 adaptor protein Crk, to the detergent insoluble fraction was detected.
Regulation of PKL Focal Adhesion Localization by PAK
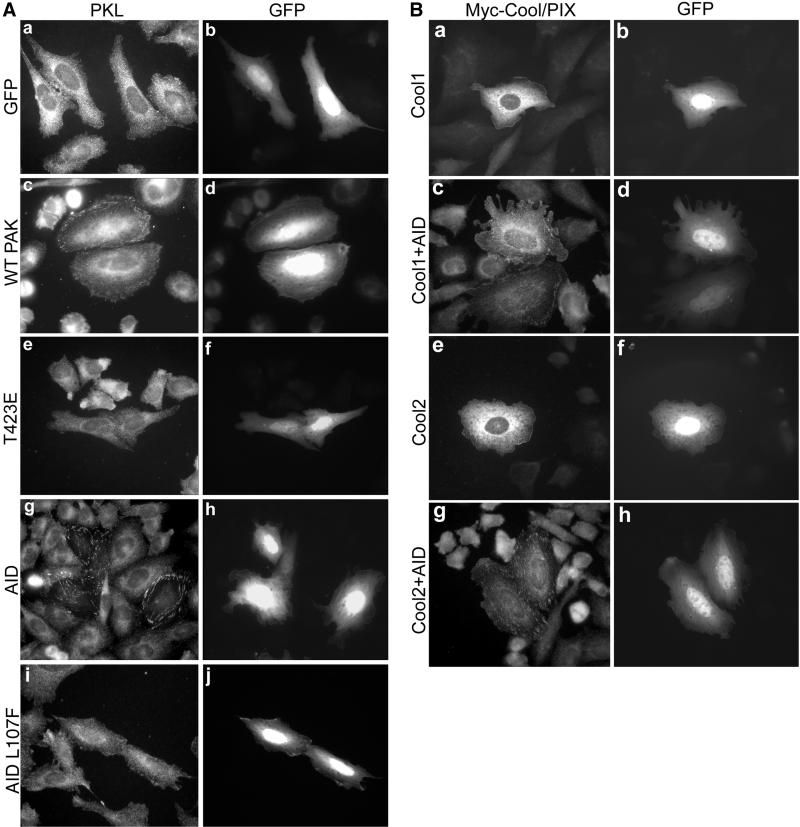
PAK is a primary downstream effector of Cdc42 and Rac; thus, the capacity of PKL localization to be regulated by PAK was examined. CHO.K1 cells were transfected with GFP. PKL was diffusely distributed within both GFP-transfected cells and nontransfectants (Figure 3A, a). Expression of myc-tagged-WT PAK1 or WT PAK3 in CHO.K1 cells resulted in only a very modest stimulation (2–3% of transfectants) of endogenous PKL focal adhesion localization (Figure 3A, c), perhaps due to a lack of PAK activation (Sells et al., 1997, 1999). To determine whether the catalytic activity or the amino-terminal regulatory/scaffold domain of PAK regulated PKL focal adhesion localization, CHO.K1 cells were transfected with myc-PAK1 T423E, a constitutively active form, or with GFP-PAK1 83–149, the kinase AID of PAK, that eliminates PAK autophosphorylation and full activation (Frost et al., 1998; Zhao et al., 1998; Zenke et al., 1999). Expression of PAK1 T423E was ineffective at inducing PKL focal adhesion localization (Figure 3A, e). However, expression of GFP-AID in CHO.K1 cells resulted in the induction of endogenous PKL localization to focal adhesions in 90% of transfectants (Figure 3A, g). A similar effect of AID expression has been described for the PKL-related Arf-GAP family member GIT1 in HeLa cells (Zhao et al., 2000b), suggesting a generalized mechanism for the regulation of PKL/GIT family Arf-GAP localization. Expression of GFP-AID L107F, which is inactive, failed to induce PKL localization to focal adhesions (Figure 3A, i).
Figure 3.
Role for PAK scaffold function in the stimulation of PKL focal adhesion localization. (A) CHO.K1 cells were transfected with GFP only (a and b) or WT PAK1 and GFP (to identify cotransfectants, c and d) followed by examination of endogenous PKL (a and c) by immunofluorescence microscopy; 2 ± 1% (n = 3) of WT PAK1 (c) and 3.75 ± 2.5% (n = 3) of WT PAK3 (our unpublished data) transfectants showed PKL in focal adhesions vs. none with GFP alone (a). Constitutively active PAK1 T423E expression did not stimulate PKL focal adhesion localization (e). However, expression of GFP-AID (g and h) but not inactive GFP-AID L107F (i and j) resulted in the induction of endogenous PKL localization to focal adhesions (g) in 90 ± 6.5% (n = 5) of transfectants. (B) Cool1–2/βα-PIX localize to focal adhesions upon GFP-AID expression. CHO.K1 cells were cotransfected with myc-Cool-1/β-PIX and GFP (a and b), myc-Cool-2/α-PIX and GFP (e and f), myc-Cool-1/β-PIX and GFP-AID (c and d), or myc-Cool-2/α-PIX and GFP-AID (h and i) followed by immunofluorescence examination of myc-Cool localization.
If PAK is mediating the localization of PKL to focal adhesions, the intermediary PIX/Cool should show a similar localization. Currently available PIX antibodies were unsuitable for immunofluorescence analysis of endogenous PIX, thus myc-tagged Cool-1/β-PIX or myc-Cool-2/α-PIX was used. Neither myc-Cool-1/β-PIX or myc-Cool-2/α-PIX was found to localize to focal adhesions in spread CHO.K1 cells as examined by cotransfection with GFP (Figure 3B, a and e). However, cotransfection with GFP-AID triggered focal adhesion localization of these PAK- and PKL-binding proteins (Figure 3B, c and g), similar to PKL (Figure 3A, g). These data implicate the amino-terminal regulatory/scaffold function of PAK, rather than the catalytic activity, in controlling PKL focal adhesion localization.
Role for PAK Scaffold Domain in PKL Localization
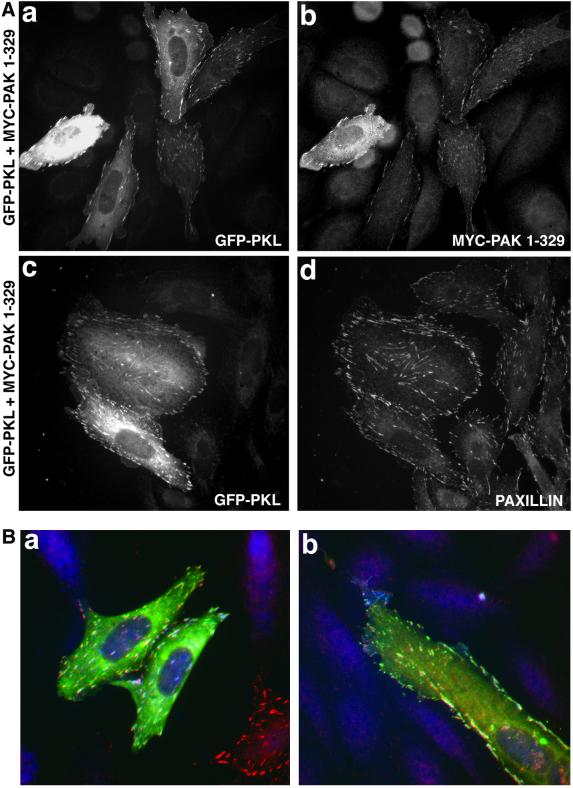
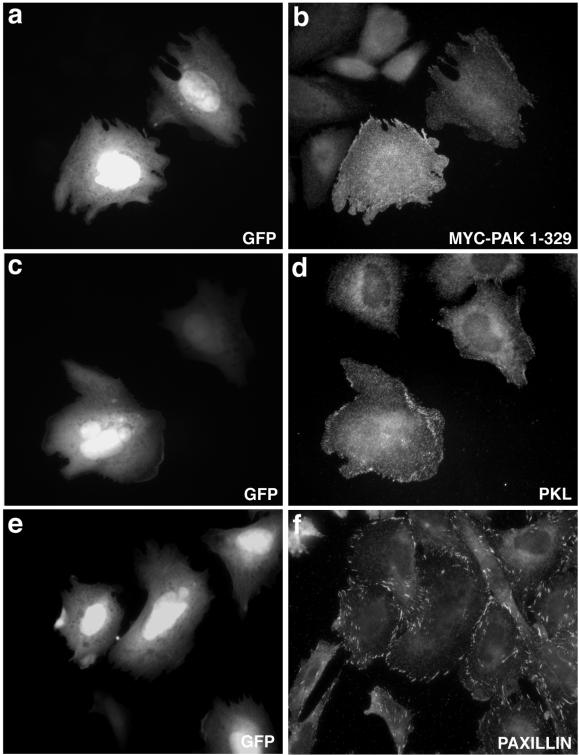
Previous studies have demonstrated that expression of the amino-terminal regulatory domain of PAK, in the absence of the catalytic domain and thus conformational repression, results in constitutive focal adhesion localization of this region of PAK (Manser et al., 1997). To confirm a role for the amino-terminal scaffold function of PAK in PKL targeting to focal adhesions we transfected CHO.K1 cells with a myc-PAK1 construct encompassing aa 1–329 (PAK1–329) containing the NCK- and PIX-binding sites but lacking the kinase domain. Cells were cotransfected with PAK1–329 and GFP full-length wild-type PKL (GFP-PKL) to allow for coincident examination of subcellular localization (Figure 4A). WT PKL and PAK1–329 colocalized to focal adhesions in 86% of cotransfectants (Figure 4A, a and b) confirming the importance of the PAK amino-terminal scaffold function in mediating PKL focal adhesion localization. GFP-PKL (Figure 3A, c) also colocalized with paxillin (Figure 4A, d) when cotransfected with PAK1–329. That these three proteins colocalize was substantiated as evidenced by the white focal adhesions generated by colocalization visualized through three-channel microscopy performed by cotransfecting CHO.K1 cells with GFP-PKL (green), PAK1–329 (blue), and WT avian paxillin (red) (Figure 4B, a and b).
Figure 4.
Scaffold domain of PAK triggers PKL and PAK focal adhesion localization. (A) GFP-WT PKL (a) and myc-PAK1–329 (b) were cotransfected into CHO.K1 cells and focal adhesion localization observed by immunofluorescence microscopy; 86 ± 9% (n = 5) of transfectants showed PAK and PKL in focal adhesions. GFP-WT PKL (c) colocalizes with paxillin within focal adhesions (d) when coexpressed with PAK1–329. (B) GFP-WT PKL and myc-PAK1–329 colocalize (white) with paxillin within focal adhesions in CHO.K1 (a and b). The cell at the bottom right with red focal adhesion staining (a) is expressing only WT avian paxillin.
PIX, but Not NCK Binding to PAK, Is Required for PKL and PAK Localization to Focal Adhesions
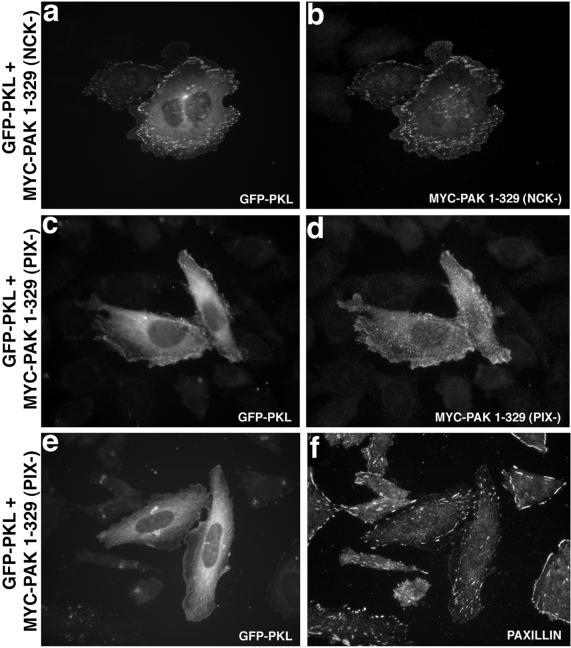
NCK–PAK–PIX–PKL interact in a linear array (Turner et al., 1999). The capacity of the PAK amino terminus to stimulate de novo localization of PKL to focal adhesions raises the question as to whether NCK or PIX binding to PAK are necessary for this effect. A role for NCK binding in the localization of PAK1–329 and PKL to focal adhesions was examined after cotransfection of GFP-WT PKL with a myc-PAK1–329/P13A (NCK−) mutant, a mutation that has been shown previously to eliminate NCK binding (Bokoch et al., 1996; Galisteo et al., 1996). No significant change in PAK or PKL focal adhesion localization was observed relative to PAK1–329 and WT PKL, indicating NCK binding to PAK was not essential (Figure 5, a and b).
Figure 5.
PIX- but not NCK-binding is required for PAK1–329 or PKL localization to focal adhesions. CHO.K1 cells were cotransfected with GFP-WT PKL (a) and myc-PAK1–329/P13A (NCK-binding defective, NCK−) (b), followed by immunofluorescence microscopy that confirmed focal adhesion localization (84 ± 6.9%, n = 4). However, expression of myc-PAK1–329 P191G/R192A (PIX binding defective, PIX−) with GFP-WT PKL resulted in an attenuation in the capacity of PKL (c) and PAK (d) to localize efficiently to focal adhesions, 28% (myc-PAK1–329 P191G/R192A) vs. 86% (myc-PAK1–329, n = 5. e shows the weak capacity of GFP-PKL to localize to focal adhesions when cotransfected with myc-PAK1–329 (PIX−). The presence of paxillin-containing focal adhesions in these cotransfectants was confirmed (f).
We generated a P191G/R192A myc-PAK1–329 (PIX−) mutant defective in PIX binding (Manser et al., 1998) to characterize the effect of perturbation of the PAK–PIX association on PAK and GFP-PKL focal adhesion localization. Consistent with previous reports, mutation of the PIX binding site significantly attenuated PIX binding to PAK1–329 as tested by coimmunoprecipitation (our unpublished data), and also resulted in an inhibition of the capacity of both PKL and PAK to colocalize to focal adhesions, with 28% of PAK1–329 (PIX−) transfectants vs. 86% for PAK1–329 (Figure 5, c and d) showing localization. The presence of paxillin-containing focal adhesions in GFP-PKL/MYC-PAK1–329 (PIX−) cotransfectants was confirmed (Figure 5e,f). Note the weak localization of PKL to focal adhesions in the cell on the right (e). Thus, efficient localization of PAK and PKL to focal adhesions requires productive binding of the intermediary PIX to PAK.
PKL Is Necessary for PAK Localization to Focal Adhesions
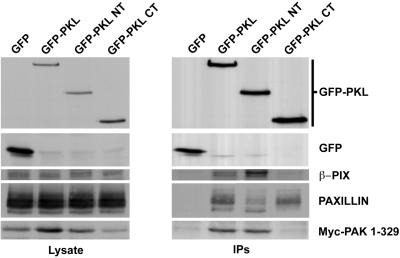
PKL is comprised of several putative functional domains, including an amino-terminal PIX-binding site and two potential paxillin-binding subdomains, PBS1 (aa 119–155) within the amino terminus and PBS2 (aa 643–679) within the carboxyl terminus (Turner et al., 1999). We have recently identified PBS2 as the principal paxillin-binding site (West et al., 2001). Consistent with this, full-length wild-type PKL coimmunoprecipitated PAK1–329, β-PIX, and paxillin (Figure 6), demonstrating the in vivo formation of this complex, whereas the PKL amino terminus (aa 1–576, GFP-PKL NT) only associated with PAK1–329 and β-PIX, and the PKL carboxyl terminus (aa 448–757, GFP-PKL CT) coimmunoprecipitated paxillin, but not β-PIX or PAK1–329. This agrees with our previous findings (Turner et al., 1999), but differs from a recent assertion that PAK can bind directly to paxillin (Hashimoto et al., 2001).
Figure 6.
In vivo formation of a PAK–PIX–PKL–paxillin complex. CHO.K1 cells were cotransfected with GFP, GFP-PKL, GFP-PKL NT, or GFP-PKL CT and myc-PAK1–329 followed by immunoprecipitation with anti-GFP and blotting for GFP, β-PIX, paxillin, and myc. Full-length PKL associates with β-PIX, paxillin, and PAK, whereas the amino terminus binds efficiently to β-PIX and PAK, and the carboxyl terminus binds to paxillin but not β-PIX or PAK.
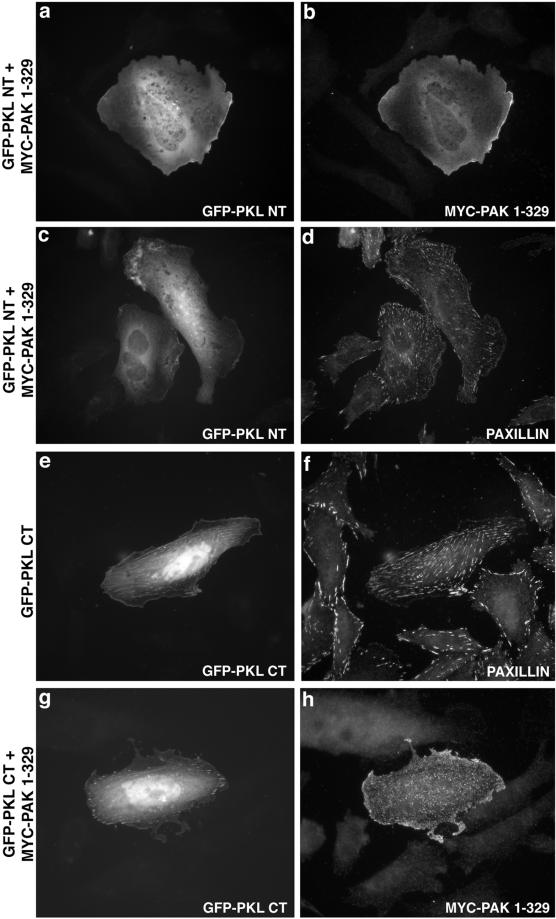
Thus, to determine whether PAK recruits PKL, or PKL recruits PAK to focal adhesions through a paxillin interaction we examined the capacity of GFP-PKL NT, containing PBS1 and the PIX-binding site, to localize to focal adhesions when coexpressed with PAK1–329 (Figure 7). Not only was the PKL amino terminus unable to localize to focal adhesions (Figure 7a) but also coexpressed PAK1–329 was prevented from localizing to focal adhesions in the presence of this PKL mutant (Figure 7b). These molecules, although unable to localize to focal adhesions, exhibited some localization to the membrane. In contrast, expression of the GFP-PKL CT, containing only PBS2, constitutively localized to paxillin-containing focal adhesions (Figure 7, e and f), suggesting that PBS2 is necessary and sufficient for PKL focal adhesion localization. In addition, this result may indicate that PKL, as with PAK (Manser et al., 1997), is conformationally constrained and requires an activation event to expose a functional PBS domain. Notably, the PKL CT was not only restricted to the points of paxillin localization but also exhibited additional localization, perhaps along stress fibers at those paxillin-containing focal adhesions. Interestingly, expression of the PKL carboxyl terminus (Figure 7g) also blocked the ability of PAK1–329 (Figure 7h) to localize to focal adhesions.
Figure 7.
PKL amino terminus cannot support PKL or PAK localization to focal adhesions, whereas the PKL carboxyl terminus is unmasked and is constitutively in focal adhesions. CHO.K1 cells were cotransfected with GFP-PKL NT and myc-PAK1–329 followed by immunofluorescence analysis of GFP-PKL (a) and myc-PAK1–329 (b). Both PKL and PAK were diffusely distributed. Paxillin localization (d) to focal adhesions in cotransfected cells (GFP-PKL NT, c) was confirmed. PKL CT is constitutively localized (e) to paxillin-containing (f) focal adhesions; further, coexpression (g) prevents PAK1–329 localization to focal adhesions (h).
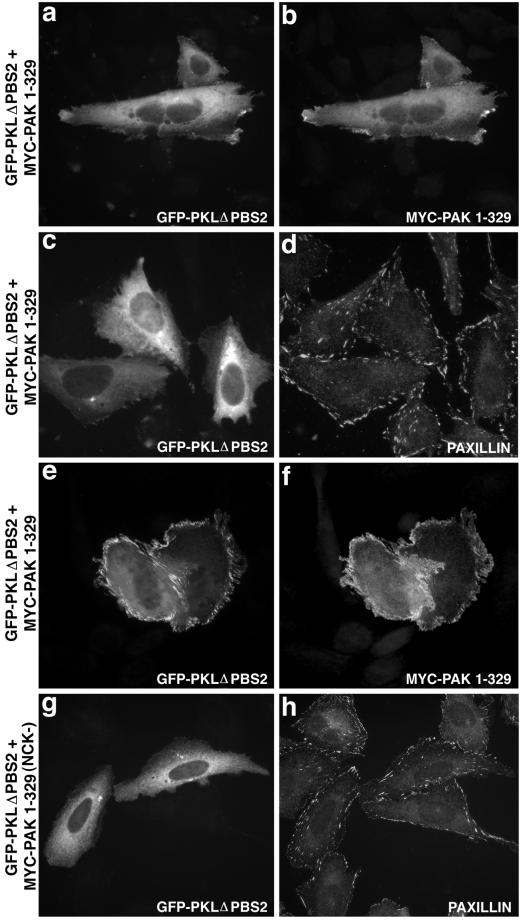
Finally, further evidence for the importance of the PKL PBS2 domain in mediating PAK–PIX–PKL localization to focal adhesions was provided by the demonstration that expression of a PBS2 deletion mutant of PKL abrogated the ability of both PKL and PAK1–329 to localize to focal adhesions (Figure 8, a and b). Notably, although a majority of PKL and PAK showed a diffuse localization, a small percentage of cells (25%) exhibited a striking peripheral plasma membrane concentration (Figure 8, e and f). Interestingly, this membranous localization was not observed upon coexpression of GFP-PKLΔPBS2 and myc-PAK1–329/P13A (Figure 8, g and h), suggesting NCK binding to PAK may mediate this phenotype.
Figure 8.
Deletion of PKL PBS2 abrogates localization of PKL and PAK to focal adhesions. CHO. K1 cells cotransfected with GFP-PKLΔPBS2 (a) and myc-PAK1–329 (b) were examined by immunofluorescence, demonstrating a diffuse as well as discrete dorsal peripheral membrane localization. Paxillin localization (d) to focal adhesions in cotransfected cells (GFP-PKLΔPBS2, c) was confirmed. A striking dorsal peripheral membrane localization of GFP-PKL ΔPBS2 (e) and myc-PAK1–329 (f) was observed in 25% of cotransfected cells. Mutation of the NCK-binding site (NCK−) on PAK (myc-PAK1–329 P13A) abrogated this membranous localization of GFP-PKL ΔPBS2 (g). Normal paxillin staining was observed as is shown in h.
Paxillin Is Essential for PAK and PKL Focal Adhesion Localization
Final confirmation of a requirement for a paxillin LD4–PKL PBS2 interaction in the localization of PKL and PAK to focal adhesions was tested by cotransfecting myc-PAK1–329 with GFP (to identify transfectants; Figure 9, a, c, and e) into CHO.K1ΔLD4 clones stably expressing paxillin lacking LD4. These cells have been previously characterized and seem to have down-regulated endogenous paxillin (West et al., 2001). The distribution of endogenous PKL and myc-tagged PAK1–329 in CHO.K1ΔLD4 cells was examined (Figure 9). The inability of PKL to bind paxillin LD4 motif resulted in the exclusion of both PKL (d) and PAK (b) from focal adhesions (f), although colocalization of PKL and PAK to a distinct membranous compartment was observed. No immunoreactive paxillin was apparent in such a compartment (f). Thus, an interaction between PKL PBS2 and paxillin LD4 motif is essential for the recruitment of PAK, as well as PKL to focal adhesions.
Figure 9.
PKL and PAK cannot localize to focal adhesions in cells expressing paxillin lacking the LD4 motif confirming paxillin-dependent recruitment of the complex to focal adhesions. CHO.K1 paxillinΔLD4 cells were cotransfected with GFP (to identify transfectants; a, c, and e) and myc-PAK1–329 followed by immunofluorescence microscopy to characterize endogenous PKL (d) and myc-PAK1–329 (b) localization. Both PKL and PAK demonstrated a diffuse as well as peripheral membrane compartmentalization distinct from the focal adhesion localization of paxillin (f).
In total, these data suggest a complex, multistage activation process to induce the localization of the NCK–PAK–PIX–PKL complex to focal adhesions. We propose the trigger is Cdc42/Rac activation of PAK scaffold function to transmit a signal through PIX to PKL, allowing the PKL PBS2 domain to interact productively with paxillin LD4, thereby facilitating recruitment of the complex to focal adhesions.
DISCUSSION
The p21-activated serine-threonine kinase PAK is a principal mediator of Cdc42- and Rac-dependent signaling to the cytoskeleton and nucleus. Regulation of PAK function is complex, consistent with the importance of maintaining precise temporal and spatial control of this signal transduction effector. Binding of PAK to the SH2-SH3 adaptor protein NCK has been implicated in PAK membrane compartmentalization, whereas binding to the Rac guanine nucleotide exchange factor PIX/Cool is necessary for localization to focal complexes. We previously identified a paxillin- and PIX/Cool-binding protein named PKL, a GIT2 Arf-GAP family member, and hypothesized that this protein may mediate PAK focal adhesion localization (Turner et al., 1999). In this report, we provide evidence for a GTP-Cdc42/GTP-Rac–triggered multistep activation cascade leading to the activation of the adaptor function of PAK, which through PIX binding leads to a conformational unmasking of the PKL PBS2 and consequent recruitment of the PAK–PIX–PKL complex to focal adhesions through the paxillin LD4 motif. Expression of the PAK amino-terminal regulatory domain (PAK1–329) or the PAK AID is sufficient for the localization of PAK and PKL to focal adhesions, demonstrating active scaffold but not catalytic function of PAK is required. In addition, PAK binding to PIX but not NCK is required for focal adhesion targeting. Importantly, although PAK activation is required, we show PKL recruits PAK to focal adhesions. The PKL amino terminus, containing PBS1 and the PIX-binding site but lacking PBS2, cannot localize to focal adhesions and blocks PAK focal adhesion localization. Furthermore, the PKL carboxyl terminus, containing PBS2 but not the PIX-binding site, localizes constitutively to focal adhesions and effectively blocks PAK targeting. Similar inhibition was observed after expression of a nontargeting PKL molecule containing a deletion of PBS2. Finally, we demonstrate that neither PAK nor PKL can localize to focal adhesions in cells overexpressing a paxillin molecule lacking the PKL binding site (LD4). This provides confirmation of a requirement for a paxillin LD4–PKL PBS2 interaction in the localization of PKL and PAK to focal adhesions.
Analysis of PKL localization in asynchronously growing, basal state cells revealed primarily a diffuse compartmentalization (Figures 1 and 3) similar to that described for PAK (Manser et al., 1997, 1998). However, PKL targeted to Cdc42 complexes and Rac1 adhesions but was excluded from RhoA focal adhesions (Figure 1) and thus exhibited an identical small p21 GTPase bias as described for PAK (Manser et al., 1997). We have found that PKL localization to focal adhesions is similarly regulated by physiological mediators of Cdc42 and Rac activation. Accordingly, Rac activation associated with respreading on fibronectin-coated coverslips stimulates PKL focal adhesion localization (Price et al., 1998; Turner et al., 1999; Cox et al., 2001; West et al., 2001), as does stimulation of CHO.K1 cells expressing bradykinin B2, epidermal growth factor, or AT1 receptors with their respective agonists (Kozma et al., 1995; Mackay and Hall, 1998; Schmitz et al., 1998; Boshans et al., 2000) (Brown, Turner, and Faussner, unpublished observations).
PAK is a primary effector of Cdc42 and Rac. Although we found expression of either WT PAK1 or WT PAK3 only modestly induced the capacity of endogenous PKL to localize to focal adhesions (Figure 3), cells with “scaffold-active” PAK (AID or 1–329 transfectants) but not kinase active PAK T423E induced efficient and robust accumulation of PKL in focal adhesions (Figures 3 and 4). Full-length PAK is generally conformationally constrained and the focal adhesion localization capacity is masked (Manser et al., 1997). Active Cdc42 or Rac binding to the PAK PBD dissociates the PAK dimer, resulting in a conformational opening and elimination of autoinhibition of PAK, in effect permitting stepwise activation and ultimately full kinase activation (Lei et al., 2000). The cycle closes upon PAK autophosphorylation, causing loss of NCK and PIX binding and a return of PAK to an inactive state (Zhao et al., 2000a; Howe, 2001). PAK functions upstream of Rac as well as downstream, presumably through stimulation of the GEF activity of PIX (Manser et al., 1998; Obermeier et al., 1998; Daniels et al., 1999; Yoshii et al., 1999). The PAK amino terminus, in the absence of the carboxyl-terminal catalytic domain, is competent for constitutive focal adhesion localization (Manser et al., 1997). In the absence of a kinase-terminating signal, PAK1–329 may provide a positive feed-forward loop of Rac activation, generating an environment allowing the maintenance of PKL and PAK in focal adhesions similar to a constitutively active Rac phenotype (Figure 1). In fact, we found that cells expressing PAK1–329 had robust levels of GTP-Rac as assessed by PBD assay (our unpublished data). However, based on prior studies, PAK1–329, in addition to being free of conformational constraint, may be expected to function similarly to the AID in blocking kinase activity (Zenke et al., 1999). The AID function may cause dissociation of the autoinhibited PAK dimer, allowing conformational opening as well as blocking PAK kinase activity. Indeed, the AID (aa 83–149) is effective in triggering PKL focal adhesion localization (Figure 2), as has been reported for the PKL-related protein GIT1 (Zhao et al., 2000b). The AID, through direct binding to and activation of endogenous PAK, may obviate the necessity for Rac activation/binding to stimulate PAK scaffold function, PKL PBS2 domain unmasking, and subsequent focal adhesion localization. PAK1–329, unlike GFP-AID, localizes to focal adhesions, probably due to the absence of the PIX-binding site in GFP-AID; however, we cannot rule out that these two molecules regulate PAK and PKL through fundamentally different mechanisms. The role for Rac activation in PAK1–329– and GFP-AID–induced PKL focal adhesion localization was examined by coexpression of dominant negative Rac. No effect on PKL localization was observed consistent with PAK adaptor function acting downstream of Rac (Sells et al., 1997, 1999; Daniels et al., 1998; Zhao et al., 1998). Further work is required to understand the precise means by which the PAK amino terminus and the AID trigger PKL localization.
NCK binding to the cytoplasmic domains of tyrosine phosphorylated growth factor receptors or perhaps to FAK (Schlaepfer et al., 1994; Bokoch et al., 1996; Galisteo et al., 1996; Lu et al., 1997; Sells et al., 1997; Lu and Mayer, 1999) has been implicated in PAK targeting to the membrane and stimulating PAK kinase activity. Formation of unipolar lamellipodia and directional motility of fibroblasts and endothelial cells, but not neurite extension, also require NCK binding (Daniels et al., 1998; Kiosses et al., 1999; Sells et al., 1997, 1999). However, mutation of the NCK binding site on PAK1–329 did not block localization of PKL or PAK to focal adhesions (Figure 5), although a role for NCK in membrane targeting is suggested by the loss of plasma membrane localization of PAK1–329/PKLΔPBS2 (Figure 8). Conversely, PAK binding to PIX is required for PAK localization to Cdc42-stimulated peripheral complexes (Manser et al., 1998) and consistent with that study, mutation of the PIX binding site severely attenuated localization of PAK as well as PKL (Figure 5). Residual PIX binding to the P191G/R192A PAK1–329 mutant was observed, which may explain the weak PKL and PAK focal adhesion localization. The inability to completely block PKL localization also may be due to the capacity of ectopic PAK1–329 P191G/R192A to activate WT PAK, perhaps through AID-like function. In addition, recent reports have noted the capacity of both PIX/Cool and PAK to dimerize, probably forming heterotetramers (Feng et al., 2001; Kim et al., 2001; Koh et al., 2001). Nonetheless, the substantial reduction in PAK1–329 P191G/R192A and PKL localization, as well as the capacity of the PKLΔPBS2 mutant to completely eliminate localization of the complex (Figure 8), suggests a PAK–PIX–PKL–paxillin array is the primary mechanism of localization of PAK to focal adhesions.
A recent report detailed the ability of GST-PAK expressed in COS7 cells to bind to hemagglutinin-paxillin expressed in insect cells, potentially bypassing a requirement for a PIX-PKL link to target PAK to focal contacts (Hashimoto et al., 2001). Although purified insect-expressed paxillin also precipitated functional serine/threonine kinase activity of unknown identity, these data were taken to indicate direct binding of PAK to paxillin, contrary to our previous report (Turner et al., 1999). GFP-PKL NT exhibited a diffuse cytoplasmic distribution and was unable to localize to focal adhesions (Figure 7). Furthermore, when coexpressed, PAK1–329 remained in the cytosol, whereas paxillin focal adhesion staining was unaffected (Figure 7). The PKL amino terminus contains PBS1, which is completely conserved in KIAA0148/GIT2short (Turner et al., 1999). It has been reported that PBS1 of GIT2short supports weak paxillin binding (Mazaki et al., 2001), and overexpression eliminates paxillin perinuclear and focal adhesion localization (Mazaki et al., 2001). However, although the PKL amino terminus exhibited some detectable paxillin binding (Figure 6), neither PKL amino terminus nor putative PAK binding to paxillin was sufficient for focal adhesion localization of these proteins nor do they promote loss of paxillin from focal adhesions in this context (Figure 6).
Evidence for the function of the carboxyl-terminal PBS2 in mediating PKL binding to paxillin was obtained by expression of GFP-PKL CT and the observation that it binds to paxillin (Figure 6) and constitutively localizes to paxillin-containing focal adhesions (Figure 7). In addition to the focal adhesion colocalization with paxillin, PKL CT also seems to extend partially along actin stress fibers at these sites of focal adhesion, suggesting the PKL amino terminus is necessary for restriction of PKL to focal adhesions. This is similar to the role of the amino-terminal paxillin LD motifs in restricting paxillin to focal adhesions (Brown et al., 1996). These localization data also indicate that full-length PKL, as with PAK, is constrained in a basal state, with the PKL focal contact localization motif inaccessible. PIX binding to GIT1 increases the affinity of GIT1 for paxillin, leading to the prescient hypothesis that the GIT1 PBS may be masked (Zhao et al., 2000b). This is also consistent with the inability of GIT2 to coprecipitate with paxillin in unstimulated cells (Premont et al., 2000). Significantly, coexpression of the PKL CT with PAK1–329 blocked the ability of PAK1–329 to localize to focal adhesions. We reason that PKL CT binding to paxillin LD4 via the PBS2 domain competes with the capacity of PAK1–329, PIX and endogenous full-length PKL to be recruited to focal adhesions. Confirmation of the necessity of PKL PBS2 for recruitment of both PKL and PAK to focal adhesions was obtained by expression of GFP-PKL lacking PBS2 (Figure 8). Whether functional unmasking of the PKL PBS2 domain involves a conformational change and/or displacement of another PKL-binding protein remains to be determined.
Finally, we demonstrate the essential nature of paxillin LD4–PKL PBS2 association in mediating PAK and PKL localization to focal adhesions by overexpressing a paxillin molecule lacking LD4 (paxillinΔLD4). Neither PAK1–329 nor PKL was capable of localizing to focal adhesions in the paxillinÆLD4 cells, confirming a requirement for this motif in recruitment of the PAK–PIX–PKL complex to focal adhesions (Figure 9).
Several reports have detailed a role for Arf-GAP family proteins in focal adhesion disassembly and recruitment of paxillin to focal adhesions that, although discordant with the role we have hypothesized for PKL, probably indicates that the diversity of Arf-GAPs reflects their discrete and specific roles in cellular regulation. The SH3- and PH-domains containing Arf-GAPs ASAP and PAPα have been reported to induce translocation of paxillin to platelet-derived growth factor-stimulated dorsal ruffles, and to inhibit paxillin recruitment to the membrane, respectively (Kondo et al., 2000; Randazzo et al., 2000). Expression of the Arf1-GAP GIT2short causes a loss of paxillin from a perinuclear compartment as well as focal adhesions (Mazaki et al., 2001), consistent with a previous study detailing a role for Arf1 in targeting paxillin to focal adhesions and potentiating Rho function (Norman et al., 1998). Similar to our data demonstrating PKL function, the related Arf-GAP protein GIT1 has been hypothesized to mediate PAK and PIX localization to focal adhesions through direct binding to paxillin (Zhao et al., 2000b). They further show that recruitment of the GIT1 complex causes the specific loss of paxillin from focal adhesions, by an unknown mechanism, to facilitate motility. In contrast, the avian ortholog of GIT1, APP1, has been reported to be involved in paxillin delivery to the membrane, to promote a similar motile phenotype, as well as Arf6-mediated membrane recycling (Di Cesare et al., 2000). We have not observed a profound loss of paxillin from focal adhesions upon expression of PKL with the PAK scaffold domain. This may reflect the nature of the focal adhesion generated by expression of (kinase-deficient) PAK1–329, i.e., arresting an adhesion at the point of PAK-mediated induction of focal adhesion formation or transition of a Rho adhesion to a Rac adhesion. Alternatively, the lack of PAK kinase activity may preclude PAK-mediated focal adhesion disassembly. Furthermore, it has been reported that GIT1 binds FAK, which mediates in part the increased cell motility observed upon overexpression of GIT1 perhaps by antagonizing Rho (Ren et al., 2000; Zhao et al., 2000b). Thus, these Arf-GAP family members may have unique roles in cytoskeletal regulation. Further studies will be required to completely understand the specific role of GIT1 and PKL/GIT2 in the PAK-paxillin axis.
What then is the role for paxillin recruitment of a PAK–PIX–PKL complex to focal adhesions? We have found that cells expressing paxillin lacking the LD4 motif exhibit persistent Rac activation, increased membrane protrusion, lamellipodia formation, cell spreading, random motility, and decrease in directional motility (West et al., 2001). These effects are recapitulated by expression of PKL lacking the PBS2 domain, whereas WT PKL has no effect. Also, it has been reported that a PAK–PIX–PKL complex is essential for upstream activation of PAK kinase activity in response to T-cell receptor stimulation; and that expression of paxillin LD4 blocks this activation (Ku et al., 2001). Although localization of PAK to the membrane is sufficient for PAK kinase activation (Lu et al., 1997; Manser et al., 1997; Lu and Mayer, 1999), the context is critical. For instance, targeting PAK to focal adhesions through an FAK focal adhesion-targeting motif carboxyl-terminal-NCK SH3 fusion that would preclude paxillin association (Hildebrand et al., 1993) did not support PAK activation (Lu et al., 1997). Together, by preventing PAK from localizing to focal adhesions in the proper context, full PAK (kinase?) activation may not be realized. Accordingly, perturbation of PAK localization to focal adhesions may prevent the autophosphorylation of sites adjacent to the NCK- and PIX-binding sites that result in shedding of NCK and PIX from PAK (Zhao et al., 2000a; Howe, 2001), and cycling of PAK out of focal adhesions. Interestingly, expression of PAK1 AID also reduces directed cell motility similar to the effects of overexpression of paxillin LD4 motif or paxillinΔLD4 (Turner et al., 1999; Zhao et al., 2000b; West et al., 2001). Blocking localization of the complex, or stabilizing the complex without normal catalytic function, may cause constitutive maintenance of the active complex and PIX-GEF activity, elevation in Rac, and prevention of a Rac-to-Rho transition by the loss of PAK targeting and/or Rac antagonism of Rho (Moorman et al., 1999; Rottner et al., 1999; Sander et al., 1999; Cox et al., 2001).
Finally, PAK kinase activity has been shown to be critical for stabilization of a dominant lamellipodium, coordination of rear tail release, and resulting directional motility (Kiosses et al., 1999; Sells et al., 1999, 2000). These effects require the coordinated cycling of Rac and Rho activities (Clark et al., 1998; Nobes and Hall, 1999). We speculate that the PAK scaffold may stimulate the formation of peripheral focal complexes and stimulate the transition of Rho focal adhesions to Cdc42/Rac focal adhesions to facilitate membrane protrusion and directional motility. The normal cycle of PAK phosphorylation events and autophosphorylation then leads to loss of focal adhesion localization, Rac-to-Rho transition to stabilize the protrusion and allow rear tail release followed by reinitiation of the cycle as required for persistent cell migration. The coordination with PAK of specific and transient targeting of an Arf GAP (PKL) to focal adhesions and consequent effects on Arf-regulated protein/membrane delivery (Radhakrishna et al., 1999; Zhang et al., 1999; Al-Awar et al., 2000; Boshans et al., 2000; Di Cesare et al., 2000) may also be expected to contribute to cell motility. Now that we have identified the mechanism of PAK targeting to focal adhesions we can begin studies aimed at the elucidation of the function of PAK/PKL cycling through focal adhesions on complex cell behaviors.
ACKNOWLEDGMENTS
We thank Drs. Rick Cerione, Jonathan Chernoff, Pam Silver, and Marc Symons for generous gifts of reagents; Dr. Rick Horwitz and Mykola Kovalenko for valuable discussions and sharing unpublished results; and Brian Bouverat for excellent technical assistance. This research was supported by grants from the National Institutes of Health General Medical Sciences Institute and the American Heart Association.
Footnotes
Article published online ahead of print. Mol. Biol. Cell 10.1091/mbc.02–02–0015. Article and publication date are at www.molbiolcell.org/cgi/doi/10.1091/mbc.02–02–0015.
REFERENCES
- Al-Awar O, Radhakrishna H, Powell NN, Donaldson JG. Separation of membrane trafficking and actin remodeling functions of ARF6 with an effector domain mutant. Mol Cell Biol. 2000;20:5998–6007. doi: 10.1128/mcb.20.16.5998-6007.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aplin AE, Howe AK, Juliano RL. Cell adhesion molecules, signal transduction and cell growth [see comments] Curr Opin Cell Biol. 1999;11:737–744. doi: 10.1016/s0955-0674(99)00045-9. [DOI] [PubMed] [Google Scholar]
- Bagrodia S, Cerione RA. PAK to the future. Trends Cell Biol. 1999;9:350–355. doi: 10.1016/s0962-8924(99)01618-9. [DOI] [PubMed] [Google Scholar]
- Bagrodia S, Taylor SJ, Jordon KA, Van Aelst L, Cerione RA. A novel regulator of p21-activated kinases. J Biol Chem. 1998;273:23633–23636. doi: 10.1074/jbc.273.37.23633. [DOI] [PubMed] [Google Scholar]
- Bishop AL, Hall A. Rho GTPases, and their effector proteins. Biochem J. 2000;348:241–255. [PMC free article] [PubMed] [Google Scholar]
- Bokoch GM, Wang Y, Bohl BP, Sells MA, Quilliam LA, Knaus UG. Interaction of the Nck adapter protein with p21-activated kinase (PAK1) J Biol Chem. 1996;271:25746–25749. doi: 10.1074/jbc.271.42.25746. [DOI] [PubMed] [Google Scholar]
- Boshans RL, Szanto S, van Aelst L, D'Souza-Schorey C. ADP-ribosylation factor 6 regulates actin cytoskeleton remodeling in coordination with Rac1 and RhoA. Mol Cell Biol. 2000;20:3685–3694. doi: 10.1128/mcb.20.10.3685-3694.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown MC, Perrotta JA, Turner CE. Identification of LIM3 as the principal determinant of paxillin focal adhesion localization and characterization of a novel motif on paxillin directing vinculin and focal adhesion kinase binding. J Cell Biol. 1996;135:1109–1123. doi: 10.1083/jcb.135.4.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown MC, Perrotta JA, Turner CE. Serine and threonine phosphorylation of the paxillin LIM domains regulates paxillin focal adhesion localization and cell adhesion to fibronectin. Mol Biol Cell. 1998;9:1803–1816. doi: 10.1091/mbc.9.7.1803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchwald G, Hostinova E, Rudolph MG, Kraemer A, Sickmann A, Meyer HE, Scheffzek K, Wittinghofer A. Conformational switch and role of phosphorylation in PAK activation. Mol Cell Biol. 2001;21:5179–5189. doi: 10.1128/MCB.21.15.5179-5189.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark EA, King WG, Brugge JS, Symons M, Hynes RO. Integrin-mediated signals regulated by members of the rho family of GTPases. J Cell Biol. 1998;142:573–586. doi: 10.1083/jcb.142.2.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox EA, Sastry SK, Huttenlocher A. Integrin-mediated adhesion regulates cell polarity and membrane protrusion through the Rho family of GTPases. Mol Biol Cell. 2001;12:265–277. doi: 10.1091/mbc.12.2.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels RH, Hall PS, Bokoch GM. Membrane targeting of p21-activated kinase 1 (PAK1) induces neurite outgrowth from PC12 cells. EMBO J. 1998;17:754–764. doi: 10.1093/emboj/17.3.754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniels RH, Zenke FT, Bokoch GM. αPix stimulates p21-activated kinase activity through exchange factor-dependent and -independent mechanisms. J Biol Chem. 1999;274:6047–6050. doi: 10.1074/jbc.274.10.6047. [DOI] [PubMed] [Google Scholar]
- Di Cesare A, Paris S, Albertinazzi C, Dariozzi S, Andersen J, Mann M, Longhi R, de Curtis I. p95-APP1 links membrane transport to Rac-mediated reorganization of actin. Nat Cell Biol. 2000;2:521–530. doi: 10.1038/35019561. [DOI] [PubMed] [Google Scholar]
- Donaldson JG, Jackson CL. Regulators and effectors of the ARF GTPases. Curr Opin Cell Biol. 2000;12:475–482. doi: 10.1016/s0955-0674(00)00119-8. [DOI] [PubMed] [Google Scholar]
- Feng Q, Albeck JG, Cerione RA, Yang W. Regulation of the cool/pix proteins: key binding partners of the Cdc42/Rac-targets, the p21-activated kinases (PAKs) J Biol Chem. 2002;277:5644–5658. doi: 10.1074/jbc.M107704200. [DOI] [PubMed] [Google Scholar]
- Frost JA, Khokhlatchev A, Stippec S, White MA, Cobb MH. Differential effects of PAK1-activating mutations reveal activity-dependent and -independent effects on cytoskeletal regulation. J Biol Chem. 1998;273:28191–28198. doi: 10.1074/jbc.273.43.28191. [DOI] [PubMed] [Google Scholar]
- Galisteo ML, Chernoff J, Su YC, Skolnik EY, Schlessinger J. The adaptor protein Nck links receptor tyrosine kinases with the serine-threonine kinase Pak1. J Biol Chem. 1996;271:20997–21000. doi: 10.1074/jbc.271.35.20997. [DOI] [PubMed] [Google Scholar]
- Hall A. Rho GTPases and the actin cytoskeleton. Science. 1998;279:509–514. doi: 10.1126/science.279.5350.509. [DOI] [PubMed] [Google Scholar]
- Hashimoto S, Tsubouchi A, Mazaki Y, Sabe H. Interaction of paxillin with p21-activated kinase (PAK). Association of paxillin α with the kinase-inactive and the Cdc42-activated forms of PAK3. J Biol Chem. 2001;276:6037–6045. doi: 10.1074/jbc.M005854200. [DOI] [PubMed] [Google Scholar]
- Hildebrand JD, Schaller MD, Parsons JT. Identification of sequences required for the efficient localization of the focal adhesion kinase, pp125FAK, to cellular focal adhesions. J Cell Biol. 1993;123:993–1005. doi: 10.1083/jcb.123.4.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotchin NA, Hall A. The assembly of integrin adhesion complexes requires both extracellular matrix and intracellular rho/rac GTPases. J Cell Biol. 1995;131:1857–1865. doi: 10.1083/jcb.131.6.1857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe AK. Cell adhesion regulates the interaction between Nck and p21-activated kinase. J Biol Chem. 2001;276:14541–14544. doi: 10.1074/jbc.C000797200. [DOI] [PubMed] [Google Scholar]
- Jackson TR, Brown FD, Nie Z, Miura K, Foroni L, Sun J, Hsu VW, Donaldson JG, Randazzo PA. ACAPs are arf6 GTPase-activating proteins that function in the cell periphery. J Cell Biol. 2000a;151:627–638. doi: 10.1083/jcb.151.3.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson TR, Kearns BG, Theibert AB. Cytohesins and centaurins: mediators of PI 3-kinase-regulated Arf signaling. Trends Biochem Sci. 2000b;25:489–495. doi: 10.1016/s0968-0004(00)01644-3. [DOI] [PubMed] [Google Scholar]
- Jockusch BM, Bubeck P, Giehl K, Kroemker M, Moschner J, Rothkegel M, Rudiger M, Schluter K, Stanke G, Winkler J. The molecular architecture of focal adhesions. Annu Rev Cell Dev Biol. 1995;11:379–416. doi: 10.1146/annurev.cb.11.110195.002115. [DOI] [PubMed] [Google Scholar]
- Kim S, Lee SH, Park D. Leucine zipper-mediated homodimerization of the p21-activated kinase-interacting factor, beta Pix. Implication for a role in cytoskeletal reorganization. J Biol Chem. 2001;276:10581–10584. doi: 10.1074/jbc.C000806200. [DOI] [PubMed] [Google Scholar]
- Kiosses WB, Daniels RH, Otey C, Bokoch GM, Schwartz MA. A role for p21-activated kinase in endothelial cell migration. J Cell Biol. 1999;147:831–844. doi: 10.1083/jcb.147.4.831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knaus UG, Bokoch GM. The p21Rac/Cdc42-activated kinases (PAKs) Int J Biochem Cell Biol. 1998;30:857–862. doi: 10.1016/s1357-2725(98)00059-4. [DOI] [PubMed] [Google Scholar]
- Koh CG, Manser E, Zhao ZS, Ng CP, Lim L. Beta1PIX, the PAK-interacting exchange factor, requires localization via a coiled-coil region to promote microvillus-like structures and membrane ruffles. J Cell Sci. 2001;114:4239–4251. doi: 10.1242/jcs.114.23.4239. [DOI] [PubMed] [Google Scholar]
- Kondo A, Hashimoto S, Yano H, Nagayama K, Mazaki Y, Sabe H. A new paxillin-binding protein, PAG3/Papα/KIAA0400, bearing an ADP-ribosylation factor GTPase-activating protein activity, is involved in paxillin recruitment to focal adhesions and cell migration. Mol Biol Cell. 2000;11:1315–1327. doi: 10.1091/mbc.11.4.1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozma R, Ahmed S, Best A, Lim L. The Ras-related protein Cdc42Hs and bradykinin promote formation of peripheral actin microspikes and filopodia in Swiss 3T3 fibroblasts. Mol Cell Biol. 1995;15:1942–1952. doi: 10.1128/mcb.15.4.1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ku GM, Yablonski D, Manser E, Lim L, Weiss A. A PAK1-PIX-PKL complex is activated by the T-cell receptor independent of Nck, Slp-76 and LAT. EMBO J. 2001;20:457–465. doi: 10.1093/emboj/20.3.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei M, Lu W, Meng W, Parrini MC, Eck MJ, Mayer BJ, Harrison SC. Structure of PAK1 in an autoinhibited conformation reveals a multistage activation switch. Cell. 2000;102:387–397. doi: 10.1016/s0092-8674(00)00043-x. [DOI] [PubMed] [Google Scholar]
- Lu W, Katz S, Gupta R, Mayer BJ. Activation of Pak by membrane localization mediated by an SH3 domain from the adaptor protein Nck. Curr Biol. 1997;7:85–94. doi: 10.1016/s0960-9822(06)00052-2. [DOI] [PubMed] [Google Scholar]
- Lu W, Mayer BJ. Mechanism of activation of Pak1 kinase by membrane localization. Oncogene. 1999;18:797–806. doi: 10.1038/sj.onc.1202361. [DOI] [PubMed] [Google Scholar]
- Mackay DJ, Hall A. Rho GTPases. J Biol Chem. 1998;273:20685–20688. doi: 10.1074/jbc.273.33.20685. [DOI] [PubMed] [Google Scholar]
- Manser E, Huang HY, Loo TH, Chen XQ, Dong JM, Leung T, Lim L. Expression of constitutively active alpha-PAK reveals effects of the kinase on actin and focal complexes. Mol Cell Biol. 1997;17:1129–1143. doi: 10.1128/mcb.17.3.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manser E, Lim L. Roles of PAK family kinases. Prog Mol Subcell Biol. 1999;22:115–133. doi: 10.1007/978-3-642-58591-3_6. [DOI] [PubMed] [Google Scholar]
- Manser E, Loo TH, Koh CG, Zhao ZS, Chen XQ, Tan L, Tan I, Leung T, Lim L. PAK kinases are directly coupled to the PIX family of nucleotide exchange factors. Mol Cell. 1998;1:183–192. doi: 10.1016/s1097-2765(00)80019-2. [DOI] [PubMed] [Google Scholar]
- Mazaki Y, Hashimoto S, Okawa K, Tsubouchi A, Nakamura K, Yagi R, Yano H, Kondo A, Iwamatsu A, Mizoguchi A, Sabe H. An ADP-ribosylation factor GTPase-activating protein git2-short/kiaa0148 is involved in subcellular localization of paxillin and actin cytoskeletal organization. Mol Biol Cell. 2001;12:645–662. doi: 10.1091/mbc.12.3.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moorman JP, Luu D, Wickham J, Bobak DA, Hahn CS. A balance of signaling by Rho family small GTPases RhoA, Rac1 and Cdc42 coordinates cytoskeletal morphology but not cell survival. Oncogene. 1999;18:47–57. doi: 10.1038/sj.onc.1202262. [DOI] [PubMed] [Google Scholar]
- Nikolopoulos SN, Turner CE. Actopaxin, a new focal adhesion protein that binds paxillin LD motifs and actin and regulates cell adhesion. J Cell Biol. 2000;151:1435–1448. doi: 10.1083/jcb.151.7.1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobes CD, Hall A. Rho GTPases control polarity, protrusion, and adhesion during cell movement. J Cell Biol. 1999;144:1235–1244. doi: 10.1083/jcb.144.6.1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobes CD, Hall A. Rho, rac, and cdc42 GTPases regulate the assembly of multimolecular focal complexes associated with actin stress fibers, lamellipodia, and filopodia. Cell. 1995;81:53–62. doi: 10.1016/0092-8674(95)90370-4. [DOI] [PubMed] [Google Scholar]
- Norman JC, Jones D, Barry ST, Holt MR, Cockcroft S, Critchley DR. ARF1 mediates paxillin recruitment to focal adhesions and potentiates Rho-stimulated stress fiber formation in intact and permeabilized Swiss 3T3 fibroblasts. J Cell Biol. 1998;143:1981–1995. doi: 10.1083/jcb.143.7.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obermeier A, Ahmed S, Manser E, Yen SC, Hall C, Lim L. PAK promotes morphological changes by acting upstream of Rac. EMBO J. 1998;17:4328–4339. doi: 10.1093/emboj/17.15.4328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petit V, Boyer B, Lentz D, Turner CE, Thiery JP, Valles AM. Phosphorylation of tyrosine residues 31 and 118 on paxillin regulates cell migration through an association with CRK in NBT-II cells. J Cell Biol. 2000;148:957–970. doi: 10.1083/jcb.148.5.957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Premont RT, Claing A, Vitale N, Perry SJ, Lefkowitz RJ. The GIT Family of ADP-ribosylation Factor GTPase-activating proteins. Functional diversity of git2 through alternative splicing. J Biol Chem. 2000;275:22373–22380. doi: 10.1074/jbc.275.29.22373. [DOI] [PubMed] [Google Scholar]
- Price LS, Leng J, Schwartz MA, Bokoch GM. Activation of Rac and Cdc42 by integrins mediates cell spreading. Mol Biol Cell. 1998;9:1863–1871. doi: 10.1091/mbc.9.7.1863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radhakrishna H, Al-Awar O, Khachikian Z, Donaldson JG. ARF6 requirement for Rac ruffling suggests a role for membrane trafficking in cortical actin rearrangements. J Cell Sci. 1999;112:855–866. doi: 10.1242/jcs.112.6.855. [DOI] [PubMed] [Google Scholar]
- Randazzo PA, Andrade J, Miura K, Brown MT, Long YQ, Stauffer S, Roller P, Cooper JA. The Arf GTPase-activating protein ASAP1 regulates the actin cytoskeleton [see comments] Proc Natl Acad Sci USA. 2000;97:4011–4016. doi: 10.1073/pnas.070552297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren XD, Kiosses WB, Sieg DJ, Otey CA, Schlaepfer DD, Schwartz MA. Focal adhesion kinase suppresses Rho activity to promote focal adhesion turnover. J Cell Sci. 2000;113:3673–3678. doi: 10.1242/jcs.113.20.3673. [DOI] [PubMed] [Google Scholar]
- Rottner K, Hall A, Small JV. Interplay between Rac and Rho in the control of substrate contact dynamics. Curr Biol. 1999;9:640–648. doi: 10.1016/s0960-9822(99)80286-3. [DOI] [PubMed] [Google Scholar]
- Rozengurt E. Convergent signaling in the action of integrins, neuropeptides, growth factors and oncogenes. Cancer Surv. 1995;24:81–96. [PubMed] [Google Scholar]
- Sander EE, ten Klooster JP, van Delft S, van der Kammen RA, Collard JG. Rac downregulates Rho activity: reciprocal balance between both GTPases determines cellular morphology and migratory behavior. J Cell Biol. 1999;147:1009–1022. doi: 10.1083/jcb.147.5.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sastry SK, Burridge K. Focal adhesions: a nexus for intracellular signaling and cytoskeletal dynamics. Exp Cell Res. 2000;261:25–36. doi: 10.1006/excr.2000.5043. [DOI] [PubMed] [Google Scholar]
- Sastry SK, Lakonishok M, Wu S, Truong TQ, Huttenlocher A, Turner CE, Horwitz AF. Quantitative changes in integrin and focal adhesion signaling regulate myoblast cell cycle withdrawal. J Cell Biol. 1999;144:1295–1309. doi: 10.1083/jcb.144.6.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlaepfer DD, Hanks SK, Hunter T, van der Geer P. Integrin-mediated signal transduction linked to Ras pathway by GRB2 binding to focal adhesion kinase. Nature. 1994;372:786–791. doi: 10.1038/372786a0. [DOI] [PubMed] [Google Scholar]
- Schmitz AA, Govek EE, Bottner B, Van Aelst L. Rho GTPases. signaling, migration, and invasion. Exp Cell Res. 2000;261:1–12. doi: 10.1006/excr.2000.5049. [DOI] [PubMed] [Google Scholar]
- Schmitz U, Ishida T, Ishida M, Surapisitchat J, Hasham MI, Pelech S, Berk BC. Angiotensin II stimulates p21-activated kinase in vascular smooth muscle cells: role in activation of JNK. Circ Res. 1998;82:1272–1278. doi: 10.1161/01.res.82.12.1272. [DOI] [PubMed] [Google Scholar]
- Schwartz MA, Baron V. Interactions between mitogenic stimuli, or, a thousand and one connections. Curr Opin Cell Biol. 1999;11:197–202. doi: 10.1016/s0955-0674(99)80026-x. [DOI] [PubMed] [Google Scholar]
- Schwartz MA, Schaller MD, Ginsberg MH. Integrins: emerging paradigms of signal transduction. Annu Rev Cell Dev Biol. 1995;11:549–599. doi: 10.1146/annurev.cb.11.110195.003001. [DOI] [PubMed] [Google Scholar]
- Sells MA, Boyd JT, Chernoff J. p21-activated kinase 1 (Pak1) regulates cell motility in mammalian fibroblasts. J Cell Biol. 1999;145:837–849. doi: 10.1083/jcb.145.4.837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sells MA, Knaus UG, Bagrodia S, Ambrose DM, Bokoch GM, Chernoff J. Human p21-activated kinase (Pak1) regulates actin organization in mammalian cells. Curr Biol. 1997;7:202–210. doi: 10.1016/s0960-9822(97)70091-5. [DOI] [PubMed] [Google Scholar]
- Sells MA, Pfaff A, Chernoff J. Temporal and spatial distribution of activated Pak1 in fibroblasts. J Cell Biol. 2000;151:1449–1458. doi: 10.1083/jcb.151.7.1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner CE. Paxillin. Int J Biochem Cell Biol. 1998;30:955–959. doi: 10.1016/s1357-2725(98)00062-4. [DOI] [PubMed] [Google Scholar]
- Turner CE. Paxillin and focal adhesion signaling. Nat Cell Biol. 2000a;2:E231–E236. doi: 10.1038/35046659. [DOI] [PubMed] [Google Scholar]
- Turner CE. Paxillin interactions. J Cell Sci. 2000b;113:4139–4140. doi: 10.1242/jcs.113.23.4139. [DOI] [PubMed] [Google Scholar]
- Turner CE, Brown MC, Perrotta JA, Riedy MC, Nikolopoulos SN, McDonald AR, Bagrodia S, Thomas S, Leventhal PS. Paxillin LD4 motif binds PAK and PIX through a novel 95-kD ankyrin repeat, ARF-GAP protein: a role in cytoskeletal remodeling. J Cell Biol. 1999;145:851–863. doi: 10.1083/jcb.145.4.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner CE, Miller JT. Primary sequence of paxillin contains putative SH2 and SH3 domain binding motifs and multiple LIM domains: identification of a vinculin and pp125Fak-binding region. J Cell Sci. 1994;107:1583–1591. doi: 10.1242/jcs.107.6.1583. [DOI] [PubMed] [Google Scholar]
- Turner CE, West KA, Brown MC. Paxillin-ARF GAP signaling, and the cytoskeleton. Curr Opin Cell Biol. 2001;13:593–599. doi: 10.1016/s0955-0674(00)00256-8. [DOI] [PubMed] [Google Scholar]
- Van Aelst L, D'Souza-Schorey C. Rho GTPases and signaling networks. Genes Dev. 1997;11:2295–2322. doi: 10.1101/gad.11.18.2295. [DOI] [PubMed] [Google Scholar]
- West KA, Zhang H, Brown MC, Nikolopoulos SN, Riedy MC, Horwitz AF, Turner CE. The LD4 motif of paxillin regulates cell spreading and motility through an interaction with paxillin kinase linker (PKL) J Cell Biol. 2001;154:161–176. doi: 10.1083/jcb.200101039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshii S, Tanaka M, Otsuki Y, Wang DY, Guo RJ, Zhu Y, Takeda R, Hanai H, Kaneko E, Sugimura H. αPIX nucleotide exchange factor is activated by interaction with phosphatidylinositol 3-kinase. Oncogene. 1999;18:5680–5690. doi: 10.1038/sj.onc.1202936. [DOI] [PubMed] [Google Scholar]
- Zenke FT, King CC, Bohl BP, Bokoch GM. Identification of a central phosphorylation site in p21-activated kinase regulating autoinhibition and kinase activity. J Biol Chem. 1999;274:32565–32573. doi: 10.1074/jbc.274.46.32565. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Calafat J, Janssen H, Greenberg S. ARF6 is required for growth factor- and rac-mediated membrane ruffling in macrophages at a stage distal to rac membrane targeting. Mol Cell Biol. 1999;19:8158–8168. doi: 10.1128/mcb.19.12.8158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao ZS, Manser E, Chen XQ, Chong C, Leung T, Lim L. A conserved negative regulatory region in αPAK: inhibition of PAK kinases reveals their morphological roles downstream of Cdc42 and Rac1. Mol Cell Biol. 1998;18:2153–2163. doi: 10.1128/mcb.18.4.2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao ZS, Manser E, Lim L. Interaction between PAK and NCK: a template for NCK targets and role of PAK autophosphorylation. Mol Cell Biol. 2000a;20:3906–3917. doi: 10.1128/mcb.20.11.3906-3917.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao ZS, Manser E, Loo TH, Lim L. Coupling of PAK-interacting exchange factor PIX to GIT1 promotes focal complex disassembly. Mol Cell Biol. 2000b;20:6354–6363. doi: 10.1128/mcb.20.17.6354-6363.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]