Abstract
von Willebrand factor (vWF) is a large, multimeric protein secreted by endothelial cells and involved in hemostasis. When expressed in AtT-20 cells, vWF leads to the de novo formation of cigar-shaped organelles similar in appearance to the Weibel-Palade bodies of endothelial cells in which vWF is normally stored before regulated secretion. The membranes of this vWF-induced organelle, termed the pseudogranule, are uncharacterized. We have examined the ability of these pseudogranules, which we show are secretagogue responsive, to recruit membrane proteins. Coexpression experiments show that the Weibel-Palade body proteins P-selectin and CD63, as well as the secretory organelle membrane proteins vesicle-associated membrane protein-2 and synaptotagmin I are diverted away from the endogenous adrenocorticotropic hormone-containing secretory granules to the vWF-containing pseudogranules. However, transferrin receptor, lysosomal-associated membrane protein 1, and sialyl transferase are not recruited. The recruitment of P-selectin is dependent on a tyrosine-based motif within its cytoplasmic domain. Our data show that vWF pseudogranules specifically recruit a subset of membrane proteins, and that in a process explicitly driven by the pseudogranule content (i.e., vWF), the active recruitment of at least one component of the pseudogranule membrane (i.e., P-selectin) is dependent on residues of P-selectin that are cytosolic and therefore unable to directly interact with vWF.
INTRODUCTION
The mechanisms of dense-cored secretory granule (DCG) formation have long been of interest, and recent progress has been made toward solving this problem such that there is now an emerging consensus as to those processes involved (reviewed in Thiele et al., 1997; Arvan and Castle, 1998; Tooze, 1998; Dannies, 1999; Glombik and Gerdes, 2000). Sorting of secretory proteins between regulated and constitutive pathways is thought to occur in the trans-Golgi network (TGN) and the immature DCG (iDCG). In the TGN, there is a calcium- and pH-dependent aggregation of certain regulated secretory proteins. This ultimately leads to formation of the dense core and serves to both concentrate and segregate the soluble proteins destined for regulated secretion from those of the constitutive pathway. This newly formed protein core buds from the TGN as an iDCG, from which residual miss-sorted proteins can be subsequently removed.
How the forming granule acquires its distinct set of membrane proteins is less clear. This is a particularly interesting issue, because conventional sorting of membrane proteins into the budding granule via the activity of specific adaptors/coats is not thought to take place; the clathrin patches on the iDCG are implicated in removal of miss-sorted, nongranule proteins rather than in the selection of proteins for entry into the iDCG itself (reviewed in Tooze, 1998).
Two nonmutually exclusive mechanisms for sorting of membrane proteins into regulated secretory granules have been proposed, both of which are consistent with the observation that many regulated secretory proteins can be found in a form that is tightly associated with the lipid bilayer (Pimplikar and Huttner, 1992) (reviewed in Thiele and Huttner, 1998). In the first mechanism both this membrane association and also the sorting are due to direct physical interaction (possibly even coaggregation) between the proteins of the forming core and the lumenal domains of secretory granule membrane proteins (Colomer et al., 1996; Rindler, 1998). In the second, the association of regulated secretory proteins with the inner leaflet of the lipid bilayer results in a distinct lipid composition of the membrane surrounding the newly forming core. This distinct lipid composition then facilitates selection of the appropriate membrane proteins through lateral protein–lipid and protein–protein interactions within the plane of the lipid bilayer (Thiele and Huttner, 1998; Blazquez et al., 2000).
However, the mechanisms outlined above may not be able to account for the sorting of all DCG membrane proteins. Heterologously expressed P-selectin is found both within the immature as well as the mature neuroendocrine granule, implying that it enters the DCG at the level of the TGN (Blagoveshchenskaya et al., 1999). However, DCG-targeting information is present within its cytoplasmic domain; the lumenal domain makes no contribution in sorting to DCG (Modderman et al., 1998; Blagoveshchenskaya et al., 1999). This is also the case for synaptobrevin 2 (vesicle-associated membrane protein-2, VAMP2) (Regazzi et al., 1996). The question thus arises, when a protein is targeted to a forming granule via its cytoplasmic domain, is the granule core still involved in its recruitment?
To answer this question, we have exploited the discovery that when von Willebrand factor (vWF) is expressed in neuroendocrine AtT-20 cells, this content protein of endothelial Weibel-Palade bodies (WPBs) and of platelet α-granules (Jaffe et al., 1973; Nachman et al., 1977) is excluded from the endogenous DCG and instead forms cigar-shaped structures reminiscent of WPBs (Wagner et al., 1991). Thus, by expressing vWF alone, a “core” can be introduced into cells, allowing independent manipulation of this component of granule formation. Because P-selectin is normally found within WPBs, this should provide an excellent system for examination of the sorting of this protein to granules. Indeed, Hop et al. (2000) show that in T24 cells vWF can influence the behavior of P-selectin. Whether this occurs by altering trafficking is as yet unclear. Moreover, whether other proteins are affected has not been addressed, nor the targeting signals involved. Finally, although the origin of T24 cells is obscure, they exhibit some endothelial characteristics (Brown et al., 2000). We have therefore examined targeting of P-selectin and other membrane proteins to vWF-containing structures in the mouse pituitary neuroendocrine cell-line AtT-20, where no endothelial-specific factors can influence their targeting.
MATERIALS AND METHODS
Antibodies and Reagents
Rabbit polyclonal anti-human vWF, anti-human vWF conjugated with horseradish peroxidase (HRP), and anti-HRP were purchased from DAKO (Cambridgeshire, United Kingdom). Sheep polyclonal and mouse monoclonal anti-human vWFs were from Serotec (Oxford, United Kingdom). Rabbit polyclonal anti-human adrenocorticotropic hormone (ACTH) was obtained from Sigma-Aldrich (St. Louis, MO). Mouse monoclonal (clone 2H11) was purchased from Insight Biotechnology (Middlesex, United Kingdom). Mouse monoclonal antibody (mAb) against the lumenal domain of human P-selectin (clone AK-6) was from AMS Biotechnology (Oxon, United Kingdom). Mouse monoclonal anti-synaptotagmin I was purchased from Synaptic Systems (Göttingen, Germany). Affinity-purified rabbit polyclonal antibody against the cytoplasmic domain of lysosomal-associated membrane protein-1 (LAMP1) was generously provided by C.R. Hopkins (Imperial College, London, United Kingdom). Mouse mAb (219.6) to rat chromogranin B (CgB) was generously provided by W.B. Huttner (Max Planck Institute of Molecular Cell Biology and Genetics, Dresden, Germany). Rabbit polyclonal serum raised against the peptide GTYGVFTNAAFDPSPC from the cytoplasmic domain of P-selectin was obtained from S. Maxwell (Imperial College, London, United Kingdom) and affinity purified using Affi-Gel 10 gel (Bio-Rad, Hemel Hempstead, United Kingdom) coupled with the peptide. Secondary antibodies conjugated either with fluorescein-isothiocyanate or with Texas-Red were from Stratech Scientific (Luton, Beds, United Kingdom).
[3H]Acetic anhydride (50 mCi/mmol) was purchased from PerkinElmer Life Sciences (Boston, MA). Affinity-purified 125I-protein A was from Amersham Biosciences UK (Little Chalfont, Buckinghamshire, United Kingdom). Other reagents unless otherwise specified were from Sigma-Aldrich.
Constructs
Human full-length vWF in pSV7D (Wagner et al., 1991) was cut with EcoRI and subcloned into pCIneo (Promega, Southampton, United Kingdom). ssHRPP-selectin and a chimera with a deletion of the entire cytoplasmic domain, ssHRPP-selectin763, were described previously (Norcott et al., 1996). The HRP–P-selectin chimera with a tetra-alanine substitution of YGVF within the cytoplasmic domain was constructed as described previously (Blagoveshchenskaya et al., 1998). HRP-transferrin receptor (TrnR) and HRP-sialyl transferase (ST) were described previously (Stinchcombe et al., 1995; Hopkins et al., 2000). cDNA encoding HRP-LAMP1 was kindly provided by C.R. Hopkins (Imperial College). cDNA encoding enhanced green fluorescent protein (EGFP)-CD63 was kindly provided by P. Luzio (Wellcome Trust Center for Molecular Mechanisms in Disease, Cambridge, United Kingdom). cDNA encoding green fluorescent protein (GFP)-VAMP2 was a generous gift from N. Thompson and R. Solari (GlaxoSmithKline, Uxbridge, Middlesex, United Kingdom).
Cell Lines and Transfections
Mouse pituitary neuroendocrine AtT-20 cells were generously provided by S. Tooze (ICRF, London, United Kingdom) and by M. Shipston (University of Edinburgh, Edinburgh, United Kingdom) and were maintained in DMEM supplemented with 10% fetal bovine serum and 50 μg/ml gentamicin (Invitrogen, Carlsbad, CA). The cells were grown at 5% CO2. Eighteen hours before transfection, cells were plated to 70% confluence. Transfections were performed using either fuGENE 6 (Roche Applied Science, Indianapolis, IN) or TransFast (Promega) according to the manufacturers' instructions. The following ratios of DNA/transfection reagent were found to be optimal (scale up for 35-mm dish): 97 μl of serum-free medium + 3 μl of fuGENE 6 + 2 μl of DNA (1 mg/ml) or 840 μl of serum-free medium + 3.2 μl of DNA (1 mg/ml) + 9.7 μl of TransFast. The DNA in this mix included either two vectors containing cDNA in a 1:1 ratio for coexpression experiments, or an empty vector and a vector encoding cDNA in the same ratio used when only one protein was to be expressed.
Subcellular Fractionation
Cells growing on 90-mm dishes were rinsed twice with homogenization buffer (HB) (0.25 M sucrose, 1 mM EDTA, 10 mM HEPES-KOH, pH 7.3), scraped in 1.5 ml of HB, and homogenized by 10 passages through a ball-bearing homogenizer with a 0.009-mm clearance (EMBL, Heidelberg, Germany). The cell homogenate was centrifuged at 800 × g for 10 min and 1.3 ml of post-nuclear supernatant (PNS) layered on top of a 12-ml preformed 0.7–1.75 M sucrose gradient made in 10 mM HEPES-KOH, pH 7.3. The gradients were centrifuged for 24 h at 35000 rpm in a SW40Ti rotor (Beckman Coulter, Fullerton, CA) and collected in 0.5-ml fractions from the top of the tube.
Stimulated vWF Release
Cells expressing vWF were rinsed with phosphate-buffered saline (PBS) and then incubated for 1 h at 37°C in Ca2+-free Hanks' balanced salt solution (125 mM NaCl, 4.75 mM KCl, 1.4 mM MgCl2, 10 mM glucose, 0.07% bovine serum albumin, 25 mM HEPES-NaOH, pH 7.3) in the absence or presence of 3 mM BaCl2. The incubation medium was then collected, centrifuged for 5 min at 1500 rpm, and immunoreactive material in the media and cell lysates quantitated by enzyme-linked immunosorbent assay (ELISA).
vWF ELISA
vWF in releasates, cell lysates, and gradient fractions was measured by a solid-phase sandwich ELISA. Briefly, polystyrene 96-well plates were coated overnight with 200 μl/well of rabbit polyclonal anti-vWF diluted in PBS (9.7 μg/ml) at room temperature. The antibody solution was removed and the plates were then incubated for 1 h at room temperature to block nonspecific binding by using 300 μl/well of 1× TEB (1% Triton X-100, 0.2% gelatin, 1 mM EDTA in PBS). The 1× TEB was removed, each well was filled with 100 μl of 2× TEB plus 100 μl of sample, and the plates were incubated while rotating for 1 h. The plates were washed three times with 250 μl of 1× TEB and incubated (200 μl/well) with rabbit polyclonal anti-vWF conjugated with HRP diluted in 1× TEB to 1.3 μg/ml for 1 h with rotation. After three washes with 250 μl/well of 1× TEB and one rinse with PBS, HRP activity was visualized using a standard o-phenylenediamine assay (see below).
Confocal Immunofluorescence Microscopy
AtT-20 cells plated on glass coverslips were transfected as described above and used for experiments 2–4 d after transfection. Cells were washed with PBS and fixed with 4% paraformaldehyde in PBS for 15 min at room temperature, washed with PBS, and quenched with 50 mM NH4Cl in PBS for 10 min. Cells were then permeabilized with 0.2% saponin, 3% gelatin in PBS for 30 min and incubated with primary antibodies in permeabilization buffer for 45 min. Primary antibodies were detected with appropriate fluorophore-conjugated secondary antibodies and viewed in an MRC-1000 confocal microscope (Bio-Rad, Hercules, CA).
Enzyme Assays
N-Acetyl-β-d-glucosaminidase (NAGA) and HRP activities were determined as described previously [Kornilova et al. (1992) and Norcott et al. (1996), respectively].
The activity of cathepsin D was evaluated by the degradation of [3H]acetyl-hemoglobin used as a substrate. [3H]Acetyl-hemoglobin was synthesized in a substitution reaction between bovine hemoglobin and [3H]acetic anhydride. Then 2 mCi of [3H]acetic anhydride was added to 3 ml of 20 mg/ml hemoglobin solution in PBS and allowed to incubate for 1 h at 0°C. Free anhydride was removed by gel filtration on Sephadex G-50 (Pharmacia, Peapack, NJ) by using 50 mM sodium acetate, pH 3.5, containing 1% NaCl as an equilibrating and eluating buffer. [3H]Acetyl-hemoglobin was then precipitated with 3% trichloroacetic acid at 4°C for 1 h, resuspended in 1 ml of 0.1 M sodium borate pH 8.5, and dialyzed against PBS. Dialyzed [3H]acetyl-hemoglobin was aliquoted and stored at −20°C. Measurement of cathepsin D activity was performed according to a modified method described previously (Dingle et al., 1977). Briefly, to 0.15 ml of subcellular fraction was added 5 μl of [3H]acetyl-hemoglobin, 250 μl of 0.2 M acetate buffer pH 3.5, and 0.1 ml of subcellular fraction buffer (1 mM NaHCO3, 1 mM EDTA, 0.01% Triton X-100). The mix was incubated for 30–60 min at 37°C and the reaction halted by adding 0.25 ml of ice-cold 30% trichloroacetic acid. Precipitation was carried out for 20 min on ice followed by centrifugation at 5000 × g for 20 min. The activity of cathepsin D was estimated by the amount of 3H radioactivity present in the supernatant.
Quantitation of ACTH Immunoreactivity
The distribution of ACTH across gradients was determined by dot-blot analysis. Gradient fractions were solubilized by boiling for 3 min in reducing sample buffer (62.5 mM Tris-HCl pH 6.8, 2% SDS, 5% β-mercaptoethanol, 0.001% bromphenol blue) and 20 μl of each fraction in quadruplicate was then immobilized on nitrocellulose membrane by using a Bio-Dot apparatus (Bio-Rad). The membranes were blocked with 5% skimmed milk in TBS (20 mM Tris-HCl pH 7.6, 50 mM NaCl) for 1 h and subsequently incubated with rabbit polyclonal anti-ACTH (diluted 1:1000 in TBS) followed by detection with 125I-protein A (diluted 1:1000 in TBS). ACTH immunoreactivity was quantitated using PhosphorImager exposure and Molecular Analyst software (Bio-Rad).
RESULTS
Pseudogranules Containing vWF Are Secretagogue-responsive Organelles Separate from ACTH-containing Endogenous Granules
In some cell lines, heterologously expressed vWF is found within rod/cigar-shaped organelles resembling endothelial WPBs (Wagner et al., 1991; Voorberg et al., 1993). However, to provide a model system with which to analyze secretory granule biogenesis, it is important to establish that these vWF-containing organelles, which we called pseudogranules, are secretagogue responsive. Previously, Wagner et al. (1991) had concluded that the heterologously expressed vWF was not secretagogue responsive, whereas Hop et al. (1997) have reported stimulated release from Madin-Darby canine kidney cells. We therefore analyzed the secretion of vWF from AtT-20 cells transiently expressing vWF. We have based our choice of transient over stable expression on findings from the Wagner group (Wagner et al., 1991) and our own unpublished observations that the level of vWF and the number of cigar-shaped organelles decrease rapidly in stable cell lines over a few passages. We took advantage of new lipid-based transfection reagents, such as TransFast or fuGENE 6, which allowed us to get 30–50% of AtT-20 cells transiently expressing the protein of interest with ∼70% coexpression of two proteins in the same cell after double transfections (see below).
To assay for secretagogue sensitivity, AtT-20 cells transiently expressing vWF at 2–3 d posttransfection were treated with BaCl2 for 1 h at 37°C. The incubation medium was then collected and the amount of vWF both in the medium and in the cell lysates was determined by ELISA. Treatment with BaCl2 led to a near doubling in vWF secretion compared with that from the control cells (Figure 1A). This pattern of release of heterologously expressed vWF from AtT20 cells is very similar to the difference between stimulated and unstimulated release of endogenous vWF from human umbilical vein endothelial cells (HUVECs) as measured in our laboratory (our unpublished data).
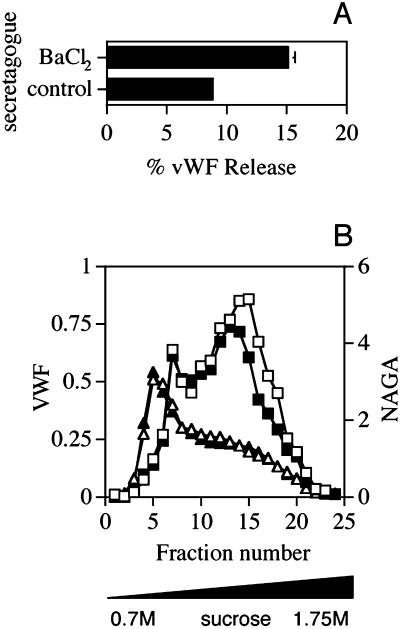
Figure 1.
Effects of secretagogue stimulation on secretion (A) and intracellular distribution (B) of vWF in AtT-20 cells. (A) vWF secretion. Duplicate dishes of AtT-20 cells transiently expressing vWF incubated in the presence or absence of 3 mM BaCl2 for 1 h at 37°C. The incubation medium was then collected and the amounts of vWF both in the medium and in the cell lysates were determined by an ELISA. The extent of stimulated vWF release was calculated as the amount of vWF released divided by cell-associated plus released vWF and expressed as a percentage. Each bar represents the mean ± SE of three independent experiments. (B) Intracellular distribution of vWF. AtT-20 cells expressing vWF were incubated in the presence or absence of 3 mM BaCl2 as described in A, rinsed with HB, homogenized, and a PNS obtained. The PNS was centrifuged on linear 0.7–1.75 M sucrose gradients to equilibrium in an SW40Ti rotor (Beckman Coulter) for 24 h at 35000 rpm, which was then collected in 0.5-ml fractions. The distribution of vWF across the gradients from control cells (□) or from cells treated with BaCl2 (∗) was monitored by ELISA (see MATERIALS AND METHODS). The distribution of NAGA activity across the same gradients was determined as described in MATERIALS AND METHODS and is shown for control cells (▴) and those treated with BaCl2 (▵). The data from one representative experiment out of two are shown.
We also examined the effects of secretagogue action on the intracellular distribution of vWF by subcellular fractionation. Transfected cells, treated with secretagogue as described above, were washed with HB, homogenized, and the PNS centrifuged to equilibrium on 0.7–1.75 M sucrose gradients. After fractionation, the distribution of vWF across the gradient was determined by ELISA. As seen in Figure 1B, vWF was localized to two distinct peaks (fractions 7–8 and fractions 12–17), the relative proportions of which were variable between experiments (Figures 4, A–C; 7B; and 8, B and C). Of these two, only the denser peak comprising fractions 12–17 diminished after secretagogue treatment of the cells. The denser peak thus contains the secretagogue-responsive population of vWF-positive secretory granules, whereas the more buoyant (i.e., less dense) material (peaking in fraction 7) probably corresponds to vWF within the early secretory pathway. This seems likely because a very similar distribution of vWF on density gradients has been demonstrated in HUVECs (Ewenstein et al., 1987). In HUVECs, electron microscopy analysis of the subcellular fractions revealed that the more buoyant peak of vWF includes the components of rough endoplasmic reticulum, plasma membrane, and Golgi apparatus, whereas the denser peak is comprised of elongated structures filled with longitudinally arranged dense filaments characteristic of WPBs (Ewenstein et al., 1987).
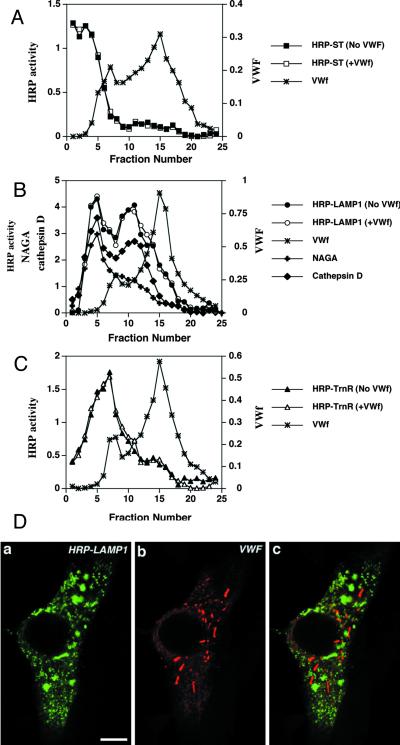
Figure 4.
vWF does not affect the localization of HRP-ST, HRP-LAMP1, and HRP-TrnR in AtT-20 cells. (A–C) Cells transiently expressing either HRP-ST, HRP-LAMP1, or HRP-TrnR in the absence or presence of vWF expression were fractionated on 0.7–1.75 M sucrose equilibrium gradients followed by measurement of HRP activity across the gradient (see MATERIALS AND METHODS). The amounts of vWF (∗), NAGA activity (+), and cathepsin D (♦) across the gradient were measured as described in MATERIALS AND METHODS. (A) Distribution of ST-HRP expressed on its own (▪) or coexpressed with vWF (□). (B) Distribution of HRP-LAMP1 expressed on its own (●) or coexpressed with vWF (○). (C) Distribution of HRP-TrnR expressed on its own (▴) or coexpressed with vWF (▵). (D) Immunofluorescence labeling of cells transiently coexpressing HRP-LAMP1 and vWF. Cells were fixed and permeabilized as described in MATERIALS AND METHODS and then colabeled with rabbit polyclonal anti-HRP (a) and sheep polyclonal anti-vWF (b). A merger of two composite channels is shown in c. Bar, 5 μm.
In the AtT-20 cells, the dense peak of vWF was not homogenous with respect to secretagogue responsiveness: vWF present within denser fractions of this broad peak was more BaCl2 responsive, whereas the lighter fractions were less affected (Figure 1B). Given that secretory granules, including WPBs (Vischer and Wagner, 1994) generally increase in density as they mature, this phenomenon is in agreement with recent findings from AtT-20 cells that newly formed immature granules are less secretagogue responsive (Eaton et al., 2000) and with documented evidence that BaCl2 stimulates Ca2+-triggered exocytosis of mature granules (von Ruden et al., 1993; von Ruden and Neher, 1993). Importantly, treatment of AtT-20 cells expressing vWF with BaCl2 did not cause any fall in amounts of a lysosomal marker NAGA, which sedimented within fractions 4–6, thus indicating the specificity of the secretagogue action on exocytic organelles (Figure 1B).
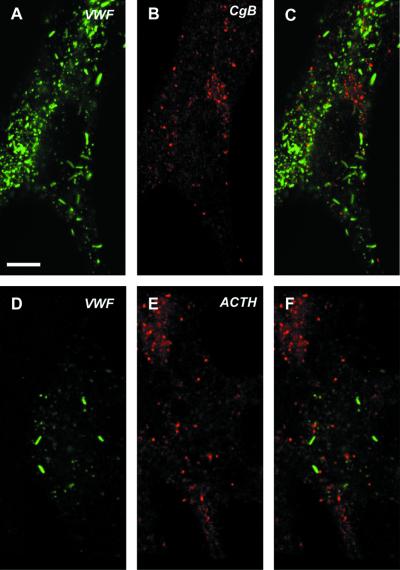
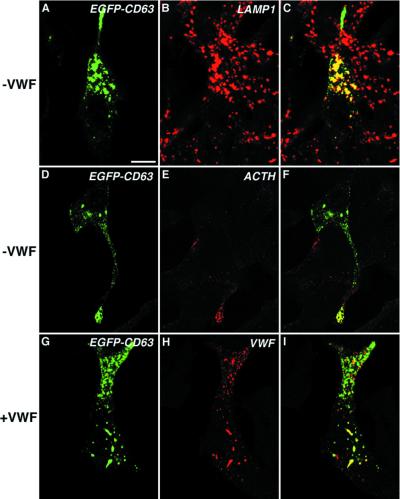
A second critical aspect of the system was whether vWF was forming organelles separate from the endogenous secretory granules as originally described (Wagner et al., 1991). We therefore examined the distribution of vWF relative to both ACTH (to confirm the observations of Wagner et al., 1991) and to CgB as markers for the endogenously regulated secretory granules. Our data (Figure 2) show that there is no staining of either of these two markers within the vWF-positive structures, confirming that the vWF is indeed differentially packaged from endogenous granule content markers.
Figure 2.
vWF pseudogranules do not contain endogenous ACTH or CgB. AtT-20 cells transiently expressing vWF (3 d posttransfection) were double labeled with either rabbit anti-vWF (A and C) and mouse monoclonal anti-CgB (B and C) or mouse monoclonal anti-vWF (D and F) and rabbit anti-ACTH (E and F). Fixation, permeabilization, and immunofluorescent labeling were performed as described in MATERIALS AND METHODS. The images shown in C and F represent the two channels merged. Bar, 5 μm.
Specific Subset of Membrane Proteins Is Sorted into Pseudogranules
For the pseudogranules to become secretagogue responsive, they must have acquired membrane proteins that confer this function. The pseudogranules could either have acquired a highly specific subset or a random sample of the membrane proteins produced in AtT-20 cells. We have examined the intracellular location of representative membrane proteins to determine whether they colocalize with vWF expressed in AtT-20 cells. Although many of the membrane proteins described below are endogenously expressed in AtT-20 cells, in this work we evaluated the effect of vWF on their localization by using transient double transfections. This was designed to match the kinetics of expression of the two proteins, thereby reducing the possibility of underestimating the diversion of membrane proteins to pseudogranules.
To determine whether membrane proteins likely to be involved in generating the secretagogue responsiveness of the pseudogranules could be diverted to vWF-containing structures, we have examined the sorting behavior of VAMP2 and synaptotagmin I when coexpressed with vWF in AtT-20 cells. These two proteins, both closely involved in regulated exocytosis, are found on secretagogue-responsive mature secretory granules of AtT-20 cells (Eaton et al., 2000) as well as in other well-studied cell types such as PC12 cells. They are therefore likely candidates for recruitment to the secretagogue-responsive pseudogranules. At present, the WPBs in endothelial cells are poorly characterized; and exactly which membrane proteins are present within these organelles, including those likely to be involved in exocytosis, has yet to be established.
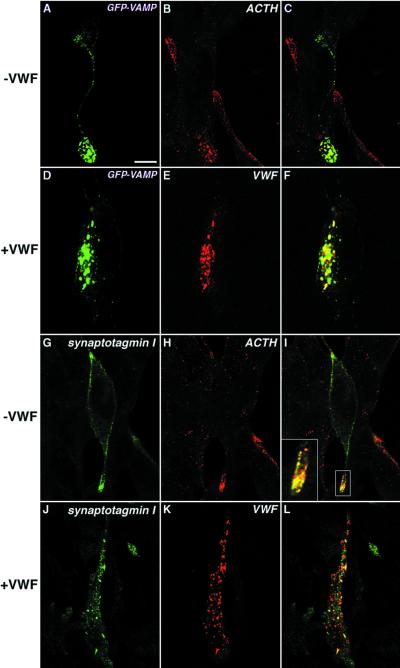
When exogenously expressed in AtT-20 cells, GFP-VAMP is colocalized with ACTH at the process tips (Figure 3, A–C). However, when coexpressed together with vWF, GFP-VAMP codistributes with vWF to the rod-shaped WPB-like granules and is no longer seen at the tips of processes where ACTH-containing DCGs are concentrated (Figure 3, D–F). Similarly, in the absence of vWF, a significant proportion of expressed full-length synaptotagmin I colocalized with ACTH at the tips of processes extending from the cell body of AtT-20 cells (Figure 3, G–I, plus inset), although some immunoreactivity was also seen associated with the plasma membrane. This latter phenomenon has been previously described for synaptotagmin, which is particularly prone to surface accumulation after overexpression (Feany and Buckley, 1993).
Figure 3.
vWF alters the localization of GFP-VAMP and synaptotagmin I in AtT-20 cells. Fixation and permeabilization of cells were carried out as described in MATERIALS AND METHODS. Cells transfected with corresponding cDNAs to transiently express GFP-VAMP (A–C) or full-length synaptotagmin I (G–I) on their own were labeled with rabbit polyclonal anti-ACTH (B and C and E and F) plus mouse monoclonal anti-synaptotagmin I (G and I). Cells coexpressing vWF plus GFP-VAMP (D–F) or vWF plus synaptotagmin I (J–L) were stained with rabbit polyclonal anti-vWF (E and F and K and L) plus mouse monoclonal anti-synaptotagmin I (J and L). C, F, I, and L show merges of the two channels. Bar, 5 μm.
In cells overexpressing either synaptotagmin I or GFP-VAMP alone, there is some ACTH staining that is not duplicated by the membrane protein. The ACTH-positive/synaptotagmin I-negative or ACTH-positive/GFP-VAMP-negative structures could result from the transient expression used, i.e., this is ACTH made before or after the wave of heterologous expression. Alternatively, they could result from the likely ability of the anti-ACTH to recognize proopiomelanocortin, which is mainly secreted constitutively. There is a small peak of ACTH immunoreactivity copurifying on our gradients with the vWF that we believe to be biosynthetic (Figure 7B), which this may represent. We were easily able to distinguish those cells overexpressing the untagged synaptotagmin I by the intensity of immunofluorescence (Figure 3, G and J). In the presence of vWF, a significant proportion of expressed synaptotagmin I was found within WPB-like organelles containing vWF (Figure 3, J–L). Thus, VAMP2 and synaptotagmin I redistribute with vWF in pseudogranules in AtT-20 cells.
Figure 7.
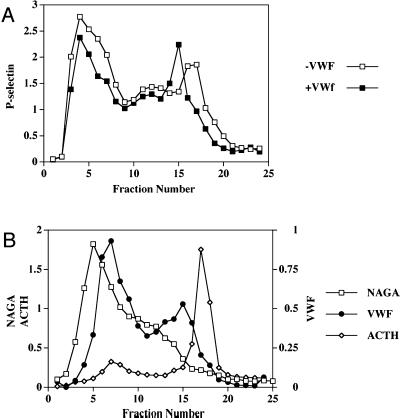
ssHRPP-selectin accumulating within ACTH-containing granules and lysosomes is diverted to pseudogranules in cells expressing vWF. AtT-20 cells transiently expressing either ssHRPP-selectin on its own or coexpressing ssHRPP-selectin plus vWF were processed by subcellular fractionation, as detailed in the legend for Figure 1B at 4 d posttransfection. (A) Distribution of HRP activity along 0.7–1.75 M sucrose equilibrium gradients from cells expressing ssHRPP-selectin on its own (□) and those coexpressing ssHRPP-selectin and vWF (▪). (B) Distribution of vWF (●), NAGA activity (□), and ACTH immunoreactivity (♦) were monitored by ELISA, by NAGA enzymatic assay, and by quantitative densitometry as described in MATERIALS AND METHODS. The data from one of three representative independent experiments are shown.
To demonstrate the specificity of the effect of vWF on the redistribution of membrane proteins, we have determined whether membrane proteins of the TGN, endosomes, or lysosomes are also found within pseudogranules. We analyzed the subcellular distribution of HRP-ST, HRP-TrnR, and HRP-LAMP1 when expressed on their own or in combination with vWF. After subcellular fractionation on 0.7–1.75 M sucrose gradients, most of the HRP-ST sedimented within the top fractions (2–4) in the absence of vWF and did not move to pseudogranules in cells cotransfected with vWF, as monitored by the distribution of HRP activity and an ELISA for vWF across the gradient (Figure 4A). Likewise, HRP-TrnR, which accumulated within fractions 5–8, was not diverted to vWF-containing pseudogranules (Figure 4C).
The HRP-LAMP1 chimera when expressed alone was found within two compartments: the lysosomes (fractions 4–6), the distribution of which coincided with that of NAGA and cathepsin D; and the late endosomes, as seen by the distribution of the second peak of cathepsin D (fractions 9–12) (Figure 4B). Such a differential accumulation of NAGA and cathepsin D within lysosomes and late endosomes has been described previously (Blagoveshchenskaia et al., 1995). No shift of HRP-LAMP1 to pseudogranules from either compartment was seen in the cells expressing vWF (Figure 4B). We have also confirmed these data by immunofluorescence double labeling of cells coexpressing HRP-LAMP1 and vWF. As shown in Figure 4D, HRP-LAMP1 was found in a variety of structures distributed throughout the cytoplasm and did not appear in rod-shaped WPB-like granules. Together, these findings demonstrate that the pseudogranules acquire a specific subset of membrane proteins, which are normally residents of the regulated secretory pathway.
Two Membrane Proteins of Weibel-Palade Bodies Are Diverted to Pseudogranules
Having established that only a subset of membrane proteins become colocalized with vWF in AtT-20 cells, we examined the sorting of the two membrane proteins known to be present in the WPB within endothelial cells, P-selectin and CD63.
CD63 is a ubiquitous membrane glycoprotein of the late endosomal/lysosomal system (Fukuda, 1991). However, in specialized cell lines it is also localized to secretory organelles such as secretory lysosomes in hematopoetic cells (Dell'Angelica et al., 2000) and WPBs in endothelial cells (Vischer and Wagner, 1993). When an EGFP-CD63 chimera is expressed on its own in AtT-20 cells, a significant proportion of the chimera was seen in LAMP1-positive organelles throughout the cytoplasm (Figure 5, A–C). In addition, a second pool of EGFP-CD63 was colocalized with ACTH at the tips of cells where the secretory granules are known to accumulate (Burgess et al., 1987; Tooze and Burke, 1987) (Figure 5, D–F). In AtT-20 cells coexpressing vWF and EGFP-CD63, some of the latter was seen in rod-shaped pseudogranules along with the vWF (Figure 5, G–I). These data therefore show that CD63, a membrane protein of bona fide endothelial WPB, is capable of entering pseudogranules in AtT-20 cells.
Figure 5.
Effect of vWF on the localization of EGFP-CD63 in AtT-20 cells. AtT-20 cells expressing EGFP-CD63 on its own (A–F) were fixed, permeabilized as described in text, and labeled either with rabbit polyclonal anti-LAMP1 (B and C) or with rabbit polyclonal anti-ACTH (E and F). Cells coexpressing EGFP-CD63 and vWF were fixed, permeabilized as described in MATERIALS AND METHODS, and then stained with rabbit polyclonal anti-vWF (H and I). C, F, and I show the two channels merged. Bar, 5 μm.
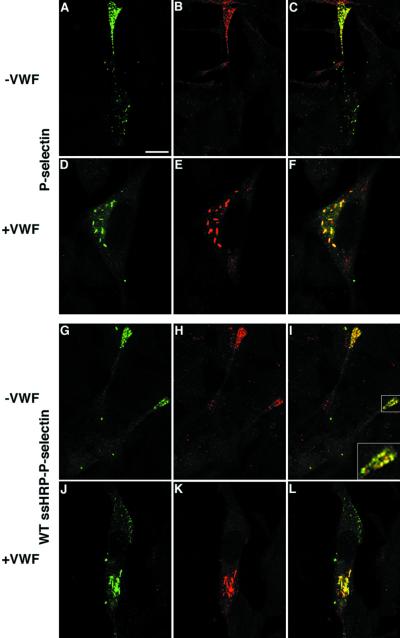
To characterize the distribution of P-selectin in the absence of vWF, cells were transfected with full-length P-selectin and analyzed by double-label confocal microscopy. Except for the occasional staining of structures located in the cell body, most likely representing endosomes/lysosomes (see below), the majority of P-selectin is seen at the tips of processes protruding from AtT-20 cells (Figure 6A). Indeed, double-label immunostaining revealed a partial colocalization of P-selectin and ACTH, a marker of the regulated secretory pathway in AtT-20, in these areas (Figure 6, A–C). These data are in agreement with previous findings that documented the accumulation of heterologously expressed P-selectin in DCG within AtT-20 cells (Koedam et al., 1992; Modderman et al., 1998). In contrast, in the cells expressing vWF, P-selectin is found in the juxta-nuclear region of the cell body where it colocalizes with vWF within rod-shaped WPB-like pseudogranules (Figure 6, D–F). These data suggest that vWF diverts P-selectin away from the existing regulated secretory pathway used by ACTH into the pseudogranules.
Figure 6.
Localization of full-length P-selectin and ssHRPP-selectin in the presence or absence of vWF in AtT-20 cells. Cells transiently expressing either full-length P-selectin (A–C) or ssHRPP-selectin (G–I) alone were double labeled with either mouse monoclonal AK-6 (A and C) and rabbit polyclonal anti-ACTH (B and C) or with mouse monoclonal 2H11 (G and I) and rabbit polyclonal anti-ACTH (H and I). AtT-20 cells transiently coexpressing vWF plus full-length P-selectin (D and F) were costained with mouse monoclonal AK-6 (D and F) and rabbit polyclonal anti-vWF (E and F), whereas cells coexpressing vWF plus ssHRPP-selectin (J–L) were double labeled with mouse monoclonal 2H11 (J and L) and rabbit polyclonal anti-vWF (K and L). Fixation, permeabilization, and immunofluorescent labeling were performed as described in MATERIALS AND METHODS. The images shown in C, F, I, and L represent the two channels merged. Bar, 5 μm.
To further analyze the trafficking of P-selectin, we have used the HRP–P-selectin chimerae that we have previously analyzed in a variety of experiments (Norcott et al., 1996; Blagoveshchenskaya et al., 1998, 1999; Blagoveshchenskaya and Cutler, 2000a,b). We first investigated whether this chimera in which the lumenal domain of P-selectin has been replaced with HRP is diverted to the pseudogranules. Double labeling revealed that the intracellular distribution of ssHRPP-selectin and full-length P-selectin in the presence or absence of vWF was indistinguishable (Figure 6, A–F and G–L). Both immunofluorescence microscopy and subcellular fractionation suggested that in the absence of vWF, P-selectin was to be found both within the ACTH-containing granules and within late endosomes/lysosomes (Figure 6, A–C and G—I; and Figure 7A), whereas vWF expression led to a significant amount of P-selectin appearing in the pseudogranules. One important question then arises: Is P-selectin within the pseudogranules being diverted from other destinations, such as endogenous ACTH-containing granules and lysosomes? Previously, Hop et al. (2000) found that despite the presence of mRNA encoding P-selectin, the protein could not be found in the absence of vWF. Their data thus imply that vWF could be affecting synthesis or degradation of P-selectin rather than its trafficking. If vWF diverts P-selectin to a new destination then we should be able to determine by subcellular fractionation whether there is a reduction of this protein within the ACTH granules and the lysosomes concomitant with its appearance in the de novo-formed pseudogranule compartment.
In the absence of vWF, ssHRPP-selectin can be seen cosedimenting with NAGA-containing lysosomes (fractions 3–5), with fractions that are enriched in endosomes (fractions 10–13), and with ACTH-containing granules (fractions 16–19) (Figure 7, A and B), in agreement with the immunofluorescence analyses. Coexpression with vWF led to a major reduction of the amount of HRP activity within ACTH-containing DCG, as well as a smaller reduction of the HRP activity within lysosomes and endosomes (Figure 7A), whereas there was an increase in HRP activity within fractions 14–16, which coincides with the secretagogue-responsive peak of vWF (Figure 1). The simplest explanation for these data is that P-selectin that would otherwise appear in the DCG (and to a lesser extent within the lysosomal/endosomal pool) was diverted to the vWF-containing organelles (Figure 7, A and B, fractions 13–16).
Sorting of P-selectin to vWF-containing Pseudogranules Is Dependent on a Tyrosine-based Signal Located within Its Cytoplasmic Domain
The previous data established that heterologously expressed vWF can influence the trafficking of specific membrane proteins, including P-selectin. We have exploited this system to determine whether in a heterologous system, where we are monitoring an explicitly core-driven process, the targeting of P-selectin to the pseudogranules is dependent on a cytoplasmic sequence that cannot interact directly with vWF.
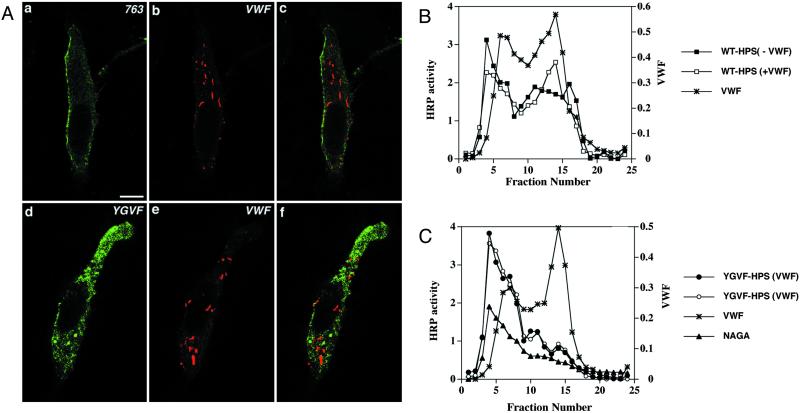
The accumulation of P-selectin within the pseudogranules could result from either active targeting or passive accumulation in this compartment. We have shown previously that delivery of this protein to the secretory granules of PC12 cells was a signal-dependent process occurring at the level of the TGN rather than a failure of removal during maturation of iDCG (Blagoveshchenskaya et al., 1999). We and others have also shown that a tyrosine-based sorting signal, 777YGVF780, within the cytoplasmic domain of P-selectin was required for targeting to DCG (Modderman et al., 1998; Blagoveshchenskaya et al., 1999). To test whether this is also the case with vWF-containing pseudogranules, AtT-20 cells were transfected with vWF plus either ssHRPP-selectin763, a chimera with a deletion of the entire cytoplasmic domain; or with ssHRPP-selectinYGVF, in which the tyrosine-based targeting determinant was replaced by tetra-alanine. Double-label indirect immunofluorescence confocal microscopy revealed that ssHRPP-selectin763 accumulated on the plasma membrane in AtT-20 cells as previously reported for PC12 cells (Norcott et al., 1996), and did not codistribute with vWF in rod-shaped pseudogranules (Figure 8A, a–c). Likewise, no colocalization was found for vWF and ssHRPP-selectinYGVF, most of which was localized to internal compartments (Figure 8A, d–f), as would be expected from our analysis in PC12 cells where it was found both in early and late endosomes as well as in lysosomes (Blagoveshchenskaya et al., 1999).
Figure 8.
vWF has no effect on the distribution of ssHRPP-selectin763 and ssHRPP-selectinYGVF in AtT-20 cells. (A) Double immunofluorescence labeling of AtT-20 cells transiently coexpressing ssHRPP-selectin763 (763) and vWF (a–c) as well as ssHRPP-selectinYGVF (YGVF) and vWF (d–f). After fixation and permeabilization, the cells were stained with mouse monoclonal 2H11 (a and c and d and f) and rabbit polyclonal anti-vWF (b and c and e and f). c and f represent a merger of two composite channels. (B and C) Subcellular compartmentalization of ssHRPP-selectin, WT-HPS (B), and ssHRPP-selectinYGVF (YGVF-HPS) (C) in the cells expressing or mock-expressing vWF. AtT-20 cells transiently expressing ssHRPP-selectin (▪), ssHRPP-selectin plus vWF (□), ssHRPP-selectinYGVF (●), and ssHRPP-selectinYGVF plus vWF (○) were processed by subcellular fractionation and the distribution of HRP activity across the gradients was monitored as described in the legend for Figure 3. The distributions of vWF (∗) and NAGA activity (▴) are shown. Data from one of two representative experiments are displayed.
We further examined the intracellular distribution of ssHRPP-selectinYGVF in parallel with wild-type ssHRPP-selectin by using subcellular fractionation. In the absence of vWF expression, ssHRPP-selectinYGVF (YGVF-HPS) predominantly accumulated within the NAGA-enriched lysosomal peak (Figure 8C, fractions 4–7), whereas its targeting to the ACTH-containing DCG (fractions 15–17) was very much reduced compared with a wild-type chimera (WT-HPS) (Figure 8B). In agreement with the immunofluorescence data (Figure 8A, d–f), vWF coexpressed with ssHRPP-selectinYGVF did not cause a rise of HRP activity within the pseudogranule peak (Figure 8C, fractions 13–15), as seen for wild-type ssHRPP-selectin (Figure 8B). Together, these data suggest that the tyrosine-based signal, which functions as a targeting signal to ACTH-containing DCGs, is also needed for delivery of P-selectin to pseudogranules.
DISCUSSION
In this study, we have investigated the acquisition of membrane proteins by a heterologously introduced endothelial secretory granule core containing vWF in neuroendocrine AtT-20 cells. Previous findings (Wagner et al., 1991; Voorberg et al., 1993; Hop et al. 1997) and the present work demonstrate that heterologously expressed vWF induces the formation of organelles of similar morphology and density to the WPB of endothelial cells (Weibel and Palade, 1964). Importantly, these pseudogranules segregate away from endogenous secretory granules (Wagner et al., 1991; this study) and from proteins of other TGN-derived pathways (Hop et al., 1997), indicating that vWF is packaged into its own unique cigar-shaped organelle.
One of the hallmarks of bona fide secretory granules is the ability to undergo exocytosis after external stimulation with secretagogue. Wagner et al. (1991) previously concluded that the vWF expressed in AtT-20 cells was not secretagogue responsive. Under the experimental conditions used herein, we found that vWF release into the medium can be stimulated twofold, as can ACTH by treatment with BaCl2 (Xiang et al., 2000). Because this is a quantitatively similar response to the stimulation of WPBs in HUVECs, we conclude that the pseudogranules are secretagogue responsive. These data, coupled with the diminution of intracellular pseudogranule vWF after BaCl2 treatment as monitored by subcellular fractionation (Figure 1), provide evidence that the vWF-induced pseudogranules are indeed bona fide exocytic organelles. The differences between the results obtained by Wagner and coworkers and us presumably reflect differences in cell culture, expression systems, and the particular secretagogue used.
We have found that the vWF pseudogranules recruit two groups of membrane proteins: those normally localized to bona fide endothelial WPB, such as P-selectin and CD63; and proteins that are part of the exocytic machinery, such as VAMP2 and synaptotagmin I, variants of which have been found in all regulated secretory organelles. For P-selectin, a tyrosine-based targeting determinant within its cytoplasmic tail directs this protein to both the endogenous ACTH-containing DCGs and the vWF-containing pseudogranules, whereas the lumenal domain of P-selectin was not required for its delivery to either. Importantly, membrane proteins not normally found in the membrane of secretory granules within either neuroendocrine or endothelial cells such as sialyl transferase, transferrin receptor, or LAMP1 did not accumulate within the pseudgranules. These data therefore rule out the possibility of nonspecific membrane flow to the pseudogranules and emphasize that the acquisition of their membrane proteins is selective. Furthermore, the signal dependence of P-selectin targeting strongly implies that these membrane proteins are being actively targeted to the vWF structures rather than failing to be removed.
We have therefore discovered that in a heterologous system where we are monitoring an explicitly core-driven process, the targeting of P-selectin to the pseudogranules is actively dependent on a cytoplasmic sequence that cannot interact directly with vWF.
In a previous work, Pannekoek and coworkers (Hop et al., 2000) showed that in T24 cells, expression of vWF caused the endogenously expressed P-selectin to colocalize with the core protein. Because, in the absence of vWF, P-selectin was not detected at the protein level, these data did not allow distinction between a number of possible explanations for this phenomenon. Although our data are in agreement with that study they also provide a likely explanation for the appearance of P-selectin in the pseudogranules only in the presence of vWF. We suggest that in the absence of vWF, P-selectin is synthesized, delivered to the lysosome, and degraded in cells lacking a regulated secretory pathway, as observed previously (Green et al., 1994; Blagoveshchenskaya et al., 1998). When vWF is expressed, P-selectin is diverted to the pseudogranules, and therefore escapes degradation, allowing its detection by immunofluorescence.
Role for Cytoplasmic Domain in Targeting Membrane Proteins to Pseudogranules
Although for some membrane proteins direct interaction between their lumenal domain and the core proteins might account for their targeting to granules (Colomer et al., 1996; Rindler, 1998), the lumenal domain of P-selectin is not involved in the recruitment of this protein to vWF-containing pseudogranules, because its replacement by HRP does not affect the appearance of HRP–P-selectin within these organelles. Importantly, this phenomenon is not restricted to P-selectin, because VAMP2 is also targeted to granules by a sequence within its cytoplasmic domain (Regazzi et al., 1996). Another mechanism implicated in granule formation involves interactions between proteins and the lipids of the granule membrane. Lipid microdomains are capable of interacting with some membrane-associated proteins and therefore may be involved in directing them to secretory granules (Blazquez et al., 2000; Dhanvantari and Loh, 2000). Although in those examples described, association of these proteins with lipid microdomains must have occurred within the lumen of endomembrane system, such interactions could in principle also take place between the transmembrane or cytoplasmic domains of membrane proteins and lipid microdomains. Given that the transmembrane domain of P-selectin is known to facilitate sorting of this protein to granules (Fleming et al., 1998), a role for the lipid environment may well be involved in lateral redistribution of P-selectin into microdomains, which might, in turn, provide a membrane for the budding granule (Thiele and Huttner, 1998). However, because the transmembrane domain of P-selectin is not sufficient for sorting but only serves to increase its efficiency (Fleming et al., 1998), this mechanism can only be part of the overall process by which P-selectin is sorted into granules.
An alternative hypothesis to account for the involvement of a cytoplasmic tyrosine-based motif (YGVF) in delivery of P-selectin to secretory granules arises from a consideration of where along the secretory pathway sorting of P-selectin to pseudogranules is occurring. Sorting of P-selectin for delivery to lysosomes or ACTH granules most likely takes place at or before the TGN, although sorting of some proteins to secretory granules may occur earlier in the secretory pathway (reviewed in Dannies, 1999). Indeed, recent three-dimensional reconstructions of the Golgi complex by using high-voltage electron microscopy provide strong circumstantial evidence that proteins traveling to different post-Golgi destinations can be sorted before the TGN. This study suggests that proteins may be delivered in separate transport vesicles to particular structures at the trans-Golgi, each of which then produces only one type of vesicle, presumably bound for one particular destination (Ladinsky et al., 1999).
If this is extrapolated to the trafficking of P-selectin to pseudogranules, we hypothesize that delivery to the membrane that gives rise to pseudogranules may use conventional coat/adaptor-dependent cytoplasmic signal-mediated selection for inclusion in the appropriate transport vesicle earlier in the secretory pathway than the point at which the pseudogranules bud. This could also account for the tyrosine signal-based delivery of P-selectin to the iDCG of PC12 cells. A full analysis of this hypothesis will require a detailed electron microscopy survey of the transfected AtT-20 cells; a suitable future project.
Acquisition of Membrane Proteins by vWF Pseudogranules
The differential packaging of vWF and ACTH in the same cell might have suggested some fundamental difference in the formation of WPBs and ACTH-containing granules, resulting in differential membrane recruitment. Indeed, the unimpaired formation of WPBs in CPE-deficient mice with defects in the biogenesis of ACTH-containing secretory granules (Methia et al., 1999) as well as in patients with gray platelet syndrome who possess abnormal platelet α-granules, which normally contain vWF (Gebrane-Younes et al., 1993), suggests that formation of WPBs might be significantly different from that of other secretory granules.
However, our data show that these two very different secretory granule cores, that of the ACTH-containing secretory granules or the vWF-containing pseudogranules, recruit the same proteins from among those that we expressed in AtT-20 cells. In addition, we found that the same tyrosine-based sorting signal, 777YGVF780, within the cytoplasmic domain of P-selectin is needed for recruitment of P-selectin to both the pseudogranules and the endogenous ACTH-containing granules, reinforcing the argument that the cellular machinery delivering membrane proteins to granules is recognizing the pseudogranules as bona fide secretory organelles.
We have previously shown that in PC12 cells the same tyrosine-based sorting signal promotes targeting of P-selectin not only to the DCG but also to another type of regulated secretory organelle, synaptic-like microvesicles (Blagoveshchenskaya et al., 1999). A similar phenomenon has also been seen for VAMP2, in which an amphipathic α-helix was found to be a key determinant in targeting this protein to both insulin-containing secretory granules and synaptic-like microvesicles in pancreatic β-cells (Regazzi et al., 1996). Together, these data show that the appearance of a membrane protein in multiple regulated secretory organelles can be dependent on the same targeting signal
Advantages of Heterologous Introduction of vWF Core for Analyses of Granule Formation
Most previous studies using cDNA expression to delineate the mechanisms of granule formation focused on the sorting of heterologously expressed proteins to endogenous secretory granules in cells with a regulated secretory pathway. In the current work, we took an inverted approach, involving introduction of a separate endothelial secretory granule core into neuroendocrine AtT-20 cells. This has provided a graphic demonstration of the extent to which granule formation is core driven as well as novel insights into the universality of acquisition of membrane proteins during their biosynthesis. This new approach might also allow for a more stringent test for the specificity of granule targeting of membrane proteins than has hitherto been achieved, because preferential targeting to different types of secretory granules within the same cell could now be analyzed. For instance, in those individual cells with a low level of vWF expression only a modest proportion of P-selectin was seen within pseudogranules, with significant amounts of this protein remaining associated with ACTH-containing DCGs. However, almost all of the P-selectin redistributed to pseudogranules from ACTH-containing granules in individual cells with a high level of vWF expression (our unpublished data). Interestingly, in platelets, P-selectin is found within both types of secretory granules at steady state, α-granules and dense bodies, but only α-granules contain vWF (Stenberg et al., 1985; Israels et al., 1992), thus implying that the effect of vWF on P-selectin traffic is not absolute when examined in a physiological context. Further study is needed for a clearer understanding of these complex relationships.
ACKNOWLEDGMENTS
We thank the following for the kind gift of cells, antibodies, or plasmids: W.B. Huttner (Max Planck Institute for Molecular Cell Biology and Genetics, Dresden, Germany); N. Thompson and R. Solari (GlaxoSmithKline, Uxbridge, Middlesex, United Kingdom); C.R. Hopkins and S. Maxwell (Imperial College, London, United Kingdom); P. Luzio (Wellcome Trust Center for Molecular Mechanisms in Disease, Cambridge, United Kingdom); and S. Tooze (ICRF, London, United Kingdom) and M. Shipston (University of Edinburgh, Edinburgh, United Kingdom). This work was funded by a Medical Research Council program grant (to D.F.C.).
Abbreviations used:
- CgB
chromogranin B
- DCG
dense-cored secretory granule
- HB
homogenization buffer
- iDCG
immature dense-cored secretory granule
- NAGA
N-acetyl-β-d-glucosaminidase
- ST
sialyl transferase
- TrnR
transferrin receptor
- vWF
von Willebrand factor
- WPB
Weibel-Palade body
Footnotes
DOI: 10.1091/mbc.01–09–0462.
REFERENCES
- Arvan P, Castle D. Sorting and storage during secretory granule biogenesis: looking backward and looking forward. Biochem J. 1998;332:593–610. doi: 10.1042/bj3320593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blagoveshchenskaya AD, Cutler DF. Biochemical analyses of trafficking with horseradish peroxidase-tagged chimeras. Methods Enzymol. 2000a;327:45–60. doi: 10.1016/s0076-6879(00)27266-2. [DOI] [PubMed] [Google Scholar]
- Blagoveshchenskaya AD, Cutler DF. Sorting to synaptic-like microvesicles from early and late endosomes requires overlapping but not identical targeting signals. Mol Biol Cell. 2000b;11:1801–1814. doi: 10.1091/mbc.11.5.1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blagoveshchenskaya AD, Hewitt EW, Cutler DF. A complex web of signal-dependent trafficking underlies the triorganellar distribution of P-selectin in neuroendocrine PC12 cells. J Cell Biol. 1999;145:1419–1433. doi: 10.1083/jcb.145.7.1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blagoveshchenskaia AD, Kornilova ES, Nikol'skii NN. The endocytosis of the epidermal growth factor (EGF) in EGF-dependent cells of the HC11 mouse mammary epithelium line. Tsitologiia. 1995;37:883–892. [PubMed] [Google Scholar]
- Blagoveshchenskaya AD, Norcott JP, Cutler DF. Lysosomal targeting of P-selectin is mediated by a novel sequence within its cytoplasmic tail. J Biol Chem. 1998;273:2729–2737. doi: 10.1074/jbc.273.5.2729. [DOI] [PubMed] [Google Scholar]
- Blazquez M, Thiele C, Huttner WB, Docherty K, Shennan KI. Involvement of the membrane lipid bilayer in sorting prohormone convertase 2 into the regulated secretory pathway. Biochem J. 2000;349:843–852. doi: 10.1042/bj3490843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J, Reading SJ, Jones S, Fitchett CJ, Howl J, Martin A, Longland CL, Michelangeli F, Dubrova YE, Brown CA. Critical evaluation of ECV304 as a human endothelial cell model defined by genetic analysis and functional responses: a comparison with the human bladder cancer derived epithelial cell line T24/83. Lab Invest. 2000;80:37–45. doi: 10.1038/labinvest.3780006. [DOI] [PubMed] [Google Scholar]
- Burgess TL, Craik CS, Matsuuchi L, Kelly RB. In vitro mutagenesis of trypsinogen: role of the amino terminus in intracellular protein targeting to secretory granules. J Cell Biol. 1987;105:659–668. doi: 10.1083/jcb.105.2.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colomer V, Kicska GA, Rindler MJ. Secretory granule content proteins and the luminal domains of granule membrane proteins aggregate in vitro at mildly acidic pH. J Biol Chem. 1996;271:48–55. doi: 10.1074/jbc.271.1.48. [DOI] [PubMed] [Google Scholar]
- Dannies PS. Protein hormone storage in secretory granules: mechanisms for concentration and sorting. Endocr Rev. 1999;20:3–21. doi: 10.1210/edrv.20.1.0354. [DOI] [PubMed] [Google Scholar]
- Dell'Angelica EC, Mullins C, Caplan S, Bonifacino JS. Lysosome-related organelles. FASEB J. 2000;14:1265–1278. doi: 10.1096/fj.14.10.1265. [DOI] [PubMed] [Google Scholar]
- Dhanvantari S, Loh YP. Lipid raft association of carboxypeptidase E is necessary for its function as a regulated secretory pathway sorting receptor. J Biol Chem. 2000;275:29887–29893. doi: 10.1074/jbc.M005364200. [DOI] [PubMed] [Google Scholar]
- Dingle JT, Blow AM, Barrett AJ, Martin PE. Proteoglycan-degrading enzymes. A radiochemical assay method and the detection of a new enzyme cathepsin F. Biochem J. 1977;167:775–785. doi: 10.1042/bj1670775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaton BA, Haugwitz M, Lau D, Moore HP. Biogenesis of regulated exocytotic carriers in neuroendocrine cells. J Neurosci. 2000;20:7334–7344. doi: 10.1523/JNEUROSCI.20-19-07334.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewenstein BM, Warhol MJ, Handin RI, Pober JS. Composition of the von Willebrand factor storage organelle (Weibel-Palade body) isolated from cultured human umbilical vein endothelial cells. J Cell Biol. 1987;104:1423–1433. doi: 10.1083/jcb.104.5.1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feany MB, Buckley KM. The synaptic vesicle protein synaptotagmin promotes formation of filopodia in fibroblasts. Nature. 1993;364:537–540. doi: 10.1038/364537a0. [DOI] [PubMed] [Google Scholar]
- Fleming JC, Berger G, Guichard J, Cramer EM, Wagner DD. The transmembrane domain enhances granular targeting of P-selectin. Eur J Cell Biol. 1998;75:331–343. doi: 10.1016/s0171-9335(98)80066-6. [DOI] [PubMed] [Google Scholar]
- Fukuda M. Lysosomal membrane glycoproteins. Structure, biosynthesis, and intracellular trafficking. J Biol Chem. 1991;266:21327–21330. [PubMed] [Google Scholar]
- Gebrane-Younes J, Cramer EM, Orcel L, Caen JP. Gray platelet syndrome. Dissociation between abnormal sorting in megakaryocyte alpha-granules and normal sorting in Weibel-Palade bodies of endothelial cells. J Clin Invest. 1993;92:3023–3028. doi: 10.1172/JCI116926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glombik MM, Gerdes HH. Signal-mediated sorting of neuropeptides and prohormones: secretory granule biogenesis revisited. Biochimie. 2000;82:315–326. doi: 10.1016/s0300-9084(00)00195-4. [DOI] [PubMed] [Google Scholar]
- Green SA, Setiadi H, McEver RP, Kelly RB. The cytoplasmic domain of P-selectin contains a sorting determinant that mediates rapid degradation in lysosomes. J Cell Biol. 1994;124:435–448. doi: 10.1083/jcb.124.4.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hop C, Fontijn R, van Mourik JA, Pannekoek H. Polarity of constitutive and regulated von Willebrand factor secretion by transfected MDCK-II cells. Exp Cell Res. 1997;230:352–361. doi: 10.1006/excr.1996.3431. [DOI] [PubMed] [Google Scholar]
- Hop C, Guilliatt A, Daly M, de Leeuw HP, Brinkman HJ, Peake IR, van Mourik JA, Pannekoek H. Assembly of multimeric von Willebrand factor directs sorting of P-selectin. Arterioscler Thromb Vasc Biol. 2000;20:1763–1768. doi: 10.1161/01.atv.20.7.1763. [DOI] [PubMed] [Google Scholar]
- Hopkins C, Gibson A, Stinchcombe J, Futter C. Chimeric molecules employing horseradish peroxidase as reporter enzyme for protein localization in the electron microscope. Methods Enzymol. 2000;327:35–45. doi: 10.1016/s0076-6879(00)27265-0. [DOI] [PubMed] [Google Scholar]
- Israels SJ, Gerrard JM, Jacques YV, McNicol A, Cham B, Nishibori M, Bainton DF. Platelet dense granule membranes contain both granulophysin and P- selectin (GMP-140) Blood. 1992;80:143–152. [PubMed] [Google Scholar]
- Jaffe EA, Nachman RL, Becker CG, Minick CR. Culture of human endothelial cells derived from umbilical veins. Identification by morphologic and immunologic criteria. J Clin Invest. 1973;52:2745–2756. doi: 10.1172/JCI107470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koedam JA, Cramer EM, Briend E, Furie B, Furie BC, Wagner DD. P-selectin, a granule membrane protein of platelets and endothelial cells, follows the regulated secretory pathway in AtT-20 cells. J Cell Biol. 1992;116:617–625. doi: 10.1083/jcb.116.3.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornilova ES, Taverna D, Hoeck W, Hynes NE. Surface expression of erB-2 protein is post-transcriptionally regulated in mammary epithelial cells by epidermal growth factor and by the culture density. Oncogene. 1992;7:511–519. [PubMed] [Google Scholar]
- Ladinsky MS, Mastronarde DN, McIntosh JR, Howell KE, Staehelin LA. Golgi structure in three dimensions: functional insights from the normal rat kidney cell. J Cell Biol. 1999;144:1135–1149. doi: 10.1083/jcb.144.6.1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Methia N, Denis CV, Wagner DD. Carboxypeptidase E does not mediate von Willebrand factor targeting to storage granules. Eur J Cell Biol. 1999;78:884–891. doi: 10.1016/S0171-9335(99)80090-9. [DOI] [PubMed] [Google Scholar]
- Modderman PW, Beuling EA, Govers LA, Calafat J, Janssen H, Von dem Borne AE, Sonnenberg A. Determinants in the cytoplasmic domain of P-selectin required for sorting to secretory granules. Biochem J. 1998;336:153–161. doi: 10.1042/bj3360153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nachman R, Levine R, Jaffe EA. Synthesis of factor VIII antigen by cultured guinea pig megakaryocytes. J Clin Invest. 1977;60:914–921. doi: 10.1172/JCI108846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norcott JP, Solari R, Cutler DF. Targeting of P-selectin to two regulated secretory organelles in PC12 cells. J Cell Biol. 1996;134:1229–1240. doi: 10.1083/jcb.134.5.1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pimplikar SW, Huttner WB. Chromogranin B (secretogranin I), a secretory protein of the regulated pathway, is also present in a tightly membrane-associated form in PC12 cells. J Biol Chem. 1992;267:4110–4118. [PubMed] [Google Scholar]
- Regazzi R, Sadoul K, Meda P, Kelly RB, Halban PA, Wollheim CB. Mutational analysis of VAMP domains implicated in Ca2+-induced insulin exocytosis. EMBO J. 1996;15:6951–6959. [PMC free article] [PubMed] [Google Scholar]
- Rindler MJ. Carboxypeptidase E, a peripheral membrane protein implicated in the targeting of hormones to secretory granules, co-aggregates with granule content proteins at acidic pH. J Biol Chem. 1998;273:31180–31185. doi: 10.1074/jbc.273.47.31180. [DOI] [PubMed] [Google Scholar]
- Stenberg PE, McEver RP, Shuman MA, Jacques YV, Bainton DF. A platelet alpha-granule membrane protein (GMP-140) is expressed on the plasma membrane after activation. J Cell Biol. 1985;101:880–886. doi: 10.1083/jcb.101.3.880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stinchcombe JC, Nomoto H, Cutler DF, Hopkins CR. Anterograde and retrograde traffic between the rough endoplasmic reticulum and the Golgi complex. J Cell Biol. 1995;131:1387–1401. doi: 10.1083/jcb.131.6.1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiele C, Gerdes HH, Huttner WB. Protein secretion: puzzling receptors. Curr Biol. 1997;7:R496–R500. doi: 10.1016/s0960-9822(06)00247-8. [DOI] [PubMed] [Google Scholar]
- Thiele C, Huttner WB. Protein and lipid sorting from the trans-Golgi network to secretory granules-recent developments. Semin Cell Dev Biol. 1998;9:511–516. doi: 10.1006/scdb.1998.0259. [DOI] [PubMed] [Google Scholar]
- Tooze SA. Biogenesis of secretory granules in the trans-Golgi network of neuroendocrine and endocrine cells. Biochim Biophys Acta. 1998;1404:231–244. doi: 10.1016/S0167-4889(98)00059-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tooze J, Burke B. Accumulation of adrenocorticotropin secretory granules in the midbody of telophase AtT20 cells: evidence that secretory granules move anterogradely along microtubules. J Cell Biol. 1987;104:1047–1057. doi: 10.1083/jcb.104.4.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vischer UM, Wagner DD. CD63 is a component of Weibel-Palade bodies of human endothelial cells. Blood. 1993;82:1184–1191. [PubMed] [Google Scholar]
- Vischer UM, Wagner DD. von Willebrand factor proteolytic processing and multimerisation precede the formation of Weibel-Palade bodies. Blood. 1994;83:3536–3544. [PubMed] [Google Scholar]
- von Ruden L, Garcia AG, Lopez MG. The mechanism of Ba(2+)-induced exocytosis from single chromaffin cells. FEBS Lett. 1993;336:48–52. doi: 10.1016/0014-5793(93)81606-z. [DOI] [PubMed] [Google Scholar]
- von Ruden L, Neher E. A Ca-dependent early step in the release of catecholamines from adrenal chromaffin cells. Science. 1993;262:1061–1065. doi: 10.1126/science.8235626. [DOI] [PubMed] [Google Scholar]
- Voorberg J, Fontijn R, Calafat J, Janssen H, van Mourik JA, Pannekoek H. Biogenesis of von Willebrand factor-containing organelles in heterologous transfected CV-1 cells. EMBO J. 1993;12:749–758. doi: 10.1002/j.1460-2075.1993.tb05709.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner DD, Saffaripour S, Bonfanti R, Sadler JE, Cramer EM, Chapman B, Mayadas TN. Induction of specific storage organelles by von Willebrand factor propolypeptide. Cell. 1991;64:403–413. doi: 10.1016/0092-8674(91)90648-i. [DOI] [PubMed] [Google Scholar]
- Weibel ER, Palade GE. New cytoplasmic components in arterial endothelia. J Cell Biol. 1964;23:101–112. doi: 10.1083/jcb.23.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang Y, Molloy SS, Thomas L, Thomas G. The PC6B cytoplasmic domain contains two acidic clusters that direct sorting to distinct trans-Golgi network/endosomal compartments. Mol Biol Cell. 2000;11:1257–1273. doi: 10.1091/mbc.11.4.1257. [DOI] [PMC free article] [PubMed] [Google Scholar]